Abstract
The binding of human immunodeficiency virus type 1 (HIV-1) (Hx10) virions to two different cell lines was analyzed by using a novel assay based on the detection, by anti-HLA-DR-specific antibodies, of HLA-DR+ virus binding to HLA-DR− cells. Virion attachment to the CD4+-T-cell line A3.01 was highly CD4 dependent in that it was potently inhibited by CD4 monoclonal antibodies (MAbs), and little virus binding to the CD4− sister A2.01 line was observed. By contrast, virion binding to HeLa cells expressing moderate or high levels of CD4 was equivalent to, or lower than, binding to wild-type CD4− HeLa cells. Moreover, several CD4 MAbs did not reduce, but enhanced, HIV-1 attachment to HeLa-CD4 cells. CD4 was required for infection of HeLa cells, however, demonstrating a postattachment role for this receptor. MAbs specific for the V2 and V3 loops and the CD4i epitope of gp120 strongly inhibited virion binding to HeLa-CD4 cells, whereas MAbs specific for the CD4bs and the 2G12 epitopes enhanced attachment. Despite this, all gp120- and gp41-specific MAbs tested neutralized infectivity on HeLa-CD4 cells. HIV-1 attachment to HeLa cells was only partially inhibited by MAbs specific for adhesion molecules present on the virus or target cells but was completely blocked by polyanions such as heparin, dextran sulfate, and pentosan sulfate. Treatment of HeLa-CD4 cells with heparinases completely eliminated HIV attachment and infection, strongly implicating cell surface heparans in the attachment process. CD4 dependence for HIV-1 attachment to target cells is thus highly cell line specific and may be replaced by other ligand-receptor interactions.
Human immunodeficiency virus type 1 (HIV-1) cellular tropism is determined, with few exceptions, both in vitro and in vivo by expression of the cellular receptor molecule, CD4 (reviewed in references 12 and 71). The physiological target cells for HIV-1 infection, CD4+ T cells, monocytes/macrophages, and some populations of dendritic cells, all express CD4 (reviewed in reference 41). Most CD4− cells of human or nonhuman primate origin can be rendered susceptible to HIV infection by transfection of CD4 (4, 20, 44). HIV-1 binds a 20-amino-acid loop in the first domain of CD4 via an interaction with the virus surface glycoprotein, the gp120 molecule (reviewed in reference 75). Additional interactions take place between HIV-1 and the recently described coreceptor molecules, members of the seven-transmembrane-domain, G-protein-coupled chemokine receptor family (recently reviewed in references 7, 26, and 55). A number of these chemokine receptors function in HIV infection and HIV-induced syncytium formation. The CXCR4 molecule is the receptor for the chemokine SDF-1, and its expression confers susceptibility to T-cell line-adapted (TCLA) and syncytium-inducing primary isolate HIV-1 viruses (8, 30, 72, 88). CCR5 is the principal coreceptor for macrophage-tropic, non-syncytium-inducing HIV-1 (1, 19, 27, 28) and is important in HIV transmission, since individuals homozygous for an inactivating deletion in the CCR5 gene are relatively resistant to HIV infection (63, 68). It is thought that HIV binding to CD4 induces conformational changes in the HIV envelope glycoproteins that result in the exposure of a coreceptor binding site on gp120 (39, 77, 81, 85). The interaction of gp120 and perhaps gp41 with CD4 and the coreceptor molecules results ultimately, by a largely unknown mechanism, in the fusion of virus and cell membranes (reviewed in references 10 and 49).
Measurements of the affinity between soluble CD4 (sCD4) and soluble gp120 (sgp120) reveal a high-affinity interaction in the low-nanomolar range, with the precise value depending on the viral origin and method of production of the gp120 (13, 37, 40, 53). On the surface of the virion, each molecule of gp120 is noncovalently associated with a molecule of the transmembrane glycoprotein gp41, and these heterodimers are organized into trimers (18, 83, 84). The affinity between sCD4 and virion-associated, trimeric gp120 is often lower than that measured for the monomeric forms of gp120 (51, 70); for certain primary-isolate gp120s this can be as much as 200-fold lower (50). The dynamics of the association between HIV and cell-associated CD4 have not been well studied, and we do not have estimates for the avidity of this interaction. Moreover, it seems likely that a variety of factors influence the efficiency of virion-cell binding. For example, molecules of cellular origin, such as HLA-DR and adhesion molecule LFA-1 and its ligands ICAM-1, -2, and -3, are incorporated into HIV virions (3, 6, 33, 60) and can, in certain systems, increase virus infectivity (17, 33, 66), probably by increasing the avidity between the virion and the cell. Cell surface polyanions are also thought to participate in HIV infection of T cells; proteoglycan-anchored heparan sulfate interacting with the V3 loop of TCLA HIV-1 gp120 facilitates infection of T cells (59, 62, 67). It has nonetheless been assumed that CD4 plays a central role in HIV attachment to its target cells and that interactions between other ligand-receptor pairs serve only to reinforce the gp120-CD4 interaction.
HeLa cells stably transfected with human CD4 (HeLa-CD4 cells) are permissive for TCLA HIV-1 infection, since they express the coreceptor CXCR4, and have been used extensively for virus production and to measure virus infectivity and its inactivation. It has previously been shown that TCLA HIV-1 infection of HeLa-CD4 cells is inefficient, in that only a very small fraction of the total infectious virus population was able to infect these cells, and infection was independent of CD4 expression level (38). In order to investigate the role of HIV-1 attachment in this phenomenon, we tested the binding of the TCLA, CXCR4-dependent HIV-1 molecular clone Hx10 to HeLa and HeLa-CD4 cells and compared this with virion binding to the T-cell line A3.01 (CD4+) and its sister line A2.01 (CD4−). Virion attachment to cells was measured by using a novel assay based on the detection of virion-associated HLA-DR molecules by indirect immunofluorescence and flow cytometric analysis (80). Since HeLa, HeLa-CD4, A3.01, and A2.01 cells are all HLA-DR−, bound virus particles will yield an HLA-DR+ signal. We show that whereas HIV-1 attachment to a CD4+-T-cell line is highly CD4 dependent, attachment to wild-type HeLa or HeLa-CD4 cells is CD4 independent. By contrast, infection of HeLa-CD4 cells by HIV-1 requires CD4. Virus binding to, and infection of, HeLa-CD4 cells was potently inhibited by certain monoclonal antibodies (MAbs) specific for gp120, by sulfated polysaccharides, and by treatment of the cells with heparinases. These results demonstrate that HIV-1 binding to HeLa-CD4 cells is CD4 independent but requires other interactions between gp120 and cell surface heparans. Moreover, the data show that the requirement for CD4 for virion-cell binding is highly cell type dependent and imply that the intrinsic affinity between cell surface CD4 and TCLA virion-associated, native gp120 may be low.
MATERIALS AND METHODS
Antibodies and recombinant proteins.
The anti-CD4 mouse MAbs were obtained as follows: Q4120 and Q425 (from H. Holmes and the Medical Research Council [MRC] AIDS Reagent Project, Potters Bar, United Kingdom) were previously mapped to the first and third domains of CD4, respectively (36); L120 (36) and L222 (24) were produced and characterized at Becton Dickinson Immunocytochemistry Systems (San Jose, Calif.), were obtained from D. Buck, and map to the fourth domain and the first-domain CDR-2-like loop of CD4, respectively; 5A8 binds the second CD4 domain and was obtained from L. Burkly (Biogen Inc, Cambridge, Mass.) (14); and 13B.8.2, which binds the CDR-3-like loop of CD4 domain 1, was kindly provided by M. Hirn (Immunotech SA, Marseille, France) (69). Q4120, 13B.8.2, and L222 interfere with sgp120-CD4 binding and inhibit HIV infection and syncytium formation, 5A8 and Q425 inhibit HIV infection and syncytium formation but not sgp120-CD4 binding, and L120 does not influence sgp120-cell binding and only weakly interferes with infection and syncytium formation (36, 80). The CXCR4-specific MAb 12G5 was a kind gift from J. Hoxie (University of Pennsylvania, Philadelphia) (29). Biotinylated anti-HLA-DR MAb B8.12.2 and CD26-specific MAb BA5 were from Immunotech SA. The anti-adhesion molecule MAbs, listed in Table 1 (see Results), specific for CD2, CD11a, CD11b, CD11c, CD18, CD29, CD49a, CD49b, CD49c, CD49d, CD49e, CD50, CD58, CD59, and CD102, were obtained from the Fifth Leucocyte Typing Workshop (Boston, Mass.) (73). The human anti-V3 loop MAb 447-52D (23, 35) was purchased from Cellular Products Inc., Buffalo, N.Y. The chimpanzee anti-gp120/V2 MAb C108G, obtained from S. Tilley (Public Health Research Institute, New York, N.Y.), was prepared and characterized as previously described (82). The human MAbs specific for the CD4bs, F91, the CD4-induced epitope (CD4i) 48d (76, 87), and the V3 loop, 19b (56), were from J. Robinson (University of Connecticut, Storrs). Recombinant anti-CD4bs monospecific antibody IgG1b12 (15) and the anti-V3 loop monospecific antibody Loop 2 and its Fab were from D. Burton (Scripps Research Institute, La Jolla, Calif.) and were prepared as previously described (5, 25). The human anti-gp120 MAb 2G12 (78, 79) and the anti-gp41 MAb 2F5 (57, 58) were prepared by H. Katinger and were obtained from the MRC AIDS Reagent Project. Sheep anti-HIV-1 gp120 antibody D7324 (52), raised against a peptide synthesized from a highly conserved sequence in the gp120 COOH terminus, was from Aalto BioReagents Ltd. (Dublin, Ireland). Recombinant sCD4 (32) was from L. Burkly (Biogen, Cambridge, Mass.). HIV-1IIIB (CHO cell produced)-derived purified recombinant sgp120 was obtained from the MRC AIDS Reagent Project.
TABLE 1.
Inhibition of HIV-1–cell binding and infection by anti-adhesion molecule MAbsa
| MAb clusterb | MAb name | Phenotypec
|
Inhibition on HeLa-CD4 cellsd of:
|
||
|---|---|---|---|---|---|
| PM1 cells | HeLa-CD4 cells | Virus binding | Virus infection | ||
| CD4 | Q4120 | ++ | + | × | +++ |
| CD11b (Mac-1) | CC1.7 | +++ | − | − | + |
| TMG6-5 | NDe | ND | − | + | |
| CD18 (integrin β2) | MAY.017 | +++ | − | − | ++ |
| CD29 (integrin β1) | 5D9 | + | +++ | × | − |
| CD44 (H-CAM) | NIH44-1 | +++ | ++++ | + | + |
| CD49a (VLA-1) | IB3.1 | + | +++ | − | − |
| SR84 | + | +++ | − | − | |
| CD49b (VLA-2) | 12F1 | − | +++ | + | − |
| CD49c (VLA-3) | A3-IIF5 | + | +++ | ++ | ++ |
| CD49d (VLA-4) | HP2/1 | + | − | + | ++ |
| CD49e (VLA-6) | SAM1 | − | ++ | + | + |
MAbs specific for the following adhesion molecules were tested and found to be negative for inhibition of both virus attachment and infection: CD2 (LFA-2), CD11a (LFA-1), CD11c (p150), CD50 (ICAM-3), CD54 (ICAM-1), and CD102 (ICAM-2).
The CD clustering was as defined at the Fifth Leucocyte Typing Workshop, Boston, Mass., 1995.
Phenotyping was carried out by labeling the cells with the indicated MAbs followed by indirect immunofluorescence staining and flow cytometric analysis. −, MFI of <10; +, MFI of 11 to 40; ++, MFI of 41 to 100; +++, MFI of 101 to 500; ++++, MFI of >500.
×, >21% enhancement; −, <20% enhancement and <20% inhibition (considered to be within the experimental error range for a nonsignificant effect); +, 21 to 40% inhibition; ++, 41 to 70% inhibition, +++, >71% inhibition.
ND, not done.
Cell culture and virus infection.
The HLA-DR−/CD4+ line A3.01 and its CD4− derivative A2.01 were obtained from T. Folks (Center for Disease Control, Atlanta, Ga.) and were cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), here termed growth medium (GM). The HLA-DR+/CD4+ PM1 cell line derived from HUT78 by P. Lusso and R. Gallo (43), was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Md. HeLa cells were obtained from the MRC AIDS Reagent Project, and HeLa-CD4-LTR-LacZ cells (HeLa-P4 cells [21]) were from P. Charneau (Pasteur Institute, Paris, France). HeLa clone 15 cells were obtained by stably transfecting HeLa cells with the human CD4 cDNA by PMV7-based retroviral transfection as previously described (44) and then selecting a high-expressing clone. The HIV-1 molecular clone Hx10 (31) was obtained from A. Fisher (Royal Postgraduate Medical School, London, United Kingdom). The preparation of concentrated virus for the virus-cell attachment assay was carried out essentially as described previously (80). Briefly, PM1 cells infected overnight with Hx10 at a multiplicity of infection of about 0.1 were cultured in GM which was renewed every 24 h to prevent the accumulation of heat-inactivated virus particles (45) and to allow culture of the cells at high density. The production of viral p24 protein was monitored by p24 enzyme-linked immunosorbent assay (52), and the supernatants containing the peak production of p24 were harvested. After removal of the supernatant, the infected cells were vortexed for 1 min and remixed with the supernatant, the cells were again pelleted, and the supernatant was harvested; we have found that vortexing the cells increases the virus titer by up to fivefold. Virus-containing supernatant was clarified by centrifugation at 3,000 × g and filtration through a 0.45-μm-pore-size filter and then concentrated by about 10-fold with a 300-kDa-cutoff Macrosep centrifugal concentrator (Filtron Technology Corp. Northborough, Mass.). The concentrated virus was aliquoted and stored at −80°C until used. Mock-infected supernatants from uninfected PM1 cells were prepared in the same way.
Virus neutralization assays.
Virus neutralization determined with HeLa-CD4-LTR-LacZ cells (here termed HeLa-CD4 cells) was carried out as previously described (65). Briefly, 6 × 105 HeLa-CD4 cells, pretreated or not with anti-CD4 MAbs (10 μg/ml), anti-adhesion molecule MAbs (25 μg of purified immunoglobulin G [IgG] per ml or a 1/200 dilution of ascites), or heparinases as described below, were incubated with virus pretreated or not with MAbs or polyanions, either for 30 min at 37°C (subsequently used for the virus binding test [see below]) or for 2 h at 37°C (for inhibition-of-infection analysis). Subsequently the cells were washed, trypsinised, cultured for 36 h in GM, and lysed, the soluble substrate chlorophenol red β-d-galactopyranoside was added, and the optical density at 550 nm (OD550) was determined. Percent inhibition was calculated by the formula 100 − [(t − c)/(m − c) × 100], where t represents the OD signal for the test sample, c represents the background signal in the absence of virus, and m represents the maximum signal obtained with virus but no inhibitor. Inhibition of virus infectivity measured with A3.01 cells was carried out as follows. Virus was preincubated with anti-Env MAbs for 1 h at 37°C before addition of 5 × 104 A3.01 cells. Cells and virus were incubated together for 2 h at 37°C, washed, and cultured for 48 h. The supernatant was harvested and tested for cell-free p24 protein as described previously (52). The results are expressed as percent inhibition of HIV infection, calculated on the basis of p24 concentration.
Virus binding assay.
The virus-cell attachment assay was carried out essentially as previously described (80) by incubation of 50 μl of concentrated virus, or dilutions thereof, with 5 × 105 cells for 30 min at 37°C in a total volume of 70 μl. HeLa and HeLa-CD4 cells were detached from monolayers with phosphate-buffered saline (PBS)–10 mM EDTA and washed prior to addition of virus. Subsequently, the cells were washed twice in PBS–1% FCS–0.02% sodium azide (wash buffer [WB]) and then resuspended in 50 μl of either the biotinylated HLA-DR MAb B8.12.2 or, in one experiment (see Fig. 2B), the HIV-1 gp120bs-specific MAb IgG1b12 at 10 μg/ml for 1 h at 4°C. Another variation of this protocol was to preincubate the virus stock with the biotinylated B8.12.2 detection antibody for 1 h at 4°C before addition to the cells (see Fig. 3B). The cells were washed twice in WB and then fixed overnight in 0.5% formaldehyde in WB. No vortexing was carried out during the washing steps to avoid removing virus bound weakly to the cell surface. After being washed three times in WB, the cells were incubated for 1 h at 4°C in WB containing either a 1/50 dilution of streptavidin-phycoerythrin or a 1/50 dilution of anti-human IgG–phycoerythrin (Immunotech). Bound virus was detected by flow cytometry with a FACScan with Lysis II software (Becton Dickinson, San Jose, Calif.), and analysis was carried out on 104 accumulated events, gated on side- and forward-angle light scatter. Inhibition of virus binding by CD4 MAbs and poly-l-lysine was carried out by preincubating cells for 1 h at 4°C before addition of virus, whereas inhibition by anti-Env MAbs or polyanions was carried out by preincubating virus with the ligands for 2 h at 37°C before addition of cells; virus neutralization and inhibition of binding were carried out with the same virus preparation (see “Virus neutralization assays” above). Dextran, dextran sulfate, pentosan polysulfate, heparin, chondroitin sulfate, poly-l-lysine, and heparinases I, II, and III were obtained from Sigma-Aldrich. Heparinases were diluted in 10 mM phosphate buffer (pH 7.4) containing 0.15 M NaCl, 3 mM KCl, 0.5 mM MgCl2, 1 mM CaCl2, 0.1% glucose, 1% FCS, and 0.5% bovine serum albumin and were used at a final concentration of 10 U/ml. Cells were incubated with the enzymes for 1 h at 37°C before washing and addition of virus.
FIG. 2.
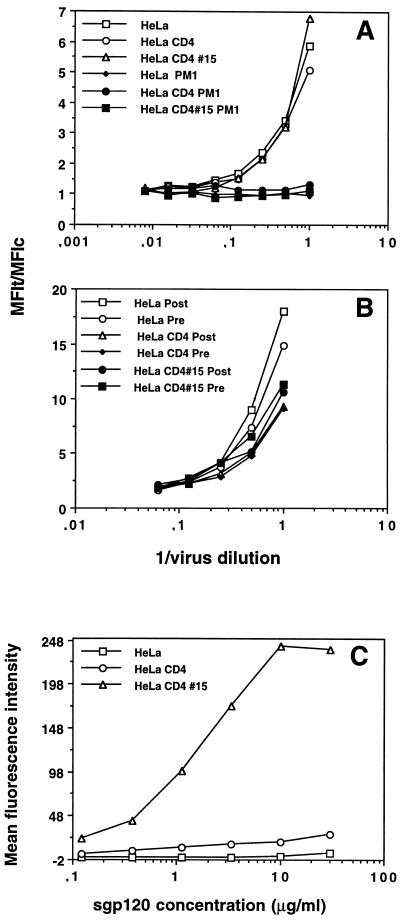
Hx10 attachment to HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells. Cells in suspension were incubated with undiluted concentrated virus or mock virus for 30 min at 37°C, washed, labeled with biotinylated anti-HLA-DR MAb (A) or gp120 CD4bs-specific MAb IgG1b12 (B), and fixed overnight. The cells were then stained with the appropriate phycoerythrin conjugate, washed, and analyzed by flow cytometry as described for Fig. 1. C-HeLa represents HeLa cells incubated with the phycoerythrin conjugate alone; HeLa-CD4 and HeLa-CD4 clone 15 cells yielded very similar background signals (not shown).
FIG. 3.
Concentration dependence of Hx10 and sgp120IIIB binding to HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells. HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells were incubated with serial dilutions of concentrated virus, mock virus, sgp120 and stained and analyzed as described for Fig. 1. (A) Detection of prebound virus with the biotinylated anti-HLA-DR MAb. Open symbols, incubation with virus; closed sympols, incubation with mock virus. (B) Another experiment in which virus was labeled with anti-HLA-DR MAb prior to cell binding (Pre) or subsequent to cell binding (Post). Results are expressed as the ratio of the test signal to the background signal (MFIt/MFIc) to normalize for variation between the different cell clones in the background staining. (C) HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells were incubated with sgp120 for 4 h at 4°C before being labeled with anti-gp120, washed, and stained with fluorescein conjugate. Binding was analyzed by flow cytometry as described for Fig. 1.
Phenotyping of cells by indirect immunofluorescence and flow cytometry.
Cells (3 × 105), either grown as a suspension culture (PM1) or detached from a monolayer with 10 mM EDTA in PBS (HeLa, HeLa-CD4, and HeLa-CD4 clone 15) were stained with anti-CD4 MAb Q4120 (10 μg/ml), anti-HLA-DR MAb DA6 (10 μg/ml), or anti-adhesion molecule MAbs (25 μg/ml or a 1/200 dilution of ascites) for 1 h at 4°C with agitation. After two washes in WB, cells were incubated for a further 1 h at 4°C in anti-mouse IgG–phycoerythrin (Immunotech) before washing and analysis by flow cytometry as described above. The results are expressed as the test signal minus the background signal (phycoerythrin conjugate alone), since the nonspecific staining for HeLa-CD4 and PM1 cells was very similar.
sgp120-cell binding assay.
HeLa, HeLa-CD4, or HeLa-CD4 clone 15 cells (3 × 105) were incubated for 4 h at 4°C with agitation with different concentrations of sgp120IIIB in a total volume of 30 μl in a 96-well plate. The cells were then washed twice with WB, resuspended in 50 μl of 2-μg/ml D7324, and incubated with agitation for 1 h at 4°C. Anti-sheep IgG–fluorescein isothiocyanate (Sigma-Aldrich) was added to the cell pellet at a 1/64 dilution in 50 μl of WB, and the cells were agitated at 4°C for 1 h and washed twice in WB. Labeled cells were analyzed by flow cytometry as described above.
RESULTS
CD4 expression on HeLa-CD4 cells.
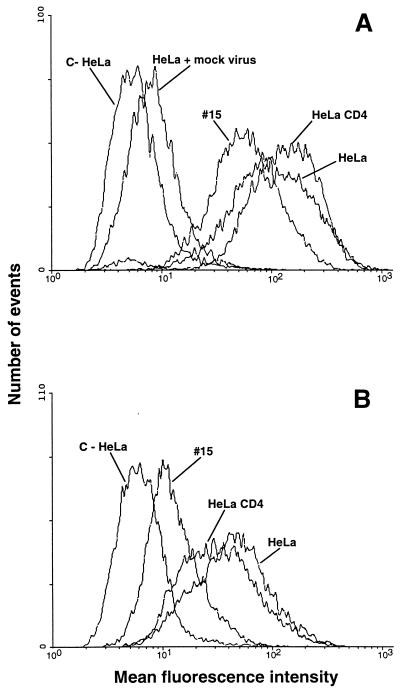
HeLa, HeLa-CD4-LTR-LacZ (HeLa-CD4), HeLa-CD4 clone 15, A3.01, and A2.01 cells were analyzed for CD4 expression by indirect immunofluorescence staining with anti-CD4 domain 1-specific MAb Q4120. The T-cell line A3.01 and its CD4− sister line were chosen for comparison with the HeLa lines, as we had previously demonstrated that HIV-1 binding to A3.01 cells is highly CD4 dependent, and A2.01 cells are an appropriate control line. Figure 1 indicates the relative CD4 levels for each line; HeLa and A2.01 cells were negative, whereas HeLa-CD4 cells demonstrated a moderate level of CD4 staining (mean fluorescence intensity [MFI], ≈70) and HeLa-CD4 clone 15 and A3.01 cells demonstrated high levels (MFI, ≈700 and 1,400, respectively).
FIG. 1.
Measurement of CD4 levels on the cell lines used. (A) HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells; (B) A3.01 and A2.01 cells. Cells in suspension were labeled or not with the CD4 MAb Q4120 for 1 h at 4°C and then washed and stained with anti-mouse IgG–phycoerythrin. Peaks marked C− represent the background staining in the absence of anti-CD4 MAb or CD4− cell lines (A2.01 and wild-type HeLa cells) in the presence of anti-CD4 MAb. Fluorescence was analyzed by flow cytometry; 104 gated events were acquired for each datum point, and these are expressed as the MFI.
HIV-1 binding to HeLa and HeLa-CD4 cells.
We next measured Hx10 binding to the different cell lines. We previously demonstrated that this virus binds in a CD4-dependent manner to A3.01 cells by using a test based on the detection of HLA-DR present in the virion membrane (80). Since neither A3.01 nor HeLa cells express HLA-DR, an HLA-DR+ signal on these cells should represent virus bound to the cell surface. The PM1 cells used to generate the virus stocks produce HLA-DR+ membrane vesicles in the culture supernatant irrespective of whether they are infected (9, 34). In order to control for this material of cellular origin, mock-infected supernatants from PM1 cells were used as specificity controls for virus binding. Figure 2A shows flow cytometric histograms from a representative experiment, in which Hx10 or mock virus binding to HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells was detected by an anti-HLA-DR MAb. In the absence of virus, all cell types gave background signals similar to that of wild-type HeLa cells, with an MFI of approximately 7. The addition of the mock-infected PM1 supernatant increased the detection signal to an MFI of 10 on all cell types, indicating that a small proportion of the signal obtained with virus-containing supernatants is due to cellular material contaminating the virus stock. The signal obtained for wild-type HeLa cells in the presence of virus was ≈140, approximately 14-fold higher than that obtained in the presence of the mock-infected preparation. A slightly higher signal was obtained for HeLa-CD4 cells (MFI, ≈160), but a substantially lower signal (MFI, ≈70) was obtained for HeLa CD4 clone 15. These data imply that there is variation between different clones of HeLa but that the virus binding does not relate to the expression of CD4. As a further control for specificity, detection by the anti-HLA-DR MAb was replaced by detection by the gp120 CD4bs-specific MAb IgG1b12; the advantage of this MAb over other gp120-specific MAbs is that sgp120 shed from the virus during the assay and subsequently bound to CD4 will not be detected, since occupation of the CD4bs on gp120 prevents IgG1b12 binding. Figure 2B shows that although the signal was lower than that obtained by detection with the anti-HLA-DR MAb, it was nevertheless specific since the mock virus gave background levels of staining. Under these conditions, the virus gave a higher signal on HeLa cells (MFI, ≈56) than on the HeLa-CD4 (MFI, ≈45) or HeLa-CD4 clone 15 (MFI, ≈15) cells. The reduced signal obtained with IgG1b12 when virus was bound to HeLa-CD4 cells and particularly to HeLa-CD4 clone 15 cells is probably the result of CD4 molecules binding to the virion gp120 after virion attachment to the cells; this would mask the gp120 CD4bs and prevent IgG1b12 binding. The data obtained with IgG1b12 reinforce the idea that CD4 does not contribute to the initial virus attachment to these cells and confirm that the signal obtained by detection of HLA-DR is representative of virion binding. Titration of Hx10 on the different HeLa clones was carried out to determine whether binding was concentration dependent. The amount of virus bound, detected by anti-HLA-DR MAbs (Fig. 3A), was dose dependent but unrelated to the expression of CD4, further supporting the idea that CD4 is not required for the initial attachment of virus to these cells. One factor that might alter the detection of bound virions is the interaction between CD4 on the target cell and HLA-DR on the virus; this could partially mask HLA-DR epitopes on the virion and reduce the detection signal on CD4+ cells as opposed to CD4− cells. To exclude this possibility, we precoated the virus with the anti-HLA-DR MAb before incubation with the target cells. As shown in Fig. 3B, there is little obvious difference between detection of bound virus by using precoated virions or by binding the HLA-DR MAb after virion attachment to the cells, suggesting that CD4–HLA-DR interactions do not substantially influence virus binding or detection. Moreover, in this experiment the HeLa cells bound more virus than the HeLa-CD4 or HeLa-CD4 clone 15 cells, confirming the lack of CD4 dependence in virus attachment to these cells. To determine whether the CD4 independence of virion attachment was shared by monomeric sgp120, sgp120 derived from the same virus isolate (HIV-1IIIB), was titrated on HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells. The values obtained for HeLa, HeLa-CD4, and HeLa-CD4 clone 15 cells at saturation (30 μg/ml) were ≈5, 27, and 240, respectively (Fig. 3C). The approximately 9-fold-greater binding of sgp120 to HeLa-CD4 clone 15 cells compared to HeLa-CD4 cells reflects the 10-fold difference in CD4 expression as determined by CD4 MAb staining. Thus, sgp120IIIB binding appears to be proportional to CD4 expression on HeLa cells. We were unable to compare directly virion binding and sgp120 binding to the different HeLa clones, since sgp120 binding analysis was carried out at equilibrium with relatively high concentrations of ligand whereas virion binding was not. However, if the initial portions of the curves for sgp120 binding are compared with those for virion binding, it is clear that sgp120 shows CD4 dependency at all concentrations whereas virus does not under any conditions, demonstrating that sgp120-CD4 binding is not representative of virion-CD4 binding.
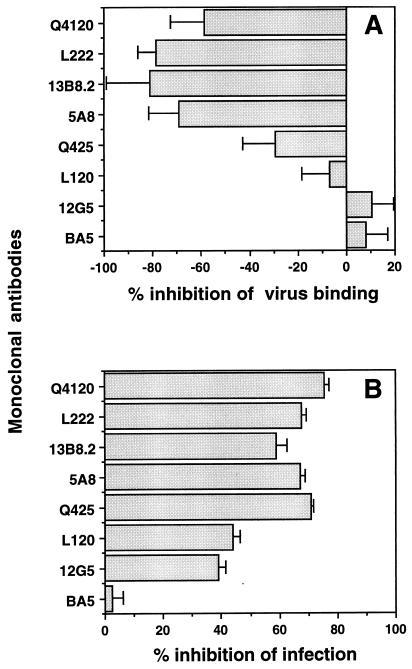
Inhibition of Hx10 binding to A3.01 cells, but not to HeLa-CD4 cells, by CD4 MAbs.
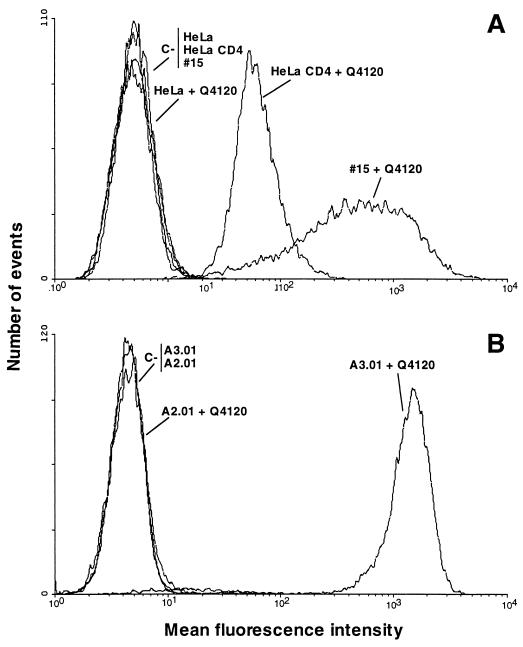
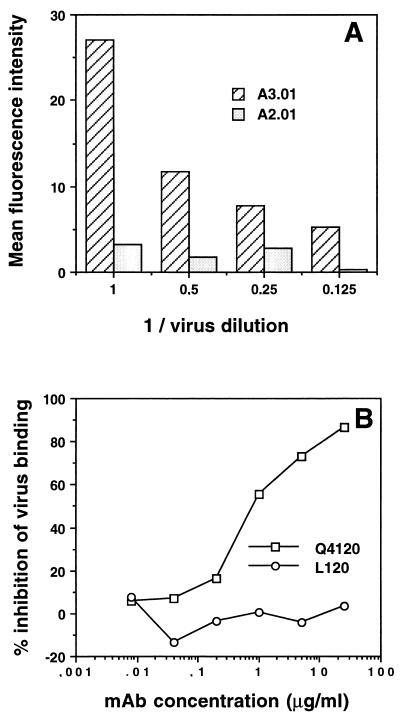
We previously demonstrated that Hx10 attachment to A3.01 cells is CD4 dependent, in that only very low levels of binding to the CD4− sister line A2.01 were detected compared to the signal obtained with A3.01, and CD4 MAbs directed to CD4 domain 1 potently inhibited binding to A3.01 cells (80). Results of similar experiments are shown in Fig. 4; HLA-DR staining increased with decreasing dilution of the virus stock on A3.01 cells but not on A2.01 cells, and the CD4 domain 1-specific MAb Q4120 efficiently prevented virus binding to A3.01, whereas the domain 4-specific MAb L120 had no effect. We tested these and other CD4 MAbs for their ability to inhibit Hx10 binding to HeLa-CD4 cells. As shown in Fig. 5A, at a saturating concentration of 10 μg/ml, none of the CD4 MAbs tested reduced virus-cell binding; indeed, the domain 1-specific MAbs Q4120, L222, and 13B.8.2, the domain 2-specific MAb 5A8, and the domain 3-specific MAb Q425 enhanced virus attachment by 30 to 80%. This enhancement was not observed with wild-type HeLa cells (results not shown), demonstrating that it is CD4 specific. The reason for this enhancement is unclear, but it suggests a qualitatively different interaction between HIV-1 and CD4 on HeLa cells than on A3.01 cells. Neither the domain 4-specific CD4 MAb L120 nor the control MAbs BA5 (anti-CD26) and 12G5 (anti-CXCR4) significantly influenced HIV attachment. By contrast to the results obtained with virus attachment, all of the CD4-specific MAbs reduced HIV infection of the HeLa-CD4 cells by 40 to 75% (Fig. 5B). BA5 had no effect on infectivity, whereas the anti-CXCR4 MAb inhibited HIV infection by about 40%. These results confirm the absence of dependence on CD4 for Hx10 binding to HeLa-CD4 cells but also confirm the requirement for CD4 for HIV infection at a step subsequent to virion attachment.
FIG. 4.
Concentration dependence of Hx10 binding to A3.01 and A2.01 cells and inhibition by CD4 MAbs. (A) A3.01 and A2.01 cells were incubated with serial dilutions of Hx10 for 30 min at 37°C before being washed, labeled with biotinylated anti-HLA-DR, washed, and fixing in formaldehyde. After being stained with streptavidin-phycoerythrin, cells were analyzed by flow cytometry as described for Fig. 1. (B) A3.01 cells were preincubated with CD4 MAbs for 1 h at 4°C before addition of virus. Subsequent steps were as described for panel A.
FIG. 5.
(A) HeLa-CD4 cells were preincubated with CD4 MAbs for 1 h at 4°C before treatment with Hx10 for 30 min at 37°C. Cells were washed, labeled with anti-HLA-DR, fixed overnight, and subsequently stained with streptavidin-phycoerythrin and analyzed as described for Fig. 1. Results are means and standard deviations for triplicates, expressed as percent inhibition of virus binding. (B) HeLa-CD4 cells pretreated with CD4 MAbs and incubated with virus as described for panel A were cultured for 36 h before lysis and addition of substrate to detect activation of β-galactosidase activity. The color change was read as OD550, and results are expressed as percent inhibition of HIV infection.
Inhibition of Hx10 binding to and infection of HeLa-CD4 cells by viral ligands.
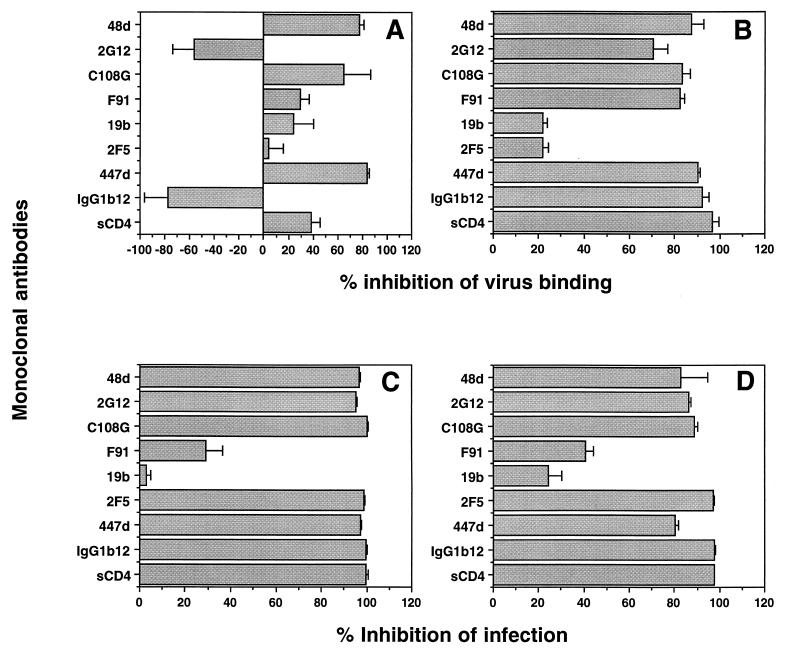
We have previously shown that neutralizing antibodies to HIV-1 gp120 and sCD4 interfere with HIV attachment to A3.01 cells. We therefore decided to test a panel of HIV-1-specific ligands for their ability to interfere with Hx10 binding to HeLa-CD4 cells. Three MAbs substantially inhibited virion binding; the V3 loop MAb 447-52D, the V2 MAb C108G, and the CD4-induced epitope-specific MAb 48d decreased binding by 80, 65, and 80%, respectively (Fig. 6A). Weaker inhibition was achieved by sCD4 (40%) and the CD4bs-specific MAb F91 (30%), and a low level of nonspecific inhibition was observed for the control V3-loop specific MAb 19b, which does not bind with detectable affinity to Hx10 gp120. The anti-gp41 MAb 2F5 had no effect on virus attachment, confirming our earlier results suggesting that gp41 is not involved in this process. Interestingly, MAb 2G12 and the CD4bs-specific MAb IgG1b12 enhanced virus binding by approximately 50 and 75%, respectively. The same pattern of inhibition or enhancement by these MAbs was also seen on wild-type (CD4−) HeLa cells (data not shown), indicating that CD4 did not obviously influence the gp120 MAb-sensitive interactions taking place between gp120 and the cell surface. By contrast to the results obtained with HeLa and HeLa-CD4 cells, all gp120-specific neutralizing MAbs except 19b, the negative control (since this MAb does not bind Hx10 gp120), efficiently inhibited Hx10 attachment to A3.01 cells (Fig. 6B), confirming our previous report (80). All of the MAbs tested that are known to be neutralizing for Hx10 inhibited infection of HeLa-CD4 cells by 95 to 100%, with the exception of F91, which only weakly neutralized (≈30%), and the control MAb 19b (Fig. 6C). As observed with the HeLa-CD4 cells, the gp120- and gp41-specific MAbs potently neutralized (>80%, with the exception of F91 and 19b) infection of A3.01 cells as demonstrated by a diminution of cell-free p24 core protein (Fig. 6D).
FIG. 6.
(A and B) HIV was pretreated with neutralizing anti-Env MAbs at 10 μg/ml or with sCD4 at 3 μg/ml for 1 h at 37°C before incubation with HeLa-CD4 (A) or A3.01 (B) cells for 30 min. After washing, bound virus was detected with anti-HLA-DR MAb as described for Fig. 2A. (C and D) HIV was pretreated with neutralizing anti-Env MAbs at 10 μg/ml or with sCD4 at 3 μg/ml for 1 h at 37°C before incubation with HeLa-CD4 (C) or A3.01 (D) cells for 2 h at 37°C. After culture for 36 h, HeLa-CD4 cells were lysed and analyzed for β-galactosidase activity as described for Fig. 5B, and supernatants from A3.01 cultures were analyzed for cell-free p24 protein by enzyme-linked immunosorbent assay as described in Materials and Methods. Results for both cell types are means and standard deviations for triplicates, expressed as percent inhibition of virus attachment (A and B) and of HIV infection (C and D).
Contribution of adhesion molecules to HIV attachment and infection.
Molecules other than CD4 have been implicated directly or indirectly in increasing HIV-1 binding to target cells. The presence of cell-derived adhesion molecules in the virion envelope has been demonstrated to increase HIV-1 infectivity, presumably by increasing the overall avidity of attachment between the virion and the target cell. In order to investigate the possibility that HIV-HeLa cell attachment was mediated by adhesion molecules, we initially screened PM1 cells (in which the Hx10 was produced) and HeLa-CD4 cells for their expression of adhesion molecules by using a large panel of adhesion molecule-specific MAbs obtained from the Fifth Leucocyte Typing Workshop. The results from this are summarized in Table 1; HeLa-CD4 cells expressed CD29, CD44, CD49a, CD49b, CD49c, CD49e, CD54, CD58, and CD59, whereas PM1 cells expressed all markers tested except CD2, CD11c, CD49b, and CD49e. We tested at least one representative MAb from each cluster for inhibition of HIV attachment to and infection of HeLa-CD4 cells, and we preselected the MAbs based on their ability to interfere with the adhesion interaction between ligand-receptor pairs, as reported in the manual for the Fifth Leucocyte Typing Workshop. Table 1 lists only those MAbs that inhibited in either the virus attachment or the infectivity assay; the others that were tested are noted in the footnotes to Table 1. As shown, only MAbs specific for CD44 and CD49 significantly, albeit weakly, inhibited Hx10 binding to HeLa CD4 cells, whereas MAbs to CD4 and CD29 enhanced binding. By contrast, several MAbs inhibited HIV infection of the same cells, suggesting that this assay may be somewhat more sensitive to MAb-mediated interference than the attachment assay. As expected, a MAb to CD4 was strongly inhibitory for HIV infection. MAbs specific for CD44, CD11b, CD18, and three isotypes of CD49 (c, d, and e) interfered weakly with infection. Taken together, these data suggest that only the receptor-ligand adhesion pairs consisting of ICAM-1 with Mac-1 and CD49 with extracellular matrix may contribute, albeit weakly, to the HIV-cell interactions involved in attachment to and infection of HeLa cells.
Role of cell surface heparans in HIV attachment to and infection of HeLa cells.
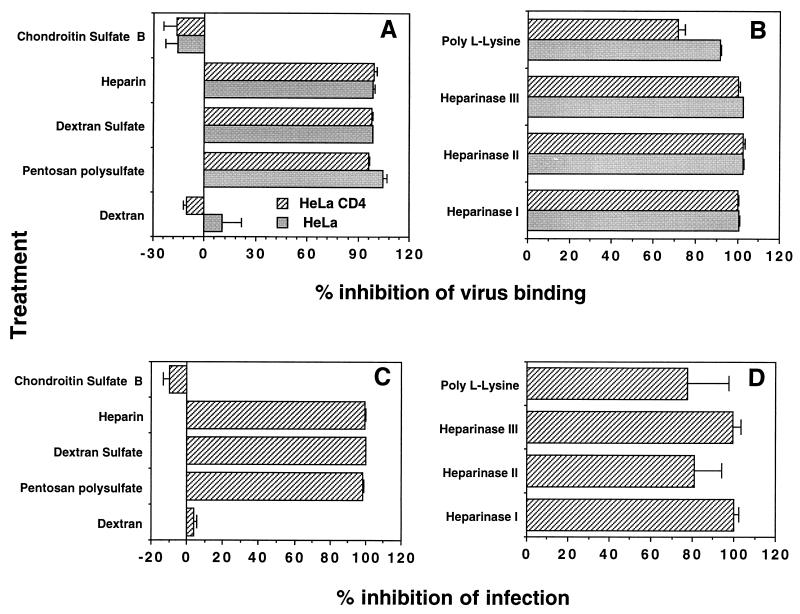
Cell surface heparans have been demonstrated previously to play a role in increasing the infectivity of HIV-1 for certain T-cell lines, and this was shown to be a result of increased virus attachment to cells carrying these polyanionic molecules. We therefore tested a series of polyanions for their ability to prevent Hx10 binding to HeLa or HeLa CD4 cells. Pretreatment of the virus with heparin, dextran sulfate, and pentosan polysulfate at 20 μg/ml inhibited Hx10 attachment (Fig. 7A) and infection (Fig. 7C) by 95 to 100%, whereas the uncharged (dextran) or more weakly charged (chondroitin sulfate) negative controls had no effect at the same concentration. These results suggest that the dominant interactions mediating HIV attachment to HeLa and HeLa-CD4 cells are charge based. To complement these results obtained by treatment of the virus with the sulfated polysaccharides, we pretreated HeLa or HeLa-CD4 cells with the polycation poly-l-lysine at 10 μg/ml for 1 h at 37°C. This treatment inhibited HIV attachment to both cell types by 70 to 90% (Fig. 7B) and reduced infection of HeLa-CD4 cells by ≈75% (Fig. 7D). To determine whether cell surface heparans were mediating virus attachment, we treated the cells with heparinase type I, II, or III at concentrations of 10, 2.5, 1, and 0.4 U/ml for 1 h at 37°C. The results obtained with the enzymes at 10 U/ml are shown in Fig. 7B and D; binding was eliminated completely by treatment with all three enzymes, and this corresponded to reductions in infectivity of approximately 100, 80, and 100% for heparinase types I, II, and III respectively. Treatment with these enzymes did not alter CD4 expression at the cell surface (results not shown). Taken together, these results provide a strong argument for the requirement of cell surface heparans in HIV attachment to HeLa cells.
FIG. 7.
Inhibition of Hx10 binding to HeLa and HeLa-CD4 cells and infection of HeLa-CD4 cells by polyanions, a polycation, and heparinases. (A and B) Hx10 was preincubated with polyanions and control molecules at 10 μg/ml for 1 h at 37°C before addition of HeLa or HeLa-CD4 cells (A), or HeLa and HeLa-CD4 cells were preincubated with poly-l-lysine (10 μg/ml) or heparinases (10 U/ml) for 1 h at 37°C before washing and treatment with virus for 30 min at 37°C (B). Virus binding was detected by biotinylated anti-HLA-DR MAb as described for Fig. 2A. (C and D) Virus was preincubated with polyanions or control molecules for 1 h at 37°C before addition of HeLa-CD4 cells (C), or HeLa-CD4 cells were preincubated with poly-l-lysine or heparinases before washing and addition of virus (D). Cells were cultured for 36 h at 37°C before detection of β-galactosidase activity as described for Fig. 5. Results are means and standard deviations for triplicates, expressed as percent inhibition of virus attachment (A and B) and of HIV infection (C and D).
DISCUSSION
We show in this study that HIV-1 attachment to target cells may be either CD4 dependent, as in the case of the A3.01 T-cell line, or CD4 independent, as in the case of wild-type and CD4+ HeLa cells. Adhesion molecules appear to compensate only weakly for the lack of CD4-mediated virion binding to HeLa cells, whereas charge-based interactions appear to be critical. HIV attachment via a CD4-gp120 interaction is therefore highly cell type dependent and can be replaced with other intermolecular interactions of apparently higher avidity. This finding strongly suggests that the avidity of association between cell surface CD4 and virion-associated gp120 is very likely to be considerably lower than originally thought and shows that the virus may rely to a large extent on other forces to bring it into close contact with the cell membrane. Despite this, CD4 is clearly required for the fusion process, since both CD4-specific and anti-gp120 CD4bs-specific MAbs efficiently inhibit HIV infection of HeLa-CD4 cells.
The binding of sgp120 to cell surface CD4 does not appear to mimic virion attachment to the same cells, since we observed that sgp120 binding was found to be strictly CD4 dependent on HeLa cells, whereas virion binding was CD4 independent. These data are consistent with the findings of Roderiquez and colleagues (67), who demonstrated that the binding of sgp120 to CD4+ T cells was not influenced by heparan sulfate expression, whereas virion binding was. It is unclear from our results whether HIV attachment is absolutely independent of CD4 or whether there may be a low level of gp120-CD4 interaction that is masked by a more avid gp120-heparan sulfate interaction. Several observations argue against a role for CD4 in binding HIV-1 to HeLa cells, however. First, enzymatic removal of cell surface heparans completely eliminated virus binding to HeLa CD4 cells, suggesting that if a residual gp120-CD4 interaction remained, it was below the limit of detection. Second, CD4-specific MAbs known to prevent HIV binding to CD4+ T cells increased HIV attachment to HeLa-CD4 cells. Third, a MAb specific for the CD4bs on gp120 that eliminated HIV binding to A3.01 cells enhanced HIV binding to HeLa-CD4 cells. The molecular mechanisms by which MAbs to CD4 and gp120 CD4bs enhance virion binding to HeLa-CD4 cells are obscure, but these results suggest qualitative differences in the interaction between HIV gp120 and CD4 that are cell type dependent.
The attachment of Hx10 virions to wild-type and CD4+ HeLa cells is likely to be mediated by the gp120 molecule, since MAbs to certain neutralizing epitopes, most notably the V2 and V3 loops and the CD4-induced epitope 48d, prevented this process. It may be that positively charged regions of the V3 loop interact directly with cell surface heparins, as has previously been described (62, 67). Those workers showed that negatively charged polyanions such as dextran sulfate partially inhibited HIV-1 infection of T-cell lines (16) and that this effect was probably mediated at the level of HIV attachment, since virus binding was partially inhibited by polyanions and removal of cell surface heparans (62). Moreover, it was shown that HIV can bind heparan sulfate in the absence of CD4 (62). Our findings are fully consistent with these data. Moreover, the enhanced binding of Hx10 to HeLa-CD4 cells in the presence of MAb 2G12 could be explained by the fact that the binding of this MAb to Hx10 virions partially increases the exposure of the gp120 V3 loop (54), potentially allowing greater contact between V3 and the polyanionic surfaces. Similarly, 2G12 and IgG1b12 may expose other, as yet undefined surfaces required for contact with either polyanions or coreceptor molecules. Taken together with these previous studies, our data are consistent with a model in which preliminary docking of HIV-1 virions with HeLa and, to a lesser extent, T-cell lines would be by gp120-cell surface polyanion interactions, followed by recruitment of CD4 and coreceptor molecules into a fusion-competent complex. Such a model is reminiscent of the binding of herpes simplex virus (HSV) to cells via glycosaminoglycans (86). This initial attachment step is followed by a secondary interaction between HSV and a novel member of the tumor necrosis factor-nerve growth factor receptor family termed HSV entry mediator (46). Since the HIV-1 gp120 V3 loop is thought to form an important component of the coreceptor binding domain (22, 85), this region may subsequently be liberated for interaction with coreceptor molecules, or different segments of the V3 loop may interact simultaneously with the two ligands, as previously suggested (67). In this model, fusion could be inhibited at a post-virus attachment stage by CD4-specific antibodies, a finding that has indeed been made previously with T-cell lines (74). Moreover, postattachment neutralization by anti-V3 loop MAbs (2, 42, 64) may be mediated in part by reversal of virion association with cell surface polyanions and/or by interference with coreceptor binding (77, 85).
The lack of dependence on CD4 of HIV-1 attachment to CD4+ HeLa cells is consistent with the observation that infection of these cells by TCLA HIV-1 is independent of the cell surface CD4 expression level (38). Interpreting these findings in the light of our data, it seems probable that the subsequent recruitment of CD4 molecules by TCLA HIV-1 into a fusion complex is not rate-limiting for infection. Moreover, Kabat et al. (38) showed that only a small fraction of HIV-1 infectivity could be adsorbed onto CD4+ HeLa cells from virus-containing supernatants, suggesting that this may be the rate-limiting step in HIV infection. This conclusion is in accord with a previous study that proposed that virion attachment, as opposed to penetration, is rate-limiting for HIV infection (61). If it is considered that virion-associated gp120 interactions with cellular CD4 are multivalent, then individual interactions between virion-associated gp120 and cell surface CD4 may be very weak indeed. Unlike virion attachment to HeLa cells, binding of sgp120 derived from the same virus isolate was dependent on the level of CD4 expression and had an affinity determined by 50% binding to HeLa CD4 cells in the low-nanomolar range. This estimate corresponds well with previous measurements of the gp120IIIB affinity for sCD4 (40, 47). How can the difference in affinity between monomeric sgp120-monomeric sCD4 interactions and virion-associated gp120-cell surface CD4 interactions be explained? Clearly, there will be geometric and steric constraints on both the virion surface and the cell surface that will reduce the possibility of multiple interactions between CD4 and gp120. Moreover, there is likely to be charge repulsion between the virion and cell membranes at close proximity, and hydration forces will need to be overcome at a very close range. The oligomerization of gp41-associated gp120 into trimers has been shown to reduce affinity for sCD4 by up to 20-fold for TCLA viruses (51), and this is thought to be even more dramatic in the case of primary HIV-1 isolates (50). It has been proposed that primary virus sacrifices CD4 binding affinity in return for a relative increase in resistance to antibody-mediated neutralization (48). Direct binding studies with primary HIV-1 isolates and CD4+ cells have not been carried out, either in the present study or elsewhere. It seems likely that such viruses bind with even lower efficiency to both T cells and HeLa cells than TCLA viruses, since not only may they have a lower intrinsic avidity for CD4 (50), but macrophage-tropic primary viruses also have a reduced positive charge on, and more limited exposure of, the V3 loop (11). Thus, charge-based, V3 loop-dependent interactions are likely to be less efficient for CCR5-using viruses than for CXCR4-using viruses, as has been previously proposed (59, 67). A further implication of the CD4-independent attachment of HIV-1 to cells via heparans is the possibility that this may be a mechanism of virus clearance in vivo, in that the efficient binding of virus to different CD4− cell types would not lead to infection but would rapidly remove virus from the circulation. If CXCR4-using viruses attach more avidly than CCR5-using viruses to negatively charged surfaces, then this might be a mechanism for selective elimination of virus populations in vivo. Further studies comparing CXCR4- and CCR5-using isolates should help to dissect the dynamics of the earliest HIV-cell interactions and may pave the way to development of effective inhibitors of virus attachment.
ACKNOWLEDGMENTS
We thank J. Robinson, D. Burton, J. P. Moore, and S. Tilley for MAbs and L. Burkly for sCD4 and MAb 5A8.
This study was supported by the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Agence Nationale de Recherches sur le SIDA, and the EC Concerted Action Programme “Antibody-Mediated Enhancement and Neutralization of Lentivirus Infections.”
REFERENCES
- 1.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a rantes, MIP-1a, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong S J, McInerney T L, McLain L, Wahren B, Hinkula J, Levi M, Dimmock N J. Two neutralizing anti-V3 monoclonal antibodies act by affecting different functions of human immunodeficiency virus type 1. J Gen Virol. 1996;77:2931–2941. doi: 10.1099/0022-1317-77-12-2931. [DOI] [PubMed] [Google Scholar]
- 3.Arthur L O, Bess J W, Jr, de Sowder R C, Benveniste R E, Mann D L, Chermann J C, Henderson L E. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science. 1992;258:1935–1938. doi: 10.1126/science.1470916. [DOI] [PubMed] [Google Scholar]
- 4.Ashorn P A, Berger E A, Moss B. Human immunodeficiency virus envelope glycoprotein/CD4-mediated fusion of nonprimate cells with human cells. J Virol. 1990;64:2149–2156. doi: 10.1128/jvi.64.5.2149-2156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbas C F I, Collett T A, Amberg W, Roben P, Binley D, Hoekstra D, Cababa D, Jones T M, Williamson R A, Pilkington G R, Haigwood N L, Satterthwait A C, Sanz I, Burton D R. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol. 1993;230:812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 6.Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger E A. HIV entry and tropism: the chemokine receptor connection. AIDS. 1997;11:S3–S16. [PubMed] [Google Scholar]
- 8.Berson J F L, Doranz B J, Rucker J, Jirik F R, Doms R W. A seven-transmembrane-domain receptor involved in fusion and entry of T-cell-tropic human immunodeficiency virus type 1 strains. J Virol. 1996;70:6288–6295. doi: 10.1128/jvi.70.9.6288-6295.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bess J W, Gorelick R J, Henderson L E, Arthur L O. Microvesicles are a source of contaminating cellular proteins found in purified HIV-1 preparations. Virology. 1997;230:134–144. doi: 10.1006/viro.1997.8499. [DOI] [PubMed] [Google Scholar]
- 10.Binley J, Moore J P. The viral mousetrap. Nature. 1997;387:346–348. doi: 10.1038/387346a0. [DOI] [PubMed] [Google Scholar]
- 11.Bou-Habib D C, Roderiquez G, Oravecz T, Berman P W, Lusso P, Norcross M A. Cryptic nature of envelope V3 region epitopes protects primary monocytotropic human immunodeficiency virus type 1 from antibody neutralization. J Virol. 1994;68:6006–6013. doi: 10.1128/jvi.68.9.6006-6013.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bour S, Geleziunas R, Wainberg M A. The human immunodeficiency virus type 1 (HIV-1) CD4 receptor and its central role in promotion of HIV-1 infection. Microbiol Rev. 1995;59:63–93. doi: 10.1128/mr.59.1.63-93.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brighty D W, Rosenberg M, Chen I S Y, Ivey-Hoyle M. Envelope proteins from clinical isolates of HIV-1 which are refractory to neutralisation by sCD4 posses high affinity for the CD4 receptor. Proc Natl Acad Sci USA. 1991;88:7802–7805. doi: 10.1073/pnas.88.17.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkly L C, Olson D, Shapiro R, Winkler G, Rosa J J, Thomas D W, Williams C, Chisholm P. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J Immunol. 1992;149:1779–87. [PubMed] [Google Scholar]
- 15.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W, Sawyer L S, Hendry R M, Dunlop N, Nara P L, Burton D. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 16.Callahan L N, Phelan M, Mallinson M, Norcross M A. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J Virol. 1991;65:1543–1550. doi: 10.1128/jvi.65.3.1543-1550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantin R, Fortin J-F, Lamontagne G, Tremblay M. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J Virol. 1997;71:1922–1930. doi: 10.1128/jvi.71.3.1922-1930.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 19.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 20.Clapham P R, Blanc D, Weiss R A. Specific cell surface requirements for the infection of CD4-positive cells by human immunodeficiency virus types 1 and 2 and by simian immunodeficiency virus. Virology. 1991;181:703–715. doi: 10.1016/0042-6822(91)90904-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clavel F, Charneau P. Fusion from without directed by human immunodeficiency virus particles. J Virol. 1994;68:1179–1185. doi: 10.1128/jvi.68.2.1179-1185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cocci F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 23.Conley A J, Gorny M K, Kessler J A, Boots L J, Ossorio-Castro M, Koenig S, Lineberger D W, Emini E A, Williams C, Zolla-Pazner S. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J Virol. 1994;68:6994–7000. doi: 10.1128/jvi.68.11.6994-7000.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis S, Schockmel G, Somoza C, Buck D, Healey D, Rieber E, Reiter C, Williams A. Antibody and HIV-1 gp120 recognition of CD4 undermines the concept of mimicry between antibodies and receptors. Nature. 1992;358:76–79. doi: 10.1038/358076a0. [DOI] [PubMed] [Google Scholar]
- 25.Ditzel H J, Parren P W H I, Binley J, Sodroski J, Moore J P, Barbas C F, Burton D R. Mapping the protein surface of HIV-1 gp120 using human monoclonal antibodies from phage display libraries. J Mol Biology. 1997;267:684–689. doi: 10.1006/jmbi.1997.0912. [DOI] [PubMed] [Google Scholar]
- 26.Doms R W, Peiper S C. Unwelcomed guests with master keys: how HIV uses chemokine receptors for cellular entry. Virology. 1997;235:179–190. doi: 10.1006/viro.1997.8703. [DOI] [PubMed] [Google Scholar]
- 27.Doranz B, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Doms R W. A dual-tropic primary HIV-1 isolate that uses fusin and the b-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 28.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 29.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis-Turner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Landau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by Fusin/CXCR-4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 30.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 31.Fisher A G, Ensoli B, Looney D, Rose A, Gallo R C, Saag M S, Shaw G M, et al. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988;334:444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- 32.Fisher R A, Bertonis J M, Meier W, Johnson V A, Costopoulos D S, Liu T, Tizard R, Walker B D, et al. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988;331:76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- 33.Fortin J-F, Cantin R, Lamontagne G, Tremblay M. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J Virol. 1997;71:3588–3596. doi: 10.1128/jvi.71.5.3588-3596.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gluschankof P, Mondor I, Gelderblom H R, Sattentau Q J. Cell membrane vesicles are a major contaminant of gradient-enriched human immunodeficiency virus type 1 (HIV-1) preparations. Virology. 1997;230:125–133. doi: 10.1006/viro.1997.8453. [DOI] [PubMed] [Google Scholar]
- 35.Gorny M K, Conley S, Karwowska A, Buchbinder A, Xu J-Y, Emini E A, Koenig S, Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992;66:7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Healey D, Dianda L, Moore J P, McDougal J S, Moore M J, Estess P, Buck D, Kwong P D, Beverley P C L, Sattentau Q J. Novel anti-CD4 monoclonal antibodies separate human immunodeficiency virus infection and fusion of CD4+ cells from virus binding. J Exp Med. 1990;172:1233–1242. doi: 10.1084/jem.172.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ivey-Hoyle M, Culp J S, Chaikin M A, Hellmig B D, Matthews T J, Sweet R W, Rosenberg M. Envelope glycoproteins from biologically diverse isolates of human immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci USA. 1991;88:512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabat D, Kozak S L, Wehrly K, Chesebro B. Differences in CD4 dependence for infectivity of laboratory-adapted and primary patient isolates of human immunodeficiency virus type 1. J Virol. 1994;68:2570–2577. doi: 10.1128/jvi.68.4.2570-2577.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapham C K, Ouyang J, Chandrasekhar B, Kguyen N Y, Dimitrov D S, Golding H. Evidence for cell-surface association between fusin and the CD4-gp120 complex in human cell lines. Science. 1996;274:602–605. doi: 10.1126/science.274.5287.602. [DOI] [PubMed] [Google Scholar]
- 40.Lasky L A, Nakamura G, Smith D H, Fennie C, Shimasaki C, Patzer E, Berman P, Gregory T, Capon D J. Delineation of a region of the human immunodeficiency virus type 1 gp120 glycoprotein critical for interaction with the CD4 receptor. Cell. 1987;50:975–985. doi: 10.1016/0092-8674(87)90524-1. [DOI] [PubMed] [Google Scholar]
- 41.Levy J. HIV and the pathogenesis of AIDS. Washington, D.C: American Society for Microbiology; 1994. [Google Scholar]
- 42.Lu S, Putney S D, Robinson H L. Human immunodeficiency virus type 1 entry into T cells: more-rapid escape from an anti-V3 loop than from an antireceptor antibody. J Virol. 1992;66:2457–2550. doi: 10.1128/jvi.66.4.2547-2550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lusso P, Cocchi F, Balotta C, Markham P D, Louie A, Farci P, Pal R, Gallo R C, C R J M. Growth of macrophage-tropic and primary human immunodeficiency virus type 1 (HIV-1) isolates in a unique CD4+ T-cell clone (PM1): failure to downregulate CD4 and to interfere with cell line-tropic HIV-1. J Virol. 1995;69:3712–3720. doi: 10.1128/jvi.69.6.3712-3720.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maddon P J, Dalgleish A G, McDougal J S, Clapham P R, Weiss R A, Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986;47:333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- 45.McKeating J A, McKnight A, Moore J P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991;65:852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Montgomery R I, Warner M S, Lum R J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 47.Moore J P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990;4:297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Moore, J. P., and D. D. Ho. 1995. HIV neutralization: the consequence of viral adaptation to growth on transformed T cells. AIDS 9(Suppl. A):S117–S136. [PubMed]
- 49.Moore J P, Jameson B A, Weiss R A, Sattentau Q J. The HIV-cell fusion reaction. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press; 1993. pp. 233–291. [Google Scholar]
- 50.Moore J P, McKeating J A, Huang Y, Ashkenazi A, Ho D D. Virions of primary human immunodeficiency virus type 1 isolates resistant to soluble CD4 (sCD4) neutralization differ in sCD4 binding and glycoprotein gp120 retention from sCD4-sensitive isolates. J Virol. 1992;66:235–243. doi: 10.1128/jvi.66.1.235-243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moore J P, McKeating J A, Norton W A, Sattentau Q J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991;65:1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moore J P, McKeating J A, Weiss R A, Sattentau Q J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990;250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- 53.Moore J P, Morikawa Y, Jones I M. Binding of recombinant HIV-1 and HIV-2 SU glycoproteins to sCD4. J Acquired Immune Defic Syndr. 1991;4:442–449. [PubMed] [Google Scholar]
- 54.Moore J P, Sodroski J. Antibody cross competition analysis of the human immunodeficiency virus type 1 gp120 exterior envelope glycoprotein. J Virol. 1996;70:1863–1872. doi: 10.1128/jvi.70.3.1863-1872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moore J P, Trkola A, Dragic T. Co-receptors for HIV-1 entry. Curr Opin Immunol. 1997;9:551–562. doi: 10.1016/s0952-7915(97)80110-0. [DOI] [PubMed] [Google Scholar]
- 56.Moore J P, Trkola A, Korber B, Boots L J, Kessler J A N, McCutchan F E, Mascola J, Ho D D, Robinson J, Conley A J. A human monoclonal antibody to a complex epitope in the V3 region of gp120 of human immunodeficiency virus type 1 has broad reactivity within and outside clade B. J Virol. 1995;69:122–30. doi: 10.1128/jvi.69.1.122-130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muster T, Guinea R, Trkola A, Purtscher M, Klima A, Steindl F, Palese P, Katinger H. Cross-neutralizing activity against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J Virol. 1994;68:4031–4034. doi: 10.1128/jvi.68.6.4031-4034.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohshiro Y, Murakami K, Nishioka K, Yoshida K, Yamamoto N. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type 1 (HIV-1) infection. Microbiol Immunol. 1996;40:827–835. doi: 10.1111/j.1348-0421.1996.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 60.Orentas R J, Hildreth J E K. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses. 1993;9:1157–1165. doi: 10.1089/aid.1993.9.1157. [DOI] [PubMed] [Google Scholar]
- 61.Orloff G M, Orloff S L, Kennedy M S, Maddon P J, McDougal J S. Penetration of CD4 T cells by HIV-1. The CD4 receptor does not internalize with HIV, and CD4-related signal transduction events are not required for entry. J Immunol. 1991;146:2578–2587. [PubMed] [Google Scholar]
- 62.Patel M, Ynangishita M, Rodriquez G, Bou Habib D C, Oravecz T, Hascall V C, Norcross M A. Cell surface heparan sulfate proteoglycan mediates HIV-1 infection of T cell lines. AIDS Res Human Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]
- 63.Paxton W A, Martin S R, Tse D, O’Brien T R, Skurnik J, VanDevanter N L, Padian N, Braun J F, Kotler D P, Wolinsky S M, Koup R A. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nat Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 64.Pelchen-Matthews A, Clapham P, Marsh M. Role of CD4 endocytosis in human immunodeficiency virus infection. J Virol. 1995;69:8164–8168. doi: 10.1128/jvi.69.12.8164-8168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Poignard P, Fouts T, Naniche D, Moore J P, Sattentau Q J. Neutralizing antibodies to human immunodeficiency virus type-1 gp120 induce envelope glycoprotein subunit dissociation. J Exp Med. 1996;183:473–484. doi: 10.1084/jem.183.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzuto C D, Sodroski J G. Contribution of virion ICAM-1 to human immunodeficiency virus infectivity and sensitivity to neutralization. J Virol. 1997;71:4847–4851. doi: 10.1128/jvi.71.6.4847-4851.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roderiquez G, Oravecz T, Yanagishita M, Bou-Habib D C, Mostowski H, Norcross M A. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J Virol. 1995;69:2233–2239. doi: 10.1128/jvi.69.4.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C-M, Saragosti S, Lapoumerouli C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 69.Sattentau Q J, Arthos J, Deen K, Hanna N, Healey D, Beverley P C, Sweet R, Truneh A. Structural analysis of the human immunodeficiency virus-binding domain of CD4. Epitope mapping with site-directed mutants and anti-idiotypes. J Exp Med. 1989;170:1319–1334. doi: 10.1084/jem.170.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sattentau Q J, Moore J P, Vignaux F, Traincard F, Poignard P. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J Virol. 1993;67:7383–7393. doi: 10.1128/jvi.67.12.7383-7393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sattentau Q J, Weiss R A. The CD4 antigen: physiological ligand and HIV receptor. Cell. 1988;52:631–632. doi: 10.1016/0092-8674(88)90397-2. [DOI] [PubMed] [Google Scholar]
- 72.Simmons G, Wilkinson D, Reeves J D, Dittmar M T, Beddows S, Weber J, Carnegie G, Desselberger U, Gray P W, Weiss R A, Clapham P R. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer T A, Luther E, Klickstein L B. Adhesion structures: section report. In: Schlossman S F, Boumsell L, Gilks W, Harlan J M, Kishimoto T, Morimoto C, Ritz J, Shaw S, Silverstein R, Springer T, Tedder T F, Todd R F, editors. Leukocyte typing V. Vol. 2. Oxford, United Kingdom: Oxford University Press; 1995. pp. 1443–1740. [Google Scholar]
- 74.Srivastava K K, Fernandez-Larsson R, Zinkus D M, Robinson H L. Human immunodeficiency virus type 1 NL4-3 replication in four T-cell lines: rate and efficiency of entry, a major determinant of permissiveness. J Virol. 1991;65:3900–3902. doi: 10.1128/jvi.65.7.3900-3902.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweet R A, Truneh A, Hendrickson W A. CD4: its structure, role in immune function and AIDS pathogenesis, and potential as a pharmacological target. Curr Opin Biotechnol. 1991;2:622–633. doi: 10.1016/0958-1669(91)90089-n. [DOI] [PubMed] [Google Scholar]
- 76.Thali M, Moore J P, Furman C, Charles M, Ho D D, Robinson J, Sodroski J. Characterization of conserved human immunodeficiency virus type 1 gp120 neutralization epitopes exposed upon gp120-CD4 binding. J Virol. 1993;67:3978–3988. doi: 10.1128/jvi.67.7.3978-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 78.Trkola A, Pomales A B, Yuan H, Korber B, Maddon P J, Allaway G P, Katinger H, Barbas III C F, Burton D, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore J, Katinger H. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ugolini S, Mondor I, Parren P W H I, Burton D R, Tilley S A, Klasse P-J, Sattentau Q J. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp Med. 1997;186:1287–1298. doi: 10.1084/jem.186.8.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ugolini S, Moulard M, Mondor I, Barois N, Demandolx D, Hoxie J, Brelot A, Alizon M, Davoust J, Sattentau Q J. HIV-1 gp120 binding to CD4+ cells induces an interaction between CD4, gp120 and the chemokine receptor CXCR4. J Immunol. 1997;159:3000–3008. [PubMed] [Google Scholar]
- 82.Warrier S V, Pinter A, Honnen W J, Girard M, Muchmore E, Tilley S A. A novel, glycan-dependent epitope in the V2 domain of human immunodeficiency virus type 1 gp120 is recognized by a highly potent, neutralizing chimpanzee monoclonal antibody. J Virol. 1994;68:4636–4642. doi: 10.1128/jvi.68.7.4636-4642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Weissenhorn W, Wharton S A, Calder L J, Earl P L, Moss B, Aliprandis E, Skehel J J, Wiley D C. The ectodomain of HIV-1 env subunit gp41 forms a soluble, α-helical, rod-like oligomer in the absence of gp120 and the N-terminal fusion peptide. EMBO J. 1996;15:1507–1514. [PMC free article] [PubMed] [Google Scholar]
- 84.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 85.Wu L, Geradr N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 86.WuDunn D, Spear P G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989;63:52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol. 1995;69:5723–5733. doi: 10.1128/jvi.69.9.5723-5733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang L, Huang Y, He T, Cao Y, Ho D D. HIV-1 subtype and second-receptor use. Nature. 1996;383:768. doi: 10.1038/383768a0. [DOI] [PubMed] [Google Scholar]