Abstract
Livestock workers experience an increased burden of bioaerosol-induced respiratory disease including high prevalence of rhinosinusitis. Dairy operations generate bioaerosols spanning the inhalable size fraction (0–100 μm) containing bacterial constituents such as endotoxin. Particles with an aerodynamic diameter between 10–100 μm are known to deposit in the nasopharyngeal region and likely affect the upper respiratory tract. We evaluated the effectiveness of a hypertonic saline nasal lavage in reducing inflammatory responses in dairy workers from a high-volume dairy operation. Inhalable personal breathing zone samples and pre-/post-shift nasal lavage samples from each participant over five consecutive days were collected. The treatment group (n=5) received hypertonic saline while the control group (n=5) received normotonic saline. Personal breathing zone samples were analyzed for particulate concentrations and endotoxin using gravimetric and enzymatic methods, respectively. Pro- and anti-inflammatory cytokines (i.e., IL-8, IL-10, and TNF-α) were measured from nasal lavage samples using a multiplex assay. Inhalable dust concentrations ranged from 0.15 to 1.9 mg/m3. Concentrations of both pro- and anti-inflammatory cytokines, specifically IL-6, IL-8, and IL-10, were significantly higher in the treatment group compared to the control group (p < 0.02, p < 0.04, and p < 0.01 respectively). Further analysis of IL-10 anti-inflammatory indicates a positive association between hypertonic saline administration and IL-10 production. This pilot study demonstrates that hypertonic saline nasal lavages were successful in upregulating anti-inflammatory cytokines to support larger interventional studies.
Keywords: Agricultural health, bioaerosols, cytokines, inflammation, nasal lavage
INTRODUCTION
Dairy workers have a high risk for exposure to bioaerosols, which have been consistently associated with inflammatory airway diseases (Arteaga et al. 2015; Burch et al. 2009; Davidson et al. 2018; Eduard et al. 2009; M. Iversen et al. 2000; Nonnenmann et al. 2017; Schaeffer et al. 2017). Bioaerosols are complex mixtures containing particulates (primarily 10–100 μm in aerodynamic diameter) with an abundance of bacterial communities and associated inflammagens (e.g., endotoxin and muramic acid) that can impact in the nasopharyngeal region (Moscato et al. 2008; Moscato & Siracusa 2009; Schaeffer et al. 2017). Previous studies have reported bioaerosol exposure concentrations as a high as 7.38 mg/m3 – an exposure that is more than three times higher than occupational guidelines recommended by Reynolds et al. over 25 years ago (2.4 mg/m3) (Reynolds et al. 1996, 2012). Further, concentrations of endotoxin, a cell wall component of gram negative bacteria well-recognized to elicit inflammatory responses, were found to be in excess of the recommended occupational exposure limits including the former Dutch standard (90 EU/m3) in 89% of dairy workers across 30 high plains dairies (Davidson et al. 2018; Dutch Expert Committee on Occupational Safety 2010). Consequently, dairy workers experience a disproportionate burden of respiratory disease that includes asthma, chronic obstructive pulmonary disease, and decreased lung functionality (Dalphin et al. 1998; Eduard et al. 2009; M. Iversen et al. 2000; Marx et al. 1990). Moreover, symptoms of upper airway disease are largely overlooked (e.g., rhinitis), as 60–70% of agricultural workers report upper respiratory disease symptoms (Akpinar-Elci et al. 2016). These upper respiratory symptoms often serve as precursors to asthma (Moscato et al. 2008; Moscato & Siracusa 2009). Increased surveillance could be important in prevention or early detection of lower airway symptoms and disease.
The dairy industry is unique when compared to other agricultural sectors due to its 24-hour production schedule, contact with large animals, operation of heavy equipment, and long work shifts (Douphrate et al. 2013; USDA 2011). Moreover, dairy operations in the U.S. have transitioned to larger herds (>500 head) and shifted toward immigrant labor (Douphrate et al. 2013; Jenkins et al. 2009; Maloney & Grusenmeyer 2005; USDA 2011). These dramatic changes to production, work organization and tasks have altered bioaerosol exposure patterns among dairy workers (Douphrate et al. 2013; USDA 2011). For example, to meet increasing demand, workers now have more specialized tasks (e.g.: milking, bedding, and birthing), which we hypothesize has led to greater potential of sustained exposure to bioaerosols. The dairy workforce in the U.S. is estimated to be >90% Hispanic/Latino with no prior experience on a farm; this population is considered immunologically naïve to these complex bioaerosols (Douphrate et al. 2013; Jenkins et al. 2009; Maloney & Grusenmeyer 2005; Schenker & Gunderson 2013). As such, these workers may experience a more pronounced physiological response than those that have adapted to the environment. Many workers also have limited access to or interaction with medical care providers, highlighting the need for effective interventions to aid in health and welfare vulnerabilities of this at-risk population.
Interventions that follow a hierarchical approach to reduce respiratory illness among dairy workers have shown promise, but feasibility and effectiveness remain largely untested (Choudhry et al. 2012; Lee et al. 2005). While engineering controls may be the most efficacious in reducing exposures, these solutions may not be economically feasible or practical. For example, reduction in personal exposure to respirable dust was observed after doubling the frequency of parlor washing, endotoxin concentrations were not reduced (Choudhry et al. 2012). Further, costs associated with the large increase in water use was deemed a substantial adoption barrier. Personal protective equipment (e.g., N95 respirator) is a low-cost control; however, compliance is difficult to maintain and protection against bioaerosols has been shown to be inadequate (Lee et al. 2005). In summary, the adoption and effectiveness of interventions to reduce worker inhalation exposures on dairies so far has been limited.
To address the limitations of past interventions, we pilot tested the effectiveness of a low-cost, low-burden nasal rinse among dairy workers exposed to high concentrations of bioaerosols. A potential therapeutic role for hypertonic saline solutions (HTS; a solution with elevated salt concentrations greater than physiological conditions) has been proposed as various in vitro and in vivo studies demonstrate its effectiveness in reducing pro-inflammatory markers in the lung when compared to a normotonic saline solution (a solution with physiologically normal salt concentrations) (Cuschieri et al. 2002; Mitra et al. 2017; Oreopoulos et al. 2000; Rizoli et al. 2006; Theobaldo et al. 2012; Wohlauer et al. 2012). However, its hygienic influence in the nasal passageway is untested and its efficacy on bioaerosol-exposed dairy workers remains unknown. We hypothesized that workers receiving the HTS nasal rinse would experience greater attenuation of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokines than workers receiving a normotonic control with cumulative effects over time to inform interventional studies in these vulnerable populations.
METHODS
Participant recruitment
A representative modern dairy operation in the southwest United States was identified and recruited through the HICAHS Dairy Advisory board. Study participation was offered to all workers employed at the dairy facility and based on their interest and availability to participate during the sampling period. From a pool of 20 eligible dairy workers, ten study participants with variable shift start times agreed to participate and were randomly assigned to the intervention or control group. All study protocols were approved by Colorado State University’s (CSU) Institutional Review Board and informed consent was obtained in person prior to all data collection. Workers job tasks included milking, birthing, animal transport, mixing feed, re-bedding, animal medical care, and hoof maintenance. Worker personal protective equipment was limited (e.g., pants, long sleeves, and boots). After completion of the informed consent, participants were also administered a medical history questionnaire in either English or Spanish to determine if they met exclusion criteria (e.g., no use of steroidal nasal sprays, immune-suppressant medication, auto-immune medications, chemotherapy medications, recent surgery, recent chest injury, and history of stroke or heart disease). Screening questionnaires were based on the American Thoracic Society (ATS) standard questionnaire with modifications specific to dairy populations (Ferris 1978; Martenies et al. 2020; Reynolds et al. 2012).
Sample Collection
Inhalable Dust
Full eight-hour work-shift personal breathing zone (PBZ) inhalable dust samples were collected over all five sampling days using the SKC Button sampler equipped with 25mm PVC filters with 5um pore size (SKC Inc., Eighty-Four, PA). Samplers were connected to SKC XR5000 personal sampling pumps (SKC Inc., Eighty-Four, PA) that were calibrated to a flow rate of 4 L/min using a primary standard (DryCal Defender 510 Mesa Labs, Butler, NJ). A difference of +/−5% between pre- and post-sample flow rates were considered acceptable. Filters were desiccated for 24 hr before and after sampling prior to gravimetric analysis, which was performed using a Mettler MT5 balance (Mettler-Toledo, Columbus, OH). Field blanks were averaged and subtracted from sample weights before calculating airborne concentrations. Filters were stored at −80°C for subsequent endotoxin analysis.
Endotoxin
Filters were extracted in pyrogene-free water with 0.05% Tween-20. Filter extracts were analyzed using the Recombinant Factor C (rFC) assay (Lonza, Basel, Switzerland) on a Biotek reader (Biotek Instruments FLX800TBIE, Winooski, VT) as previously reported (Burch et al. 2009). Endotoxin was quantified based on standard curve and fluorescence and reported as endotoxin units per cubic meter of air (EU/m3). Reagent blanks, filter blanks, and quality control spikes were used to ensure accuracy of results.
Nasal Lavage and Pro-Inflammatory Cytokines
Pre- and post-shift nasal lavages were administered to participants across all five days of sampling. Participants in the interventional treatment group received normotonic saline solution (308mOsm) pre-shift and hypertonic saline (HTS) (400mOsm) post-shift to assess the effect of HTS post-exposure and not interfere with pre-exposure flora. Participants in the control group received normotonic saline solution pre- and post-shift. Once collected, a protease inhibitor was added (1% v/v) to lavage samples before storage on ice until processing. Lavages were centrifuged, aliquoted into 1ml supernatants, and stored in a −80°C prior to cytokine measurement. Meso Scale Discovery (MSD) technology was used to measure 10 cytokines and chemokines, including interleukin (IL)- IL-1β, IL-2, IL-4, IL-6, IL-8, IL-12p70, IL-13, interferon gamma, and tumor necrosis factor (TNF)-α, as well as IL-10 (an anti-inflammatory cytokine). Samples were analyzed and quantified with the MSD V-PLEX assay and MSD QuickPlex SQ 120 (MESO Scale Diagnostics, Rockville, MD).
Statistical Analysis
An exclusion criterion was applied to each analyte to mitigate the impact of non-detects on data analysis. Analytes with greater than 70% of measurements above the LOD (i.e., detectable measurements) were included in downstream analysis, while analytes with less than 70% detectable measurements were excluded. Analytes meeting this criterion had all non-detect measurements assigned a value equal to the LOD divided by the square root of two.
Based on the log normal distribution of the data, the bioaerosol exposure measurements were log transformed for statistical analysis. Descriptive measures [i.e., geometric means (GM) and geometric standard deviations (GSD]) were calculated and compared to suggested occupational exposure limits (Donham et al. 2000; Dutch Expert Committee on Occupational Safety 2010; Reynolds et al. 1996). A statistical model was then used to relate exposure measurements (i.e., inhalable dust & endotoxin) to the measured outcome (cytokines) and determine the effect of the lavage intervention.
For each of the dust, endotoxin, a linear mixed effects model was used to compare the response between HTS treatment and control groups (fixed effect for group) across the five days of the study (fixed effect for day), and where applicable (i.e., the cytokine measures) the time of day (i.e., pre-shift, post-shift; fixed effect). Two-way interaction effects were included in all models and for cytokine measures, three-way interaction between group, day, and time of day was included in the model. A random effect for participant was included to account for repeated measures of the participants over the five days of the study. Log (base 10) was used to transform each of the measures to overcome heteroskedasticity of the residuals. Specifically, the model for dust can be expressed as:
| (1) |
where is the log exposure level of the participant in the group (treatment, control) on day . The random effect for participant is represented by which follows a normal distribution with mean 0 and variance . represents the random error term that follows a normal distribution with mean 0 and variance . The model for the cytokines is the same as in (1) but also includes the fixed effect terms , and where time of day has two levels of am and pm. The variance components covariance structure was used in the estimation of the random effects. While this is a simplistic structure, due to small sample size, adding more parameters was not feasible.
SAS PROC MIXED with Kenward-Rogers degrees of freedom adjustment was used to fit each of the models. Tukey’s Honestly Significant Difference (HSD) was used to account for multiple comparisons. All tests were conducted using a significance level of 0.05 in SAS (Version 9.4, Cary, NC) and R (Version 3.5.1., R core team, Vienna, Austria).
RESULTS
A total of 100 nasal lavages and 50 PBZ samples were collected from a total of 10 participants across a five-day sampling campaign before and after worker shifts. Geometric mean dust concentrations for treatment and control groups were 0.45 mg/m3 and 0.41 mg/m3, respectively. Geometric mean endotoxin concentrations for treatment and control groups were 2.12 EU/m3 and 1.59 EU/m3 respectively. Daily inhalable dust and endotoxin concentrations separated by treatment group are presented in Table 1.
Table 1.
Geometric mean (geometric standard deviation) dust measures for HTS treatment (T) and control groups (C) on each of the five days of the study. Mean based on n=5 for each group.
| Original Scale | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | T | C | T | C | T | C | T | C | T | C |
| mg/m3 | 0.793 (1.46) | 0.394 (2.11) | 0.203 (6.26) | 0.318 (1.49) | 0.498 (2.34) | 0.531 (1.84) | 0.512 (2.15) | 0.524 (1.72) | 0.399 (1.93) | 0.317 (1.33) |
| EU/m3 | 2.36 (4.69) | 3.41 (4.14) | 1.33 (8.10) | 0.945 (2.37) | 1.57 (3.63) | 0.994 (2.94) | 5.92 (3.14) | 2.10 (3.70) | 1.64 (2.30) | 1.53 (2.47) |
Among the ten chemokines and cytokines selected for analysis in the nasal lavage samples, five (TNF-α, IL-4, IL-12p70, and IL-13) were removed due to greater than 30% of values being below the limit of detection. Group level comparisons revealed the treatment group had significantly higher concentrations of IL-1β, IL-2, IL-6, and IL-8 pro-inflammatory cytokines (all p<0.05) as well as anti-inflammatory IL-10 (p<0.01). Log geometric mean concentrations by day, time, and treatment group are presented in picograms per milliliter (pg/ml) in the figures below. Results from the linear mixed effects model fits, including tests of the fixed effects and estimates of the random effects are presented in Table 2.
Table 2.
Linear mixed effects model fits for fixed effects and random effect estimates.
| Group (df=1) | Day (df=4) | Group*Day (df=4) | Time of Day (df=1) | Group* Time of Day (df=1) | Day * Time of Day (df=4) | Group*Day* Time of Day (df=4) | |||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Dust (mgm3) | F=0.07 (p=0.802) | F=1.95 (p=0.127) | F=0.84 (p=0.513) | 0.013 (0.02) | 0.100 (0.03) | ||||
| Endotoxin (eum3) | F=0.25 (p=0.634) | F=2.04 (p=0.114) | F=0.47 (p=0.754) | 0.080 (0.07) | 0.228 (0.06) | ||||
| Il10 | F=14.74 (p=0.005) | F=0.85 (p=0.496) | F=2.41 (p=0.057) | F=2.55 (p=0.115) | F=2.71 (p=0.104) | F=1.21 (p=0.313) | F=3.66 (p=0.009) | 0.007 (0.01) | 0.082 (0.01) |
| Il1b | F=0.88 (p=0.376) | F=0.84 (p=0.507) | F=1.89 (p=0.121) | F=18.19 (p<0.001) | F=0.74 (p=0.392) | F=0.75 (p=0.563) | F=1.61 (p=0.181) | 0.175 (0.10) | 0.174 (0.03) |
| Il6 | F=8.49 (p=0.020) | F=1.75 (p=0.149) | F=1.78 (p=0.141) | F=15.85 (p<0.001) | F=0.32 (p=0.574) | F=1.55 (p=0.197) | F=2.22 (p=0.075) | 0.063 (0.04) | 0.131 (0.02) |
| Il8 | F=5.97 (p=0.040) | F=0.24 (p=0.914) | F=0.94 (p=0.447) | F=1.41 (p=0.238) | F=0.26 (p=0.611) | F=0.67 (p=0.615) | F=1.49 (p=0.214) | 0.028 (0.03) | 0.367 (0.06) |
There was no evidence of an interaction effect on average log mg/m3 between treatment (HTS and control) and day (p=0.513), in average log mg/m3 between HTS treatment and control groups (p=0.802), or among days (p=0.127), indicating similar exposures between groups. Additionally, there was no evidence of an interaction effect on average log EU/m3 between treatment (hypertonic and control) and day (p=0.754), average log EU/m3 between HTS treatment and control groups (p=0.634) or among days (p=0.114), also indicating similar exposures between groups.
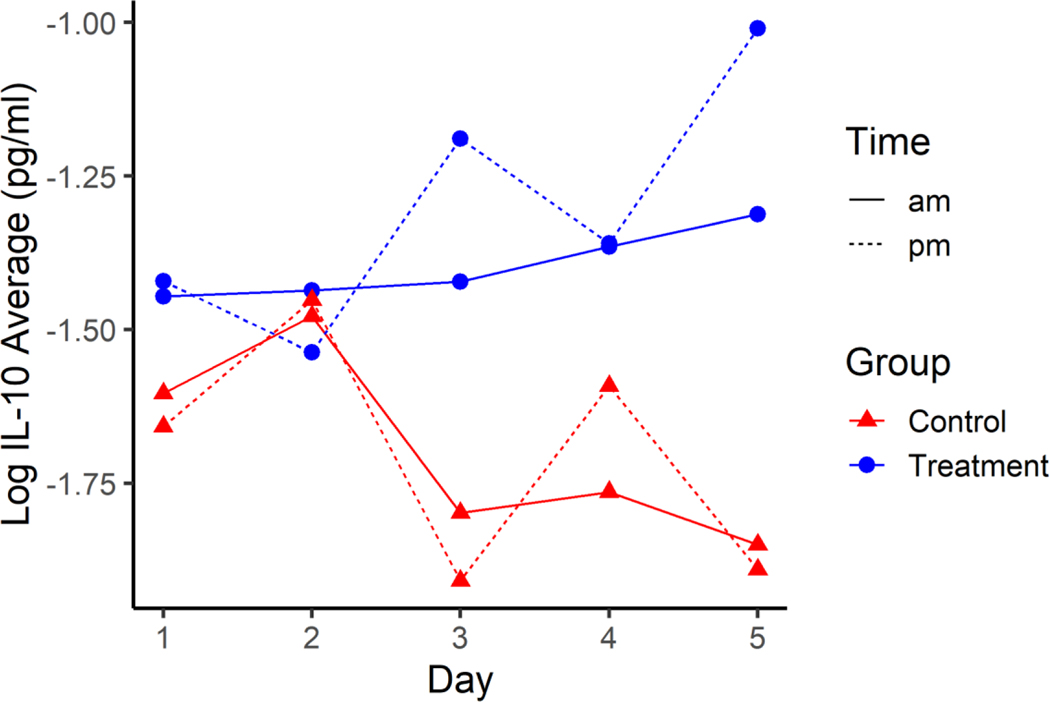
There was evidence of an interaction effect on average log IL-10 between treatment, day, and time of day (p=0.009, Figure 1). After adjusting for multiple comparisons using Tukey’s Honestly Significant Difference, there was evidence that the average IL-10 concentration in the treatment group was higher than the control groups after administration of HTS on two different days. Briefly, those concentrations observed on Day 3 (p=0.035/2=0.018; 95% confidence interval for average log IL-10 0.343, 1.10) and on Day 5 (p=0.002/2=0.001; 95% confidence interval for average log IL-10: 0.504, 1.26) indicate a positive association between HTS administration and IL-10 production.
Figure 1.
Average Log IL-10 across the five days for HTS Treatment (blue) and Normal Control groups (red) pre-shift (solid line) and post-shift (dotted line). Standard error for all estimates are 0.1339.
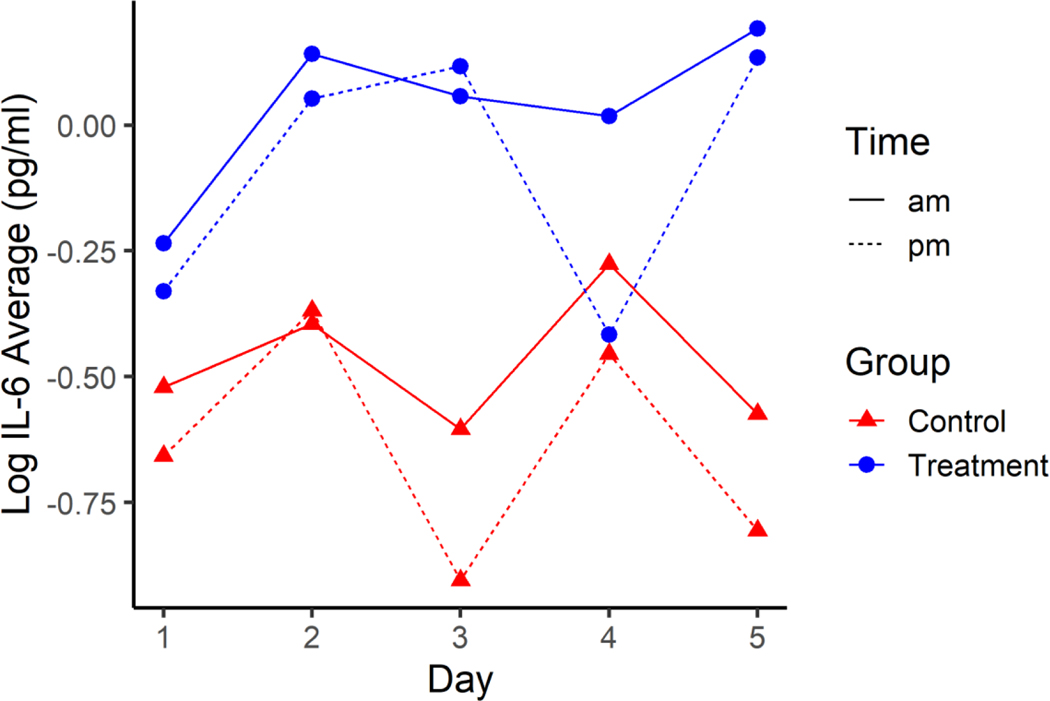
There was evidence of differences in average log IL-6 between treatment and control groups (p=0.020), with higher average concentrations being 0.106 to 0.912 units higher in treatment than control. There was also evidence of differences in average log IL-6 between pre- and post-shift measurements (p<0.001), with pm average log IL-6 being between 0.144 and 0.432 units higher than am average log IL-6 (Figure 2). There was not evidence of an interaction effect on average log IL-6 between treatment, day, and time of day (p=0.075), day and time (p=0.197), time and treatment (p=0.574), or day by treatment (p=0.141). There was not evidence of differences in average log IL-6 among days (p=0.150).
Figure 2.
Average Log IL-6 across the five days for HTS Treatment (blue) and Normal Control groups (red) pre-shift (solid line) and post-shift (dotted line). Standard error for all estimates are 0.1236.
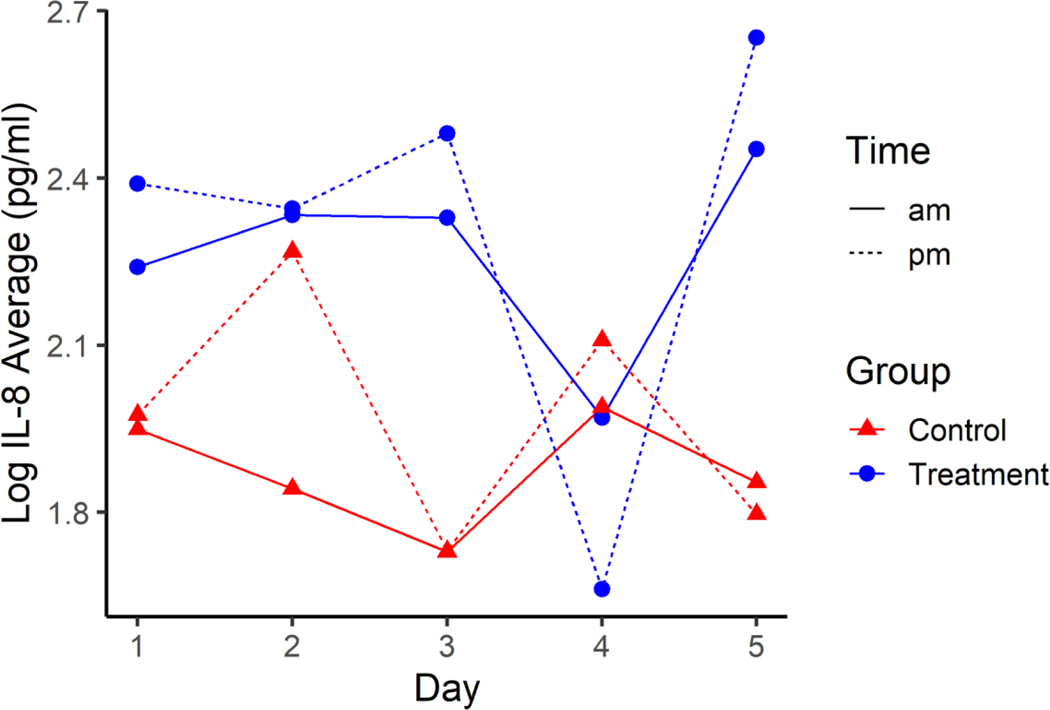
There was a difference in average log IL-8 between HTS treatment and control groups (p=0.040), with the estimated average log IL-8 for HTS treatment being between 0.022 and 0.763 higher than average log IL-8 for the control group (Figure 3). There was not evidence of an interaction effect on average log IL-8 between treatment, day, and time of day (p=0.214), day and time (p=0.615), time and treatment (p=0.611), or day by treatment (p=0.447). There was not evidence of differences in average log IL-8 among days (p=0.914) or between pre-shift and post-shift (p=0.238).
Figure 3.
Average Log IL-8 across the five days for HTS Treatment (blue) and Normal Control groups (red) pre-shift (solid line) and post-shift (dotted line). Standard error for all estimates are 0.1135.
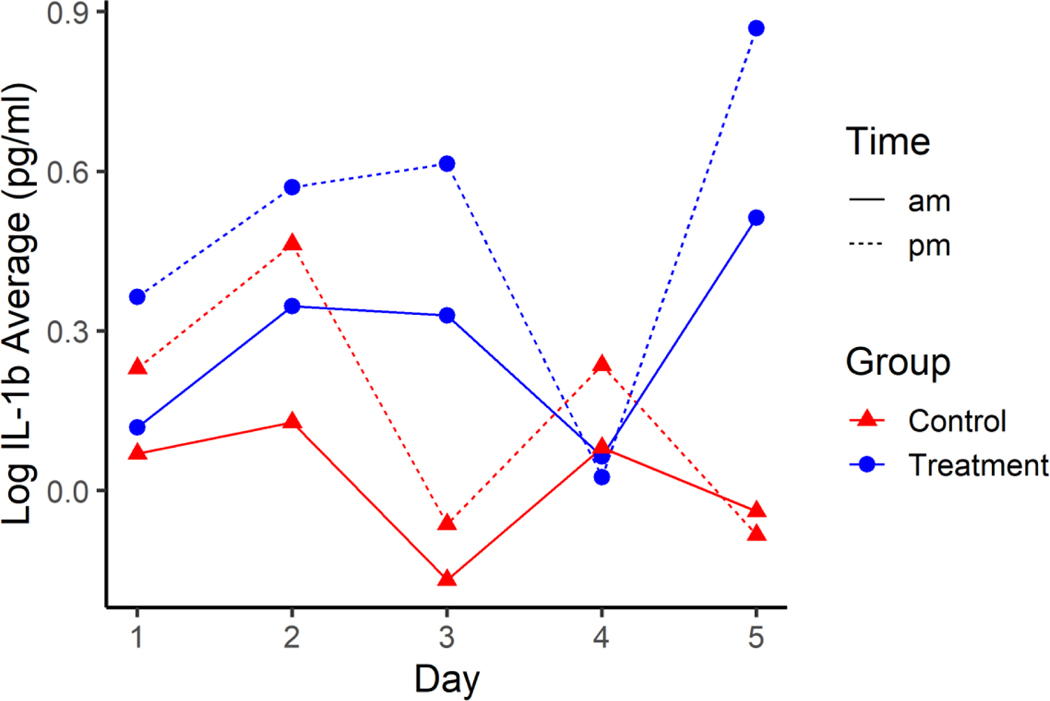
There was no evidence of an interaction effect on average log IL-1β between treatment, day, and time of day (p=0.181), day and time (p=0.563), time and treatment (p=0.392), or day by treatment (p=0.121). There was also not evidence of a difference in average log IL-1β between treatments (p=0.376) or among days (p=0.507). However, there was evidence of a difference in average log IL-1β between times of day (i.e., pre- and post-shift; p<0.001), with the pre-shift average IL-1β estimated to be between 0.190 and 0.523 units higher than the post-shift average log IL-1β (Figure 4).
Figure 4.
Average Log IL-1β across the five days for HTS Treatment (blue) and Normal Control groups (red) pre-shift (solid line) and post-shift (dotted line). Standard error for all estimates are 0.1448.
There were no differences in average log IL-2 between treatment and control groups, among days, or pre- and post-shift (all p>0.05). Therefore, IL-2 results are presented in supplementary Figure 1.
DISCUSSION
Bioaerosols in large animal farming operations such as dairy facilities are associated with increase prevalence of both upper and lower airway diseases with limited therapeutic options. In this pilot study, hypertonic saline nasal lavages post-work exposure demonstrated potentially advantageous effects. The main interaction effects were observed among IL-10 concentrations, day, and time, supporting an increased anti-inflammatory effect with hypertonic saline irrigations as compared to normotonic saline lavages. However, there was no interaction effect for any of the analyzed pro-inflammatory cytokines. Moreover, IL-6 and IL-8 pro-inflammatory cytokine levels were observed to be lower in controls than in the treatment group. It is important to note that higher baseline cytokine concentrations in the treatment group can be attributed to natural variation in immune response from participant to participant. For that reason, each participant served as their own control our analysis.
Our findings confirm previous clinical studies recently published on the immunomodulatory effects of hypertonic saline on anti-inflammatory cytokine production (Oreopoulos et al. 2000; Rizoli et al. 2006). For example, adult hemorrhagic trauma patients treated with HTS plus dextran observed significant increases in production of anti-inflammatory cytokines IL-10 and IL-ra (Rizoli et al. 2006). Others have demonstrated that peritoneal exudative macrophages preconditioned with HTS prior to lipopolysaccharide exposure exhibit a two-fold increase in IL-10 production as compared to isotonic pretreatment (Oreopoulos et al. 2000). Collectively, our results and other studies strongly suggest that hypertonic saline treatment promotes upregulation of anti-inflammatory IL-10 release.
In contrast, HTS nasal lavage did not reduce commonly measured pro-inflammatory cytokine levels as has been reported in earlier studies. The aforementioned studies also observed decreases in IL-6 and TNF-α production with HTS while our results indicate an increase in IL-6 and partially in IL-8 (Oreopoulos et al. 2000; Rizoli et al. 2006). IL-6 has been previously recognized as a pro-inflammatory mediator. Increased IL-6 levels in the serum and/or bronchoalveolar lavage fluid have been associated with asthma and COPD severity (Ferrari et al. 2013; Rincon & Irvin 2012). However, increases in IL-6 occur in active asthmatics even when TNF-α and IL-1β were not increased, which has led others to propose that IL-6 is not simply a pro-inflammatory marker but may be the result of “activated” airway epithelial cells (Rincon & Irvin 2012). Moreover, it has also been shown that airway inflammatory consequences induced by swine confinement facility organic dust extract exposure, which included airway neutrophil influx, cytokine/chemokine release and lung pathology, were not reduced in IL-6 deficient mice as compared to wild type (Wells et al. 2017). Thus, the significance of increased IL-6 following hypertonic saline solution administration necessitates further evaluation to fully understand its significance, particularly following large animal farming dust exposures. It is possible that a decrease in TNF-α existed in the treatment group, but too many samples were below the analytical limit of detection to adequately analyze.
In general, the concentrations of dust (0.46 mg/m3) and endotoxin (1.94 EU/m3) were overall lower than previously reported values and were also well below suggested exposure guidelines generated from studies of other agricultural environments (Donham et al. 2000; Reynolds et al. 1996). These lower exposures could be caused by wet sampling conditions during the sampling period or cleaning measures used by this dairy facility. In either case, low exposure levels could be muting the effect size of HTS treatment, specifically in the pro-inflammatory cytokines directly associated with endotoxin exposure.
Alongside efficacy, it is also important to evaluate interventions through the context of adoption and use. A previous intervention introducing the use of N95 respirators was not well received by workers and inadequate compliance was noted (Lee et al. 2005). Because the nasal rinse can be completed within ~10 seconds before and after a shift, the barriers for adoption are much lower. The rinse will not interfere with job tasks, worker comfort, or require the worker to invest significant time before and after a work shift.
When interpreting the results from this study there are clear limitations to consider. First, sample size was small comparatively as only 10 dairy workers were enrolled. While repeated measures were utilized to achieve good sample depth, it is evident in the statistical analysis that the study did not have the power required to reveal small effects of additional inflammatory mediator responses. Additionally, this study used a convenience sample by collecting exposure data from the first ten workers who volunteered. This could have led our study to be subject to healthy worker bias as workers who were on medication for underlying respiratory health problems were not eligible to participate due to testing requirements. We were also limited by the fact that this was a pilot study and the first to test the potential of a HTS nasal rinse as an intervention in the agricultural field. Nonetheless, these findings are an important proof-of-concept for optimizing sampling and analysis procedures. A lack of data on other immunologically reactive inflammagens (i.e., peptidoglycans) and health outcomes from the observed changes in pro- and anti-inflammatories is another limitation of this study. Future studies should characterize additional exposures and address the impacts of HTS on health-based outcomes like lung function and changes to the nasal microbiome. Finally, sampling from one dairy over a short time period in one season introduces generalizability limitations. Due to the lack of research on the application of HTS outside of laboratory and hospital settings, this study offers valuable insight for future studies despite these limitations.
CONCLUSION
Livestock workers experience an increased burden of bioaerosol exposure and subsequent respiratory disease with limited efficacious treatment options. The novel application of HTS nasal lavage in dairy workers following their work shift over a short period of time demonstrated advantageous results consistent with emerging data using HTS. Workers receiving the HTS rinse experienced significantly higher levels of the anti-inflammatory cytokine IL-10 than the control group. However, nasal levels of IL-6 and to a lesser degree IL-8 were increased in the treatment group. Consequently, there remains uncertainty regarding the effectiveness of HTS as a viable treatment option for respiratory disease in dairy workers. Based on these results, normotonic saline rinses alone may provide health benefits and adequate attenuation against inflammation. Future studies are warranted with increased worker sample size as well as a pre- and post-shift HTS administration (as opposed to post-shift only) to improve statistical power and better quantify the practicality of the intervention. This study provides an important step towards utilizing low-cost, non-invasive approaches including this HTS nasal lavage strategy to improve the health of at-risk agriculture workers.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank the HICAHS Dairy Advisory board, the producers and dairy workers who participated in data collection. We thank the Human Immune Monitoring Shared Resource at the CU Anschutz Medical Campus for their expert assistance in analysis of multiplex cytokine arrays. This project was supported by HICAHS and NIOSH U01 grant #1U01OH010840 and the CDC/NIOSH Mountain and Plains Education and Research Center (T42OH009229-07). JAP is funded by NIOSH (1RO1OH012-45) and the Department of Defense (PR200793). Analysis supported by the Cancer Center Support Grant (P30CA04634). JAP has received funding unrelated to this project from AstraZeneca and Takeda.
DATA AVAILABILITY
The data that support the findings of this study are openly available in Mountain Scholar at http://dx.doi.org/10.25675/10217/234559
REFERENCES
- Akpinar-Elci M, Pasquale DK, Abrokwah M, Nguyen MN, & Elci OC (2016). United Airway Disease Among Crop Farmers. Journal of Agromedicine, 21(3), 217–223. 10.1080/1059924X.2016.1179239 [DOI] [PubMed] [Google Scholar]
- Arteaga VE, Mitchell DC, Matt GE, Quintana PJE, Schaeffer J, Reynolds SJ, Schenker MB, & Mitloehner FM (2015). Occupational Exposure to Endotoxin in PM<sub>2.5</sub> and Pre- and Post-Shift Lung Function in California Dairy Workers. Journal of Environmental Protection, 06(05), 552–565. 10.4236/jep.2015.65050 [DOI] [Google Scholar]
- Burch JB, Svendsen E, Siegel PD, Wagner SE, Von Essen S, Keefe T, Mehaffy J, Serrano Martinez A, Bradford M, Baker L, Cranmer B, Saito R, Tessari J, Linda P, Andersen C, Christensen O, Koehncke N, & Reynolds SJ (2009). Endotoxin Exposure and Inflammation Markers Among Agricultural Workers in Colorado and Nebraska. Journal of Toxicology and Environmental Health, Part A, 73(1), 5–22. 10.1080/15287390903248604 [DOI] [PubMed] [Google Scholar]
- Choudhry AH, Reynolds SJ, Mehaffy J, Douphrate DI, Gilmore K, Levin JL, & Nonnenmann MW (2012). Evaluation of parlor cleaning as an intervention for decreased occupational exposure to dust and endotoxin among dairy parlor workers-A pilot study. Journal of Occupational and Environmental Hygiene, 9(7). 10.1080/15459624.2012.691410 [DOI] [PubMed] [Google Scholar]
- Cuschieri J, Gourlay D, Garcia I, Jelacic S, & Maier RV (2002). Hypertonic Preconditioning Inhibits Macrophage Responsiveness to Endotoxin. The Journal of Immunology, 168(3), 1389–1396. 10.4049/jimmunol.168.3.1389 [DOI] [PubMed] [Google Scholar]
- Dalphin JC, Dubiez A, Monnet E, Cora D, Westeel V, Pernet D, Polio JC, Gibey R, Laplante JJ, & Depierre A. (1998). Prevalence of asthma and respiratory symptoms in dairy farmers in the French province of the doubs. American Journal of Respiratory and Critical Care Medicine, 158(5 PART I), 1493–1498. 10.1164/ajrccm.158.5.9709108 [DOI] [PubMed] [Google Scholar]
- Davidson ME, Schaeffer J, Clark ML, Magzamen S, Brooks EJ, Keefe TJ, Bradford M, Roman-Muniz N, Mehaffy J, Dooley G, Poole JA, Mitloehner FM, Reed S, Schenker MB, & Reynolds SJ (2018). Personal exposure of dairy workers to dust, endotoxin, muramic acid, ergosterol, and ammonia on large-scale dairies in the high plains western United States. Journal of Occupational and Environmental Hygiene, 15(3), 182–193. 10.1080/15459624.2017.1403610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham K, Cumro D, Reynolds S, & & Merchant J. (2000). Dose-Response Relationships Between Occupational Aerosol Exposures and Cross-Shift Declines of Lung Function in Poultry Workers: Recommendations for Exposure Limits. Journal of Occupational and Environmental Medicine, 42(3), 260–269. [DOI] [PubMed] [Google Scholar]
- Douphrate DI, Hagevoort GR, Nonnenmann MW, Lunner Kolstrup C, Reynolds SJ, Jakob M, & Kinsel M. (2013). The Dairy Industry: A Brief Description of Production Practices, Trends, and Farm Characteristics Around the World. Journal of Agromedicine, 18(3), 187–197. 10.1080/1059924X.2013.796901 [DOI] [PubMed] [Google Scholar]
- Dutch Expert Committee on Occupational Safety. (2010). Endotoxins: Health-Based Recommended Occupational Exposure Limit. Den Haag: Health Council of the Netherlands. [Google Scholar]
- Eduard W, Pearce N, & Douwes J. (2009). Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest, 136(3), 716–725. 10.1378/chest.08-2192 [DOI] [PubMed] [Google Scholar]
- Ferrari R, Tanni SE, Caram LMO, Corrêa C, Corrêa CR, & Godoy I. (2013). Three-year follow-up of Interleukin 6 and C-reactive protein in chronic obstructive pulmonary disease. Respiratory Research, 14(1), 1–7. 10.1186/1465-9921-14-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris B. (1978). Epidemiology standardization project II: Recommended respiratory disease questionnaires for use with adults and children in epidemiological research. American Journal of Respiratory and Critical Care Medicine ISSN: 1073–449X EISSN: 1535–4970, 118, 7–53. [Google Scholar]
- Jenkins PL, Stack SG, May JJ, & Earle-Richardson G. (2009). WAGR growth of the spanish-speaking workforce in the northeast dairy industry. Journal of Agromedicine, 14(1), 58–65. 10.1080/10599240802623387 [DOI] [PubMed] [Google Scholar]
- Lee SA, Adhikari A, Grinshpun SA, McKay R, Shukla R, Zeigler HL, & Reponen T. (2005). Respiratory protection provided by N95 filtering facepiece respirators against airborne dust and microorganisms in agricultural farms. Journal of Occupational and Environmental Hygiene, 2(11), 577–585. 10.1080/15459620500330583 [DOI] [PubMed] [Google Scholar]
- Iversen M, Kirychuk S, Drost H, & Jacobson L. (2000). Human Health Effects of Dust Exposure in Animal Confinement Buildings. Journal of Agricultural Safety and Health, 6(4), 283–288. 10.13031/2013.1911 [DOI] [PubMed] [Google Scholar]
- Maloney TR, & Grusenmeyer DC (2005). Survey of Hispanic Dairy Workers in New York State (Issue February). www.sri.cornell.edu [Google Scholar]
- Martenies SE, Schaeffer JW, Erlandson G, Bradford M, Poole JA, Wilson A, Weller Z, Reynolds SJ, & Magzamen S. (2020). Associations between Bioaerosol Exposures and Lung Function Changes among Dairy Workers in Colorado. Journal of Occupational and Environmental Medicine, 62(6), 427–430. 10.1097/JOM.0000000000001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx JJ, Guernsey J, Emanuel DA, Merchant JA, Morgan DP, & Kryda M. (1990). Cohort studies of immunologic lung disease among Wisconsin dairy farmers. American Journal of Industrial Medicine, 18(3), 263–268. 10.1002/ajim.4700180304 [DOI] [PubMed] [Google Scholar]
- Mitra S, Schiller D, Anderson C, Gamboni F, D’Alessandro A, Kelher M, Silliman CC, Banerjee A, & Jones KL (2017). Hypertonic saline attenuates the cytokine-induced pro-inflammatory signature in primary human lung epithelia. PLoS ONE, 12(12), 1–20. 10.1371/journal.pone.0189536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscato G, & Siracusa A. (2009). Rhinitis guidelines and implications for occupational rhinitis. Current Opinion in Allergy and Clinical Immunology, 9(2), 110–115. [DOI] [PubMed] [Google Scholar]
- Moscato G, Vandenplas O, Van Wijk RG, Malo JL, Quirce S, Walusiak J, Castano R, De Groot H, Folletti I, Gautrin D, Yacoub MR, Perfetti L, & Siracusa A. (2008). Occupational rhinitis. Allergy: European Journal of Allergy and Clinical Immunology, 63(8), 969–980. 10.1111/j.1398-9995.2008.01801.x [DOI] [PubMed] [Google Scholar]
- Nonnenmann MW, Gimeno Ruiz de Porras D, Levin J, Douphrate D, Boggaram V, Schaffer J, Gallagher M, Hornick M, & Reynolds S. (2017). Pulmonary function and airway inflammation among dairy parlor workers after exposure to inhalable aerosols. American Journal of Industrial Medicine, 60(3), 255–263. 10.1002/ajim.22680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreopoulos GD, Hamilton J, Rizoli SB, Fan J, Lu Z, Li YH, Marshall JC, Kapus A, & Rotstein OD (2000). In vivo and in vitro modulation of intercellular adhesion molecule (ICAM)-1 expression by hypertonicity. Shock (Augusta, Ga.), 14(3), 405–409. 10.1097/00024382-200014030-00029 [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Clark ML, Koehncke N, Von Essen S, Prinz L, Keefe TJ, Mehaffy J, Bradford M, Cranmer B, Davidson ME, Yang IV, & Burch JB (2012). Pulmonary function reductions among potentially susceptible subgroups of agricultural workers in Colorado and Nebraska. Journal of Occupational and Environmental Medicine, 54(5), 632–641. 10.1097/JOM.0b013e31824d2e1c [DOI] [PubMed] [Google Scholar]
- Reynolds SJ, Donham KJ, Whitten P, Merchant JA, Burmeister LF, & Popendorf WJ (1996). Longitudinal evaluation of dose-response relationships for environmental exposures and pulmonary function in swine production workers. American Journal of Industrial Medicine, 29(1), 33–40. [DOI] [PubMed] [Google Scholar]
- Rincon M, & Irvin CG (2012). Role of IL-6 in asthma and other inflammatory pulmonary diseases. International Journal of Biological Sciences, 8(9), 1281–1290. 10.7150/ijbs.4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizoli SB, Rhind SG, Shek PN, Inaba K, Filips D, Tein H, Brenneman F, & Rotstein O. (2006). The immunomodulatory effects of hypertonic saline resuscitation in patients sustaining traumatic hemorrhagic shock: A randomized, controlled, double-blinded trial. Annals of Surgery, 243(1), 47–57. 10.1097/01.sla.0000193608.93127.b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer JW, Reynolds S, Magzamen S, Vandyke A, Gottel NR, Gilbert JA, Owens SM, Hampton-Marcell JT, & Volckens J. (2017). Size, Composition, and Source Profiles of Inhalable Bioaerosols from Colorado Dairies. Environmental Science and Technology, 51(11), 6430–6440. 10.1021/acs.est.7b00882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenker M, & Gunderson P. (2013). Occupational Health in the Dairy Industry Needs to Focus on Immigrant Workers, the New Normal. Journal of Agromedicine, 18(3), 184–186. 10.1080/1059924X.2013.797375 [DOI] [PubMed] [Google Scholar]
- Theobaldo MC, Barbeiro HV, Barbeiro DF, Petroni R, & Soriano FG (2012). Hypertonic saline solution reduces the inflammatory response in endotoxemic rats. Clinics, 67(12), 1463–1468. 10.6061/clinics/2012(12)18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USDA. (2011). Farms, Land in Farms, and Livestock Operations 2011 Summary. February. [Google Scholar]
- Wells A, Romberger DJ, Thiele GM, Wyatt TA, Staab E, Heires AJ, Klassen LW, Duryee MJ, Mikuls TR, Dusad A, West WW, Wang D, & Poole JA (2017). Systemic IL-6 effector response in mediating systemic bone loss following inhalation of organic dust. Journal of Interferon and Cytokine Research, 37(1), 9–19. 10.1089/jir.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlauer M, Moore EE, Silliman C. ., Fragoso M, Gamboni F, Harr J, Accurso F, Wright F, Haenel J, Fullerton D, & Banerjee A. (2012). Nebulised hypertonic saline attenuates acute lung injury following trauma and hemorrhagic shock. Critical Care Medicine, 40(9), 2647–2653. 10.1097/CCM.0b013e3182592006.NEBULIZED [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in Mountain Scholar at http://dx.doi.org/10.25675/10217/234559