Abstract
Intestinal inflammatory fibrosis is a severe consequence of inflammatory bowel diseases (IBDs). There is currently no cure for the treatment of intestinal fibrosis in IBD. Although inflammation is necessary for triggering fibrosis, the anti-inflammatory agents used to treat IBD are ineffective in preventing the progression of intestinal fibrosis and stricture formation once initiated, suggesting that inflammatory signals are not the sole drivers of fibrosis progression once it is established. Among multiple mechanisms involved in the initiation and progression of intestinal fibrosis in IBD, stromal cells play critical roles in mediating the process. In this review, we summarize recent progress on how stromal cells regulate intestinal fibrosis in IBD and how they are regulated by focusing on immune regulation and gut microbiota. We also outline the challenges moving forward in the field.
Keywords: Stromal cells, Inflammatory bowel diseases, Intestinal fibrosis, Myofibroblast, Fibroblasts
Summary.
This review elucidates the effect of different intestinal stromal cells on intestinal inflammatory fibrosis. Unveiling the complex interplay among stromal cells, immune responses, and gut microbiota offers valuable insights for understanding inflammatory bowel disease–related fibrosis.
Intestinal inflammatory fibrosis refers to a pathologic condition in which the excessive extracellular matrix (ECM) components deposit in the intestinal wall, which develops in response to chronic inflammation within the gastrointestinal tract, mainly seen in inflammatory bowel diseases (IBDs).1 Crohn’s disease (CD) and ulcerative colitis (UC) are 2 major types of IBD that are characterized by chronic and recurrent gut inflammation and occur in genetically susceptible individuals caused by an abnormal immune response to gut microbiota.2 Chronic recurrent intestinal inflammation and the resulting tissue injury in IBD, accompanied by defective resolution and repair processes, results in the exaggerated synthesis and deposition of ECM components.3, 4, 5 This leads to intestinal fibrosis in up to 50% of CD patients.6 The development of intestinal fibrosis is associated with fistula development and the need for surgery, and many patients with CD needing surgery experience stricture recurrence.7 Although underappreciated in patients with UC, fibrosis also leads to bowel wall stiffening with accompanying reductions in compliance and impaired quality of life.8 The features of intestinal fibrosis in CD and UC are not identical. Similar to inflammation, fibrosis also is transmural in CD, characterized by increased thickness of all intestinal layers, including lamina propria, muscularis mucosa, submucosa, and muscularis propria.9,10 Intestinal fibrosis in UC is relatively superficial and confined mostly to mucosal and submucosal layers of the colon, but it might affect deeper layers if ulceration is profound.11,12 Current anti-inflammatory biologics and small-molecule therapies for IBD patients are ineffective in treating fibrosis onset and progression.13 Despite the availability of these novel treatments, the incidence of intestinal fibrosis has not changed, and the development of different therapeutic strategies is required. Emerging evidence suggests that the inflammation-independent mechanisms also might be involved in sustaining and perpetuating intestinal fibrosis in IBD.4
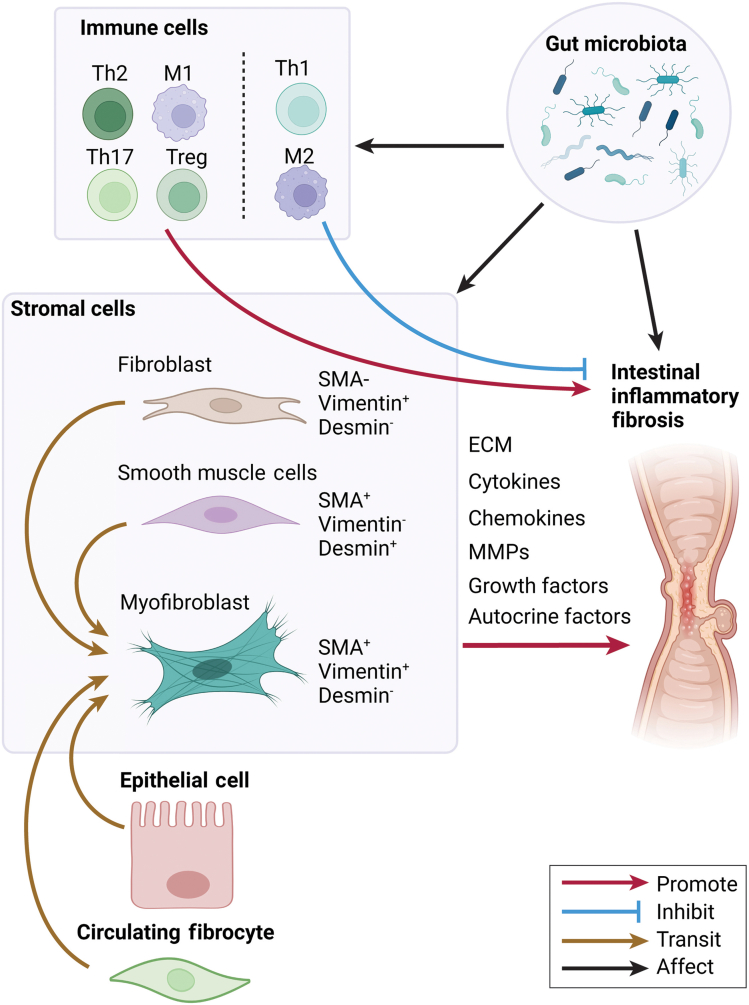
Fibrosis is a physiological process responsible for tissue repair that is triggered by inflammation and injury and involves the deposition of ECM proteins. During the normal tissue repair response, inflammation and injury initiate a cascade of events, including the activation of stromal cells, mainly intestinal myofibroblast (MF), which triggers the secretion of proteases and ECM components.3, 4, 5 This process usually is controlled and resolves appropriately by limiting intestinal MF proliferation, migration, and ECM production, and is followed by the resolution of inflammation and fibrotic signaling.14 Pathogenic tissue remodeling is observed when an imbalance between production and degradation of ECM proteins occurs, which leads to increased tissue stiffness and altered organ function.15 In patients with CD, MFs are thought to be the major source of increased collagen production and collagen deposition in the muscularis.10,16 Activated MFs can migrate and proliferate to facilitate the synthesis and deposition of collagen-rich ECM to aid in the reparative processes within damaged tissues. The increase in intestinal MF numbers is driven primarily by enhanced proliferation and aberrant survival cues/responses. With the increasing attention to intestinal fibrosis complications in IBD patients, understanding its mechanisms is essential for identifying potential molecular targets and developing treatments to prevent and treat fibrosis in the intestine. In this review, we focus on the role of different stromal cells in regulating intestinal inflammatory fibrosis and how they are regulated by the immune responses and gut microbiota (Figure 1).
Figure 1.
Scheme of immune cell regulation of stromal cells in intestinal inflammatory fibrosis.
Role of Stromal Cells in Intestinal Inflammatory Fibrosis
Stromal cells are a group of nonhematopoietic cells with phenotypic and functional heterogeneity found in the connective tissues, including the intestine.17,18 They provide essential support for tissue structure and tissue homeostasis in the healthy intestine.17 Upon injury or inflammation, stromal cells are involved in complex tasks, including wound healing, tissue regeneration, and immune regulation.19 In addition, stromal cells have been found to play an important role in the development and progression of intestinal fibrosis in the context of IBD.
Stromal cells, also called mesenchymal cells, contribute to the pathogenesis of intestinal fibrosis, mainly including myofibroblasts, fibroblasts, and smooth muscle cells (SMCs).14 These cells can be identified by the expression of 3 markers: alpha-smooth muscle actin (α-SMA), vimentin, and desmin.15 Fibroblasts express vimentin but not α-SMA and desmin, whereas SMCs are positive for α-SMA and desmin without the expression of vimentin. Myofibroblasts are α-SMA+ vimentin+, but desmin- stromal cells. Myofibroblasts have an intermediate phenotype between fibroblasts and SMCs. These cells are the major cells producing ECM in the intestine and can differentiate from each other under different contexts.20 Expansion and activation of stromal cells and increased deposition of ECM are the hallmarks of intestinal inflammatory fibrosis. In addition, nonstromal cells, including intestinal epithelial and endothelial cells, contribute to intestinal fibrogenesis via epithelial-to-mesenchymal transition (EMT) and endothelial-to-mesenchymal transition.14,21,22 Furthermore, bone marrow–derived circulating fibrocytes are also the source of myofibroblasts.
Myofibroblasts
Myofibroblasts, which express vimentin and α-SMA, are effector cells for intestinal fibrosis that produce the end products deposited in the extracellular space.23 MFs are characterized by specific cellular markers that give them an intermediate phenotype between fibroblasts and smooth muscle cells and their ability to produce large quantities of ECM components when activated. MFs are considered the key players in intestinal inflammatory fibrosis.14 In the context of recurrent and chronic inflammation, myofibroblasts can be multiplied and remain activated by continuous stimulus from cytokines and chemokines in the surrounding microenvironment. Activated myofibroblasts migrate to the inflammatory sites and synthesize excess production of ECM, including collagen, fibronectin, proteoglycans, and other components. ECM replaces the damaged tissues and restores the continuity of the affected areas by accumulating within the intercellular space. The secretion of profibrotic factors by myofibroblasts is a critical step in the development of intestinal fibrosis. Several key molecules produced by myofibroblasts that contribute to fibrosis have been identified. For example, transforming growth factor-β (TGF-β), a potent profibrotic cytokine, stimulates myofibroblasts to produce collagen and fibronectin.24 In addition, myofibroblasts produce matrix metalloproteinases (MMPs) that can degrade the extracellular matrix, promoting tissue remodeling. The dysregulated balance between profibrotic and antifibrotic factors leads to the accumulation of fibrotic tissue in the intestine. In experimental models of intestinal fibrosis, the role of myofibroblasts has been demonstrated. It has been shown that the ablation or inhibition of myofibroblasts can ameliorate fibrosis. In an experimental colitis-induced model of intestinal fibrosis, deletion of α-SMA+ myofibroblasts resulted in less fibrosis, indicating their importance in the pathogenesis of intestinal fibrosis.25
Although the origin of MFs in intestinal fibrosis still is unknown, there are several potential sources, including fibroblasts, SMCs, and epithelial cells, through EMT.14
Fibroblasts
In response to inflammation, fibroblasts can transform into myofibroblasts.26 The activation of fibroblasts in intestinal fibrosis is driven by a complex interplay of various signaling pathways and cytokines.15 TGF-β is a central mediator in this process, which stimulates fibroblasts to differentiate into myofibroblasts, acquiring contractile properties and synthesizing large amounts of ECM proteins.27 Fibroblasts in intestinal fibrosis produce collagen and secrete other ECM proteins such as fibronectin, contributing to tissue stiffness and scarring. Moreover, fibroblasts actively participate in tissue remodeling by mediating the degradation of the extracellular matrix through the production of MMPs, which break down the ECM components.28 In fibrotic tissues, there is often an imbalance between the production of MMPs and their inhibitors, favoring the accumulation of extracellular matrix and fibrosis.29 This imbalance results from the altered function of fibroblasts in fibrotic conditions.
In addition to their role in extracellular matrix regulation, fibroblasts also can influence the immune response in intestinal fibrosis. They produce chemokines and cytokines that recruit and activate immune cells, further contributing to inflammation and fibrosis in the affected tissues.30,31 It has been shown that activated fibroblasts can induce the recruitment of lymphocytes via C-X-C motif chemokine ligand 12 (Cxcl12), the recruitment of monocytes and macrophages via C-C motif chemokine ligand 2 (Ccl2) and colony stimulating factor 1 (Csf1), and the recruitment of neutrophils via Cxcl1 and Cxcl8.32, 33, 34 These immune cells, in turn, release cytokines that perpetuate the fibrotic response.
Smooth Muscle Cells
Multiple mechanisms are involved in the contributions of SMCs to fibrosis. SMCs produce large amounts of ECM, such as collagen, fibronectin, and vitronectin.35,36 Similar to fibroblasts and myofibroblasts, SMCs produce more collagen in intestinal fibrosis than in control tissue.37 Interleukin (IL)6 and TGF-β are primary cytokines that stimulate SMC production of collagen.38 MSCs also can produce cytokines and growth factors to regulate myofibroblasts and fibroblasts. In addition, some autocrine factors produced by SMCs have profibrotic effects on myofibroblasts.39 Under inflammatory stimulation, SMCs can transform into myofibroblasts to further promote intestinal fibrosis.14 Although fibrosis, featured as stromal cell proliferation with ECM deposition in stroma, has been considered the essential factor for the development of fibrostenosis in IBD patients, 1 study challenged the conventional view by highlighting the complexity of histologic changes contributing to stricturing in CD patients.40 Smooth muscle hyperplasia/hypertrophy plays a more crucial role in contributing to the stricturing phenotype in CD-associated fibrostenosis, suggesting that the inflammation-smooth muscle hyperplasia axis may be an important factor in the pathogenesis of strictures in CD.40
Epithelial-Mesenchymal Transition
EMT is an important hallmark of fibrogenesis through which epithelial cells lose their epithelial phenotype and transform into mesenchymal cells.41 The EMT process is critical for cellular conversion from epithelial cells to mesenchymal phenotypes. Under various stimulations, epithelial cells gradually lose their epithelial markers, such as E-cadherin and cytokeratin, and de novo express some mesenchymal markers, typically vimentin and fibroblast-specific protein 1. Fibroblasts in fibrotic areas of IBD patients with fibrosis show strong nuclear β-catenin staining.42 Concomitantly, a number of subepithelial cells in and around fibrotic areas also show expression of E-cadherin, and large numbers of α-SMA–positive cells are present in the fibrotic area, indicating the presence of EMT in IBD patients with fibrosis. EMT plays a crucial role in fibrosis and fistula formation in IBD.43 Several factors have been identified to mediate the EMT process, including both profibrotic molecules and antifibrotic molecular mechanisms. Activation of the Wingless-related integration site (Wnt)–β-catenin pathway has been shown to promote EMT, and blockade of the Wnt/β-catenin pathway inhibits intestinal fibrosis.44 Hedgehog and Notch signaling promote EMT, myofibroblast activation, and ECM production.45,46 Emerging evidence has indicated that epigenetic regulation plays a crucial role in EMT. Several EMT-related genes are modified epigenetically in IBD patients, including hypermethylation of the promoters of cadherin 1 (CDH1), cadherin 13 (CDH13), neurogenin 1 (NEUROG1), and caudal type homeobox 1 (CDX1). Sirtuin 1 (Sirt1), an epigenetic regulator that deacetylates several transcription factors, inhibits EMT through deacetylating mothers against decapentaplegic homolog 4 (SMAD4) to block TGF-β signaling.47
Circulating Fibrocytes
Circulating fibrocytes are derived from bone morrow and possess characteristics of both immune cells and fibroblasts. They express immune cell marker CD45 and fibroblast marker collagen I. Upon inflammatory or injury, fibrocytes migrate into the inflammatory or injured sites and contribute to tissue healing, repair, and fibrosis by producing collagens, fibrogenic cytokines, and growth factors.48 TGF-β1 drives circulating fibrocyte-to-myofibroblast differentiation, which also participates in the fibrotic process.49 It has been reported that circulating fibrocytes correlate with the fibrostenotic phenotype in CD patients.50 chemokine receptor type 2 (CCR2)+ fibrocytes were reported to promote colon fibrosis by inhibiting collagen degradation.
The Heterogeneity of the Stromal Compartment in Response to Inflammation
Through single-cell transcriptomic analysis, the heterogeneity of colonic stromal cells was investigated in both mice and human beings in health and colitis.51 In addition to established cells, including myofibroblasts and pericytes, 4 additional distinct fibroblast-like populations were identified. Of note, a colonic niche stromal population, which is located in proximity to epithelial crypts, expresses WNT genes crucial for stem cell self-renewal. This population is dysregulated in colitis, potentially contributing to an impaired intestinal barrier in IBD. An activated mesenchymal population, which gains lymph node fibroblastic reticular cell–like features, is increased in colitis. This study highlights that the functionally divergent stromal remodeling in response to inflammation is in a subset-specific manner.51 In addition, inflammatory activated stromal cells with other specific immune cells are associated with resistance to anti–tumor necrosis factor (TNF) therapy in ileal CD patients.34 The heterogeneity of stromal cells in response to inflammation is complicated and delicate, and needs to be investigated further.
Immune Regulation of Stromal Cells in Intestinal Inflammatory Fibrosis
Intestinal fibrosis is associated commonly with IBD. Because the immune response plays a significant role in the development of IBD, it also is crucial in regulating intestinal fibrosis, mainly through affecting stromal cells. Immune regulation of stromal cells in intestinal fibrosis is a complex and dynamic process involving interactions between immune cells, stromal cells (including fibroblasts), various cytokines, and signaling pathways. The initial trigger for intestinal fibrosis often is chronic inflammation in the intestine, typically associated with conditions such as CD. Immune cells, particularly those involved in the adaptive and innate immune responses, produce cytokines and chemokines in response to ongoing inflammation, which directly or indirectly can stimulate stromal cell activation and transformation of fibroblasts/smooth muscle cells (MSCs) into myofibroblasts. Because of the space limit in this review, we focus only on the following 2 types of immune cells: T cells and macrophages, as well as cytokines.
T Cells
Among adaptive immune cells, T cells play a pivotal role in the intricate regulation of stromal cells in intestinal fibrosis. There are several subsets of T cells, including interferon γ (IFNγ)-producing Th1 cells, IL4 and IL13 producing Th2 cells, IL17 producing T helper (Th)17 cells, and TGF-β producing regulatory T (Treg) cells. All subsets of T cells have been shown to regulate stromal cells in intestinal fibrosis at different levels.52 Although Th17 and Th1 cells are involved in intestinal inflammation, Th17 cells promote intestinal fibrosis by producing IL17 and amphiregulin (Areg).53 Th1 cells can be pro-intestinal or anti-intestinal fibrosis, depending on different contexts through the production of IFNγ. Th2 cells promote intestinal fibrosis through the production of IL13.54 Although Treg cells inhibit intestinal inflammation, they promote intestinal fibrosis, both mediated by TGF-β.55 The roles of B cells in intestinal fibrosis are unclear. A recent report showed an expansion of an IFN-induced B-cell subset during experimental mucosal healing, which was located in damaged areas and associated with colitis severity but inhibited interactions between stromal and epithelial cells.56 Investigating whether such expanded B cells also inhibit intestinal fibrosis development after intestinal inflammation will be interesting.
Macrophages
Macrophages, an innate cell population, regulate tissue repair, inflammation, and fibrosis, including intestinal fibrosis.57 They can adopt different activation states with M1-like proinflammatory and M2-like anti-inflammatory macrophages.58 As classically activated macrophages, M1-like macrophages produce proinflammatory cytokines such as TNF-α, IL1b, and various fibrogenic mediators such as TGF-β and platelet-derived growth factor, which can stimulate the proliferation of fibroblasts and collagen production.59 M1-like macrophages also can recruit fibroblasts to the site of inflammation through the secretion of chemokines.60,61 M2-like macrophages inhibit intestinal fibrosis in general, mainly through efferocytosis to clear apoptotic cells to prevent secondary necrosis and inflammation, as well as through promoting fibrosis resolution by producing enzymes such as MMPs, which help degrade excess ECM proteins.62 A recent study identified a broadly CD9+ triggering receptor expressed on myeloid cells 2 (TREM)2+ fibrogenic macrophage subset that expresses secreted phosphoprotein 1 (SPP1), glycoprotein non-metastatic protein B (GPNMB), fatty acid binding protein 5 (FABP5), and CD63 in lung and liver fibrosis induced by type 3 inflammation.63,64 It will be very interesting to determine whether those fibrogenic macrophages also are present in intestinal fibrosis and their roles in intestinal fibrosis.
Cytokines
Various cytokines produced by T cells, innate cells, and stromal cells have been implicated in regulating fibrosis. We focus on a few of them, which are relatively well studied.
Proinflammatory Cytokines
TNF-like ligand 1A
TNF-like ligand 1A (TL1A) is a member of the TNF superfamily. TL1A interacts with death receptor-3 to create the TL1A/death receptor-3 costimulatory system.65 CD patients with increased serum TL1A levels have been shown to have an increased susceptibility to developing intestinal strictures.66 Genetic predisposition TL1A genotype rs6478108 is a potential risk factor for the development of intestinal fibrosis in CD patients.67 Treatment with anti-TL1A antibodies not only attenuates but also reverses the established fibrosis in animal models,68,69 providing a new therapeutic target for intestinal fibrotic diseases.
IL13
IL13, often associated with Th2-type immune responses, can stimulate fibroblasts to produce collagen and other ECM components, contributing to tissue remodeling.70 These effects are driven primarily by the capacity of IL13 to induce the up-regulation of downstream TGF-β, promote myofibroblast differentiation, and enhance collagen production within the intestinal stroma.71 IL13 also promotes EMT.72
IL17A
IL17A, mainly produced by Th17 cells and innate lymphoid cell 3 (ILC3), dynamically regulates stromal cells through complex signaling pathways, impacting intestinal fibrotic development.73,74 IL17A induces the expression of MMPs and tissue inhibitors of metalloproteinases by stromal cells, thus influencing ECM degradation and synthesis.73
IL36
IL36, a member of the IL1 superfamily, is a cytokine that has diverse functions. It consists of 5 distinct isoforms: IL36α, IL36β, IL36γ, IL36Ra, and IL38.75 In the context of inflammatory conditions, it has been shown that both IL36α and IL36γ are increased in the mucosa of IBD patients.76, 77, 78 In addition, IL36α is especially prominent in the tissues of CD-associated fibrostenosis.79 IL36α and IL36γ induce fibroblast activation and epithelial cell proliferation,80 which, in turn, is associated with increased collagen production, leading to the development of intestinal fibrosis.79
Anti-Inflammatory Cytokines
TGF-β
TGF-β, produced by many types of cells, including Treg cells and macrophages, is one of the most potent cytokines against intestinal inflammation and driving intestinal fibrosis.81 Through activating SMAD2, SMAD3, and SMAD4, TGF-β activates fibroblasts and promotes their differentiation into myofibroblasts.24 TGF-β stimulates stromal cell production of ECM proteins such as collagen, contributing to tissue fibrosis.82 In addition, TGF-β is the primary cytokine to promote EMT.83
Areg
Areg, an epidermal growth factor–like molecule, promotes human intestinal myofibroblast proliferation and motility by activating mammalian target of rapamycin (mTOR) and mitogen-activated protein kinase kinase (MEK).53 Areg expression is increased in CD patients with fibrosis compared with CD patients without fibrosis.53 Interestingly, Th17 cells produce high levels of Areg.53 Areg-/- Th17 cells induce more severe intestinal inflammation but less severe intestinal fibrosis,53 indicating a crucial role of Areg in the induction of intestinal fibrosis independent of intestinal inflammation.
Microbiota–Stromal Cell Interactions in Intestinal Inflammatory Fibrosis
The gut microbiota plays a critical role in maintaining intestinal homeostasis, and dysregulation of microbiota is implicated in the pathogenesis of IBD and its complications, including intestinal fibrosis.84,85 The dysfunction of bacterial sensing by nucleotide-binding oligomerization domain-containing protein 2 (NOD2) is suggested to trigger intestinal fibrosis because CD patients carrying single or 2 NOD2 mutations are at an increased risk for developing strictures and fistulae.86 Increased serum antibodies to flagellin (anti-CBir1) in CD patients suggest aberrant adaptive immunity to commensal bacteria.87 Furthermore, serum anti-CBir1 is associated independently with fibrostenosing disease feature in CD patients.88
Several studies have shown that mice fail to develop intestinal fibrosis in germ-free conditions or after antibiotics pretreatment,89, 90, 91, 92, 93 which indicate that commensal bacteria participate in the development of intestinal fibrosis. Furthermore, certain pathogens could induce intestinal fibrosis, such as adherent-invasive Escherichia coli94,95 and Salmonella enterica serovar Typhimurium.96 Aberrant immune responses to microbial stimuli regulate stromal cells as discussed earlier, contributing to tissue damage and intestinal fibrosis. Antibiotic treatment decreases profibrotic cytokine TGF-β1 production in the intestine,93 which is critical in stromal cell activation and collagen deposition. Several other cytokines also are involved in microbiota–immune cell–stromal cell interactions. For instance, various gut microbiota differentially induce the differentiation of T-cell subsets.97 These T cells then regulate stromal cells by producing their feature cytokines. In addition, microbiota could affect the activation, differentiation, and functions of stromal cells directly, leading to intestinal fibrosis. Stromal cells express Toll-like receptors (TLRs) and Nod-like receptors (NLRs) to recognize bacteria-derived pathogen-associated molecular patterns. Lipopolysaccharide, the ligand for TLR4, activates myofibroblasts98 and increases collogen contraction in intestinal fibroblasts.99 Flagellin, which is present in flagellated bacteria and activates TLR5 signaling, promotes fibronectin and collagen production in human intestinal myofibroblasts.100 Mice deficient in the pregnane X receptor, the receptor for microbiota-derived indole-3-propionic acid, showed exacerbated intestinal fibrosis, and myofibroblast lacking the pregnane X receptor were hyperresponsive to stimulation to produce proinflammatory cytokines,101 suggesting that microbiota-derived metabolites also may mediate microbiota–stromal interaction.
The Challenges and Future Directions
Many challenges remain in the research on the roles of stromal cells in IBD-associated intestinal fibrosis. Because intestinal fibrosis often is associated with severe intestinal inflammation, inflammation has been considered a crucial factor in driving intestinal fibrosis. However, current anti-inflammatory biologics and small-molecule therapies for IBD patients are ineffective in treating fibrosis, suggesting that additional inflammation-independent mechanisms also contribute to fibrosis in IBD, which need to be understood for targeting to develop new therapeutic strategies to target and reverse these fibrotic complications. It will be interesting to investigate such inflammation-independent pathways in mediating intestinal fibrosis. Furthermore, although many immune responses regulate intestinal inflammation in IBD, the mechanisms remain largely unclear. Understanding such mechanisms will provide novel insights into developing new therapies targeting immune responses affecting fibrosis pathways, potentially offering more effective treatments.
Acknowledgments
CRediT Authorship Contributions
Wenjing Yang, MD, PhD (Conceptualization: Equal; Data curation: Lead; Investigation: Lead; Methodology: Lead; Writing – original draft: Equal; Writing – review & editing: Equal)
Tianming Yu, MD, PhD (Conceptualization: Equal; Data curation: Equal; Investigation: Equal; Methodology: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Yingzi Cong, PhD (Conceptualization: Lead; Funding acquisition: Lead; Writing – original draft: Lead; Writing – review & editing: Lead)
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK135193, DK124132, and DK125011.
References
- 1.Rieder F., Fiocchi C. Intestinal fibrosis in IBD—a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol. 2009;6:228–235. doi: 10.1038/nrgastro.2009.31. [DOI] [PubMed] [Google Scholar]
- 2.Ramos G.P., Papadakis K.A. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamias G., Pizarro T.T., Cominelli F. Immunological regulation of intestinal fibrosis in inflammatory bowel disease. Inflamm Bowel Dis. 2021;28:337–349. doi: 10.1093/ibd/izab251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rieder F., Fiocchi C. Intestinal fibrosis in inflammatory bowel disease — current knowledge and future perspectives. J Crohns Colitis. 2008;2:279–290. doi: 10.1016/j.crohns.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Wang J., Lin S., Brown J.M., van Wagoner D., Fiocchi C., Rieder F. Novel mechanisms and clinical trial endpoints in intestinal fibrosis. Immunol Rev. 2021;302:211–227. doi: 10.1111/imr.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan W.P.W., Mourad F., Leong R.W. Crohn's disease associated strictures. J Gastroenterol Hepatol. 2018;33:998–1008. doi: 10.1111/jgh.14119. [DOI] [PubMed] [Google Scholar]
- 7.Hwang J.M., Varma M.G. Surgery for inflammatory bowel disease. World J Gastroenterol. 2008;14:2678–2690. doi: 10.3748/wjg.14.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon I.O., Agrawal N., Willis E., et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment Pharmacol Ther. 2018;47:922–939. doi: 10.1111/apt.14526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin X., Wang Y., Liu Z., et al. Intestinal strictures in Crohn's disease: a 2021 update. Therap Adv Gastroenterol. 2022;15 doi: 10.1177/17562848221104951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon I.O., Bettenworth D., Bokemeyer A., et al. International consensus to standardise histopathological scoring for small bowel strictures in Crohn’s disease. Gut. 2022;71:479–486. doi: 10.1136/gutjnl-2021-324374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bruyn J.R., Meijer S.L., Wildenberg M.E., et al. Development of fibrosis in acute and longstanding ulcerative colitis. J Crohns Colitis. 2015;9:966–972. doi: 10.1093/ecco-jcc/jjv133. [DOI] [PubMed] [Google Scholar]
- 12.Magro F., Sousa H.T. Editorial: ulcerative colitis submucosal fibrosis and inflammation: more than just strictures. Aliment Pharmacol Ther. 2018;47:1033–1034. doi: 10.1111/apt.14575. [DOI] [PubMed] [Google Scholar]
- 13.Baumgart D.C., Le Berre C. Newer biologic and small-molecule therapies for inflammatory bowel disease. N Engl J Med. 2021;385:1302–1315. doi: 10.1056/NEJMra1907607. [DOI] [PubMed] [Google Scholar]
- 14.Li C., Kuemmerle J.F. The fate of myofibroblasts during the development of fibrosis in Crohn's disease. J Dig Dis. 2020;21:326–331. doi: 10.1111/1751-2980.12852. [DOI] [PubMed] [Google Scholar]
- 15.Lawrance I.C., Rogler G., Bamias G., et al. Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. 2017;11:1491–1503. doi: 10.1016/j.crohns.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera J., Henke C.A., Bitterman P.B. Extracellular matrix as a driver of progressive fibrosis. J Clin Invest. 2018;128:45–53. doi: 10.1172/JCI93557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owens B.M.J., Simmons A. Intestinal stromal cells in mucosal immunity and homeostasis. Mucosal Immunol. 2013;6:224–234. doi: 10.1038/mi.2012.125. [DOI] [PubMed] [Google Scholar]
- 18.Keating A. Mesenchymal stromal cells. Curr Opin Hematol. 2006;13:419–425. doi: 10.1097/01.moh.0000245697.54887.6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnhoorn M.C., Hakuno S.K., Bruckner R.S., et al. Stromal cells in the pathogenesis of inflammatory bowel disease. J Crohns Colitis. 2020;14:995–1009. doi: 10.1093/ecco-jcc/jjaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roulis M., Flavell R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92:116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Lovisa S., Genovese G., Danese S. Role of epithelial-to-mesenchymal transition in inflammatory bowel disease. J Crohns Colitis. 2019;13:659–668. doi: 10.1093/ecco-jcc/jjy201. [DOI] [PubMed] [Google Scholar]
- 22.Rieder F., Kessler S.P., West G.A., et al. Inflammation-induced endothelial-to-mesenchymal transition: a novel mechanism of intestinal fibrosis. Am J Pathol. 2011;179:2660–2673. doi: 10.1016/j.ajpath.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valatas V., Filidou E., Drygiannakis I., et al. Stromal and immune cells in gut fibrosis: the myofibroblast and the scarface. Ann Gastroenterol. 2017;30:393–404. doi: 10.20524/aog.2017.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim K.K., Sheppard D., Chapman H.A. TGF-β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol. 2018;10:a022293. doi: 10.1101/cshperspect.a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grim C., Noble R., Uribe G., et al. Impairment of tissue-resident mesenchymal stem cells in chronic ulcerative colitis and Crohn's disease. J Crohns Colitis. 2021;15:1362–1375. doi: 10.1093/ecco-jcc/jjab001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J., Mao R., Kurada S., et al. Pathogenesis of fibrostenosing Crohn's disease. Transl Res. 2019;209:39–54. doi: 10.1016/j.trsl.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Frangogiannis N. Transforming growth factor-β in tissue fibrosis. J Exp Med. 2020;217 doi: 10.1084/jem.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi T., Hattori S., Shinkai H. Matrix metalloproteinases-2 and -9 are secreted from human fibroblasts. Acta Derm Venereol. 2003;83:105–107. doi: 10.1080/00015550310007436. [DOI] [PubMed] [Google Scholar]
- 29.Giannandrea M., Parks W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidson S., Coles M., Thomas T., et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21:704–717. doi: 10.1038/s41577-021-00540-z. [DOI] [PubMed] [Google Scholar]
- 31.Chalkidi N., Paraskeva C., Koliaraki V. Fibroblasts in intestinal homeostasis, damage, and repair. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.924866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stzepourginski I., Nigro G., Jacob J.M., et al. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A. 2017;114:E506–E513. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jasso G.J., Jaiswal A., Varma M., et al. Colon stroma mediates an inflammation-driven fibroblastic response controlling matrix remodeling and healing. PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin J.C., Chang C., Boschetti G., et al. Single-cell analysis of Crohn's disease lesions identifies a pathogenic cellular module associated with resistance to anti-TNF therapy. Cell. 2019;178:1493–1508.e20. doi: 10.1016/j.cell.2019.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Graham M.F., Diegelmann R.F., Elson C.O., et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology. 1988;94:257–265. doi: 10.1016/0016-5085(88)90411-8. [DOI] [PubMed] [Google Scholar]
- 36.Flynn R.S., Murthy K.S., Grider J.R., et al. Endogenous IGF-I and alphaVbeta3 integrin ligands regulate increased smooth muscle hyperplasia in stricturing Crohn's disease. Gastroenterology. 2010;138:285–293. doi: 10.1053/j.gastro.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Severi C., Sferra R., Scirocco A., et al. Contribution of intestinal smooth muscle to Crohn's disease fibrogenesis. Eur J Histochem. 2014;58:2457. doi: 10.4081/ejh.2014.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C., Iness A., Yoon J., et al. Noncanonical STAT3 activation regulates excess TGF-β1 and collagen I expression in muscle of stricturing Crohn's disease. J Immunol. 2015;194:3422–3431. doi: 10.4049/jimmunol.1401779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meier J.K., Scharl M., Miller S.N., et al. Specific differences in migratory function of myofibroblasts isolated from Crohn's disease fistulae and strictures. Inflamm Bowel Dis. 2011;17:202–212. doi: 10.1002/ibd.21344. [DOI] [PubMed] [Google Scholar]
- 40.Chen W., Lu C., Hirota C., et al. Smooth muscle hyperplasia/hypertrophy is the most prominent histological change in Crohn's fibrostenosing bowel strictures: a semiquantitative analysis by using a novel histological grading scheme. J Crohns Colitis. 2017;11:92–104. doi: 10.1093/ecco-jcc/jjw126. [DOI] [PubMed] [Google Scholar]
- 41.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lewis A., Sánchez S., Berti G., et al. Small-molecule Wnt inhibitors are a potential novel therapy for intestinal fibrosis in Crohns disease. Clin Sci (Lond) 2022;136:1405–1423. doi: 10.1042/CS20210889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang H., Shen J., Ran Z. Epithelial–mesenchymal transition in Crohn’s disease. Mucosal Immunol. 2018;11:294–303. doi: 10.1038/mi.2017.107. [DOI] [PubMed] [Google Scholar]
- 44.Li S.S., Sun Q., Hua M.R., et al. Targeting the Wnt/β-catenin signaling pathway as a potential therapeutic strategy in renal tubulointerstitial fibrosis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.719880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu B., Phan S.H. Notch in fibrosis and as a target of anti-fibrotic therapy. Pharmacol Res. 2016;108:57–64. doi: 10.1016/j.phrs.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu L., Lin X., Lu H., et al. An overview of Hedgehog signaling in fibrosis. Mol Pharmacol. 2015;87:174–182. doi: 10.1124/mol.114.095141. [DOI] [PubMed] [Google Scholar]
- 47.O'Callaghan C., Vassilopoulos A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell. 2017;16:1208–1218. doi: 10.1111/acel.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao T., Rajasingh S., Rajasingh J. Circulating fibrocytes serve as a marker for clinical diagnosis. Ann Transl Med. 2016;4(Suppl 1):S38. doi: 10.21037/atm.2016.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suga H., Rennert R.C., Rodrigues M., et al. Tracking the elusive fibrocyte: identification and characterization of collagen-producing hematopoietic lineage cells during murine wound healing. Stem Cells. 2014;32:1347–1360. doi: 10.1002/stem.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueno A., Jijon H.B., Peng R., et al. Association of circulating fibrocytes with fibrostenotic small bowel Crohn's disease. Inflamm Bowel Dis. 2022;28:246–258. doi: 10.1093/ibd/izab157. [DOI] [PubMed] [Google Scholar]
- 51.Kinchen J., Chen H.H., Parikh K., et al. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386.e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang M., Zhang S.T. Cells in fibrosis and fibrotic diseases. Front Immunol. 2020;11:1142. doi: 10.3389/fimmu.2020.01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao X., Yang W., Yu T., et al. Th17 cell-derived amphiregulin promotes colitis-associated intestinal fibrosis through activation of mTOR and MEK in intestinal myofibroblasts. Gastroenterology. 2023;164:89–102. doi: 10.1053/j.gastro.2022.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gieseck R.L., Wilson M.S., Wynn T.A. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18:62–76. doi: 10.1038/nri.2017.90. [DOI] [PubMed] [Google Scholar]
- 55.Stolfi C., Troncone E., Marafini I., et al. Role of TGF-beta and Smad7 in gut inflammation, fibrosis and cancer. Biomolecules. 2020;11:17. doi: 10.3390/biom11010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frede A., Czarnewski P., Monasterio G., et al. B cell expansion hinders the stroma-epithelium regenerative cross talk during mucosal healing. Immunity. 2022;55:2336–2351.e12. doi: 10.1016/j.immuni.2022.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Yao H., Tang G. Macrophages in intestinal fibrosis and regression. Cell Immunol. 2022;381 doi: 10.1016/j.cellimm.2022.104614. [DOI] [PubMed] [Google Scholar]
- 58.Cox N., Pokrovskii M., Vicario R., et al. Origins, biology, and diseases of tissue macrophages. Annu Rev Immunol. 2021;39:313–344. doi: 10.1146/annurev-immunol-093019-111748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pierce G.F., Mustoe T.A., Lingelbach J., et al. Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Biol. 1989;109:429–440. doi: 10.1083/jcb.109.1.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wynn T.A., Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–257. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Braga T.T., Agudelo J.S., Camara N.O. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin-Rodriguez O., Gauthier T., Bonnefoy F., et al. Pro-resolving factors released by macrophages after efferocytosis promote mucosal wound healing in inflammatory bowel disease. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.754475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramachandran P., Dobie R., Wilson-Kanamori J.R., et al. Resolving the fibrotic niche of human liver cirrhosis at single-cell level. Nature. 2019;575:512–518. doi: 10.1038/s41586-019-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fabre T., Barron A.M.S., Christensen S.M., et al. Identification of a broadly fibrogenic macrophage subset induced by type 3 inflammation. Sci Immunol. 2023;8 doi: 10.1126/sciimmunol.add8945. [DOI] [PubMed] [Google Scholar]
- 65.Siakavellas S.I., Bamias G. Tumor necrosis factor-like cytokine TL1A and its receptors DR3 and DcR3: important new factors in mucosal homeostasis and inflammation. Inflamm Bowel Dis. 2015;21:2441–2452. doi: 10.1097/MIB.0000000000000492. [DOI] [PubMed] [Google Scholar]
- 66.Barrett R., Zhang X., Koon H.W., et al. Constitutive TL1A expression under colitogenic conditions modulates the severity and location of gut mucosal inflammation and induces fibrostenosis. Am J Pathol. 2012;180:636–649. doi: 10.1016/j.ajpath.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang D.H., Yang S.K., Song K., et al. TNFSF15 is an independent predictor for the development of Crohn's disease-related complications in Koreans. J Crohns Colitis. 2014;8:1315–1326. doi: 10.1016/j.crohns.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 68.Shih D.Q., Zheng L., Zhang X., et al. Inhibition of a novel fibrogenic factor Tl1a reverses established colonic fibrosis. Mucosal Immunol. 2014;7:1492–1503. doi: 10.1038/mi.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jacob N., Kumagai K., Abraham J.P., et al. Direct signaling of TL1A-DR3 on fibroblasts induces intestinal fibrosis in vivo. Sci Rep. 2020;10 doi: 10.1038/s41598-020-75168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fichtner-Feigl S., Young C.A., Kitani A., et al. IL-13 signaling via IL-13R alpha2 induces major downstream fibrogenic factors mediating fibrosis in chronic TNBS colitis. Gastroenterology. 2008;135:2003–2013. doi: 10.1053/j.gastro.2008.08.055. 13.e1–7. [DOI] [PubMed] [Google Scholar]
- 71.Fichtner-Feigl S., Fuss I.J., Young C.A., et al. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 72.Cao H., Zhang J., Liu H., et al. IL-13/STAT6 signaling plays a critical role in the epithelial-mesenchymal transition of colorectal cancer cells. Oncotarget. 2016;7:61183–61198. doi: 10.18632/oncotarget.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Biancheri P., Pender S.L., Ammoscato F., et al. The role of interleukin 17 in Crohn's disease-associated intestinal fibrosis. Fibrogenesis Tissue Repair. 2013;6:13. doi: 10.1186/1755-1536-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramani K., Biswas P.S. Interleukin-17: friend or foe in organ fibrosis. Cytokine. 2019;120:282–288. doi: 10.1016/j.cyto.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhou L., Todorovic V. Interleukin-36: structure, signaling and function. Adv Exp Med Biol. 2021;21:191–210. doi: 10.1007/5584_2020_488. [DOI] [PubMed] [Google Scholar]
- 76.Boutet M.A., Bart G., Penhoat M., et al. Distinct expression of interleukin (IL)-36α, β and γ, their antagonist IL-36Ra and IL-38 in psoriasis, rheumatoid arthritis and Crohn's disease. Clin Exp Immunol. 2016;184:159–173. doi: 10.1111/cei.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Russell S.E., Horan R.M., Stefanska A.M., et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 2016;9:1193–1204. doi: 10.1038/mi.2015.134. [DOI] [PubMed] [Google Scholar]
- 78.Nishida A., Hidaka K., Kanda T., et al. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:303–314. doi: 10.1097/MIB.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 79.Scheibe K., Kersten C., Schmied A., et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology. 2019;156:1082–1097.e11. doi: 10.1053/j.gastro.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 80.Scheibe K., Backert I., Wirtz S., et al. IL-36R signalling activates intestinal epithelial cells and fibroblasts and promotes mucosal healing in vivo. Gut. 2017;66:823–838. doi: 10.1136/gutjnl-2015-310374. [DOI] [PubMed] [Google Scholar]
- 81.Yun S.M., Kim S.H., Kim E.H. The molecular mechanism of transforming growth factor-β signaling for intestinal fibrosis: a mini-review. Front Pharmacol. 2019;10:162. doi: 10.3389/fphar.2019.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ignotz R.A., Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 83.Xu J., Lamouille S., Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nishida A., Inoue R., Inatomi O., et al. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11:1–10. doi: 10.1007/s12328-017-0813-5. [DOI] [PubMed] [Google Scholar]
- 85.Watanabe D., Kamada N. Contribution of the gut microbiota to intestinal fibrosis in Crohn's disease. Front Med (Lausanne) 2022;9 doi: 10.3389/fmed.2022.826240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Adler J., Rangwalla S.C., Dwamena B.A., et al. The prognostic power of the NOD2 genotype for complicated Crohn's disease: a meta-analysis. Am J Gastroenterol. 2011;106:699–712. doi: 10.1038/ajg.2011.19. [DOI] [PubMed] [Google Scholar]
- 87.Lodes M.J., Cong Y., Elson C.O., et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Targan S.R., Landers C.J., Yang H., et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 89.Crawford P.A., Gordon J.I. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao Z., Cheng W., Qu W., et al. Antibiotic alleviates radiation-induced intestinal injury by remodeling microbiota, reducing inflammation, and inhibiting fibrosis. ACS Omega. 2020;5:2967–2977. doi: 10.1021/acsomega.9b03906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rigby R.J., Hunt M.R., Scull B.P., et al. A new animal model of postsurgical bowel inflammation and fibrosis: the effect of commensal microflora. Gut. 2009;58:1104–1112. doi: 10.1136/gut.2008.157636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jacob N., Jacobs J.P., Kumagai K., et al. Inflammation-independent TL1A-mediated intestinal fibrosis is dependent on the gut microbiome. Mucosal Immunol. 2018;11:1466–1476. doi: 10.1038/s41385-018-0055-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mourelle M., Salas A., Guarner F., et al. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998;114:519–526. doi: 10.1016/s0016-5085(98)70535-9. [DOI] [PubMed] [Google Scholar]
- 94.Imai J., Kitamoto S., Sugihara K., et al. Flagellin-mediated activation of IL-33-ST2 signaling by a pathobiont promotes intestinal fibrosis. Mucosal Immunol. 2019;12:632–643. doi: 10.1038/s41385-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu Y., Qian W., Huang L., et al. Crohn's disease-associated AIEC inhibiting intestinal epithelial cell-derived exosomal let-7b expression regulates macrophage polarization to exacerbate intestinal fibrosis. Gut Microbes. 2023;15 doi: 10.1080/19490976.2023.2193115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Grassl G.A., Valdez Y., Bergstrom K.S., et al. Chronic enteric salmonella infection in mice leads to severe and persistent intestinal fibrosis. Gastroenterology. 2008;134:768–780. doi: 10.1053/j.gastro.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 97.Ivanov, Tuganbaev T., Skelly A.N., et al. T cell responses to the microbiota. Annu Rev Immunol. 2022;40:559–587. doi: 10.1146/annurev-immunol-101320-011829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Otte J.M., Rosenberg I.M., Podolsky D.K. Intestinal myofibroblasts in innate immune responses of the intestine. Gastroenterology. 2003;124:1866–1878. doi: 10.1016/s0016-5085(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 99.Burke J.P., Cunningham M.F., Watson R.W., et al. Bacterial lipopolysaccharide promotes profibrotic activation of intestinal fibroblasts. Br J Surg. 2010;97:1126–1134. doi: 10.1002/bjs.7045. [DOI] [PubMed] [Google Scholar]
- 100.Zhao S., Dejanovic D., Yao P., et al. Selective deletion of MyD88 signaling in α-SMA positive cells ameliorates experimental intestinal fibrosis via post-transcriptional regulation. Mucosal Immunol. 2020;13:665–678. doi: 10.1038/s41385-020-0259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Flannigan K.L., Nieves K.M., Szczepanski H.E., et al. The pregnane X receptor and indole-3-propionic acid shape the intestinal mesenchyme to restrain inflammation and fibrosis. Cell Mol Gastroenterol Hepatol. 2023;15:765–795. doi: 10.1016/j.jcmgh.2022.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]