Abstract
Cerebrovascular reactivity (CVR) deficits may index vulnerability to vascular brain injury and cognitive impairment, but findings on age-related changes in CVR have been mixed, and no studies to date have directly compared age-related changes in CVR to hypercapnia vs. hypocapnia. The present study compared CVR in 31 cognitively unimpaired older adults (ages 55–87) and 30 healthy younger adults (ages 18–28). Breath control tasks induced CVR to hypocapnia (0.1Hz paced breathing) and hypercapnia (15s breath holds) during pseudo-continuous arterial spin labeling MRI. Relative to younger adults, cognitively unimpaired older adults displayed lower levels of global CVR under both hypocapnia and hypercapnia. In region-of-interest analyses, older adults exhibited attenuated CVR to hypocapnia in select frontal and temporal regions, and lower CVR to hypercapnia in all cortical, limbic, and subcortical regions examined, relative to younger adults. Results indicate age-related deficits in CVR are detectible even in cognitively unimpaired older adults and are disproportionately related to vasodilatory (hypercapnia) responses relative to vasoconstrictive (hypocapnia) responses. Findings may offer means for early detection of cerebrovascular dysfunction.
Keywords: cerebrovascular reactivity, hypocapnia, hypercapnia, older adults, cerebral blood flow, cerebrovascular reserve
1. Introduction
Vascular dysfunction has been increasingly implicated in age-related cognitive decline and dementia (Arvanitakis et al., 2016; Qiu and Fratiglioni, 2015; Snyder et al., 2015; Toth et al., 2017). Despite advances in neuroimaging approaches to detection of cerebrovascular lesions (Wardlaw et al., 2019), further studies are needed to establish cerebrovascular markers that may predate irreversible lesion formation and cognitive impairment. Cerebrovascular reactivity (CVR) is one candidate marker that represents the ability of cerebral vessels to modulate cerebral blood flow (CBF) through dilation or constriction in response to vasoactive stimuli (e.g., CO2) (Liu et al., 2019; Urback et al., 2017). In the largest study of CVR to date, Sur and colleagues evaluated CVR to hypercapnia using blood oxygen level-dependent (BOLD) signal response to CO2 gas inhalation in 72 older adults with normal or impaired cognition (Sur et al., 2020). Findings indicated significant attenuation of CVR in older adults with cognitive impairment relative to those without cognitive impairment. There was also a positive association between CVR and cognition, after accounting for covariates such as white matter lesion burden. Despite these promising findings regarding CVR as a potentially valuable marker of cognitive dysfunction, it remains unclear whether CVR deficits are observable during the preclinical period, when aging individuals are cognitively unimpaired. Thus, studies of age-related CVR deficits in cognitively unimpaired individuals may inform preclinical CVR assessment. Further characterization of age-related changes in CVR may also provide relevant data for investigations of various disorders, such as Moyamoya disease and carotid artery stenosis, as impairments in CVR have significant implications in clinical outcomes (Markus and Cullinane, 2001; Han et al., 2011).
Previous research on age-related alterations in CBF response to CO2 have yielded mixed results. Both BOLD-fMRI and transcranial doppler (TCD) investigations instigating hypercapnia via gas challenge and rebreathing have shown both decreased (McKetton et al., 2018; Peng et al., 2018) and increased (Tomoto et al., 2019; Zhu et al., 2013) CVR with aging. Apparent “improvements” in CBF response may, however, reflect a rightward shift in the reactivity curve prompted by general decreases in resting CBF with aging (Zhu et al., 2013). Notably, a number of studies have failed to detect any CBF changes in response to CO2 manipulation with aging. Null findings in studies of age differences in CVR may be due to differential effects of hypercapnic stimuli in older versus younger adults (Ances et al., 2009), underscoring the importance of capnographic monitoring during CVR experiments. Nevertheless, the pattern of mixed findings from prior studies leaves open the question of whether aging itself may impact CVR, even in cognitively unimpaired older adults.
It is also unclear whether aging may differentially impact vasodilatory versus vasoconstrictive responses, as the overwhelming majority of reactivity studies have focused on hypercapnia exclusively (Liu et al., 2019). In the few studies that examined age-related changes in hypocapnic response, there has been a similarly mixed pattern of findings. For example, TCD investigations have variously found decreased (Tomoto et al., 2019; Zhu et al., 2013), increased (Stefanidis et al., 2019), and equivalent (Minhas et al., 2019) CBF response to hyperventilation-induced hypocapnia in older adults, relative to younger adult controls.
The literature on age-related changes in hypocapnia responses is also methodologically limited, as most studies have relied on TCD to measure CBF. Although this approach offers excellent temporal precision, it lacks the spatial resolution of MRI-based methods and is limited to assessment of flow through larger intracranial arteries in neuroanatomically superficial regions (van Beek et al., 2008). Arterial spin labeling MRI provides a measure directly proportional to cerebral perfusion in every voxel in the brain and offers distinct information to complement findings from TCD or BOLD fMRI studies (Liu et al., 2019). Given that many neurovascular changes underpinning age-related declines in cerebrovascular autoregulation appear to occur in smaller vascular compartments (Iadecola, 2004), pseudo-continuous arterial spin labeling (pCASL) perfusion MRI may be particularly adept at assessing age-related changes in cerebrovascular function.
The present study evaluated age-related differences in CVR to gain further insight into the potential for CVR as a preclinical marker. CVR comparisons were made between older adults screened for cognitive impairment and healthy younger adults to address whether age-related CVR changes are detectible even in cognitively unimpaired older adults. CVR experiments utilized pCASL MRI to observe cerebral perfusion responses to both hypocapnia and hypercapnia in order to compare age-related changes in vasoconstrictive and vasodilatory responses. Capnography was employed during all experiments to ensure compliance with breathing manipulations and equivalence of end-tidal CO2 (etCO2) responses across age groups, and to calculate CVR maps indicative of perfusion changes per unit changes in etCO2. Regional CVR values were examined in cortical and limbic regions previously shown to have age-related changes in resting CBF and susceptibility to dementia (Zhang et al. 2017).
2. Material and Methods
2.1. Participants
Participants were drawn from the Vascular Senescence and Cognition (VaSC) Study at the University of Southern California (USC). The VaSC Study enrolled individuals from the community via word-of-mouth, flyers, and hosted events through the USC School of Gerontology. Participants provided informed consent to the VaSC study protocol, approved by the USC Institutional Review Board, and were financially compensated for their participation. Exclusion criteria included diagnosis of cognitive impairment or dementia, known family history of a genetic mutation that produces dementia, insulin-dependent diabetes, MRI contraindication(s) (e.g., weight exceeding 270 pounds), current organ failure or major systemic illness, history of stroke, myocardial infarction, major neurological or psychiatric disorder impacting cognition, head injury with loss of consciousness exceeding 15 minutes, substance abuse resulting in hospitalization, B12 deficiency, and/or hypothyroidism. Based on self-reported medical history, no individual in the current sample had a diagnosis of vascular or pulmonary diseases known to have a major impact on CVR (e.g., carotid artery stenosis, Moyamoya angiopathy). One participant had a history of pulmonary embolism but was able to complete breathing exercises, with their average resting etCO2 within normal range (40.20 mmHg).
2.2. Neuropsychological Screening
Older adults underwent comprehensive neuropsychological testing to screen for mild cognitive impairment (MCI) by sensitive neuropsychological criteria (Bondi et al., 2014). Briefly, participants were excluded if they exhibited impaired performance on two or more tests within a cognitive domain or three or more tests across cognitive domains. Thus, all participants were determined to be cognitively unimpaired at the time of the evaluation. Specifically, the following cognitive domains and tests were considered for diagnosis of MCI (Bondi et al., 2014): Attention and executive function (Trail Making Test: A and B), memory (Rey Auditory Verbal Learning Test: Long Delay Recall and Recognition), and language (Verbal Fluency Test: Animals and Boston Naming Test/Multilingual Naming Test).
2.3. Breathing Paradigms
Participants completed paced breathing and breath hold exercises during separate pCASL acquisitions. All participants engaged in a training protocol for breathing exercises before entering the scanner. Each breathing exercise was preceded by verbal and written instructions. Participants were also provided with in-scanner visual stimuli to guide breath control and ensure accurate completion of each exercise.
2.3.1. Paced breathing (0.1Hz): hypocapnia
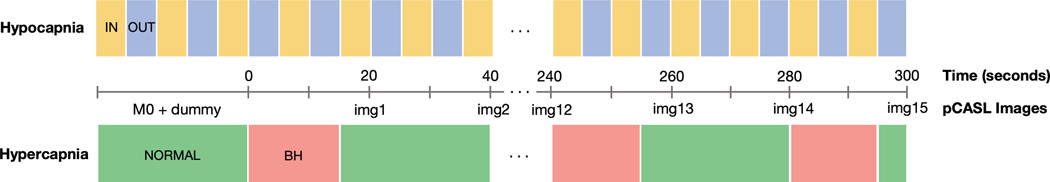
Participants were presented with a circle that filled with color over a 5-second interval. The color of the filling circle alternated between yellow and blue. Participants were instructed to “inhale while the circle is yellow” and “exhale while the circle is blue.” Perfusion images were acquired continuously throughout the 0.1Hz paced breathing modulation at two exhale-inhale cycle intervals (Fig. 1).
Fig. 1.
Schematic illustration of temporal synchronizations between breathing paradigms and pseudo-continuous arterial spin labeling (pCASL) MRI acquisitions. IN = breathe in, OUT = breathe out, NORMAL = breathe normally, BH = breath hold, img = perfusion image.
2.3.2. Breath hold: hypercapnia
Participants were shown a circle that filled with color (green) over a 25-second interval. Once full, the circle “restarted” and began filling with another color (red) over a 15-second interval. Participants were instructed to, “breathe normally while the circle is green” and “hold your breath while the circle is red.” Participants were also instructed to exhale prior to each breath hold to equate initial lung volume across participants and to maximize hypercapnic effect given the breath hold duration (Urback et al., 2017). At the end of each breath hold, participants were instructed to exhale in order to synchronize capnographic sampling across participants and to measure etCO2 approximating increased CO2 level induced by breath hold (Murphy et al., 2011). Each cycle of normal breathing and breath hold was synchronized with pCASL acquisition such that cerebral perfusion sampling occurred at the end of normal breathing period/ beginning of breath hold and at 5s after the participant stopped the breath hold and was breathing normally again (i.e., during peak perfusion response) (Fig. 1).
2.4. Neuroimaging and Physiological Measures
All MRI scans were conducted on a 3T scanner (Siemens MAGNETOM Prisma System) using a 20-channel head coil at the USC Dana and David Dornsife Cognitive Neuroimaging Center. Anatomical images were collected using a 3D high-resolution T1-weighted magnetization-prepared rapid gradient-echo (MPRAGE) scan (TR = 2300ms; TE = 2.98ms; TI = 900ms; resolution = 1.0×1.0×1.2mm3). Cerebral perfusion was measured by a 3D gradient and spin-echo (GRASE) pCASL scan with background suppression and the following sequence parameters: TR = 5000ms; TE = 36.3ms; FOV = 240mm; resolution = 2.5×2.5×3.4mm3; slice thickness = 3.42mm; number of slices = 24; labeling duration = 1517ms; post-labeling delay = 2000ms; 32 total acquisitions (1 M0 image + 1 dummy image + 30 alternating tag and control images); total scan time = 5 minutes 25 seconds. Altogether, each pCASL scan for 0.1Hz paced breathing and breath hold yielded 15 tag-control pair images.
The pCASL scans were pre-processed using the ASLtbx pipeline, implemented in SPM12 within MATLAB (Wang et al., 2008; Wang, 2012). Pre-processing steps for pCASL scans included motion correction, co-registration to individual subject’s structural T1-weighted image, spatial smoothing with a 6 mm full-width at half-maximum Gaussian kernel, and tag-control subtraction resulting in 15 tag-control pairs for each subject with values for absolute CBF (mL/100g tissue/min). All CBF images were thresholded below 10 or above 150mL/100g/min to exclude CBF outside the expected physiological range of gray matter (Nation et al., 2013; Clark et al, 2020). Tag-control pairs were warped to MNI space and averaged to create mean CBF maps for each subject. Resulting mean CBF maps were visually inspected for quality and gross abnormalities (i.e., large signal dropout). Partial volume correction was performed by applying subject-specific gray matter masks derived from the gray matter tissue class segmentation of T1-weighted structural images (Petr et al., 2018). Segmented gray matter maps were thresholded at 0.3, binarized, and multiplied by the mean CBF maps to ensure CBF was limited to gray matter.
Capnography indexed etCO2 during MRI acquisition using an M3015A sidestream carbon dioxide extension module (Philips Medical Systems) connected to a nasal cannula into which participants breathed. To correct for sampling tubing latency, etCO2 time series were shifted by a pre-calibrated duration of time (i.e., 10 seconds in our set-up). For 0.1Hz paced breathing exercise (hypocapnia), etCO2 was extracted from the raw time series in accordance with the breathing rate (i.e., at every expiration). For breath hold exercise (hypercapnia), maximum etCO2 was sampled for each pCASL image (i.e., maximum positive peak across the acquisition interval for each tag-control pair) from the raw time series, to effectively capture increases in etCO2 induced by breath hold. For each breathing paradigm, participants were excluded from analysis if they failed to adhere to breathing instructions (e.g., lack of positive peaks in raw data – participant might be breathing through the mouth).
2.5. CVR Maps
CVR was conceptualized as the percent change in CBF per unit change in etCO2 (Liu et al., 2019; Marshall et al., 2014). Specific computational approach was adapted from the method used by Marshall et al. (2014), which evaluated CVR in hypercapnia via CO2 gas administration. In the present study, participant’s individual CVR maps were generated for each breathing paradigm by calculating the following (1) in every voxel:
| (1) |
Regional mean CVR values were extracted for our regions of interest (ROI), including inferior frontal gyrus (IFG), orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), amygdala, hippocampus, parahippocampal gyrus (PHG), entorhinal cortex (EC), perirhinal cortex (PRhC), medial temporal lobe (MTL), inferior temporal gyrus (ITG), inferior parietal cortex (IPC), posterior cingulate cortex (PCC), precuneus, caudate, and thalamus (Zhang et al., 2017).
2.6. Statistical Analyses
Analyses were performed using SAS 9.4 (Cary, NC). Age (young versus old) comparisons of etCO2 responses (i.e., etCO2 maximum − etCO2 minimum) for each breathing paradigm were performed using independent samples t-tests. Chi-square tests were conducted to compare sex distribution between younger and older adults. Analysis of covariance (ANCOVA) models tested the difference between younger and older adults in their regional CVR values, adjusting for sex. The Benjamini-Hochberg method was used to correct for multiple comparisons, with false discovery rate (FDR) controlled at p < .05.
3. Results
3.1. Sample Characteristics and Effects of Breathing Manipulations
The overall sample consisted of 30 younger (age 18–28) and 31 older (age 55–87) adults. From this overall sample, participants successfully completing at least one of the breathing exercises were included in the analysis. For 0.1Hz paced breathing (hypocapnia), participants were excluded due to non-adherence to breathing instructions (n=2), no pCASL scan (n=3), and physio equipment failure (n=1). Thus, in the final analytic sample, 29 younger (mean age = 21.79, SD = 2.64; 27.6% female) and 26 older (mean age = 70.27, SD = 8.61; 26.9% female) adults were included. For breath hold (hypercapnia), participants were excluded due to non-adherence to breathing instructions (n=5) and physio equipment failure (n=1). Thus, in the final analytic sample, 26 younger (mean age = 22.00, SD = 2.67; 30.8% female) and 29 older (mean age = 69.62, SD = 8.38; 27.6% female) adults were included. Chi-square tests revealed no difference in sex distributions between younger and older adults in hypocapnia (χ2 = .00, p = .96) or hypercapnia (χ2 = .07, p = .80) conditions.
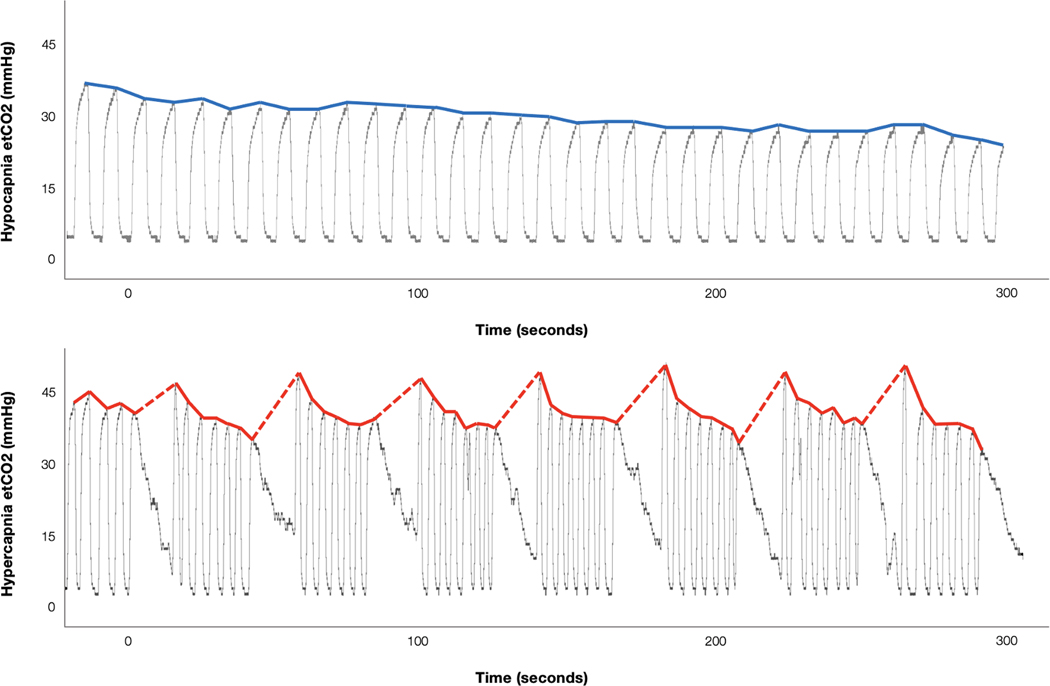
Older adults did not differ from younger adults in their etCO2 responses to either 0.1Hz paced breathing (hypocapnia: younger adults mean = 6.07 mmHg, SD = 2.57; older adults mean = 6.16 mmHg, SD = 2.29; t = .14, p = .89) or breath hold (hypercapnia: younger adults mean = 9.36 mmHg, SD = 4.23; older adults mean = 10.43 mmHg, SD = 2.11; t = 1.21, p = .23). Fig. 2 shows representative raw capnography recordings and etCO2 time series data for 0.1Hz paced breathing (hypocapnia) and breath hold (hypercapnia) exercises.
Fig. 2.
Representative end-tidal CO2 (etCO2) timeseries data across the duration of pseudo-continuous arterial spin labeling (pCASL) scans for 0.1Hz paced breathing (top) and breath hold (bottom) conditions. Raw capnography recordings of etCO2 are shown as gray traces. Extracted etCO2 values are shown in bold colored lines. Top (blue): EtCO2 levels are gradually decreasing during 0.1Hz paced breathing (hypocapnia). Bottom (red): Dashed lines indicate breath holds. The exhale immediately following each breath hold shows an increase in etCO2 (hypercapnia).
3.2. Age Differences in CVR
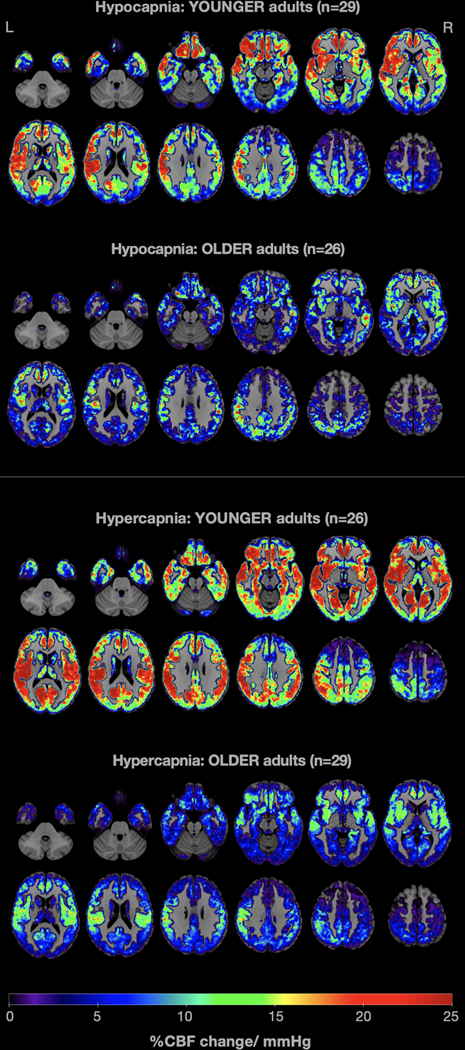
Visual comparison of mean CVR maps of younger and older adults in 0.1Hz paced breathing (hypocapnia) and breath hold (hypercapnia) conditions indicate attenuated CVR responses in older adults relative to younger adults (Fig. 3).
Fig. 3.
Average whole-brain gray-matter cerebrovascular reactivity (CVR) maps contrasting young and older adults in 0.1Hz paced breathing (top: hypocapnia) and breath hold (bottom: hypercapnia) conditions. CVR was computed as the percent change in CBF per mmHg change in etCO2. Warm colors indicate higher CVR values, while cold colors show lower CVR values. It is easily noticeable that older adults demonstrated attenuated CVR in both conditions, compared with younger adults.
Global CVR was attenuated in older adults during 0.1Hz paced breathing (hypocapnia) and breath hold (hypercapnia) relative to younger adults (Table 1). Older adults demonstrated reduced CVR to hypocapnia in the OFC, ACC, and ITG relative to younger adults, as well as reduced CVR to hypercapnia in all regions examined (Table 1).
Table 1.
Comparisons of mean (S.E.) regional CVR values for younger and older adults in hypocapnia (0.1Hz paced breathing) and hypercapnia (breath hold), adjusting for sex
| Hypocapnia |
Hypercapnia |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Brain regions | Younger (n = 29) | Older (n = 26) | F | p | ηp2 | Younger (n = 26) | Older (n = 29) | F | p | ηp2 |
| Whole brain | 14.48 (1.41) | 9.64 (1.50) | 4.69 | .02 | .09 | 21.78 (1.41) | 8.80 (1.33) | 22.93 | <.0001 | .50 |
| IFG | 19.16 (2.77) | 15.60 (2.92) | 4.54 | .37 | .02 | 26.02 (2.08) | 12.06 (1.97) | 12.68 | <.0001 | .32 |
| OFC | 26.64 (3.35) | 12.35 (3.41) | 4.61 | <.01 | .15 | 27.75 (2.58) | 12.74 (2.40) | 9.16 | <.0001 | .26 |
| ACC | 21.85 (2.57) | 12.15 (2.71) | 6.33 | .01 | .12 | 26.64 (2.42) | 14.81 (2.29) | 6.34 | <.01 | .20 |
| Amygdala | 17.47 (3.99) | 12.36 (4.15) | 1.04 | .37 | .02 | 32.65 (3.46) | 13.66 (3.21) | 8.97 | <.01 | .23 |
| Hippocampus | 13.61 (2.50) | 6.38 (2.64) | 2.57 | .05 | .07 | 25.89 (2.03) | 10.48 (1.92) | 15.95 | <.0001 | .37 |
| PHG | 13.69 (2.59) | 8.40 (2.73) | 2.11 | .16 | .04 | 24.76 (1.85) | 8.86 (1.75) | 20.45 | <.0001 | .42 |
| EC | 9.43 (2.36) | 3.41 (2.54) | 1.76 | .08 | .06 | 23.70 (2.11) | 9.63 (2.00) | 11.92 | <.0001 | .31 |
| PRhC | 14.47 (3.09) | 5.96 (3.26) | 2.80 | .06 | .07 | 20.68 (1.88) | 6.36 (1.78) | 16.69 | <.0001 | .37 |
| MTL | 11.99 (2.39) | 6.35 (2.52) | 2.62 | .11 | .05 | 21.88 (1.68) | 7.64 (1.59) | 19.81 | <.0001 | .42 |
| ITG | 13.06 (2.25) | 3.14 (2.37) | 4.61 | <.01 | .15 | 19.10 (2.23) | 4.97 (2.11) | 11.69 | <.0001 | .28 |
| IPC | 10.83 (2.42) | 11.12 (2.56) | .53 | .93 | .00 | 19.10 (1.77) | 8.96 (1.67) | 9.97 | <.01 | .24 |
| PCC | 10.95 (2.40) | 7.96 (2.54) | .58 | .40 | .01 | 19.72 (2.32) | 8.66 (2.16) | 6.73 | <.01 | .20 |
| Precuneus | 11.48 (2.64) | 10.42 (2.78) | .19 | .78 | .00 | 20.93 (2.13) | 8.27 (2.01) | 9.38 | <.0001 | .26 |
| Caudate | 20.79 (4.38) | 8.17 (4.63) | 2.89 | .05 | .07 | 22.43 (2.11) | 9.74 (1.92) | 9.94 | <.0001 | .28 |
| Thalamus | 18.31 (4.29) | 15.89 (4.53) | .17 | .70 | .00 | 21.74 (2.32) | 9.20 (2.20) | 7.81 | <.01 | .23 |
Abbreviations: IFG=inferior frontal gyrus; OFC=orbitofrontal cortex; ACC=anterior cingulate cortex; PHG=parahippocampal gyrus; EC=entorhinal cortex; PRhC=perirhinal cortex; MTL=medial temporal lobe; ITG=inferior temporal gyrus; IPC=inferior parietal cortex; PCC=posterior cingulate cortex.
Values in bold indicate statistically significant results at FDR p < .05
4. Discussion
The present study found that cognitively unimpaired older adults exhibit attenuated whole-brain CVR in response to both hypocapnia and hypercapnia relative to younger adults. These findings are consistent with some prior studies utilizing a variety of methodologies to characterize age group differences in CVR (McKetton et al., 2018; Peng et al., 2018), but are contrary to other studies suggesting either no age group difference (Ances et al., 2009) or an increase in CVR in older adults (Tomoto et al., 2019; Zhu et al., 2013). Several methodological differences across studies, including CVR paradigm, method of perfusion estimation, and monitoring and incorporation of etCO2, may contribute to variability across studies. For the present study, examination of both hypocapnia and hypercapnia, utilization of pCASL-MRI markers of cerebral perfusion, and calculation of CVR normalized to etCO2, all support clear interpretation of the findings indicating attenuated cerebrovascular function with aging.
The majority of prior efforts have examined vasomotor effects of hypercapnia, while few have focused on hypocapnia. For MRI-based studies of CVR, non-invasive induction of hypocapnia through hyperventilation can cause participant discomfort and movement artifacts that obscure interpretation of experimental results (Pinto et al., 2021). The present study’s novel use of a paced breathing paradigm reduced etCO2 and consequently induced vasoconstrictive CVR responses with minimal reported participant discomfort or observed motion artifact. Utilization of short 15s interval breath holds similarly allowed for increased etCO2 levels with minimal reported discomfort in our older adult participants. Examination of CVR to both hypocapnia and hypercapnia allowed for comparison of vasoconstrictive and vasodilatory CVR deficits in older adults in the current study. Findings indicated a clear difference in the magnitude and anatomical distribution of aging effects. Older adults displayed significant global CVR deficits in vasoconstriction (hypocapnic stimuli), with specific regional vasoconstrictive deficits in the frontal and inferior temporal regions. Age-related CVR deficits in vasodilation (hypercapnic stimuli) were of large effect size and showed statistically significant effects in both the global CVR analysis and in all ROIs, including frontal, temporal, parietal, and limbic regions. These findings suggest that vasodilatory capacity may be disproportionately impacted by aging, with more subtle and circumscribed changes in vasoconstrictive capacity also being detectible. This pattern of age-related CVR differences is consistent with a net vasoconstrictive phenotype suggested by studies of age-related changes in the cerebrovasculature (Yew and Nation, 2017; Kisler et al., 2017). It is possible these age-related changes in vasodilatory versus vasoconstrictive capacities may convey vulnerability to cerebral hypoperfusion, but further longitudinal studies are needed.
Deficits in CVR to hypercapnia have been linked to cognitive impairment (Cantin et al., 2011; McKetten et al., 2018; Peng et al., 2018; Sur et al., 2020) and dementia risk (Silvestrini et al., 2006). However, few studies have focused on cognitively unimpaired older adults, confirmed by comprehensive neuropsychological testing. A key goal of CVR research is to develop preclinical markers of vascular brain injury and risk for future cognitive decline, but achieving this goal will require improved understanding of age-related changes in CVR. Thus, the present study findings add to the existing literature on age-related CVR changes by establishing CVR deficits in cognitively unimpaired older adults relative to younger adults. Interestingly, very large aging effects were observed in response to hypercapnic stimuli despite the fact that participants were cognitively unimpaired. These findings suggest that decline in vasodilatory reserve may begin to attenuate at an earlier age than the age range of the present study (55–87 years). This is consistent with one of the few longitudinal studies of CVR change over the lifespan, which found that vasodilatory CVR declined most rapidly during midlife (Peng et al., 2018). This is also consistent with prior studies suggesting the importance of midlife (versus latelife) vascular risk factors with respect to neurocognitive decline (Armstrong et al., 2019).
To our knowledge, no study to date has examined the longitudinal trajectory of vasoconstrictive CVR. Although the present study is cross-sectional, our findings provide further insight into age-related changes in vasoconstrictive capacity, as our comparisons of younger versus older adults detected milder, anatomically circumscribed age-related differences in vasoconstrictive responses than those of the more well-studied vasodilatory responses. These findings suggest that vasoconstrictive responses may be more preserved than vasodilatory responses into late life, or that significant declines in vasoconstrictive responses may only occur in the context of cerebrovascular disease. Evidently, more data from longitudinal and clinical studies are needed to confirm these hypotheses. Yet, the present study underscores a value in evaluating dynamic changes in vasoconstrictive responses in the older adult population, where vasodilatory changes may already be substantial.
The ROI analysis in the present study indicated widespread age group differences in vasodilatory response in all cortical, limbic, and subcortical regions with no clear anatomical pattern. In contrast, age group differences in vasoconstrictive response involved specific frontal and temporal regions, including the orbitofrontal, anterior cingulate, and inferior temporal cortices. It remains unclear why these particular regions would show age group differences in vasoconstriction to hypocapnia. However, the temporal lobe finding is consistent with prior work indicating that temporal regions are the earliest to show declines in CVR (Peng et al., 2018). Age-related declines in CVR to hypocapnia in frontal and anterior limbic regions may explain relatively increased perfusion found in these anterior regions in cognitively normal older adults (Lee et al., 2009). Researchers have posited that the frontal lobes and associated circuitry are particularly vulnerable to the effects of aging (“frontal lobe hypothesis”; Cabeza and Dennis, 2013), to explain functional deficits mediated by the prefrontal regions and compensatory mechanisms. However, our findings also indicated age-related deficits in temporal regions. Longitudinal studies of CVR to hypocapnia will provide further clarity regarding the spatiotemporal pattern of age-related changes in cerebral vasoconstriction. Moreover, future studies may investigate into possible hemispheric differences in CVR, about which little is known in the literature.
Study strengths include investigation of a well-tolerated, non-invasive approach to indexing relative cerebrovascular responses to both hypocapnia and hypercapnia in older and younger adults, correction for etCO2 changes, and focus on a rigorously screened sample of cognitively unimpaired older adults. Study limitations include the relatively small sample size. The use of pCASL acquisition represents a strength, in that pCASL captures perfusion changes in response to CO2 levels more directly than BOLD-fMRI signals, which are influenced by other factors including cerebral blood volume and metabolic rate of oxygen consumption (Liu et al., 2019). However, the low signal-to-noise ratio of individual tag-control pairs, low temporal resolution, and limited ability to assess white matter perfusion represent widely recognized limitations to pCASL studies. Tradeoffs exist in terms of the strengths and limitations of any given approach to CVR studies, which warrants the continued investigation of multiple alternative approaches that may yield different information about cerebrovascular function in the older adult population, such as investigation of perfusion and CVR in the white matter. Additionally, our methods were able to detect patterns of age-related differences in CVR to hypocapnia and hypercapnia despite limitations inherent to pCASL methodology, such as differences in labeling efficiency during hypocapnia and hypercapnia (Aslan et al., 2010).
In sum, our findings and methodological approach present important clinical benefits. Firstly, the disproportionate pattern of results contrasting age-related CVR deficits in vasodilation vs. vasoconstriction provides implications for models of age-related diseases, such as Alzheimer’s disease, which has been characterized by researchers as a “hypercontractile” phenotype (Kim et al., 2013; Kisler et al., 2017). Secondly, utilization of CVR as a clinical biomarker of disease in older populations benefits from more refined understanding of age-related changes in cerebral hemodynamics. For instance, vasoconstrictive CVR might be expected to be normal if an older patient is presumed healthy, but the same may not hold true of vasodilatory CVR. Lastly, the present study provides a paradigm for clinical studies that is well-tolerated by older adults and allows evaluation of both vasodilatory and vasoconstrictive capacity, particularly under circumstances where gas inhalation is contraindicated or unavailable.
5. Conclusions
Our findings indicate that cognitively unimpaired older adults exhibit attenuated CVR to hypocapnia and hypercapnia, compared with younger adults. Further, the effects of age-related CVR deficits are greater for hypercapnia across a range of cortical, limbic, and subcortical regions than for hypocapnia, circumscribed to specific frontal and temporal regions. Aging may have a disproportionate impact on vasodilatory versus vasoconstrictive CVR, and this pattern of changes may support the utility of CVR assessment in estimating the risk for cerebrovascular injury and cognitive impairment in older adults. Further, considerations of aging effects may facilitate better characterization of CVR changes as a predictive and treatment-monitoring tool for clinical populations, such as individuals with reduced vascular reserve (e.g., Moyamoya angiopathy).
Acknowledgements
This work was supported by the National Institutes of Health (grant numbers R01AG064228, R01AG060049, P50AG016573, P01AG052350); the National Science Foundation (grant number DGE1418060); and the Alzheimer’s Association (grant number AARG-17-532905).
Footnotes
Disclosure Statement
The authors declare that there is no conflict of interest.
References
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB, 2009. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum. Brain. Mapp. 30 1120–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong NM, Bangen KJ, Au R, Gross AL, 2019. Associations between midlife (but not late-life) elevated coronary heart disease risk and lower cognitive performance: results from the Framingham Offspring Study. Am. J. Epidemiol. 188, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA, 2016. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 15, 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan S, Xu F, Wang PL, Uh J, Yezhuvath US, van Osch M, Lu H, 2010. Estimation of labeling efficiency in pseudocontinuous arterial spin labeling. Magn Reson Med. 63, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, Nation DA, Libon DJ, Au R, Galasko D, Salmon DP, 2014. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J. Alzheimer’s Dis. 42, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Dennies NA Frontal lobes and aging: deterioration and compensation. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2013. p.628–652. [Google Scholar]
- Cantin S, Villien M, Moreaud O, Tropres I, Keignart S, Chipon E, Le Bas JF, Warnking J, Krainik A, 2011. Impaired cerebral vasoreactivity to CO2 in Alzheimer’s disease using BOLD fMRI. NeuroImage 58, 579–587. [DOI] [PubMed] [Google Scholar]
- Clark AL, Weigand AJ, Bangen KJ, Merritt VC, Bondi MW, Delano-Wood L, 2021. Repetitive mTBI is associated with age-related reductions in cerebral blood flow but not cortical thickness. J. Cereb. Blood Flow Metab. 41, 431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Abou-Hamden A, Mandell DM, Poublanc J, Crawley AP, Fisher JA, Mikulis DJ, Tymianski M, 2011. Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke. 42, 3047–3054. [DOI] [PubMed] [Google Scholar]
- Iadecola C, 2004. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat. Rev. Neurosci. 5, 347–360. [DOI] [PubMed] [Google Scholar]
- Kim SM, Kim MJ, Rhee HY, Ryu C-W, Kim EJ, Petersen ET, Jahng G-H, 2013. Regional cerebral perfusion in patients with Alzheimer’s disease and mild cognitive impairment: Effect of APOE Epsilon4 allele. Neuroradiology. 55, 25–34. [DOI] [PubMed] [Google Scholar]
- Kisler K, Nelson AR, Montagne A, Zlokovic BV, 2017. Cerebral blood flow regulation and neurovascular dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 18, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Lopez OL, Becker JT, Raji C, Dai W, Kuller LH, Gach HM, 2009. Imaging cerebral blood flow in the cognitively normal aging brain with arterial spin labeling: implications for imaging of neurodegenerative disease. J. Neuroimaging 19, 344–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, De Vis JB, Lu H, 2019. Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. NeuroImage 187, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xu C, Lin Z, Sur S, Li Y, Yasar S, Rosenberg P, Albert M, Lu H, 2020. Cerebrovascular reactivity mapping using intermittent breath modulation. NeuroImage 215, 116787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus H, Cullinane M, 2001. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 124, 457–467. [DOI] [PubMed] [Google Scholar]
- Marshall O, Lu H, Brisset JC, Xu F, Liu P, Herbert J, Grossman RI, Ge Y, 2014. Impaired cerebrovascular reactivity in multiple sclerosis. JAMA neurol. 71, 1275–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetton L, Sobczyk O, Duffin J, Poublanc J, Sam K, Crawley AP, Venkatraghavan L, Fisher JA, Mikulis DJ, 2018. The aging brain and cerebrovascular reactivity. NeuroImage 181, 132–141. [DOI] [PubMed] [Google Scholar]
- Minhas JS, Haunton VJ, Robinson TG, Panerai RB, 2019. Determining differences between critical closing pressure and resistance-area product: responses of the healthy young and old to hypocapnia. Pflugers Arch. 471, 1117–1126. [DOI] [PubMed] [Google Scholar]
- Murphy K, Harris AD, Wise RG, 2011. Robustly measuring vascular reactivity differences with breath-hold: normalising stimulus-evoked and resting state BOLD fMRI data. NeuroImage 54, 369–379. [DOI] [PubMed] [Google Scholar]
- Nation DA, Wierenga CE, Clark LR, Dev SI, Stricker NH, Jak AJ, Salmon DP, Delano-Wood L, Bangen KJ, Rissman RA, Liu TT, 2013. Cortical and subcortical cerebrovascular resistance index in mild cognitive impairment and Alzheimer’s disease. J.Alzheimer’s Dis. 36, 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S-L, Chen X, Li Y, Rodrigue KM, Park DC, Lu H, 2018. Age-related changes in cerebrovascular reactivity and their relationship to cognition: a four-year longitudinal study. NeuroImage 174, 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petr J, Mutsaerts HJ, De Vita E, Steketee RM, Smits M, Nederveen AJ, Hofheinz F, van Den Hoff J, Asllani I, 2018. Effects of systematic partial volume errors on the estimation of gray matter cerebral blood flow with arterial spin labeling MRI. MAGMA 31, 725–734. [DOI] [PubMed] [Google Scholar]
- Pinto J, Bright MG, Bulte DP Figueiredo, P., 2021. Cerebrovascular reactivity mapping without gas challenges: a methodological guide. Front. Physiol. 11, 1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C, Fratiglioni L, 2015. A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 12, 267–277. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Pasqualetti P, Baruffaldi R, Bartolini M, Handouk Y, Matteis M, Moffa F, Provinciali L, Vernieri F, 2006. Cerebrovascular reactivity and cognitive decline in patients With Alzheimer disease. Stroke 37, 1010–1015. [DOI] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC, 2015. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers. Dement. 11, 710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanidis KB, Askew CD, Klein T, Lagopoulos J, Summers MJ, 2019. Healthy aging affects cerebrovascular reactivity and pressure-flow responses, but not neurovascular coupling: a cross-sectional study. PLoS One 14, e0217082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S, Lin Z, Li Y, Yasar S, Rosenberg P, Moghekar A, Hou X, Kalyani R, Hazel K, Pottanat G, Xu C, van Zijl P, Pillai J, Liu P, Albert M, Lu H, 2020. Association of cerebrovascular reactivity and Alzheimer pathologic markers with cognitive performance. Neurology 95, e962–e972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoto T, Riley J, Turner M, Zhang R, Tarumi T, 2019. Cerebral vasomotor reactivity during hypo- and hypercapnia across the adult lifespan. J. Cereb. Blood Flow Metab. 40, 600–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P, Tarantini S, Csiszar A, Ungvari Z, 2017. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am. J. Physiol. Heart. Circ. Physiol. 312, H1–H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urback AL, MacIntosh BJ, Goldstein BI, 2017. Cerebrovascular reactivity measured by functional magnetic resonance imaging during breath-hold challenge: a systematic review. Neurosci. Biobehav. Rev. 79, 27–47. [DOI] [PubMed] [Google Scholar]
- van Beek AHEA, Claassen JAHR, Rikkert MGMO, Jansen RWMM, 2008. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J. Cereb. Blood Flow Metab. 28, 1071–1085. [DOI] [PubMed] [Google Scholar]
- Wang Z, Aguirre GK, Rao H, Wang J, Fernández-Seara MA, Childress AR, Detre JA, 2008. Empirical optimization of ASL data analysis using an ASL data processing toolbox: ASLtbx. Magn. Reson. Imaging 26, 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, 2012. Improving cerebral blood flow quantification for arterial spin labeled perfusion MRI by removing residual motion artifacts and global signal fluctuations. Magn. Reson. Imaging 30, 1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Smith C, Dichgans M, 2019. Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696. [DOI] [PubMed] [Google Scholar]
- Yew B, Nation DA, Alzheimer’s Disease Neuroimaging Initiative, 2017. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 140, 1987–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gordon ML Goldberg TE., 2017. Cerebral blood flow measured by arterial spin labeling MRI at resting state in normal aging and Alzheimer’s disease. Neurosci. Biobehav. Rev. 72, 168–175. [DOI] [PubMed] [Google Scholar]
- Zhu Y-S, Tarumi T, Tseng BY, Palmer DM, Levine BD, Zhang R, 2013. Cerebral vasomotor reactivity during hypo- and hypercapnia in sedentary elderly and Masters athletes. J. Cereb. Blood Flow Metab. 33, 1190–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]