Abstract
Cigarette smoking remains a leading cause of preventable disease and death worldwide. Due to the devastating negative health effects of smoking, many users attempt to quit, but few are successful in the long-term. Thus, there is a critical need for novel therapeutic approaches. In these investigations, we sought to examine whether cannabidiol (CBD) has the potential to be repurposed as a nicotine cessation therapeutic. In the first study, male and female mice were trained to respond for intravenous nicotine infusions at either a low or moderate nicotine dose and then were pretreated with CBD prior to their drug-taking session. We found that CBD produced a significant decrease in the number of nicotine rewards earned, and this effect was evidenced across CBD doses and with both the low and moderate levels of nicotine intake. These effects on drug intake were not due to general motor-related effects, since mice self-administering food pellets did not alter their behavior with CBD administration. The potential effects of CBD in mitigating nicotine withdrawal symptoms were then investigated. We found that CBD attenuated the somatic signs of nicotine withdrawal and prevented nicotine’s hyperalgesia-inducing effects. Taken together, these results demonstrate that modulation of cannabinoid signaling may be a viable therapeutic option as a smoking cessation aid.
Keywords: Nicotine, Cannabidiol, Intravenous nicotine self-administration, Tobacco addiction, E-cigarette, Therapeutic, Withdrawal
1. Introduction
Cigarette smoking is a leading cause of preventable disease and death worldwide (CDC, 2014). While over 50% of adult smokers desire to quit the tobacco smoking habit, less than 8% are successful in the long term (FDA, 2019a). Current methods available for those who desire to quit tobacco smoking include nicotine replacement therapeutics, such as nicotine gum and nasal spray. Varenicline tartrate and bupropion hydrochloride are considered the current first-line therapeutics for tobacco cessation (Fava et al., 2005; FDA, 2019b). However, moderately efficacious outcomes have been found in the long term, with patient compliance reduced due to side-effects (FDA, 2019a; Le Foll et al., 2022). Furthermore, new products that infer addiction liability for nicotine continue to increase in the commercial marketplace, including e-cigarettes, nicotine pouches, and quick dissolving nicotine lozenges. Indeed, while cigarette use has decreased from 31.4% in 2000 to 5.7% in 2019 among high school seniors, nicotine e-cigarette vaping has risen in popularity, with 25.5% of high school seniors reporting recent use of nicotine vape products in 2019, an increase from 12.4% reported in 2017 (CDC, 2017, 2021). These findings not only highlight the changing marketplace, but also indicate that a growing youth population have the potential to become dependent on nicotine products, which will lead to a continued need for more efficacious nicotine cessation aids.
Cannabis Sativa has long been used for its medicinal properties and is also widely available as a recreational drug across the US (Bridgeman and Abazia, 2017). Many compounds have been isolated from the cannabis plant, of which more than 100 have been identified as cannabinoids (Pertwee, 2008). Of these extractions, delta (9)-tetrahydrocannabinol (THC) and cannabidiol (CBD) are two of the main constituents. THC is considered the main psychoactive component. In contrast, CBD has not exhibited common indications of addiction liability in pre-clinical models, as evidenced with conditioned place preference, withdrawal assessments, and locomotor analyses (Viudez-Martínez et al., 2019). In the US, the Agriculture Improvement Act (2018) was passed to decriminalize the use of hemp, which is the part of the cannabis plant that contains less than 0.3% THC, and this has resulted in CBD expanding into a wide variety of commercially available products, including foods, dietary supplements, veterinary products, and cosmetics (FDA, 2019c). While CBD is currently being marked for a variety of maladies, including chronic pain, anxiety, inflammation, insomnia, among other indications (Scuderi et al., 2009), rigorous scientific research has yet to systemically validate many of the beneficial health claims. Of further concern, studies show that commercially available CBD products have highly inaccurate labeling of CBD concentrations, including 18 of 84 samples tested containing THC although not labeled (Gurley et al., 2020). Thus, controlled dosing will be needed for therapeutic indications. Indeed, in 2018, the FDA approved CBD for the treatment of epileptic seizures attributed to Dravet and Lennox-Gastaut syndromes (Biosciences, 2021). This sesame oil formulation has been shown to be well tolerated with no serious side effects or toxicity found in patients (Cunha et al., 1980). In the validation process for this FDA-approved formulation, CBD was found to not be self-administered by rodents, and doses between 10 and 60 mg/kg elicited positive ‘drug-liking’ evaluations in humans that were similar to placebo (60 mg/kg highest dose examined) (Biosciences, 2021). At doses <125 mg/kg, oral administration in pregnant rats did not induce maternal or developmental effects, and after long-term treatment (2 years), carcinogenesis was not observed (Biosciences, 2021). However, other studies have found that at doses >125 mg/kg, toxicity in animal models has been observed, notably with intravenous CBD administration (Huestis et al., 2019; Rosenkrantz et al., 1981). In human studies, other adverse effects, such as diarrhea, loss of appetite, somnolence and sedation, have been recorded, but these studies were confounded by the co-administration other psychiatric mediations that could have contributed to the side effects (Devinsky et al., 2017; McGuire et al., 2018; Thiele et al., 2018).
A few studies have begun to demonstrate a potential for CBD’s effectiveness with nicotine dependence. For instance, CBD administration following overnight nicotine abstinence reduced the self-reported subjective pleasantness of cues for cigarettes (Hindocha et al., 2018), and ad-hoc use of a CBD, but not placebo, inhaler reduced cigarette consumption in a preliminary study (Morgan et al., 2013). More recently, a small, open label study suggested that CBD could potentially mitigate withdrawal symptoms and anxiety state following e-cigarette use in humans (Gournay et al., 2023). In a preclinical study, repeated injections of CBD were also found to attenuate both the somatic signs and hyperalgesia during nicotine withdrawal in rats (Smith et al., 2021). However, it is unknown as to whether oral CBD leads to beneficial outcomes in controlled pre-clinical studies. Indeed, oral intake is highly desirable due to convenience and accuracy of within-subject dosing (MacCallum and Russo, 2018) and in consideration of the potential adverse effects of inhaled CBD on lung health (Leigh and Goniewicz, 2020a, 2020b). Furthermore, it has not yet been shown whether CBD would alter other aspects of the drug use trajectory, for instance nicotine reinforcement and intake.
In these studies, we sought to examine whether orally dosed CBD would alter nicotine self-administration and mitigate somatic withdrawal symptomology. As described above, CBD was administered orally to mimic the most common current method of use by humans (MacCallum and Russo, 2018). In the first series of studies, mice were implanted with intravenous catheters and examined for their reinforcement related responding to obtain nicotine infusions. Given that individuals may experience differing levels of nicotine intake, we examined two different doses of nicotine, for which mice titrate their baseline intake at either a low or moderate level. In the second series of studies, mice were examined for CBD’s effectiveness in altering spontaneous nicotine withdrawal, with a focus on anxiety-like behavior, hyperalgesia, and somatic signs of nicotine withdrawal. Taken together, these studies provide evidence for the effectiveness of orally administered CBD to dose dependently alter nicotine intake and dependence behaviors, thereby supporting further development as a cessation therapeutic.
2. Materials and methods
2.1. Animals
Male and female C57BL/6J mice ≥8 weeks of age (Jackson Laboratory, Bar Harbor, ME) were used for all studies. At the University of California, Irvine, animals were housed in a reversed light-dark cycle (12h:12h), with water proved ad libitum; food was provided ad libitum and then restricted to 85–90% of free-feeding weights during the self-administration studies. For withdrawal studies, mice were housed in standard environmental conditions with the light-dark cycle (12h:12h) and food and water available ad libitum at Virginia Commonwealth University. Housing conditions were maintained at a constant temperature (22 °C) and humidity (50–60%) at both institutions. Subjects were observed and weighed daily to ensure well-being. All animal care and experimental procedures complied with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees at the University of California Irvine and Virginia Commonwealth University.
2.2. Drugs
(−)-Nicotine hydrogen tartrate salt [(−)-1-methyl-2-(3-pyridyl) pyrrolidine (+)-bitartrate salt] was purchased from Sigma-Aldrich or MP Biomedicals. Nicotine was dissolved in physiological saline (0.9% sodium chloride). All doses of nicotine refer to the free-base form. In self-administration studies, nicotine was adjusted to pH 7.4. Cannabidiol (CBD) was purchased from Cayman Chemicals. CBD was dissolved in sesame oil (Sigma-Aldrich) and administered by oral gavage at a volume of 0.05 ml.
2.3. Food self-administration
Mice were mildly restricted to 85–95% of their free-feeding body weight and were then trained to lever press for food pellet rewards (grain-based, 20 mg, 5TUM, Test Diet) on a two-lever operant task across ascending fixed ratio (FR) schedules from one up to five lever presses, as previously described (Fowler and Kenny, 2011). The left and right lever extend into the chamber at the beginning of the session. Responses on the active lever that reached the FR criteria resulted in the delivery of a food pellet and a cue light that remained active for a 20-s time-out period. Mice were required to achieve a FR5TO20 s schedule above criterion (>30 pellets per hr session) for three consecutive days prior to testing. Inactive lever responses were recorded but had no scheduled consequences. Testing was conducted 6 d per week, and responses were recorded with the MedPC interface (Med Associates). Mice were then habituated to the oral gavage procedure prior to sessions to ensure that this route of administration did not alter baseline responding prior to testing with CBD doses. Thereafter, mice (n = 20, 14 males, 6 females) were examined for changes in responding following p.o. gavage administration of vehicle (0 mg/kg), 40 mg/kg, or 100 mg/kg CBD presented in a Latin square design, 45 min before the session. Mice were required to return to baseline responding prior to the next CBD dose administration, which necessitated a minimum of 2 d in between each CBD dosage day. One mouse was barbered by its cagemate, so it was removed from the study prior to completion of the dosing schedule and sacrificed.
2.4. Intravenous nicotine self-administration
Mice were first trained for food self-administration until achieving criterion, as described above. Thereafter, subjects were anesthetized with 1–3% isoflurane/oxygen mixture and catheterized as previously described (Chen et al., 2018; Fowler and Kenny, 2011). A 6 cm catheter tubing was passed subcutaneously from the animal’s back to the right jugular vein. Approximately 1 cm of the catheter tip was inserted into the vein and tied with a surgical silk suture. Following surgery, animals were provided ≥72 h to recover and then were reinstated for food responding to ensure full recovery. Thereafter, mice were transitioned to respond for intravenous nicotine under the FR5TO20 s schedule with 1 h daily sessions, 6 d per week, at the training dose of nicotine (0.03 mg/kg per infusion) for ~8 sessions. For all doses, nicotine (0.03 ml per infusion volume) was delivered through tubing into the intravenous catheter by a Razel syringe pump (Med Associates). Based on prior findings, mice typically achieve stable responses for nicotine after ~5 d of acquisition14, so the acquisition period was provided to allow for consistency with baseline responding across subjects. Subjects were also administered vehicle with oral gavage during baseline sessions and were required to exhibit stable responding prior to dose testing, which served to ensure that this route of administration did not alter baseline behavior. Mice (n = 16 total) were then observed for changes in responding following administration of CBD (0, 40, or 100 mg/kg, p.o.) 45 min prior to the session. This pretreatment time point was selected given that it has been shown to occur on the ascending limb of brain accumulation and in consideration of subsequent high levels across the next hr of testing during the session (Xu et al., 2019). Since it was unknown if the mice would be able to restabilize responding following CBD administration, one cohort of mice were examined for vehicle and either 40 or 100 mg/kg CBD (7 males, 2 females) in a cross-over design. After we found that mice could re-established baseline responding with 2+ days between doses, another cohort of mice were examined with all three doses (0, 40 or 100 mg/kg) in a Latin square design (5 males, 2 females). For the second experiment with the moderate dose of nicotine, mice first underwent acquisition at the 0.03 mg/kg/infusion dose, and then were provided access to the moderate 0.1 mg/kg/infusion dose for at least 5 days to stabilize responding. Thereafter, mice (n = 12 total) began CBD dose administration (0, 40, or 100 mg/kg, p.o.). As above, the first cohort were examined for vehicle and either 40 or 100 mg/kg CBD (1 male, 1 female) in a cross-over design. After we found that mice could re-established baseline responding with 2+ days between doses, a second cohort of mice were examined with all three doses (0, 40 or 100 mg/kg) in a Latin square design (n = 10, 6 males, 4 females). For all of the experiments described above, mice were required to reestablish baseline responding for nicotine infusions, which occurred with at least 2 d of baseline sessions before receiving the next CBD dose in the sequence. Catheters were flushed daily with heparin (100 units/ml). Catheter integrity was verified with the ultra-short-acting barbiturate anesthetic Brevital (2%, methohexital sodium, Eli Lilly) at the end of the study. Mice (n = 6) were excluded from the data analysis due to failed catheters.
2.5. Chronic nicotine administration to induce withdrawal
Male and female mice were anesthetized with inhalation of an isoflurane/oxygen vapor mixture (1–3%). Alzet osmotic minipumps (model 2000; Alzet Corporation), containing either nicotine or saline, were then surgically implanted subcutaneously. The minipumps were kept at a constant flow rate to deliver 24 mg nicotine bitartrate/kg animal body weight/day for 14 days which has been show to maintain stable nicotine level and elicit nicotine withdrawal symptoms upon removal (Damaj et al., 2003; Jackson et al., 2008).
2.6. Spontaneous nicotine withdrawal
Nicotine or vehicle minipumps were removed from mice the evening of day 14 exposure, and behavioral observations were performed on day 15, ~18–20 h after removal. Mice (n = 60; 10 per group with 5 males, 5 females) were pretreated with either vehicle or CBD (15, 30, 60 mg/kg, p.o.) 2 h before behavioral observations to assess the effects of CBD on spontaneous nicotine withdrawal. This pretreatment time was chosen based on previous time course kinetics (Xu et al., 2019) and in consideration of the testing time for withdrawal behavioral measures. Behavioral testing was performed by an observer blinded to the experimental treatment and in a specific testing sequence known to produce the most consistent results with minimal within-group variability (Jackson et al., 2008). Specifically, animals were habituated to the room for 30 min before testing. Mice were first evaluated for 5 min in the light-dark box (LDB) test for anxiety-related behavior; the total time spent in the light compartment was recorded for 5 min by a video monitoring technique and ANY-MAZE software (Stoelting Co.). We then followed the LDB test with a 30-min observation of somatic signs measured as paw and body tremors, head shakes, backing, jumps, curls, and ptosis. The total number of signs was measured. Hyperalgesia was evaluated in the hot-plate test (52 °C) immediately following the somatic sign observation period as previously described (Jackson et al., 2008).
2.7. Data analysis
Data were analyzed by a one-way or two-way ANOVA or mixed-effects analysis with Prism 10 software (GraphPad, La Jolla, CA, USA), as appropriate. Significant main or interaction effects were followed by Tukey or Sidak post-hoc comparison with correction for multiple comparisons. The criterion for significance was set at α = 0.05.
3. Results
3.1. CBD attenuates nicotine intake
Mice were assessed for the effects of CBD treatment on intravenous nicotine self-administration (Fig. 1A). Our first study examined nicotine intake at a low infusion dose (0.03 mg/kg/infusion), which resulted in a mean level of ~0.4 mg per hr session with vehicle dosing (Fig. 1B). We found significant differences with CBD pretreatment in the number of nicotine infusions earned (F(2,21) = 13.17, p = 0.0002) (Fig. 1B). The post-hoc test revealed a decrease in nicotine infusions for both the 40 mg/kg (p = 0.0015) and 100 mg/kg (p = 0.0004) CBD doses compared to vehicle treatment. When comparing the latency to reward, CBD treatment had no significant effect on seconds to obtain the first earned nicotine infusion (F(2,36) = 1.683, p = 0.2001) (Fig. 1C). The number of lever presses also differed based on dose and lever (Dose, F(2,30) = 9.820, p = 0.0005; Lever, F(1,15) = 56.15, p < 0.0001; Interaction, F(2,12) = 12.38, p = 0.0012) (Fig. 1D). The post hoc test revealed significantly less active lever presses following both the 40 mg/kg (p < 0.0001) and 100 mg/kg (p < 0.0001) CBD doses compared to vehicle. No differences were found in the number of inactive lever presses across treatment conditions. Further, when comparing the latency to lever press, CBD treatment had no significant effect on the time to first press the active lever following the session initiation (F(2,36) = 1.251, p = 0.2983) (Fig. 1E).
Fig. 1. CBD attenuates nicotine intake at a low self-administered nicotine dose.
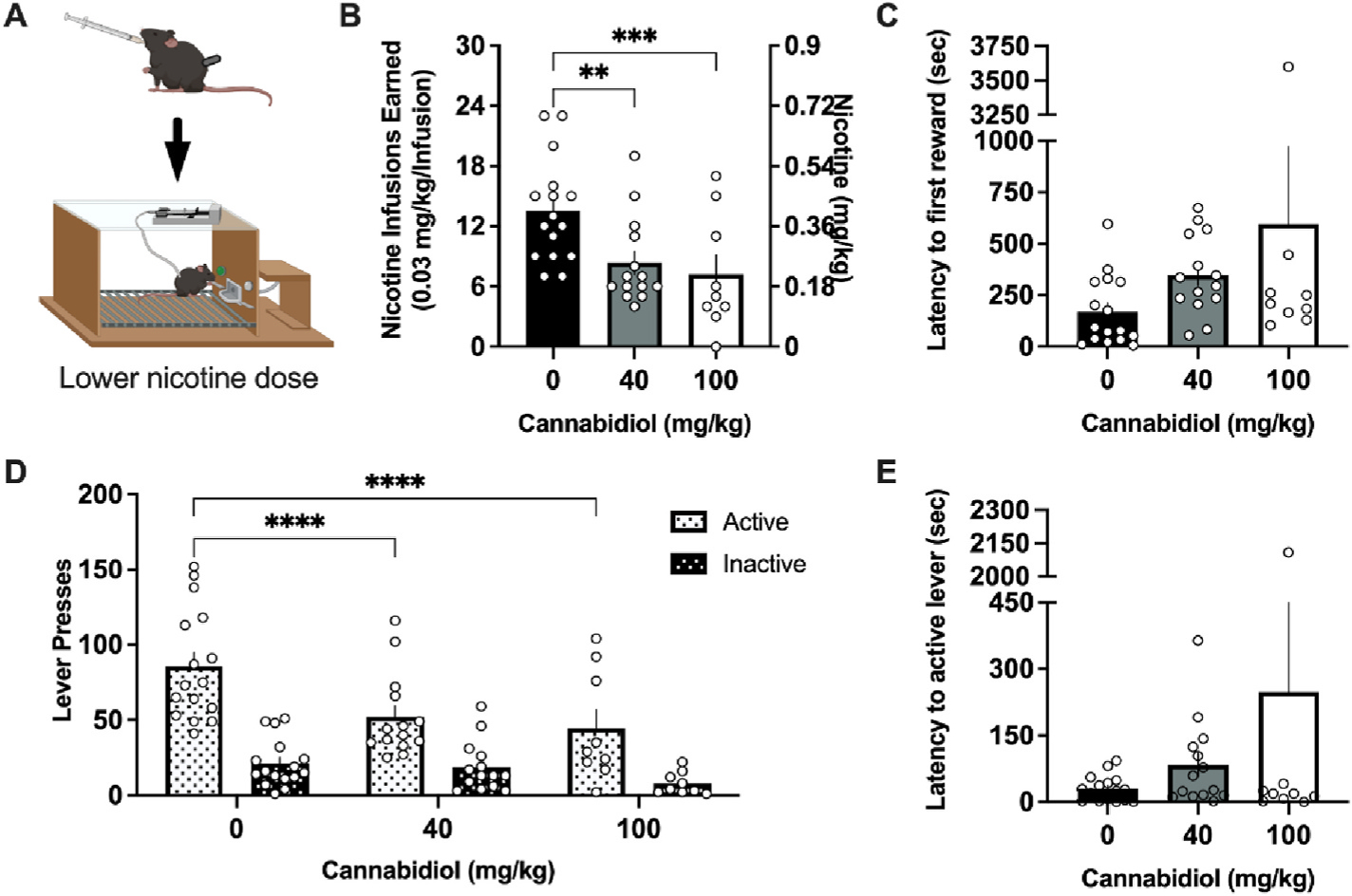
(A) Schematic of the experimental design in which mice received vehicle, 40 mg/kg, or 100 mg/kg cannabidiol (CBD) perioral 45 min prior to an intravenous nicotine self-administration session; image created with Biorender.com. (B) Male and female mice self-administering a lower dose of nicotine significantly reduced the number of infusions earned following treatment with 40 or 100 mg/kg CBD. (C) Mice did not differ in the latency to the first nicotine infusion earned following CBD treatment. (D) CBD treatment decreased active lever pressing behavior at both the 40 and 100 mg/kg CBD doses. No statistical differences were observed in inactive lever pressing behavior across treatments. (E) Mice did not differ following CBD treatment in the latency to the first active lever press. Individual data points shown on graphs for each subject. Bar graphs represent mean ± SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001.
Next, we sought to examine whether CBD would maintain effectiveness at a moderate dose of nicotine (0.1 mg/kg/infusion) (Fig. 2A), which resulted in a mean level of ~1.4 mg per hr session with vehicle dosing. At this moderate level of nicotine intake, we found a significant reduction in the number of infusions earned (F(2,20) = 10.56, p = 0.0007) (Fig. 2B). The post-hoc analysis revealed decreased infusions for both the 40 mg/kg (p = 0.0026) and 100 mg/kg (p = 0.0010) CBD dose, compared to vehicle. When comparing the latency to reward, CBD treatment had no significant effect on seconds to obtain the first earned nicotine infusion (F(2, 20) = 1.874, p = 0.1795) (Fig. 2C). Lever pressing behavior also differed based on dose and lever (Dose, F(2,22) = 8.675, p = 0.0017; Lever, F(1,11) = 38.20, p <0.0001; Interaction, F(2,18) = 7.539, p = 0.0042) (Fig. 2D). Post hoc analysis revealed that CBD significantly reduced the active lever responding at 40 mg/kg (p < 0.0001) and 100 mg/kg (p < 0.0001) doses compared to vehicle. No differences were observed in inactive lever responding. Finally, when comparing the latency to lever press, CBD treatment had no significant effect on the time to first press the active lever following the session initiation (F(2,20) = 1.642, p = 0.2186) (Fig. 2E). Of note, no sex differences were found in any of the above nicotine self-administration assessments (Sup Table 1).
Fig. 2. CBD attenuates nicotine intake at a moderate self-administered nicotine dose.
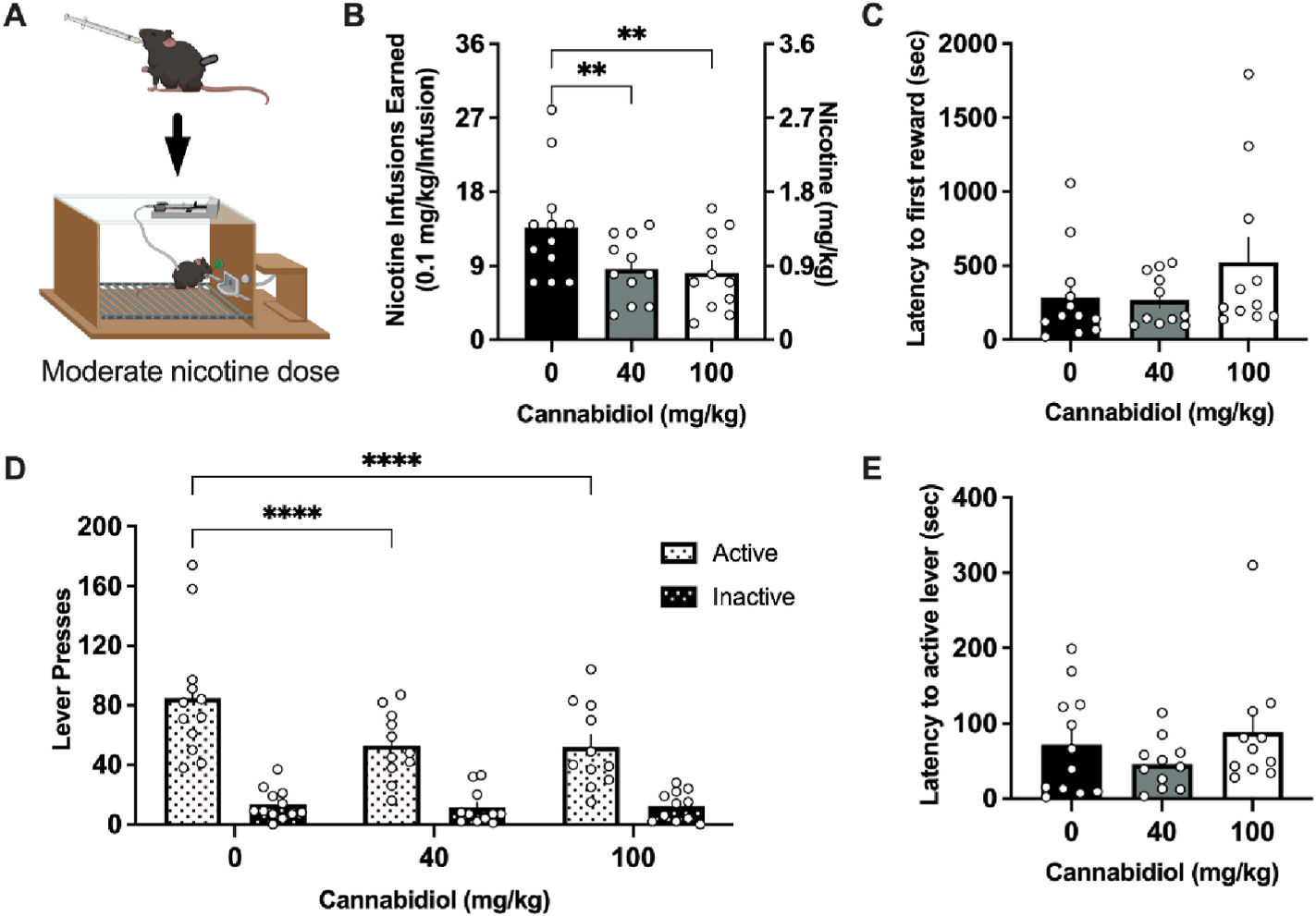
(A) Schematic of the experimental design with the moderate 0.1 mg/kg/infusion dose of nicotine, generated with Biorender.com (B) Male and female mice self-administering the moderate dose of nicotine significantly reduced the number of rewards earned following treatment with 40 and 100 mg/kg CBD. (C) Mice did not differ in the latency to the first nicotine infusion earned following CBD treatment. (D) Analysis of lever pressing behavior demonstrates a reduction in active lever responding at both the 40 and 100 mg/kg CBD doses. No statistical differences were observed in inactive lever pressing behavior across treatment. (E) Mice did not differ following CBD treatment in the latency to the first active lever press. Individual data points shown on graphs for each subject. Bar graphs represent mean ± SEM. **p < 0.01, ****p < 0.0001.
3.2. CBD treatment does not affect operant responding for food reward
Mice were examined with operant food self-administration to determine if CBD’s effects were specific to nicotine or perhaps affected overall lever-pressing behavior (Fig. 3A). Importantly, no significant differences were found among groups in the number of food pellets earned (F(2,38) = 0.3057, p = 0.7384) (Fig. 3B). When comparing the latency to reward, CBD treatment had a main effect on seconds to obtain the first earned food pellet (F(2,38) = 3.648, p = 0.0356), but no differences were found in the post-hoc test when comparing CBD doses to the vehicle control (Fig. 3C). Active and Inactive lever responding also revealed no significant differences following CBD administration (Dose, F(2,76) = 0.6164, p = 0.5426; Lever, F(1,38) = 71.27, p < 0.0001; Interaction, F(2,76) = 0.5433, p = 0.5831) (Fig. 3D). When comparing the latency to lever press, CBD treatment had no significant effect on the time to first press the active lever following the session initiation (F(2,38) = 1.663, p = 0.2030) (Fig. 3E). Variability was noted in the number of rewards earned and lever presses within each session for food, and this was attributed to sex differences in the number of food pellets earned and active lever presses, independent of CBD drug treatment (Sup Table 1). Specifically, males exhibited higher levels of active lever responding and rewards earned for food compared to females (Sup Fig. 1A–C), which was consistent with differences in body weight (female: 19.32 g mean ± 0.4454 SEM, male: 20.33 ± 0.2656; t(19) = 2.004, p = 0.0298). There was also a slightly longer latency noted in females with the 40 mg/kg CBD dose for the first active lever press and first reward (Sup Fig. 1B–D), but this was likely attributable to overall higher female variability in this measure and lower number of females subjects, as compared to males.
Fig. 3. CBD does not alter operant responding for food reward.
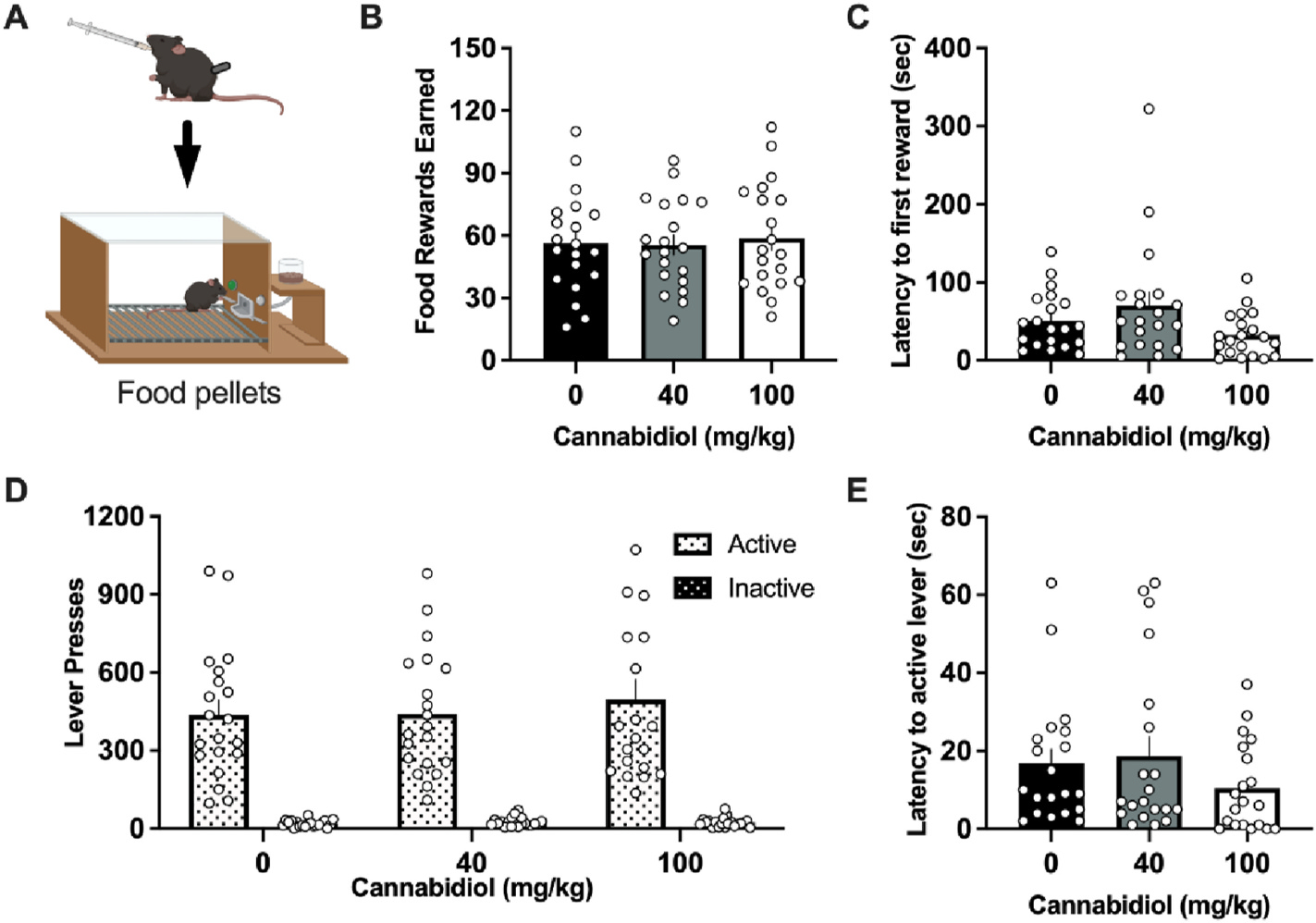
(A) Schematic of the experimental design for food self-administration testing, generated with Biorender.com. (B) Male and female mice self-administering a grain-based food reward did not differ in the number of food pellets earned following CBD pre-treatment. (C) Mice did not differ in the latency to the first earned food reward following CBD, as compared to vehicle control. (D) No significant differences were observed with CBD treatment for the number of active or inactive lever presses. (E) Mice did not statistically differ following CBD treatment in the latency to the first active lever press. Individual data points shown on graphs for each subject. Bar graphs represent mean ± SEM.
3.3. CBD attenuates nicotine withdrawal symptomology
Spontaneous nicotine withdrawal was induced in male and female mice via the removal of minipumps and several withdrawal signs were observed following a 2 h pre-treatment of CBD or vehicle (Fig. 4A). Withdrawal signs include affective (anxiety-like behavior), somatic, and hyperalgesia behaviors. An effect of nicotine treatment was found on anxiety-like behavior in the light-dark-box test (F(1, 55) = 11.35, p = 0.0014). Compared to saline, nicotine induced a significant decrease in the time spent in the light compartment (p = 0.0281), but this difference was ameliorated by CBD treatment at doses 30 and 60 mg/kg (Fig. 4B). Analyses of the total number of crosses revealed no differences between groups (F(1,43) = 0.0031, p = 0.9557) (Fig. 4C), indicating no significant changes in locomotor activity. For the somatic signs of withdrawal, a main effect of both nicotine (F(1,55) = 66.92, p < 0.0001) and CBD (F(3,55) = 9.619, p < 0.0001) was found (Fig. 4D). The post-hoc analysis showed that 30 mg/kg (p = 0.0015) and 60 mg/kg (p = 0.0007) CBD significantly reduced the number of somatic signs of withdrawal in mice treated with nicotine. Finally, for hyperalgesia, nicotine altered the behavioral response (F(1,55) = 22.48, p < 0.0001), with a significant decrease in paw withdrawal latency compared to saline (p = 0.0004) (Fig. 4E). CBD attenuated nicotine withdrawal induced hyperalgesia (F(3,55) = 11.81, p < 0.0001), with the post-hoc revealing an effect at the 30 mg/kg (p = 0.0045) and 60 mg/kg (p < 0.0001) CBD doses comparing to vehicle for nicotine withdrawal. Overall, minimal differences were found between females and males in the nicotine withdrawal assessments (Sup Table 1). One significant difference was noted with higher somatic signs exhibited by the females following nicotine minipump exposure (Sup Fig. 1E), suggesting that there may be brain circuitry differences involved in somatic signs versus anxiety-like behavior and thermal hyperalgesia. However, this sex difference was not found with CBD treatment, indicating that CBD was effective in both male and female subjects to reduce withdrawal signs.
Fig. 4. CBD attenuates nicotine withdrawal.
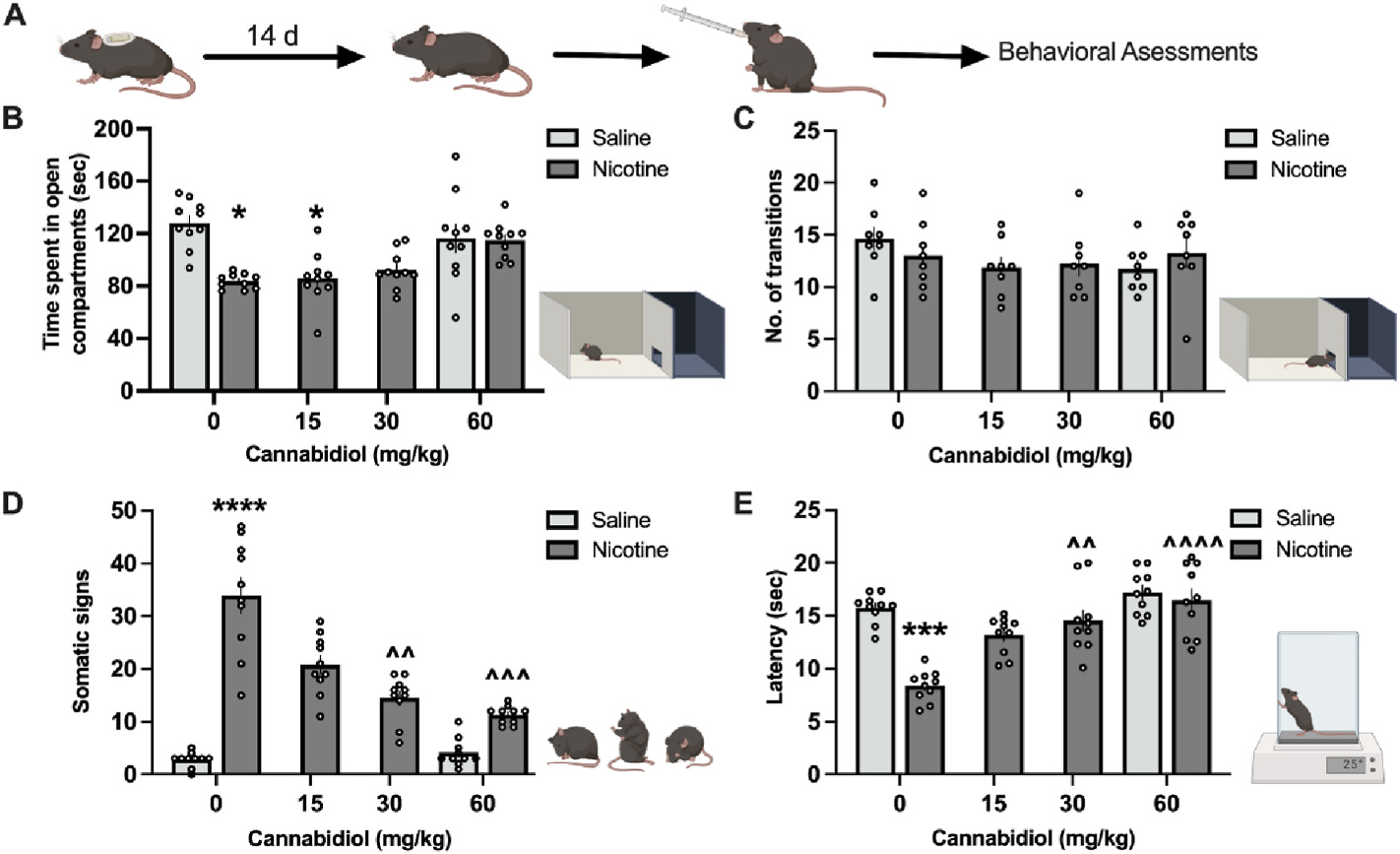
(A) Schematic illustration of the light-dark box generated with Biorender.com. Mice were chronically treated with nicotine (24 mg/kg/day) for 14 days. On day 14, minipumps were removed, and on day 15, mice were administered CBD perioral at the dose of 0, 15, 30, or 60 mg/kg. Two hours later, mice were tested for anxiety-associated behavior, signs of withdrawal, and hyperalgesia. (B) With vehicle administration, nicotine induced anxiolytic behavior, which was evidenced by significantly less time spent in the light compartment. CBD pretreatment did not alter behavior for the saline control. (C) No significant differences were found in the number of crosses between the light and dark compartments with CBD pretreatment. (D) Nicotine withdrawal induced a significant increase in somatic symptoms, and 60 mg/kg of CBD significantly attenuated these withdrawal behaviors. (E) Nicotine withdrawal induced hyperalgesia in the tail-flick assay, and 60 mg/kg CBD reversed this enhanced pain sensitivity. Individual data points shown on graphs for each subject. Bar graphs represent mean ± SEM. *p < 0.05, ****p < 0.0001 saline vehicle vs nicotine, ^^p < 0.01, ^^^p < 0.001, ^^^^p < 0.0001 nicotine vehicle vs nicotine CBD.
4. Discussion
In these studies, we found that acute oral administration of CBD decreases nicotine intake at both a low and moderate level of self-administered nicotine. Given that smokers can differ in their levels of daily drug use (Le Foll et al., 2022), these results provide important evidence to support the potential effectiveness of CBD for wide range of individuals with varying daily intake levels. We also demonstrated that CBD attenuates the somatic signs of nicotine withdrawal, as well as withdrawal-induced hyperalgesia and anxiety-associated behaviors. Taken together, these preclinical studies support the further development of CBD as a smoking cessation therapeutic.
4.1. CBD’s effects during nicotine intake and withdrawal
CBD has been shown to act on multiple targets which could mitigate its effects on drug reinforcement and withdrawal behaviors. First, CBD is a negative allosteric modulator on the cannabinoid 1 receptor (CB1R), as well as an inverse agonist on CB2Rs at nanomolar concentrations (Deiana et al., 2012). CB1Rs and nicotinic acetylcholine receptors (nAChRs), the main receptor mitigating nicotine’s actions, exhibit overlapping expression patterns within brain regions implicated in drug reinforcement, aversion and withdrawal (Renard et al., 2014; Tuesta et al., 2011). On the cellular level, CB1Rs and nAChRs are expressed on presynaptic axon terminals, and both can function to modulate release of neurotransmitters (Fowler et al., 2011; Renard et al., 2014). For instance, CBD has been shown inhibit nicotine-mediated norepinephrine release in rat hippocampus, likely by interacting with α7 nAChRs (Mahgoub et al., 2013). Further, endocannabinoid and cholinergic signaling mechanisms can reciprocally mediate each another. For instance, exogenous cannabinoids can modulate cholinergic neurotransmission in the brain (Acquas et al., 2001), and similarly, nicotine administration can alter endocannabinoid signaling (Solinas et al., 2007b). The rewarding effects of nicotine or cannabinoids can also be altered with pharmacological modulation of either CB1Rs or nAChRs (Gamaleddin et al., 2015; Solinas et al., 2007a). Secondly, CBD has been shown to have an inhibitory effect on serotonin 5-HT1A and TRPV1 receptor signaling (Galaj et al., 2020; Martinez-Aguirre et al., 2020; Russo et al., 2005), and both of these receptors have been implicated in nicotine’s effects (Dao et al., 2011; Liu et al., 2004). Further, CBD appears to interact with GPR55 to increase intracellular calcium, and interestingly, modulation of GPR55 can influence the rewarding properties of nicotine (Liu et al., 2021; Ryberg et al., 2007). Finally, nicotine has been shown to modulate microglial activity in the brain (Adeluyi et al., 2019; Guan et al., 2015; Shytle et al., 2004), which could potentially be affected by CBD administration (Kozela et al., 2010). Thus, given that nicotine and CBD may interact at varying circuit and molecular levels, it will be interesting in further studies to determine the specificity of action with integration across levels.
Recently, CBD’s enzyme inducing effects have also been proposed to lead to a reduction in nicotine metabolism (Nasrin et al., 2023), which based on our prior studies (Chen et al., 2020), would be expected to decrease nicotine consumption. Specifically, CBD and its metabolite 7-OH-CBD have been shown inhibit the activity of CYP2A6 in vitro, which is critical for nicotine metabolism in vivo (Chen et al., 2020; Nasrin et al., 2023). However, in our studies, we examined CBD’s actions following acute treatment, which would have been during high blood and brain concentrations of CBD in vivo (Xu et al., 2019), and subjects were required to return to the baseline levels of nicotine intake following each dose, thereby verifying that the CBD dose did not exert longer term effects on nicotine intake with this dosing regimen. Similarly, with the withdrawal studies, CBD was administered ~18–20 h after nicotine minipump removal, a time point in which nicotine would have been fully metabolized and excreted (Petersen et al., 1984). Thus, based on our evidence, CBD’s actions on nicotine self-administration and withdrawal were likely due to an aforementioned effect at the cellular and/or circuit level, rather than metabolic enzyme induction. Nevertheless, it will be important in further research to determine whether chronic CBD dosing regimens can support nicotine cessation in the longer-term through metabolic mechanisms.
4.2. Specificity of CBD’s actions on nicotine behaviors
While we found effects of CBD on nicotine-specific behavioral outcomes, we did not find any effects on general behavioral responding in the absence of nicotine. Specifically, CBD had no effect on food self-administration in the operant task at both the lower and higher doses, as compared to the vehicle control. While clinical studies with CBD have reported lack of appetite as a side effect (Pinto and Martel, 2022), this was not revealed in the mice with the acute dosing paradigm employed. However, it should be noted that we used a grain-based food pellet, and a prior study did find that CBD decreased responding for sucrose pellets in rats (Bi et al., 2020), thereby suggesting potentially dissociable effects on reinforcement responding based on the value of the reward. In the withdrawal studies, we also found that CBD did not induce any significant differences in mice only treated with the saline minipumps. Moreover, CBD did not have any significant differences on the latency to the first active lever press or reward with both nicotine infusions and food pellets. In addition, during nicotine withdrawal, no differences were found with locomotion in the light-dark box. Therefore, taken together, the current findings demonstrate that CBD’s actions were specific to mitigating nicotine intake and the expression of withdrawal symptoms, rather than a generalized effect on locomotor or behavioral function.
5. Conclusions
The current findings reveal that acute CBD administration decreases nicotine intake and ameliorates withdrawal symptomology. While further studies are needed to determine the precise mechanism of action and duration of effectiveness after acute and chronic dosing, these findings represent an important step in substantiating CBD as a viable therapeutic approach for the cessation of nicotine-containing products, including tobacco cigarettes and e-cigarettes.
Supplementary Material
Funding and disclosures
The authors have no competing financial interests in relation to the work described. This work was supported by grants from the NIH National Institute on Drug Abuse (R01 DA051831 to CDF and DA032246 to MID) and Tobacco-Related Disease Research Program (TRDRP T32IR4866 to CDF). BB was supported in part by a NIDA T32 DA007027, SNC was supported by a NIDA DA051831 Diversity Supplement, and AM was supported by a NIDA T32 DA050558.
Footnotes
Declaration of competing interest
The authors have no competing financial interests in relation to the work described.
CRediT authorship contribution statement
Samantha N. Cheeks: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. Belle Buzzi: Data curation, Formal analysis, Validation, Visualization, Writing – original draft, Writing – review & editing. Ashley Valdez: Data curation, Investigation, Writing – review & editing. Allison S. Mogul: Data curation, Formal analysis, Investigation, Writing – review & editing. M. Imad Damaj: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. Christie D. Fowler: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neuropharm.2023.109833.
Data availability
Data will be made available on request.
References
- Acquas E, Pisanu A, Marrocu P, Goldberg SR, Di Chiara G, 2001. Delta9-tetrahydrocannabinol enhances cortical and hippocampal acetylcholine release in vivo: a microdialysis study. Eur. J. Pharmacol. 419, 155–161. [DOI] [PubMed] [Google Scholar]
- Adeluyi A, Guerin L, Fisher ML, Galloway A, Cole RD, Chan SSL, Wyatt MD, Davis SW, Freeman LR, Ortinski PI, Turner JR, 2019. Microglia morphology and proinflammatory signaling in the nucleus accumbens during nicotine withdrawal. Sci. Adv. 5, eaax7031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi GH, Galaj E, He Y, Xi ZX, 2020. Cannabidiol inhibits sucrose self-administration by CB1 and CB2 receptor mechanisms in rodents. Addiction Biol. 25, e12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biosciences G, 2021. Epidolex Prescribing Information. Greenwich Biosciences, Carlsbad, CA. [Google Scholar]
- Bridgeman MB, Abazia DT, 2017. Medicinal cannabis: history, pharmacology, and implications for the acute care setting. Pharm. Therapeut. 42, 180–188. [PMC free article] [PubMed] [Google Scholar]
- CDC, 2014. The health consequences of smoking—50 Years of progress: a report of the surgeon general. Accessed at: https://www.ncbi.nlm.nih.gov/books/NBK294320/.CentersforDisease. Control and Prevention (US), Atlanta (GA). [Google Scholar]
- CDC, 2017. Use of selected substances in the past 30 days among 12th graders, 10th graders, and 8th graders, by sex and race: United States, selected years 1980–2016. Accessible at: https://www.cdc.gov/nchs/data/hus/2017/051.pdf.
- CDC, 2021. Use of selected substances in the past 30 days among 12th graders, 10th graders, and 8th graders, by sex and race. Accessed at: https://www.cdc.gov/nchs/hus/data-finder.htm?year=2020-2021&table=Table%20SubUseTn.
- Chen E, Lallai V, Sherafat Y, Grimes NP, Pushkin AN, Fowler JP, Fowler CD, 2018. Altered baseline and nicotine-mediated behavioral and cholinergic profiles in ChAT-Cre mouse lines. J. Neurosci. 38, 2177–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Fowler JP, Wang J, Watson CJW, Sherafat Y, Staben A, Lazarus P, Denton TT, Fowler CD, 2020. The novel CYP2A6 inhibitor, DLCI-1, decreases nicotine self-administration in mice. J. Pharmacol. Exp. Therapeut. 372, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, Sanvito WL, Lander N, Mechoulam R, 1980. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology 21, 175–185. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR, 2003. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J. Pharmacol. Exp. Therapeut. 307, 526–534. [DOI] [PubMed] [Google Scholar]
- Dao JM, McQuown SC, Loughlin SE, Belluzzi JD, Leslie FM, 2011. Nicotine alters limbic function in adolescent rat by a 5-HT1A receptor mechanism. Neuropsychopharmacology 36, 1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, Platt B, Riedel G, 2012. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive–compulsive behaviour. Psychopharmacology (Berl) 219, 859–873. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, Scheffer IE, Thiele EA, Wright S, Cannabidiol in Dravet Syndrome Study, G, 2017. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N. Engl. J. Med. 376, 2011–2020. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA, 2005. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim. Care Companion J. Clin. Psychiatry 7, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, 2019a. FDA Drug Safety Communication: FDA revises description of mental health side effects of the stop-smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. Accessed at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-description-mental-health-side-effects-stop-smoking. FDA.
- FDA, 2019b. FDA in Brief: FDA updates label for Chantix with data underscoring it’s not effective in children 16 and younger. Accessed at: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-updates-label-chantix-data-underscoring-its-not-effective-children-16-and-younger. FDA.
- FDA, 2019c. Hemp production and the 2018 farm bill. Accessed at: https://www.ams.usda.gov/sites/default/files/media/2018FarmBill.pdf. Senate Committee on Agriculture, Nutrition, and Forestry.
- Fowler CD, Kenny PJ, 2011. Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology 61, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ, 2011. Habenular alpha5 nicotinic receptor subunit signalling controls nicotine intake. Nature 471, 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Bi GH, Yang HJ, Xi ZX, 2020. Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology 167, 107740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamaleddin IH, Trigo JM, Gueye AB, Zvonok A, Makriyannis A, Goldberg SR, Le Foll B, 2015. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front. Psychiatr. 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gournay LR, Petry J, Bilsky S, Hill MA, Feldner M, Peters E, Bonn-Miller M, Leen-Feldner E, 2023. Cannabidiol Reduces Nicotine Withdrawal Severity and State Anxiety during an Acute E-Cigarette Abstinence Period: A Novel, Open-Label Study. Cannabis Cannabinoid Res. [DOI] [PubMed] [Google Scholar]
- Guan YZ, Jin XD, Guan LX, Yan HC, Wang P, Gong Z, Li SJ, Cao X, Xing YL, Gao TM, 2015. Nicotine inhibits microglial proliferation and is neuroprotective in global ischemia rats. Mol. Neurobiol. 51, 1480–1488. [DOI] [PubMed] [Google Scholar]
- Gurley B, Murphy T, Gul W, Walker L, ElSohly MA, 2020. Content versus label claims in cannabidiol (CBD)-Containing products obtained from commercial outlets in the state of Mississippi. J. Diet. Suppl. 17, 599–607. [DOI] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Grabski M, Stroud JB, Crudgington H, Davies AC, Das RK, Lawn W, Morgan CJA, Curran HV, 2018. Cannabidiol reverses attentional bias to cigarette cues in a human experimental model of tobacco withdrawal. Addiction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardo FP, 2019. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 17, 974–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KJ, Martin BR, Changeux JP, Damaj MI, 2008. Differential role of nicotinic acetylcholine receptor subunits in physical and affective nicotine withdrawal signs. J. Pharmacol. Exp. Therapeut. 325, 302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozela E, Pietr M, Juknat A, Rimmerman N, Levy R, Vogel Z, 2010. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 283, 1616–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Piper ME, Fowler CD, Tonstad S, Bierut L, Lu L, Jha P, Hall WD, 2022. Tobacco and nicotine use. Nat. Rev. Dis. Prim. 8. [DOI] [PubMed] [Google Scholar]
- Leigh NJ, Goniewicz ML, 2020a. Acute effect of electronic cigarette-generated aerosol from flavored CBD-containing refill solutions on human bronchial epithelial cells. Front. Physiol. 11, 592321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh NJ, Goniewicz ML, 2020b. Effect of aerosolized nicotine on human bronchial epithelial cells is amplified after co-administration with cannabidiol (CBD): a pilot in vitro study. BMC Pharmacol Toxicol 21, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhu W, Zhang Z-S, Yang T, Grant A, Oxford G, Simon SA, 2004. Nicotine inhibits voltage-dependent sodium channels and sensitizes vanilloid receptors. J. Neurophysiol. 91, 1482–1491. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yu J, Li X, Guo Y, Sun T, Luo L, Ren J, Jiang W, Zhang R, Yang P, Yang Q, 2021. Cannabinoid receptor GPR55 activation blocks nicotine use disorder by regulation of AMPAR phosphorylation. Psychopharmacology (Berl) 238, 3335–3346. [DOI] [PubMed] [Google Scholar]
- MacCallum CA, Russo EB, 2018. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 49, 12–19. [DOI] [PubMed] [Google Scholar]
- Mahgoub M, Keun-Hang SY, Sydorenko V, Ashoor A, Kabbani N, Al Kury L, Sadek B, Howarth CF, Isaev D, Galadari S, Oz M, 2013. Effects of cannabidiol on the function of alpha7-nicotinic acetylcholine receptors. Eur. J. Pharmacol. 720, 310–319. [DOI] [PubMed] [Google Scholar]
- Martinez-Aguirre C, Carmona-Cruz F, Velasco AL, Velasco F, Aguado-Carrillo G, Cuellar-Herrera M, Rocha L, 2020. Cannabidiol acts at 5-HT1A receptors in the human brain: relevance for treating temporal lobe epilepsy. Front. Behav. Neurosci. 14, 611278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire P, Robson P, Cubala WJ, Vasile D, Morrison PD, Barron R, Taylor A, Wright S, 2018. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am. J. Psychiatr. 175, 225–231. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Das RK, Joye A, Curran HV, Kamboj SK, 2013. Cannabidiol reduces cigarette consumption in tobacco smokers: preliminary findings. Addict. Behav. 38, 2433–2436. [DOI] [PubMed] [Google Scholar]
- Nasrin S, Coates S, Bardhi K, Watson C, Muscat JE, Lazarus P, 2023. Inhibition of nicotine metabolism by cannabidiol (CBD) and 7-hydroxycannabidiol (7-OH-CBD). Chem. Res. Toxicol. 36, 177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 2008. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 153, 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA, 1984. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab. Dispos. 12, 725–731. [PubMed] [Google Scholar]
- Pinto JS, Martel F, 2022. Effects of cannabidiol on appetite and body weight: a systematic review. Clin. Drug Invest. 42, 909–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard J, Krebs MO, Le Pen G, Jay TM, 2014. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front. Neurosci. 8, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkrantz H, Fleischman RW, Grant RJ, 1981. Toxicity of short-term administration of cannabinoids to rhesus monkeys. Toxicol. Appl. Pharmacol. 58, 118–131. [DOI] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK, 2005. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res. 30, 1037–1043. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ, 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol. 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G, 2009. Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders. Phytother Res. 23, 597–602. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR, Tan J, 2004. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J. Neurochem. 89, 337–343. [DOI] [PubMed] [Google Scholar]
- Smith LC, Tieu L, Suhandynata RT, Boomhower B, Hoffman M, Sepulveda Y, Carrette LLG, Momper JD, Fitzgerald RL, Hanham K, Dowling J, Kallupi M, George O, 2021. Cannabidiol reduces withdrawal symptoms in nicotine-dependent rats. Psychopharmacology (Berl) 238, 2201–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR, 2007a. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J. Neurosci. 27, 5615–5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Scherma M, Tanda G, Wertheim CE, Fratta W, Goldberg SR, 2007b. Nicotinic facilitation of delta9-tetrahydrocannabinol discrimination involves endogenous anandamide. J. Pharmacol. Exp. Therapeut. 321, 1127–1134. [DOI] [PubMed] [Google Scholar]
- Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, Lyons PD, Taylor A, Roberts C, Sommerville K, Group GS, 2018. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 391, 1085–1096. [DOI] [PubMed] [Google Scholar]
- Tuesta LM, Fowler CD, Kenny PJ, 2011. Recent advances in understanding nicotinic receptor signaling mechanisms that regulate drug self-administration behavior. Biochem. Pharmacol. 82, 984–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viudez-Martínez A, García-Gutiérrez MS, Medrano-Relinque J, Navarrón CM, Navarrete F, Manzanares J, 2019. Cannabidiol does not display drug abuse potential in mice behavior. Acta Pharmacol. Sin. 40, 358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Chang T, Du Y, Yu C, Tan X, Li X, 2019. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ. Toxicol. Pharmacol. 70, 103202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


