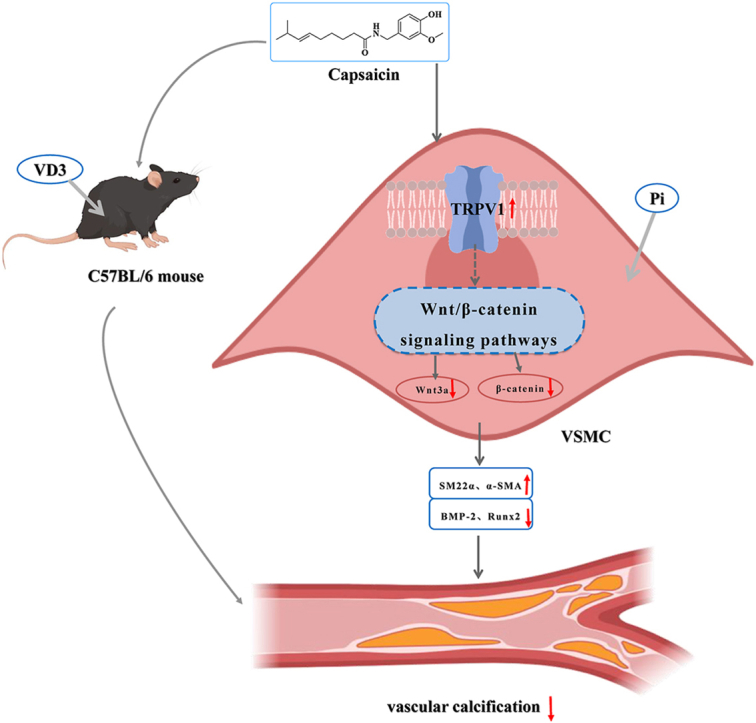
Abstract
Vascular calcification (VC) is an accurate risk factor and predictor of adverse cardiovascular events; however, there is currently no effective therapy to specifically prevent VC progression. Capsaicin (Cap) is a bioactive alkaloid isolated from Capsicum annuum L., a traditional medicinal and edible plant that is beneficial for preventing cardiovascular diseases. However, the effect of Cap on VC remains unclear. This study aimed to explore the effects and related mechanisms of Cap on aortic calcification in a mouse and on Pi-induced calcification in vascular smooth muscle cells (VSMCs). First, we established a calcification mouse model with vitamin D3 and evaluated the effects of Cap on calcification mice using von Kossa staining, calcium content, and alkaline phosphatase activity tests. The results showed that Cap significantly improved calcification in mice. VSMCs were then cultured in 2.6 mM Na2HPO4 and 50 μg/mL ascorbic acid for 7 days to obtain a calcification model, and we investigated the effects and mechanisms of Cap on VSMCs calcification by assessing the changes of calcium deposition, calcium content, and subsequent VC biomarkers. These results showed that Cap alleviated VSMCs calcification by upregulating the expressions of TRPV1. Moreover, Cap reduced the expression of Wnt3a and β-catenin, whereas DKK1 antagonised the inhibitory effect of Cap on VSMC calcification. This study is the first to offer direct evidence that Cap inhibits the Wnt/β-catenin signaling pathway by upregulating the expression of the TRPV1 receptor, resulting in the decreased expression of Runx2 and BMP-2, thereby reducing VSMC calcification. Our study may provide novel strategies for preventing the progression of VC. This could serve as a theoretical basis for clinically treating VC with spicy foods.
Keywords: Vascular calcification, Capsaicin, TRPV1, Wnt/β-catenin signaling pathway
Graphical abstract
1. Introduction
Cardiovascular disease (CVD) is the leading cause of mortality worldwide, with a relatively high prevalence (37.5% of the average population), and vascular calcification (VC) is generally observed in chronic diseases, such as chronic kidney disease (CKD). It is an accurate risk factor and predictor of adverse cardiovascular events, particularly in patients with CKD [1]. The osteogenic transformation of VSMCs plays a key role in VC progression [2]. VSMCs undergo phenotypic transition from a contractile to a synthetic and osteochondrogenic phenotype under stimulation by high calcium and phosphate levels, oxidative stress, and inflammatory factors [3]. This transition is characterised by an increased expression of osteogenesis-related genes such as runt-related transcription factor 2 (Runx2) and bone morphogenetic protein-2 (BMP-2), along with a decrease in markers associated with the contractile phenotype, such as smooth muscle 22 alpha (SM22α) and α-smooth muscle actin (α-SMA) [4]. Despite the high prevalence and severe clinical consequences of CVD, there is no effective therapy to prevent or halt VC progression. The current management of VC includes adjusting the diet to limit the intake of phosphate, calcium, and vitamin D [5], but these strategies have minimal impact on mortality. Therefore, the development of new drugs to suppress VC is essential.
In recent years, natural bioactive products have attracted widespread attention owing to their low toxicity and health benefits. Modern nutritional science has found that a high intake of dietary phytochemicals may reduce the risk of CVD [6]. Capsicum annuum L. (chili pepper), a traditional medicinal and edible plant, has been reported to have different pharmacological activities [7], and an emerging study has demonstrated that individuals who consume chili pepper may live longer and have a significantly lower risk of CVD-related mortality [8]. Hence, studies on the importance of dietary phytochemicals in CVD have suggested that the use of chilli peppers is a potential therapeutic strategy for VC.
Capsaicin (Cap), a bioactive vanilla amide alkaloid component of chilli peppers, is extensively used as a food additive. It has been demonstrated to display a variety of pharmacological effects, including anti-inflammatory, antioxidant, analgesic, and antihypertensive effects [9]. Cap is an agonist of transient receptor potential vanilloid channel 1 (TRPV1), and some evidence suggests that Cap-mediated activation of TRPV1 effectively regulates metabolic disorders and improves cardiovascular function [10]. TRPV1 is an ion channel receptor closely associated with pain transmission and is considered a promising therapeutic target for the development of novel anti-inflammatory and analgesic drugs [11]. Many studies have demonstrated that TRPV1 overexpression by Cap is closely related to the development and progression of CVD; however, the relationship between Cap and VC is relatively weak. A study by Zhou et al. found that Cap-mediated activation of TRPV1 inhibited VSMC phenotypic switching through upregulation of PPARα expression [12]. Luo et al. found that Cap facilitates deacetylation and degradation of Hif1α by upregulating SIRT6, which inhibits osteogenic transdifferentiation and protects against arterial calcification [13]. In addition, no study has reflected on the relationship between Cap and VC. The Wnt/β-catenin signaling pathway has been shown to play a critical role in the process of VC both in vivo and in vitro. Studies have delineated that this pathway are engaged in VSMC osteogenesis under various pro-calcifying stimulations [14,15]. However, whether Cap could improve VC by inhibiting the Wnt/β-catenin signaling pathway remains unclear.
Therefore, the present study aimed to investigate the effects and mechanisms of action of Cap on calcification in mice and VSMCs. First, von Kossa staining and calcium content and alkaline phosphatase (ALP) activity tests were performed to assess the effect of Cap on calcification in mice. To investigate the effect of Cap on the calcification of VSMCs, we used Alizarin Red staining and quantification of calcium content to assess changes in calcium deposition and calcium content. Reverse transcription-polymerase chain reaction (RT-PCR) and western blotting were performed to assess the expression of osteogenic transcription factors and phenotype-switching factors in VSMCs. In addition, capsazepine (CPZ; a TRPV1 antagonist) and Dickkopf-related protein 1 (DKK1; an antagonistic inhibitor of the Wnt signaling pathway) were used to determine whether Cap could activate TRPV1 and inhibit Wnt/β-catenin signaling pathway. Our study provides new targets and strategies to prevent or halt the progression of VC, providing a theoretical basis for the clinical treatment of VC using spicy foods.
2. Materials and methods
2.1. Animals and reagents
2.1.1. Animals
A total of 24 male SPF-grade C57BL/6 mice (8-week-old, weight: 20–25 g) were purchased from the Laboratory Animal Centre of the Air Force Medical University (Xi'an, Shaanxi, China) (Permit No. SYXK-2019-001). All animal experiments were approved by the Laboratory Animal Management and Ethics Committee of Northwestern University (Ethics approval number: NWU-AWC-20231103M). Before the experiment, the animals were housed in a specific pathogen-free environment with standard conditions (50 ± 10% humidity, 23 ± 3 °C temperature, a 12 h dark/light cycle, and free access to food and water).
2.1.2. Chemicals and reagents
Cap (211275), CPZ (C191), Na2HPO4 (S9763), ascorbic acid (PHR1008), phenylmethanesulfonyl fluoride (PMSF, PMSF-RO), phosphatase inhibitors (524624), RIPA lysis buffer (20–188), dimethyl sulfoxide (D2447) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco's modified Eagle's medium (DMEM, 11965084), foetal bovine serum (FBS, 16140071), streptomycin/penicillin (15070063), and trypsin (25200072) were obtained from Hyclone company (South Logan, UT, USA), and Alizarin Red S solution (MB4623) was purchased from Meilunbio (Dalian, China). The von Kossa solution (G1043) was obtained from Servicebio (Wuhan, China). TRIzol reagent was purchased from Takara Bio Inc. (Shiga, Japan). Kits, including one step RT-PCR kit (FP313) and SuperRealPreMix (SYBR Green) (FP215), were purchased from Tiangen Biotech (Beijing) Co., Ltd. The calcium colorimetric assay kit (S1063S) and alkaline phosphatase assay kit (P0321S) were purchased from Beyotime (Shanghai, China), and BCA protein assay kit (PC0020) was obtained from Solarbio (Beijing, China). Antibodies against BMP-2 (PRP100126) and Runx2 (ABP53087) were purchased from Abbkine (Wuhan, Hubei, China); SM22α (AF9266) and Wnt3a (DF6113), Affinity Biosciences LTD; β-actin (81115-1-RR), proteintech (Hubei, China); β-catenin (ab32572), Abcom (USA); and DKK1 (HY-P73305), MCE China (Beijing, China).
2.2. Animal experiments
After acclimatization for 1 week, the mice were randomly divided into control, Model, and Model + Cap groups (n = 8 per group). Prior to inducing the calcification, mice in the Model + Cap group were subjected to intragastric administration of 1 mg/kg Cap (200 μL/20 g/d) for 1 week, while the other group received an equivalent volume of saline. To induce the calcification model, VD3 (14.575 mg) was added to ethanol (70 μL) and mixed with 500 μL hydrogenated castor oil for 15 min at 25 °C, and the solution was mixed with 6.2 mL sterile water containing 250 mg glucose for 15 min at room temperature. Mice in Model and Model + Cap groups were subcutaneously injected with VD3 (150 μL/25 g/d) for the next 3 consecutive days [16]. All animals were intragastrically administered the same dose of Cap or saline for the next 4 days until the end of the experiment [17]. The mice were euthanised to obtain aortic samples, and the complete aortas in each group were excised and carefully dissected for further analysis, including von Kossa staining, calcium content, and ALP activity tests.
2.3. VSMC culture and treatment
VSMCs (rat thoracic aorta smooth muscle cells, A7R5) were kindly provided by the Cell Bank, Chinese Academy of Sciences (Shanghai, China). As recommended, the cells were cultured in complete DMEM (containing 10% FBS supplemented with 1% penicillin-streptomycin) in a humidified atmosphere with 5% CO2. The incubator temperature was set at 37 °C. Calcification of VSMCs was induced as described by Pang et al. with some modifications [18]. Briefly, VSMCs were plated in triplicate in 6-well culture plates at a density of 4 × 104 cells/well for 24 h. VSMCs were then cultured with complete DMEM containing 2.6 mM Na2HPO4 and 50 μg/mL ascorbic acid (pro-calcifying medium). In the Cap-treated group, different concentrations of Cap (1, 5, 10, and 20 μM) were added. To explore the potential action of Cap in preventing VC, CPZ and DKK1 were added to the pro-calcifying medium. The medium was refreshed every 3–4 days. After 7 days of treatment, VSMCs were collected for further analysis. Each experiment was performed at least three times. In this study, VSMCs from passages three to eight were utilised.
2.4. Alizarin Red staining assay
Calcium deposition was assessed using the Alizarin Red staining assay, as previously described [19]. Briefly, VSMCs were cultured in control medium or calcifying medium for 7 days and then washed three times with 0.1 M phosphate buffer (pH 7.2) for 3 min each time. Next, the cells were fixed in 4% paraformaldehyde at room temperature for 30 min and washed with distilled water. The VSMCs were then exposed to Alizarin Red S staining solution (pH 4.2, 1%) at room temperature for 0.5 h. Finally, the stained cells were washed again with distilled water and visualised under a light microscope (Olympus BX 50; Olympus Optical Tokyo, Japan).
2.5. Von Kossa staining assay
For von Kossa staining, aortic samples were fixed with 4% paraformaldehyde, embedded in OCT-Gel, and frozen in liquid nitrogen. They were then cut into thin sections (10 μm) with a cryomicrotome (Leica CM1950) at −20 °C and pasted on the glass slides. After complete attachment to slides, von Kossa staining was performed using von Kossa solution (Servicebio) according to the manufacturer's instructions. Briefly, samples were incubated with von Kossa solution under ultraviolet light for 4 h. The tissue sections were washed with ddH2O and then counterstained with haematoxylin and eosin solution. Next, they were dehydrated with alcohol, rendered transparent in xylene, and embedded in neutral gum. Finally, the aortic samples were observed and photographed under a light microscope (Olympus BX 50; Olympus Optical Tokyo, Japan).
2.6. Quantification of calcium content
The effects of Cap on calcium content were assessed using a calcium colorimetric assay kit (Beyotime), according to the manufacturer's instructions [20]. A standard curve was plotted. VSMCs or aortic tissues were lysed using a lysis reagent (Beyotime). The sample lysates were centrifuged (12000×g) at 4 °C for 5 min, and the supernatant was used to detect calcium content. Calcium content was normalised to the cell protein content using a BCA protein assay kit (Solarbio).
2.7. ALP activity determination
To determine ALP activity, an ALP assay kit (Beyotime) was used, following the manufacturer's instructions. Aortic tissues were homogenised with a tissue homogeniser (Beyotime) and then centrifuged at 12000×g for 5 min at 4 °C. Finally, tissue supernatants were used to detect ALP activity.
2.8. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) assay
mRNA expression was determined through RT-qPCR as previously described [21], with some modifications. Total RNA was extracted from VSMCs using the TRIzol reagent according to the manufacturer's protocol. For the reverse transcription reaction, cDNA was synthesised with a FastKing gDNA Dispelling RT SuperMix kit provided by Tiangen Biotech (Beijing, China) Co., Ltd. and stored at −20 °C before use. RT-qPCR was performed using the SuperReal PreMix Plus (SYBR Green) kit (Tiangen Biotech Co., Ltd., Beijing, China) according to the manufacturer's instructions. The PCR reaction was carried out as follows: initial activation, 95 °C for 30 s; denaturation, 95 °C for 5s; annealing, 60 °C for 34 s; and extension, 95 °C for 30 s (40 cycles). The expression level of the target mRNA was normalised to the level of the GAPDH mRNA in each sample, and the 2−ΔΔCt method [22] was then used to calculate relative quantification. Each sample was tested at least three times. The PCR primers used in this study are listed in Table 1.
Table 1.
The PCR primers used for RT-qPCR in this study.
| Gene name | Primer sequences |
|
|---|---|---|
| Forward (5′-3′) | Reverse (5′-3′) | |
| SM22α | CTGGAGGAGCGGCTAGTGGAG | GGCACCTTCACTGGCTTGGATC |
| a-SMA | GCGTGGCTATTCCTTCGTGACTAC | CCATCAGGCAGTTCGTAGCTCTTC |
| Runx2 | CTTCGTCAGCGTCCTATCAGTTCC | TCCATCAGCGTCAACACCATCATTC |
| BMP-2 | GTDACTTTTGGCCACGG | GACGCTTCCGCTTTGTGT |
| TRPV1 | CTTGTACTAGCTGTCCTGTAG | CTCACAGACAACGAGTTCAAAG |
| GAPDH | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
| Wnt3a | GTTCTTCTCTGGTCCTTGGCTGTG | GGCATGATCTCCACGTAGTTCCTG |
| β-catenin | ACAAGCCACAGGACTACAAGAAACG | TCAGCAGTCTCATTCCAAGCCATTG |
2.9. Western blot analysis
Western blotting was used to determine the expression levels of proteins related to VC [23]. The cultured cells were quickly washed three times with ice-cold PBS for 5 min each time. RIPA buffer supplemented with PMSF (100:1) and a phosphatase inhibitor cocktail (100:1) was used to lyse cells on ice. They were centrifuged at 12000×g for 5 min at 4 °C to extract total protein, and the protein concentrations were then measured with a BCA protein assay kit (Solarbio). Next, the protein samples were mixed with 1.5 × loading buffer and heat denatured at 100 °C for 10 min. Equal amounts of protein were separated using 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% skim milk for 1 h at room temperature and then were incubated with primary antibodies overnight at 4 °C. After washing thrice with PBST, the membranes were incubated with secondary antibodies for 2 h at room temperature. Finally, protein bands were detected using an ECL reagent (Advansta, United States). For quantitative analysis, band intensities were quantified using ImageJ software and normalised to β-actin levels. All experiments were repeated at least three times.
2.10. Statistical analysis
All experiments were performed at least thrice. The data were reported as means ± standard deviations, and significant differences between mean values were evaluated with Tukey's multiple range test, followed by one-way ANOVA (*p < 0.05, **p < 0.01, ***p < 0.001). Bar graphs were plotted using GraphPad Prism (version 8.0.2) for Windows (GraphPad Software Inc.), and all statistical analyses were performed using SPSS software version 24.0.
3. Results
3.1. Cap treatment attenuates VC in mice
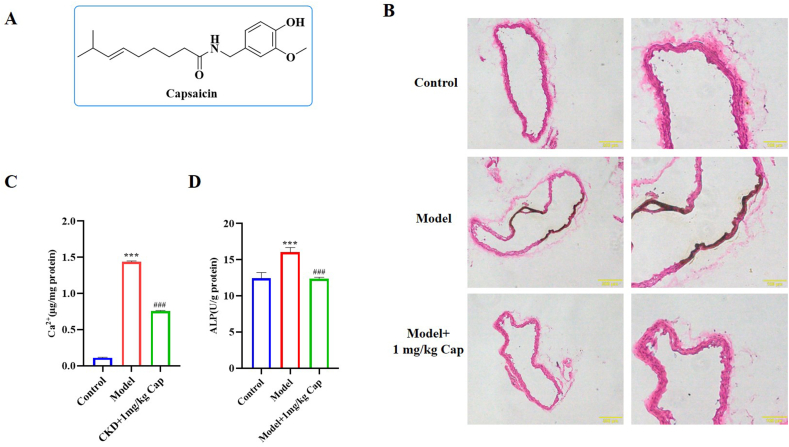
VC increases the risk of cardiovascular mortality in patients with chronic kidney disease. In this study, we established a calcification mouse model to evaluate the effects of Cap on VC in vivo. Von Kossa staining was used for the histological visualisation of calcium deposits in the sections. The effects of Cap on aortic calcification in mice were confirmed with von Kossa staining of the calcified area. The molecular structure of Cap is shown in Fig. 1A. As shown in Fig. 1B, there was no calcification in the control group; however, aortic calcification indicated by von Kossa staining was severe in the Model group (black area). Nonetheless, it dramatically improved in the Model +1 mg/kg Cap group. Meanwhile, the aortic calcium content in the Model group was 1.44 μg/mg protein, which was significantly higher than that of the control group (0.11 μg/mg protein). Interestingly, the aortic calcium content was decreased to 0.76 μg/mg protein in the Model + Cap group, which was also significantly lower than that of the Model group (Fig. 1C). In addition, aortic ALP activity in the control group was 12.45 U/g protein, which was significantly lower than that in the Model group (16.05 U/g protein). The aortic ALP activity in the Model + Cap group (12.38 U/g protein) was significantly lower than that of the Model group and was the same as that of the control group (Fig. 1D). Taken together, these results revealed that Cap significantly improved calcification in mice.
Fig. 1.
Cap treatment attenuates VC in mice; A) Chemical structure of Cap. B) Von Kossa staining assay showing the effect of Cap on calcium deposition in calcification mice. C) Effect of the Cap on calcium content in calcification mice assessed with a calcium colorimetric assay kit. D) Effect of the Cap on ALP activity in calcification mice assessed using an alkaline phosphatase assay kit; Data are mean ± SD (n = 3); #: p < 0.05, ##: p < 0.01, ###: p < 0.001 vs Control; *: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Model; ns: p > 0.05 vs Control; p > 0.05 vs Model.
3.2. Cap treatment suppresses VSMC calcification induced by high Pi concentrations
3.2.1. Effect of cap on calcium deposition and calcium content in VSMCs
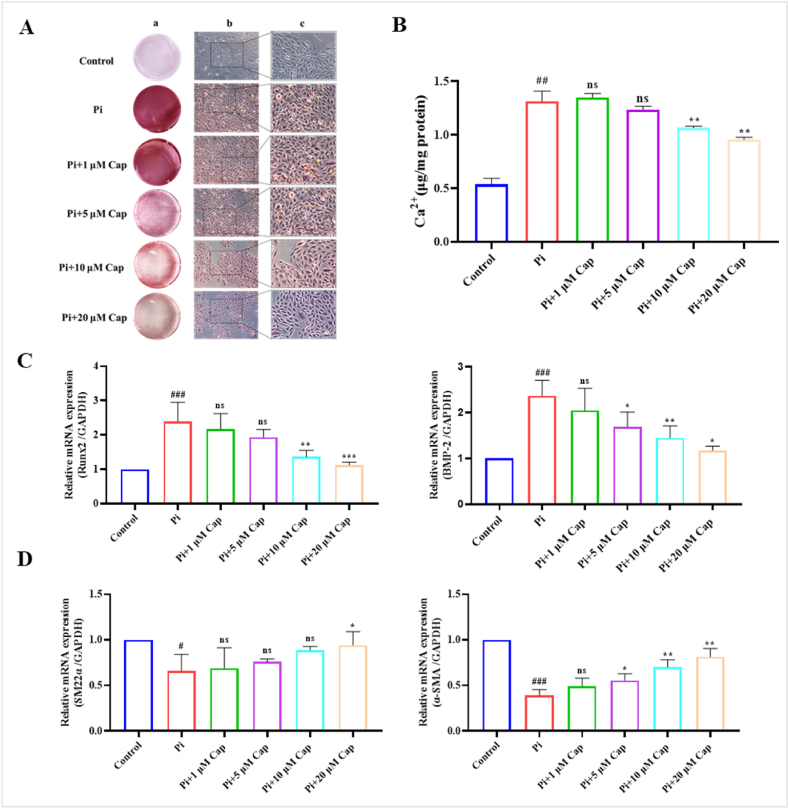
Alizarin Red staining is commonly used to detect and visualise calcium deposits in cells or tissues [24]. In this study, it was used to visualise the effect of Cap on VSMCs mineralization. The results showed no calcification in the control group but numerous orange-red calcification nodules in the high Pi treatment group (pro-calcifying medium: with 2.6 mmol/L Na2HPO4 and 50 μg/mL ascorbic acid). Following the culture of VSMCs with different concentrations of Cap (1, 5, 10, and 20 μM), calcium nodules exhibited a significant decrease compared to those in the control group, and the amount of calcium deposition decreased with increasing Cap concentration (Fig. 2A). These results revealed that cap significantly attenuated mineralised nodule formation in a concentration-dependent manner.
Fig. 2.
Cap treatment suppresses VSMC calcification induced by high Pi concentrations. A) Alizarin Red staining assay showing the effect of Cap on calcium deposition in VSMCs. B) Effect of the Cap on calcium content in VSMCs assessed using a calcium colorimetric assay kit. C) RT-qPCR revealing the effect of Cap on the expression of osteogenesis-specific markers in VSMC calcification. D) RT-qPCR illustrating the effect of Cap on the expression of phenotypic markers in VSMC calcification. VSMCs cultured in the complete and pro-calcifying medium were defined as the control and Pi group, respectively, and VSMCs treated with different concentrations of Cap in the pro-calcifying medium were used as the treatment group (Pi+1 μM, 5 μM, 10 μM, 20 μM Cap); Data are mean ± SD (n = 3); #: p < 0.05, ##: p < 0.01, ###: p < 0.001 vs Control; *: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Pi; ns: p > 0.05 vs Control; p > 0.05 vs Pi. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The effect of Cap on the calcium content of the VSMCs was measured using a calcium colorimetric assay kit. The calcium content of the VSMCs was normalised to the protein content and expressed as μg/mg protein. After being cultured in the pro-calcifying medium (Pi group) for 7 days, the calcium content in VSMCs reached 1.32 μg/mg protein, which was significantly increased compared to that of the control group (0.54 μg/mg protein). Interestingly, the calcium content was reduced in the presence of different concentrations of Cap compared with that in the Pi group. When the concentration of Cap was 20 μM, calcium content decreased to 0.96 μg/mg protein, which was significantly lower than that in the Pi group (Fig. 2B).
3.2.2. Effect of cap on the expression of biomarkers in VSMC calcification
To further verify the suppressive effects of Cap on VSMC calcification, we investigated the effect of various concentrations of Cap on the osteogenic differentiation of VSMCs by examining the expression of osteogenesis-specific markers, such as Runx2 and BMP-2. The mRNA expression levels of Runx2 and BMP-2 in the Pi group were approximately 2.39-fold and 2.37-fold, respectively, higher than those in the control group. Similarly, after treatment with 20 μM of Cap, the mRNA expression of these genes was approximately 1.28-fold and 1.18-fold higher compared to the control group, exhibiting a significant decrease than those in the Pi group (Fig. 2C). These results revealed that Cap downregulated Runx2 and BMP-2 mRNA expression in a concentration-dependent manner. We also evaluated the mRNA expression of phenotypic markers in VSMCs. Both the relative mRNA expression levels of SM22α and α-SMA were downregulated in the Pi group relative to the control group. However, with the increase of the Cap concentration, the relative mRNA expression levels of SM22α and α-SMA in VSMCs were upregulated (Fig. 2D). Taking all these data into consideration, the calcification of VSMCs induced by a high Pi concentration was improved after Cap treatment within the concentration range of 1 μM–20 μM, and the most effective concentration of Cap was 20 μM. Therefore, the concentration of 20 μM Cap was deemed optimal, yielding maximum effectiveness, and was consequently used in subsequent experiments.
3.3. Cap inhibits Pi-induced calcification of VSMCs by activating the TRPV1 receptor
3.3.1. Expression of TRPV1 in Pi-induced calcification of VSMCs was reduced
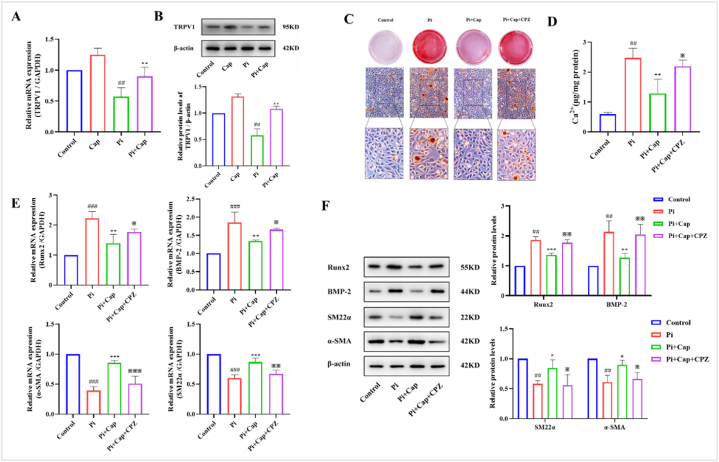
TRPV1 is an ion channel receptor, and Cap-mediated activation of TRPV1 can effectively regulate metabolic disorders and improve cardiovascular function. Thus, we tested the expression of TRPV1 in VSMCs using RT-qPCR and western blotting. Fig. 3A shows that the relative mRNA expression of TRPV1 was 0.59-fold lower in the Pi group but 1.24-fold higher in the Cap group than in the control group. Interestingly, mRNA expression in the Pi + Cap group was 0.89-fold lower than that in the control group and still increased significantly compared to that in the Pi group (p < 0.05). Furthermore, we detected the protein expressions of TRPV1, which were found to be upregulated in the Cap group and downregulated in the Pi group compared to those in the control group. However, this upregulation was significantly suppressed in the Pi + Cap group (Fig. 3B). These results demonstrated that the expression of TPRV1 in VSMCs was inhibited under high Pi conditions and that Cap could upregulate the expression of TRPV1 in high Pi-induced calcification of VSMCs.
Fig. 3.
Cap inhibits Pi-induced calcification of VSMCs by activating the TRPV1 receptor. A) RT-qPCR showing the mRNA levels of TRPV1; B) Western blot depicting the protein expression levels of TRPV1; C) Alizarin Red staining assay; D) Calcium content in VSMCs assessed using a calcium colorimetric assay kit. E) RT-qPCR illustrating the expression of osteogenesis-specific and phenotypic markers in VSMCs calcification. F) Western blot revealing the protein expression levels of osteogenesis-specific and phenotypic markers in VSMCs calcification. VSMCs cultured in the complete and pro-calcifying medium were defined as the control and Pi group, respectively; Pi + Cap: VSMCs cultured in the pro-calcifying medium with 20 μM Cap; Pi + Cap + CPZ: VSMCs cultured in the pro-calcifying medium with 20 μM Cap and 10 μM CPZ; Data are mean ± SD (n = 3); #: p < 0.05, ##: p < 0.01, ###: p < 0.001 vs Control; *: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Pi; ※: p < 0.05, ※※: p < 0.01, ※※※: p < 0.001 vs Pi + Cap; ns: p > 0.05 vs Control; p > 0.05 vs Pi; p > 0.05 vs Pi + Cap. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3.2. Cap inhibits VSMCs calcification by activating TRPV1
Since the expression levels of TRPV1 were upregulated in the Cap group and downregulated in the Pi + Cap group, we further studied the relationship between TRPV1 and Pi-induced calcification of VSMCs by assessing the calcification indicators. As shown in Fig. 3C, more red-calcified nodules were observed after treatment with CPZ, a TRPV1 antagonist, than in the Pi + Cap group. Similarly, the calcium content increased to 2.19 μg/mg protein after the treatment of CPZ, which was almost equal to the control group (Fig. 3D). The mRNA and protein expression levels of VSMC calcification biomarkers were studied in the presence of CPZ. The RT-qPCR results showed that the mRNA expressions of Runx2 and BMP-2 in Pi + Cap + CPZ were upregulated and those of SM22α and α-SMA were significantly downregulated, compared to those in the Pi + Cap group. The mRNA expression levels of all markers were similar to those in the Pi group (Fig. 3E). Western blot analysis further revealed that Cap significantly reduced the protein expressions of BMP-2 and Runx2, as well as increased the protein expression levels of SM22α and α-SMA. However, these effects were attenuated when induced by CPZ treatment (Fig. 3F). These results suggest that Cap inhibits Pi-induced calcification of VSMCs by activating the TRPV1 receptor.
3.4. Cap inhibits Pi-induced calcification of VSMCs by inhibiting the Wnt/β-catenin signaling pathway by upregulating the TRPV1 receptor
3.4.1. Cap inhibits the Wnt/β-catenin signaling pathway-related proteins via the TRPV1 receptor
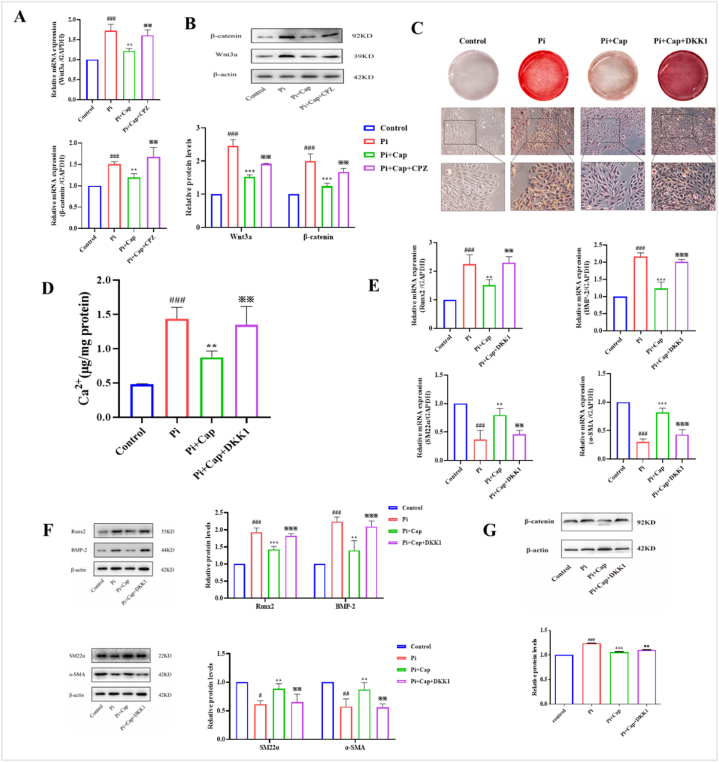
The Wnt/β-catenin signaling pathway plays a critical role in VSMC calcification, as it promotes VSMC calcification by directly modulating the expression of Runx2. In this study, we investigated the effects of Cap on related proteins (Wnt3a and β-catenin) of the Wnt/β-catenin signaling pathway. Fig. 4A and B shows the mRNA and protein expression levels of Wnt3a and β-catenin after being incubated with different culture mediums. In the Pi group, both the mRNA and protein expression levels of Wnt3a and β-catenin were increased significantly compared to those in the control group. In the Pi + Cap group, their expression decreased sharply compared with that in the Pi group. However, in the presence of CPZ, both the expressions of mRNA and protein of Wnt3a and β-catenin were upregulated compared to those in the Pi + Cap group. The mRNA expression levels of Wnt3a and β-catenin were 1.61-fold and 1.68-fold higher, respectively, compared to those in the control group, which is approximately equal to the expressions in the Pi group (1.73-fold and 1.57-fold, respectively). These results suggest that Cap inhibits the expression of related proteins in the Wnt/β-catenin signaling pathway by upregulating the expression of TRPV1.
Fig. 4.
Cap inhibits Pi-induced calcification of VSMCs by inhibiting the Wnt/β-catenin signaling pathway by upregulation of TRPV1 receptor expression. A) RT-qPCR showing the mRNA levels of Wnt3a and β-catenin; B) Western blot illustrating the protein expression levels of Wnt3a and β-catenin; C) Alizarin Red staining assay; D) Calcium content in VSMCs assessed using a calcium colorimetric assay kit. E) RT-qPCR showing the expression of osteogenesis-specific and phenotypic markers in VSMC calcification. F) Western blot depicting the protein expression levels of osteogenesis-specific and phenotypic markers in VSMC calcification; G) Western blot showing the protein expression of β-catenin after treatment with DKK1. VSMCs cultured in the complete and pro-calcifying medium were defined as the control and Pi group, respectively; Pi + Cap: VSMCs cultured in the pro-calcifying medium with 20 μM Cap; Pi + Cap + CPZ: VSMCs cultured in the pro-calcifying medium with 20 μM Cap and 10 μM CPZ; Pi + Cap + DKK1: VSMCs cultured in the pro-calcifying medium with 20 μM Cap and 20 ng/mL DKK1; Data are mean ± SD (n = 3); #: p < 0.05, ##: p < 0.01, ###: p < 0.001 vs Control; *: p < 0.05, **: p < 0.01, ***: p < 0.001 vs Pi; ※: p < 0.05, ※※: p < 0.01, ※※※: p < 0.001 vs Pi + Cap; ns: p > 0.05 vs Control; p > 0.05 vs Pi; p > 0.05 vs Pi + Cap. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.4.2. Cap inhibits Pi-induced calcification of VSMCs by inhibiting the Wnt/β-catenin signaling pathway
DKK1 is an antagonistic inhibitor of the Wnt signaling pathway, which was introduced to investigate whether Cap improves Pi-induced calcification of VSMCs by inhibiting the Wnt/β-catenin signaling pathway. As shown in Fig. 4C, numerous orange-red calcified nodules were observed in the Pi + Cap + DKK1 group. The calcium content in the Pi + Cap + DKK1 group was 1.34 μg/mg protein (Fig. 4D), which was increased significantly compared to that in the Pi + Cap group (0.87 μg/mg protein) and was almost equal to that in the Pi group (1.44 μg/mg). Next, the expression of osteogenesis-specific and phenotypic markers was evaluated, and the results showed that both the mRNA and protein expression levels of Runx2 and BMP-2 were significantly upregulated in the Pi + Cap + DKK1 group compared to those in the Pi + Cap group. Simultaneously, the expression levels of SM22α and α-SMA of both mRNA and protein were severely reduced in the Pi + Cap + DKK1 group compared to those in the Pi + Cap group. The expression levels of all four markers in the Pi + Cap + DKK1 group were similar to those in the Pi group (Fig. 4E and F). Meanwhile, the protein expression of β-catenin was significantly increased in Pi + Cap + DKK1 group compared with that in the Pi + Cap group (Fig. 4G). These results suggest that Cap inhibits Pi-induced calcification of VSMCs by inhibiting the Wnt/β-catenin signaling pathway through upregulation of the TRPV1 receptor.
4. Discussion
As the active ingredient of chili pepper, Cap exhibits a variety of pharmacological activities, including analgesic, anticancer, anti-inflammatory, anti-obesity, and antioxidant [25]. Current studies have shown that Cap can significantly improve the function of the vascular endothelium, reduce blood pressure in spontaneously hypertensive rats, and slow atherosclerosis [26]. It also inhibits the proliferation and migration of VSMCs. The osteogenic phenotypic transformation of VSMCs is the most important aspect of VC; however, the specific role and mechanism of Cap in VC remain unclear. In the present study, C57BL/6 mice were injected subcutaneously with VD3 for 3 consecutive days to obtain calcification mice in vivo, and the results showed that Cap could weaken calcification in mice. VSMCs were then cultured in a pro-calcifying medium for 7 days to obtain a calcification model. Next, we confirmed that Cap alleviated Pi-induced calcification of VSMCs by upregulating the expressions of phenotypic markers (SM22α and α-SMA) and downregulating the expressions of osteoblastic markers (Runx2 and BMP-2) via activation of TRPV1. Moreover, we found that Cap reduced the expression of Wnt3a and β-catenin and that DKK1 antagonised the inhibitory effect of Cap on VSMC calcification. This study for the first time provided direct evidence that Cap inhibits Pi-induced calcification of VSMCs by inhibiting the Wnt/β-catenin signaling pathway by upregulating the TRPV1 receptor.
Capsicum annuum L. is a traditional edible and medicinal plant, serving as a globally popular vegetable and spice [27]. An epidemiological survey in 2020 showed a noteworthy difference in the prevalence of obesity, hypertension, acute myocardial infarction, coronary heart disease, and the annual interventional treatment of acute coronary syndrome between the southwest and north of China; the southwest region, owing to their higher consumption of spicy food, exhibited significantly lower rates of these health conditions [28]. In 2019, Bonaccio et al. found that in a large adult Mediterranean population, regular consumption of chili pepper was associated with a lower risk of total and CVD mortality, independent of CVD risk factors [29]. In addition, a systematic review and meta-analysis found that regular consumption of spicy food was associated with a 12% lower risk of all-cause mortality than infrequent consumption, and consumption of spicy food was associated with a significant reduction in the risk of death from cardiac diseases [8]. These studies showed that chilli pepper may protect against CVD.
VC is a complex process involving the active deposition of calcium phosphate on the blood vessel wall, which is highly correlated with increased mortality and morbidity of CVD [30]. It occurs in both the intimal and medial layers of the vasculature and shares many similarities with bone formation and mineralization [31]. A previous study demonstrated that intimal calcification involves increased inflammatory cell infiltration and lipid deposition, whereas medial calcification is associated with a phenotypic transformation of VSMCs in the arterial media [32]. VC increases in patients with CKD and is associated with significant morbidity and mortality [33]. Cap is a bioactive component of chili peppers with various biological activities. The authors found that Cap promoted the phenotypic switching of VSMCs, inhibited their proliferation and migration, and ultimately reduced vascular remodelling [34]. However, limited studies have examined the effects of Cap on VC. To better understand the effect of Cap on VC, we induced calcification in C57BL/6 mice by subcutaneously injecting VD3 for 3 consecutive days, and the results showed that Cap could weaken the calcification of mice. We also cultured VSMCs in a pro-calcifying medium with various concentrations of Cap for 7 days to assess the calcification of VSMCs. The results showed that high Pi-treatment significantly increased the calcium content of VSMCs, whereas treatment with Cap resulted in a decrease, and the expressions of osteogenesis-specific markers (Runx2 and BMP-2) were inhibited while that of the phenotypic markers (including SM22α and α-SMA) were increased after being treated with different concentrations of Cap. This suggests that Cap can inhibit the osteogenic transformation of VSMCs and further alleviate their calcification, which is consistent with a previous report [13]. These results provide experimental data on the benefits of a spicy diet on VC.
TRPV1 is a nonselective cation channel activated by Cap, and is widely distributed in vascular endothelial cells and VSMCs [35]. Many studies have suggested that TRPV1 is an important factor in the progression of CVD, including atherosclerosis, hypertension, and myocardial ischemia-reperfusion injury [36,37]. Emerging evidence suggests that TRPV1 activation by Cap improves intracranial arteriole remodelling by inhibiting the phenotypic modulation of VSMCs during hypertension through the PI3K/Akt signaling pathway [38]. Another study showed that activation of TRPV1 by Cap could rescue autophagy impaired by oxidised low-density lipoprotein by activating the AMPK signaling pathway and finally inhibiting foam cell formation [39]. However, the relationship between TRPV1 and VC remains unclear. Luo et al. found that the expression of TPRV1 in VSMCs decreased after 7 days of induction with 2.8 mM sodium phosphate [13], and our study found the same result after culturing VSMCs in pro-calcifying medium for 7 days. These results collectively confirm that high Pi levels downregulate the expression of TPRV1. Further studies found that Cap significantly increased the expression of TRPV1 in high Pi-induced calcification of VSMCs, which is consistent with a previous report [40]. To further clarify the role of TRPV1 in Pi-induced calcification of VSMCs, CPZ (a TRPV1 antagonist) was added. The results showed that calcium deposition and calcium content in VSMCs significantly increased after the inhibition of TRPV1. In addition, upon inhibition of TRPV1, there was a notable down-regulation in the expressions of SM22α and α-SMA, coupled with an upregulation of Runx2 and BMP-2 compared to the Pi group. These results suggest that Cap improves Pi-induced calcification of VSMCs by upregulating the expression of TRPV1, thereby inhibiting the osteogenic transformation of VSMCs.
TGF-β signaling pathway, BMP signaling pathway, Notch signaling pathway, and the Wnt/β-catenin signaling pathway play crucial roles in osteogenic differentiation and calcification [41]. According to the findings of domestic and foreign scholars, the Wnt/β-catenin signaling pathway has a decisive effect on VC. Guo et al. demonstrated that blocking the Wnt/β-catenin pathway significantly attenuated VC [42]. A study by Park et al. found that silencing the expression of Neuromedin B hindered the Pi-induced osteogenic differentiation of VSMCs by inhibiting the Wnt/β-catenin signaling pathway [43]. Chen et al. revealed that melatonin attenuated VSMC calcification by inhibiting the Wnt1/β-catenin system [44]. In addition, a study from Sun et al. found that the activated Wnt/β-catenin signaling could play a vital role in promoting osteogenic transdifferentiation and arterial medial calcification [45]. In this study, we found that the expressions of Wnt3a, β-catenin, and their osteogenesis-specific markers such as Runx2 and BMP-2 were increased in Pi-induced calcification of VSMCs, which was consistent with the previous research [46]. The expressions of Wnt3a, β-catenin, Runx2, and BMP-2 were downregulated after being treated with Cap, which indicates that Cap regulated VSMC osteogenic transformation through the Wnt/β-catenin signaling pathway. CPZ is an antagonist of TRPV1, which blocked the binding of Cap to TRPV1. We found that the inhibitory effect of Cap in Pi-induced calcification of VSMCs was reduced after the addition of CPZ, and the expression of Wnt3a and β-catenin and their downstream osteogenesis-specific markers were increased after being treated with CPZ. These results further confirmed that Cap inhibited the Wnt/β-catenin signaling pathway by upregulating the expression of the TRPV1 receptor, resulting in the decrease in the expression of Runx2 and BMP-2, thereby reducing VSMC calcification. Furthermore, the reversal of this effect by DKK1 aligns with results observed by other researchers.
However, this study had some limitations. We did not investigate the effect of knocking down TRPV1 and key molecules in the Wnt/β-catenin signaling pathway, which would further validate our results. In addition, we only focused on the effect of Cap on the Wnt/β-catenin signaling pathway, and other signaling pathways involved in the regulation of Cap-mediated inhibition of VC may be excluded. Thus, it is crucial to identify the mechanisms underlying the inhibition of VC by Cap using high-throughput sequencing methods, such as RNA sequencing.
In conclusion, this study confirmed that Cap attenuated calcification in mice, inhibited VSMC osteogenic phenotype transition, and reduced VSMC calcification. Additionally, Cap inhibited the Wnt/β-catenin signaling pathway by upregulating the expression of the TRPV1 receptor, which led to the reduction of the expression of osteogenic markers Runx2 and BMP-2, thereby reducing VSMC calcification. These results provide an experimental foundation supporting the use of a spicy diet to enhance VC. It could potentially offer a novel clinical strategy for the prevention and treatment of CVD.
Data availability statement
Data will be made available on request.
Ethics statement
Animal experimental procedures were approved by the Laboratory Animal Management and Ethics Committee of Northwestern University (Ethics approval number: NWU-AWC-20231103M).
CRediT authorship contribution statement
Yin-Fang Yan: Writing – review & editing, Writing – original draft, Validation, Supervision, Software, Methodology, Investigation, Data curation, Conceptualization. Yue Feng: Validation, Supervision, Software, Methodology, Investigation, Data curation, Conceptualization. Si-Min Wang: Visualization, Methodology. Fei Fang: Visualization, Methodology. Hong-Yan Chen: Methodology. Ming-Xia Zhen: Visualization. Yu-Qiang Ji: Writing – review & editing, Validation, Software, Conceptualization. Song-Di Wu: Writing – review & editing, Validation, Software.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported financially by Key Research and Development Program of Shaanxi (No.2021SF-151, 2022SF-548) and the Science and Technology Planning Project of Xi'an (No.21YXYJ0028 and No.23YXYJ0035); Support was also supplied by Shaanxi Administration of Traditional Chinese Medicine (No. 2022-SLRH-LJ-013).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28021.
Contributor Information
Yu-Qiang Ji, Email: jiyuqiang112299@126.com.
Song-Di Wu, Email: wusongdi@gmail.com.
Appendix ASupplementary data
The following is the Supplementary data to this article:
References
- 1.Ryu J., Ahn Y., Kook H., Kim Y.K. The roles of non-coding RNAs in vascular calcification and opportunities as therapeutic targets. Pharmacol. Ther. 2021;218 doi: 10.1016/j.pharmthera.2020.107675. [DOI] [PubMed] [Google Scholar]
- 2.Wang P., Pan Y., Yang C., Zhang L., Zhao Z., Ye K., Li L., Xia S., Lu X., Shi H., Li W., Yin M. TNFα activation and TGFβ blockage act synergistically for smooth muscle cell calcification in patients with venous thrombosis via TGFβ/ERK pathway. J. Cell Mol. Med. 2022;26(16):4479–4491. doi: 10.1111/jcmm.17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angbohang A., Huang L., Li Y., Zhao Y., Gong Y., Fu Y., Mao C., Morales J., Luo P., Ehteramyan M., Gao Y., Margariti A., Gu W., Zhang M., Smith A., Shah A.M., Li T., Kong W., Zeng L. X-box binding protein 1-mediated COL4A1s secretion regulates communication between vascular smooth muscle and stem/progenitor cells. J. Biol. Chem. 2021;296 doi: 10.1016/j.jbc.2021.100541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erlandsson H., Qureshi A.R., Ripsweden J., Haugen Löfman I., Söderberg M., Wennberg L., Lundgren T., Bruchfeld A., Brismar T.B., Stenvinkel P. Scoring of medial arterial calcification predicts cardiovascular events and mortality after kidney transplantation. J. Intern. Med. 2022;291(6):813–823. doi: 10.1111/joim.13459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shioi A., Morioka T., Shoji T., Emoto M. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients. 2020;12(2):583. doi: 10.3390/nu12020583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Ma Y.Y., Zhao X.N., Hua F. Phytochemicals as potential target on thioredoxin-interacting protein (TXNIP) for the treatment of cardiovascular diseases. Inflammopharmacology. 2023;31(1):207–220. doi: 10.1007/s10787-022-01130-8. [DOI] [PubMed] [Google Scholar]
- 7.Mandal S.k., Rath S.K., Logesh R., Mishra S.K., Devkota H.P., Das N. Capsicum annuum L. and its bioactive constituents: a critical review of a traditional culinary spice in terms of its modern pharmacological potentials with toxicological issues. Phytother Res. 2023;37(3):965–1002. doi: 10.1002/ptr.7660. [DOI] [PubMed] [Google Scholar]
- 8.Ofori-Asenso R., Mohsenpour M.A., Nouri M., Faghih S., Liew D., Mazidi M. Association of spicy chilli food consumption with cardiovascular and all-cause mortality: a meta-analysis of prospective cohort studies. Angiology. 2021;72(7):625–632. doi: 10.1177/0003319721995666. [DOI] [PubMed] [Google Scholar]
- 9.Wang F., Xue Y., Fu L., Wang Y., He M., Zhao L., Liao X. Extraction, purification, bioactivity and pharmacological effects of capsaicin: a review. Crit. Rev. Food Sci. Nutr. 2022;62(19):5322–5348. doi: 10.1080/10408398.2021.1884840. [DOI] [PubMed] [Google Scholar]
- 10.Braga Ferreira L.G., Faria J.V., Dos Santos J.P.S., Faria R.X. Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 2020;887 doi: 10.1016/j.ejphar.2020.173356. [DOI] [PubMed] [Google Scholar]
- 11.Iftinca M., Defaye M., Altier C. TRPV1-targeted drugs in development for human pain conditions. Drugs. 2021;81(1):7–27. doi: 10.1007/s40265-020-01429-2. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y., Wang X., Guo L., Chen L., Zhang M., Chen X., Li J., Zhang L. TRPV1 activation inhibits phenotypic switching and oxidative stress in vascular smooth muscle cells by upregulating PPARα. Biochem. Biophys. Res. Commun. 2021;545:157–163. doi: 10.1016/j.bbrc.2021.01.072. [DOI] [PubMed] [Google Scholar]
- 13.Luo D., Li W., Xie C., Yin L., Su X., Chen J., Huang H. Capsaicin attenuates arterial calcification through promoting SIRT6-mediated deacetylation and degradation of Hif1α (hypoxic-inducible factor-1 alpha) Hypertension. 2022;79(5):906–917. doi: 10.1161/HYPERTENSIONAHA.121.18778. [DOI] [PubMed] [Google Scholar]
- 14.Ren S.C., Mao N., Yi S., Ma X., Zou J.Q., Tang X., Fan J.M. Vascular calcification in chronic kidney disease: an update and perspective. Aging Dis. 2022;13(3):673–697. doi: 10.14336/AD.2021.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang J.H., Kawano T., Murata M., Toita R. Vascular calcification and cellular signaling pathways as potential therapeutic targets. Life Sci. 2023;336 doi: 10.1016/j.lfs.2023.122309. [DOI] [PubMed] [Google Scholar]
- 16.Ahn B.Y., Jeong Y., Kim S., Zhang Y., Kim S.W., Leem Y.E., Kang J.S. Cdon suppresses vascular smooth muscle calcification via repression of the Wnt/Runx2 Axis. Exp. Mol. Med. 2023;55(1):120–131. doi: 10.1038/s12276-022-00909-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwon D.H., Eom G.H., Ko J.H., Shin S., Joung H., Choe N., Nam Y.S., Min H.K., Kook T., Yoon S., Kang W., Kim Y.S., Kim H.S., Choi H., Koh J.T., Kim N., Ahn Y., Cho H.J., Lee I.K., Park D.H., Suk K., Seo S.B., Wissing E.R., Mendrysa S.M., Nam K.I., Kook H. MDM2 E3 ligase-mediated ubiquitination and degradation of HDAC1 in vascular calcification. Nat. Commun. 2016;7 doi: 10.1038/ncomms10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pang Q., Wang P., Pan Y., Dong X., Zhou T., Song X., Zhang A. Irisin protects against vascular calcification by activating autophagy and inhibiting NLRP3-mediated vascular smooth muscle cell pyroptosis in chronic kidney disease. Cell Death Dis. 2022;13(3):283. doi: 10.1038/s41419-022-04735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan Z., Chen A., Li L., Ye Y., Liang Q., Dong Q., Wang S., Fu M., Li Y., Liu X., Zhu Z., Ou J.S., Qiu X., Lu L., Yan J.A. Downregulation of HDAC9 by the ketone metabolite β-hydroxybutyrate suppresses vascular calcification. J. Pathol. 2022;258(3):213–226. doi: 10.1002/path.5992. [DOI] [PubMed] [Google Scholar]
- 20.Li X., Chen M., Wang P., Yao Y., Han X., Liang J., Jiang Q., Sun Y., Fan Y., Zhang X. A highly interweaved HA-SS-nHAp/collagen hybrid fibering hydrogel enhances osteoinductivity and mineralization. Nanoscale. 2020;12(24):12869–12882. doi: 10.1039/d0nr01824d. [DOI] [PubMed] [Google Scholar]
- 21.Miyazaki-Anzai S., Keenan A.L., Blaine J., Miyazaki M. Targeted disruption of a proximal tubule-specific TMEM174 gene in mice causes hyperphosphatemia and vascular calcification. J. Am. Soc. Nephrol. 2022;33(8):1477–1486. doi: 10.1681/ASN.2021121578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matilla L., Roncal C., Ibarrola J., Arrieta V., García-Peña A., Fernández-Celis A., Navarro A., Álvarez V., Gainza A., Orbe J., Cachofeiro V., Zalba G., Sádaba R., Rodríguez J.A., López-Andrés N. A role for MMP-10 (matrix metalloproteinase-10) in calcific aortic valve stenosis. Arterioscler. Thromb. Vasc. Biol. 2020;40(5):1370–1382. doi: 10.1161/ATVBAHA.120.314143. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z., Wang Y., Liu F., Zhu D., Chen Y., Yim W.Y., Hu K., Rao Z., Pan X., Li F., Dong N. Long noncoding TSI attenuates aortic valve calcification by suppressing TGF-β1-induced osteoblastic differentiation of valve interstitial cells. Metabolism. 2023;138 doi: 10.1016/j.metabol.2022.155337. [DOI] [PubMed] [Google Scholar]
- 24.Bernar A., Gebetsberger J.V., Bauer M., Streif W., Schirmer M. Optimization of the alizarin red S assay by enhancing mineralization of osteoblasts. Int. J. Mol. Sci. 2022;24(1):723. doi: 10.3390/ijms24010723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang K.C., Chiang Y.F., Huang T.C., Chen H.Y., Lin P.H., Ali M., Hsia S. Capsaicin alleviates cisplatin-induced muscle loss and atrophy in vitro and in vivo. J. Cachexia Sarcopenia Muscle. 2023;14(1):182–197. doi: 10.1002/jcsm.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong B., Ma S., Wang D.H. Protease-activated receptor 2 protects against myocardial ischemia-reperfusion injury through the lipoxygenase pathway and TRPV1 channels. Exp. Ther. Med. 2019;18(5):3636–3642. doi: 10.3892/etm.2019.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kariagina A., Doseff A.O. Anti-inflammatory mechanisms of dietary flavones: tapping into nature to control chronic inflammation in obesity and cancer. Int. J. Mol. Sci. 2022;23(24) doi: 10.3390/ijms232415753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li X., Wu C., Lu J., Chen B., Li Y., Yang Y., Hu S., Li J. Cardiovascular risk factors in China: a nationwide population-based cohort study. Lancet Public Health. 2020;5(12):672–681. doi: 10.1016/S2468-2667(20)30191-2. [DOI] [PubMed] [Google Scholar]
- 29.Bonaccio M., Di Castelnuovo A., Costanzo S., Ruggiero E., De Curtis A., Persichillo M., Tabolacci C., Facchiano F., Cerletti C., Donati M.B., de Gaetano G., Iacoviello L. Chili pepper consumption and mortality in Italian adults. J. Am. Coll. Cardiol. 2019;4(25):3139–3149. doi: 10.1016/j.jacc.2019.09.068. [DOI] [PubMed] [Google Scholar]
- 30.Bhat O.M., Yuan X., Camus S., Salloum F.N., Li P.L. Abnormal lysosomal positioning and small extracellular vesicle secretion in arterial stiffening and calcification of mice lacking mucolipin 1 gene. Int. J. Mol. Sci. 2020;21(5):1713. doi: 10.3390/ijms21051713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang P., Troncone L., Augur Z.M., Kim S.S.J., McNeil M.E., Yu P.B. The role of bone morphogenetic protein signaling in vascular calcification. Bone. 2020;141 doi: 10.1016/j.bone.2020.115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi B., Kim E.Y., Kim J.E., Oh S., Park S.O., Kim S.M., Choi H., Song J.K., Chang E.J. Evogliptin suppresses calcific aortic valve disease by attenuating inflammation, fibrosis, and calcification. Cells. 2021;10(1):57. doi: 10.3390/cells10010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang J.H., Kawano T., Murata M., Toita R. Vascular calcification and cellular signaling pathways as potential therapeutic targets. Life Sci. 2023;336 doi: 10.1016/j.lfs.2023.122309. [DOI] [PubMed] [Google Scholar]
- 34.Liu R., Heiss E.H., Guo D., Dirsch V.M., Atanasov A.G. Capsaicin from chili (Capsicum spp.) inhibits vascular smooth muscle cell proliferation. F1000Res. 2015;4:26. doi: 10.12688/f1000research.6007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korishettar A.M., Nishijima Y., Wang Z., Xie Y., Fang J., Wilcox D.A., Zhang D.X. Endothelin-1 potentiates TRPV1-mediated vasoconstriction of human adipose arterioles in a protein kinase C-dependent manner. Br. J. Pharmacol. 2021;178(3):709–725. doi: 10.1111/bph.15324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong B., Ma S., Wang D.H. Protective effects of TRPV1 activation against cardiac ischemia/reperfusion injury is blunted by diet-induced obesity. Cardiovasc. Hematol. Disord. Drug Targets. 2020;20(2):122–130. doi: 10.2174/1871529X19666190912152041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C., Ye L., Zhang Q., Wu F., Wang L. The role of TRPV1 channels in atherosclerosis. Channels. 2020;14(1):141–150. doi: 10.1080/19336950.2020.1747803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang M.J., Liu Y., Hu Z.C., Zhou Y., Pi Y., Guo L., Wang X., Chen X., Li J.C., Zhang L.L. TRPV1 attenuates intracranial arteriole remodeling through inhibiting VSMC phenotypic modulation in hypertension. Histochem. Cell Biol. 2017;147(4):511–521. doi: 10.1007/s00418-016-1512-x. [DOI] [PubMed] [Google Scholar]
- 39.Li B.H., Yin Y.W., Liu Y., Pi Y., Guo L., Cao X.J., Gao C.Y., Zhang L.L., Li J.C. TRPV1 activation impedes foam cell formation by inducing autophagy in oxLDL-treated vascular smooth muscle cells. Cell Death Dis. 2014;5(4):1182. doi: 10.1038/cddis.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang F., Zheng J. Understand spiciness: mechanism of TRPV1 channel activation by capsaicin. Protein Cell. 2017;8(3):169–177. doi: 10.1007/s13238-016-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan W., Jie W.A., Huang H. Vascular calcification: molecular mechanisms and therapeutic interventions. MedComm. 2020;4(1) doi: 10.1002/mco2.200. (2023) 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo L., Wang Y., Li S., Zhou L., Li D. GALNT3 protects against phosphate-induced calcification in vascular smooth muscle cells by enhancing active FGF23 and inhibiting the wnt/β-catenin signaling pathway. Cell. Signal. 2022;100 doi: 10.1016/j.cellsig.2022.110477. [DOI] [PubMed] [Google Scholar]
- 43.Park H.J., Kim M.K., Kim Y., Kim H.J., Bae S.K., Bae M.K. Neuromedin B modulates phosphate-induced vascular calcification. BMB Rep. 2021;54(11):569–574. doi: 10.5483/BMBRep.2021.54.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W.R., Zhou Y.J., Yang J.Q., Liu F., Zhao Y.X., Sha Y. Melatonin attenuates β-glycerophosphate-induced calcification of vascular smooth muscle cells via a Wnt1/β-catenin signaling pathway. BioMed Res. Int. 2019;2019 doi: 10.1155/2019/3139496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai T., Sun D., Duan Y., Wen P., Dai C., Yang J., He W. WNT/β-catenin signaling promotes VSMCs to osteogenic transdifferentiation and calcification through directly modulating Runx2 gene expression. Exp. Cell Res. 2016;345(2):206–217. doi: 10.1016/j.yexcr.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Rong S., Zhao X., Jin X., Jin Z., Zhang Z., Chen L., Zhu Y., Yuan W. Vascular calcification in chronic kidney disease is induced by bone morphogenetic protein-2 via a mechanism involving the Wnt/β-catenin pathway. Cell. Physiol. Biochem. 2014;34(6):2049–2060. doi: 10.1159/000366400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.