No effective treatment of systemic sclerosis-associated interstitial lung disease (SSc-ILD) exists. Numerous evidences support the importance of adaptive immunity in SSc-ILD. A basket trial showed higher efficacy of mycophenolate when combined with rituximab in non-specific interstitial pneumonia (NSIP), the most frequent pattern in SSc-ILD.1 Deep B-cell depletion outperforms rituximab in connective tissue diseases,2 3 prompting us to combine these principles in SSc-ILD.
We here report on a 38-year-old woman suffering from Scl70+SSc with rapid progressive NSIP in which we added CD19.CAR-T-cells to a pre-existing therapy with mycophenolate/nintedanib (figure 1A). Stably elevated highly sensitive troponin T (hsTNT) levels were interpreted as mild myocardial involvement, pulmonary arterial hypertension was excluded. Except for mild obesity and a long-ago history of smoking, the medical history was empty. The disease progressed despite cyclophosphamide, mycophenolate and nintedanib. The latter was initiated because the criteria for progressive pulmonary fibrosis (PPF) were met.4
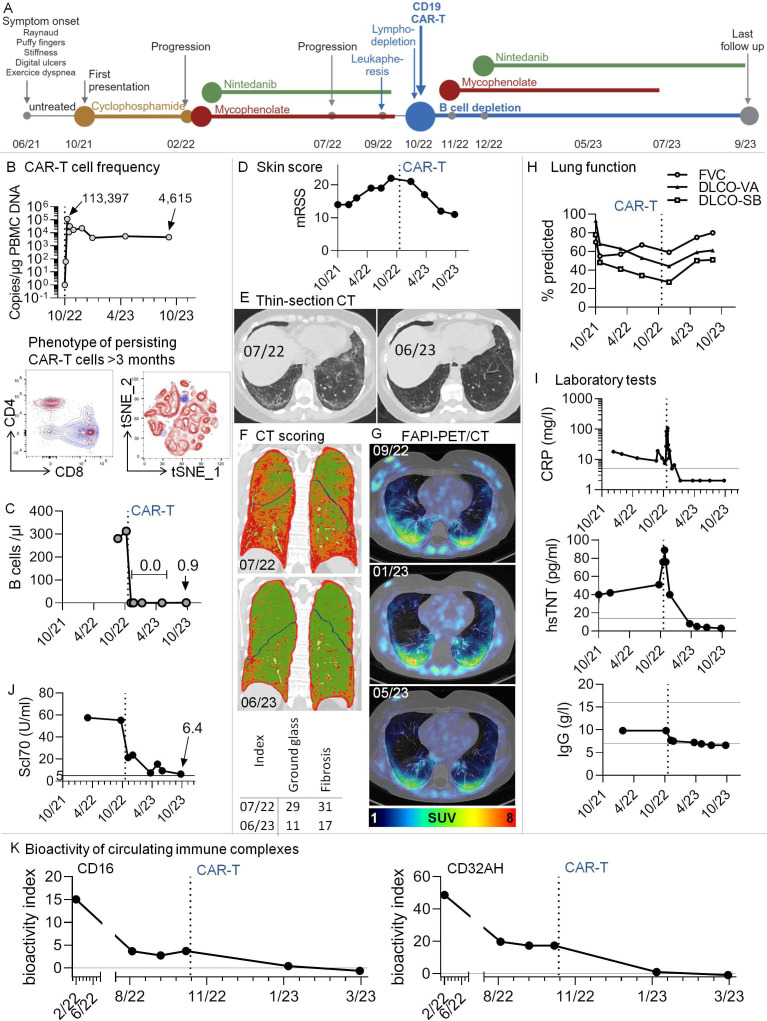
Figure 1.
Multimodal therapy including third-generation CD19.CAR-T-cells in a patient with Scl70+systemic sclerosis and progressive pulmonary fibrosis. (A) Graphical overview of disease course and therapeutic regimen. (B) Top: CAR-T-cells rapidly expanded in vivo and were still detectable after 11 months. The graph shows copy numbers of the CAR construct related to the amount of DNA in peripheral blood mononuclear cells (PBMCs), as determined by PCR. Bottom: High-dimensional spectral flow cytometry was used to phenotype persisting CAR-T-cells at months 3 and 7 after infusion. An antibody against the CD19-CAR construct was used to identify CAR-T-cells. Data from the two time points were in silico concatenated and shown together in single graphs. The dot plots show overlays of persisting CAR-T-cells (blue) with non-CAR total (left) and CD8+ (right) T cells after gating on non-doublet, viable, CD45+CD3+ lymphocytes. (C) B-cell counts were determined by standard conventional flow cytometry. No B-cells could be detected after CAR-T therapy. In 10/23, very low levels of B-cells were redetectable (0.9 /µL). (D) Modified Rodnan Skin Score (mRSS). The degree of skin sclerosis was evaluated by the same treating rheumatologist over time. (E) Thin-section CT. Ground glass opacification and signs of pulmonary fibrosis decreased dramatically after CAR-T-cell therapy. Example axial non-enhanced images before/after CAR-T-cell therapy; (F) CT scoring. Colour-coded coronal images at the indicated time points before/after CAR-T therapy. Based on radiological densities in CT scans (Hounsfield units), cut-off values were chosen to separate lung areas into ‘normal’, ‘ground glass’ and‘fibrosis’ using an algorithm, similar to previously described.6 These areas were imaged by a colour-code (green: normal lung, orange: ground glass, red: fibrosis). Bottom: For volumetric quantification, summarising ground glass and fibrosis indices were calculated, with indices giving the relative volume of affected lung parenchyma. (G) Low-dose CT scans were combined with fibroblast activation protein-inhibitor positron emission tomography (68Ga-FAPI-PET/CT), an imaging modality to assess fibroblast activation in vivo. Pulmonary areas with 68Ga-FAPI-uptake showed a tendency for regression. SUV, standardised uptake value; HU, Hounsfield units. (H) Lung function tests were performed at several time points before and after CAR-T-cell infusion. (I) Selected clinical laboratory test results over time. Additional horizontal lines denote normal ranges. (J) Anti-Scl70 (topoisomerase) autoantibodies over time. The horizontal line denotes the cut-off of normal. (K) Longitudinal measurement of the bioactivity of circulating immune complexes at Fcɣ-receptor-IIIA and -IIA (CD16 and CD32AH) indicating the disappearance of bioactive immune complexes after CAR-T-cell therapy. A functional reporter cell-based assay was applied as reported earlier.16 The bioactivity index was calculated by (mean sample−mean negative control)/(mean positive control−mean negative control)×100, leading to results between 0 and 100. The bioactivity index is both a surrogate of the presence of soluble immune complexes in serum and proof of their bioactivity via Fcɣ-receptor engagement. DLCO-SB/-VA, single-breath/ventilation-adapted diffusion capacities; FVC, forced vital capacity; CRP, C reactive protein; hsTNT, highly sensitive troponin T.
Given her poor prognosis, our interdisciplinary team offered the compassionate use of third-generation CD19.CAR-T-cells, for the first time in a non-cancer patient. We generated CD19.CAR-T-cells in our Good Manufactory Practice facility by transducing autologous T-lymphocytes retrovirally with a CAR containing intracellular coactivating domains of CD3ζ, CD28 and 41BB.5 Mycophenolate/nintedanib was stopped before leukapheresis and lymphodepletion chemotherapy (500 mg/m2 cyclophosphamide+30 mg/m2 fludarabine on days −4 to –3, −2).5 On day −3, fever and elevated C reactive protein (CRP) occurred, requiring an empirical antibiotic therapy. On day 0 (October 2022), the patient received 400×106 (5×106/kg of body weight) CD19.CAR-T-cells (figure 1B).5 The same day, she developed a short phase of hypotension (100/50 mm Hg) and a dry cough. A CT scan indicated mild exacerbation of interstitial pneumonia. These CAR-T-cell infusion-associated findings were interpreted as cytokine release syndrome I°. Tocilizumab was not required. CAR-T-cells expanded rapidly and B-cells vanished (figure 1B, C). After day 10, the general condition, cough and CRP regressed to levels prior to chemotherapy. In contrast to systemic lupus,3 CAR-T-cells in our patient did not induce immediate amelioration. Therefore, and according to the will of the patient, mycophenolate and nintedanib were reinitiated.
Skin fibrosis (Modified Rodnan Skin Score) regressed (figure 1D); the fingers appeared less puffy, despite persisting contractions and Raynaud’s phenomenon. No digital ulcers occurred. By March 2023, dyspnoea regressed gradually. According to CT scans, pulmonary affection improved dramatically, including indices of ground glass opacification and fibrosis6 (figure 1E,F). 68Ga-fibroblast activation protein-inhibitor positron emission tomography (68Ga-FAPI-PET/CT) confirmed regressing, yet not completely resolved, pulmonary fibroblast activation at month 6 (figure 1G).7 8 Lung function improved (figure 1H). In July, the patient could climb 2–3 floors without pause, while, during her worst phases, she had suffered from speech dyspnoea. Despite the withdrawal of mycophenolate in July, this improved condition persisted until last follow-up (September).
CRP, hsTNT and Scl70 normalised or strongly decreased (figure 1I J). ANA titres gradually dropped from 1:5120 to 1:320 at last follow-up. CAR-T-cells persisted during the follow-up (figure 1B), being >90% CD8-positive and highly expressing PD1, CD57 and TIGIT, while CD8 and CD27 were lowly expressed, indicating chronic stimulation and exhaustion. Scattered B-cells reappeared at the last follow-up.
Additionally, we observed a disappearance of Fcɣ-receptor-activating immune complexes, which had persisted before the addition of CAR-T-cells (figure 1K).
Together, we report on the first non-cancer patient in whom third-generation CD19.CAR-T-cells were applied.5 The combination regimen in this SSc-case lead to a sustained amelioration of lung function, aligned by a dramatic regression of imaging findings. This treatment success suggests that pulmonary improvement is an achievable goal in SSc-ILD/PPF. While effects through chemotherapy may also have contributed to short-term effects, the 11-month lasting benefit was confirmed by continuously normalised CRP and hsTNT and regressing autoantibody levels.
This study is also the first to describe the disappearance of Fcɣ-receptor-activating immune complexes by CD19.CAR-T-cell-containing therapy in autoimmune disease. Immune complexes in Scl70+SSc have been suggested to contribute to pathology via activating Fcɣ-receptors.9–11
While PPF is clinically defined,4 the pathophysiology of ILD and lung fibrosis is extremely complex, with ‘early’ injury and ‘late’ fibroblast-driven phases being closely intertwined.12 The clinical aspects demonstrate an improvement of ILD and PPF in our patient. Beyond this and despite the absence of repeated lung biopsies, it can be assumed that also late fibroblast-driven pathophysiology, pulmonary fibrosis in the narrower sense, has improved. This assumption is based on slowly reduced fibroblast pathology in vivo in FAPI-PET/CTs which paralleled clinical amelioration and reduced autoimmune features. In short, all investigated aspects of autoimmunity-related ILD and lung fibrosis have improved.
Our case builds on other CAR-T-cell reports in rheumatology.3 13 14 Previous reports studied second-generation CAR-T-cells. Further differences include higher numbers of infused cells, persistence of CAR-T-cells and long-term B-cell depletion. As this is the first report of long-term persistence of CD19.CAR-T cells in a patient with rheumatic/fibrotic disease, the reasons and consequences need to be considered. Reasons may include the higher cell numbers infused, but also potentially persisting CD19+cells, as antigen-recognition is pivotal for CAR-T-cell persistence. Whether CAR-T-cell persistence is necessary for the delayed clinical effect in SSc or is rather a risk that should be avoided, must be addressed in larger studies.
No severe adverse event occurred, but we critically question fludarabine in ILD, given rare fludarabine-associated pneumonitis15 and the temporary deterioration of lung pathology in our patients.
Overall, CD19.CAR-T-cell-combination led to a hitherto unachieved pulmonary improvement in autoimmune PPF.
Acknowledgments
We thank Anja Funkert, Stefan Krienke and further staff of the Medizinische Klinik V and Thoraxklinik, Heidelberg, including the team of the GMP Core Facility and all physicians, involved in the manufacturing of the 3rd generation CD19.CAR-T-cells, in the treatment of the patient and the sampling of materials or data.
Footnotes
Handling editor: Josef S Smolen
Contributors: Served as scientific advisors: VF, CW, AS, TS, CM-T, PD, MC, PK and MP; biomaterial sampling, experimental design, conduct, interpretation: WM, PK, MC, MF, LR, FD, IA, L-OT and TT; critically reviewed the manuscript: all authors; collected data/performed diagnostic procedures: WM, CPH, MP, MS and MR; cared for study patient: WM, MS, H-ML, AS, PD and TS; overseeing the project, data handling, writing the manuscript: WM.
Funding: Deutsche Gesellschaft für Innere Medizin e.V., German Research Foundation (DFG, TR 466-4-1).
Competing interests: L-OT has received research support from Pfizer. WM has received consulting fees, speaking fees, support for meetings and/or travel and/or honoraria from Novartis, Roche, UCB, BMS and Galapagos and unrestricted third-party funds from Roche between 2015 and 2018. H-ML has received grants from Abbvie, Novartis, Pfizer, Roche/Chugai and consulting fees, honoraria, meeting support from Abbvie, Astra-Zeneca, Actelion, Amgen, Bayer Vital, Boehringer Ingelheim, BMS, Celgene, GSK, Gilead/Galapagos, Janssen-Cilag, Lilly, Medac, MSD, Novartis, Pfizer, Roche/Chugai, Sanofi, UCB. NB has received honoraria and meeting support from SOBI, Novartis and Boehringer. MS: research grants from Apogenix, Hexal and Novartis. Travel grants from Hexal and Kite. Financial support for educational activities and conferences from bluebird bio, Kite and Novartis. Advisory board member of MSD. (Co‐)PI of clinical trials of MSD, GSK, Kite and BMS. Co‐Founder and shareholder of TolerogenixX. PD: consultancy AbbVie, AstraZeneca, Gilead, Janssen, Novartis, Riemser, Roche; speakers bureau AbbVie, Gilead, Novartis, Riemser, Roche; research support from Neovii and Riemser. None of the mentioned sources supported the work described within this manuscript. TS: Consultant AbbVie, Takeda, Astellas, Amgen, Bristol‐Myers Squibb, Gilead, Ridgeline Discoveries. Honorarium: Pfizer, AbbVie, Jazz Pharmaceuticals. Financial support congress participation: AbbVie, Jazz Pharmaceuticals., CM-T: research support from Bayer AG. Advisory board member Pfizer, Janssen‐Cilag. Grants and/or provision of investigational medicinal products from Pfizer, Daiichi Sankyo, BiolineRx. MP: payment or honoraria for lectures, presentations, speakers’ bureaus from AstraZeneca and Boehringer Ingelheim. AS: Travel grants from Hexal and Jazz Pharmaceuticals. Research grant from Therakos/Mallinckrodt. Consultancy BMS, Janssen‐Cilag. Co‐founder and part‐time employee of TolerogenixX of TolerogenixX. CH: see last page on uploaded COI file. The remaining coauthors declared no competing interests (TT, MF, FD, LR, IA, CW, PK, VF and MR).
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves a human participant and was approved by clinical ethic committee University clinic Heidelberg, 20220808. The participant gave informed consent to participate in the study before taking part.
References
- 1. Mankikian J, Caille A, Reynaud-Gaubert M, et al. Rituximab and mycophenolate mofetil combination in patients with interstitial lung disease (EVER-ILD): a double-blind, randomised, placebo-controlled trial. Eur Respir J 2023;61. 10.1183/13993003.02071-2022 [DOI] [PubMed] [Google Scholar]
- 2. Kvacskay P, Merkt W, Günther J, et al. Obinutuzumab in connective tissue diseases after former Rituximab-non-response: a case series. Ann Rheum Dis 2022;81:744–6. 10.1136/annrheumdis-2021-221756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mackensen A, Müller F, Mougiakakos D, et al. Anti-Cd19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat Med 2022;28:2124–32. 10.1038/s41591-022-02017-5 [DOI] [PubMed] [Google Scholar]
- 4. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022;205:e18–47. 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schubert M-L, Schmitt A, Hückelhoven-Krauss A, et al. Treatment of adult ALL patients with third-generation Cd19-directed CAR T cells - results of a pivotal trial. J Hematol Oncol 2023;16:79. 10.1186/s13045-023-01470-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Colombi D, Dinkel J, Weinheimer O, et al. Visual vs fully automatic Histogram-based assessment of idiopathic pulmonary fibrosis (IPF) progression using sequential Multidetector computed tomography (MDCT). PLoS One 2015;10:e0130653. 10.1371/journal.pone.0130653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Röhrich M, Leitz D, Glatting FM, et al. Fibroblast activation protein-specific PET/CT imaging in Fibrotic interstitial lung diseases and lung cancer: A Translational exploratory study. J Nucl Med 2022;63:127–33. 10.2967/jnumed.121.261925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rosenkrans ZT, Massey CF, Bernau K, et al. 68) Ga]Ga-FAPI-46 PET for non-invasive detection of pulmonary fibrosis disease activity. Eur J Nucl Med Mol Imaging 2022;49:3705–16. 10.1007/s00259-022-05814-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lande R, Lee EY, Palazzo R, et al. Cxcl4 assembles DNA into liquid crystalline complexes to amplify Tlr9-mediated interferon-Α production in systemic sclerosis. Nat Commun 2019;10:1731. 10.1038/s41467-019-09683-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. French MA, Harrison G, Penning CA, et al. Serum immune complexes in systemic sclerosis: relationship with precipitating nuclear antibodies. Ann Rheum Dis 1985;44:89–92. 10.1136/ard.44.2.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Penning CA, Cunningham J, French MA, et al. Antibody-dependent cellular cytotoxicity of human vascular Endothelium in systemic sclerosis. Clin Exp Immunol 1984;57:548–56. [PMC free article] [PubMed] [Google Scholar]
- 12. Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet 2022;400:769–86. 10.1016/S0140-6736(22)01052-2 [DOI] [PubMed] [Google Scholar]
- 13. Müller F, Boeltz S, Knitza J, et al. Cd19-targeted CAR T cells in refractory Antisynthetase syndrome. Lancet 2023;401:815–8. 10.1016/S0140-6736(23)00023-5 [DOI] [PubMed] [Google Scholar]
- 14. Bergmann C, Müller F, Distler JHW, et al. Treatment of a patient with severe systemic sclerosis (SSC) using Cd19-targeted CAR T cells. Ann Rheum Dis 2023;82:1117–20. 10.1136/ard-2023-223952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helman DL, Byrd JC, Ales NC, et al. Fludarabine-related pulmonary toxicity: a distinct clinical entity in chronic lymphoproliferative syndromes. Chest 2002;122:785–90. 10.1378/chest.122.3.785 [DOI] [PubMed] [Google Scholar]
- 16. Zhao S, Grieshaber-Bouyer R, Rao DA, et al. Effect of JAK inhibition on the induction of proinflammatory HLA-DR+Cd90+ rheumatoid arthritis Synovial fibroblasts by interferon-Γ. Arthritis Rheumatol 2022;74:441–52. 10.1002/art.41958 [DOI] [PMC free article] [PubMed] [Google Scholar]