Abstract
In recent years the role of diagnostic imaging by pelvic ultrasound in the diagnosis and staging of gynecological cancers has been growing exponentially. Evidence from recent prospective multicenter studies has demonstrated high accuracy for pre-operative locoregional ultrasound staging in gynecological cancers. Therefore, in many leading gynecologic oncology units, ultrasound is implemented next to pelvic MRI as the first-line imaging modality for gynecological cancer. The work herein is a consensus statement on the role of pre-operative imaging by ultrasound and other imaging modalities in gynecological cancer, following European Society guidelines.
Keywords: cervical cancer, ovarian cancer, vulvar and vaginal cancer, uterine cancer, cross-sectional studies
INTRODUCTION
During the last decade, the use of ultrasound in the diagnosis and locoregional staging of gynecological malignancies has increased. Several ultrasound biomarkers are already established in clinical practice for detection of gynecologic cancer, prediction of the risk of disease or therapeutic outcome, prediction of oncological outcome, and evaluation of treatment response. An example of a diagnostic biomarker is the Ovarian-Adnexal Reporting and Data System (O-RADS) risk stratification and management system1 based on the International Ovarian Tumor Analysis Assessment of Different NEoplasias in the AdneXa (IOTA ADNEX) risk prediction model,2 or morphologic descriptors.3 A prognostic biomarker is the American Joint Committee on Cancer (AJCC) TNM (Tumor, Node, Metastasis) staging system,4 and an example of a response biomarker is the Response Evaluation in Solid Tumors (RECIST) criteria.5 Ultrasound provides both morphology-based (eg, tumor echogenicity and size) and functional biomarkers (eg, tumor perfusion based on power Doppler assessment or using contrast agents).6 7 With emerging machine-learning approaches, the applicability of precision ultrasound diagnostics will potentially further expand.8
The introduction of the high-resolution endovaginal probe allows detailed depiction of the pelvic anatomy, comparable to that achieved by pelvic MRI.9–14 Transabdominal ultrasound using a convex array probe provides detailed views of the abdominal organs, visceral and retroperitoneal lymph nodes, and peritoneum, guiding the prediction of disease resectability.15–17 Lastly, the use of a linear probe allows direct, high-resolution visualization of superficial structures, such as the peripheral lymph nodes (Figure 1).18 19 This has led to the implementation of ultrasound next to pelvic MRI as the first-line modality in the assessment of locoregional stage in gynecologic cancers, according to the European Society of Gynecological Oncology (ESGO) guidelines.9–14
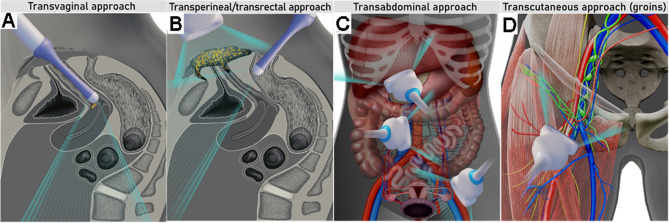
Figure 1.
Ultrasound approaches for gynecological cancer staging: transvaginal (A) and transperineal in combination with transrectal (B) approach for local staging; transabdominal approach using a convex array probe for evaluation of abdominal infiltrated visceral and retroperitoneal lymph nodes, peritoneal, or parenchymal metastases (C); transcutaneous approach with linear array probe for evaluation of inguinofemoral lymph nodes (D).
Ultrasound has the advantages of having low cost with high availability, involving no radiation exposure and minimal discomfort to the patient.20 21 Furthermore, ultrasound is an ideal tool for guiding core-needle biopsies to establish the histologic diagnosis, prior to expedited start of tailored therapy.22 23 Ultrasound may also be useful intra-operatively, such as to guide surgeons during fertility-sparing surgeries to preserve uninvolved ovarian tissue24 or to delineate the free margins during trachelectomy.25 Ultrasound-guided drainage of fluid collections (eg, lymphoceles, abscesses, etc) or palliative insertion of permanent catheters can help to avoid unnecessary surgeries. Furthermore, its wide availability makes it a useful bedside test for early detection and monitoring of surgical complications. Ultrasound scanners are relatively inexpensive compared with other modalities such as MRI, CT and positron emission tomography-CT (PET-CT) (Table 1).26
Table 1.
Comparison of different imaging methods for application in gynecologic oncology
| Expert ultrasound | MRI | CECT | FDG-PET-CT | |
| Costs | 1x | 4x | 2x | 6x |
| Availability | Specialized centers | Most hospitals | Most hospitals | Specialized centers |
| Range of examination | Abdomen and pelvis, peripheral lymph nodes | Whole body | Whole body | Whole body |
| Examination duration (min) | 15–20 | 60 | 5 | 30 |
| Dynamic evaluation* | Yes | No | No | No |
| Preparation before imaging | None | Antiperistaltic agents | None | 4 hours fasting and 1 hour rest prior to scanning |
| Contrast agent | None | Gadolinium-based† | Iodine-based | FDG-radiotracer and iodine-based |
| Radiation exposure | None | None | 10–20 mSv | 20–25 mSv |
| Limitations for application and factors impacting diagnostic quality | No contraindications. Limited depiction of abdominal deeper structures when overlying bowel gas/air | Contraindication if severe claustrophobia, and for some metal- or cochlear implants/cardiac pacemakers. Image artifacts from implants |
Contraindication for iodine-based contrast agent:
Image artifacts from implants |
Contraindication for iodine-based contrast agent:
Image artifacts from implants |
| Dependent on expertise | Yes | Yes | Yes | Yes |
Radiation exposure of 10–20 mSv from CECT (thorax, abdomen, pelvis) equals ~3–7 years of average background radiation and radiation exposure of 20–25 mSV from FDG-PET-CT (whole body) equals ~7–8 years of average background radiation (Dose-Reference-Card.pdf (acr.org)).
*Ultrasound imaging provides information about site-specific tenderness and visualizes how pelvic structures move in relation to each other (positive sliding sign or negative sliding sign in adhesions).
†In patients with renal insufficiency gadolinium contrast media must be used with caution.
CECT, contrast-enhanced CT; FDG-PET-CT, 18F-fluorodeoxyglucose positron emission tomography combined with CT; MRI, magnetic resonance imaging; mSv, millisievert.
Some reservations regarding widespread implementation of ultrasound stem from lack of training that may lead to ultrasound-related misdiagnosis. The inter-rater agreement for primary staging parameters between less and more experienced sonographers has been assessed for various gynecologic cancers.27 28 For example, more experienced sonographers had higher detection rates for cervical stromal invasion in endometrial cancer .27 Similarly, experienced sonographers had higher inter-observer agreement than less experienced sonographers for diagnosing parametrial invasion in cervical cancer.28 Data from an ultrasound study in ovarian cancer (Imaging Study on Advanced ovArian Cancer, ISAAC) suggest almost perfect agreement among sonographers to stage advanced ovarian cancer, when at least 6 months' ultrasound training is provided in a specialized center.29 The introduction of ultrasound training into the gynecologic oncology curriculum, and the development of trusted certification- and accreditation systems by the scientific societies, may increase its widespread use and acceptance. Sonographers should perform detailed scanning using a systematic approach and standardized terminology for the relevant staging parameters. The use of checklists is recommended to guarantee uniformity and reproducibility of the reported staging results (Online Supplemental Appendices S1–S5). The applications of ultrasound and other imaging methods on gynecologic cancer staging are outlined in Table 2.
Table 2.
Applications of ultrasound and other diagnostic imaging methods for primary staging of gynecologic cancers according to current international guidelines
| Cancer type | Local/locoregional staging | Distant spread |
| Vulvar cancer 2023 updated ESGO guidelines for the management of patients with vulvar cancer9 |
Local staging (≥T2 tumor or if the finding is equivocal)
Inguinofemoral lymph nodes (>T1 a)
|
Multifocal tumors and/or ≥4 cm and/or cN1
|
| Vaginal cancer 2023 ESTRO/ESGO/SIOPE guidelines for the management of patients with vaginal cancer10 |
Local (locoregional) staging
|
Locally advanced disease and/or cN1
|
| Cervical cancer 2018 and updated 2023 ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer11 |
Local (locoregional) staging
|
Locally advanced disease and/or cN1
|
| Endometrial cancer 2021 ESGO/ESTRO/ESP guidelines for the management of patients with endometrial cancer12 |
Abnormal uterine bleeding
Local (locoregional) staging
|
Risk factors for N1 or M1‡
|
| Tubo-ovarian cancer 2021 ESGO/ISUOG/IOTA/ESGE consensus statement on pre-operative diagnosis of ovarian tumors13 2019 and updated 2023 ESGO/ESMO consensus conference recommendations on ovarian cancer14 2024 ESGO/ISUOG consensus statement on ultrasound-guided biopsy in gynecologic oncology |
Primary tumor characterization
For pelvic and abdominal staging
Image-guided core needle biopsy
|
Risk factors for extra-abdominal spread§
|
*Ultrasound is an option for staging in specialized centers with trained sonographers, using all approaches (transvaginal/transrectal/perineal/transcutaneous/transabdominal) as appropriate.
†Whole-body FDG-PET-CT is recommended before treatment (eg, chemoradiotherapy or exenteration) with curative intent.
‡The main risk factors for nodal spread or metastatic disease are poorly differentiated tumors or those with non-endometrioid histology, p53 abnormal tumors (if available molecular profiling pre-operatively) and/or tumors with anteroposterior diameter >20 mm and/or deep myometrial invasion (>50%) and/or cervical stroma infiltration).
§Extensive retroperitoneal nodal involvement, massive diaphragmatic carcinomatosis, pleural effusion/pleural parietal carcinomatosis etc.
ADNEX, Assessment of Different NEoplasias in the adnexa; CECT, contrast-enhanced CT; cN1, suspicious lymph node/-s involvement on pre-operative imaging or palpation; DWI, diffusion-weighted imaging; ESGE, European Society for Gynecological Endoscopy; ESGO, European Society of Gynecological Oncology; ESMO, European Society for Medical Oncology; ESP, European Society of Pathology; ESTRO, European SocieTy for Radiotherapy and Oncology; EUROCAN, European Cancer Patient Coalition; FDG-PET-CT, 18F-fluorodeoxyglucose positron emission tomography combined with CT; IOTA, the International Ovarian Tumor Analysis; ISUOG, International Society of Ultrasound in Obstetrics and Gynecology; MRI, magnetic resonance imaging; SIOPE, European Society of Pediatric Oncology; WB, whole body.
ijgc-2023-004609supp001.pdf (151.8KB, pdf)
ijgc-2023-004609supp002.pdf (7MB, pdf)
ijgc-2023-004609supp003.pdf (8.3MB, pdf)
ijgc-2023-004609supp005.pdf (17.9MB, pdf)
ijgc-2023-004609supp004.pdf (17.7MB, pdf)
ijgc-2023-004609supp006.pdf (7.7MB, pdf)
This review on ultrasound imaging in gynecologic cancers follows this structure: (1) vulvar, (2) vaginal, (3) cervical, (4) endometrial, and (5) tubo-ovarian cancers.
VULVAR CANCER
Introduction
Vulvar cancer accounts for 4% of all gynecological cancers, affecting predominantly elderly women. More than 90% of cases are squamous cell carcinoma and its variants. Some variants (basaloid and warty) are more frequent in younger women and are related to human papillomavirus (HPV) infection.30 Metastatic involvement of the inguinofemoral lymph nodes at diagnosis is the major prognostic factor and affects the surgical approach and the need for adjuvant therapy.31–33 Furthermore, large primary tumor size, stromal invasion, and positive resection margins significantly predict recurrent disease.32–34 Currently, there is a limited alignment between the eighth edition of TNM and the International Federation of Gynecology and Obstetrics (FIGO) 2021 staging systems (Online Supplemental Appendix S1), and lack of evidence to base treatment on the 2021 FIGO staging system.30 35 36 A further version of TNM classification for vulvar cancer (ninth version) aligned with the 2021 FIGO staging system is expected to be available in 2024. In the meantime, the eighth TNM classification is advised for staging and treatment planning.9 The 2021 FIGO staging system allows incorporation of findings from all cross-sectional imaging methods into the FIGO stage.
Imaging provides information on tumor size, the extent of local involvement of the surrounding structures (vagina, uterus, anus, rectum, urethra, bladder), and the inguinofemoral, distant lymphatic and hematogenous spread, most commonly to the liver or lungs. For local staging, MRI is the modality of choice to assess invasion of vulvar cancer into septa, vagina, urethra, anus, and/or rectum due to its excellent soft tissue resolution.31 However, in gynecologic oncology centers with available expertise, ultrasound assessment can also be used (Figure 2, Online Supplemental Video S1).
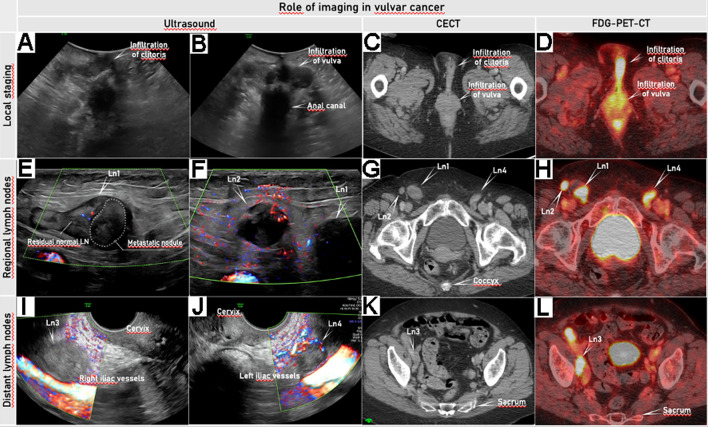
Figure 2.
Imaging by ultrasound, CECT, and FDG-PET-CT (depicting locoregional spread and distant spread in a patient in her 80s, diagnosed with squamous cell vulvar cancer FIGO stage IVB. For local staging, transperineal ultrasound using a convex array probe in the transverse plane allows visualization of tumor infiltration in the clitoris (A) and labia majora bilaterally (B). The same vulvar pathology is depicted as hyperdense tissue on CECT (C) with high FDG-avidity on FDG-PET-CT (D). Regional lymph nodes in the groins are evaluated by transcutaneous ultrasound using a linear array probe (according to the Vulvar International Tumor Analysis (VITA) Group consensus for the evaluation of inguinofemoral lymph nodes) (E, F).18 Two pathologic lymph nodes (Ln1 and Ln2) above the fascia lata and the femoral vessels on the right side (E, F) are seen: Ln1 is partially infiltrated while Ln2 shows complete infiltration. The same pathology is depicted as enlarged inguinal lymph nodes on CECT (G), highly FDG-avid on FDG-PET-CT (H). Pelvic lymph nodes are assessable by ultrasound using an endoluminal probe inserted transvaginally and metastatic lymph nodes are seen around the right (Ln3) and left (Ln4) (I, J) iliac vessels. The enlarged iliac lymph node is clearly visualized on the right side (Ln3) on CECT (K) and as highly FDG-avid on FDG-PET-CT (L). The location and size of this vulvar lesion made a transvaginal ultrasound approach possible. Online Supplemental Video S1 shows ultrasound and other imaging methods in vulvar cancer. CECT, contrast-enhanced CT; FDG-PET-CT, 18F-fluorodeoxyglucose positron emission tomography combined with CECT. FIGO, International Federation of Gynecology and Obstetrics; Ln, lymph node.
ijgc-2023-004609supp007.mp4 (76.2MB, mp4)
For regional nodal staging, expert ultrasound is the method of choice for pre-operative assessment of inguinofemoral lymph nodes,18 with sensitivity of 76–90% and specificity of 60–96%, allowing a detailed evaluation of nodal architecture and perfusion (Figure 2).37–39 The methodologic assessment of vulvar lymph nodes by ultrasound was previously reported in the Vulvar International Tumor Analysis (VITA) consensus.18 The use of ultrasound guidance for fine needle aspiration or core needle biopsy improved the detection of metastases in lymph nodes with altered morphology.40 41 Core needle biopsy should be preferred whenever possible to obtain sufficient material for histological analysis, although fine needle aspiration may be appropriate for small suspicious lymph nodes.31 MRI is currently considered an alternative imaging method for lymph node staging, with variable sensitivity (ranging from 40% to 89%) depending on the diagnostic criteria used.42–46 However, novel MRI techniques (eg, DWI (diffusion-weighted imaging); DCE (dynamic contrast-enhanced)-, and high-resolution T2WI (T2-weighted imaging) series) are promising for improving locoregional staging.31 High-quality ultrasound or MRI examination for local (loco-regional) staging purposes should be complemented by a structured imaging report to communicate clinically relevant information to the referring physician.
Patients who are not candidates for sentinel lymph node biopsy (if they have multifocal tumors, unifocal tumors with size ≥4 cm, and/or suspicious inguinofemoral nodes in pre-operative evaluation) should undergo further imaging in addition to the ultrasound or pelvic MRI assessment to exclude distant metastases. Thoracic and abdominal contrast-enhanced CT (CECT) or whole-body 18F-fluorodeoxyglucose positron emission tomography combined with CT (FDG-PET-CT) should be performed to exclude pelvic lymph node involvement and other distant metastases (Figure 2).31 47 48 New MRI sequences such as T2WI ultrafast spin echo sequences and whole-body DWI may be useful for assessing the upper abdomen and diagnosing distant nodal metastases.31
The location of the primary tumor and any suspicious regional and distant lymph nodes should be documented in a schematic drawing within a standardized systematic checklist (Online Supplemental Appendix S1).
Current Guidelines and the Role of Imaging in Vulvar Cancer Staging
Following the updated 2023 ESGO guidelines for the management of patients with vulvar cancer9:
Pre-operative work-up includes a medical history; general assessment of co-morbidities; frailty assessment; clinical examination; biopsy of all suspicious areas followed by pathologic review; and imaging as indicated.
For pT1a tumors (tumor ≤2 cm confined to the vulva and/or perineum, with stromal invasion ≤1 mm), no further imaging is required.
In patients considered eligible for a sentinel lymph node biopsy procedure, imaging of inguinofemoral lymph nodes by ultrasound or MRI is recommended. Suspicious inguinofemoral nodes on imaging should be assessed by ultrasound-guided fine needle aspiration or core needle biopsy if this would alter primary treatment.
In all other cases, systemic staging (including pelvic lymph nodes and distant organs) by CECT (chest/abdomen/pelvis) or FDG-PET-CT is recommended.
If the invasive tumor clinically involves surrounding tissues (≥T2 FIGO staging) or if clinical findings are equivocal, evaluation of extra-vulvar structures (urethra, bladder, vagina, cervix, and anal canal) with MRI is recommended. In specialized centers with an available trained ultrasound examiner, transvaginal/transrectal/perineal ultrasound can be an option in determining local staging.
Equivocal distant metastasis should be biopsied (if possible) to avoid inappropriate treatment.
VAGINAL CANCER
Introduction
Primary vaginal cancer is rare, constituting only 2% of all genital tract malignancies in women.49 Squamous cell carcinoma is the most common histologic type, with an incidence of 80–95%. Rare tumor types typically occur in young children (mean age at diagnosis 2 years) and include embryonal rhabdomyosarcoma and germ cell tumors, especially yolk sac tumor.50 In adults, it is estimated that only 10% of all vaginal malignancies originate from the vagina; the majority are metastatic spread from other sites (ie, cervix, endometrium, vulva, rectum). When a vaginal tumor extends to the vulva it should be classified as a vulvar cancer, and when a vaginal tumor extends into the cervical ostium it should be classified as a cervical cancer.51
Main prognostic factors for vaginal cancer are the location, nodal status, histologic tumor type, and presence of lymphovascular space invasion.52 Tumor size is known to be a prognostic factor and differentiates between substages FIGO 1A (≤2 cm) and FIGO 1B (>2 cm).53 Tumors involving the lower third of the vagina or the full vaginal length have a poorer prognosis.54–56 Tumors located in the upper third of the vagina will typically spread through lymphatic pathways to the iliac lymph nodes, whereas tumors in the lower third typically spread to the inguinofemoral lymph nodes. Tumors of the middle third may spread to either or both lymph node regions.51 57 58
Vaginal cancer staging is defined by the 2016 TNM classification and the 2021 FIGO staging system (Online Supplemental Appendix S2).51 53 To determine the stage of the disease, a complete work-up should be performed, including clinical examination with biopsies and imaging.
To determine local tumor extent, pelvic MRI is recommended, given its superior soft tissue resolution.59 Ultrasound performed by an expert sonographer can be used as a complementary imaging method in the primary work-up for locoregional staging (Figure 3, Online Supplemental Video S2).57 Also, regular ultrasound evaluation is recommended for assessing the response to neoadjuvant treatment of non-squamous rare cancers in childhood and adolescence (ie, germ cell cancer) and for follow-up in cases with complete remission (together with serum α-fetoprotein evaluation).10 CECT (chest/abdomen/pelvis) or whole-body FDG-PET-CT should be added especially in node-positive or locally and metastatic advanced disease (Figure 3, Online Supplemental Video S2). FDG-PET-CT is recommended for treatment planning before chemoradiotherapy or exenterative surgery with curative intent, or in the evaluation of recurrent disease.10 57 60 Because of the low sensitivity of any imaging method for detecting lymph node micro-metastases (≤2 mm) or small-volume (<5 mm) metastases, surgical staging of regional lymph nodes may be reasonable. The use of sentinel lymph node biopsy in vaginal cancer, in contrast to cervical cancer, is not yet established. In patients with positive pelvic nodes and negative para-aortic nodes on FDG-PET-CT, laparoscopic para-aortic lymph node surgical staging may be added to guide the external beam radiotherapy field. Suspicious inguinofemoral nodes on imaging should be sampled by ultrasound-guided fine needle aspiration or core needle biopsy if this would alter the primary treatment.61
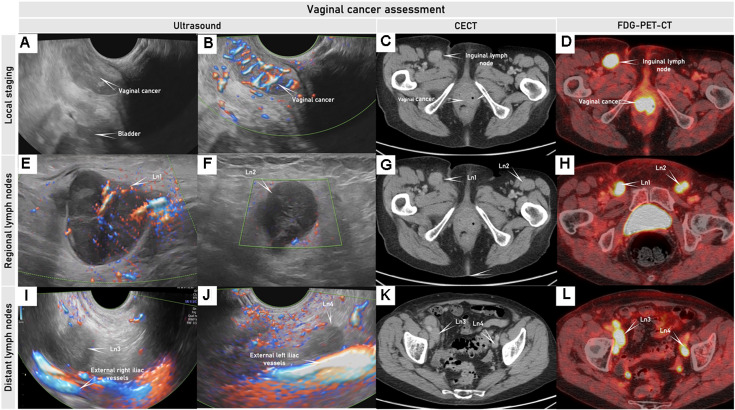
Figure 3.
Ultrasound, CECT and FDG-PET-CT in woman in her 70 s diagnosed with squamous cell vaginal cancer FIGO stage III. Transrectal ultrasound depicts a hypoechogenic to isoechogenic, richly vascularized tumor in the lower part of the anterior vaginal wall (A, B); corresponding pathologic mass depicted on axial CECT (C) is slightly contrast-enhancing and is highly FDG-avid on 18FFDG-PET-CT (D).Right and left hypoechogenic enlarged metastatic inguinal lymph nodes (Ln1 and Ln2) were seen on ultrasound (E, F), axial CECT (G), and as FDG-avid lesions on FDG-PET-CT (H). Right and left external iliac lymph nodes (Ln3 and Ln4) demonstrated similar appearance to the involved inguinal lymph nodes on transrectal ultrasound (I, J), axial CECT (K), and FDG-PET-CT (L), indicating metastatic lymph nodes. CECT, contrast-enhanced CT; FDG-PET-CT, 18F-fluorodeoxyglucose positron emission tomography combined with CECT; FIGO, International Federation of Gynecology and Obstetrics; ln, lymph nodes. The Online Supplemental Video S2 shows this tumor in the lower part of the vagina.
ijgc-2023-004609supp008.mp4 (120.3MB, mp4)
The location of the primary tumor and any metastatic regional and distant lymph nodes should be documented by preoperative imaging using a standardized systematic checklist including the use of schematic drawings as appropriate (Online Supplemental Appendix S2).
Current Guidelines and the Role of Imaging in Vaginal Cancer Staging
Following the upcoming 2023 ESGO-ESTRO (European Society for Radiotherapy and Oncology) – SIOPE (European Society of Pediatric Oncology) guidelines for the management of patients with vaginal cancer10:
Pelvic and vaginal examination with histologic confirmation of the disease is the first step in the diagnosis of vaginal cancer. Colposcopy is recommended, especially in stage I disease, for exact mapping of any (pre-)invasive disease.
Pelvic MRI is the standard imaging method to determine local extent. Expert pelvic ultrasound may be complementary.
CECT (chest/abdomen/pelvis) is recommended to assess the presence of nodal and distant disease.
FDG-PET-CT is recommended in node-positive or locally advanced disease before chemoradiotherapy or exenterative surgery with curative intent, or in the evaluation of recurrent disease.
CERVICAL CANCER
Introduction
Cervical cancer is the fourth most common cancer in women. Most of the cases are squamous cell carcinoma (75–90%) and adenocarcinomas (5–25%), with variable distribution across patient populations and countries. HPV plays a crucial role in the carcinogenesis, and is responsible for over 90% of all squamous cell carcinomas and 80–85% of adenocarcinomas.50 Main prognostic factors are described by the TNM classification and the FIGO staging system (maximum tumor size, depth of cervical stromal invasion, the maximum thickness of uninvolved stroma, extracervical extension, nodal involvement and distant metastases, pathological tumor type including HPV status, and presence of lymphovascular space involvement).62 63
Cervical cancer staging has undergone several updates in recent years, highlighting the necessity of accurate imaging for adequate treatment planning based on tumor size and location, parametrial involvement, lymph nodes status, and distant metastases (Figure 4). The 2021 version of the AJCC and the Union for International Cancer Control (UICC) TNM cervical cancer classification was recently aligned with the latest 2018 FIGO staging system of cervical cancer (Online Supplemental Appendix S3).62 63 All of them now emphasize and incorporate imaging findings in stage allocation and prognostication.
Figure 4.
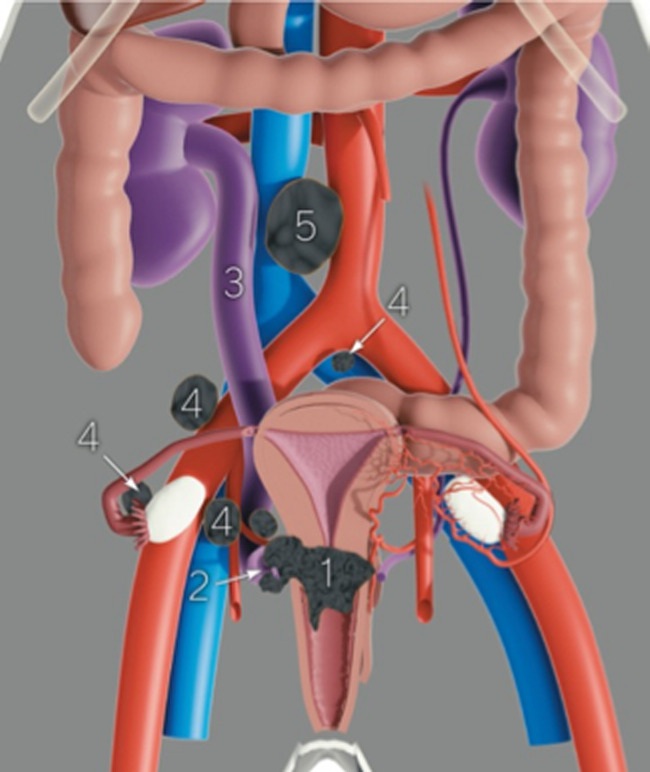
Pre-operative assessment of the disease extent and prognostic factors necessary for planning treatment. The size and tumor topography within the cervix (1), the parametrial spread (ventral, lateral, dorsal; right and left) (2) with the possible involvement of the urinary tract (3), the presence of metastatic pelvic (4), and para-aortic lymph nodes (5) and others. Pre-operative staging shows Online Supplemental Video S3 (available here).
To assess the local spread, clinical staging should be complemented by radiological staging as it may identify important prognostic factors that could guide the choice of treatment (Figure 4, online supplemental video S3, available here). The imaging method of choice to determine local tumor extent in the pelvis is MRI, due to its high soft tissue resolution (Figure 5, Online Supplemental Video S4). Maximum tumor size at MRI has been shown to be highly reproducible and is a strong predictor of survival.64 An MRI study on 416 patients with cervical cancer found substantial overall inter-observer agreement (among four readers) for key FIGO staging parameters (ie, tumor size categories (≤ 2 cm; >2 cm and ≤4 cm; >4 cm), parametrial invasion, vaginal invasion, and enlarged lymph nodes; κ=0.61–0.80).65 Unfortunately, access to MRI scanners is limited, particularly in low-income countries. MRI also has known contraindications and requires specific radiological expertise (Table 1). This may explain why the reportedly high MRI staging accuracy in single-unit cervical cancer studies was not reproduced in a multicenter study.66 A European multicenter trial of early-stage cervical cancer showed comparable or better accuracy of ultrasound than MRI in local staging assessment (Figure 5).67 A recent meta-analysis reported similar diagnostic performance for detecting parametrial invasion in cervical cancer by ultrasound/MRI, reporting pooled sensitivities and specificities of 78%/68% and 96%/91%, respectively (p=0.55).68 In a multicenter trial by Pálsdóttir et al, the reliability of ultrasound and MRI to define local tumor extension by readers with different levels of experience was also documented, reporting only moderate inter-observer agreement for transvaginal ultrasound as opposed to moderate-to-substantial agreement for MRI.28 Interestingly, similar agreement for tumor extension was seen between experienced and less experienced observers, both for ultrasound and MRI, except for parametrial invasion by ultrasound. Importantly, inter-observer agreement is likely to improve with dedicated training.
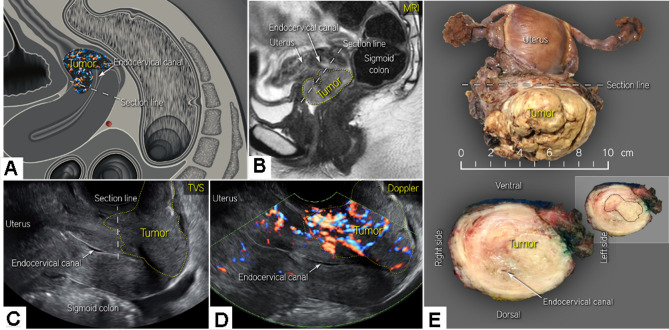
Figure 5.
Cervical cancer on the anterior lip of the cervix (A) visualized by MRI (C) and transvaginal ultrasound C,D) in a patient in her 30s diagnosed with squamous cell carcinoma of the cervix, FIGO stage IB3 on radiologic staging. A bulky tumor infiltrating the anterior lip of the cervix and cervical stroma up to the internal os (A) is depicted as hyperintense on T2-weighted MRI (B) and hypoechoic on transvaginal ultrasound (C) with high vascularity depicted by color Doppler (D). Visualization of the tumor vessels supported the drawing of the tumor boundaries (yellow dotted line) and measurements of tumor size (B, D). Bulky cervical tumor clearly visible in the hysterectomy specimen (upper panel) and after transverse sectioning (lower panel) of the tumor at the level of the section line (indicated in A-C) (E). FIGO, International Federation of Gynecology and Obstetrics; MRI, magnetic resonance imaging; TVS, transvaginal scan. The Online Supplemental Video S4 shows an ultrasound of a cervical cancer on the anterior lip.
ijgc-2023-004609supp010.mp4 (29.6MB, mp4)
Ultrasound examination allows the assessment of predictive imaging markers such as tumor size, echogenicity or vascular (Doppler) features.7 Abundant perfusion in the primary tumor has been linked to an aggressive clinical phenotype and poor treatment response.69 70 In studies on locally advanced cervical cancer, tumor ultrasound-derived 3D vascular indices prior to chemoradiotherapy were adversely associated with treatment response.71–73 Low vascular indices observed in patients with poor treatment response were probably linked to tumor hypoxia, which is known to induce therapy resistance in various solid tumors.7
For assessing lymph node involvement, ultrasound has poor sensitivity (38–43%) but high specificity (96%) for detecting nodal metastases, especially in early-stage cervical cancer.74 75 This is partly related to the small size of lymph node metastases in most cases (median maximum size of affected lymph nodes, 14 mm; median size of intranodal metastasis, 3.5 mm).74 76 On the other hand, other imaging modalities (ie, MRI, CECT, and FDG-PET-CT) also reportedly have poor sensitivities for detecting small-volume lymph node metastases.7 A multicenter prospective imaging study (Cervical Cancer Lymph Node Staging, CANNES study; https://clinicaltrials.gov/ct2/show/NCT05573451) is currently ongoing with the aim to compare the accuracy of expert ultrasound, MRI, and FDG-PET-CT for detecting pelvic and para-aortic lymph node metastases in cervical cancer.77
Thus, negative imaging findings do not rule out metastatic nodal involvement in cervical cancer, and surgical lymph node staging should be performed in early-stage cervical cancer. The use of sentinel lymph node biopsy is recommended, taking into consideration that about 10% (65/645) of early-stage cervical cancers with negative lymph nodes on pre-operative imaging have micrometastases (<2 mm), detectable by histopathological ultrastaging.78 In locally advanced cervical cancer, para-aortic lymph node dissection up to the inferior mesenteric artery may be considered for staging and treatment planning even if the nodes appear negative on imaging, to reveal otherwise undetectable small nodal metastases.79
In node-positive or locally advanced disease, CECT or FDG-PET-CT should be added. As with previously discussed gynecologic cancers, FDG-PET-CT is recommended for treatment planning before chemoradiotherapy or exenterative surgery with curative intent, or in the evaluation of recurrent disease.
Real-life data on gynecologic oncologists’ preferred primary staging modality and its diagnostic performance in early-stage cervical cancer were provided by the prospective, international SENTIX study.80 Each participating site was instructed to choose their preferred method based on their routine clinical practice. Among 690 prospectively enrolled patients with early-stage cervical cancer, 46.7% and 43.1% of patients underwent MRI and ultrasound, respectively, and 10.1% underwent both modalities. Pelvic MRI and ultrasound yielded similar diagnostic performance for predicting histologically confirmed tumor size, parametrial involvement, and macrometastatic nodal involvement.
The structured checklist is reported in Online Supplemental Appendix S3 and Online Supplemental Video S3 (available here) demonstrates pre-operative ultrasound staging.
Current Guidelines and the Role of Imaging in Cervical Cancer Staging
Following the 2018 and updated 2023 ESGO/ESTRO/ESP (European Society of Pathology) guidelines for the management of patients with cervical cancer11:
Pelvic examination and biopsy±colposcopy are mandatory to diagnose cervical cancer.
Pelvic MRI is mandatory for initial assessment of the extent of pelvic tumor and to guide treatment options (optional for stage T1a with free margins after conization). Endovaginal/transrectal ultrasonography is an option if performed by an adequately trained sonographer.
In the locally advanced cervical cancer (T1b3 and higher, except T2a1, or in early-stage disease with suspicious lymph nodes on the imaging), FDG-PET-CT or CECT (chest/abdomen/pelvis) are recommended for the assessment of nodal spread and distant metastases.
FDG-PET-CT is recommended before chemoradiotherapy with curative intent.
ENDOMETRIAL CANCER
Introduction
Endometrial cancer is the most common gynecological malignancy in Europe, with a rising incidence due to increased age and obesity in the population.49 The majority of endometrial cancers (80%) are confined to the uterus at the time of diagnosis, since post-menopausal bleeding prompts investigations and early detection.12 Deep (≥50%) myometrial invasion, cervical stromal extension, non-endometrioid histology, high tumor grade and substantial (in contrast to focal) lymphovascular space invasion are independent risk factors for lymph node metastases and poor prognosis.81–83 The distribution of lymph node involvement is also prognostic as para-aortic lymph node metastases independently predict poor outcome.82 84 Since The Cancer Genome Atlas defined four molecular subgroups of endometrial cancers in 2013 (DNA polymerase ɛ ultramutated, POLEmut; DNA mismatch repair-deficient, MMRd; no specific molecular profile, NSMP; p53-abnormal, p53abn), molecular factors are increasingly being used to define groups at risk and guide adjuvant or systemic treatment.82 85 Among the four molecular subgroups p53abn cancers have the highest risk, while POLEmut have the lowest risk.86
The updated 2023 FIGO staging system for endometrial cancer includes histological types and tumor grading and also molecular subgroups if available, in order to better reflect the underlying biologic behavior of endometrial cancers.83 If POLEmut or p53abn is detected in early stage disease (former FIGO 2009 stage I) regardless of lymphovascular space invasion status or histologic type, the 2023 FIGO stage is changed to stage IAmPOLEmut or stage IICmp53abn, respectively.83 In addition, the 2023 FIGO staging system for endometrial cancer differentiates between synchronous ovarian cancers and metastatic ovarian lesions.83 Disease limited to the endometrium and ovaries in low-grade endometrioid carcinomas (stage IA3)(Figure 6) is distinguished from extensive spread of endometrial carcinoma to the ovary (stage IIIA1) (Figure 7) by the presence of the following criteria: (1) superficial (<50%) myometrial invasion; (2) absence of extensive/substantial lymphovascular space invasion; (3) no additional metastases; and (4) unilateral ovarian tumor limited to the ovary, without capsule invasion/rupture (equivalent to pT1a ovarian cancer). Low-grade endometrioid cancers involving both the endometrium and the ovary are considered to have good prognosis, and no adjuvant treatment is recommended, while metastatic ovarian involvement by endometrial carcinoma is associated with poor prognosis (Figures 6 and 7, Online Supplemental Videos S5 and 6).87 The changes incorporated in the 2023 FIGO staging system (Online Supplemental Appendix S4) should be consistent with the TNM classification, which should also be updated accordingly.
Figure 6.
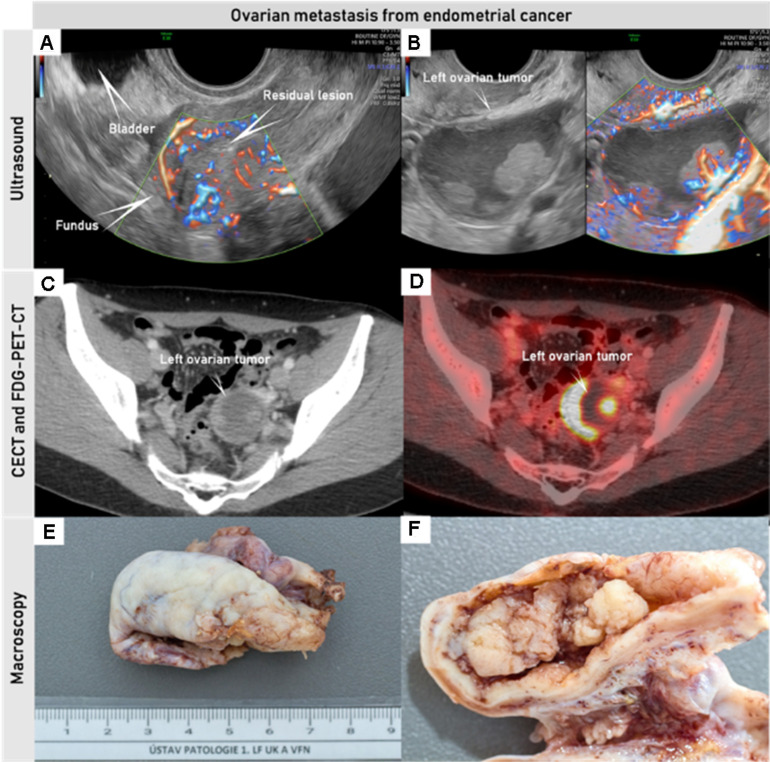
Synchronous endometrial and ovarian low-grade endometrioid cancer FIGO stage IA3 in a woman in her 30s who underwent transvaginal ultrasound, CECT, and FDG-PET-CT pre-operatively. Uterus with residual low-grade endometrial endometrioid cancer after hysteroscopic resection, with an intrauterine device in situ (A). Left ovary with unilocular-solid tumor histologically verified as low-grade endometrioid carcinoma; of note are papillary projections with smooth rounded contours, high perfusion on color Doppler and intracystic fluid with ground glass echogenicity (B). Pathologic lesion in the left ovary depicted on CECT (C) with contrast-enhancing solid components, and on PET-CT (D) with high FDG-avidity in the solid components; macroscopic appearance of the left ovary after oophorectomy (E, F). CECT, contrast-enhanced CT; FDG-PET-CT, 18F-fluorodeoxyglucose positron emission tomography combined with CECT. FIGO, International Federation of Gynecology and Obstetrics.The Online Supplemental Video S5 shows this case.
Figure 7.
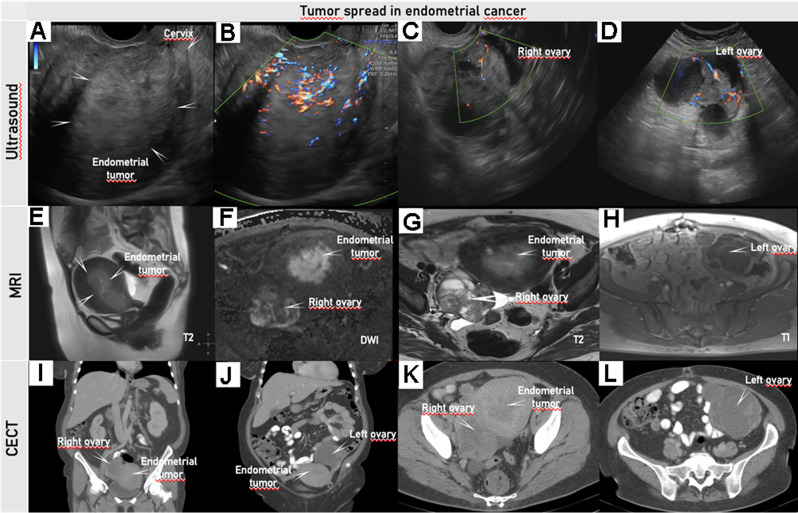
Transvaginal ultrasound (A–D), pelvic MRI (E–H), and abdominal CECT (I–L) in a woman in her 50 s diagnosed with clear cell endometrial cancer FIGO stage IIIA1. Transvaginal ultrasound depicts an isoechogenic uterine tumor infiltrating more than 50% of the myometrial wall (A); with protrusion but no invasion into the proximal endocervix, the distance from the external cervical os to the lower margin of the tumor is 22 mm (B); color Doppler depicts a moderately vascularized solid tumor of the right ovary (C); and a multilocular-solid tumor in the left ovary with moderately vascularized solid components (D). Pelvic MRI with T2-weighted sagittal (E) and T2/T1-weighted axial (G, H) series and DWI (high b-value image) (F) depicts a large, hyperintense mass in the uterus extending from the uterine fundus down to the cervix (E). The tumor exhibits restricted diffusion depicted as hyperintensity on the DWI image (F) with low apparent diffusion coefficient (ADC) value on the ADC map (not shown), indicating malignant tumor. On axial T2-weighted MRI small cysts are seen in the right ovarian tumor (G) as hyperintense regions in the anterior part. The left ovarian tumor depicted on T1-weighted MRI is hypointense due to cystic portion (H). Abdominal CECT depicts an irregular large uterine mass (I, K) and the right (I, K) and left (J, L) ovarian tumors with cystic lesions and contrast-enhancing septae. On axial CT at the level of the uterus (K) and at the level above the uterus (L) hypodense areas in the left ovary (L) and the right ovarian tumors (K) indicating cystic spaces. DWI, diffusion-weighted imaging; CECT, contrast-enhanced CT; FIGO, International Federation of Gynecology and Obstetrics. Online Supplemental Video S6 shows this case of endometrial cancer.
ijgc-2023-004609supp011.mp4 (36.3MB, mp4)
Ultrasound is the first-line imaging technique to evaluate endometrial pathology in cases of abnormal uterine bleeding and helps to triage patients for appropriate diagnostic tests. Transvaginal ultrasound plays a pivotal role in planning the management of women with abnormal uterine bleeding.6 88 The International Endometrial Tumor Analysis (IETA) group has been established to define the standardized terms, definitions, and measurements for description of sonographic features of the endometrium and uterine cavity (Online Supplemental Appendix S4).89 Based on a large amount of prospectively collected data, the IETA group defined easy-to-assess features, such as endometrial thickness <3 mm, three-layer pattern, and linear endometrial midline, all indicating low risk of endometrial cancer.90 Patients presenting with these features can be safely discharged with no further follow-up even with a history of abnormal uterine bleeding. Similarly, the presence of a single vessel without branching is associated with very low risk (1.5%) of endometrial cancer.90 For all other findings, further investigations and biopsy are recommended. The method of obtaining a histological sample (pipelle, curettage, or hysteroscopic resection under direct visualization) depends on the available resources and clinical experience. However, hysteroscopic biopsy is recommended (at least for focal lesions), since it yields higher agreement with final histological diagnosis.12 91–93
In histologically verified endometrial cancer, a trained sonographer can assess the size of the endometrial lesion (its anteroposterior diameter has key prognostic impact),94 depth of myometrial invasion, and cervical stromal invasion as well as screen for other pelvic pathology (Online Supplemental Appendix S4).12 27 95 Additionally, the identification of ultrasound features on gray-scale and power Doppler may be used to predict low-risk and high-risk endometrial cancer phenotypes (Online Supplemental Appendix S4).6 Unlike transabdominal ultrasound, transvaginal ultrasound is less limited by patient habitus (obesity) or position of the uterus.96 Ultrasound and MRI have similar accuracy in determining the local extent of endometrial cancer, although both methods may be inaccurate in 15–25% cases.88 97–103 A recent systematic review and meta-analysis from Alcázar et al confirmed very similar diagnostic performances of transvaginal ultrasound/MRI for detecting cervical stromal invasion, with reported pooled sensitivities and specificities of 69%/69% and specificities of 93%/91%, respectively.97 Another meta-analysis by the same authors found similar diagnostic performance of transvaginal ultrasound/MRI for detecting deep myometrial invasion, reporting pooled sensitivities and specificities of 75%/83% and 82%/82%, respectively.98 No statistical differences were found between ultrasound and MRI in local staging in both meta-analyses.97 98 Diagnostic performance for detecting deep myometrial invasion appears similar between expert and non-expert sonographers, whereas experts perform better in the evaluation of cervical stromal invasion.27 Thus, the training of sonographers in endometrial cancer staging is critical.
For local staging of endometrial cancer, pelvic MRI and transvaginal/transrectal ultrasound yield similar diagnostic performance, and the choice of imaging method is determined by local access to these imaging modalities and operator expertise.104 At some centers, transvaginal ultrasound is used as the first-line imaging tool, with subsequent selective use of pelvic MRI in cases with suboptimal assessment on ultrasound (eg, reduced acoustic visibility/penetration due to fibroids/bowel gas/obesity/other pathology). In other centers, pelvic MRI is used as the first-line imaging modality for pre-operative local staging.
Whole-body imaging can be considered in addition to pelvic ultrasound or MRI depending on the putative risk profile based on imaging findings, clinical features, and presence of pathologic factors, such as high tumor grade, substantial lymphovascular space invasion, non-endometrioid histology, p53abn molecular subgroup, tumor anteroposterior diameter >20 mm, deep (>50%) myometrial invasion, or cervical stroma infiltration.12 105–107 To predict high-risk cancers using ultrasound, the strategy of combining subjective assessment of cervical stromal invasion and myoinvasion with tumor grade correctly stratified 80% of patients as high-risk or low-risk cancer for the presence of lymph node metastases.108 Patients were classified as high risk based on biopsy-confirmed grade 3 endometrioid, gastrointestinal-type mucinous cancer or other non-endometrioid histotype and/or suspicion of deep myoinvasion or cervical stromal invasion on ultrasound. A similar approach was tested by Fasmer et al using pre-operative biopsy and pelvic MRI in all patients, with selective FDG-PET-CT based on high-risk MRI features (myoinvasion ≥50% and/or cervical stromal invasion and/or suspicious lymph nodes).109 Based on their findings, pre-operative FDG-PET-CT only in cases with high-risk MRI features seems to bring the most benefit in detecting distant spread while avoiding unnecessary FDG-PET-CT-related radiation in low-risk patients.
Both CECT and MRI are considered equivalent for the evaluation of nodal metastases, although neither can replace surgicopathologic lymph node assessment.104 FDG-PET-CT is considered the best imaging method to evaluate lymph node and distant metastases due to its high specificity, although sensitivity is lower.110 111 Due to limited sensitivity of imaging to detect small-volume metastases, surgical lymph node staging by sentinel lymph node biopsy remains important12 to allow the proper selection of adjuvant treatment and improve patient outcome.112
Local tumor extent, regional and distant lymph node metastases and other distant metastases should be documented by preoperative imaging using a standardized systematic checklist, including the use of schematic drawing(s) as appropriate (Online Supplemental Appendix S4).
Current Guidelines and the Role of Imaging in Endometrial Cancer
Following the 2020 ESGO/ESTRO/ESP guidelines for the management of patients with endometrial cancer12:
Pre-operative work-up includes: obtaining family history and medical history; general assessment; geriatric assessment, if appropriate; clinical examination including pelvic examination.
Expert transvaginal or transrectal pelvic ultrasound or pelvic MRI is recommended for local staging.
Depending on the clinical and pathologic risk, additional imaging modalities should be considered to assess ovarian, nodal, peritoneal, and other sites of metastatic disease. For assessing distant lymph node metastases or distant spread, CECT (chest/abdomen/pelvis) or FDG-PET-CT is recommended.
TUBO-OVARIAN CANCER
Introduction
Tubo-ovarian cancer is the leading cause of death among all gynecological cancers in developed countries, with more than two-thirds of patients being diagnosed at an advanced stage (FIGO stage III and IV) due to absence of symptoms in the initial stages of the disease.14 It is acknowledged that tubo-ovarian cancer is not a homogeneous disease, but rather a group of tumors with different epidemiologies, precursor lesions, morphologies, response to treatment, and prognosis. More than 90% of malignant tubo-ovarian tumors are of epithelial origin, with the most common and lethal being high-grade serous carcinoma.14 In 2014, FIGO revised its ovarian cancer staging system to incorporate ovarian, fallopian tube, and peritoneal cancer in the same classification.113 114 The eighth edition of the TNM staging system of cancer of the ovary, fallopian tube, and peritoneum mirrors that of 2014 FIGO staging classification (Online Supplemental Appendix S5).
Accurate pre-operative diagnosis of tubo-ovarian cancer and timely referral of patients to specialized centers is crucial for their prognosis.115 For these reasons, four scientific societies—namely, ESGO, the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), the IOTA group, and the European Society for Gynecological Endoscopy (ESGE), have issued an evidence-based consensus statement on the pre-operative diagnosis of ovarian cancer to help differentiate between benign and malignant ovarian tumors.13 The tumor should be characterized by expert sonographers (level III) subjectively, or by less experienced sonographers using the IOTA Simple Rules risk calculation116 or the IOTA ADNEX model.2 The IOTA ADNEX model uses simple predictor variables and discriminates between benign and malignant tumors, but also calculates the risk of four types of malignancy. However, a large proportion of adnexal masses (40%) can be classified as benign using the modified benign descriptors without computer support.117 Thus the IOTA group has recently validated a two-step strategy (Online Supplemental Video S7).117 For this strategy, the first step consists of applying the modified benign descriptors if applicable. If one of these applies, the mass can be classified as benign with a risk of malignancy <1%, while if none applies, the IOTA ADNEX model can then be used to estimate the risk of malignancy.13 117 In particular, patients at intermediate (10–50%) and high risk (≥50%) of malignancy should be referred to a specialized center.13 Knowledge of standardized ultrasound terms describing ovarian pathology118 is essential in order to accurately apply IOTA-based models.118 The standard IOTA terminology refers to the main characteristics of adnexal tumors, including definitions of the lesion’s solid component, intracystic content, blood flow (ie, color score), acoustic shadows, and others (Online Supplemental Appendix S5).
ijgc-2023-004609supp012.mp4 (90.5MB, mp4)
For patients diagnosed with tubo-ovarian cancer, the most important independent prognostic factor is complete surgical tumor resection (no residual tumor at the end of surgery). Therefore, accurate pre-operative identification of peritoneal and other metastatic spread is critical for prognostication and optimizing patient management. In general, all imaging modalities have high specificity for predicting residual disease (remaining visible cancer tissue at the end of debulking surgery), but low sensitivity for detecting small-volume carcinomatosis, potentially resulting in unnecessary surgical explorations due to non-resectable disease (Figure 8, Online Supplemental Video S8).119 Laparoscopy, on the other hand, offers direct visualization of the peritoneal parietal, visceral, omental, and mesenteric surfaces but may miss retroperitoneal spread, tumors behind the gastrosplenic ligament or the lesser sac, as described in the recent review by Pinto et al.119 Laparoscopy can be considered in cases of uncertain resectability or to exclude small-volume carcinomatosis which may not be seen on imaging, such as on the bowel serosa or mesentery (Figure 8).
Figure 8.
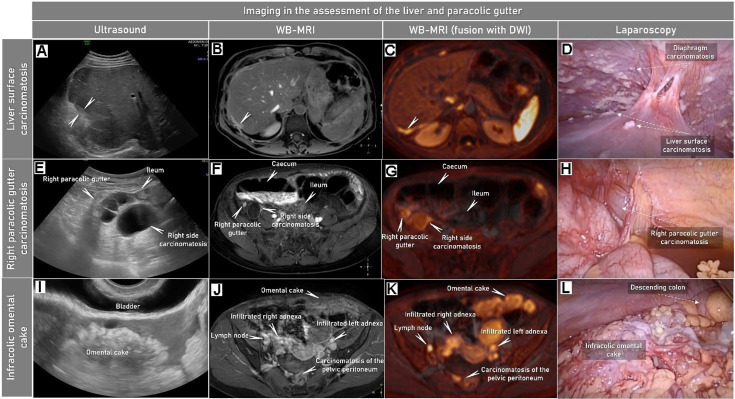
Characteristic imaging findings on abdominal ultrasound, whole body (WB)-MRI (showing the abdomen) with DWI and at laparoscopy in a woman in her 50 s diagnosed with FIGO Stage IVB high-grade serous carcinoma of the ovary. Abdominal convex array and transvaginal ultrasound (first column), axial CE-T1-weighted fat-suppressed (CE-T1WI-FS) MRI (part of WB-MRI) (second column), CE-T1WI-FS fused with DWI (high b-value images) (third column) and laparoscopy findings (fourth column). The imaging findings confirmed by laparoscopy indicate visceral hepatic carcinomatosis marked with arrows on the surface of the liver (A–D), mainly cystic carcinomatosis lesions in the lower part of right paracolic gutter (E–H) and large infracolic omental cake (I–L). Peritoneal carcinomatosis is contrast-enhancing (seen as hyperintensity on the WB-MRI series; second column) and exhibits restricted diffusion (seen as hyperintensity on the fused WB-MRI fused with DWI; third column). CE, contrast-enhanced; DWI, diffusion-weighted imaging; FIGO, International Federation of Gynecology and Obstetrics; FS, fat suppression; WB-MRI, whole-body MRI. Online Supplemental Video S8 shows this case of tubo-ovarian cancer spread.
ijgc-2023-004609supp013.mp4 (91.2MB, mp4)
The role of expert ultrasound in the pre-operative staging of tubo-ovarian cancer has been re-evaluated with recent evidence demonstrating its high accuracy in the prediction of tumor histotype and radiological staging. This includes the assessment of pelvic and abdominal peritoneal involvement, retroperitoneal lymph node metastasis, and ultimately, the prediction of non-resectability (Figure 8, Online Supplemental Video S8).15–17 120 121 In addition to diagnostic performance, patient satisfaction on ultrasound, CECT, and whole-body diffusion-weighted MRI was evaluated in the prospective international ISAAC study. Ultrasound was the preferred imaging method despite being associated with mild (or occasionally moderate) pain when compared with CECT and whole-body diffusion-weighted MRI.20 Whole-body diffusion-weighted MRI, involving long procedural time (~60 min) in the MRI scanner, was the least preferred by patients.20 As part of the same study, the reproducibility of ultrasound staging was tested.29 After a minimum of 6 months of training in a high-volume specialist center, 12 less experienced and 13 more experienced ultrasound operators assessing the presence of advanced ovarian cancer in 19 anatomic sites based on video clips acquired by one expert sonographer achieved almost perfect agreement (κ coefficient 0.88).29 CECT is usually considered the standard imaging modality for pre-operative tubo-ovarian cancer staging, despite reported variable overall pre-operative staging accuracy and poor inter-observer agreement.17 121 122 The novel but time-consuming whole-body diffusion-weighted MRI may be superior to CECT for primary tumor characterization, staging, and prediction of residual disease and reportedly yields almost perfect inter-observer staging agreement in ovarian cancer (Figure 8).123 FDG-PET-CT may serve as a problem-solving imaging modality if there is a very high risk of distant spread or indeterminate distant (eg, thoracic) metastases have been identified by CECT.124 125 Several models and imaging scoring systems have been developed for predicting surgical outcome and residual disease, but studies have frequently failed to provide sufficient external validation of their results.119 Nowadays, a thorough and structured imaging assessment of critical sites for ovarian cancer surgery remains the most useful approach (Online Supplemental Appendix S5).126 Using this approach, the results of an international ISAAC interim analysis showed that transvaginal/transabdominal ultrasound was non-inferior to both CECT (p value=0.029) or whole-body diffusion-weighted MRI (p value=0.036) for predicting surgical non-resectability.126 In an ideal setting, a woman can receive an all-in-one ultrasound-based approach at the same appointment. This includes diagnosis, staging, and prediction of non-resectability, and establishing the histopathologic diagnosis using ultrasound guided core-needle biopsy if the disease is deemed unresectable (one-stop ovarian cancer clinic concept).
As with previously discussed cancers, all imaging modalities face limitations in detecting small-volume lymph node metastases.127 128 Therefore, in early-stage ovarian cancer, systematic pelvic and abdominal lymphadenectomy are usually recommended to detect occult small-volume lymph nodes metastases, and tailor adjuvant treatment.129 In advanced ovarian cancer it is recommended to remove only clinically affected lymph nodes since the LION study showed that routine systematic pelvic and para-aortic lymphadenectomy for all patients does not improve progression-free or overall survival.130 The main role of imaging in these cases is therefore to detect suspicious or enlarged lymph nodes for selective resection.
A description of the primary tumor location and any locations of peritoneal spread, infiltrated regional and distant lymph nodes and other metastatic sites should be documented by preoperative imaging using a standardized systematic checklist (Online Supplemental Appendix S5).
Current Guidelines and the Role of Imaging in Tubo-ovarian Cancer
Following the 2021 ESGO/ISUOG/IOTA/ESGE consensus on ovarian tumor diagnosis13:
Subjective assessment by expert sonographers (level III) has the best diagnostic performance to distinguish between benign and malignant ovarian tumors.
If the above is not available, the use of ultrasound-based diagnostic models can assist clinicians to distinguish between benign and malignant ovarian tumors.
Ultrasound-based diagnostic models (IOTA Simple Rules risk model or IOTA ADNEX model) are preferable to CA 125 level, HE4 level, or Risk of Ovarian Malignancy Algorithm (ROMA) as they better distinguish between benign and malignant ovarian tumors.
The IOTA ADNEX model and the IOTA Simple Rules risk model are recommended instead of morphological scoring systems, including the Risk of Malignancy Index.
Following the 2019 and updated 2023 ESGO/ESMO (European Society for Medical Oncology) consensus conference recommendations on ovarian cancer14:
CECT, whole-body diffusion-weighted MRI, and FDG-PET-CT are considered viable options for assessing tumor extent and resectability and to detect distant metastases in ovarian cancer.
Ultrasound by an expert sonographer may also be used to assess tumor extent and resectability in the pelvic and abdominal cavity.
CONCLUSION
Ultrasound is a reliable imaging modality, which is widely available, non-invasive, low cost, and has no known contraindications or patient risks. Based on the recent evidence, expert ultrasound is recommended as an equal alternative to MRI for locoregional staging of vulvar, vaginal, cervical, and endometrial cancer and as the first-choice imaging modality for evaluation of abnormal uterine bleeding. In ovarian cancer staging, the choice of imaging modality may depend on availability of imaging method and expertise, although ultrasound is recognized as comparable to CECT, MRI, and FDG-PET-CT for abdominal staging. Similarly, for predicting non-resectability in ovarian cancer, ultrasound is recognized as non-inferior to CECT and MRI and an effective tool for guiding core needle biopsy in patients deemed unfit for surgery. Moreover, ultrasound is the imaging method of choice in primary ovarian tumor characterization. Its crucial role in gynecological oncology should be acknowledged, and financial and logistic resources need to be allocated for the training of future ultrasound experts in gynecological cancer.
ijgc-2023-004609supp009.mp4 (39.4MB, mp4)
Acknowledgments
We thank Adam Preisler (Polygoniq Studio), for providing the illustrations, Tomas Herrmann (Institute of Scientific Information, First Faculty of Medicine, Charles University), for video editing.
Footnotes
Presented at 23rd European Congress on Gynaecological Oncology (October 29, 2022): A review article was presented as an debate at 23rd European Congress on Gynaecological Oncology (October 29, 2022) Meetings/Events|ESGO eAcademy by European Society of Gynaecological Oncology (ESGO).
All authors: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; and drafting the work or revising it critically for important intellectual content; and final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This research was supported by Ministry of Health of the Czech Republic (NV19-03-00552 and NU21-03-00461) and MH CZ-DRO-VFN64165.
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Andreotti RF, Timmerman D, Strachowski LM, et al. O-RADS US risk stratification and management system: a consensus guideline from the ACR ovarian-adnexal reporting and data system committee. Radiology 2020;294:168–85. 10.1148/radiol.2019191150 [DOI] [PubMed] [Google Scholar]
- 2. Van Calster B, Van Hoorde K, Valentin L, et al. Evaluating the risk of ovarian cancer before surgery using the ADNEX model to differentiate between benign, borderline, early and advanced stage invasive, and secondary metastatic tumours: prospective multicentre diagnostic study. BMJ 2014;349:g5920. 10.1136/bmj.g5920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Andreotti RF, Timmerman D, Benacerraf BR, et al. Ovarian-adnexal reporting lexicon for ultrasound: a white paper of the ACR ovarian-adnexal reporting and data system committee. J Am Coll Radiol 2018;15:1415–29. 10.1016/j.jacr.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 4. TNM classification of malignant tumours, 8th Edn. 2016.
- 5. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 6. Epstein E, Fischerova D, Valentin L, et al. Ultrasound characteristics of endometrial cancer as defined by International Endometrial Tumor Analysis (IETA) consensus nomenclature: prospective multicenter study. Ultrasound Obstet Gynecol 2018;51:818–28. 10.1002/uog.18909 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/51/6 [DOI] [PubMed] [Google Scholar]
- 7. Haldorsen IS, Lura N, Blaakær J, et al. What is the role of imaging at primary diagnostic work-up in uterine cervical cancer Curr Oncol Rep 2019;21:77. 10.1007/s11912-019-0824-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christiansen F, Epstein EL, Smedberg E, et al. Ultrasound image analysis using deep neural networks for discriminating between benign and malignant ovarian tumors: comparison with expert subjective assessment. Ultrasound Obstet Gynecol 2021;57:155–63. 10.1002/uog.23530 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/57/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oonk MHM, Planchamp F, Baldwin P, et al. European Society of Gynaecological Oncology guidelines for the management of patients with vulvar cancer - update 2023. Int J Gynecol Cancer 2023;33:1023–43. 10.1136/ijgc-2023-004486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nout RA, Calaminus G, Planchamp F, et al. ESTRO/ESGO/SIOPe guidelines for the management of patients with vaginal cancer. Int J Gynecol Cancer 2023;33:1185–202. 10.1136/ijgc-2023-004695 [DOI] [PubMed] [Google Scholar]
- 11. Cibula D, Raspollini MR, Planchamp F, et al. ESGO/ESTRO/ESP guidelines for the management of patients with cervical cancer - update 2023. Int J Gynecol Cancer 2023;33:649–66. 10.1136/ijgc-2023-004429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Concin N, Matias-Guiu X, Vergote I, et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021;31:12–39. 10.1136/ijgc-2020-002230 [DOI] [PubMed] [Google Scholar]
- 13. Timmerman D, Planchamp F, Bourne T, et al. ESGO/ISUOG/IOTA/ESGE consensus statement on pre-operative diagnosis of ovarian tumors. Int J Gynecol Cancer 2021;31:961–82. 10.1136/ijgc-2021-002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Colombo N, Sessa C, du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer 2019:ijgc-2019-000308. 10.1136/ijgc-2019-000308 [DOI] [PubMed] [Google Scholar]
- 15. Testa AC, Ludovisi M, Mascilini F, et al. Ultrasound evaluation of intra-abdominal sites of disease to predict likelihood of suboptimal cytoreduction in advanced ovarian cancer: a prospective study. Ultrasound Obstet Gynecol 2012;39:99–105. 10.1002/uog.10100 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/39/1 [DOI] [PubMed] [Google Scholar]
- 16. Fischerova D, Zikan M, Semeradova I, et al. Ultrasound in preoperative assessment of pelvic and abdominal spread in patients with ovarian cancer: a prospective study. Ultrasound Obstet Gynecol 2017;49:263–74. 10.1002/uog.15942 Available: http://doi.wiley.com/10.1002/uog.2017.49.issue-2 [DOI] [PubMed] [Google Scholar]
- 17. Fischerova D, Pinto P, Burgetova A, et al. Preoperative staging of ovarian cancer: comparison between ultrasound, CT and whole-body diffusion-weighted MRI (ISAAC study). Ultrasound Obstet Gynecol 2022;59:248–62. [DOI] [PubMed] [Google Scholar]
- 18. Fischerova D, Garganese G, Reina H, et al. Terms, definitions and measurements to describe sonographic features of lymph nodes: consensus opinion from the Vulvar International Tumor Analysis (VITA) group . Ultrasound Obstet Gynecol 2021;57:861–79. 10.1002/uog.23617 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/57/6 [DOI] [PubMed] [Google Scholar]
- 19. Oonk MHM, Planchamp F, Baldwin P, et al. European society of gynaecological oncology guidelines for the management of patients with Vulvar cancer. Int J Gynecol Cancer 2017;27:832–7. 10.1097/IGC.0000000000000975 [DOI] [PubMed] [Google Scholar]
- 20. Pinto P, Borcinova M, Wiesnerova M, et al. Op12.05: patient satisfaction with ultrasound, CT and WB-DWI/MRI for preoperative ovarian cancer staging: a multicentre prospective survey. Ultrasound Obstet Gynecol 2023;62:78–9. 10.1002/uog.26546 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/62/S1 [DOI] [Google Scholar]
- 21. Saini S, Seltzer SE, Bramson RT, et al. Technical cost of radiologic examinations: analysis across imaging modalities. Radiology 2000;216:269–72. 10.1148/radiology.216.1.r00jl18269 [DOI] [PubMed] [Google Scholar]
- 22. Fischerova D, Cibula D, Dundr P, et al. Ultrasound-guided tru-cut biopsy in the management of advanced abdomino-pelvic tumors. Int J Gynecol Cancer 2008;18:833–7. 10.1111/j.1525-1438.2007.01015.x [DOI] [PubMed] [Google Scholar]
- 23. Zikan M, Fischerova D, Pinkavova I, et al. Ultrasound-guided tru-cut biopsy of abdominal and pelvic tumors in gynecology. Ultrasound Obstet Gynecol 2010;36:767–72. 10.1002/uog.8803 [DOI] [PubMed] [Google Scholar]
- 24. Mascilini F, Moro F, De Leo R, et al. Intraoperative ultrasound assistance for the surgical removal of lost Intrauterine device. Ultrasound Obstet Gynecol 2019;53:705–6. 10.1002/uog.19167 [DOI] [PubMed] [Google Scholar]
- 25. Pinkavova I, Dundr P, Fischerova D, et al. Intra-operative ultrasound in fertility sparing procedure for cervical cancer (Oc24.04). Ultrasound Obstet Gynecol 2012;40:51. 10.1002/uog.11379 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/40/S1 [DOI] [Google Scholar]
- 26. Bierig SM, Jones A. Accuracy and cost comparison of ultrasound versus alternative imaging modalities, including CT, MR, PET, and angiography. J Diagnost Med Sonog 2009;25:138–44. 10.1177/8756479309336240 [DOI] [Google Scholar]
- 27. Eriksson LSE, Lindqvist PG, Flöter Rådestad A, et al. Transvaginal ultrasound assessment of myometrial and cervical stromal invasion in women with endometrial cancer: interobserver reproducibility among ultrasound experts and gynecologists. Ultrasound Obstet Gynecol 2015;45:476–82. 10.1002/uog.14645 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/45/4 [DOI] [PubMed] [Google Scholar]
- 28. Pálsdóttir K, Fridsten S, Blomqvist L, et al. Interobserver agreement of transvaginal ultrasound and magnetic resonance imaging in local staging of cervical cancer. Ultrasound Obstet Gynecol 2021;58:773–9. 10.1002/uog.23662 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/58/5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fischerová D, Pesta M, Blazko M, et al. Oc12.08: observer-reality agreement for tumour spread and prediction non-resectability in ovarian cancer (ISAAC study, imaging study on advanced ovarian cancer). Ultrasound Obstet Gynecol 2023;62:29–30. 10.1002/uog.26402 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/62/S1 [DOI] [Google Scholar]
- 30. Olawaiye AB, Cuello MA, Rogers LJ. Cancer of the vulva: 2021 update. Int J Gynaecol Obstet 2021;155:7–18. 10.1002/ijgo.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nikolić O, Sousa FAE, Cunha TM, et al. Vulvar cancer staging: guidelines of the European society of Urogenital Radiology (ESUR). Insights Imaging 2021;12:131. 10.1186/s13244-021-01075-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rogers LJ, Cuello MA. Cancer of the vulva. Int J Gynaecol Obstet 2018;143 Suppl 2:4–13. 10.1002/ijgo.12609 [DOI] [PubMed] [Google Scholar]
- 33. Zapardiel I, Iacoponi S, Coronado PJ, et al. Prognostic factors in patients with vulvar cancer: the VULCAN study. Int J Gynecol Cancer 2020;30:1285–91. 10.1136/ijgc-2019-000526 [DOI] [PubMed] [Google Scholar]
- 34. Iacoponi S, Zapardiel I, Diestro MD, et al. Prognostic factors associated with local recurrence in squamous cell carcinoma of the vulva. J Gynecol Oncol 2013;24:242–8. 10.3802/jgo.2013.24.3.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olawaiye AB, Cotler J, Cuello MA, et al. FIGO staging for carcinoma of the vulva: 2021 revision. Int J Gynaecol Obstet 2021;155:43–7. 10.1002/ijgo.13880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gibb RK, Olawaiye AB, Bhosale PR, et al. Vulva. In: Amin MB, ed. TNM Classification of Malignant Tumours. Switzerland: Springer International Publishing AG Switzerland, 2016. [Google Scholar]
- 37. de Gregorio N, Ebner F, Schwentner L, et al. The role of preoperative ultrasound evaluation of Inguinal lymph nodes in patients with vulvar malignancy. Gynecol Oncol 2013;131:113–7. 10.1016/j.ygyno.2013.07.103 [DOI] [PubMed] [Google Scholar]
- 38. Verri D, Moro F, Fragomeni SM, et al. The role of ultrasound in the evaluation of Inguinal lymph nodes in patients with vulvar cancer: a systematic review and meta-analysis. Cancers (Basel) 2022;14:3082. 10.3390/cancers14133082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Virarkar M, Vulasala SS, Daoud T, et al. Vulvar cancer: 2021 revised FIGO staging system and the role of imaging. Cancers (Basel) 2022;14:2264. 10.3390/cancers14092264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Serrado MA, Horta M, Cunha TM. State of the art in Vulvar cancer imaging. Radiol Bras 2019;52:316–24. 10.1590/0100-3984.2018.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Angelico G, Santoro A, Inzani F, et al. Ultrasound-guided FNA cytology of groin lymph nodes improves the management of squamous cell carcinoma of the vulva: results from a comparative cytohistological study. Cancer Cytopathol 2019;127:514–20. 10.1002/cncy.22154 [DOI] [PubMed] [Google Scholar]
- 42. Bipat S, Fransen GA, Spijkerboer AM, et al. Is there a role for magnetic resonance imaging in the evaluation of inguinal lymph node metastases in patients with vulva carcinoma Gynecol Oncol 2006;103:1001–6. 10.1016/j.ygyno.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 43. Hawnaur JM, Reynolds K, Wilson G, et al. Identification of Inguinal lymph node metastases from vulval carcinoma by magnetic resonance imaging: an initial report. Clin Radiol 2002;57:995–1000. 10.1053/crad.2002.1057 [DOI] [PubMed] [Google Scholar]
- 44. Kataoka MY, Sala E, Baldwin P, et al. The accuracy of magnetic resonance imaging in staging of vulvar cancer: a retrospective multi-centre study. Gynecol Oncol 2010;117:82–7. 10.1016/j.ygyno.2009.12.017 [DOI] [PubMed] [Google Scholar]
- 45. Singh K, Orakwue CO, Honest H, et al. Accuracy of magnetic resonance imaging of Inguinofemoral lymph nodes in vulval cancer. Int J Gynecol Cancer 2006;16:1179–83. 10.1111/j.1525-1438.2006.00456.x [DOI] [PubMed] [Google Scholar]
- 46. Sohaib SAA, Richards PS, Ind T, et al. MR imaging of carcinoma of the vulva. AJR Am J Roentgenol 2002;178:373–7. 10.2214/ajr.178.2.1780373 [DOI] [PubMed] [Google Scholar]
- 47. Rufini V, Garganese G, Ieria FP, et al. Diagnostic performance of preoperative [18F]FDG-PET/CT for lymph node staging in vulvar cancer: a large single-centre study. Eur J Nucl Med Mol Imaging 2021;48:3303–14. 10.1007/s00259-021-05257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lakhman Y, Vargas HA, Reinhold C, et al. ACR appropriateness criteria® staging and follow-up of vulvar cancer. J Am Coll Radiol 2021;18:S212–28. 10.1016/j.jacr.2021.02.016 [DOI] [PubMed] [Google Scholar]
- 49. Sung H, Ferlay J, Siegel RL, et al. Global cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 50. Board WCoTE . Female genital tumours: International agency for research on cancer. 2020.
- 51. Adams TS, Rogers LJ, Cuello MA. Cancer of the vagina: 2021 update. Int J Gynaecol Obstet 2021;155:19–27. 10.1002/ijgo.13867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Beller U, Benedet JL, Creasman WT, et al. Carcinoma of the vagina. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet 2006;95 Suppl 1:S29–42. 10.1016/S0020-7292(06)60029-5 [DOI] [PubMed] [Google Scholar]
- 53. Gibb RK, Olowaiye AB, Bosale PR, et al. Vagina. In: Amin MB, ed. AJCC Cancer Staging Manual. Switzerland: Springer International Publishing AG Switzerland, 2016. [Google Scholar]
- 54. Wolfson AH, Reis IM, Portelance L, et al. Prognostic impact of clinical tumor size on overall survival for subclassifying stages I and II vaginal cancer: a SEER analysis. Gynecol Oncol 2016;141:255–9. 10.1016/j.ygyno.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 55. Yang J, Delara R, Magrina J, et al. Management and outcomes of primary vaginal cancer. Gynecol Oncol 2020;159:456–63. 10.1016/j.ygyno.2020.08.036 [DOI] [PubMed] [Google Scholar]
- 56. Prameela CG, Ravind R, Gurram BC, et al. Prognostic factors in primary vaginal cancer: a single Institute experience and review of literature. J Obstet Gynaecol India 2016;66:363–71. 10.1007/s13224-015-0697-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rajaram S, Maheshwari A, Srivastava A. Staging for vaginal cancer. Best Pract Res Clin Obstet Gynaecol 2015;29:822–32. 10.1016/j.bpobgyn.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 58. Frumovitz M, Gayed IW, Jhingran A, et al. Lymphatic mapping and sentinel lymph node detection in women with vaginal cancer. Gynecol Oncol 2008;108:478–81. 10.1016/j.ygyno.2007.12.001 [DOI] [PubMed] [Google Scholar]
- 59. Gardner CS, Sunil J, Klopp AH, et al. Primary vaginal cancer: role of MRI in diagnosis, staging and treatment. Br J Radiol 2015;88:20150033. 10.1259/bjr.20150033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lamoreaux WT, Grigsby PW, Dehdashti F, et al. FDG-PET evaluation of vaginal carcinoma. Int J Radiat Oncol Biol Phys 2005;62:733–7. 10.1016/j.ijrobp.2004.12.011 [DOI] [PubMed] [Google Scholar]
- 61. Nout R, Calaminus G, Planchamp F, et al. ESTRO/ESGO/SIOPe guidelines for the management of patients with vaginal cancer. Radiother Oncol 2023;186:109662. 10.1016/j.radonc.2023.109662 [DOI] [PubMed] [Google Scholar]
- 62. Olawaiye AB, Baker TP, Washington MK, et al. The new (version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J Clin 2021;71:287–98. 10.3322/caac.21663 [DOI] [PubMed] [Google Scholar]
- 63. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019;145:129–35. 10.1002/ijgo.12749 [DOI] [PubMed] [Google Scholar]
- 64. Lura N, Wagner-Larsen KS, Forsse D, et al. What MRI-based tumor size measurement is best for predicting long-term survival in uterine cervical cancer Insights Imaging 2022;13:105. 10.1186/s13244-022-01239-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wagner-Larsen KS, Lura N, Salvesen Ø, et al. Interobserver agreement and prognostic impact for MRI-based 2018 FIGO staging parameters in uterine cervical cancer. Eur Radiol 2022;32:6444–55. 10.1007/s00330-022-08666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mitchell DG, Snyder B, Coakley F, et al. Early invasive cervical cancer: tumor delineation by magnetic resonance imaging, computed tomography, and clinical examination, verified by pathologic results, in the ACRIN 6651/GOG 183 Intergroup study. J Clin Oncol 2006;24:5687–94. 10.1200/JCO.2006.07.4799 [DOI] [PubMed] [Google Scholar]
- 67. Epstein E, Testa A, Gaurilcikas A, et al. Early-stage cervical cancer: tumor delineation by magnetic resonance imaging and ultrasound - a European multicenter trial. Gynecol Oncol 2013;128:449–53. 10.1016/j.ygyno.2012.09.025 [DOI] [PubMed] [Google Scholar]
- 68. Alcazar JL, García E, Machuca M, et al. Magnetic resonance imaging and ultrasound for assessing parametrial infiltration in cervical cancer. A systematic review and meta-analysis. Med Ultrason 2020;22:85–91. 10.11152/mu-2361 [DOI] [PubMed] [Google Scholar]
- 69. Jurado M, Galván R, Martinez-Monge R, et al. Neoangiogenesis in early cervical cancer: correlation between color Doppler findings and risk factors. A prospective observational study. World J Surg Oncol 2008;6:126. 10.1186/1477-7819-6-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Testa AC, Di Legge A, De Blasis I, et al. Imaging techniques for the evaluation of cervical cancer. Best Pract Res Clin Obstet Gynaecol 2014;28:741–68. 10.1016/j.bpobgyn.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 71. Qin J, Cheng X, Chen X, et al. Value of three-dimensional power Doppler to predict clinical and histological response to neoadjuvant chemotherapy in locally advanced cervical carcinoma. Ultrasound Obstet Gynecol 2012;39:226–34. 10.1002/uog.10071 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/39/2 [DOI] [PubMed] [Google Scholar]
- 72. Alcazar JL, Arribas S, Martinez-Monge R, et al. Three-dimensional power Doppler ultrasound for predicting response and local recurrence after concomitant chemoradiation therapy for locally advanced carcinoma of the cervix. Int J Gynecol Cancer 2016;26:534–8. 10.1097/IGC.0000000000000641 [DOI] [PubMed] [Google Scholar]
- 73. Csutak C, Badea R, Bolboaca SD, et al. Multimodal endocavitary ultrasound versus MRI and clinical findings in pre- and post-treatment advanced cervical cancer. Med Ultrason 2016;18:75–81. 10.11152/mu.2013.2066.181.csk [DOI] [PubMed] [Google Scholar]
- 74. Fischerova D, Zikan M, Pinkavova I, et al. The role of ultrasound in planning fertility sparing surgery and individual treatment in early stage cervical cancer. Ultrasound Obstet Gynecol 2012;40:51. 10.1002/uog.11380 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/40/S1 [DOI] [Google Scholar]
- 75. Pálsdóttir K, Fischerova D, Franchi D, et al. Preoperative prediction of lymph node metastasis and deep stromal invasion in women with invasive cervical cancer: prospective multicenter study using 2D and 3D ultrasound. Ultrasound Obstet Gynecol 2015;45:470–5. 10.1002/uog.14643 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/45/4 [DOI] [PubMed] [Google Scholar]
- 76. Cibula D, Kocian R, Plaikner A, et al. Sentinel lymph node mapping and intraoperative assessment in a prospective, International, multicentre, observational trial of patients with cervical cancer: the SENTIX trial. Eur J Cancer 2020;137:69–80. 10.1016/j.ejca.2020.06.034 [DOI] [PubMed] [Google Scholar]
- 77. ClinicalTrials.gov NCT05573451 - General University Hospital P, Charles University CR . Comparison of the Accuracy of US, MRI and PET/CT in the Assessment of LNs in Cervical Cancer. 2023. [Google Scholar]
- 78. Cibula D, Abu-Rustum NR, Dusek L, et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol Oncol 2012;124:496–501. 10.1016/j.ygyno.2011.11.037 [DOI] [PubMed] [Google Scholar]
- 79. Cibula D, Pötter R, Planchamp F, et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Int J Gynecol Cancer 2018;28:641–55. 10.1097/IGC.0000000000001216 [DOI] [PubMed] [Google Scholar]
- 80. Cibula D, Jarkovsky J, Kocian R, et al. Magnetic resonance or expert ultrasound in preoperative local staging of patients with early-stage cervical cancer: final results of the SENTIX prospective, single-arm, international trial (CEEGOG CX-01; ENGOT-Cx2). Int J Gynecol Cancer 2023;33:A2. [Google Scholar]
- 81. Stålberg K, Kjølhede P, Bjurberg M, et al. Risk factors for lymph node metastases in women with endometrial cancer: a population-based, nation-wide register study-on behalf of the Swedish Gynecological Cancer Group. Int J Cancer 2017;140:2693–700. 10.1002/ijc.30707 [DOI] [PubMed] [Google Scholar]
- 82. Koskas M, Amant F, Mirza MR, et al. Cancer of the corpus uteri: 2021 update. Int J Gynecol Obstet 2021;155:45–60. 10.1002/ijgo.13866 Available: https://obgyn.onlinelibrary.wiley.com/toc/18793479/155/S1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Berek JS, Matias-Guiu X, Creutzberg C, et al. FIGO staging of endometrial cancer: 2023. Int J Gynaecol Obstet 2023;162:383–94. 10.1002/ijgo.14923 [DOI] [PubMed] [Google Scholar]
- 84. Paño B, Sebastià C, Ripoll E, et al. Pathways of lymphatic spread in gynecologic malignancies. Radiographics 2015;35:916–45. 10.1148/rg.2015140086 [DOI] [PubMed] [Google Scholar]
- 85. The Cancer Genome Atlas Research Network, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature 2013;497:67–73. 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. León-Castillo A, de Boer SM, Powell ME, et al. Molecular classification of the PORTEC-3 trial for high-risk endometrial cancer: impact on prognosis and benefit from adjuvant therapy. J Clin Oncol 2020;38:3388–97. 10.1200/JCO.20.00549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Stewart CJR, Crum CP, McCluggage WG, et al. Guidelines to aid in the distinction of endometrial and endocervical carcinomas, and the distinction of independent primary carcinomas of the endometrium and adnexa from metastatic spread between these and other sites. Int J Gynecol Pathol 2019;38:S75–92. 10.1097/PGP.0000000000000553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Valentin L. Ultrasound deserves to play a prominent role in the diagnosis and management of endometrial cancer. Ultrasound Obstet Gynecol 2014;43:483–7. 10.1002/uog.13371 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/43/5 [DOI] [PubMed] [Google Scholar]
- 89. Leone FPG, Timmerman D, Bourne T, et al. Terms, definitions and measurements to describe the sonographic features of the endometrium and Intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound Obstet Gynecol 2010;35:103–12. 10.1002/uog.7487 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/35/1 [DOI] [PubMed] [Google Scholar]
- 90. Van Den Bosch T, Verbakel JY, Valentin L, et al. Typical ultrasound features of various endometrial pathologies described using International endometrial tumor analysis (IETA) terminology in women with abnormal uterine bleeding . Ultrasound Obstet Gynecol 2021;57:164–72. 10.1002/uog.22109 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/57/1 [DOI] [PubMed] [Google Scholar]
- 91. Di Spiezio Sardo A, Saccone G, Carugno J, et al. Endometrial biopsy under direct hysteroscopic visualisation versus blind endometrial sampling for the diagnosis of endometrial hyperplasia and cancer: systematic review and meta-analysis. Facts Views Vis Obgyn 2022;14:103–10. 10.52054/FVVO.14.2.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Hanegem N, Prins MMC, Bongers MY, et al. The accuracy of endometrial sampling in women with postmenopausal bleeding: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2016;197:147–55. 10.1016/j.ejogrb.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 93. Rodolakis A, Scambia G, Planchamp F, et al. ESGO/ESHRE/ESGE guidelines for the fertility-sparing treatment of patients with endometrial carcinoma Hum Reprod Open 2023;2023:hoac057. 10.1093/hropen/hoac057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Green RW, Heremans R, Fischerová D, et al. Oc19.01 prediction of outcome in endometrial cancer improved by combining traditional with sonographic and demographic parameters: IETA4 cohort follow-up. Ultrasound Obstet Gynecol 2023;62:43–4. 10.1002/uog.26440 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/62/S1 [DOI] [Google Scholar]
- 95. Epstein E, Blomqvist L. Imaging in endometrial cancer. Best Pract Res Clin Obstet Gynaecol 2014;28:721–39. 10.1016/j.bpobgyn.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 96. Fischerova D, Frühauf F, Zikan M, et al. Factors affecting sonographic preoperative local staging of endometrial cancer. Ultrasound Obstet Gynecol 2014;43:575–85. 10.1002/uog.13248 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/43/5 [DOI] [PubMed] [Google Scholar]
- 97. Alcazar JL, Carazo P, Pegenaute L, et al. Preoperative assessment of cervical involvement in endometrial cancer by transvaginal ultrasound and magnetic resonance imaging: a systematic review and meta-analysis. Ultraschall Med 2023;44:280–9. 10.1055/a-1408-2292 [DOI] [PubMed] [Google Scholar]
- 98. Alcázar JL, Gastón B, Navarro B, et al. Transvaginal ultrasound versus magnetic resonance imaging for preoperative assessment of myometrial infiltration in patients with endometrial cancer: a systematic review and meta-analysis. J Gynecol Oncol 2017;28:e86. 10.3802/jgo.2017.28.e86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Christensen JW, Dueholm M, Hansen ES, et al. Assessment of myometrial invasion in endometrial cancer using three-dimensional ultrasound and magnetic resonance imaging. Acta Obstet Gynecol Scand 2016;95:55–64. 10.1111/aogs.12806 [DOI] [PubMed] [Google Scholar]
- 100. Wong M, Amin T, Thanatsis N, et al. A prospective comparison of the diagnostic accuracies of ultrasound and magnetic resonance imaging in preoperative staging of endometrial cancer. J Gynecol Oncol 2022;33:e22. 10.3802/jgo.2022.33.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Palmér M, Åkesson Å, Marcickiewicz J, et al. Accuracy of transvaginal ultrasound versus MRI in the preoperative diagnostics of low-grade endometrial cancer (PODEC) study: a prospective multicentre study. Clin Radiol 2023;78:70–9. 10.1016/j.crad.2022.09.118 [DOI] [PubMed] [Google Scholar]
- 102. Antonsen SL, Jensen LN, Loft A, et al. MRI, PET/CT and ultrasound in the preoperative staging of endometrial cancer - a multicenter prospective comparative study. Gynecol Oncol 2013;128:300–8. 10.1016/j.ygyno.2012.11.025 [DOI] [PubMed] [Google Scholar]
- 103. Rodríguez-Trujillo A, Martínez-Serrano MJ, Martínez-Román S, et al. Preoperative assessment of myometrial invasion in endometrial cancer by 3D ultrasound and diffusion-weighted magnetic resonance imaging: a comparative study. Int J Gynecol Cancer 2016;26:1105–10. 10.1097/IGC.0000000000000724 [DOI] [PubMed] [Google Scholar]
- 104. Haldorsen IS, Salvesen HB. What is the best preoperative imaging for endometrial cancer? Curr Oncol Rep 2016;18:25. 10.1007/s11912-016-0506-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Mariani A, Webb MJ, Keeney GL, et al. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary Am J Obstet Gynecol 2000;182:1506–19. 10.1067/mob.2000.107335 [DOI] [PubMed] [Google Scholar]
- 106. Ytre-Hauge S, Husby JA, Magnussen IJ, et al. Preoperative tumor size at MRI predicts deep myometrial invasion, lymph node metastases, and patient outcome in endometrial carcinomas. Int J Gynecol Cancer 2015;25:459–66. 10.1097/IGC.0000000000000367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dybvik JA, Fasmer KE, Ytre-Hauge S, et al. MRI-assessed tumor-free distance to serosa predicts deep myometrial invasion and poor outcome in endometrial cancer. Insights Imaging 2022;13:1. 10.1186/s13244-021-01133-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Verbakel JY, Mascilini F, Wynants L, et al. Validation of ultrasound strategies to assess tumor extension and to predict high-risk endometrial cancer in women from the prospective IETA (International Wndometrial Tumor Analysis)-4 cohort. Ultrasound Obstet Gynecol 2020;55:115–24. 10.1002/uog.20374 [DOI] [PubMed] [Google Scholar]
- 109. Fasmer KE, Gulati A, Dybvik JA, et al. Preoperative pelvic MRI and 2-[(18)F]FDG PET/CT for lymph node staging and prognostication in endometrial cancer-time to revisit current imaging guidelines? Eur Radiol 2023;33:221–32. 10.1007/s00330-022-08949-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hu J, Zhang K, Yan Y, et al. Diagnostic accuracy of preoperative (18)F-FDG PET or PET/CT in detecting pelvic and para-aortic lymph node metastasis in patients with endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet 2019;300:519–29. 10.1007/s00404-019-05207-8 [DOI] [PubMed] [Google Scholar]
- 111. Bollineni VR, Ytre-Hauge S, Bollineni-Balabay O, et al. High diagnostic value of 18F-FDG PET/CT in endometrial cancer: systematic review and meta-analysis of the literature. J Nucl Med 2016;57:879–85. 10.2967/jnumed.115.170597 [DOI] [PubMed] [Google Scholar]
- 112. Raimond E, Ballester M, Hudry D, et al. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: results of a retrospective multicenter study. Gynecol Oncol 2014;133:506–11. 10.1016/j.ygyno.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 113. Prat J, Olowaiye AB, Bermudez A, et al. Ovary, Fallopian tube, and primary peritoneal carcinoma. In: Amin MB, ed. AJCC Cancer Staging Manual. Switzerland: Springer International Publishing AG Switzerland, 2016. [Google Scholar]
- 114. Prat J, FIGO Committee on Gynecologic Oncology . Staging classification for cancer of the ovary, Fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014;124:1–5. 10.1016/j.ijgo.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 115. Woo YL, Kyrgiou M, Bryant A, et al. Centralisation of services for gynaecological cancers - a Cochrane systematic review. Gynecol Oncol 2012;126:286–90. 10.1016/j.ygyno.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 116. Timmerman D, Van Calster B, Testa A, et al. Predicting the risk of malignancy in adnexal masses based on the simple rules from the International Ovarian Tumor Analysis Group. Am J Obstet Gynecol 2016;214:424–37. 10.1016/j.ajog.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 117. Landolfo C, Bourne T, Froyman W, et al. Benign descriptors and adnex in two-step strategy to estimate risk of malignancy in ovarian tumors: retrospective validation on IOTA 5 multicenter cohort. Ultrasound Obstet Gynecol 2023;61:231–42. 10.1002/uog.26080 Available: https://obgyn.onlinelibrary.wiley.com/toc/14690705/61/2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Timmerman D, Valentin L, Bourne TH, et al. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) group. Ultrasound Obstet Gynecol 2000;16:500–5. 10.1046/j.1469-0705.2000.00287.x [DOI] [PubMed] [Google Scholar]
- 119. Pinto P, Burgetova A, Cibula D, et al. Prediction of surgical outcome in advanced ovarian cancer by imaging and laparoscopy: a narrative review. Cancers (Basel) 2023;15:1904. 10.3390/cancers15061904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Moruzzi MC, Bolomini G, Esposito R, et al. Diagnostic performance of ultrasound in assessing the extension of disease in advanced ovarian cancer. Am J Obstet Gynecol 2022;227:601. 10.1016/j.ajog.2022.05.029 [DOI] [PubMed] [Google Scholar]
- 121. Alcázar JL, Caparros M, Arraiza M, et al. Pre-operative assessment of intra-abdominal disease spread in epithelial ovarian cancer: a comparative study between ultrasound and computed tomography. Int J Gynecol Cancer 2019;29:227–33. 10.1136/ijgc-2018-000066 [DOI] [PubMed] [Google Scholar]
- 122. Vargas HA, Huang EP, Lakhman Y, et al. Radiogenomics of high-grade serous ovarian cancer: multireader multi-institutional study from the cancer genome Atlas ovarian cancer imaging research group. Radiology 2017;285:482–92. 10.1148/radiol.2017161870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Michielsen K, Dresen R, Vanslembrouck R, et al. Diagnostic value of whole body diffusion-weighted MRI compared to computed tomography for pre-operative assessment of patients suspected for ovarian cancer. Eur J Cancer 2017;83:88–98. 10.1016/j.ejca.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 124. Kumar Dhingra V, Kand P, Basu S. Impact of FDG-PET and -PET/CT imaging in the clinical decision-making of ovarian carcinoma: an evidence-based approach. Womens Health (Lond) 2012;8:191–203. 10.2217/whe.11.91 [DOI] [PubMed] [Google Scholar]
- 125. De Iaco P, Musto A, Orazi L, et al. FDG-PET/CT in advanced ovarian cancer staging: value and pitfalls in detecting lesions in different abdominal and pelvic quadrants compared with laparoscopy. Eur J Radiol 2011;80:e98–103. 10.1016/j.ejrad.2010.07.013 [DOI] [PubMed] [Google Scholar]
- 126. Pinto P, Chiappa V, Alcazar JL, et al. 2022-RA-891-ESGO preoperative assessment of non-resectability in patients with ovarian cancer using imaging (ISAAC study) – an interim analysis. ESGO 2022 Congress 2022. 10.1136/ijgc-2022-ESGO.584 [DOI] [Google Scholar]
- 127. Fischerova D, Burgetova A. Imaging techniques for the evaluation of ovarian cancer. Best Pract Res Clin Obstet Gynaecol 2014;28:697–720. 10.1016/j.bpobgyn.2014.04.006 [DOI] [PubMed] [Google Scholar]
- 128. Fischerova D, Cibula D. Ultrasound in gynecological cancer: is it time for re-evaluation of its uses Curr Oncol Rep 2015;17:28. 10.1007/s11912-015-0449-x [DOI] [PubMed] [Google Scholar]
- 129. González-Martín A, Harter P, Leary A, et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol 2023;34:833–48. 10.1016/j.annonc.2023.07.011 [DOI] [PubMed] [Google Scholar]
- 130. Harter P, Sehouli J, Lorusso D, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med 2019;380:822–32. 10.1056/NEJMoa1808424 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ijgc-2023-004609supp001.pdf (151.8KB, pdf)
ijgc-2023-004609supp002.pdf (7MB, pdf)
ijgc-2023-004609supp003.pdf (8.3MB, pdf)
ijgc-2023-004609supp005.pdf (17.9MB, pdf)
ijgc-2023-004609supp004.pdf (17.7MB, pdf)
ijgc-2023-004609supp006.pdf (7.7MB, pdf)
ijgc-2023-004609supp007.mp4 (76.2MB, mp4)
ijgc-2023-004609supp008.mp4 (120.3MB, mp4)
ijgc-2023-004609supp010.mp4 (29.6MB, mp4)
ijgc-2023-004609supp011.mp4 (36.3MB, mp4)
ijgc-2023-004609supp012.mp4 (90.5MB, mp4)
ijgc-2023-004609supp013.mp4 (91.2MB, mp4)
ijgc-2023-004609supp009.mp4 (39.4MB, mp4)