Abstract
Background
Joint stiffness, lameness and reduced activity levels are common inflammatory responses observed in canines and have significant impact on quality of life (QOL). The symptoms are often ascribed to osteoarthritis (OA), for which the standard treatment is systemic anti‐inflammatories, but pharmacologic intervention can have significant short‐term and long‐term side effects.
Objectives
Test the efficacy of a Food and Drug Administration (FDA)‐cleared pulsed shortwave therapy (PSWT) device as a means to modulate vagus nerve activity and initiate a systemic anti‐inflammatory response to determine its ability to improve functionality and the QOL of canines with inflammatory symptoms commonly associated with OA.
Methods
A randomized, double‐blinded, placebo‐controlled 14‐day study of 60 dogs with a presumptive prior diagnosis of OA in at least one limb joint. Two outcomes assessing changes in the dog's QOL and functionality were measured: subjectively determined changes in eight behaviours associated with discomfort and objectively determined changes in passive range of motion (PROM). The device was secured near the cervico‐thoracic region of the dog's spine. PROM measures were taken at baseline and at the end of study. Behavioural measures were taken daily.
Results
Forty‐nine animals completed the study. No negative side effects were reported. Average subjective discomfort scores for the treatment group (N = 26) were reduced from 3.74 to 2.10 (44%), compared to no improvement in the placebo group (N = 23) over the study period (p = 0.0001). Average PROM scores increased by 5.51 (4.59–6.23) degrees relative to the placebo group (p < 0.01). Ninety‐six per cent of the treatment group showed either increased PROM or improved behavioural changes or both, compared to 4% for the placebo group (p < 0.01). Most changes occurred within the first 8 days of treatment.
Conclusions
PSWT applied at the level of the cervico‐thoracic spine to target the vagus nerve may have the potential to improve QOL in dogs manifesting behaviours commonly associated with OA.
Keywords: arthritics and degenerative joint disease, canine, quality of life, range of motion
This paper investigates the efficacy of an FDA‐cleared pulsed shortwave therapy (PSWT) device to modulate vagus nerve activity and initiate a systemic anti‐inflammatory response to improve the functionality of canines diagnosed with OA. Significant quality of life changes were observed relative to the placebo effect, both in terms of behavioural changes and passive range of motion over the course of 14 days of continued use of the medical device.
1. INTRODUCTION
Lameness, joint stiffness and declines in physical activity levels are commonly observed in canines, resulting in decreased quality of life (QOL). These symptoms are often attributed to osteoarthritis (OA), even in the absence of imaging evidence. The most prevalent interventions in such cases are pharmacologic, often in the form of non‐specific, systemic, non‐steroidal anti‐inflammatory drugs (NSAIDs). Although well established, a pharmacologic treatment regimen often needs veterinary prescription, can be expensive and can have both short‐ and long‐term side effects (Grubb, 2023; Johnston & Budsberg, 1997; Moore, 2016).
Although NSAIDs can be effective in improving QOL for animals with symptoms commonly associated with OA, alternative approaches which have systemic anti‐inflammatory effects may be equally effective (Grubb, 2023; Pinna et al., 2013). One such approach is biophysical intervention, specifically exposure to electromagnetic fields (Pinna et al., 2013). Biophysical therapies date back as long as modern pharmacologic therapies, though they tend not to be as well accepted due, in part, to the lack of well understood mechanisms of action (Gaynor et al., 2018). More recently, electric stimulation of the vagus nerve has received substantial interest as a therapeutic intervention as it has been shown to produce anti‐inflammatory effects in a number of animal models (Falvey et al., 2021).
Although vagus nerve stimulation commonly relies upon the introduction of low‐frequency (10–1000 Hz) electrical currents in the vicinity of the nerve through either surface or implanted electrodes (Johnson & Wilson, 2018), this approach introduces a number of potential complications. Alternatively, non‐invasive electromagnetic stimulation has also been shown to be capable of modulating nerve activity (Koneru et al., 2022). This approach utilizes much higher frequency stimuli (megaHertz [MHz]) at very low magnetic field intensities (microTesla [µT]). Devices using this approach are referred to as pulsed shortwave therapy (PSWT) devices. This therapeutic approach appears to rely on quantum magneto‐biologic effects (Koneru et al., 2016). A number of studies have shown that PSWT devices can provide relief for acute post‐operative (Brook et al., 2012; Khooshideh et al., 2017) and chronic pain (Brook et al., 2012; Bagnato et al., 2016; Koneru et al., 2019; Mohammad et al., 2021) in humans, and these devices have received clearance for both conditions from the Food and Drug Administration (FDA). Two recently completed human clinical studies specifically looked at OA and showed that PSWT improved physical functionality, reduced pain and reduced the need for pharmacotherapy (Bagnato et al., 2016; Mohammad et al., 2021). In addition, one prospective 6‐month study showed these effects were long lasting for subjects who reported initial pain reduction within the first 7 days of use and continued to use the device in an ‘as needed basis’ (Staelin et al., 2019). We propose that these positive results and the lack of adverse effects make PSWT attractive as an intervention modality for the treatment of dogs with inflammatory symptoms, by using vagus nerve stimulation to affect a systemic anti‐inflammatory response.
The goal of this randomized, placebo‐controlled, double‐blind study was to test if using one specific, commercially available PSWT device for 14 days would improve, relative to a placebo group, the QOL in canines demonstrating behavioural symptoms commonly associated with OA.
2. METHODS AND MATERIALS
2.1. Study design
The experimental protocol was designed according to the guidelines of the current European and UK laws on the protection of animals used for scientific purposes (Directive 2010/63/EU) and was approved by the Research Ethical Committee of Plumpton College. Prior to enrolment, each owner was briefed about the aims of the study. This briefing included answering questions to assess suitability for enrolment and ensuring that safety measures were explicitly communicated. Owners and the supervising veterinarians were then asked to sign a consent form, in which the veterinarian confirmed his/her belief that the ‘dog had arthritis in one or more of their joints’. In addition, owners completed both pre‐ and post‐assessment forms detailing information about the dog's demographics, medication use prior to the trial and any changes in medication during the trial as well as any planned hydrotherapy and/or unusual activities during the trial.
The selected treatment duration was 14 days, consistent with previous studies on humans, which found that those who reported relief indicated it occurred within 7 days. Treatment duration of 24 h per day for each dog was selected. Following the recommendations of the 2017 Pain in Animal Workshop (Lascelles et al., 2019), both subjective behavioural and objective outcome measures were obtained. The start of the trial for dogs on bedinvetmab was timed 2 weeks after injection, so the end of the trial preceded the next injection.
2.2. Animal recruitment
Dogs were recruited from established hydrotherapy, veterinary physiotherapy and veterinarian facilities, as well as from local dog walking groups via posters deployed at various dog walking parks across the United Kingdom.
The inclusion criteria included that the dog was adult, had an owner willing to comply with the study protocol and the owner's primary veterinarian asserting that, in his/her professional opinion, the [subject] dog had ‘arthritis in one or more joints’, although the evaluation did not typically include imaging evidence. Exclusion criteria included pregnancy, cancer, infections and the dog's medication use being altered during the trial.
2.3. Treatment device
The PSWT device used in this study was the model 088 medical device manufactured by BioElectronics Corporation, an over‐the‐counter product cleared by the US FDA for human use (Figure 1). This device uses a 5.5 cm flexible loop antenna (magnetic dipole). The device produces a pulsed, radio frequency magnetic field at 27.1 MHz, with a pulse width of 100 ms and a pulse repetition frequency of 1 kHz. This device produces a peak incident spatial power density of 73 µW/cm2, which translates into a specific absorption rate (SAR) of approximately 0.35 µW/cm3. This SAR is roughly three orders of magnitude lower than the FDA‐approved exposure levels for cell phones Federal Communications Comission, but the peak flux density of about 2.25 µT is more than two orders of magnitude above levels shown to influence quantum biological phenomenon. Thus, although the magnetic flux density levels are too low to be perceived, these flux density levels are adequate to produce magneto‐biological effects which have been proposed to arise through quantum mechanical processes (Binhi & Rubin, 2022). Additionally, the low‐intensity exposure might result in the effects of the device being slow to develop, and so the manufacturer suggests that the device be utilized at least 12 hours/day with the expected time until the subject experiences results being up to 4 days or more.
FIGURE 1.
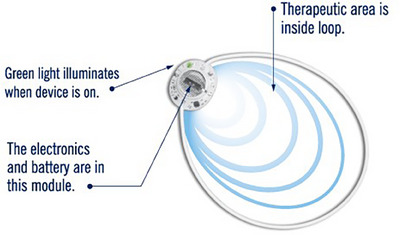
Medical device showing the location of the battery and the field of therapeutic area within the antenna.
BioElectronics Corporation, upon request from the lead researcher, supplied 60 devices, 30 being placebo devices (inactive) and 30 being treatment devices (electromagnetically active). The placebo devices were identical to the treatment units in appearance, but the power source was disconnected from the antenna. Consequently, when the device was turned on, no current flowed through the antenna, and thus there was no magnetic field generated. However, as the treatment device is sensation‐free, it was not possible for the user (owner) or the lead researcher to determine if the device was active or non‐active. The codes to the device identity were released to the lead researcher after the conclusion of the study and recording of the data, blinding the lead researcher and owners to treatment assignment.
2.4. Placement of the device
The most accessible site for vagus nerve stimulation is the neck region, where the vagus nerve descends posterior to the carotid sheath. Unlike stimulation using electric fields, which can be blocked by bone and fatty tissue, magnetic fields readily pass through all biological tissues. The field intensity of the device declines as the square of the radius of the device, so for the 5.5 cm device, the maximum depth of field penetration would be about 10 cm. Correspondingly, in order to ensure exposure of the vagus nerve and to limit the ability of the dogs to damage the device, the PSWT device was positioned over the cervico‐thoracic region of the spine by attaching it to the dog's collar and positioning it on the dorsal aspect of the dog's neck (Figure 2).
FIGURE 2.

Typical attachment of pulsed shortwave therapy (PSWT) device to the dog's collar in order to expose the cervical region of the spine to the magnetic field therapy. The device was turned on and worn continuously for 14 days.
2.5. Subjective behavioural assessment
The primary outcome measure was a subjective behavioural assessment of the dog's QOL. We refer to this QOL assessment as ‘discomfort‐associated behaviour’ (DAB) and derived it from the BEAP measurement scale developed by Dr. Shea Cox and shown in Figure 3. Designed for use in the home or hospice settings, it is primarily a QOL assessment tool. This assessment utilizes a set of eight behavioural indicators thought to be associated with discomfort; specifically, these involve breathing, eyes, ambulation, activity, appetite, attitude, posture and palpation. Although not validated as a pain assessment tool, this measurement tool taps into many of the same behaviours found in the Helsinki Chronic Pain Index, Liverpool Osteoarthritis in Dogs measure, the functionality part of the Canine Brief Pain Inventory (CBPI) instrument and the Chronic pain assessment used in Pinna et al. (2013). This measurement instrument is easily understood, uses images and relies extensively on easily observable animal behaviours, thereby allowing owners to reliably assess their animal's QOL. These behaviours include activities, such as walking, playing, getting up from a sitting position, postures, eating and interacting with others. The BEAP instrument results in a dog being classified into one of six levels of discomfort, starting with the term ‘no discomfort/pain’ and progressing up to the ‘worst discomfort/pain possible’. These six levels are assigned with scores ranging from 0 to 10, values that correspond to the often used 0–10 Visual Analogue Scale (VAS) found in human and many canine pain studies. We followed the recommended scoring convention to form our DAB measure, where 0 is assigned to the lowest level and reflects behaviours associated with no discomfort, and the remaining scores are the average of the assigned scores found in Figure 3, for example mild is the average of 1–2 or 1.5 and moderate is 3.5, so the sixth level reflects behaviours associated with worst discomfort/pain possible and is scored 9.5.
FIGURE 3.

Discomfort‐associated behaviour (DAB) scoring sheet. Source: Adapted from 2021 Shea Cox, BluePearl Pet Hospice BEAP scale.
This subjective QOL assessment was completed by the dog's owner just prior to the start of the trial period and used as a baseline measure. Specifically, the lead researcher went through the form in detail with the owner, the intent being to insure consistency across the different owners. Then this researcher asked the owners how they would grade their dog at the present moment in time, highlighting things they should be looking for. Subsequently, the owners filled out daily BEAP assessment sheets independently. At the conclusion of the trial, the lead researcher collected these daily evaluations and tabulated the individual measures to form the DAB measure. In a few instances, the owner scored some behaviours in one category and the remaining behaviours in another category. In these instances, the score assigned was the average of two categories.
Change in DAB level was calculated relative to the dog's baseline. Successful intervention was defined as a decrease of at least 2 units on the DAB scale.1 This success measure is analogous to the definition of success of a two‐point reduction in the pain‐related measure for assessing the effectiveness of bedinvetmab (Corral et al., 2021). Success determination was done using data collected for days 7 and 14.
2.6. Objective behavioural assessment
In order to capture the impact of joint stiffness on the dog's functionality, the passive range of motion (PROM) was measured in the affected joints of the animal with the expectation that increased PROM would indicate increased functionality and thus increased QOL. PROM angles were measured in degrees using a digital EasyAngle goniometer, following the manufacturer's guidelines (Gait and Motion Technology Ltd., 2021). When tested on human subjects, this device yielded reliable measurements with an inter‐rater correlation coefficient between assessors of 0.994 and a standard error of mean (SEM) of differences within an individual over a short time period of between 1.15 and 1.48 degrees (Svensson et al., 2019). Testing using goniometry on healthy Labrador Retrievers by comparing goniometer measurements to radiographic measurements found goniometry ‘is a reliable and objective method of determining range of motion of joints’ (Jaegger et al., 2002). Another study using eight canine cadaver's hind limbs reported the correlation between the radiographic (true) measures, and the standard goniometer was 0.97 (Freund et al., 2016).
Measurements were obtained either in lateral recumbency or in a standing position. All measurements were taken by the lead researcher in both the initial and the 14‐day follow‐up appointment. To ensure consistency, for each dog, both the locations where the measurements were taken and the posture used were the same across the two time periods. Measurements were taken from all joints with restricted movement and also from the contralateral limb, whether restricted or not. Carpus, elbow and tarsal were measured in flexion, whereas shoulder, stifle and hip were measured in extension.
Change in PROM over the trial period was measured at the joint level by first averaging the two readings for a given joint for a given point in time and then subtracting these two joint averages, that is one for baseline and the other for the final measure. For extension, the baseline was subtracted from the final reading, whereas for flexion, the final reading was subtracted from the baseline, so that a positive value always indicates improvement. These differences were also averaged over all of the measured joints to obtain a measure of average change (in degrees) for the dog. Successful intervention was defined as an increase in the PROM measure greater than three SEMs of the measurement instrument, as reported in Svensson et al. (2019), that is 3 × 1.48 = 4.5 degrees or more.
2.7. Statistical analysis plan
A statistical power calculation was performed using the G* Power programme (Faul et al., 2007), assuming an effective effect size of 0.25 (based on human trials) with repeated measures. The necessary sample size was determined to be 36; thus, an initial sample size of 60 was planned to allow for a 30% dropout rate.
The basic strategy for analysis was to compare before and after measures within a dog, thereby removing all fixed effects, including such factors as medication use, breed and age. In this initial analysis addressing the null hypothesis of no change, a paired t test was undertaken to evaluate the magnitude of change in the DAB scores over 14 weeks. In addition, Excel was used to calculate the measures of daily per cent change in baseline DAB score and the percent of subjects successfully showing a decrease of at least one category (two DAB points) from baseline baseline, both at 7 and 14 days. Similarly, a change in the 14‐day PROM measures from the initial PROM measures was calculated, both with respect to a specific joint and also the patient's average improvement in degrees over all measured joints. Changes over time, in distribution, but within a group, for a given measure were analysed using Wilcoxon signed rank tests and determined using Python.
In addition, the overall effect of the device on the difference in the response time paths of DAB for each group was investigated by a regression model using a SAS PROC MIXED (SAS Institute, Inc., Cary, NC, USA), which uses iterative optimization methods that maximize the likelihood function. Individual time dummies were interacted with treatment, and the effect was measured via these interaction terms. Subjects were included in the time path model as a random effect to allow consideration of both within and among group variances. The effects of intervention on the aggregate PROM measure were also investigated using a regression model and the SAS PROC MIXED procedure. Given our expectation that treatment group scores would be superior to those in the placebo group, all tests were one‐tailed test with significance set at p = 0.05. Standard deviations (SD) are provided in parenthesis.
3. RESULTS
Sixty client‐owned dogs, ranging in age between 1 and 18 years (average 9.9 ± 3.2), were enrolled into the study from five locations around the United Kingdom. This intent‐to‐treat sample size of 60 was evenly divided between treatment and placebo. During the 14‐day period, 11 dogs chewed on, and destroyed, the PSWT device. Seven of these were in the placebo group and four in the treatment group, leaving a per‐protocol sample size of 49, 26 in the treatment group (15 M; 11 F) and 23 (15 M; 8 F) in the placebo group. All but seven of the animals had been neutered. Over half of the dogs at the start of the study were receiving either OA‐related prescription medications, hydrotherapy or both (69% and 65% for the treatment and placebo groups, respectively; Table 1). Of the 26 dogs in the treatment group, 18 (69%) were receiving medications, 10 (38%) were receiving hydrotherapy, 2 (7.8%) were receiving supplements and 8 (31%) were receiving no additional treatment. Of the 23 dogs in the placebo group, 9 (39%) were receiving medications, 8 (35%) were receiving hydrotherapy, 3 (13%) were receiving supplements and 8 (35%) were receiving no additional therapy. None had their medication or special activities altered during the study period. Fourteen dogs were receiving monthly bedinvetmab, so it was timetabled to start the trial 2‐week post‐injection to minimize any effect of bedinvetmab on differences of measures across the trial period. Table 1 also shows the baseline data for age, body condition score (BCS) and gender, by group. The sample means and percentages on age, BCS, gender, medication and hydrotherapy use were considered approximately equivalent across the two groups.
TABLE 1.
Average baseline demographics, medication and hydrotherapy use group means, medians and standard deviations (in parentheses).
|
Placebo n = 23 Number, % Or Mean/Median (SD) |
Treatment n = 26 Number, % Or Mean/Median (SD) |
|
|---|---|---|
| Age in years | 8.91/9 (3.23) | 10.7/10.5 (3.40) |
| BCS | 3.12/3 (0.58) | 3.29/3 (0.75) |
| Male | n = 14, 62% | n = 17, 65% |
| Female | n = 9, 38% | n = 9, 35% |
| Medication | ||
| Bedinvetmab | n = 6, 26% | n = 8, 31% |
| Meloxicam | n = 3, 13% | n = 6, 23% |
| Oclacitinib | n = 0, 0% | n = 4, 15% |
| Hydrotherapy | n = 8, 35% | n = 10, 38% |
| Medication and/or hydrotherapy | n = 15, 65% | n = 18, 69% |
Abbreviations: BCS, body condition score; SD, standard deviation.
The 49 dogs completing the study represented 35 breeds, including purebreds and crossbreeds, and came from 5 different parts of the United Kingdom. Each dog was either visited at their home address or a familiar clinic (hydrotherapy centre or physiotherapy clinic). The nine‐point BCS was obtained by the lead researcher, and the average over the sample was 3.21 (median 3), ranging from 2.5 to 4.75. Thirty‐nine dogs had two joint locations with apparent OA (and thus four readings per time period, including the contralateral limbs); five had only one joint location with apparent OA, whereas five had three or four joints which were assessed for PROM.
Table 2 reports the mean, median and SD baseline measures for DAB scores and degrees of motion by joint by group. The last column provides the average PROM by joint for a dog without any restrictive range of motion, as previously reported (Prydie & Hewitt, 2015) and can be used to aid assessment of the possible magnitude of improvement in range of motion. Over the five joints considered, the average possible improvement ranges from about 20 degrees for the elbow to about 70 degrees for the hip. The baseline DAB scores and average possible improvements were considered approximately equal for both groups.
TABLE 2.
Average baseline group discomfort‐associated behaviour (DAB) and passive range of motion (PROM) means, medians and standard deviations (in parentheses) compared with typical normal PROM in degrees.
|
Placebo n = 23 Mean/Median (SD) |
Treatment n = 26 Mean/Median (SD) |
Normal PROM Based on Prydie and Hewitt (2015) |
|
|---|---|---|---|
| DAB score | 3.66/3.5 (2.37) | 3.74/3.5 (2.54) | |
| PROM carpus (flexion) in degrees | 82.6/79.5 (29.5) | 87.6/101.5 (40.9) | 33 |
| PROM elbow (flexion) in degrees | 49.1, 50.5 (14.4) | 53.7, 61 (16.3) | 30 |
| PROM shoulder (extension) in degrees | 101.4, 85 (31.5) | 110.7, 118.5 (27.2) | 163 |
| PROM tarsal (flexion) in degrees | 72.1, 73 (10.3) | 81.5, 81.5 (13.8) | 34 |
| PROM hip (extension) in degrees | 85.9, 69.75 (29.3) | 91.8, 96.5 (28.3) | 158 |
Abbreviation: SD, standard deviation.
Initial DAB levels ranged from 1.5 (mild discomfort) to 7.5 (severe discomfort) for both the treatment and placebo groups with means of 3.74 and 3.66, medians of 3.5 and 3.5 and SDs of 2.54 and 2.37 for the treatment and placebo groups, respectively. These mean and median DAB scores for both groups thus equated to a clinical presentation of intermittent panting, dullness of the eyes and/or slightly furrowed brow, being slower to lie down or rise up, possibly exhibiting lameness when walking, possibly being slightly unsettled and more restless, having difficulty getting comfortable, being a finicky eater, being subdued and/or less engaged in play, having difficulty squatting or lifting a leg to urinate, exhibiting subtle changes in posture, tail more tucked under and ears more flattened and resenting being touched on specific areas of the body (see Figure 3 for the moderate discomfort/pain classification).
Daily average DAB scores (mean ± SD) are shown in Figure 4. The scores for the placebo group remained essentially constant at 3.6 (only one placebo dog was recorded as having any change in DAB levels over the course of the study, although variation in behaviour was observed for a few dogs across the 14 days). In contrast, the average DAB scores for the treatment group decreased over time from 3.74 to 2.10 (p = 0.0001 by paired t test), representing an average reduction of 44% from initial levels. The generalized linear model (GLM) regression results show that the time × treatment effect was highly significant (p < 0.001), and the individual contrasts between the treatment and placebo were significant after day 8. This difference in the time path of DAB levels was also found when comparing the percentage of subjects who showed at least two DAB point reduction calculated at days 7 and 14. For the treatment group, the figures were 27% and 65%, respectively, compared to 9% and 4%, respectively, for the placebo group. The 7‐day difference in per cent improvement approached significance (p = 0.054). The 14‐day difference was highly significant (p < 0.01).
FIGURE 4.
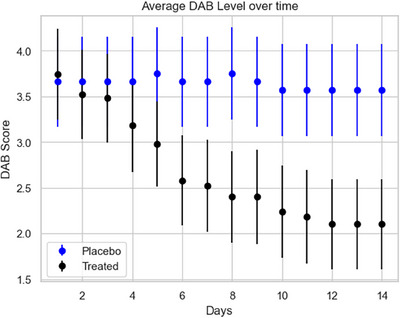
Daily average discomfort‐associated behaviour (DAB) scores for the treatment and placebo groups over the 14‐day trial along with one standard deviation measures.
Figure 5 augments the Figure 4 results by showing where the change in the DAB scores for the treatment dogs occurred based on the dog's starting condition. For example, there were 12 treated dogs that initially were scored to have behaviours associated with mild discomfort (DAB score of 1.5). Of that group, seven were reported as having behaviours associated with no discomfort (DAB score of 0) by the end of the trial, whereas five were still reported to have mild discomfort. Similarly, there were six dogs that initially showed behaviour associated with severe pain (DAB score of 7.5). Three of these dogs improved. One showed behaviour associated with no discomfort, one with moderate discomfort and one with moderate to severe discomfort. In total, 17 treatment dogs (65%) showed a decrease in DAB. Eleven (42%) of the treatment dogs were reported to have experienced a two‐point decrease, and six (23%) were reported to have improved by four or more points. In comparison, only one placebo dog (4%) was found to have reduced discomfort levels over the trial. Importantly, by the end of the study, 42% (i.e., 11 of the 26 treatment dogs) were reported to be showing behaviours associated with no discomfort compared to no dogs in the placebo group.
FIGURE 5.
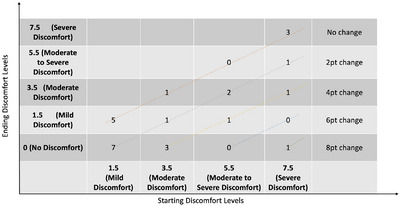
Change in discomfort‐associated behaviour (DAB) scores for treatment dogs as a function of their baseline DAB score. Numbers reflect the number of dogs who started with a particular DAB score and by the end of the trial were in the relevant DAB level. All dogs below the top diagonal line showed improved DAB scores.
The objective PROM assessments were evaluated by individual Wilcoxon signed tests on the before and after distributions for both groups separately for each of the five OA joints. In the treatment group, the distribution of PROM scores increased significantly over the trial period for all joints except the tarsal, where the sample size was only 3. In the placebo group, only the distribution of PROM scores for the left shoulder increased significantly over time. Tests comparing the distribution of average changes in PROM for the measured joints between the two groups were highly significant (p < 0.001), with the treatment group averaging a 5.66 (1.80) degree increase compared to 0.050 (1.56) degree increase for the placebo group. The GLM regression results, where the dependent value was the average PROM improvement in degrees, found the treatment effect to be significant at the 0.001 level. The coefficient for the treatment variable was 5.51 (0.46), indicating that the treatment group experienced an increase of 5.51 degrees relative to the placebo group, all else equal. GLM regressions that also included demographics yielded similar results.
The correlation between the 14‐day improvement for DAB and overall PROM measure for the treatment group was found to be 0.12, which is not significant p > 0.2.
Two post hoc analyses were conducted. The first compared the improvements for the fore and hind limb joints separately. The average improvement for the forelimb joints was 6.84 (3.40) degrees for the treatment group and 0.02 (1.77) degrees for the placebo group. The figures for the hind limb joints were 3.56 (2.65) and 0.22 (1.37), respectively. The second analysis determined the percentage of subjects who experienced success in either PROM or DAB or both after 14 days. For the treatment group, this percentage was 96% compared to 4% for the placebo group.
4. DISCUSSION
Our findings indicate that, in dogs with symptoms commonly associated with OA, PSWT intervention at the level of the cervico‐thoracic spine exerted a positive effect on animal behaviour and improved range of motion in affected limbs. This is particularly noteworthy because 69% of the treatment study participants were concurrently receiving anti‐inflammatory medication and/or hydrotherapy, indicating that a robust response was observed over and above that of traditional interventions. Nonetheless, the results raise a number of interesting questions.
The highly significant improvement in QOL behaviours as measured by our DAB score and the increase in mobility as measured by PROM compare favourably to those found in the recent clinical trial for bedinvetmab, an anti‐inflammatory medication intended to treat canine OA. In that trial, 45% of the treatment group reported having at least a 2 scale‐point reduction on the used 11‐point pain scale within 14 days (Corral et al., 2021). As per Figure 5, the current study shows at least a 2‐point reduction in our 11‐point DAB scale for 65% for the treatment group over the same period of time.
We note that 14 dogs were being treated with bedinvetmab, 6 in the placebo group and 8 in the treatment group. All of these dogs had been on this treatment regimen for at least 2 months. Given that the peak effect of this drug is reported to be 42 days after the start of the treatment (Corral et al., 2021), any difference in DAB scores for these dogs cannot be attributed to an initial increase in the effectiveness of this medication.
Also encouraging was the finding that 96% of the treatment group experienced success in reducing DAB scores or improvement in PROM or both within 14 days. We attribute this large combined effect to the empirical observation that improvements in these two measures were not significantly correlated and thus were tapping different aspects of QOL. We acknowledge that we were initially surprised by this lack of association between our subjective measure of discomfort and objective range of motion measures. However, similar findings were reported in Brown et al. (2013), where the changes in the objective gait force measures and the subjective pain interference score were not significantly correlated. One possible explanation is that increased mobility may lead dogs to be more active, thereby increasing the noxious stimulation and negating any discomfort reduction. Another is that our two measures were tapping different aspects of the dog's behaviour. In any case, the vast majority of the treated dogs showed improvement in one or both measures by the end of the trial.
In contrast to these results, a recent human study of cervical spine pain utilizing the same therapeutic technology (Mohammad et al., 2021) showed a relatively small effect size (0.6–0.8; using Cohen's d test), where self‐reported pain was the measured outcome. In the current study, we observe a Cohen's d effect size of 1.64 (utilizing the average SD of change in DAB scores for two groups). This difference may be due to the fact that the human clinical study involved the use of prescription strength level NSAIDs in the control group, whereas in the current study, the placebo group received no additional intervention. Alternatively, these results may be another indication that pain and QOL behaviours are, physiologically, only weakly associated.
We also observed a robust effect of PSWT therapy on range of motion measures for both the front and hind limb joints. These results are consistent with the effect of stimulation being systemic, rather than localized, and similar to that observed with pharmacologic interventions. These responses are also consistent with reports going back more than 20 years that vagus nerve stimulation attenuates inflammatory responses (Borovikova et al., 2000), although the pathways by which vagus nerve stimulation influences inflammation are still being investigated (Falvey, 2022).
Prior studies of this PSWT device on humans found that the vast majority of users reported relief within 4–5 days. We found in the GLM analysis that it took 8 days for the results to show statistically significant differences between the two canine groups. The slower response observed may be due to the placement of the device and, therefore, the nerves being modulated. In this study, we were targeting the vagus nerve to achieve a systemic response, whereas in prior human studies, the focus was on obtaining a localized response by modulating afferent nerve activity near an injury site. Other explanations are that the behavioural changes occur more slowly than changes in pain perception, the owner not being able to quickly determine behavioural changes or our measure is less sensitive to changes than the VAS pain scale.
An interesting observation was the lack of any placebo response, both in terms of improvement in PROM and DAB reduction. Although one might not expect the dogs to be aware they were being treated, the owners were well aware that their pets might have been given an active device, and this knowledge might be expected to influence their BEAP assessments. Therefore, we expected a significant placebo effect for this measure in our study, but none was observed. It remains unclear why the DAB data do not exhibit some placebo effect.
Closely related to the question of the lack of placebo response was the lack of variability in the placebo data. In subjective assessment studies, it is often common to see both random recovery and worsening of the condition over the course of an experiment. One explanation might be the coarseness of our BEAP measurement instrument. Dog owners were asked to score their dogs daily on eight behavioural measures that could vary from hour to hour depending on the dog's activities. Owners very possibly just integrated their observations over the day to come up with average assessments which are less likely to change. In this way, our measure is similar to the average severity of pain measure used in the CBPI index. If true, this explanation also implies that the observed reduction in DAB for the treatment group was not just a momentary improvement but instead an enduring change.
The dropout rate was somewhat lower than anticipated, which was encouraging. The fact that 18% of the units ‘failed’ due to the dog removing and/or destroying the device should be an easy issue to address for future trials. One could envisage a small pouch into which the device could be placed and then attached to the collar, better protecting it from the dog removing it. Another possible design change would be to increase the antenna loop size and allow the dog to wear the device circumferentially around the neck, similar to a collar. This approach would also enhance the stimulus exposure intensity at the site of the vagus nerve.
We note that although the owner's veterinarian indicated the dog had arthritis in one or more joints, this diagnosis was not done using radiography. Although this could be viewed as a limitation, the focus of this study was not on reducing arthritic pain but on increasing the QOL for canines who showed symptoms commonly associated with OA. Future studies might incorporate more specific measures of an ongoing inflammatory response and validated measures of QOL. Another possible limitation is the short length of the trial. A future study might extend the study period beyond the 14‐day time frame to confirm that the anti‐inflammatory response is sustained. However, a 6‐month prospective study in humans found that users who reported getting relief within the first 7 days reported sustained relief with continued use of the device on an ‘as needed basis’ over the remainder of the 6‐month trial (Staelin et al., 2019). Thus, there is already some supporting evidence that continued use of the device should keep or even increase both types of observed behavioural improvements. Extending the trial period could be readily implemented as the device utilized in this study has a 30‐day life with 24 h/day use. Finally, although the sample sizes exceeded the power calculation recommendations, it would be good to replicate the study using larger sample sizes.
Regardless of these caveats, the results of this pilot study promote the possibility that PSWT applied at the cervico‐thoracic spine level to modulate vagus nerve activity may have the potential to significantly improve QOL in dogs with inflammatory conditions such as OA. We encourage others to continue with this line of research.
AUTHOR CONTRIBUTIONS
Tanya Ella Sprunks initiated the project, was involved in the study design, collected and coded all the raw data and read the paper for accuracy. Kenneth J. McLeod edited the paper and was involved in the data analysis. Richard Staelin was involved in the study design and providing the medical devices, was primarily responsible for analysing the data, wrote the initial drafts of the paper, participated in the editing and led the revision of the manuscript.
CONFLICT OF INTEREST STATEMENT
TS declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as her having a potential conflict of interest. KM is a consultant for BioElectronics Corporation. RS is an investor and on the Board of Directors of BioElectronics Corporation.
FUNDING INFORMATION
Plumpton College, Lewis, United Kingdom; BioElectronics Corporation.
ETHICS STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. The experimental protocol was designed according to the guidelines of the current European and UK laws on the protection of animals used for scientific purposes.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.1408.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
The authors would like to acknowledge the helpful comments of Sree Koneru, Julie Edell and Jack Merrifield.
Sprunks, T. E. , McLeod, K. J. , & Staelin, R. (2024). Pulsed shortwave electromagnetic field therapy increases quality of life in canines with symptoms of osteoarthritics. Veterinary Medicine and Science, 10, e1408. 10.1002/vms3.1408
Sree Koneru and Jack Merrifield contributed equally to this study.
Footnotes
If a dog was initially placed in the mild discomfort/pain category and finally placed in the no pain category, technically this results in only a 1.5‐point reduction. However, this particular reduction is also treated as a success.
DATA AVAILABILITY STATEMENT
Data available via a request from the corresponding author.
REFERENCES
- Bagnato, G. L. , Miceli, G. , Marino, N. , Sciortino, D. , & Bagnato, G. F. (2016). Pulsed electromagnetic fields in knee osteoarthritis: A double blind, placebo‐controlled, randomized clinical trial. Rheumatology (Oxford, England), 55(4), 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binhi, V. N. , & Rubin, A. B. (2022). Theoretical concepts in magnetobiology after 40 years of research. Cells, 11(2), 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova, L. V. , Ivanova, S. , Zhang, M. , Yang, H. , Botchkina, G. I. , Watkins, L. R. , Wang, H. , Abumrad, N. , Eaton, J. W. , & Tracey, K. J. (2000). Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature, 405(6785), 458–462. [DOI] [PubMed] [Google Scholar]
- Brook, J. , Dauphinee, D. M. , Korpinen, J. , & Rawe, I. M. (2012). Pulsed radiofrequency electromagnetic field therapy: A potential novel treatment of plantar fasciitis. The Journal of Foot and Ankle Surgery: Official Publication of the American College of Foot and Ankle Surgeons, 51(3), 312–316. [DOI] [PubMed] [Google Scholar]
- Brown, D. C. , Boston, R. C. , & Farrar, J. T. (2013). Comparison of force plate gait analysis and owner assessment of pain using the canine brief pain inventory in dogs with osteoarthritis. Journal of Veterinary Internal Medicine, 27(1), 22–30. [DOI] [PubMed] [Google Scholar]
- Cell phones and specific absorption rate (2024). Federal Communications Comission. https://www.fcc.gov/general/cell‐phones‐and‐specific‐absorption‐rate [Google Scholar]
- Corral, M. J. , Moyaert, H. , Fernandes, T. , Escalada, M. , Tena, J. K. S. , Walters, R. R. , & Stegemann, M. R. (2021). A prospective, randomized, blinded, placebo‐controlled multisite clinical study of bedinvetmab, a canine monoclonal antibody targeting nerve growth factor, in dogs with osteoarthritis. Veterinary Anaesthesia and Analgesia, 48(6), 943–955. [DOI] [PubMed] [Google Scholar]
- Falvey, A. (2022). Vagus nerve stimulation and inflammation: Expanding the scope beyond cytokines. Bioelectronic Medicine, 8(1), 19. 10.1186/s42234-022-00100-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvey, A. , Metz, C. , Tracey, K. , & Pavlov, V. (2021). Peripheral nerve stimulation and immunity: The expanding opportunities for providing mechanistic insight and therapeutic intervention. International Immunology, 34(2), 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Lang, A. G. , & Buchner, A. (2007). G*power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. [DOI] [PubMed] [Google Scholar]
- Freund, K. , Kieves, N. , Hart, J. , Foster, S. , Jeffery, U. , & Duerr, F. (2016). Assessment of novel digital and smartphone goniometers for measurement of canine stifle joint angles. American Journal of Veterinary Research, 77(7), 749–755. [DOI] [PubMed] [Google Scholar]
- Gaynor, J. S. , Hagberg, S. , & Gurfein, B. T. (2018). Veterinary applications of pulsed electromagnetic field therapy. Research in Veterinary Science, 119, 1–8. [DOI] [PubMed] [Google Scholar]
- Grubb, T. (2023). Select drugs and compounds for canine osteoarthritis management. Today's Veterinary Practice, 36–41.
- Jaegger, G. , Marcellin‐Little, D. , & Levine, D. (2002). Reliability of goniometry in labrador retrievers. American Journal of Veterinary Research, 63(7), 979–986. [DOI] [PubMed] [Google Scholar]
- Johnson, R. , & Wilson, C. (2018). A review of vagus nerve stimulation as a therapeutic intervention. Journal of Inflammation Research, 11, 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, S. A. , & Budsberg, S. C. (1997). Nonsteroidal anti‐inflammatory drugs and corticosteroids for the management of canine osteoarthritis. Veterinary Clinics of North America: Small Animal Practice, 27(4), 841–862. [DOI] [PubMed] [Google Scholar]
- Khooshideh, M. , Latifi Rostami, S. S. , Sheikh, M. , Ghorbani Yekta, B. , & Shahriari, A. (2017). Pulsed electromagnetic fields for postsurgical pain management in women undergoing cesarean section: A randomized, double‐blind, placebo‐controlled trial. The Clinical Journal of Pain, 33(2), 142–147. [DOI] [PubMed] [Google Scholar]
- Koneru, S. N. , Staelin, R. , & Rawe, I. M. (2019). Chronic pain intervention using pulsed shortwave therapy: The relationship between pain demographics and central sensitization inventory. Pain Management, 9(3), 283–296. [DOI] [PubMed] [Google Scholar]
- Koneru, S. N. , Westgate, C. R. , & McLeod, K. J. (2016). Rectification of RF fields in load dependent coupled systems: Application to non‐invasive electroceuticals. Journal of Biomedical Science and Engineering, 09(02), 112–121. [Google Scholar]
- Koneru, S. , McLeod, K. J. , & Staelin, R. (2022). How electroceuticals treat chronic pain . US Pain Foundation. [Google Scholar]
- Lascelles, B. D. X. , Brown, D. C. , Conzemius, M. G. , Gill, M. , Oshinsky, M. L. , & Sharkey, M. (2019). Measurement of chronic pain in companion animals: Discussions from the Pain in Animals Workshop (PAW) 2017. Veterinary Journal, 250, 71–78. [DOI] [PubMed] [Google Scholar]
- Mohammad, E. , Rachid, A. , Koneru, S. , Staelin, R. , McLeod, K. , Tabbouche, O. , & Rawe, I. M. (2021). Pulsed shortwave therapy in cervical osteoarthritis: An NSAID‐controlled, randomized clinical trial. SN Comprehensive Clinical Medicine, 3, 166–175. [Google Scholar]
- Moore, S. A. (2016). Managing neuropathic pain in dogs. Frontiers in Veterinary Science, 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinna, S. , Landucci, F. , Tribuiani, A. , Carli, F. , & Venturini, A. (2013). The effects of pulsed electromagnetic field in the treatment of osteoarthritis in dogs: Clinical study. Pakistan Veterinary Journal, 33(1), 96–100. [Google Scholar]
- Prydie, D. , & Hewitt, I. (2015). Practical physiotherapy for small animal practice. John Wiley & Sons Ltd. [Google Scholar]
- Staelin, R. , Koneru, S. N. , & Rawe, I. M. (2019). A prospective six‐month study of chronic pain sufferers: A novel OTC neuromodulation therapy. Pain Research & Management, 2019, 3154194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson, M. , Lind, V. , & Harringe, M. L. (2019). Measurement of knee joint range of motion with a digital goniometer: A reliability study. Physiotherapy Research International, 24(2), e1765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
Data available via a request from the corresponding author.


