Abstract
HVEM (for herpesvirus entry mediator) is a member of the tumor necrosis factor receptor superfamily and mediates entry of many strains of herpes simplex virus (HSV) into normally nonpermissive Chinese hamster ovary (CHO) cells. We used sucrose density centrifugation to demonstrate that purified HSV-1 KOS virions bind directly to a soluble, truncated form of HVEM (HVEMt) in the absence of any other cell-associated components. Therefore, HVEM mediates HSV entry by serving as a receptor for the virus. We previously showed that soluble, truncated forms of HSV glycoprotein D (gDt) bind to HVEMt in vitro. Here we show that antibodies specific for gD, but not the other entry glycoproteins gB, gC, or the gH/gL complex, completely block HSV binding to HVEM. Thus, virion gD is the principal mediator of HSV binding to HVEM. To map sites on virion gD which are necessary for its interaction with HVEM, we preincubated virions with gD-specific monoclonal antibodies (MAbs). MAbs that recognize antigenic sites Ib and VII of gD were the only MAbs which blocked the HSV-HVEM interaction. MAbs from these two groups failed to coprecipitate HVEMt in the presence of soluble gDt, whereas the other anti-gD MAbs coprecipitated HVEMt and gDt. Previous mapping data indicated that site VII includes amino acids 11 to 19 and site Ib includes 222 to 252. The current experiments indicate that these sites contain residues important for HSV binding to HVEM. Group Ib and VII MAbs also blocked HSV entry into HVEM-expressing CHO cells. These results suggest that the mechanism of neutralization by these MAbs is via interference with the interaction between gD in the virus and HVEM on the cell. Group Ia and II MAbs failed to block HSV binding to HVEM yet still neutralized HVEM-mediated entry, suggesting that these MAbs block entry at a step other than HVEM binding.
The envelope of herpes simplex virus (HSV) contains at least 10 virus-encoded glycoproteins (52). The initial adsorption of HSV to glycosaminoglycan chains (GAGs) of cell surface proteoglycans is mediated by glycoprotein C (gC) and/or gB (23, 24, 58). This is followed by the interaction of gD with cellular receptors (5, 28, 29, 31, 55). Then, gB, gD, and the complex of gH and gL (gH/gL) act individually or in combination to trigger pH-independent fusion of the viral envelope with the host cell plasma membrane (52).
Recently, expression cloning was used to isolate and identify a HeLa cell gene product, which when expressed in normally nonpermissive Chinese hamster ovary (CHO) cells, allows for entry of many HSV strains (35). The gene product, called HVEM (for herpes virus entry mediator) is a 283-amino-acid type I integral membrane protein and is a member of the tumor necrosis factor receptor superfamily (1, 26, 30, 33, 35, 50). HSV-1 variants rid1 and ANG have changes in gD sequence (11) and infect HVEM-expressing cells with markedly reduced efficiency, suggesting that HVEM might interact directly with gD (35).
Subsequently, we demonstrated that soluble, truncated gD (gDt) from the KOS strain binds directly to a soluble, truncated form of HVEM (HVEMt) in vitro (55). This binding is dependent on the native conformation of gD but is independent of its asparagine-linked oligosaccharides. Soluble gDt from the rid1 or ANG strains was unable to bind to HVEMt. Thus, the inability of rid1 and ANG to infect HVEM-expressing cells may be due to the failure of these virion gDs to bind to HVEM on the cell. A variant gD protein, gD-1(Δ290–299t), showed enhanced binding to HVEMt relative to the binding of wild-type gDt (55, 57). Also, gD-1(Δ290–299t) and wild-type gDt competed for binding to HVEMt.
It is well established that gD can induce potent virus-neutralizing antibodies (Abs) (7, 9, 25, 34, 40, 43, 49). Monoclonal Abs (MAbs) against gD have been demonstrated to block a postadsorption step in virus entry prior to virus-cell fusion (20, 21, 25, 52). Although the role of gD as a receptor binding protein has been well documented (3, 5, 28, 29, 31, 55), blocking of virion gD binding to a specific cellular receptor has not been demonstrated as a mechanism of neutralization.
The purpose of this study was to characterize the interaction between the intact virus and HVEM and to begin to identify regions of gD which are important for binding to HVEM. Here we demonstrate that (i) HSV virions bind specifically and directly to HVEMt, providing formal proof that HVEM is a viral receptor; (ii) anti-gD Abs completely block the interaction between the virus and HVEM; (iii) specific antigenic sites on gD (Ib and VII) contain residues important for HVEM binding; and (iv) group Ib and VII MAbs neutralize HSV entry into HVEM-expressing CHO cells, suggesting they may neutralize entry by blocking virus binding to a gD-specific receptor.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (Vero) cells were grown in Dulbecco’s minimal essential medium supplemented with 5% fetal calf serum (FCS) at 37°C. Sf9 (Spodoptera frugiperda) cells (GIBCO BRL) used for producing recombinant baculoviruses and recombinant proteins were propagated in Sf900II medium (GIBCO BRL) (56). CHO-K1 cells expressing HVEM are designated CHO(250-2) (58a) and were grown in Ham’s F-12 medium (BioWhittaker) supplemented with 10% FCS and neomycin (250 μg/ml). HSV-1 strains KOS, KOS(rid1) (11) (abbreviated rid1), and KOS(tk12) (which contains the Escherichia coli lacZ gene in place of the HSV thymidine kinase gene [53a]) were grown on Vero cells, and titers were determined.
Production and purification of baculovirus-produced soluble proteins.
HVEM is 283 amino acids in length (35). A soluble form of HVEM truncated just prior to the transmembrane region (HVEMt) was produced from recombinant baculovirus-infected insect cells and purified by nickel-affinity chromatography as described previously (55). HSV-1 gD is 369 amino acids in length (54). Baculovirus-derived gD-1(Δ290–299t) (referred to as gDt in this study) is truncated at residue 306 just prior to the transmembrane region; has amino acids 290 to 299 deleted, with R replacing I at residue 290; and has amino acids KIFL inserted after R (6, 38, 39). gD-1(Δ290–299t) was purified by immunoaffinity chromatography with MAb DL6 (39, 56). This form of gD competes with wild-type gD for binding to the same site on HVEM and is used in this study because of its enhanced binding activity (55, 57).
Polyclonal Abs and MAbs.
Anti-gD (R7) (27), anti-VP5 (NC1) (10), and anti-HVEM (R140) polyclonal rabbit sera were used for Western blotting. The following anti-gD MAbs were used for immunoprecipitations and/or blocking of virus binding: HD1 (group Ia) (36, 43), LP2 (group Ia) (34), DL11 (group Ib) (8, 36), 114-4 (group Ib) (40), ABD (group III) (46), 99-1 (group III) (40), 11S (group III) (49), and DL2 (group VI) (8), which recognize discontinuous epitopes; MAbs DL6 (group II) (15, 27), 1D3 (group VII) (19), LP14 (group VII) (2, 34), 110S (group VII) (49), and H170 (group VII) (12, 42) recognize continuous epitopes. Anti-gB, gC, and gH-gL Abs were also used for blocking of virus binding. The anti-gB MAbs used were SS10, DL16, and DL21 (44), along with polyclonal rabbit serum R69 (17). The anti-gC MAbs used were 1C8 (19), MP1, and MP5 (45), along with polyclonal rabbit serum R46 (17). The anti-gH/gL MAbs used were LP11 (4), 53S (49), and H6 (13), along with polyclonal rabbit serum R137 (41).
SDS-PAGE analysis.
Samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and analyzed by Western blotting as described previously (55).
Virus binding assay.
Sucrose gradient-purified (22) HSV-1 KOS or rid1 virions (107 PFU) were incubated with 150 μg of HVEMt at 4°C for 2 h. Samples were layered onto a 10%-30%-60% sucrose–phosphate-buffered saline step gradient and centrifuged for 4.5 h at 16,000 × g with an SW41 swinging bucket rotor (Beckman). The virus-containing band at the 30%-60% interface was collected via side puncture and then concentrated by centrifugation for 1 h at 35,000 × g with an SW50.1 swinging bucket rotor (Beckman). The pellets were dissolved in SDS-sample buffer, separated on SDS–12% polyacrylamide gels, and transferred to nitrocellulose. Western blots were probed with NC1 and R7 to detect virion proteins VP5 and gD, respectively, and R140 was used to detect HVEM.
Immunoprecipitation of the gD-HVEM complex.
Fifty-microliter reactions containing 3 μg of gD-1(Δ290–299t) and 16 μg of HVEMt per ml were incubated in binding buffer (10 mM Tris [pH 8.0], 100 mM NaCl, 0.1% Nonidet P-40, 0.05% bovine serum albumin [BSA], 0.05% chicken egg albumin) on ice for 1 h. MAb ascites (0.1 μl) was added for 1 h, followed by 50 μl of protein A-agarose (GIBCO BRL) (50 mg/ml) for 1 h. Bound material was collected by centrifugation at 13,000 × g for 3 min. Pellets were washed four times with high-salt buffer (10 mM Tris [pH 8.0], 500 mM NaCl, 0.1% Nonidet P-40, 0.05% BSA, 0.05% chicken egg albumin) and then boiled in SDS sample buffer for 3 min. Following SDS-PAGE (12% polyacrylamide), Western blots were probed with R7 and R140.
Neutralization of virus entry.
Sucrose gradient-purified HSV-1 KOS(tk12) was incubated with twofold dilutions of different MAb immunoglobulin G (IgG) for 1 h at 37°C. Confluent CHO(250-2) cell monolayers on 96-well plates were infected with virus-Ab mixtures (4 × 104 PFU per well or a multiplicity of infection of approximately 1) for 1 h at 4°C and then shifted to 37°C for 7 h to allow for virus entry. Cell lysates (0.1% Nonidet P-40 in Ham’s F-12 medium) were prepared, CPRG (chlorophenol red-β-d-galactopyranoside [Boehringer Mannheim]) substrate was then added, and β-galactosidase activity (milli-optical density units per minute) was read at 560 nm with a microtiter plate reader (Dynatech).
RESULTS
HSV binds directly to HVEM.
Three major pieces of evidence support the concept that HVEM interacts with HSV and, along with other cellular molecules such as GAGs, mediates virus entry: (i) by definition, HVEM mediates HSV entry into normally nonpermissive CHO cells (35); (ii) anti-HVEM serum can block the entry of virus into HVEM-expressing cells without much effect on the amount of virus that adsorbs to cells via GAGs (35); and (iii) soluble, truncated (HVEMt) forms of HVEM can block virus entry (35, 55). The latter suggests that soluble HVEM binds to the virus and competes with the cellular form of HVEM. However, direct binding of intact HSV virions to HVEM has not been demonstrated.
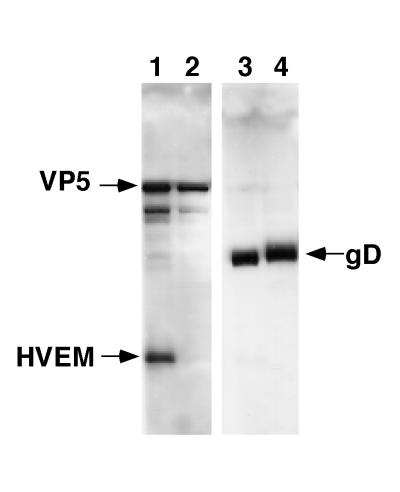
To directly detect virus-HVEM interactions, we tested the ability of soluble HVEMt to cosediment with purified HSV-1 KOS through a sucrose gradient. The virus band was collected from the 30 to 60% sucrose interface and analyzed by Western blotting. This fraction contained intact virions, as evidenced by the presence of both the major capsid protein VP5 (Fig. 1, lane 1) and envelope glycoprotein gD (Fig. 1, lane 3). HVEMt was also present in this fraction (lane 1), indicating that purified KOS virions associated with HVEMt. HVEMt was also found at the top of the gradient (not shown), indicating that only a portion of the added protein cosedimented with the virus. Since HSV-1 KOS binds directly to HVEMt, this is formal proof that HVEM mediates entry by serving as a receptor for the virus. The mutant KOS virus rid1 has a Gln27Pro mutation in gD (11) and does not infect HVEM-expressing CHO cells (35). Here we found that HVEMt did not cosediment with purified rid1 virions (Fig. 1, lanes 2 and 4). Thus, the inability of rid1 virus to utilize HVEM for entry (35) is due to a defect in virus binding to HVEM.
FIG. 1.
Cosedimentation of HSV-1 with soluble HVEMt. HVEMt (150 μg) and purified HSV-1 KOS (lanes 1 and 3) or rid1 (lanes 2 and 4) virions (107 PFU) were incubated for 2 h at 4°C and then passed through a 10%-30%-60% sucrose step gradient. The virus-containing band was collected from the 30%-60% sucrose interface and analyzed by SDS-PAGE (12% polyacrylamide) followed by Western blotting with anti-VP5 and anti-HVEM polyclonal Abs (lanes 1 and 2) or anti-gD polyclonal Ab (lanes 3 and 4). Secondary Abs were then added, and enhanced chemiluminescence was used to visualize the bands.
gD-specific antibodies block HSV binding to HVEM.
Previous studies showed that HVEM interacts with gD. For example, rid1 virions which contain a single-amino-acid change in gD (11) cannot utilize HVEM for entry (35) and, as shown in Fig. 1, cannot bind directly to HVEMt. Further, soluble gD binds directly to soluble HVEM in vitro (55). To determine which envelope glycoprotein or proteins mediate HSV-1 binding to HVEM, individual samples of purified KOS virions were pretreated with a cocktail of mouse MAbs and rabbit polyclonal Abs to either gB, gC, gD, or gH/gL. Virions were then incubated with HVEMt, and the mixtures were sedimented through a sucrose gradient. The virus band was recovered from each gradient and subjected to SDS-PAGE and Western blotting. The blots were probed with antibodies to both HVEM and VP5 as in the previous experiment. In each case, the control sample consisted of virus and soluble HVEM incubated in the absence of antibody (Fig. 2A and B, lanes 1). The results for two independent experiments are presented in Fig. 2. Abs to gB were not inhibitory in either experiment (Fig. 2A and B, lanes 2). In both experiments, anti-gD Abs efficiently blocked cosedimentation of HVEMt with HSV-1 as evidenced by the absence of HVEMt from the virus-containing fraction of the sucrose gradient in one experiment (Fig. 2A, lane 4) and the greatly reduced level of HVEM observed in a second experiment (Fig. 2B, lane 4). Because the virus-containing bands were removed by side puncture of the tube, there was some unavoidable variability in recovery. Thus, in one experiment (Fig. 2A, lane 5), there was less HVEM recovered in the sample incubated with anti-gH/gL antibodies. However, this sample contained less virus, as evidenced by the reduced amount in the VP5 band compared with that in the control (Fig. 2A, compare lanes 1 and 5). Moreover, this reduction was not seen in two repeat experiments, one of which is shown in Fig. 2A, lane 5. Variations in HVEM binding were also seen in samples incubated with anti-gC antibodies. In the first experiment there was more HVEM present (Fig. 2A, lane 3); however, in the second experiment, there appeared to be less HVEM (Fig. 2B, lane 3). Again, these variations appear to correlate with differences in the virus recovered. Our interpretation of the data is that virion gD is clearly the principal mediator of specific binding of HSV to HVEM.
FIG. 2.
Inhibition of HSV-1 binding to HVEMt by Abs specific for HSV envelope glycoproteins. Gels in panels A and B represent two separate experiments carried out in the same way. Purified HSV-1 KOS virions (107 PFU) were preincubated for 1 h at 37°C with the following Abs or left untreated (−). The Abs specific for gB were SS10 (0.5 μl of ascites), DL16 (5 μg of IgG), DL21 (5 μg of IgG), and R69 (0.5 μl of sera) (lane 2). The Abs specific for gC were MP1 (0.5 μl of ascites), MP5 (5 μg of IgG), 1C8 (5 μg of IgG), and R46 (0.5 μl of sera) (lane 3). The Abs specific for gD were 1D3 (0.5 μl of ascites), DL2 (5 μg of IgG), DL11 (5 μg of IgG), and R7 (0.5 μl of sera) (lane 4). The Abs specific for gH/gL were LP11 (0.5 μl of ascites), 53S (5 μg of IgG), H6 (5 μg of IgG), and R137 (0.5 μl of sera) (lane 5). Samples were then subjected to sedimentation with HVEMt as described in the legend to Fig. 1. Samples were analyzed by SDS-PAGE (12% polyacrylamide) followed by Western blotting with anti-VP5 and anti-HVEM polyclonal Abs. Secondary Abs were then added, and enhanced chemiluminescence was used to visualize the bands.
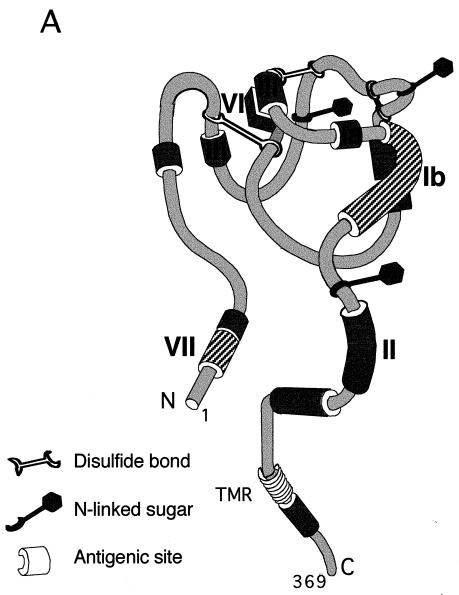
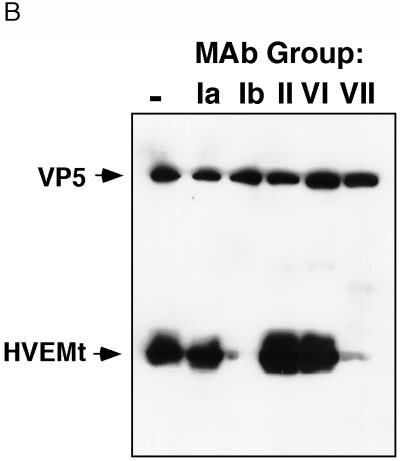
To identify regions of virion gD which are important for HVEM binding, individual samples of HSV-1 virions were pretreated with purified MAb IgGs that recognize distinct antigenic sites on the gD molecule (Fig. 3A), and then assayed for binding to purified HVEMt (Fig. 3B). MAbs HD1, DL6, and DL2, which recognize antigenic sites Ia, II, and VI, respectively (Fig. 3A) (8, 15, 27, 36, 43), did not inhibit cosedimentation of HVEMt with HSV virions (Fig. 3B). In contrast, anti-gD MAbs DL11 and 1D3, representing groups Ib and VII, respectively (8, 19, 36), effectively blocked the binding of HSV-1 KOS to HVEMt (Fig. 3B). These results suggest that antigenic sites Ib and VII on virion gD (Fig. 3A) overlap regions important for receptor binding.
FIG. 3.
Model of gD antigenic structure and inhibition of HSV-1 binding to HVEMt by a panel of anti-gD MAbs. (A) Hypothetical folded model of gD based on epitope mapping studies (37) and solution of the disulfide bond arrangement (32). Antigenic sites relevant to this study are indicated by Roman numerals. The approximate amino acid location of each site is as follows. Site Ia includes residues 216 to 234, site Ib includes residues 222 to 252, site II includes residues 272 to 279, site III includes residues 21 to 226, site VI includes residues 21 to 226, and site VII includes residues 11 to 19. Sites with hatch marks (Ib and VII) are important for HSV binding to HVEM. (B) Purified HSV-1 KOS virions were preincubated with 50 μg of the MAb IgGs HD1 (group Ia), DL11 (group Ib), DL6 (group II), DL2 (group VI), and 1D3 (group VII) or left untreated (lane 1) for 1 h at 37°C. Samples were then subjected to sedimentation with HVEMt as described in the legend to Fig. 1. Samples were analyzed by SDS-PAGE (12% polyacrylamide) followed by Western blotting with anti-VP5 and anti-HVEM polyclonal Abs. Secondary Abs were then added, and enhanced chemiluminescence was used to visualize the bands. TMR, transmembrane region.
Coimmunoprecipitation of gD and HVEM with anti-gD MAbs.
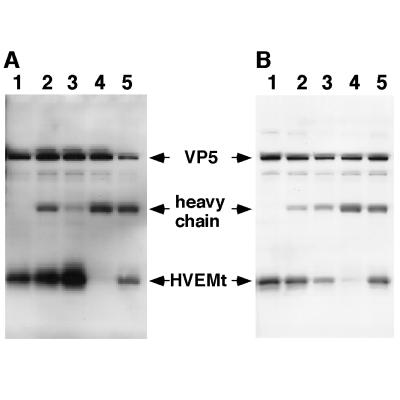
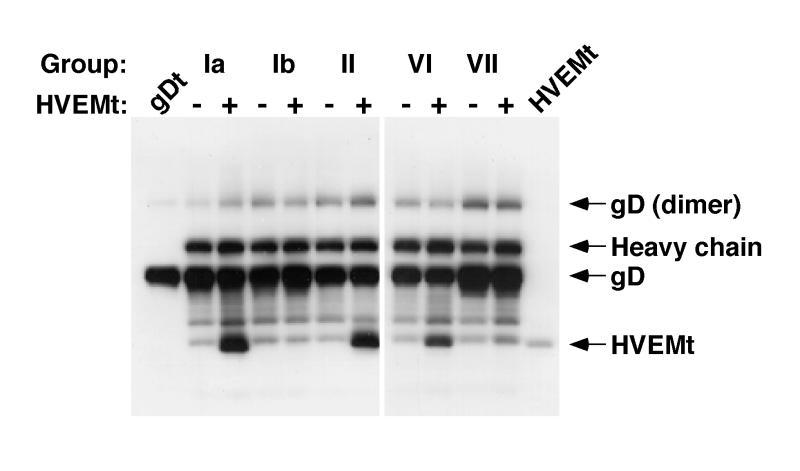
We previously showed by gel filtration chromatography that gDt binds to HVEMt in solution (55). Here, we used immunoprecipitation with a panel of anti-gD MAbs to map regions of gD important for the gD-HVEM interaction. We reasoned that an anti-gD MAb could only coimmunoprecipitate HVEMt with gDt if the MAb epitope on gD was not obscured as a result of the interaction with HVEM. When the MAbs were reacted with gDt in the absence of HVEMt (Fig. 4), 20% of the total available gD was precipitated (data not shown). When gDt was preincubated with HVEMt, MAbs from groups Ia, II, and VI coimmunoprecipitated the proteins (Fig. 4). This suggests that these sites on gD are accessible to the corresponding Ab when the glycoprotein is bound to HVEM. In contrast, MAbs that recognize sites Ib and VII (DL11 and 1D3, respectively) failed to precipitate the gD-HVEM complex (Fig. 4). This suggests that sites Ib and VII are inaccessible to Ab as a result of the interaction of gD with HVEM. Alternatively, these MAbs may disrupt the gD-HVEM complex.
FIG. 4.
Coimmunoprecipitation of HVEMt by a panel of anti-gD MAbs. Fifty-microliter reaction mixtures of 3 μg of gDt per ml in the presence (+) or absence (−) of 16 μg of HVEMt per ml were incubated for 1 h on ice. MAb ascites (0.1 μl) of HD1 (group Ia), DL11 (group Ib), DL6 (group II), DL2 (group VI), or 1D3 (group VII) were added for 1 h, followed by 50 μl of protein A-agarose (50 mg/ml) for 1 h. Bound material was collected by centrifugation at 13,000 × g for 3 min. Pellets were washed and then analyzed by SDS-PAGE (12% polyacrylamide). Western blots were probed with anti-gD and anti-HVEM polyclonal Abs. Secondary Abs were then added, and enhanced chemiluminescence was used to visualize the bands. Lane 1, gDt alone as a standard; lane 12, HVEMt alone as a standard.
Other MAbs that have been classified in this grouping scheme (14, 37) yielded results similar to the prototype MAb shown in Fig. 4 for a given group (not shown). We tested three other group VII MAbs (H170, LP14, and 110S) and one other group Ia (LP2) and Ib (114-4) MAb with similar results. Also, all group III MAbs tested, including ABD, 99-1, and 11S, also coprecipitated HVEMt and gDt (not shown). Together with the data showing the blocking of virus binding (Fig. 3B), these results support the idea that antigenic sites Ib and VII of gD (depicted in Fig. 3A) contain residues which are important for HSV binding to HVEM.
Neutralization of HSV entry into HVEM-expressing cells.
Many anti-gD MAbs neutralize virus to high titers in the absence of complement (reviewed in reference 37). For example, the group Ia, Ib, and VII antibodies are potent neutralizers of HSV infection of cultured cells (14, 25, 34, 37, 40, 43, 49). Interestingly, only the group Ib and VII MAbs blocked virus association with HVEM in vitro (Fig. 3B). We next determined whether the ability of a specific MAb to block HSV binding to HVEM correlated with its ability to neutralize virus entry into HVEM-expressing CHO cells. The CHO cell line used for these experiments, designated CHO(250-2), expresses HVEM constitutively. As reported for the HVEM-expressing CHO cell line A12 (35), CHO(250-2) cells allow entry of many HSV strains, but not the rid1 or ANG variants (58a).
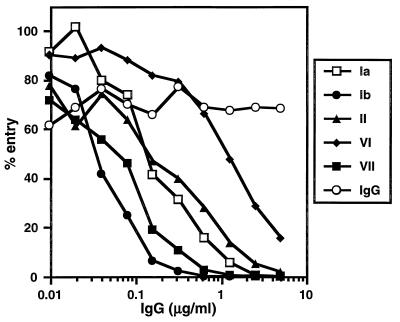
MAbs DL11 (group Ib) and 1D3 (group VII) were effective in neutralizing HSV-1 KOS(tk12) entry into CHO(250-2) cells (Fig. 5). Since antibodies in both of these groups blocked binding of soluble receptor to virus, it is possible that they block virus entry by blocking receptor binding. In contrast, DL2, a group VI MAb, was unable to block virus entry at low concentrations and only partially inhibited entry at concentrations above 1 μg/ml (Fig. 5). Interestingly, HD1 (group Ia) and DL6 (group II) MAbs neutralized virus entry, even though they failed to block binding of soluble receptor to the virus. One possibility is that these MAbs neutralized entry into the transformed CHO cells at a step other than HVEM binding. It is also possible that MAbs in groups Ib and VII block virus entry into HVEM-expressing CHO cells at both the receptor binding step as well as at a subsequent step.
FIG. 5.
Neutralization of HSV-1 entry by anti-gD MAb IgG. Purified HSV-1 KOS(tk12) was incubated with twofold dilutions of the MAb IgGs HD1 (group Ia), DL11 (group Ib), DL6 (group II), DL2 (group VI), and 1D3 (group VII) or nonimmune mouse IgG for 1 h at 37°C. Confluent CHO(250-2) cell monolayers on 96-well plates were infected with virus-Ab mixtures (4 × 104 PFU per well) for 1 h at 4°C and then shifted to 37°C for 7 h to allow for virus entry. Nonidet P-40 (0.1%) cell lysates were prepared, and then substrate was added, and β-galactosidase activity (milli-optical density units per minute) was read at 560 nm. One hundred percent entry corresponds to β-galactosidase activity in the absence of IgG. Each point represents the average of duplicate wells. Shown are the results of one representative experiment. The experiment was repeated three times with similar results.
DISCUSSION
HVEM is a receptor for HSV.
The first step in HSV entry is attachment to GAGs on the cell surface, mediated principally by gC (24, 58). This interaction, although not strictly required for entry, enhances infectivity (20, 24, 48, 51, 53), perhaps by enhancing binding to a subsequent receptor and/or by enhancing virus-cell fusion (47, 51). Following attachment, the virus binds more stably to a gD-specific receptor (5, 20, 28, 29, 31). Montgomery et al. (35) propose that GAGs and cell surface proteins such as HVEM are cofactors required for virus binding and entry into cells. Here we show that HSV-1 binds directly to HVEM in the absence of any other cellular factors, indicating it is a bona fide virus receptor. In the context of the cell, however, a role for GAGs in facilitating binding to HVEM cannot be excluded.
Anti-HVEM Ab inhibits HSV entry into human lymphocytes (35), so it is likely that HVEM functions as a receptor on these cells. Since infection of these cells is not blocked completely (35), HVEM may not be strictly required for entry into lymphocytes. Alternatively, when HVEM is blocked the virus may use another receptor. In contrast, HVEM is required for entry into CHO cells that express HVEM (35), although it is not clear if HSV-GAG and HSV-HVEM interactions are sufficient for entry into these cells or if other unidentified cellular factors are also required. The relationship between HSV binding to a gD-specific receptor, such as HVEM, and subsequent penetration into the host cell needs to be examined. Also, the specific roles of gB, gD, and gH/gL in the penetration (fusion) process remain to be addressed.
Regions of gD important for HSV-HVEM binding.
Purified gDt has been shown to bind HVEMt in vitro, indicating that gD is a ligand for HVEM (55). Abs to gD, but not gB, gC, or gH/gL, completely blocked HSV-HVEMt binding. In addition, HVEMt can be chemically cross-linked to gD in the virion (unpublished data). Thus, HSV-1 binding to HVEM is principally and specifically mediated by virion gD. Several groups have investigated the effect of specific anti-gD MAbs on HSV entry (reviewed in reference 16). For example, MAbs 114-4 and 174-1 (40) (group Ib [36, 37]) are potent neutralizers of HSV infection (40) and block virus entry into HEp-2 cells at a step after adsorption and prior to virus-cell fusion (21). Also, Highlander et al. (25) proposed that MAbs D1 (group VI [37]) and D2 (group Ib [36]) neutralized HSV entry into Vero cells at a step prior to viral penetration.
In the current study, most anti-gD MAbs tested did not block HSV-1 binding to HVEMt. However, MAbs against site Ib or site VII effectively blocked virus binding to HVEMt. The same MAbs failed to coprecipitate HVEMt in the presence of gD. The epitopes of the prototype MAbs for group Ib (DL11 [8]) and group VII (1D3 [19]) include gD amino acids 222 to 252 (36, 37a) and 11 to 19, respectively. We propose that these epitopes share residues in common with receptor binding sites on gD. Identification of these regions is a useful first step in finer mapping of HVEM-binding sites. The VII and Ib epitopes overlap or are adjacent to functional regions I and III of gD (amino acids 27 to 43 and 234 to 244, respectively) which are important for virus entry into Vero cells (6, 18). This suggests that HVEM and the Vero cell receptor(s) may bind to similar sites on gD.
HSV-1 MAb-resistant (mar) mutants selected in the presence of neutralizing Abs against sites Ia, Ib, and VII (25, 34, 36) were tested for the ability to infect HVEM-expressing cells. All mar mutants tested were capable of utilizing HVEM for entry (unpublished data). Thus, while the binding sites on gD for Ib and VII MAbs overlap those of HVEM, these sites are not identical. We previously argued that antigenic site Ib overlapped a region of gD important for virus entry into Vero cells (36). Results presented here are consistent with the notion that site Ib is important for HVEM binding and subsequent entry into HVEM-expressing cells. We also demonstrate that the inability of HSV-1 rid1 to infect HVEM-expressing CHO cells (35) is due to a defect in receptor binding. Thus, the site of the rid1 mutation in gD (amino acid 27 [11]) is also important for HSV interaction with HVEM (references 35, 38, and 55 and this work).
Blocking of receptor binding as a mechanism of HSV neutralization.
We tested the ability of anti-gD MAbs to block HSV-1 entry into an HVEM-expressing cell line, CHO(250-2). Group Ib and VII MAbs neutralized HVEM-mediated entry of HSV-1 KOS into CHO(250-2) cells. We propose that these MAbs neutralize HSV infection via blocking of virion gD binding to a specific cellular receptor, in this case, HVEM. Interestingly, the group Ia and II MAbs also blocked KOS entry into CHO(250-2) cells, yet failed to block virus binding to HVEM. These MAbs likely interfere with gD function in HSV entry into the transformed CHO cells at a step other than HVEM binding, possibly by inhibiting receptor-induced conformational change and/or virus-cell fusion. It is also possible that MAbs in group Ib and VII interfere with these steps as well. It remains to be determined whether these Abs block entry of HSV into normally permissive cells at the same step or steps of gD function. It should be noted that many of these MAbs are able to neutralize infection of ANG and rid1 viruses on cell types such as Vero (11), in spite of the fact that these viruses cannot use HVEM for entry. This predicts that similar regions of gD may be involved in interaction with both HVEM and other cell surface molecules that permit virus entry.
ACKNOWLEDGMENTS
This investigation was supported by Public Health Service grants AI-18289 (G.H.C. and R.J.E.), AI-07325 (A.V.N.), AI-36293 (P.G.S.), and AI-09022 (R.I.M.) from the National Institute of Allergy and Infectious Diseases; NS-36731 and NS-30606 (R.J.E. and G.H.C.) from the National Institute of Neurological Diseases and Stroke; and DE-08239 (G.H.C. and R.J.E.) from the National Institute of Dental Research.
We thank C. Desgranges, H. Friedman, A. Minson, L. Pereira, and M. Zweig for supplying antibodies.
REFERENCES
- 1.Baker S J, Reddy E P. Transducers of life and death: TNF receptor superfamily and associated proteins. Oncogene. 1996;12:1–9. [PubMed] [Google Scholar]
- 2.Bosch D L, Geerligs H J, Weijer W J, Feijlbrief M, Welling G W, Welling-Wester S. Structural properties and reactivity of N-terminal synthetic peptides of herpes simplex virus type 1 glycoprotein D by using antipeptide antibodies and group VII monoclonal antibodies. J Virol. 1987;61:3607–3611. doi: 10.1128/jvi.61.11.3607-3611.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunetti C R, Burke R L, Kornfeld S, Gregory W, Masiarz F R, Dingwell K S, Johnson D C. Herpes simplex virus glycoprotein D acquires mannose 6-phosphate residues and binds to mannose 6-phosphate receptors. J Biol Chem. 1994;269:17067–17074. [PubMed] [Google Scholar]
- 4.Buckmaster E A, Gompels U, Minson A. Characterisation and physical mapping of an HSV-1 glycoprotein of approximately 115 × 10(3) molecular weight. Virology. 1984;139:408–413. doi: 10.1016/0042-6822(84)90387-8. [DOI] [PubMed] [Google Scholar]
- 5.Campadelli-Fiume G, Arsenakis M, Farabegoli F, Roizman B. Entry of herpes simplex virus 1 in BJ cells that constitutively express viral glycoprotein D is by endocytosis and results in degradation of the virus. J Virol. 1988;62:159–167. doi: 10.1128/jvi.62.1.159-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang H-Y, Cohen G H, Eisenberg R J. Identification of functional regions of herpes simplex virus glycoprotein gD by using linker-insertion mutagenesis. J Virol. 1994;68:2529–2543. doi: 10.1128/jvi.68.4.2529-2543.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen G, Ponce de Leon M, Nichols C. Isolation of a herpes simplex virus-specific antigenic fraction which stimulates the production of neutralizing antibody. J Virol. 1972;10:1021–1030. doi: 10.1128/jvi.10.5.1021-1030.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen G H, Isola V J, Kuhns J, Berman P W, Eisenberg R J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing (“native” gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986;60:157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen G H, Muggeridge M I, Long D, Sodora D A, Eisenberg R J. Structural and functional studies of herpes simplex virus glycoprotein D. In: Ciardi J E, editor. Genetically engineered vaccines. New York, N.Y: Plenum Press; 1992. pp. 217–228. [DOI] [PubMed] [Google Scholar]
- 10.Cohen G H, Ponce de Leon M, Diggelmann H, Lawrence W C, Vernon S K, Eisenberg R J. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J Virol. 1980;34:521–531. doi: 10.1128/jvi.34.2.521-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dean H J, Terhune S S, Shieh M, Susmarski N, Spear P G. Single amino acid substitutions in gD of herpes simplex virus 1 confer resistance to gD-mediated interference and cause cell-type-dependent alterations in infectivity. Virology. 1994;199:67–80. doi: 10.1006/viro.1994.1098. [DOI] [PubMed] [Google Scholar]
- 12.Dietzschold B, Eisenberg R J, Ponce de Leon M, Golub E, Hudecz F, Varrichio A, Cohen G H. Fine structure analysis of type-specific and type-common antigenic sites of herpes simplex virus glycoprotein D. J Virol. 1984;52:431–435. doi: 10.1128/jvi.52.2.431-435.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubin G, Jiang H. Expression of herpes simplex virus type 1 glycoprotein L (gL) in transfected mammalian cells: evidence that gL is not independently anchored to cell membranes. J Virol. 1995;69:4564–4568. doi: 10.1128/jvi.69.7.4564-4568.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg R J, Long D, Pereira L, Hampar B, Zweig M, Cohen G H. Effect of monoclonal antibodies on limited proteolysis of native glycoprotein gD of herpes simplex virus type 1. J Virol. 1982;41:478–488. doi: 10.1128/jvi.41.2.478-488.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg R J, Long D, Ponce de Leon M, Matthews J T, Spear P G, Gibson M G, Lasky L A, Berman P, Golub E, Cohen G H. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J Virol. 1985;53:634–644. doi: 10.1128/jvi.53.2.634-644.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eisenberg R J, Long D, Sodora D L, Chiang H-Y, Wilcox W C, Abrams W R, Muggeridge M I, Cohen G H. Structure and function of glycoprotein D of herpes simplex virus. In: Becker Y, Darai G, editors. Frontiers in virology. Vol. 3. Heidelberg, Germany: Springer-Verlag; 1994. pp. 43–65. [Google Scholar]
- 17.Eisenberg R J, Ponce de Leon M, Friedman H M, Fries L F, Frank M M, Hastings J C, Cohen G H. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb Pathog. 1987;3:423–435. doi: 10.1016/0882-4010(87)90012-x. [DOI] [PubMed] [Google Scholar]
- 18.Feenstra V, Hodaie M, Johnson D C. Deletions in herpes simplex virus glycoprotein D define nonessential and essential domains. J Virol. 1990;64:2096–2102. doi: 10.1128/jvi.64.5.2096-2102.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman H M, Cohen G H, Eisenberg R J, Seidel C A, Cines D B. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature (London) 1984;309:633–635. doi: 10.1038/309633a0. [DOI] [PubMed] [Google Scholar]
- 20.Fuller A O, Lee W-C. Herpes simplex virus type 1 entry through a cascade of virus-cell interactions requires different roles of gD and gH in penetration. J Virol. 1992;66:5002–5012. doi: 10.1128/jvi.66.8.5002-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuller A O, Spear P G. Anti-glycoprotein D antibodies that permit adsorption but block infection by herpes simplex virus 1 prevent virion-cell fusion at the cell surface. Proc Natl Acad Sci USA. 1987;84:5454–5458. doi: 10.1073/pnas.84.15.5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Handler C G, Eisenberg R J, Cohen G H. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J Virol. 1996;70:6067–6075. doi: 10.1128/jvi.70.9.6067-6070.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herold B C, Visalli R J, Sumarski N, Brandt C, Spear P G. Glycoprotein C-independent binding of herpes simplex virus to cells requires cell surface heparan sulfate and glycoprotein B. J Gen Virol. 1994;75:1211–1222. doi: 10.1099/0022-1317-75-6-1211. [DOI] [PubMed] [Google Scholar]
- 24.Herold B C, WuDunn D, Soltys N, Spear P G. Glycoprotein C of herpes simplex virus type 1 plays a principal role in the adsorption of virus to cells and in infectivity. J Virol. 1991;65:1090–1098. doi: 10.1128/jvi.65.3.1090-1098.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Highlander S L, Sutherland S L, Gage P J, Johnson D C, Levine M, Glorioso J C. Neutralizing monoclonal antibodies specific for herpes simplex virus glycoprotein D inhibit virus penetration. J Virol. 1987;61:3356–3364. doi: 10.1128/jvi.61.11.3356-3364.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu S, Solovyev I, Colombero A, Elliott R, Kelley M, Boyle W J. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J Biol Chem. 1997;272:13471–13474. doi: 10.1074/jbc.272.21.13471. [DOI] [PubMed] [Google Scholar]
- 27.Isola V J, Eisenberg R J, Siebert G R, Heilman C J, Wilcox W C, Cohen G H. Fine mapping of antigenic site II of herpes simplex virus glycoprotein D. J Virol. 1989;63:2325–2334. doi: 10.1128/jvi.63.5.2325-2334.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson D C, Burke R L, Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990;64:2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson D C, Ligas M W. Herpes simplex viruses lacking glycoprotein D are unable to inhibit virus penetration: quantitative evidence for virus-specific cell surface receptors. J Virol. 1988;62:4605–4612. doi: 10.1128/jvi.62.12.4605-4612.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwon B S, Tan K B, Ni J, Kwi-Ok-Oh, Lee Z H, Kim K K, Kim Y-J, Wang S, Gentz R, Yu G-L, Harrop J, Lyn S D, Silverman C, Porter T G, Truneh A, Young P R. A newly identified member of the tumor necrosis factor receptor superfamily with a wide tissue distribution and involvement in lymphocyte activation. J Biol Chem. 1997;272:14272–14276. doi: 10.1074/jbc.272.22.14272. [DOI] [PubMed] [Google Scholar]
- 31.Lee W C, Fuller A O. Herpes simplex virus type 1 and pseudorabies virus bind to a common saturable receptor on Vero cells that is not heparan sulfate. J Virol. 1993;67:5088–5097. doi: 10.1128/jvi.67.9.5088-5097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long D, Wilcox W C, Abrams W R, Cohen G H, Eisenberg R J. Disulfide bond structure of glycoprotein D of herpes simplex virus types 1 and 2. J Virol. 1992;66:6668–6685. doi: 10.1128/jvi.66.11.6668-6685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marsters S A, Ayres T M, Skubatch M, Gray C L, Rothe M, Ashkenazi A. Herpes virus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 34.Minson A C, Hodgman T C, Digard P, Hancock D C, Bell S E, Buckmaster E A. An analysis of the biological properties of monoclonal antibodies against glycoprotein D of herpes simplex virus and identification of amino acid substitutions that confer resistance to neutralization. J Gen Virol. 1986;67:1001–1013. doi: 10.1099/0022-1317-67-6-1001. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 36.Muggeridge M I, Isola V J, Byrn R A, Tucker T J, Minson A C, Glorioso J C, Cohen G H, Eisenberg R J. Antigenic analysis of a major neutralization site of herpes simplex virus glycoprotein D, using deletion mutants and monoclonal antibody-resistant mutants. J Virol. 1988;62:3274–3280. doi: 10.1128/jvi.62.9.3274-3280.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muggeridge M I, Roberts S R, Isola V J, Cohen G H, Eisenberg R J. Herpes simplex virus. In: Van Regenmortel M H V, Neurath A R, editors. Immunochemistry of viruses. II. The basis for serodiagnosis and vaccines. Amsterdam, The Netherlands: Elsevier Biochemical Press; 1990. pp. 459–481. [Google Scholar]
- 37a.Muggeridge, M. I., et al. Unpublished data.
- 38.Nicola A V, Peng C, Lou H, Cohen G H, Eisenberg R J. Antigenic structure of soluble herpes simplex virus glycoprotein D correlates with inhibition of HSV infection. J Virol. 1997;71:2940–2946. doi: 10.1128/jvi.71.4.2940-2946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nicola A V, Willis S H, Naidoo N N, Eisenberg R J, Cohen G H. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J Virol. 1996;70:3815–3822. doi: 10.1128/jvi.70.6.3815-3822.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Para M F, Parish M L, Noble A G, Spear P G. Potent neutralizing activity associated with anti-glycoprotein D specificity among monoclonal antibodies selected for binding to herpes simplex virions. J Virol. 1985;55:483–488. doi: 10.1128/jvi.55.2.483-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng T, Ponce-de-Leon M, Jiang H, Dubin G, Lubinski J M, Eisenberg R J, Cohen G H. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J Virol. 1998;72:65–72. doi: 10.1128/jvi.72.1.65-72.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira L, Dondero D V, Gallo D, Devlin V, Woodie J D. Serological analysis of herpes simplex virus types 1 and 2 with monoclonal antibodies. Infect Immun. 1982;35:363–367. doi: 10.1128/iai.35.1.363-367.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira L, Klassen T, Baringer J R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980;29:724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samanta S, Eisenberg R J, Cohen G H. Studies of monomeric and oligomeric forms of HSV gB, abstr. 29. 19th International Herpesvirus Workshop, Vancouver, British Columbia, Canada. 1994. [Google Scholar]
- 45.Seidel-Dugan C, Ponce de Leon M, Friedman H M, Fries L F, Frank M M, Cohen G H, Eisenberg R J. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J Virol. 1988;62:4027–4036. doi: 10.1128/jvi.62.11.4027-4036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seigneurin J M, Desgranges C, Seigneurin D, Paire J, Renversez J C, Jacquemont B, Micouin C. Herpes simplex virus glycoprotein D: human monoclonal antibody produced by bone marrow cell line. Science. 1983;221:173–175. doi: 10.1126/science.6304881. [DOI] [PubMed] [Google Scholar]
- 47.Shieh M-T, Spear P G. Herpesvirus-induced cell fusion that is dependent on cell surface heparan sulfate or soluble heparin. J Virol. 1994;68:1224–1228. doi: 10.1128/jvi.68.2.1224-1228.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shieh M-T, WuDunn D, Montgomery R I, Esko J D, Spear P G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Showalter S D, Zweig M, Hampar B. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect Immun. 1981;34:684–692. doi: 10.1128/iai.34.3.684-692.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith C A, Farrah T, Goodwin R G. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- 51.Spear P G. Entry of alphaherpesviruses into cells. Semin Virol. 1993;4:167–180. [Google Scholar]
- 52.Spear P G. Membrane fusion induced by herpes simplex virus. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 201–232. [Google Scholar]
- 53.Tal-Singer R, Peng C, Ponce de Leon M, Abrams W R, Banfield B W, Tufaro F, Cohen G H, Eisenberg R J. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53a.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of HSV1, HSV2 and pseudorabies virus. Submitted for publication. [DOI] [PubMed]
- 54.Watson R J, Weis J H, Salstrom J S, Enquist L W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982;218:381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- 55.Whitbeck J C, Peng C, Lou H, Xu R, Willis S H, Ponce de Leon M, Peng T, Nicola A V, Montgomery R I, Warner M S, Soulika A M, Spruce L A, Moore W T, Lambris J D, Spear P G, Cohen G H, Eisenberg R J. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J Virol. 1997;71:6083–6093. doi: 10.1128/jvi.71.8.6083-6093.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willis S H, Peng C, Ponce de Leon M, Nicola A V, Rux A H, Cohen G H, Eisenberg R J. Expression and purification of secreted forms of herpes simplex virus glycoproteins from baculovirus-infected insect cells. In: Brown M S, MacLean A R, editors. Methods in molecular medicine. Vol. 10. Totowa, N.J: Humana Press; 1997. pp. 131–156. [DOI] [PubMed] [Google Scholar]
- 57.Willis S H, Rux A H, Lou H, Eisenberg R J, Cohen G H. The 22nd International Herpesvirus Workshop, San Diego, Calif. 1997. Binding kinetics of HSV gD to the HSV entry mediator (HVEM) using surface plasmon resonance biosensors, abstr. 171; p. 171. [Google Scholar]
- 58.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58a.Xu, R., et al. Unpublished data.