Abstract
White matter hyperintensity (WMH) lesions on brain MRI images are surrogate markers of cerebral small vessel disease. Longitudinal studies examining the association between diabetes and WMH progression have yielded mixed results. Thus, in this study, we investigated the association between HbA1c, a biomarker for the presence and severity of hyperglycemia, and longitudinal WMH change after adjusting for known risk factors for WMH progression. We recruited 64 participants from South Korean memory clinics to undergo brain MRI at the baseline and a 2-year follow-up. We found the following. First, higher HbA1c was associated with greater global WMH volume (WMHV) changes after adjusting for known risk factors (β = 7.7 × 10−4; P = 0.025). Second, the association between baseline WMHV and WMHV progression was only significant at diabetic levels of HbA1c (P < 0.05, when HbA1c >6.51%), and non–apolipoprotein E (APOE) ε4 carriers had a stronger association between HbA1c and WMHV progression (β = −2.59 × 10−3; P = 0.004). Third, associations of WMHV progression with HbA1c were particularly apparent for deep WMHV change (β = 7.17 × 10−4; P < 0.01) compared with periventricular WMHV change and, for frontal (β = 5.00 × 10−4; P < 0.001) and parietal (β = 1.53 × 10−4; P < 0.05) lobes, WMHV change compared with occipital and temporal WMHV change. In conclusion, higher HbA1c levels were associated with greater 2-year WMHV progression, especially in non–APOE ε4 participants or those with diabetic levels of HbA1c. These findings demonstrate that diabetes may potentially exacerbate cerebrovascular and white matter disease.
Article Highlights
How diabetes contributes to cerebral small vessel disease and dementia has not yet been fully clarified.
We investigated the association between HbA1c, a biomarker for the presence and severity of hyperglycemia, and longitudinal white matter hyperintensity (WMH) change after adjusting for known risk factors for WMH progression.
Higher HbA1c levels were associated with greater 2-year WMH volume progression.
Introduction
Cerebral small vessel disease (CSVD) is a leading cause of cognitive decline, functional loss, and disability in older adults. White matter hyperintensity (WMH) lesions on brain MRI are surrogate markers of CSVD. It is necessary to examine the factors associated with longitudinal WMH growth to better understand the disease mechanisms and inform the strategy to prevent and/or treat cognitive decline (1). Diabetes is a chronic macrovascular risk factor for white matter change (2). Diabetes can be a target for intervention because it may induce changes in vascular integrity and function and brain structure. However, not every person with diabetes has WMH, suggesting that WMH burden might also be related to other vascular factors and genetic variations. Vascular factors affecting WMH growth include thyroid function (3), hypertension (4), obesity (5), and amyloid burden (6). Apolipoprotein E (APOE) ε4 allele, the strongest genetic risk factor for Alzheimer disease (AD), also affects the pathomechanistic pathways of CSVD and likely shares common mechanisms with diabetes (7). However, how the relationship between diabetes and APOE contributes to CSVD and even dementia has not yet been fully clarified, despite some clinical observations (7–10).
Thus, it is necessary to examine the effects of diabetes on CSVD while considering other vascular and genetic factors to inform the precise approach for the prevention and treatment of dementia. In this study, we investigated the association between HbA1c, a glycemic status marker of diabetes, and longitudinal WMH changes after controlling for other cardiovascular factors. As an exploratory analysis, we also evaluated how this relationship could be affected by the APOE genotype and how it differed according to the baseline WMH lesions.
Research Design and Methods
Participants
This study was a part of the ongoing Biobank Innovations for Chronic Cerebrovascular Disease With Alzheimer's Disease Study (BICWALZS) and the Centre for Convergence Research of Neurological Disorders (Clinical Research Information Service identifier KCT0003391). More information on the BICWALZS can be found in the Supplementary Material. Participants from the BICWALZS were recruited at the memory clinics of Ajou University Hospital and Suwon Community Geriatric Centers in South Korea. The presence or absence of diabetes, hypertension, and hyperlipidemia were based on the clinical history of being treated under the diagnosis by a physician. Patients with a history of neurological or medical conditions, such as territorial cerebral infarction, intracranial hemorrhage, Parkinson disease, heart failure, renal failure, or other conditions that could interfere with the study, were excluded. Information on the methods for clinical diagnosis criteria, blood sampling and laboratory assessments, and APOE genotyping used for this study can be found in the Supplementary Material. We used data from 64 participants in the BICWALZS cohort, including brain MRI, APOE status, and blood laboratory assessments, including HbA1c.
Amyloid Positron Emission Tomography Acquisition and Measurement of Amyloid Deposition
The participants underwent the same protocol for 18F-flutemetamol positron emission tomography (PET) scanning using a Discovery STE/690 PET/CT scanner (GE, Milwaukee, WI).18F-flutemetamol was injected into the antecubital vein as a bolus (mean dose, 185 MBq). After 90 min, a 20-min PET scan (4 × 5-min dynamic frames) was performed. Information on the methods to quantify 18F-flutemetamol retention, based on the standard uptake value ratio (SUVR), can be found in the Supplementary Material.
MRI Data Acquisition and Processing for WMHs
Participants underwent MRI scanning on GE Discovery MR750w 3T scanners, including the following two sequences: a three-dimensional, magnetization-prepared, rapid gradient echo, T1-weighted sequence and a T2-weighted fluid-attenuated inversion recovery sequence. The MRI sequence parameters are listed in Supplementary Table 1. An automated method to segment WMH on T2-weighted fluid-attenuated inversion recovery images was used that was based on our previous studies (11,12). We generated regional cortical white matter masks for the frontal, parietal, occipital, and temporal lobes. We investigated additional models of regional WMHs using lobular cortical and deep/periventricular masks. The total WMH volume (WMHV) and regional WMHV were normalized by the intracranial volume and log-transformed for analysis. WMHV change was calculated as the difference between the normalized, log-transformed WMHV at follow-up and baseline. Extended information on the methods for automated WMH segmentation and generation of regional white matter masks can be found in the Supplementary Material.
Statistical Analysis
The relationship between HbA1c and WMHV changes was tested using linear regression models. Covariates included demographics (namely, age and sex), BMI, and cardiovascular risks (namely, systolic and diastolic blood pressures, LDL and HDL levels, and smoking status). Given the collinearity among blood pressure and lipid variables, one blood pressure variable and one lipid variable (selected on the basis of its association with WMHV change) were included in the models. Age, BMI, baseline WMHV, and HbA1c data were included in the models as covariates a priori. The main analysis included three models: main effects associated with diabetes plus baseline WMHV (model 1), main effects of diabetes plus baseline WMHV and cardiovascular risk factors (model 2), and an exploratory model examining covariates strongly associated with diabetes (model 3): APOE ɛ4 status, baseline WMHV, and their interaction effects on HbA1c. We then applied the Johnson–Neyman technique and generated a Johnson–Neyman plot to probe and visualize the conditional effect of HbA1c on WMHV change versus baseline WMHV (13). For regional WMHV change analysis, we analyzed model 1 and the exploratory model (diabetes main effects plus APOE ɛ4 status; baseline WMHV). Secondary analyses were conducted to consider the effect of thyroid-stimulating hormone, amyloid burden (global 18F-flutemetamol SUVR), and scanner site on WMHV change. Statistical analyses were performed using R (https://www.R-project.org).
Data and Resource Availability
The data sets generated during and/or analyzed in the present study are available from the corresponding author upon request.
Results
The characteristics of our study participants are listed in Table 1. Among the 64 participants, 73.02% were female. Participants’ average age was 72.13 years, and the average number of years of education was 8.20. Regarding underlying diseases, 50% had hypertension, 18.75% had diabetes mellitus (and all took diabetes medication), and 32.18% had hyperlipidemia. The proportion of participants with clinical diagnoses of mild cognitive impairment or dementia (AD or other) was 75.00% or 21.88%, respectively.
Table 1.
Participant characteristics
| Characteristic | Value* |
|---|---|
| N | 64 |
| Age at first scan, mean (SD), years | 72.13 (7.54) |
| Female sex, n (%) | 46 (73.02) |
| Years of education, mean (SD) | 8.20 (4.54) |
| BMI, mean (SD) | 23.90 (3.71) |
| Cardiovascular risk factors | |
| SBP, mean (SD), mmHg | 135.52 (22.05) |
| DBP, mean (SD), mmHg | 76.13 (11.28) |
| LDL-C, mean (SD), mg/dL | 89.63 (31.37) |
| HDL-C, mean (SD), mg/dL | 55.50 (14.60) |
| Smoking status, N (%) | 12 (18.75) |
| HbA1c, mean (SD), % mmol/mol | 5.91 (0.73) |
| TSH, mean (SD), mIU/L | 2.19 (1.53) |
| Global 18F-flutemetamol SUVR, mean (SD)§ | 0.69 (0.15) |
| APOE ɛ4 positive, n (%)‡ | 19 (29.69) |
| WMHV at baseline, mean (SD)† | −5.25 (0.96) |
| Change in WMHV, mean (SD)† | 1.97 × 10−3 (7.80 × 10−4) |
| Comorbidity, n (%) | |
| Hypertension | 32 (50.00) |
| Diabetes | 12 (18.75) |
| Hyperlipidemia | 21 (32.81) |
| Clinical diagnosis, n (%) | |
| Healthy | 0 (0) |
| SCD | 2 (3.13) |
| MCI | 48 (75) |
| AD or other dementia | 14 (21.88) |
DBP, diastolic blood pressure; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MCI, mild cognitive impairment; SBP, systolic blood pressure; SCD, subjective cognitive decline; TSH, thyroid-stimulating hormone.
As indicated in row heading.
18F-flutemetamol SUVR was available for 61 of 64 participants.
APOE ɛ4 positive: 2/4, 3/4, 4/4.
WMHV expressed as log(cm3/intracranial volume).
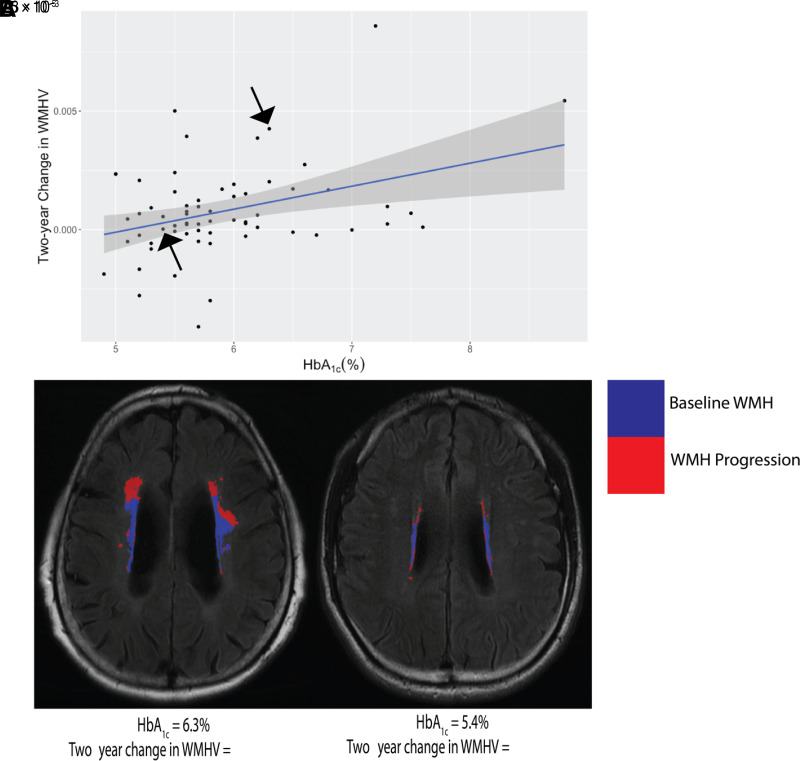
We tested the relationship between HbA1c levels and WMHV changes (Fig. 1). We first tested the relation between HbA1c and WMHV change in model 1 and observed that higher HbA1c was associated with larger WMHV change (model 1, P = 0.004) (Table 2). We then tested model 2 and found that HbA1c maintained a significant positive correlation with WMHV change (P = 0.0023). In our secondary analyses, we considered two models that tested diabetes’ main effects while controlling for thyroid-stimulating hormone, amyloid burden, or scanner site. In each model, we observed that HbA1c maintained a significant effect (P = 0.0013, 0.025, and 0.0042, respectively) (data not shown).
Figure 1.
A: HbA1c is significantly associated with 2-year WMHV progression. The two black arrows represent the two participants shown in (B). B: MRI scans of two individual participants. WMH from the first scan was registered and overlayed with the second scan and its WMH to display the WMH progression over 2 years. One participant had high HbA1c, WMHV, and WMHV change; and the other had low HbA1c, WMHV, and WMHV change. Shaded area represents the 95% CI.
Table 2.
Multiple linear regression analysis of associations of change in normalized WMHV with HbA1c
| Independent variable | Dependent variable: change in WMHV | ||
|---|---|---|---|
| β | SE | P value | |
| Model 1 (diabetes main effects)§ | |||
| HbA1c | 1.06 × 10−3 | 3.57 × 10−4 | 0.0042** |
| Model 2‡ | |||
| HbA1c | 1.10 × 10−3 | 3.46 × 10−4 | 0.0023** |
| Model 3† | |||
| HbA1c | 6.83 × 10−3 | 2.14 × 10−3 | 0.0024** |
| APOE ɛ4 positive | 1.42 × 10−2 | 4.90 × 10−3 | 0.0053** |
| WMHV at first scan | −6.04 × 10−3 | 2.4 × 10−3 | 0.015* |
| HbA1c APOEɛ4 positive* | −2.59 × 10−3 | 8.66 × 10−4 | 0.0042** |
| HbA1c WMHV at first scan* | 1.05 × 10−3 | 4.08 × 10−4 | 0.013** |
Model 1: adjusted for age at baseline, sex, BMI, and baseline WMHV. Adjusted R2 = 0.082.
Model 2: model 1 + cardiovascular risks (HDL, systolic blood pressure, and smoking status). Adjusted R2 = 0.17.
Model 3: model 1 + interaction effects between baseline WMHV, HbA1c and APOE ɛ4, HbA1c (exploratory model). Adjusted R2 = 01.19.
P < 0.05
P < 0.01.
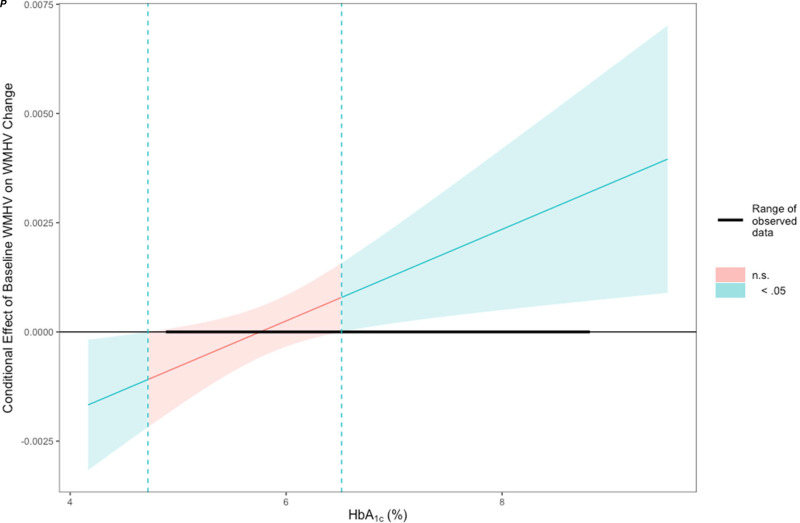
In model 3, our exploratory model examining HbA1c in relation to WMHV change, we tested for the main covariates associated with diabetes and HbA1c’s interaction effect with APOE ɛ4 status and baseline WMHV (Table 2). We observed a significant interaction between HbA1c and APOE ɛ4 status (P = 0.0042) and baseline WMHV (P = 0.013). APOE ɛ4 noncarriers had a stronger correlation between HbA1c and WMHV changes than did APOE ɛ4 carriers (Supplementary Fig. 1). The Johnson–Neyman analysis (Fig. 2) indicated that the association between WMHV change and baseline WMHV became stronger as HbA1c levels increased, becoming significant (P < 0.05) at an HbA1c of 6.51%.
Figure 2.
Baseline WMHV is significantly associated with WMHV progression but only as HbA1c approaches diabetic levels. The association between WMHV change and baseline WMHV becomes stronger as HbA1c level increases, becoming significant (P < 0.05) at an HbA1c of 6.51%. Shaded area represents the 95% CI.
Several additional analyses were conducted to observe how HbA1c spatially correlates with WMHV change (Supplementary Table 2) using model 1 and model 3. Deep WMHV change showed a significant, positive correlation in both models with HbA1c (model 1, P = 0.0024; model 3, P = 0.0004). Meanwhile, periventricular WMHV change was not significantly correlated with HbA1c in either model (model 1, P = 0.0721; model 3, P = 0.1511). In model 3, the interaction between HbA1c and APOE ɛ4 status was significant for both deep (P = 0.0037) and periventricular (P = 0.031) WMHV changes. The interaction between HbA1c and baseline WMHV was only significant for deep WMHV changes (P = 0.0026, in contrast to P = 0.3131 for periventricular changes). When testing the spatial relationship in each lobe, we observed that the frontal and parietal WMHV changes were significantly correlated with HbA1c and its interaction with APOE ɛ4 status and baseline WMHV, whereas no correlation or effects were observed in the occipital and temporal lobes.
Discussion
This study has three main findings. First, a higher HbA1c was associated with greater global WMHV expansion. This association persisted after adjusting for a range of covariates, including cardiovascular risk factors, thyroid health measures, and amyloid burden. Second, HbA1c had a significant interaction with the baseline WMHV and APOE ɛ4 allele status. The association of baseline WMHV with WMHV progression became significant only as HbA1c approached the glycemic level. In addition, APOE ɛ4 noncarriers had a stronger association between HbA1c and WMHV progression. Third, the associations between HbA1c and WMHV change were evident in deep WMH compared with periventricular WMH and were evident for the frontal and parietal WMHV change compared with the occipital and temporal WMHV change.
Our study emphasizes the importance of managing diabetic health to improve brain health outcomes. Several cross-sectional studies have reported an association between diabetes and WMHV (14–16); however, longitudinal studies investigating this association have yielded divergent findings (17–21). Our study agrees with the recent findings of a 6-year prospective study of an elderly cohort, in which researchers observed faster WMHV accumulation in prediabetes and diabetes (17). Furthermore, numerous studies have shown that a greater baseline WMHV was associated with greater WMHV progression, yet we observed that it was significant only as HbA1c approached the diabetic level (6.51%). We used a continuous measure of HbA1c as opposed to categorizing participants (healthy, prediabetic, and diabetic), yet our findings still align well with the traditional cutoff for clinical diabetes diagnosis (22). A greater WMHV is a surrogate for CSVD; thus, this interaction highlights how diabetes may exacerbate poor cerebrovascular health.
We also observed that the change in WMHV was more strongly associated with HbA1c in participants who did not carry APOE ɛ4 allele. The relationship between diabetes and the APOE genotype in contributing to CSVD and dementia has yet been clarified. In one study, APOE ɛ4 carriers were associated with long-term memory decline, a cognitive deficit strongly correlated with conversion to AD, whereas diabetes correlated with impairment of working memory and a weak correlation with conversion to AD. Diabetes did not exacerbate the risk of AD in APOE ε4 carriers (10). Another study showed that diabetes was associated with earlier deterioration of cognitive function and increased vascular pathology scores in APOE ɛ3 carriers but not in APOE ɛ4 carriers (7). Aligning with this study, we found that HbA1c had a more significant effect on CSVD progression in APOE ε4 noncarriers. Studies examining this mechanistic pathway are warranted.
This study also has some limitations. Our modest sample size (N = 64) might have not enabled us to display the full effects of each variable tested, particularly HbA1c’s effect, because there were a limited number of participants diagnosed with diabetes. Our participants were recruited from a clinical cohort with cognitive impairments and thus might not be representative of the general population. Additionally, other potential risk factors for WMHV progression and CSVD could interact on a different timescale; thus, a 2-year interval between MRI scans might have not been enough to display their effects. We did not have information on diabetes nor antihypertensive medications, which might have also affected the results. Although the Johnson–Neyman technique helps visualize the complexity of the nonlinear relationship that might occur between baseline WMHV and HbA1c on WMHV progression, the method could not capture the full extent. Finally, survival bias might have partially contributed to our findings.
In conclusion, our findings demonstrate the potential effects of hyperglycemia on cerebrovascular health. Future studies should examine how hyperglycemia affects other MRI biomarkers of CSVD and whether treatment of diabetic health can attenuate WMHV progression.
This article contains supplementary material online at https://doi.org/10.2337/figshare.24970290.
Article Information
Acknowledgments. The authors thank the staff of BICWALZS and the Suwon Geriatric Mental Health Centre for their involvement in this study.
Funding. This study was conducted using biospecimens and data from the consortium of the BICWALZS, which was funded by the Korea Disease Control and Prevention Agency for the Korea Biobank Project (grant 6637-303). This work was supported by the National Research Foundation of Korea, funded by the Ministry of Science and Information and Communication Technology (grant NRF-2019R1A5A2026045). This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HR22C1734).
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. N.S. contributed to study conception and design imaging, statistical analysis, and manuscript preparation. S.J.S. contributed to acquisition and verification of data, funding, and manuscript preparation. H.A. contributed to study conception and design as well as critical review. M.W. contributed to study conception and design as well as imaging analysis. S.Y. and B.I. contributed to manuscript preparation and critical review. C.H.H. contributed to funding and data acquisition. H.W.R. and J.Y.C. contributed to study coordination. B.P., N.-R.K., and Y.-S.A. contributed to acquisition and verification of data. J.W.C. contributed to acquisition of imaging data. S.W.S. and S.Y.M. contributed to acquisition of data. Y.H.C. contributed to data verification. S.J.H. contributed to critical review. M.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This study was conducted using biospecimens and data from the consortium of the BICWALZS, which was funded by the Korea Disease Control and Prevention Agency for the Korea Biobank Project (grant 6637-303). This work was supported by the National Research Foundation of Korea, funded by the Ministry of Science and Information and Communication Technology (grant NRF-2019R1A5A2026045). This research was also supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (HR22C1734).
Footnotes
Clinical trial reg. no. KCT0003391, https://cris.nih.go.kr/cris/index/index.do
N.S. and S.J.S. contributed equally to this work.
References
- 1. Pinter D, Enzinger C, Fazekas F.. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J Neurol 2015;262:2411–2419 [DOI] [PubMed] [Google Scholar]
- 2. Sanahuja J, Alonso N, Diez J, et al. Increased burden of cerebral small vessel disease in patients with type 2 diabetes and retinopathy. Diabetes Care 2016;39:1614–1620 [DOI] [PubMed] [Google Scholar]
- 3. Squizzato A, Gerdes VE, Brandjes DP, Büller HR, Stam J.. Thyroid diseases and cerebrovascular disease. Stroke 2005;36:2302–2310 [DOI] [PubMed] [Google Scholar]
- 4. Meissner A. Hypertension and the brain: a risk factor for more than heart disease. Cerebrovasc Dis 2016;42:255–262 [DOI] [PubMed] [Google Scholar]
- 5. Dearborn JL, Schneider ALC, Sharrett AR, et al. Obesity, insulin resistance, and incident small vessel disease on magnetic resonance imaging: Atherosclerosis Risk in Communities Study. Stroke 2015;46:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boulouis G, Charidimou A, Jessel MJ, et al. Small vessel disease burden in cerebral amyloid angiopathy without symptomatic hemorrhage. Neurology 2017;88:878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shinohara M, Tashiro Y, Suzuki K, Fukumori A, Bu G, Sato N.. Interaction between APOE genotype and diabetes in cognitive decline. Alzheimers Dement (Amst) 2020;12:e12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Son SJ, Lee KS, Lee Y, et al. Association between white matter hyperintensity severity and cognitive impairment according to the presence of the apolipoprotein E (APOE) ε4 allele in the elderly: retrospective analysis of data from the CREDOS study. J Clin Psychiatry 2012;73:1555–1562 [DOI] [PubMed] [Google Scholar]
- 9. Peila R, Rodriguez BL; Honolulu-Asia Aging Study . Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: the Honolulu-Asia Aging Study. Diabetes 2002;51:1256–1262 [DOI] [PubMed] [Google Scholar]
- 10. Ravipati K, Chen Y, Manns JR.. Reassessing diabetes and APOE genotype as potential interacting risk factors for Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2022;37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thurston RC, Wu M, Chang YF, et al. Menopausal vasomotor symptoms and white matter hyperintensities in midlife women. Neurology 2023;100:e133–e141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. D’Alonzo KT. The Johnson-Neyman procedure as an alternative to ANCOVA. West J Nurs Res 2004;26:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinstein G, Maillard P, Himali JJ, et al. Glucose indices are associated with cognitive and structural brain measures in young adults. Neurology 2015;84:2329–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin Q, Huang W-Q, Ma Q-L, et al. Incidence and risk factors of leukoaraiosis from 4683 hospitalized patients: a cross-sectional study. Medicine (Baltimore) 2017;96:e7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raffield LM, Cox AJ, Freedman BI, et al. Analysis of the relationships between type 2 diabetes status, glycemic control, and neuroimaging measures in the Diabetes Heart Study Mind. Acta Diabetol 2016;53:439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W.. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions: a population-based cohort study. Alzheimers Dement 2019;15:25–33 [DOI] [PubMed] [Google Scholar]
- 18. de Bresser J, Tiehuis AM, van den Berg E, et al. ; Utrecht Diabetic Encephalopathy Study Group . Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reitz C, Guzman VA, Narkhede A, DeCarli C, Brickman AM, Luchsinger JA.. Relation of dysglycemia to structural brain changes in a multiethnic elderly cohort. J Am Geriatr Soc 2017;65:277–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van Elderen SGC, de Roos A, de Craen AJM, et al. Progression of brain atrophy and cognitive decline in diabetes mellitus. A 3-year follow-up. Neurology 2010;75:997–1002 [DOI] [PubMed] [Google Scholar]
- 21. Gouw AA, van der Flier WM, Fazekas F, et al. ; LADIS Study Group . Progression of white matter hyperintensities and incidence of new lacunes over a 3-year period. Stroke 2008;39:1414–1420 [DOI] [PubMed] [Google Scholar]
- 22. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]