Highlights
-
•
Tisotumab vedotin is highly effective (100% complete response) in a case series of three patients with recurrent cervical cancer.
-
•
Tisotumab vedotin had a complete and durable response in a patient with recurrent HPV-independent gastric-type cervical cancer.
-
•
Ocular toxicities in our patients who live at high altitude in an arid climate are worse than those reported in the literature.
-
•
Expanding phase III and IV clinical trials to sites with different environments, including altitude and climate, will help define the environmental influence on efficacy and toxicity.
Abstract
Metastatic and recurrent cervical cancer is difficult to treat with limited options following platinum-based chemotherapy. Tisotumab vedotin (TV) is an antibody drug conjugate (ADC) targeted at a tissue factor (TF), which is a cell surface protein that is upregulated in the majority of cervical cancers. Prior clinical trials have demonstrated efficacy of TV in metastatic and recurrent cervical cancer with an objective response rate of 24–26 % with an 8.3 month duration of response. In this case series, we present 3 patients with recurrent or progressive cervical cancer of three different histologies (squamous cell, adenocarcinoma, and human papillomavirus (HPV)-independent gastric type carcinomas). We demonstrate a 100 % complete response rate with average time of complete response of 4.33 months. The duration of response was not reached as none of our patients had a confirmed progression at the time of writing this manuscript, but the mean time since the initiation of treatment was 6.1 months. In concordance with the clinical trials, our patients tolerated TV well although the grade 3 ocular toxicities were higher in our patients compared to prior data. This case series presents data confirming the efficacy and tolerability of TV in patients with recurrent cervical cancer, including an HPV-independent gastric type cervical cancer.
1. Introduction
Cervical cancer is the fourth most common cancer affecting women globally. Due to organized screening guidelines and vaccination recommendations, the disease burden falls sharply in higher income nations (Vu et al., 2018). Unfortunately, patients with locally advanced disease continue to have poor survival despite undergoing upfront chemoradiation and brachytherapy (Rose, 1999). The five-year overall survival (OS) for metastatic cervical cancer is less than 17 % (Ferlay, 2013). Once disease is recurrent, there are limited treatment options with response rates varying from 15 to 46 % for traditional chemotherapy with or without bevacizumab (Tsuda et al., 2016). Immunotherapy with pembrolizumab has a 14 % response rate in this population, but with a more durable response.
Given the lack of effective treatment options for patients with recurrent cervical cancer, tisotumab vedotin (TV), an antibody-drug conjugate (ADC) targeting tissue factor (TF), was developed. Efficacy and safety of TV was studied in a phase II single arm trial in patients with recurrent or metastatic cervical cancer with squamous cell, adenocarcinoma, or adenosquamous histology (Coleman, 2021). The ORR was 24 % and TV was well tolerated with mostly grade 1–2 toxicities.
TV was approved by the U.S. Food and Drug Administration (FDA) in September 2021 for patients with advanced or recurrent cervical cancer who have previously received chemotherapy. Since the FDA approval, we have treated 3 patients with 3 different cervical cancer histologies, including an HPV-independent gastric-type cervical cancer, with an impressive 100 % complete response (CR). We will present our small case series on the efficacy and safety of TV in our population and review the available literature.
2. Case presentations
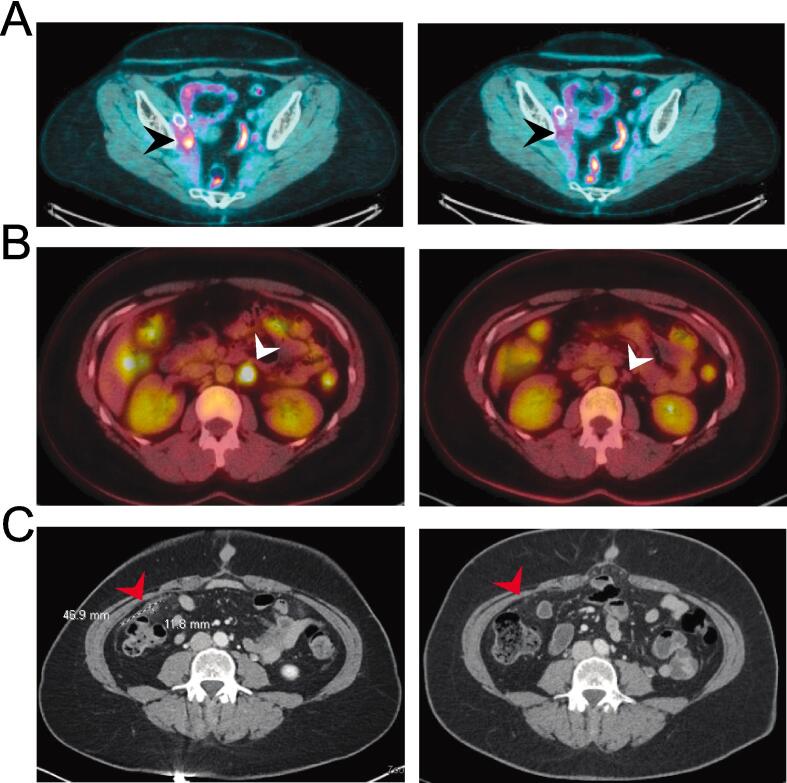
Patient 1: The first paitent is a 27-year-old female diagnosed with stage IIB human papillomavirus (HPV)-associated squamous cell carcinoma of the cervix in April 2019, initially treated with definitive chemoradiation (CRT). She completed interstitial brachytherapy on June 5, 2019 with no evidence of disease until August 2021 when she presented with a right pelvic sidewall mass with associated narrowing of the iliac vessels and right hydroureter requiring ureteral stent placement and iliac venoplasty. She completed 6 cycles of carboplatin/paclitaxel/bevacizumab on December 10, 2021, with a CR. A PET/CT was performed two months after completion of therapy showing moderate to intense FDG avidity localizing to a soft tissue density adjacent to the right iliac vessel, suspicious for recurrent disease, which was confirmed on repeat imaging at 6 weeks. The repeat PET/CT confirmed the right pelvic sidewall recurrence and increasing retrocaval nodal avidity. This marked her second disease recurrence, with disease free interval (DFI) of 3.25 months. After completion of 4 cycles of cemiplimab, an anti-PD-1 checkpoint inhibitor, she had progression of disease on PET/CT scan with disease along the right sidewall as well as in the retrocaval and right common iliac lymph nodes (LNs). She was started on TV on August 19, 2022. The initial PET scan after 3 cycles showed resolution of the FDG-avid LNs, decreased avidity of the right sidewall mass, and no FDG-avid LNs visualized. A PET/CT after six cycles demonstrated a complete response (Fig. 1A). She remains without evidence of disease recurrence at 8 months. Her main adverse effect from treatment was grade 3 punctate keratitis of both eyes, described in detail below.
Fig. 1.
Imaging findings of best response to TV. Pre-treatment imaging is shown on the left and post-treatment imaging on the right. (A) Patient 1 with HPV-associated squamous cell carcinoma - black arrow head pointing to a pretreatment right common iliac lymph node with FDG-avidity, which resolved following 6 cycles of TV. (B) Patient 2 with HPV-associated adenocarcinoma – white arrow head demonstrating enlarged left para-aortic lymph node with FDG-avidity, resolved following 3 cycles of TV. (C) Patient 3 with HPV-independent gastric-type cervical carcinoma – Red arrow shows peritoneal disease that resolved following 4 cycles of TV.
At the beginning of treatment, she had an eye exam and was started on the recommended eye drops, including dexamethasone 0.1 % prior to the infusion and then three times daily on days 1–3, brimonidine tartrate 0.2 % prior to each infusion, and artificial tears four times daily. Prior to cycle 2, she was noted to have punctate epithelial keratitis (PEK) bilaterally (1+ in the right eye and 2+ in the left eye). Fluromethanlone steroid eye drops were added to her eye care regimen and she was instructed to increase her use of artificial tears. Her eye irritation continued to increase and she had persistent PEK, now 2+ in both eyes, again managed conservatively with increasing steroids as well as adding celluvisc to her regimen. Brimonidine tartate drops were also added due to the increased steroid use to prevent elevated intraocular pressure. The PEK stabilized, but she developed pseuedomembranes in her left eye that required removal. While her exams were overall stable to improved, she continued to have symptoms of dry eye, so following cycle 8 she had punctal plugs placed. She did not require dose reductions due to the keratitis and is tolerating the treatment well.
Patient 2: The second patient is a 29-year-old female diagnosed with stage IB1 HPV-associated moderately differentiated adenocarcinoma in December 2016 who had a CR following CRT, completed March 3, 2017. She had a biopsy proven recurrence in February 2020 and a PET/CT showing increased FDG-avid left supraclavicular, mediastinal, retroperitoneal, and right iliac chain LNs and a left peritoneal mass. She received 6 cycles of carboplatin/paclitaxel/bevacizumab with post-treatment scans demonstrating a CR. She continued maintenance bevacizumab for 27 cycles. Her second recurrence was diagnosed on July 11, 2022 (8 month DFI) by PET/CT that showed increased size and avidity of a left periaortic LN. TV was initiated on July 28, 2022, and PET/CT performed after three cycles showed a CR with no FDG avidity (Fig. 1B). Unfortunately, TV was discontinued after eight cycles due to recurrent/persistent punctate epithelial erosions and grade 3 dry eye. Like the first patient, she had a baseline eye exam and was started on the recommended eye care regimen with the initiation of TV. Unfortunately, prior to C2, she was already having significant dry eye and mild punctate epithelial erosions (PEE). Despite being seen every three weeks by ophthalmology who managed symptoms with bandage contact lenses, antibiotic eye drops, corticosteroids, lid wipes, and preservative free artificial tears, she continued to have worsening dry eye and PEE and TV was discontinued. She otherwise tolerated TV well with mild toxicities including grade 2 peripheral neuropathy. Restarting TV will be considered if progression is noted in the future pending ophthalmology recommendations. She is now 9 months from initiation of TV and has no evidence of disease.
Patient 3: The third patient is a 47-year-old female diagnosed with stage IV gastric-type, HPV-independent cervical adenocarcinoma on October 19, 2021 with PET/CT showing an avid cervical mass, no definite metastatic disease, but concerning right external iliac and left pelvic sidewall LNs. At the time of a planned surgery on November 29, 2021, she was found to have more extensive disease and underwent a radical hysterectomy, bilateral salpingo-oophorectomy with debulking of pelvic LNs and a peritoneal lesion. She received six cycles of adjuvant carboplatin/paclitaxel completed on March 30, 2022, with progressive disease on CT demonstrating multiple new foci of nodularity within the peritoneum including in the right lower abdomen, left lower abdomen, and along the left paracolic parietal peritoneum. She was referred to radiation oncology, and she received IMRT to the pelvis (4500 cGY in 25 fractions) as well as a boost (2500 cGY in 5 fractions) to 2 peritoneal nodules followed by bevacizumab. CT scan 2 months after the end of treatment demonstrated progression of disease and TV was started. CT performed after four cycles of TV showed a CR (Fig. 1C).
Like our other 2 patients, she also had significant ocular toxicities. She had a normal eye exam and was started on the recommended eye drops prior to TV initiation. After cycle 1, she was found to have allergic conjunctivitis likely to brimonidine tartrate drops. Allergy relief eye drops were added to her regimen and the brimonidine tartrate drops were discontinued and substituted with Visine. With cycles 3–5, she had increased dry eye and mild increases in intraocular pressure, relieved with celluvisc gel and timolol, respectively. She also had pseudomembrane formation that required removal and 1 + PEK that responded to an increase in steroid drops. Unfortunately, prior to cycle 6, she had worsening symptoms and her exam showed 2–3 + diffuse PEK and the TV dose was reduced from 2 mg/kg to 1.3 mg/kg. Prior to cycle 7, she had continued symptoms and persistent PEK, although slightly improved, so her dose was again reduced from 1.3 mg/kg to 0.9 mg/kg. Other interventions that were used for symptom management included moisture chamber goggles, night time eye lubricant, autologous serum eye drops, visiplugs in lower eyelids, and amniotic membrane lens placement. Despite these interventions, she required an entropion repair of the right eye secondary to keratoconjunctivitis. After extensive counseling, she has elected to continue TV at the reduced dose of 0.9 mg/kg. Other toxicities included grade 1 nausea and emesis. She is on her eleventh cycle of TV with no new disease sites, but some avidity increases in a previously noted right pelvic sidewall lesion. Therefore, she had a response of at least 8 months with confirmation of disease progression pending at the time of writing this manuscript.
3. Discussion
Recurrent metastatic cervical cancer conveys a poor prognosis with limited treatment options. TV is an innovative treatment with good efficacy and tolerability in trials, which is consistent with our findings in our first 3 patients treated with the drug.
Multiple treatment regimens have been trialed for metastatic and recurrent cervical cancer, starting with cisplatin or carboplatin in combination with paclitaxel, which has a response rate of 29 % with an OS of approximately 13 months (Monk, 2009). OS was improved to 16.8 months when bevacizumab was added to platinum-based doublets (Tewari, 2017). Immunotherapy was then evaluated in recurrent or progressive disease after chemotherapy. In a phase II trial, single agent pembrolizumab had an ORR of 14.3 % with a median duration of response (DOR) that had not been reached at 24 month follow up (range 3.7–35.2 months) in patients with a PD-L1 positive [combined positive score (CPS) of 1] recurrent cervical cancer (Marabelle, 2020). Despite the relatively low ORR, the demonstrated long DOR led to the FDA approval of single-agent pembrolizumab in PD-L1 positive cervical cancer. Keynote 826 then moved pembrolizumab earlier in the treatment paradigm for advanced and recurrent cervical cancer by studying a combination of a platinum-doublet with pembrolizumab with or without bevacizumab (Colombo, 2021). This study demonstrated increased PFS (10.4 vs 8.2 months) and OS (estimated 24-month survival of 53 % vs 41.7 %) in patients with persistent, recurrent, or metastatic PD-L1 positive cervical cancer (Colombo, 2021).
Prior to the development of TV, most patients with recurrent cervical cancer had limited efficacious treatment options following chemotherapy. TV is an ADC with tissue factor (TF) as the antibody target and monomethyl auristatin E (MMAE) as the drug payload (De Bono, 2019). Most cervical cancers overexpress TF on their surface, making it an ideal target for an ADC. Additionally, TV has a bystander effect, meaning even those cells in the tumor environment that are TF negative are also exposed to the payload, increasing the clinical efficacy in heterogeneous tumors (Hong, 2020). TV was initially studied in Innova 201, a phase 1–2 open-label basket trial that included multiple solid tumors with a 15.6 % ORR rate for allcomers and an ORR of 26 % in the cervical cancer cohort (De Bono, 2019). A cervical cancer specific phase 2 study (Innova 204) was developed to evaluate TV in patients with recurrent or metastatic squamous cell, adenocarcinoma, or adenosquamous cervical cancer with progressive disease after chemotherapy (Coleman, 2021). In the study population, 68 % had squamous cell carcinoma, 60 % had recurrent disease, and 80 % had 2 prior lines of systemic therapy. Innova TV 204 demonstrated a favorable ORR of 24 % with 7 % CR and a median time to response of 1.4 months, consistent with the first imaging following initiation of treatment. The (PFS) was 4.2 months and DOR was 8.3 months (Coleman, 2021). These data confirmed TV as an exciting novel treatment for recurrent or metastatic cervical cancer.
We have presented our first three patients who received TV for recurrent or progressive metastatic cervical cancer. These patients had three different histologies including HPV-mediated squamous cell and adenocarcinoma as well as an HPV-independent gastric-type cervical cancer. We report a 100 % response rate, and more impressively, a 100 % complete response rate. Similar to published literature, the response to TV is rapid with a median time to response of 2.2 months and we noted a CR after 3–6 cycles (median 4.4 months) (Table 1). While we are unable to comment on a DOR because none of our patients have had a definitive recurrence, the mean time since the initiation of treatment is 184 days. These real-world data are encouraging in that we are observing a rapid and excellent response to TV, even in patients who have HPV-independent cervical cancer.
Table 1.
Demographics and tumor characteristics. Basic demographics of each patient as well as the clinical characteristics of each cervical cancer, prior treatments, and response to TV. Abbreviations: cisplatin (cis), carboplatin (carbo), bevacizumab (bev), external beam radiation therapy (EBRT), radiation therapy (RT), brachytherapy (brachy), squamous cell carcinoma (SCC).
| Patient 1 | Patient 2 | Patient 3 | Mean | |
|---|---|---|---|---|
| Age | 27 | 29 | 47 | 34.33 |
| Ethnicity | Black | White | White | |
| BMI | 27.1 | 28 | 26.3 | 27.13 |
| Comorbidities | N/A | N/A | N/A | |
| ECOG performance status | 0 | 0 | 0 | |
| CPS score | 0 | 0 | 0 | |
| Stage at Diagnosis | IIB | IB1 | IVB | |
| Prior Regimens | cis/RT + brachy | cis/RT + brachy | carbo/taxol + EBRT | |
| carbo/taxol/bev | carbo/taxol/bev | bev | ||
| cemiplimab | ||||
| Line of therapy | 4.00 | 3.00 | 3.00 | 3.33 |
| Histology | SCC | Adenocarcinoma | Gastric type | |
| HPV | + | 18+ | neg | |
| Time to PR (mos) | 2.00 | 2.00 | N/A | 2.00 |
| Time to CR (mos) | 4.00 | 6.00 | 3.00 | 4.33 |
For our patients, TV was well tolerated with mostly grade 1 toxicities (Table 2), although the reported toxicities differed from the most common toxicities in the Innova trials where the most common adverse events reported were alopecia (38 %), epistaxis (30 %), nausea (27 %), conjunctivitis (26 %), fatigue (26 %), and dry eyes (23 %) (De Bono, 2019, Hong, 2020) Our patients experienced worse ocular toxicities compared to trial data. InnovaTV201 reported conjunctivitis in 43 % and dry eye in 22 % of participants, with severe adverse effects of grade 3 or higher in only 3 % of participants. InnovaTV 204 noted ocular treatment related adverse events in 53 % of patients with only 2 % having grade 3 events (Hong, 2020). In sharp contrast, our three patients all experienced severe ocular toxicities requiring discontinuation of therapy in one of three patients and significant dose reduction in a second despite excellent ophthalmology care throughout treatment (Table 2).
Table 2.
Adverse events. Adverse events documented for each patient. Ocular toxicities are documented separately due to their severity leading to treatment breaks or discontinuation.
| Patient 1 | Patient 2 | Patient 3 | |
|---|---|---|---|
| Alopecia | |||
| Epistaxis | |||
| Nausea | 1 | 1 | 2 |
| Fatigue | 1 | 1 | 1 |
| Decreased appetite | 1 | 1 | |
| Constipation | 1 | ||
| Peripheral neuropathy | 2 | ||
| Vomiting | 1 | 2 | |
| Diarrhea | 1 | ||
| Abdominal pain | 1 | 1 | |
| Anemia | 2 | ||
| Hypokalemia | 1 | ||
| Pruritus | |||
| Pyrexia | |||
| Urinary Tract Infections | |||
| All Ocular AESIs: | |||
| Conjunctivities | 3 | 3 | 3 |
| Dry Eye | 2 | 3 | 3 |
| Ulcerative Keratitis | 2 | ||
| Blepharitis | |||
| Keratitis | 3 | 3 | 3 |
We can only theorize why our patient population experienced ocular toxicities at a considerably higher rate than published literature. Our first theory is that all our patients live in the metro Denver area which is a dry, arid climate at high altitude. There tends to be higher dry eye rates for people living at high altitudes compared to those living at sea level due to lower humidity and tear evaporation (Lu, 2008). This altitude-related phenomena was described in a population based prospective study from Tibet, which reported 52.4 % prevalence of dry eye syndrome (Lu, 2008). Therefore, our patients may have underlying dry eye syndrome leading to an increased risk of TV-related ocular toxicities. Another theory is that since our small population of patients experienced substantially higher response rates than those reported in the literature, there was perhaps more drug uptake increasing the off-target toxicities. However, the only adverse effects our patients experienced more than reported in InnovaTV 204 were ocular toxicities.
It will be important moving forward to aggressively manage ocular toxicities in our patients. Knowing that our patients have worse dry eye, we could consider using celluvisc at initiation of TV rather than using artificial tears as all our patients were using celluvisc within the first 2–3 treatments. Additionally, we should consider dose reductions earlier in the treatment course. All 3 of our patients developed ocular toxicities within the first 3 cycles of their treatment course and in 2 of the patients, the toxicities continued to progress despite appropriate changes in their eye care. For example, patient 2 may have benefited symptomatically from a dose reduction prior to cycle 2 when erosions were first noted, and this may have extended her time on treatment. Patient 3 did ultimately require a dose reduction, but she may have had decreased ocular symptoms if the dose reduction happened 1–2 cycles earlier.
Despite these ocular toxicities, it is notable that our first 3 cervical cancer patients treated with TV had a 100 % CR with a DOR at least as long as what is reported in the literature. Importantly, TV was also effective in a patient with HPV-independent cervical cancer. While our patients tolerated treatment well, they did have significant ocular toxicities that were worse than those reported. We hypothesize that this is due to our arid climate at altitude. While we typically do not think of different climates or altitudes as important factors in the efficacy and toxicity of drugs, there is increasing literature demonstrating that these environmental factors do affect drug metabolism (Duan, 2022). Therefore, to understand the complexity of patient and environmental interactions with drug efficacy and side effects, we are advocating for diversifying the sites included in clinical trials as well as collecting real world data in phase IV clinical trials extending to multiple sites.
Author contributions
Lisa Marie Babayan: Collected data, wrote the manuscript, performed analysis.
Catherine Bouts: contributed to the writing and editing of the manuscript.
Saketh Guntupalli: Conceived the presented idea and contributed to the writing and editing of the manuscript.
Nicole Marjon: Helped with idea conception, Designed the layout and analysis, contributed to the writing and editing of the manuscript.
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
CRediT authorship contribution statement
Lisa Marie Babayan: Writing – review & editing, Writing – original draft, Formal analysis, Data curation. Catherine Bouts: . Saketh Guntupalli: Writing – review & editing, Supervision, Conceptualization. Nicole A. Marjon: Writing – review & editing, Writing – original draft, Supervision, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coleman R.L., et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22:609–619. doi: 10.1016/S1470-2045(21)00056-5. [DOI] [PubMed] [Google Scholar]
- Colombo N., et al. Pembrolizumab for persistent, recurrent, or metastatic cervical cancer. N. Engl. J. Med. 2021;385:1856–1867. doi: 10.1056/NEJMoa2112435. [DOI] [PubMed] [Google Scholar]
- De Bono J.S., et al. Tisotumab vedotin in patients with advanced or metastatic solid tumours (InnovaTV 201): a first-in-human, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:383–393. doi: 10.1016/S1470-2045(18)30859-3. [DOI] [PubMed] [Google Scholar]
- Duan Y., et al. Exposure to high-altitude environment is associated with drug transporters change: microRNA-873-5p-mediated alteration of function and expression levels of drug transporters under hypoxia. Drug Metab. Dispos. 2022;50:174–186. doi: 10.1124/dmd.121.000681. [DOI] [PubMed] [Google Scholar]
- Ferlay J., et al. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- Hong D.S., et al. Tisotumab Vedotin in previously treated recurrent or metastatic cervical cancer. Clin. Cancer Res. 2020;26:1220–1228. doi: 10.1158/1078-0432.CCR-19-2962. [DOI] [PubMed] [Google Scholar]
- Lu P., et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008;27:545–551. doi: 10.1097/ICO.0b013e318165b1b7. [DOI] [PubMed] [Google Scholar]
- Marabelle A., et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk B.J., et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a gynecologic oncology group study. J. Clin. Oncol. 2009;27:4649–4655. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose P.G., et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N. Engl. J. Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- Tewari K.S., et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) The Lancet. 2017;390:1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda N., Watari H., Ushijima K. Department of Obstetrics and Gynecology, Kurume University School of Medicine, Kurume, Japan, & Department of Obstetrics and Gynecology, Hokkaido University Graduate School of Medicine, Sapporo, Japan. Chemotherapy and molecular targeting therapy for recurrent cervical cancer. Chin. J. Cancer Res. 2016;28:241–253. doi: 10.21147/j.issn.1000-9604.2016.02.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu M., Yu J., Awolude O.A., Chuang L. Cervical cancer worldwide. Curr. Probl. Cancer. 2018;42:457–465. doi: 10.1016/j.currproblcancer.2018.06.003. [DOI] [PubMed] [Google Scholar]