Abstract
Background
Zhuang-Gu-Fang (ZGF) has been proved to treat osteoporosis in ovariectomized rats by increasing osteogenic related factors Leptin, Ghrelin and Peptide YY(PYY). However, the mechanism of ZGF in the treatment of diabetic osteoporosis (DOP) remains unclear. The aim of this study was to explore the therapeutic effect of ZGF on DOP and its potential molecular mechanism.
Methods
Using GK rats as models, the pharmacodynamic effects of ZGF on bone loss were evaluated by hematoxylin-eosin (H&E) staining and micro-computed.
tomography (micro-CT). The expression levels of CD31 and endomucin (Emcn) were detected by immunofluorescence to assess the role of ZGF in angiogenic osteogenic coupling. Finally, real-time quantitative PCR (RT-PCR) and Western Blot (WB)were used to detect the expression levels of osteogenic and angiogenesis-related genes and proteins Notch1, Noggin and vascular endothelial growth factor (VEGF).
Results
Administration of ZGF demonstrated a significant mitigation of bone loss attributable to elevated glucose levels. H&E staining and micro-CT showed that ZGF notably improved the integrity of the trabecular and cortical bone microarchitecture. Moreover, ZGF was found to augment the density of type H vessels within the bone tissue, alongside elevating the expression levels of Osterix, a transcription factor pivotal for bone formation. Furthermore, our findings suggest that ZGF facilitates the activation of the Notch1/Noggin/VEGF pathway, indicating a potential mechanism through which ZGF exerts its osteoprotective effects.
Conclusion
Our results suggest that ZGF potentially facilitates the formation of type H vessels through the Notch1/Noggin/VEGF pathway. This action not only enhances angiogenic-osteogenic coupling but also contributes to the improvement of bone structure and density. Consequently, ZGF emerges as a promising therapeutic agent for the prevention and management of DOP, offering a novel approach by leveraging angiogenesis-dependent osteogenesis.
Keywords: Zhuang-gu-fang, Diabetic osteoporosis, Type H vessel, Notch1, Noggin, VEGF
1. Introduction
Diabetes osteoporosis (DOP) is a chronic metabolic disease, which is related to bone metabolism changes and bone microstructure damage caused by long-term exposure to the environment of diabetes [1]. This is considered to be one of the serious chronic complications of diabetes. A large number of studies have found that diabetes is positively correlated with fracture risk [[2], [3], [4]]. A meta-analysis conducted by Shah et al. showed that the risk of hip fracture in patients with type I diabetes is 4–5 times higher than that in non-diabetic patients [5]. Similarly, another study highlighted that patients with suboptimal glycemic control in type 2 diabetes exhibit a 47%–62% increased risk of fracture in compared with non-diabetic patients or those with good glycemic control [6]. With the rapid increase of diabetes population, the cost of fracture treatment for diabetic patients has increased due to complications such as prolonged wound healing time [7]. Therefore, it is necessary to formulate effective strategies to prevent bone loss and osteoporosis caused by diabetes mellitus (DM).
Bone is a tissue filled with a functional vascular network [8]. Blood vessels within bone not only provide a structural framework for bone development but also enrich the osteogenic microenvironment with key elements such as minerals, growth factors, and osteogenic progenitor cells [9]. Previous studies focused on the effect of DM on bone metabolism disorders [10]. However, more and more recent evidence supports that DM induced osteovascular disease is another important cause of diabetes osteoporosis [11,12]. Changes in the vascular supply of bones (especially cortical bones) may impair bone formation [13]. Type H vessel is a newly discovered vascular subtype, which mainly exists in bone tissue. It mediates the local growth of vascular system, provides microenvironment signals for osteoprogenitors, and can couple angiogenesis and osteogenesis [14]. There is a causal relationship between the number of type H vessels and osteoporosis [15,16], and inducing the formation of type H blood vessels can stimulate bone formation [17,18]. This suggested that enhancing type H angiogenesis and osteogenesis could be a potential molecular pathway for the therapy of diabetes-induced osteoporosis.
The theoretical system of traditional Chinese medicine (TCM) posits that the fundamental cause of osteoporosis is “kidney deficiency” [19]. The root cause of diabetes is the deficiency of “qi” and “yin” [20]. Both “Kidney deficiency” and deficiency of “qi” and “yin” can lead to blood stasis. Based on the above pathological characteristics, our research group selected six traditional Chinese medicines, including Epimedium Herb, Eucommia Bark, Rhizoma Dioscoreae, Milkvetch Root, Salvia Miltiorrhiza, and Radices Pseudoginseng, to formulate ZGF. TCM holds that Epimedium Herb and Eucommia Bark are effective in “tonifying the kidney” and “strengthening bones” [21,22]. Recent studies have found that extracts from Epimedium Herb, Eucommia Bark, and Rhizoma Dioscoreae can increase bone mineral density (BMD) and repair fractures in cancellous bone and trabeculae [[23], [24], [25]]. Milkvetch Root is not only widely used for “tonifying qi”, but also exhibits anti-diabetes activity [26,27]. Extracts from Salvia Miltiorrhiza and Radices Pseudoginseng are known to repair vascular endothelial damage and regulate blood circulation [28,29]. Therefore, ZGF complies with the treatment principles of DOP.
The previous research [30] has confirmed that ZGF can treat osteoporosis in ovariectomized rats by increasing the levels of osteogenic related factors leptin, Ghrelin and PYY. Our clinical research also demonstrates that ZGF has excellent efficacy in treating postmenopausal low bone mass populations and DOP [31,32]. However, the potential and mechanisms of ZGF in the treatment of DOP have not yet been elucidated. It has been found that the increase of leptin level can enhance the expression of vascular endothelial growth factor (VEGF) [33], the most important angiogenic factor in the body. Promoting the release of VEGF in the bone matrix is beneficial for bone angiogenesis [34]. Many findings suggest that VEGF is a vital factor in the tight integration of angiogenesis and osteogenesis during fracture repair [35]. In addition, Wang et al. found that Ghrelin may promote angiogenesis through Jagged1/Notch2/VEGF pathway [36]. Therefore, we speculate that ZGF may interfere with DOP by regulating bone angiogenesis.
The purpose of this study is to verify whether ZGF can interfere with the formation of type H vessels and the potential molecular pathway to treat high glucose induced bone loss. GK rats were treated with different doses of ZGF. After 12 weeks, we observed the morphology of rat bone tissue and the level of type H angiogenesis, and detect the expression of mRNA and protein related to osteogenesis and angiogenesis.
2. Materials and methods
2.1. Animals
Four-month-old SPF male GK rats (i.e., Goto-Kakizaki rats, a model rat in the early stage of non-obese type 2 diabetes, weighing 340–380 g) exhibit pathological characteristics similar to the clinical manifestations of human type 2 diabetes [37], making them suitable for simulating diabetic osteoporosis. 4-Month-old Wistar rats (weighing 280–320 g) were purchased from Changzhou Cavens Laboratory Animal Co., Ltd. (Laboratory animal license No. SCXK (Su) 2016-0010). Feeding environment: room temperature 25 °C–28 °C, ventilation, natural lighting, free eating and drinking of rats.
2.2. Drugs
ZGF (Table 1) was purchased from the First Affiliated Hospital of Guangxi University of Traditional Chinese Medicine, conforming to the standards of the 2015 edition of the Pharmacopoeia of the People's Republic of China. It was identified by Professor Tian Hui from the Department of Traditional Chinese Medicine Identification at Guangxi University of Traditional Chinese Medicine. The specific preparation method refers to the previous literature [30]. Calcium carbonate D3 (Beijing Zhendong Kangyuan Pharmaceutical Co., Ltd., Batch No.20190357). Calciferol α (Chongqing Yaoyou Pharmaceutical Co., Ltd., Batch No.19071820). Metformin (Shandong Shibangde Pharmaceutical Co., Ltd., Batch No.1920813).
Table 1.
Composition of ZGF.
| Latin scientific name | Chinese name | Amount used (g) |
|---|---|---|
| Epimedium sagittatum (Sieb. et Zucc.) Maxim. Berberidaceae | Yinyanghuo | 30 |
| Astragalus membranaceus (Fisch.) Bge. Leguminosae | Huangqi | 20 |
| Dioscorea opposita kunb. Dioscoreaceae | Shanyao | 15 |
| Eucommia ulmoides Oliv. Eucommiaceae | Duzhong | 15 |
| Salvia miltiorrhiza Bge. Labiatae | Dansheng | 15 |
| Panax notoginseng (Burk.) F. H. Chen. Araliaceae | Tianqi | 10 |
2.3. Animals and experimental design
Fifty 4-month-old GK rats were randomly divided into model control group (MC), positive control group (PC 85 mg kg−1 Calcium carbonate D3 and 0.1 μg kg−1 Calciferol α treatment), low dose group of ZGF (ZGF-L 5.5 g kg−1 ZGF treatment), medium dose group of ZGF (ZGF-M 11 g kg−1 ZGF treatment), and high dose group of ZGF (ZGF-H 22 g kg−1 ZGF treatment) using a random number table method, with 10 animals in each group. Additionally, ten 4-month-old Wistar rats were taken as the blank control group (BC). Both the BC and MC groups were given an equal volume of physiological saline by gavage. The other four groups received their respective anti-osteoporosis treatments on the basis of administering metformin tablets 200 mg kg−1 by gavage to reduce blood sugar. The dose administered to each rat by gavage was calculated based on the equivalent adult dose of body surface area [38]. Intragastric administration was performed once daily for 12 weeks, after which the animals were sacrificed, and tibiae were collected for further analysis.
2.4. Histological analysis
The tibiae from the right hind limbs of rats from the six groups were collected, focusing on the metaphyseal portion. The soft tissue attached to the tibia was dissected away and the bone were fixed in 4% paraformaldehyde solution for 24 h. The tibiae were then decalcified in 7% EDTA for 14 days, dehydrated step by step through ethanol, and then embedded in paraffin to make paraffin sections [37]. The sections were deparaffinized in xylene, and hydrated in decreasing concentrations of alcohol. Next, the specimens were stained with hematoxylin and eosin (Shanghai Zhanyun Chemical Co., Ltd., China), dehydrated and mounted, and images were observed and collected using a light microscope (IX71, OLYMPUS, Japan).
2.5. Micro-computed tomography (micro- CT)
After 12 weeks of treatment, GK rats and Wistar rats were euthanized, and tibial specimens from rats in each group were analyzed using micro-CT (Skyscan, Aartselaar, Belgium). The parameters for Skyscan 1176 micro-CT were set at 50 kV voltage, 800 μA current, and 12 μm scan spatial resolution. After scanning, three-dimensional image reconstruction was performed using N-Recon, software included with micro-CT. The CT-AN software was utilized to conduct a three-dimensional analysis of bone trabeculae in the region of interest [7], obtaining the following parameters: bone volume/total volume (BV/TV), trabecular thickness (Tb·Th), trabecular number (Tb·N), trabecular separation (Tb.Sp) structural model index (SMI) and BMD.
2.6. Immunofluorescence
Bone tissues from each group of rats were processed for routine paraffin embedding. The slices were incubated at 3% H2O2/PBS at room temperature for 10 min to quench endogenous peroxidase activity. After rinsing with PBS, the slices were immersed in antigen repair solution. Blocking was performed with 5% BSA. Then, anti-CD31 antibody (1:200; Abcam, ab24590, UK), anti-endomucin (Emcn) antibody (1:200, Abbkine, ABP55560, China), and anti-Osterix antibody (1:1000, Abcam, ab209484) were added. The slices were incubated overnight at 4 °C, followed by washing with PBS. Corresponding Goat Anti-Rabbit (1:200, Proteintech, SA00003-2, USA) and Anti-Mouse (1:200, Proteintech, SA00009-1) secondary antibodies were added for 1 h. At last, the slices were stained with Hochest (Solarbio, Beijing, China), dehydrated, mounted, and observed under a microscope.
2.7. RT-PCR analysis
Total RNA 5.0 μL was extracted from bone tissues using TRIzol reagent (Invitrogen, CA, USA). cDNA was obtained from total RNA using a reverse transcription kit (Thermo, USA) following the manufacturer ‘s instructions. Subsequently, RT-PCR was performed using the SYBR Green PCR kit (Thermo, USA). The PCR instrument preheat to 94 °C for 10 min, initiating the amplification cycle. In each cycle, initial denaturation was performed at 94 °C for 20 s, then followed by 55 °C for 20 s and 72 °C for 20 s. This cycle was repeated 40 times. The expression differences of the target genes between groups were analyzed by the 2-△△CT method. Gapdh (1:5000, Proteintech, 60004-1-Ig) was used as a normalization control. The RT-PCR primer sequences used in the study (Table 2).
Table 2.
Primer sequences.
| Primer | Sequence (5′-3′) |
|---|---|
| VEGF (RAT)-RT-F | GAGTATATCTTCAAGCCGTCCTGTGTG |
| VEGF (RAT)-RT-R | GTTCTATCTTTCTTTGGTCTGCATTCA |
| GAPDH (RAT)-RT-F | ACGACCCCTTCATTGACCTCAACTACA |
| GAPDH (RAT)-RT-R | GACATACTCAGCACCAGCATCACCCCA |
| Notch1 (RAT)-RT-F | ACAGTGCCGAGTGTGAGTGGGATGG |
| Notch1 (RAT)-RT-R | CAGGAAGTGGAAGGAGTTGTTGC GT |
| NOG (RAT)-RT-F | CCAGCACTATCTACACATCCGCCCA |
| NOG (RAT)-RT-R | GCGTCTCGTTCAGATCCTTCTCCTT |
2.8. Western blot analysis
Bone tissue sample were ground with liquid nitrogen and then mixed with RIPA lysate solution. After sufficient lysis, the mixture was centrifuged for 10 min, and the supernatant was collected. Measure the protein concentration using the BCA kit (KeyGEN Bio TECH, China). Then, 5 × SDS loading buffer was added to the extracted proteins, which were subsequently heated in a boiling water bath for 5 min. Equal amounts of protein lysates were separated by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene difluoride membranes. The membranes were incubated overnight at 4 °C with anti-VEGF (1:1000, Boster, BA0407), anti-Notch1 (1:1000, Abcam, ab52627) and anti-Noggin antibodies (1:1000, Abcam, ab16054). After washing with TBST buffer, horseradish peroxidase-labeled Goat Anti-rabbit (ZSGB-Bio, ZB2301) and Anti-Mouse (ZSGB-Bio, ZB2305) antibodies, diluted at 1:5000, were added and incubated for 1 h at room temperature. The membrane was then treated with an appropriate amount of ECL luminescent liquid (Beijing Dingguo Changsheng Biotechnology Co., Ltd., China) and imaged using an integrated chemiluminescence instrument (ChemiScope 5300 Pro).
(We have provided an unprocessed original version of the Western blot experimental images in Additional file 1.)
2.9. Statistical analysis
All data are presented as mean ± standard deviation (SD). Use the Normality and Lognormality Tests function of GraphPad Prism 9 software (GraphPad software, USA) to verify whether the data satisfy a normal distribution. If the distribution is normal and the variance is homogeneous, differences between multiple groups are analyzed using one-way analysis of variance (ANOVA). Subsequently, Tukey test was used to determine whether the difference between any two groups was significant. If the variance is uneven, use Brown-Forsythe and Welch ANOVA. Differences were judged to be statistically significant when P < 0.05.
3. Results
3.1. ZGF antagonizes bone loss, increases BMD and protects bone microstructure in GK rats
To evaluate the efficacy of ZGF on DOP, we conducted H&E staining and bone morphometric analysis on the tibia of rats after 12 weeks of different treatments. The data demonstrated a normal distribution and showed similar variation between groups. Micro-CT analysis results showed that the cancellous bone mass in GK rats significantly decreased, the microstructure deteriorated, and bone fragility increased (Fig. 1A–B). Treatment groups with Calcium carbonate D3 + calciferol α and ZGF improved bone loss to varying degrees after intervention (Fig. 1A–B). Compared with the model group, both the PC group and ZGF group increased BMD, BV/TV, Tb. Th, Tb. N in GK rats, and decreased Tb. Sp and SMI. The therapeutic effect of ZGF-H group was consistently better than that of the PC group. The ZGF-L group showed a therapeutic effect only on BMD and SMI (Fig. 1C). H&E staining also revealed that following ZGF therapy, the bone trabecular area in GK rats increased compared to the model group, the bone tissue structure became more compact, and the bone marrow cavity decreased (Fig. 1 D). Overall, the improvement in bone tissue structure in ZGF-H group was more obvious.
Fig. 1.
ZGF reduces bone loss in GK rats. (A–B) Representation of GK rat tibial metaphysis (2D) and bone microstructure (3D) in micro-CT images for each group. (C) Quantitative analysis of bone morphological parameters of the tibial metaphysis in rats. (D) Rats from each group were stained with H&E 12 weeks after receiving various treatments. The pink area indicated by the arrow is the trabecular bone. Scale, 50 μm *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 VS. MC group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 VS. PC group. ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, ^^^^P < 0.0001 VS. BC group, (n = 3). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. ZGF promotes type H angiogenesis and osteogenic differentiation in GK rats
A defining characteristic of type H endothelial cells is their high expression of CD31 and Emcn [14]. We assessed the level of type H angiogenesis by measuring CD31 and Emcn expression through immunofluorescence staining. According to the experimental results (Fig. 2A–C), CD31 and Emcn were positively expressed in the BC group. However, they were expressed at considerably lower levels in the GK rats. Following ZGF therapy, the expression levels of CD31 and Emcn increased, with the high-dose group exhibiting the most significant effect. Treatment with Calcium carbonate D3 and calciferol α failed to affect the expression of CD31 and Emcn. These findings indicate that ZGF possesses special functional properties in promoting the expression of CD31 and Emcn.
Fig. 2.
ZGF increases the abundance of H-type blood vessels in bone tissue of GK rats. (A) Representative immunofluorescence images of CD31 and Emcn staining in the metaphysis of Wistar and GK rats. (B–C) Analyzing CD31 and Emcn expression level quantitatively in Wistar and GK rats' metaphyses. Scale, 20 μ m. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 VS. MC group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 VS. PC group. ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, ^^^^P < 0.0001 VS. BC group, (n = 3).
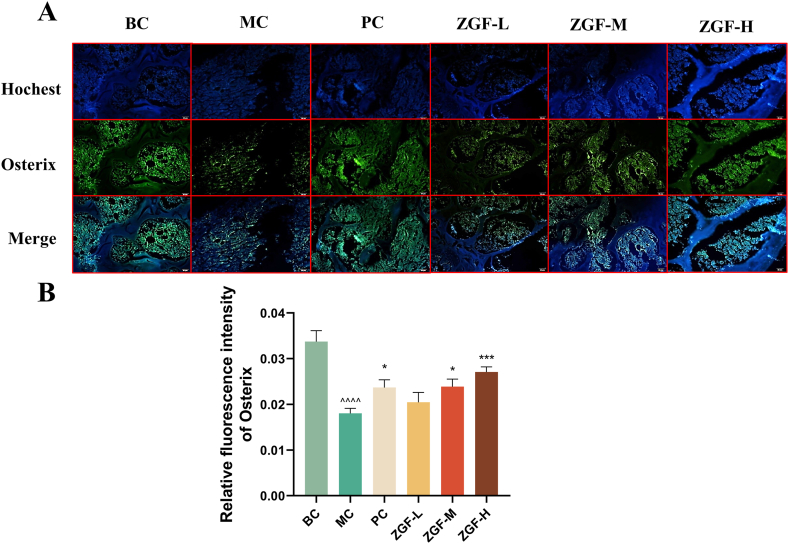
Osterix is a transcription factor related to osteoblast differentiation and bone formation. Osterix + osteoprogenitor cells are selectively localized around the H-type endothelium and are contribute to bone formation by osteoblasts and chondrocytes [39]. In Osx-deficient mice, the proliferation, maturation, and function of osteocytes are severely compromised, leading to the failure to express another osteogenic related transcription factor, Runx2 [40]. To investigate the role of ZGF in osteogenic differentiation, we utilized immunofluorescence staining to detect the expression of Osterix in bone tissue from rats in each group. We found that the expression of Osterix in the MC group was significantly reduced. After treatment with calcium carbonate D3 + calciferol α and ZGF (11 g kg−1 and 22 g kg−1), Osterix expression increased to different extents, but the effect was most pronounced in the ZGF-H (22 g kg−1) group (Fig. 3A–B). The above experimental results support the hypothesis that type H angiogenesis is accompanied by osteogenic differentiation, providing evidence that ZGF can promote osteogenic coupling of angiogenesis.
Fig. 3.
ZGF can mediate osteogenic differentiation in GK rats. (A) Representative immunofluorescence images of Osterix staining in the metaphysis of Wistar and GK rats. (B) Analyzing Osterix expression level quantitatively in Wistar and GK rats' metaphyses. Scale, 20 μ m. Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 VS. MC group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 VS. PC group. ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, ^^^^P < 0.0001 VS. BC group, (n = 3).
3.3. ZGF promotes the activation of Notch1/noggin/VEGF pathway
We showed in the above study that type H angiogenesis occurs in parallel with bone formation in GK rats treated with ZGF. To further investigate the specific mechanism by which ZGF intervenes in angiogenic osteogenic coupling, we detected the expression level of genes related to angiogenesis and osteogenesis. Research shows that the Notch1 signal, one of Notch receptors, positively influences the fracture healing process [41]. Noggin protein is a vascular endothelial secretion factor, which plays an important role in the skeletal development system [42]. VEGF, a key regulator of angiogenesis, has been widely studied in the context of bone formation [43]. These three factors are important mediators between angiogenesis and osteogenesis [3,28]. The results of the RT-PCR experiment (Fig. 4 A) showed that the target genes Noggin, Notch1, and VEGF were expressed considerably lower in GK rat models compared to the BC group. Following the intragastric administration of ZGF, the expression levels of these target genes increased to various extents, with the high-dose treatment showing the most significant effect. Compared to the GK group, treatment with calcium carbonateD3 + calciferol α also increased the mRNA and protein expression levels of Noggin, but had no significant effect on VEGF and Notch1 expression levels. In addition, western bolt analysis was consistent with the trends observed in RT-PCR data (Fig. 4 B and C).
Fig. 4.
ZGF Promotes VEGF/Notch1/Noggin Pathway Activation. (A) RT-PCR was used to detect NOG, Notch1, and VEGF mRNA expression levels in the tibial metaphysis of Wistar and GK rats. (B–C) Target proteins NOG, Notch1, and VEGF expression levels were measured by Western blot in the tibial metaphysis of Wistar and GK rats. (Uncropped Western blot images are included in Additional file 1.) Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 VS. MC group. #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 VS. PC group. ^P < 0.05, ^^P < 0.01, ^^^P < 0.001, ^^^^P < 0.0001 VS. BC group, (n = 3).
4. Discussion
The effect of diabetes on the bone marrow microenvironment has been underestimated. Several new studies conducted in mice, rats and humans have shown that diabetes can lead to a variety of bone marrow microenvironment defects, such as small vessel disease (microvascular disease), nerve endings depletion (neuropathy), and impaired stem cell mobilization (mobility disease) [44]. Peng et al. believe that in diabetes, the damage to angiogenesis is the main cause of reduced bone formation and imbalance [45]. The discovery of type H blood vessels provides strong support for vascular dysplasia as a factor in the reduction of osteogenesis. Type H vessels were mainly distributed in the metaphysis of bone, and type H endothelial cells (ECs) accounted for a very small proportion of all ECs in bone, approximately 1.77%. However, Osterix + osteoprogenitor cells accumulated around these vessels directly affected osteoblast differentiation [39,46]. Type H vessels can secrete active substances to provide a microenvironment for the osteogenic lineage, and regulate the activity of perivascular skeletal cells through the paracrine pathway, so that angiogenesis and bone formation are coupled to each other [14,47]. These results suggest that type H vessels coordinate the growth and differentiation of vascular tissue and bone tissue may be the key factors to initiate bone regeneration [16].
In this study, we compared the amounts of CD31, Emcn, and Osterix expression in the rat bone tissues of each group. It was found that the decreased expression levels of CD31, Emcn, Osterix in diabetes rats were accompanied by the decreased bone density, the number of bone trabeculae, and the osteoporosis of bone structure. Therefore, it is speculated that the damage of type H vessel coupling with osteogenesis may be one of the important reasons affecting the progression of diabetic osteoporosis. This result is consistent with Hu et al.'s finding that intraosseous vascular abnormalities, particularly type H vessels, cause angiogenesis and osteogenesis to become uncoupled in diabetic mice [11].
Currently, most anti-osteoporosis drugs mostly inhibit bone resorption, ignoring the relationship between impaired bone vascular function and certain bone diseases. There has been limited research on the use of traditional Chinese medicine to treat diabetic osteoporosis from the perspective of type H vessels. In this study, we demonstrate the effect of ZGF on the abundance of type H vessels and the coupling of angiogenesis and osteogenesis. We used the conventional anti-osteoporosis drugs calcium carbonate D3 and calciferol α, as positive controls and found that the combination of these two drugs had a certain delaying effect on high glucose-induced bone loss, but the effect was consistently lower than that of the high dose ZGF group. However, the two drugs did not significantly promote type H angiogenesis, indicating that the strategy of ZGF targeting type H vessels may be more advantageous for treating DOP.
Previous studies have demonstrated that Notch signal is involved in type H angiogenesis [14,47]. The researchers found that Notch signal was highly expressed in type H ECs. CD31 and Emcn expression decreased simultaneously with Notch signal after blocking Notch signal using blockers [48]. Type H vascular ECs secrete many growth factors associated with osteoprogenitor cell survival and proliferation, such as Noggin and VEGF, which are closely associated with bone formation and repair. Noggin is a secreted homodimeric glycoprotein that is involved in the functional regulation of osteoblasts and is closely related to cartilage repair [47]. It has been found that endothelial cell-derived noggin can relieve bone loss by regulating type H angiogenesis [49]. Notch activation in ECs is required for endothelial cell proliferation and stimulation of Noggin production. Noggin expression is significantly reduced in mutants that specifically lack Notch activation in ECs, which interferes with osteogenic and chondrocyte development via their vascular secretory function [43]. Angiogenesis is usually triggered by VEGF, and the key step is to induce proliferation and migration of ECs [50]. VEGF is one of the main mediators promoting angiogenesis, and binding with VEGF receptors 1(VEGFR-1) and 2(VEGFR-2) can promote bone repair [51]. When VEGF levels are persistently elevated, it can promote angiogenesis, restore revascularization, increase blood flow, and affect bone growth and development by affecting tissue perfusion levels [52]. The proliferation of chondrocytes in the proliferative layer of the growth plate promotes bone development, while the underlying chondrocytes can secrete VEGFA, which promotes type H blood vessels to invade the growth plate and drive bone differentiation [53]. Exogenous noggin can increase alkaline phosphatase activity, promote osteogenic differentiation, and counteract angiogenic abnormalities brought on by decreased VEGF expression in chondrocytes when used at specific dosages [47,54]. This suggests that Notch, Noggin, and VEGF are required for efficient coupling of angiogenesis and osteogenesis during skeletal development and repair.
In the current study, we found that Notch1, Noggin, and VEGF mRNA and protein expression levels were significantly decreased in the tibial metaphysis of GK rats. This suggests that impaired Notch1/Noggin/VEGF pathway may be a specific mechanism by which diabetic osteoporosis impacts type H vascular coupling to osteogenesis. In the bone tissue of diabetic rats, ZGF can enhance the expression levels of mRNA and proteins associated to osteogenesis and angiogenesis, including Notch1, Noggin, and VEGF. The above gene and protein expression levels were not significantly increased after calcium carbonate D3 combined with calciferol α treatment. These results suggest that ZGF can activate Notch/Noggin/VEGF pathway to regulate type H vascular and osteogenic coupling.
This experiment did not set up a separate metformin treatment group, which cannot completely eliminate the role of metformin in the treatment of osteoporosis. There may be a preference for therapeutic effects when comparing the ZGF treatment group and positive control group with the model group. Both the ZGF treatment group and positive control group were treated with the same dose of metformin. Therefore, the comparison of the therapeutic effects of ZGF with conventional anti osteoporosis drugs (calcium carbonate D3 and calcitonin α) was not disturbed. There is no doubt that the high-dose group of ZGF has a stronger effect on promoting type H angiogenesis than any other group, so the conclusion that ZGF may have more advantages in treating DOP through promoting type H angiogenesis is still reliable. In addition, it is not yet clear whether there is a synergistic effect between ZGF and metformin when used simultaneously. We will add the metformin alone treatment group and the ZGF alone treatment group in the next study to eliminate the additional effects.
5. Conclusions
High glucose induced type H vascular injury may be the key factor in bone loss and damage to bone formation in DOP. ZGF can enhance the coupling of type H vessels and osteogenesis by activating the Notch1/Noggin/VEGF pathway, improve bone density, enhance bone tissue structure, and delay the progression of diabetes induced osteoporosis. Our results strongly support the clinical application of ZGF. It also provides new insights into exploring early intervention targets for type H vascular injury and diabetes induced osteoporosis.
Funding statement
This research was funded by National Natural Science Foundation of China Regional Fund Project (81860784), Guangxi Natural Science Foundation Project (2020GXNSFAA297193); Guangxi University Young and Middle-Aged Teachers' Basic Ability Improvement Project for Scientific Research (2023KY1743), University level scientific research project of Faculty of Chinese Medicine Science Guangxi University of Chinese Medicine (2022MS003), Chinese Ethnic Medicine Association Scientific Research Project (2021Z1141-580302), Guangxi University of Traditional Chinese Medicine 2022 Graduate Education Innovation Program Project (YCSY2022022).
Availability of data and materials
All data generated or analyzed during this study are included within the article and available from the corresponding author upon request.
Ethics approval and consent to participate
All the above experiments have been reviewed and approved by the Ethics Committee of Guangxi Zhuoqiang Technology Co., Ltd. (No. ZQIA-2022-009).
CRediT authorship contribution statement
Xinyan Jin: Writing – review & editing, Writing – original draft, Methodology, Data curation, Conceptualization. Yuyu Sun: Software, Resources, Data curation. Rui Bai: Software, Resources, Data curation. Jun Shi: Visualization, Validation, Data curation. Linna Zhai: Visualization, Validation, Data curation. Yunxia Jiang: Visualization, Validation, Data curation. Mengchun Jiang: Visualization, Validation, Software. Jiali He: Visualization, Validation, Software. Junyu Li: Visualization, Validation, Software. Ting Wang: Visualization, Validation, Data curation. Shuanglei Li: Writing – review & editing, Supervision. Wenhui Chen: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Guangxi Zhuoqiang Co., Ltd. for providing research facilities for this study. We thank Professor Tian Hui for the identification of traditional Chinese medicine.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e28014.
Contributor Information
Shuanglei Li, Email: lslei@126.com.
Wenhui Chen, Email: chenwenhuibr@126.com.
Abbreviations
- ZGF
Zhuang-Gu-Fang
- DOP
Diabetes osteoporosis
- PYY
Peptide YY
- Emcn
Endomucin
- DM
Diabetes mellitus
- VEGF
Vascular endothelial growth factor
- BMD
Bone mineral density
- TCM
Traditional Chinese medicine
- BV
Bone volume
- TV
Total volume
- SMI
Structure model index
- Tb·N
Trabecular number
- Tb·Th
Trabecular thickness
- Tb.Sp
Trabecular separation
- EC
Endothelial cell
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Murray C.E., Coleman C.M. Impact of diabetes mellitus on bone health. Int. J. Mol. Sci. 2019;20:E4873. doi: 10.3390/ijms20194873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Ba Y., Xing Q., Du J.-L. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thong E.P., Herath M., Weber D.R., Ranasinha S., Ebeling P.R., Milat F., Teede H. Fracture risk in young and middle-aged adults with type 1 diabetes mellitus: a systematic review and meta-analysis. Clin. Endocrinol. 2018;89:314–323. doi: 10.1111/cen.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koromani F., Oei L., Shevroja E., Trajanoska K., Schoufour J., Muka T., Franco O.H., Ikram M.A., Zillikens M.C., Uitterlinden A.G., Krestin G.P., Anastassiades T., Josse R., Kaiser S.M., Goltzman D., Lentle B.C., Prior J.C., Leslie W.D., McCloskey E., Lamy O., Hans D., Oei E.H., Rivadeneira F. Vertebral fractures in individuals with type 2 diabetes: more than skeletal complications alone. Diabetes Care. 2020;43:137–144. doi: 10.2337/dc19-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah V.N., Shah C.S., Snell-Bergeon J.K. Type 1 diabetes and risk of fracture: meta-analysis and review of the literature. Diabet. Med. 2015;32:1134–1142. doi: 10.1111/dme.12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi J., Hu K.-S., Yang H.-L. Roles of TNF-α, GSK-3β and RANKL in the occurrence and development of diabetic osteoporosis. Int. J. Clin. Exp. Pathol. 2015;8:11995–12004. [PMC free article] [PubMed] [Google Scholar]
- 7.Gong W., Zhang N., Cheng G., Zhang Q., He Y., Shen Y., Zhang Q., Zhu B., Zhang Q., Qin L. Rehmannia glutinosa libosch extracts prevent bone loss and architectural deterioration and enhance osteoblastic bone formation by regulating the IGF-1/PI3K/mTOR pathway in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2019;20:E3964. doi: 10.3390/ijms20163964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson R.E., Silva M.J. Skeletal blood flow in bone repair and maintenance. Bone Res. 2013;1:311–322. doi: 10.4248/BR201304002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hankenson K.D., Dishowitz M., Gray C., Schenker M. Angiogenesis in bone regeneration. Injury. 2011;42:556–561. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Liu M., Li H., Chen Z., Liang N., Xu J., Zhang X., Zhang Y. Traditional Chinese medicine Bushen-Jianpi-Huoxue decoction prevents diabetic osteoporosis in rats via Wnt and nuclear factor-kappa B signaling pathways. Int. J. Rheum. Dis. 2017;20:941–948. doi: 10.1111/1756-185X.13050. [DOI] [PubMed] [Google Scholar]
- 11.Hu X.-F., Xiang G., Wang T.-J., Ma Y.-B., Zhang Y., Yan Y.-B., Zhao X., Wu Z.-X., Feng Y.-F., Lei W. Impairment of type H vessels by NOX2-mediated endothelial oxidative stress: critical mechanisms and therapeutic targets for bone fragility in streptozotocin-induced type 1 diabetic mice. Theranostics. 2021;11:3796–3812. doi: 10.7150/thno.50907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng J., Hui K., Hao C., Peng Z., Gao Q.X., Jin Q., Lei G., Min J., Qi Z., Bo C., Dong Q.N., Bing Z.H., Jia X.Y., Fu D.L. Low bone turnover and reduced angiogenesis in streptozotocin-induced osteoporotic mice. Connect. Tissue Res. 2016;57:277–289. doi: 10.3109/03008207.2016.1171858. [DOI] [PubMed] [Google Scholar]
- 13.Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L. IOF bone and diabetes working group, mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 14.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507:323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J., Gao Y., Cheng P., Li D., Jiang H., Ji C., Zhang S., Shen C., Li J., Song Y., Cao T., Wang C., Yang L., Pei G. CD31hiEmcnhi vessels support new trabecular bone formation at the frontier growth area in the bone defect repair process. Sci. Rep. 2017;7:4990. doi: 10.1038/s41598-017-04150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Zhou F., Zhang P., Wang H., Qu Z., Jia P., Yao Z., Shen G., Li G., Zhao G., Li J., Mao Y., Xie Z., Xu W., Xu Y., Xu Y. Human type H vessels are a sensitive biomarker of bone mass. Cell Death Dis. 2017;8:e2760. doi: 10.1038/cddis.2017.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang M., Li C.-J., Sun X., Guo Q., Xiao Y., Su T., Tu M.-L., Peng H., Lu Q., Liu Q., He H.-B., Jiang T.-J., Lei M.-X., Wan M., Cao X., Luo X.-H. MiR-497∼195 cluster regulates angiogenesis during coupling with osteogenesis by maintaining endothelial Notch and HIF-1α activity. Nat. Commun. 2017;8 doi: 10.1038/ncomms16003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie H., Cui Z., Wang L., Xia Z., Hu Y., Xian L., Li C., Xie L., Crane J., Wan M., Zhen G., Bian Q., Yu B., Chang W., Qiu T., Pickarski M., Duong L.T., Windle J.J., Luo X., Liao E., Cao X. PDGF-BB secreted by preosteoclasts induces angiogenesis during coupling with osteogenesis. Nat. Med. 2014;20:1270–1278. doi: 10.1038/nm.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J., Zhu J., Wang Y., Zhang N., Gober H.-J., Qiu X., Li D., Wang L. Chinese single herbs and active ingredients for postmenopausal osteoporosis: from preclinical evidence to action mechanism. BioSci. Trends. 2017;11:496–506. doi: 10.5582/bst.2017.01216. [DOI] [PubMed] [Google Scholar]
- 20.Dou Z., Xia Y., Zhang J., Li Y., Zhang Y., Zhao L., Huang Z., Sun H., Wu L., Han D., Liu Y. Syndrome differentiation and treatment regularity in traditional Chinese medicine for type 2 diabetes: a text mining analysis. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.728032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H., Lv Y., Zhao M., Tang R., Li Y., Fang K., Wei F., Ge W., Du W., Li C., Zhang Y. Study on the substance basis of the efficacy of eucommiae cortex before and after salt processing for the treatment of kidney-yang deficiency syndrome based on the spectrum-effect relationship. J. Ethnopharmacol. 2024;318 doi: 10.1016/j.jep.2023.116926. [DOI] [PubMed] [Google Scholar]
- 22.Qian H., Wu D., Li C., Liu X., Han X., Peng Y., Zhang H., Zhao B., Zhao Y. A systematic review of traditional uses, phytochemistry, pharmacology and toxicity of Epimedium koreanum Nakai. J. Ethnopharmacol. 2024;318 doi: 10.1016/j.jep.2023.116957. [DOI] [PubMed] [Google Scholar]
- 23.Xie F., Wu C.-F., Lai W.-P., Yang X., Cheung P.-Y., Yao X.-S., Leung P.-C., Wong M.-S. The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. Evid. base Compl. Alternative Med. 2005;2:353–361. doi: 10.1093/ecam/neh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X., Wang Y., Nie Z., Han L., Zhong X., Yan X., Gao X. Eucommia ulmoides leaf extract alters gut microbiota composition, enhances short‐chain fatty acids production, and ameliorates osteoporosis in the senescence‐accelerated mouse P6 (SAMP6) model. Food Sci. Nutr. 2020;8:4897–4906. doi: 10.1002/fsn3.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han N., Xu J., Xu F., Liu Z., Yin J. The in vivo effects of a fraction from Dioscorea spongiosa on glucocorticoid-induced osteoporosis. J. Ethnopharmacol. 2016;185:53–59. doi: 10.1016/j.jep.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Li X., Qu L., Dong Y., Han L., Liu E., Fang S., Zhang Y., Wang T. A review of recent research progress on the Astragalus genus. Molecules. 2014;19:18850–18880. doi: 10.3390/molecules191118850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong G., Yu H., Zheng Y., Qi B., He H., Yin T., Dong T.T.X., Tsim K.W.K. Astragaloside IV, a saponin from Astragalus membranaceus var. mongholicus, induces expressions of heme recycle proteins via signaling of Nrf2/ARE in cultured macrophages. J. Ethnopharmacol. 2021;265 doi: 10.1016/j.jep.2020.113389. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Cao S.-H., Cui Y.-J., Kong L.-K., Tian H., Cai H.-X., Wu Y.-P., Han J.-J., Zhao X.-M., Xia Z.-L. Salvia Miltiorrhiza Bge.f.alba ameliorates the progression of monocrotaline-induced pulmonary hypertension by protecting endothelial injury in rats. Tohoku J. Exp. Med. 2015;236:155–162. doi: 10.1620/tjem.236.155. [DOI] [PubMed] [Google Scholar]
- 29.Hu S., Wu Y., Zhao B., Hu H., Zhu B., Sun Z., Li P., Du S. Panax notoginseng saponins protect cerebral microvascular endothelial cells against oxygen-glucose deprivation/reperfusion-induced barrier dysfunction via activation of PI3K/Akt/Nrf2 antioxidant signaling pathway. Molecules. 2018;23:2781. doi: 10.3390/molecules23112781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y., Bai R., Chen W., Li S., Jiang Y. Zhuang-Gu-Fang treats osteoporosis in ovariectomized rats by increasing the osteogenesis-related factors leptin, Ghrelin, and PYY. Evid. Based Complement. Alternat. Med. 2020;2020 doi: 10.1155/2020/8164064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W., Jiang Y., Li S., Xie J., Jia H., Tian Y. Evaluation of Zhuanggufang Decoction Powder's curative effect on spleen-kidney deficiency with static blood syndrome of postmenopausal low bone mass population. China J. Tradit. Chin. Med. Pharm. 2021;36:1556–1559. [in Chinese] [Google Scholar]
- 32.Mo W., Chen W., Li S. Effect of Zhuang-Gu-Fang on the structure of osteoblasts in diabetic rats. Lishizhen Med. Mater. Med. Res. 2016;27:2065–2067. [in Chinese] [Google Scholar]
- 33.Yang W.-H., Chang A.-C., Wang S.-W., Wang S.-J., Chang Y.-S., Chang T.-M., Hsu S.-K., Fong Y.-C., Tang C.-H. Leptin promotes VEGF-C production and induces lymphangiogenesis by suppressing miR-27b in human chondrosarcoma cells. Sci. Rep. 2016;6 doi: 10.1038/srep28647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu K., Jiao H., Li S., Cao H., Galson D.L., Zhao Z., Zhao X., Lai Y., Fan J., Im H.-J., Chen D., Xiao G. ATF4 promotes bone angiogenesis by increasing vegf expression and release in the bone environment. J. Bone Miner. Res. 2013;28:1870–1884. doi: 10.1002/jbmr.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y., Berendsen A.D., Jia S., Lotinun S., Baron R., Ferrara N., Olsen B.R. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J. Clin. Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Yang L., Chen Y., Zhang L., Fei H. Ghrelin promotes angiogenesis by activating the Jagged1/Notch2/VEGF pathway in preeclampsia. J. Obstet. Gynaecol. Res. 2021;47:486–494. doi: 10.1111/jog.14555. [DOI] [PubMed] [Google Scholar]
- 37.Chen W., Jin X., Wang T., Bai R., Shi J., Jiang Y., Tan S., Wu R., Zeng S., Zheng H., Jia H., Li S. Ginsenoside Rg1 interferes with the progression of diabetic osteoporosis by promoting type H angiogenesis modulating vasculogenic and osteogenic coupling. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1010937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang Z., Yin M., Guo Y., Li W., Sun F., Guo Y., Chen Z., Zhou B. Taohong Siwu decoction promotes osteo-angiogenesis in fractures by regulating the HIF-1α signaling pathway. Evid. base Compl. Alternative Med. 2022;2022:1–10. doi: 10.1155/2022/6777447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakashima K., Zhou X., Kunkel G., Zhang Z., Deng J.M., Behringer R.R., de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X., Zhang Z., Feng J.Q., Dusevich V.M., Sinha K., Zhang H., Darnay B.G., de Crombrugghe B. Multiple functions of Osterix are required for bone growth and homeostasis in postnatal mice. Proc. Natl. Acad. Sci. U. S. A. 2010;107:12919–12924. doi: 10.1073/pnas.0912855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novak S., Roeder E., Sinder B.P., Adams D.J., Siebel C.W., Grcevic D., Hankenson K.D., Matthews B.G., Kalajzic I. Modulation of Notch1 signaling regulates bone fracture healing. J. Orthop. Res. 2020;38:2350–2361. doi: 10.1002/jor.24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J., Pan J., Jing W. Motivating role of type H vessels in bone regeneration. Cell Prolif. 2020;53 doi: 10.1111/cpr.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grosso A., Burger M.G., Lunger A., Schaefer D.J., Banfi A., Di Maggio N. It takes two to tango: coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017;5:68. doi: 10.3389/fbioe.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fadini G.P., Ferraro F., Quaini F., Asahara T., Madeddu P. Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration. Stem Cells Transl. Med. 2014;3:949–957. doi: 10.5966/sctm.2014-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng J., Qu H., Peng J., Luo T.-Y., Lv F.-J., Chen L., Wang Z.-N., Ouyang Y., Cheng Q.-F. Abnormal spontaneous brain activity in type 2 diabetes with and without microangiopathy revealed by regional homogeneity. Eur. J. Radiol. 2016;85:607–615. doi: 10.1016/j.ejrad.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 46.Saran U., Gemini Piperni S., Chatterjee S. Role of angiogenesis in bone repair. Arch. Biochem. Biophys. 2014;561:109–117. doi: 10.1016/j.abb.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 47.Ramasamy S.K., Kusumbe A.P., Wang L., Adams R.H. Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature. 2014;507:376–380. doi: 10.1038/nature13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan Z.-Q., Wang X.-K., Zhou Y., Wang Z.-G., Wang Z.-X., Jin L., Yin H., Xia K., Tan Y.-J., Feng S.-K., Xie P.-L., Tang S.-Y., Fang C.-Y., Cao J., Xie H. H-type blood vessels participate in alveolar bone remodeling during murine tooth extraction healing. Oral Dis. 2020;26:998–1009. doi: 10.1111/odi.13321. [DOI] [PubMed] [Google Scholar]
- 49.Liang S., Ling S., Du R., Li Y., Liu C., Shi J., Gao J., Sun W., Li J., Zhong G., Liu Z., Zhao D., Sun H., Li Y., Yuan X., Qu H., Jin X., Li D., Shi D., Li Y. The coupling of reduced type H vessels with unloading-induced bone loss and the protection role of Panax quinquefolium saponin in the male mice. Bone. 2021;143 doi: 10.1016/j.bone.2020.115712. [DOI] [PubMed] [Google Scholar]
- 50.Filipowska J., Tomaszewski K.A., Niedźwiedzki Ł., Walocha J.A., Niedźwiedzki T. The role of vasculature in bone development, regeneration and proper systemic functioning. Angiogenesis. 2017;20:291–302. doi: 10.1007/s10456-017-9541-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin Q., Lee S., Patel N., Walden K., Gomez-Salazar M., Levi B., James A.W. Neurovascular coupling in bone regeneration. Exp. Mol. Med. 2022;54:1844–1849. doi: 10.1038/s12276-022-00899-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu K., Olsen B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Invest. 2016;126:509–526. doi: 10.1172/JCI82585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dzamukova M., Brunner T.M., Miotla-Zarebska J., Heinrich F., Brylka L., Mashreghi M.-F., Kusumbe A., Kühn R., Schinke T., Vincent T.L., Löhning M. Mechanical forces couple bone matrix mineralization with inhibition of angiogenesis to limit adolescent bone growth. Nat. Commun. 2022;13:3059. doi: 10.1038/s41467-022-30618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hashimi S.M. Exogenous noggin binds the BMP-2 receptor and induces alkaline phosphatase activity in osteoblasts. J. Cell. Biochem. 2019;120:13237–13242. doi: 10.1002/jcb.28597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included within the article and available from the corresponding author upon request.