212Pb is a promising radionuclide for targeted alpha particle therapy for cancer. Ongoing preclinical and clinical studies are investigating the potential of 212Pb-labeled peptides and antibodies [1–5]. PSC-PEG2-TOC (VMT-α-NET) is a novel somatostatin receptor subtype 2 (SSTR2) targeting peptide for the treatment of neuroendocrine tumors (NET) that shows rapid tumor accumulation, high tumor retention, and fast renal excretion with the potential for low nephrotoxicity [6, 7].
Here, we present the case of a 75-year-old woman with an advanced G2 NET of unknown primary with liver metastases who was heavily pretreated with somatostatin analogs, various chemotherapies, multiple cycles of [177Lu]Lu-DOTA-TATE and [225Ac]Ac-DOTA-TATE, and radioembolization over 7 years. The patient received 90 MBq of [212Pb]Pb-VMT-α-NET intravenously. Whole-body scintigraphy and SPECT/CT acquisitions were performed 2, 5, and 19 h after injection on a Symbia Intevo 6 (Siemens Healthineers) using high-energy collimators. Images were obtained by detection of the characteristic X-ray emissions of 212Pb using an energy window at 79 keV (40% width). The whole-body scan speed was 8 cm/min, and SPECT/CT scans were acquired with 120 projections (60 per detector, 30 s per projection) over a non-circular 360° orbit. The SPECT/CT images showed a high accumulation of [212Pb]Pb-VMT-α-NET in liver metastases in segments III and IV, consistent with the previously acquired [68Ga]Ga-DOTA-TATE PET/CT. High tumor retention can be observed in the planar and SPECT/CT images over time. The planar images showed a high level of background noise due to down-scatter and septal penetration of high-energy photon emissions from 212Pb daughter nuclides (e.g., 2.6 MeV from 208Tl). Due to the short half-life of 212Pb (10.6 h), the images acquired after 19 h showed a relatively high level of image noise due to the low count statistics. The patient showed no early or acute adverse events.
These are the first clinical post-treatment scintigraphic images of [212Pb]Pb-VMT-α-NET and additionally the first-in-human SPECT/CT images of 212Pb.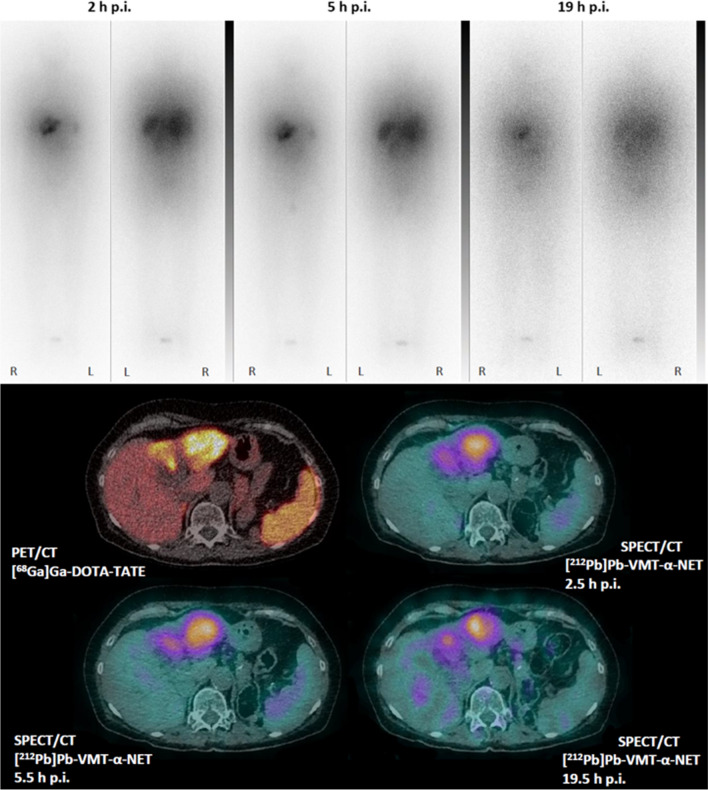
Acknowledgements
The 212Pb isotope generator for the production of the radiopharmaceutical used in this study was provided by Perspective Therapeutics, Inc.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
The data of this study are available on reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from the patient for the treatment procedure and for data publication.
Conflict of interest
The author Michael K. Schultz is the CSO (Chief Science Officer) of Perspective Therapeutics, Inc. The other authors declare no other competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Enrico Michler and David Kästner contributed equally to this work.
References
- 1.Li M, Sagastume EA, Lee D, McAlister D, DeGraffenreid AJ, Olewine KR, et al. 203/212Pb theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Curr Med Chem. 2020 doi: 10.2174/0929867327999200727190423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delpassand ES, Tworowska I, Esfandiari R, Torgue J, Hurt J, Shafie A, et al. Targeted α-emitter therapy with 212Pb-DOTAMTATE for the treatment of metastatic SSTR-expressing neuroendocrine tumors: first-in-humans dose-escalation clinical trial. J Nucl Med. 2022 doi: 10.2967/jnumed.121.263230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Durand-Panteix S, Monteil J, Sage M, Garot A, Clavel M, Saidi A, et al. Preclinical study of 212Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma. Br J Cancer. 2021 doi: 10.1038/s41416-021-01585-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapeau D, Koustoulidou S, Handula M, Beekman S, de Ridder C, Stuurman D, et al. [212Pb]Pb-eSOMA-01: a promising radioligand for targeted alpha therapy of neuroendocrine tumors. Pharmaceuticals. 2023 doi: 10.3390/ph16070985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Huang T, Hua J, Wang Q, Su Y, Chen P, et al. CD46 targeted 212Pb alpha particle radioimmunotherapy for prostate cancer treatment. J Exp Clin Cancer Res. 2023 doi: 10.1186/s13046-023-02636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller D, Herrmann H, Schultz MK, Solbach C, Ettrich T, Prasad V. 203Pb-VMT-α-NET scintigraphy of a patient with neuroendocrine tumor. Clin Nucl Med. 2023 doi: 10.1097/RLU.0000000000004464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Baumhover NJ, Liu D, Cagle BS, Boschetti F, Paulin G, et al. Preclinical evaluation of a lead specific chelator (PSC) conjugated to radiopeptides for 203Pb and 212Pb-based theranostics. Pharmaceutics. 2023 doi: 10.3390/pharmaceutics15020414. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study are available on reasonable request.


