Abstract
The morphology of facial scars shows a wide variation in terms of texture and colour. To date, there are no reliable predictors of aberrant scarring. We conducted a retrospective analysis to identify factors associated with specific scar features and types. Photographs and medical records of 428 patients with facial scars were retrospectively reviewed. Patients with keloids were excluded. The mean age of the patients was 45.43 ± 23.13 years with a male‐to‐female ratio of 1:1.36. Atrophic scars were the most common (42.8%), followed by flat scars (38.7%) and hypertrophic scars (18.5%). Scars on the forehead were more likely to be atrophic, whereas scars on the chin/jaw and around the mouth were more likely to be hypertrophic. Hypopigmentation was significantly more common in scars located on the forehead. Redness (erythema) was significantly more common in scars located on the chin/jaw. Old scars were less likely to be erythematous, and hypertrophic. Atrophic scars were more common in younger patients. Scars caused by dermatologic conditions, such as acne, were more likely to be atrophic, whereas surgical scars had the lowest risk of being atrophic or hypertrophic. In conclusion, the location, onset, and cause of facial scars were associated with specific features of scars.
Keywords: cicatrix, face, hypopigmentation, scar, wound healing
1. INTRODUCTION
Scars are the inevitable consequences of cutaneous injury caused by diverse events, including trauma, surgical procedures, and dermatologic conditions. 1 Persons with visible scars actively seek for medical and surgical treatment because of the distressing nature of scars in terms of appearance and function. Although scars can be found in any body part, facial scars cause by far the most distress for patients, often accompanying negative psychosocial effects. With the help of several modalities, scars can be prevented or treated so that they become less unsightly, and active intervention should be given ideally to all scars. 2 , 3 However, considering the time and financial cost of scar treatment, not all scars can be treated in real life. Every scar tends to show a unique clinical course, which is difficult to predict. Some scars tend to recover spontaneously over time, making the clinical decision to treat scars even trickier. Unfortunately, there is limited data and no unified guideline on which scars or cutaneous injuries require a more active intervention.
Therefore, this study aimed to analyse the morphological characteristics of facial scars according to the location and duration of scars, sex and age of patients, and causative factors. We sought to determine factors related to scars that are more cosmetically problematic so that clinicians can predict the type (flat, atrophic or depressed, and hypertrophic or elevated) and colour of scars resulting from various causes (trauma, surgery, dermatologic conditions including acne vulgaris).
2. METHODS
2.1. Study population
After Institutional Review Board approval, we searched the Asan Medical Center database for patients with facial scars who visited the outpatient clinic of the department of dermatology between January 1990 and June 2021. Cases with complete medical records, including sex, age, onset and cause of facial scars, presence of symptoms, and medical photographs of the lesions at the initial visit were included. Patients who had received scar treatment (except for wound dressing) before their visit, those with scars resulting from injury within 1 month, and patients with wounds displaying incomplete healing were excluded. All cases with missing photographs were also excluded. Furthermore, given that keloid scars have distinct pathogenesis compared to other types of scars, patients diagnosed with keloid were excluded from the study.
2.2. Variables of interest
The following clinical data were collected from patient medical records: age at diagnosis, sex, scar onset, cause of scar (trauma, surgery and other invasive procedures, acne vulgaris, and other dermatologic conditions), and presence of symptoms (pruritus and pain/tenderness). Patients with scars resulting from surgery due to trauma were classified as having scars from surgery. The anatomical location and type of scar (flat, atrophic, hypertrophic) and colour of scar (redness and hypo‐ or hyperpigmentation) were recorded on the basis of clinical photographs taken during the initial visit. Some patients had previously received surgical or medical treatment in our department for other causes (skin tumours and dermatologic conditions) before initiating scar treatment. In these cases, the date of the first scar treatment was regarded as the initial visit.
2.3. Statistical analysis
The relationships between categorical variables (location, morphology, pigment, erythema, pruritus, pain, and aetiology) were analysed using the chi‐square analysis. Post hoc analyses were conducted for all chi‐square tests. When the expected value was ≤5, Fisher's exact test was used. To examine differences in the scar morphology, occurrence of pigmentation, erythema, pruritus, and pain according to age and onset, logistic regression was used. When comparing the relationship between three categories, such as scar morphology and pigmentation, with age and onset, a logistic regression analysis was done between one category and the rest.
All statistical analyses were performed using R (version 4.2.2). For the chi‐square post hoc analysis, the chisq.posthoc.test package and the fifer package in R were used. The significance criterion was set at 0.05. The p‐values in multiple comparisons were adjusted using the Bonferroni correction.
3. RESULTS
3.1. Patient demographics and characteristics of facial scars
A total of 428 patients with facial scars were included for analysis. Patient demographics and characteristics of facial scars are summarized in Table 1. Forty‐seven of them had multiple scars located on two or more different anatomical sites, which were counted separately as individual cases. Thus, of the total 507 scars, 92 were sets of multiple similar‐looking scars and the rest were solitary scars. The mean age of the patients was 43.43 (range, 1–94) years. More female patients were identified than male patients (M:F = 1:1.36). The mean onset of scar was 27.67 ± 75.65 [mean ± standard deviation] months. The cheeks (28.6%) were the most common locations, followed by the nose (25.1%) and the forehead (14.2%).
TABLE 1.
Clinical characteristics of the included patients and scars.
| Mean ± SD or No. (%) | |
|---|---|
| Age | 45.43 ± 23.13 |
| Sex | |
| Female | 254 (57.60%) |
| Male | 187 (42.40%) |
| Onset (months) | 27.67 ± 75.65 |
| With multiple scars | 47 (10.66%) |
| Scar location | |
| Forehead | 72 (14.20%) |
| Temple | 25 (4.93%) |
| Periocular | 49 (9.66%) |
| Nose | 127 (25.05%) |
| Cheeks | 145 (28.60%) |
| Lip & perioral | 49 (9.66%) |
| Chin and jaw | 40 (7.89%) |
| Type of scar | |
| Flat | 196 (38.66%) |
| Atrophic | 217 (42.80%) |
| Hypertrophic | 94 (18.54%) |
| Erythema | 314 (61.93%) |
| Dyspigmentation | |
| Hypopigmentation | 97 (19.13%) |
| Normal pigmentation | 280 (55.23%) |
| Hyperpigmentation | 130 (25.64%) |
| Presence of symptom | |
| Pruritus | 21 (4.23%) |
| Pain or tenderness | 11 (2.21%) |
| Cause of scar | |
| Trauma | 119 (23.47%) |
| Surgery and other invasive procedures | 274 (12.23%) |
| Acne | 62 (9.86%) |
| Other dermatologic condition | 50 (9.86%) |
| Unknown | 2 (0.39%) |
Abbreviation: SD, standard deviation.
3.2. Type of scar: hypertrophic, atrophic, or flat
The most common type of scar was atrophic (42.8%), followed by flat (38.7%) and hypertrophic scars (18.54%). The proportion of each scar type was calculated according to the location (Figure 1A). In our sample of 507 scars, a chi‐squared test showed a significant association between the location and the type of scar (χ2 = 84.94, p < 0.001). As shown in the mosaic plot of Figure 1B, a significantly higher proportion of scars on the chin/jaw and perioral areas were hypertrophic, whereas scars in the periocular areas were less likely to be hypertrophic. Scars on the forehead were significantly more likely to be atrophic. In addition, scars on the nose and around the eyes were more likely to be flat. Moreover, the association between scar type and onset was evaluated (Table 2). Logistic regression revealed that as the onset duration increased, significantly more scars were atrophic compared with flat and hypertrophic scars (p < 0.001). Furthermore, scars with a shorter onset duration had a significantly higher proportion of flat scars compared to atrophic and hypertrophic scars (p = 0.036).
FIGURE 1.
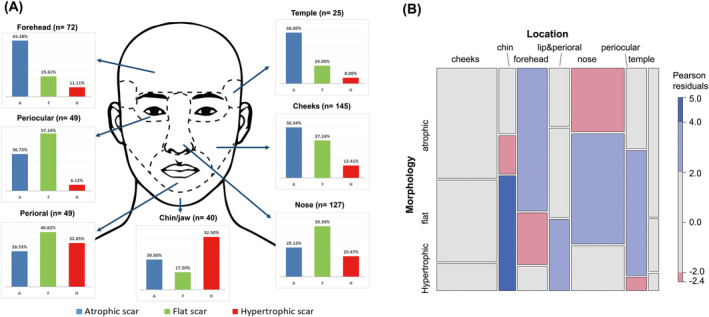
(A) Proportion of each scar type according to the location. (B) Mosaic plot comparing the distribution of scar morphology across locations.
TABLE 2.
Association between scar onset, patient age, and clinical features of facial scars.
| Onset (months, mean ± SD) | p‐value | Age (years, mean ± SD) | p‐value | |
|---|---|---|---|---|
| Morphology | ||||
| Atrophic | 53.2 ± 105.0 | <0.001* | 39.2 ± 21.0 | <0.001* |
| Flat | 21.0 ± 68.6 | 0.036* | 50.1 ± 24.1 | <0.001* |
| Hypertrophic | 20.9 ± 44.9 | 0.351 | 44.1 ± 21.4 | 1.000 |
| Pigmentation | ||||
| Hypopigmented | 48.4 ± 85.0 | 0.186 | 39.0 ± 23.8 | 0.030* |
| Normal | 27.2 ± 80.2 | 0.264 | 46.4 ± 23.3 | 0.075 |
| Hyperpigmented | 35.0 ± 84.6 | 1.000 | 43.9 ± 20.1 | 1.000 |
| Erythema | ||||
| Yes | 13.3 ± 37.3 | <0.001* | 44.7 ± 23.0 | 0.644 |
| No | 70.2 ± 121.0 | 43.7 ± 22.4 | ||
| Pruritus | ||||
| Yes | 39.6 ± 35.3 | 0.742 | 34.6 ± 15.8 | 0.046* |
| No | 33.2 ± 84.3 | 44.9 ± 23.1 | ||
| Pain | ||||
| Yes | 12.9 ± 12.5 | 0.477 | 35.8 ± 18.7 | 0.21 |
| No | 33.9 ± 83.6 | 44.7 ± 22.9 | ||
Abbreviation: SD, standard deviation.
p < 0.05.
In addition, older patients had a tendency to developing flat scars than atrophic and hypertrophic scars (p < 0.001) (Table 2). On the other hand, younger patients had a significantly higher tendency to develop atrophic scars compared to flat and hypertrophic scars (p < 0.001). Regarding the cause of scar, scars resulting from acne were most commonly atrophic (61.3%), followed by hypertrophic (22.6%) and flat (16.1%). By contrast, the vast majority (88.0%) of scars resulting from other dermatologic conditions were atrophic, with only a few hypertrophic (12.0%). Scars resulting from surgery and other invasive procedures were more likely to be flat (52.2%), with a higher proportion of atrophic scars (28.1%) than hypertrophic scars (19.7%). Approximately half (47.9%) of the scars caused by trauma not related to medical procedures were atrophic, and flat scars (31.1%) were the second most common type. Furthermore, a subgroup analysis was conducted according to sex, and no significant difference in the scar type was found between sexes.
3.3. Colour of scar: erythema, hypo‐ or hyperpigmentation
The proportion of scars with colour changes according to the location area is summarized in Figure 2A. Redness or erythema was seen in 61.9% of all the evaluated scars. Compared with scars located elsewhere, those on the chin/jaw were significantly more likely to be red (p = 0.006) (Figure 2B). In terms of pigmentation, 55.2% of the evaluated scars showed normal pigmentation, 25.6% were hyperpigmented, and 19.1% were hypopigmented. This proportion did not differ significantly according to the location of the scar, except for those located on the forehead, which were more likely to be hypopigmented (40.3%) than the others (p < 0.001) (Figure 2C). Upon categorization according to the type of scar, a significantly higher proportion (92.6%) of hypertrophic scars were erythematous compared with atrophic or flat scars (p < 0.001) (Figure 3). Furthermore, the scars with a shorter onset were significantly more likely to be erythematous (p < 0.001) (Table 2). By contrast, a significant association was found neither between the change in pigmentation and the type of facial scar, or between scar onset and change in pigmentation. A significant association was observed between pigmentation and age. Younger patients tended to develop hypopigmented scars more frequently (p = 0.01), while older patients were more likely to develop normal‐coloured scars without pigmentation changes, though the difference was not statistically significant (p = 0.075). No significant differences in pigmentation were observed according to sex.
FIGURE 2.
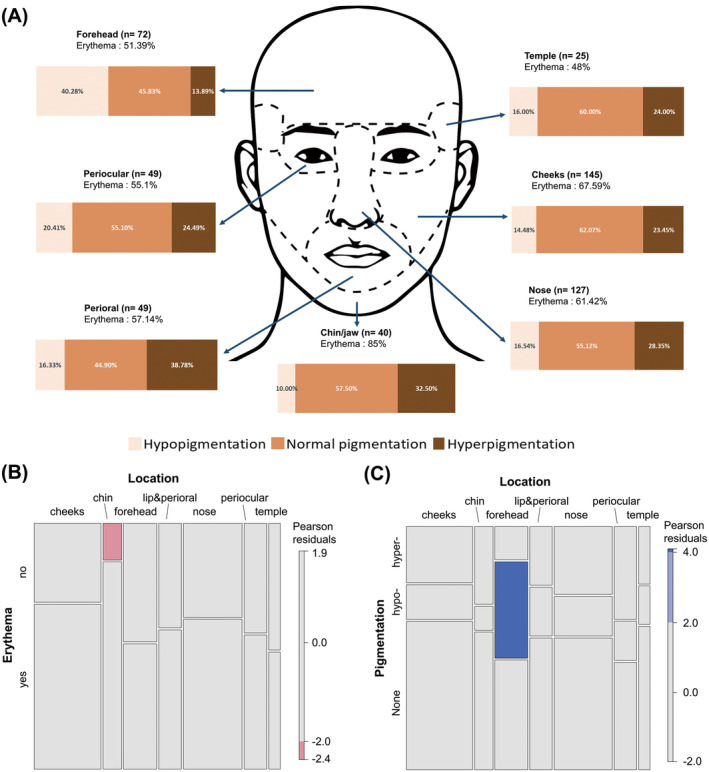
(A) Proportion of scars with colour changes according to the location. (B) Mosaic plot comparing the occurrence of erythema across locations. (C) Mosaic plot comparing the occurrence of hypo‐ or hyperpigmentation across locations.
FIGURE 3.

Mosaic plot comparing the occurrence of erythema across scar types.
There was no significant difference in the occurrence of erythema based on the cause of the scar. However, a significant association between the cause of the scar and pigmentation was observed. Scars resulting from dermatologic conditions showed a significantly higher occurrence of hyperpigmented scars, while scars caused by trauma were less frequently normo‐pigmented and more commonly hypopigmented (Figure 4).
FIGURE 4.

Mosaic plot comparing the occurrence of hypo‐ or hyperpigmentation across aetiology of scars.
3.4. Symptoms of scars: pruritus and pain
Patient‐reported symptoms related to facial scars were recorded. Figure 5 illustrates the proportion of scars with symptoms according to the location. Generally, pruritus (4.2%) was more commonly reported than pain or tenderness (2.2%). Both symptoms were more common in patients with scars located on the chin/jaw (Figure 6). As shown in Figure 7, both symptoms were more frequently reported for hypertrophic scars than other scar types (p < 0.001 for pruritus, p = 0.002 for pain). While there was no significant correlation between pruritus and onset (p = 0.742), there was a significant relationship with age. Younger patients had a higher tendency to have pruritic scars compared to older patients (p = 0.046) (Table 2). Pain did not have a meaningful correlation with either onset or age. There was no significant difference in pain based on the cause of the scar; however, there was a significant difference in the presence of pruritus. Specifically, scars from surgery had significantly less pruritus compared to those from acne and trauma (p < 0.001 and p = 0.007, respectively).
FIGURE 5.
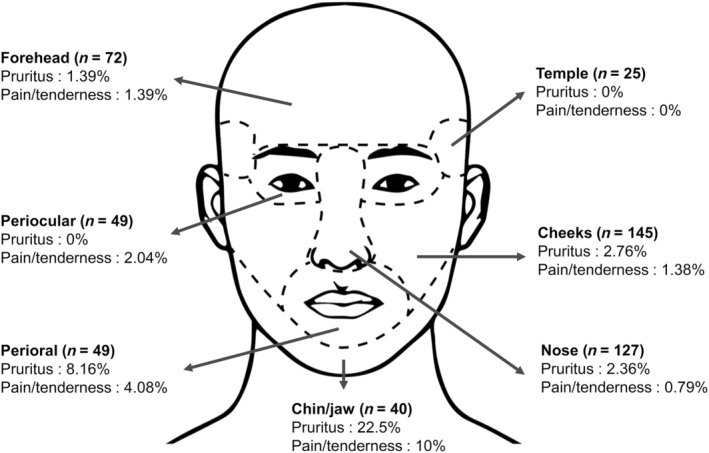
Proportion of scars with symptoms according to the location.
FIGURE 6.

Proportion of pruritic and painful scars across locations.
FIGURE 7.

Proportion of pruritic and painful scars across scar types.
4. DISCUSSION
Scarring is a natural consequence of wound healing after skin damage. Some scars can be socially and cosmetically acceptable. However, facial scars are generally disfiguring and can negatively affect one's self‐esteem. 4 Patients seek medical and surgical care to treat facial scars; however, cosmetic outcomes vary widely. Because the treatment of established scars is challenging, prevention of aberrant scarring becomes equally important. Although the face is a relatively small area, which makes up 3.5% of the entire body surface area in adults, the appearance of facial scars shows a wide variation. 5 Therefore, we aimed to determine factors associated with the clinical and morphological characteristics of facial scars so that clinicians may predict the average natural course of facial scars and provide tailored treatment to individual scars.
Cosmetic determinants of scars can be categorized into changes in texture or volume (flat, atrophic, or hypertrophic) and colour (erythema and hypo‐ or hyperpigmentation). 6 , 7 In this study, a strong association was found between the location of facial scars and the scar type. Our findings indicate that scars located on the jaw or the chin are more likely to be hypertrophic. One explanation for this could be the relatively high skin tension applied across the jawline and the chin. Neck movements, especially neck extension, and jaw movements both result in excessive stretching of the skin in the jawline and chin area. Mechanical tension across the damaged skin contributes to the formation of hypertrophic scar. 8 , 9 , 10 Thus, the high proportion of hypertrophic scars in the jaw/chin area represents excessive skin tension across this area. This is also supported by the likelihood of scars located on the nose and around the eyes to be flat and less noticeable because these areas are not subject to major movement. On the contrary, the higher proportion of atrophic scars among scars located on the forehead demonstrates the opposite; the forehead skin is rarely subjected to mechanical tension, but is rather subject to “folding” forces exerted by the frontalis muscle, possibly leading to atrophic scar formation. In addition to mechanical tension, other factors such as repeated trauma, infection due to shaving, or habitual rubbing contribute to chronic inflammation of the scar tissue, which may also determine the fate of facial scars. 9 , 11 In light of our findings, early scars located on these tension‐prone facial areas should prompt special attention and active management to prevent hypertrophic scarring.
The onset time is a major determinant of scar morphology. Facial scars that had formed long ago were less likely to be hypertrophic. In addition, erythema was uncommon in old scars. Taken together, these findings imply that older scars typically present fewer cosmetic concerns as the most noticeable and aesthetically disruptive scars are those that are hypertrophic and erythematous. Considering that only treatment‐naïve patients with facial scars were included in this study, the aforementioned association of scar onset and different characteristics of scars provided us with insight into the natural course of facial scars. Although the efficacy of active scar treatment consisting of various modalities has been evaluated in numerous clinical studies, not everyone can benefit from it owing to limited resources. Our results suggest that scar improvement can, to some extent, be expected in terms of shape, colour, and symptoms as the scar matures, even without treatment. Scar maturation is believed to reflect the successful completion of the remodelling phase, which can take several months or years. 12 , 13 Considering the collective findings of our study, surgical wounds around the eyes and nose have the highest likelihood of resulting in flat scars without noticeable colour changes.
Scars can occur as a result of various insults to the skin, including physical/chemical trauma, and inflammatory dermatoses such as acne. In this study, intentional or planned surgical procedures were less likely (47.8%) to result in aberrant scarring (hypertrophic or atrophic scars), whereas more than half of all scars caused by accidental trauma, acne, and other dermatologic conditions were either hypertrophic or atrophic. Despite substantial diversity in each etiologic category, both active wound care and appropriate reconstruction during surgery or other invasive procedures could have ameliorated the appearance of the resulting scar. 14
This study has certain limitations. First, owing to the nature of this retrospective cross‐sectional study, the clinical course of facial scars could not be analysed. In addition, many cases were referred from other clinics or hospitals after surgery or treatment for dermatologic conditions without a detailed description of the clinical course. Therefore, we could not incorporate factors affecting wound healing and scarring, such as infection, dehiscence, or ulceration, into the analysis. Second, an arbitrary cutoff of 1 month of onset was used to distinguish scars from wounds. Because wound healing is a continuous process, defining the exact timing of scar formation is difficult. During our review of clinical photographs, most wounds were completely healed by 1 month after the trauma or surgery. Hence, we decided on the 1‐month cutoff, except for some wounds that showed incomplete healing on photographs. Moreover, because the extent of the initial injury could not be determined, contractures and widening (extension) of scars could not be assessed in our analysis.
In conclusion, this study demonstrates the association between the morphology of facial scars and their location. The cause and onset were additional determinants of the morphology and symptoms of facial scars. These findings may be useful in patient counselling and treatment planning.
FUNDING INFORMATION
This study was supported by the Korean National Research Foundation (grant number: NRF‐2022R1F1A1062848).
CONFLICT OF INTEREST STATEMENT
The authors report there are no competing interests to declare regarding this study.
ACKNOWLEDGEMENTS
The authors declare that there is no conflict of interest of none of the authors regarding this publication.
Kim GH, Lee WJ, Jung JM, et al. Morphological characteristics of facial scars: A retrospective analysis according to scar location, onset, age, and cause. Int Wound J. 2024;21(4):e14453. doi: 10.1111/iwj.14453
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable requests.
REFERENCES
- 1. Marshall CD, Hu MS, Leavitt T, Barnes LA, Lorenz HP, Longaker MT. Cutaneous scarring: basic science, current treatments, and future directions. Adv Wound Care. 2018;7(2):29‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kauvar ANB, Kubicki SL, Suggs AK, Friedman PM. Laser therapy of traumatic and surgical scars and an algorithm for their treatment. Lasers Surg Med. 2020;52(2):125‐136. [DOI] [PubMed] [Google Scholar]
- 3. Seago M, Shumaker PR, Spring LK, et al. Laser treatment of traumatic scars and contractures: 2020 international consensus recommendations. Lasers Surg Med. 2020;52(2):96‐116. [DOI] [PubMed] [Google Scholar]
- 4. Ngaage M, Agius M. The psychology of scars: a mini‐review. Psychiatr Danub. 2018;30(Suppl 7):633‐638. [PubMed] [Google Scholar]
- 5. Lund CC, Browder NC. The estimation of areas of burns. Surg Gynecol Obstet. 1944;79:352‐358. [Google Scholar]
- 6. Chadwick S, Heath R, Shah M. Abnormal pigmentation within cutaneous scars: a complication of wound healing. Indian J Plast Surg. 2012;45(2):403‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. El Kinani M, Duteille F. Scar epidemiology and consequences. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, eds. Textbook on scar management. Springer; 2020:45‐49. [PubMed] [Google Scholar]
- 8. Harn HI, Ogawa R, Hsu CK, Hughes MW, Tang MJ, Chuong CM. The tension biology of wound healing. Exp Dermatol. 2019;28(4):464‐471. [DOI] [PubMed] [Google Scholar]
- 9. Ogawa R. Keloid and hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18(3):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ogawa R. Mechanobiology of cutaneous scarring. In: Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, eds. Textbook on Scar Management. Springer; 2020:11‐18. [PubMed] [Google Scholar]
- 11. Wang ZC, Zhao WY, Cao Y, et al. The roles of inflammation in keloid and hypertrophic scars. Front Immunol. 2020;11:603187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stadelmann WK, Digenis AG, Tobin GR. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176(2 Suppl):26S‐38S. [DOI] [PubMed] [Google Scholar]
- 13. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee Peng G, Kerolus JL. Management of surgical scars. Facial Plast Surg Clin North Am. 2019;27(4):513‐517. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable requests.


