Abstract
Background
Triple-negative breast cancer (TNBC) lacks effective therapeutic targets. Scutellaria barbata D.Don (SB) has been revealed to have anti-breast cancer (BC) effect, but the effect of SB extract in TNBC is still unclear. Herein, this research delves into the underlying mechanism.
Methods
SB was extracted by solvent extraction, and the main components were identified using an Agilent 6,520 HPLC-Chip/Q-TOF (Chip/Q-TOF) MS system. In vitro cell experiments were conducted. The effects of SB extract alone, SB extract plus EGF, GSK alone, GSK plus Ezrin overexpression, or SB extract plus Ezrin overexpression on cell viability, invasion, migration, and apoptosis were examined by cell function experiments. The apoptosis- and RhoA/ROCK1 pathway-related protein levels were analyzed by western blot assay.
Results
Mass spectrometry analysis exhibited that SB extract mainly contains long-chain fatty acids and ursolic acid. SB extract mitigated TNBC cell biological phenotypes, apoptosis- and RhoA/ROCK1 pathway-related marker expressions, which were reversed by EGF. The further results found that GSK obviously weakens TNBC cell biological behaviors, apoptosis- and RhoA/ROCK1 signaling-related protein levels, while oe-Ezrin treatment reverses the effect of GSK on TNBC cells. Moreover, SB extract regulated Ezrin-mediated function of TNBC cells by impeding the RhoA/ROCK1 pathway.
Conclusion
Our findings demonstrated that SB extract regulated Ezrin-mediated proliferation, migration, invasion, and apoptosis of TNBC cells via suppressing the RhoA /ROCK1 signaling. Our results offer the experimental foundation for further investigation of the anti-cancer role of SB in TNBC cells.
Highlights
SB extract inhibits the biological phenotypes of TNBC cells.
SB extract inhibits the biological behaviors of TNBC cells through the RhoA/ROCK1 pathway.
SB extract modulates Ezrin-mediated TNBC cell proliferation, migration, invasion, and apoptosis via restraining the RhoA/ROCK1 signaling.
Keywords: triple negative breast cancer, RhoA, ROCK1, Ezrin, scutellaria barbata D.Don extract
Introduction
Breast cancer (BC) is a kind of malignant tumor originating from the Epithelium of the breast, which has become the most common malignant tumor among women today.1 Triple-negative breast cancer (TNBC) refers to breast cancer with negative expression of estrogen receptor (ER), progestogen receptor (PR), and human Epidermal growth factor receptor 2 (HER2).2,3 It is the most difficult subtype to treat at present, accounting for 10% ~ 20% of BC, which has the clinical characteristics of strong invasion, rapid progress, high recurrence rate, and poor prognosis.4 Currently, the therapy methods for BC include surgery, neoadjuvant chemotherapy, adjuvant chemotherapy, endocrine therapy, and targeted therapy.5 Due to the lack of ER, PR, and HER2 expression in TNBC patients, endocrine therapy and anti-HER2 targeted therapy cannot be used.6 Therefore, it is urgent to find new therapeutic targets and therapy drugs for TNBC.
Scutellaria barbata D. Don (SB) is a commonly applied Chinese medicine, which has the effects of clearing heat and removing toxicity, promoting blood circulation for removing blood stasis, inducing diuresis to reduce edema.7 Pharmacological studies have found that SB manifests a significant role in the treatment of tumors, which can effectively weaken the proliferation of a variety of tumor cells, including hepatoma, cervical cancer, and colorectal cancer.8–10 The killing effect of SB on tumor cells is related to multiple cell pathways. Xue et al. have confirmed that SB can restrain the biological behaviors of cervical cancer cells by activating the miR-195-5p/LOXL2 pathway.9 Another study has reported that ursolic acid (one of the effective components in SB) can alleviate the proliferation, invasion, and migration of BC cells by regulating the ERK and PI3K/AKT pathways, thus playing an anti-tumor role.11 However, there are few studies on SB in TNBC. The mechanism of SB’s anti-TNBC has not been fully elucidated.
The Rho/ROCK pathway is a crucial intracellular signaling pathway, which is tightly correlated with the pathological and physiological processes of malignant tumors.12 RhoA is a key signaling molecule of Rho/ROCK pathway, which exhibits a pivotal role in cell morphogenesis, cell polarity, and cell migration by activating its downstream target molecule Lock.13 Research has indicated that RhoA, as an oncogene, is highly expressed in basal-like breast tumors, hepatocellular cancer, and lung cancer.14,15 According to the literature reports, the Rho protein family is associated with the occurrence of BC.16 Scientific research has shown that ursolic acid is involved in the modulation of the RhoA/ROCK signaling, which can reverse liver fibrosis by suppressing the activation of NOX4/ROS in hematopoietic stem cells (HSCs), and down-regulating the expression of Rac1 (a member of the RhoA Homeotic gene family).17 Therefore, we speculate whether SB extract impedes the progression of TNBC cells by regulating the RhoA/ROCK signaling.
In order to investigate the potential inhibitory effect of SB extract on TNBC cells and its underlying mechanism, this study takes human TNBC cell lines (MDA-MB-231 and BT-20) as the research objects to assess the repressive effect of SB extract on TNBC cell biological phenotypes and its molecular mechanism. This study offers a reference basis for the development of SB extract and the clinical treatment of TNBC.
Methods
Extraction of petroleum ether from SB
The dried 5 kg of SB was crushed into coarse particles, and extracted by the following process: 75% ethanol overnight three times, 1:10 of solid–liquid ratio and 1 h each time. The extract was concentrated under a vacuum to yield a crude extract of SB. The crude extract of SB was dispersed with 2,000 mL deionized water and then extracted with 60–90 °C petroleum ether (1:1 v/v) 8 times. The extraction solution was concentrated under reduced pressure and purified via silica gel (100–200 mesh size) column chromatography by applying ethyl acetate as eluent. The petroleum ether extract of SB was dissolved in methanol (10 mg/mL) and filtered by 0.22 μm filter followed by storage at −20 °C. The petroleum ether extract of SB was diluted to the desired concentration with a culture medium. SB extract was subjected to liquid chromatography-mass-spectrometry under an Agilent 6,520 HPLC-Chip/Q-TOF (Chip/Q-TOF) MS system.
Cell culture
Human TNBC cell lines (MDA-MB-231 and BT-20) were obtained from Shanghai Ruiyong Biotechnology Co., Ltd (Shanghai, China). These two cell lines are common cell lines commonly used in TNBC research. Cell passages 3-5 were grown in RPMI-1640 medium (iCell-0002, iCell Bioscience, Shanghai, China) with 10% fetal bovine serum (iCell-0500, iCell Bioscience) at 37 °C in a humidified incubator with 5% CO2.
Transfection
For the construction of Ezrin overexpression (oe-Ezrin), the full-length sequence of Ezrin was constructed from YouBio followed by insertion into pcDNA3.1 vector (VT1001, YouBio, Hunan, China). An empty vector was taken as a negative control of oe-Ezrin (oe-NC). The above plasmids were then transfected into MDA-MB-231 and BT-20 cells using Lipo6000 (C0526, Beyotime, Shanghai, China).
Treatments and groups
Experiments were evenly divided into 5 parts, where each part was as follows: Part 1 (Cell cultures were treated with different dosages of SB extract (0, 100, 200, 300, 400, and 500 μg/mL) for 24 h); 500 μg/mL of SB extract was selected according to the results of cell counting kit-8 (CCK-8) test for subsequent experiments. Part 2 (TNBC cells were stimulated with or without 500 μg/mL SB extract for 24 h); Part 3 (TNBC cells were subjected to 500 μg/mL SB extract for 24 h and then treated with or without 50 ng/mL RhoA/ROCK1 pathway activator, EGF (SRP3027, Sigma-Aldrich) for 24 h); Part 4 (TNBC cells transfected with or without oe-NC/oe-Ezrin were intervened with or without 1 μM RhoA/ROCK1 pathway inhibitor, GSK429286A (HY-11000, MedChemExpress) for 24 h); Part 5 (TNBC cells transfected with or without oe-NC/oe-Ezrin were treated with or without 500 μg/mL SB extract for 24 h).
Cell viability
After digesting and counting MDA-MB-231 and BT-20 cells, the above cells were inoculated into 96 well plates at a density of 3 × 104 cells/mL per well (100 μL per hole). After the cells adhered to the wall and processed according to the methods of Part 1 ~ 5. The each well added 10 μL of diluted CCK-8 solution (C266180, Aladdin, Shanghai, China) and continued to culture for 3 h. Optical density (OD) values of each well were then assessed with the use of a microplate reader (LX800, BioTek, Biotek Winooski, Vermont, USA) at a wavelength of 450 nm. Cell viability was calculated as follows:
 |
Where ODs, ODc, and ODb represent OD value of the experimental group, the control group, and the blank group, respectively.
Colony formation assay
MDA-MB-231 and BT-20 cells were inoculated with a cell density of 500 cells per well into a 6-well plate, with a volume of 2 mL per well. After incubation overnight, 2 mL of the supernatant was discarded and processed according to the methods of Part 1 ~ 5 and then cultivated for 7 days (the medium was changed after every 3 days in culture). Next, they were washed and then fixed with 2 mL of 4% (w/v) paraformaldehyde for 20 min. We stained the cells with 0.25% crystal violet (C8470, Solarbio, Beijing, China) for 2 min. The stained results were acquired under an optical microscope (Eclipse E100, Nikon, Tokyo, Japan).
Transwell assay
In the invasion experiment, TNBC cells (1 × 104 per well) were re-suspended in medium without FBS and plated into the upper chamber with inserts pre-coated with Matrigel, while the corresponding lower chamber was added to DMEM medium containing 10% FBS. In the migration experiment, TNBC cells (4 × 104 per well) were loaded in the upper chamber in serum-free medium, and the complete medium was injected into the lower chamber at the same time. After being cultured for 24 h, the cells on the upper chamber were eliminated using the cotton swabs, and those cells presented on the lower surface of the inserts were fixed with methanol, which was then stained with 0.1% crystal violet (C0121, Beyotime). At last, the cells that passed through the filter were photographed and counted under the optical microscope in five randomly chosen microscopic fields.
Flow cytometry
Annexin V-FITC/PI apoptosis kit (556547) was bought from BD Pharmingen (San Diego, CA, USA). The conditions of cell inoculation and sample treatment were the same as those mentioned in section 2.4. After treatment, the cells were digested and collected. The cells were centrifuged for 5 min at 2,000 rpm, and the supernatant was discarded. After adding 1 mL 1× Binding Bufer and centrifuging (4 °C, 2,000 rpm, 5 min), 100 μL 1× Binding Buffer, 10 μL Annexin V-FITC, and 5 μL propidium iodide (PI) were added. The treated cells were placed in a dark room at 37 °C for 15 min. Finally, the cells were analyzed through flow cytometry.
Western blot
After treatment, the cells were digested and harvested. The content of protein in the supernatant was determined using the Coomassie brilliant blue method. The 5% configure concentrated gel and 8% separation gel was prepared, and 20 μL protein samples were used for electrophoresis. After electrophoresis, samples were transferred to PVDF membranes and blocked for 1 h at room temperature with a 5% blocking solution. Samples were incubated overnight at 4 °C with primary antibody to Bax (1:1000, ab32503, 21 kDa, Abcam, Cambridge, MA, USA), Bcl-2 (1:1000, ab59348, 26 kDa, Abcam), Caspase3 (1:2000, ab13847, 34 kDa, Abcam), RhoA (1:2000, ab187027, 22 kDa, Abcam), p-RhoA (1:2000, ab41435, 22 kDa, Abcam), ROCK1 (1:2000, ab134181, 158 kDa, Abcam) and p-ROCK1 (1:1000, ab203273, 158 kDa, Abcam), Ezrin (1:2000, ab4069, 69 kDa, Abcam), β-actin (1:5000, ab8226, 42 kDa, Abcam). Next, all membranes were incubated with HRP-labeled secondary antibody for 2 h. Finally, membranes were visualized using an ECL kit (E266188, Aladdin) in a ChemiDoc Gel Imaging System (Bio-Rad, Hercules, CA, USA).
Statistical analysis
The experimental data are expressed by means ± standard deviation (SD), and the experimental results are analyzed by SPSS 19.0 software. The differences among multiple groups were analyzed utilizing a one-way analysis of variance followed by Tukey’s post hoc test. The comparison between the two groups was performed by Student’s t test. A p-value of <0.05 was considered significant.
Results
HPLC-Q-TOF-MS analysis
By searching the Scifinder database and related literature, using the Formulafinder function of the software, combined with the first-and second-order mass spectra of related compounds, as well as isotope distribution and cracking law of compounds, the compounds were analyzed and identified. The petroleum ether extract of SB mainly includes long-chain fatty acids and terpenoids. The TIC diagram and related data of SB petroleum ether extract are shown in Fig. S1 and Table S1.
Cell viability of MDA-MB-231 and BT20 cells treated with different concentrations’ SB extract
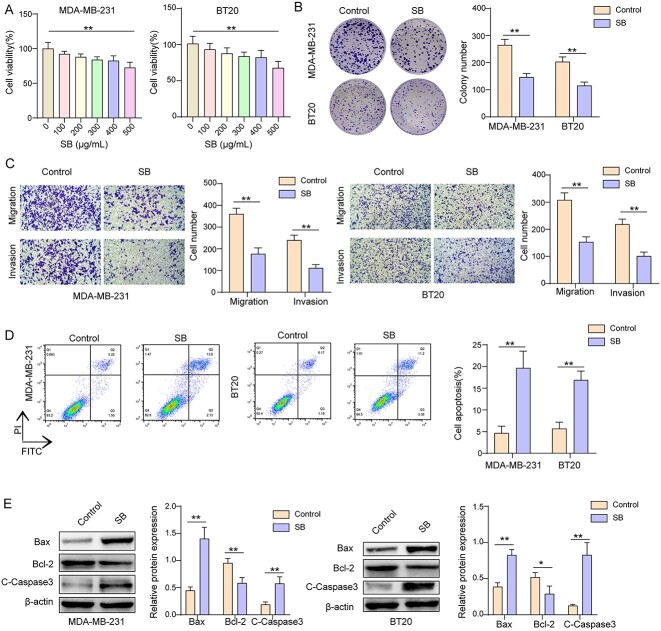
In Fig. 1A, compared with the control group, there was no statistically significant difference at SB extract concentrations in the range of 0 to 400 μg/mL, indicating that SB extract had no obvious suppressive effect on the cell viability of both cells within the concentration range (0 to 400 μg/mL) in this experiment. However, the cell viability of both cells was slightly but effectively weakened at SB extract concentration in 500 μg/mL (Fig. 1A). Therefore, 500 μg/mL was selected as the best experimental concentration of SB extract.
Fig. 1.
SB extract inhibited the biological phenotypes of TNBC cells. A) After cell cultures were treated with different dosage SB extract (0, 100, 200, 300, 400, and 500 μg/mL) for 24 h, the cell viability was detected by CCK-8. B) Clone formation was analyzed by colony formation assay. C) The numbers of migration and invasion cells were examined by transwell assay. D) The apoptosis cells were tested by flow cytometric. E) The protein expression levels of Bax, Bcl-2, and C-Capase3 were tested by western blot. n = 3. Data were displayed as mean ± SD. *P < 0.05, **P < 0.01.
SB extract inhibited the biological behaviors of TNBC cells
The effect of SB extract on the proliferation of MDA-MB-231 and BT-20 cells was assessed by functional experiments. The number of cell clones were decreased notably after SB extract treatment (Fig. 1B). The further transwell assay results presented that the numbers of cell migration and invasion are largely lessened by SB extract treatment (Fig. 1C). SB extract also extremely facilitated the apoptosis of TNBC cells (Fig. 1D). Moreover, by conducting western blot assay, we found that the expressions of Bax and C-Capase3 in TNBC cells treated with SB extract are up-regulated markedly, while the level of Bcl-2 is down-regulated prominently (Fig. 1E). In summary, SB extract obviously inhibited the proliferation, migration, and invasion but promoted apoptosis of TNBC cells.
SB extract restrained the activation of the RhoA/ROCK1 pathway
First, This study used molecular docking technology to visually analyze the docking of ursolic acid extracted from SB petroleum ether with RhoA protein receptors. Our results found that ursolic acid from SB petroleum ether extract can effectively bind to the active cavities on the surface of RhoA protein. Ursolic acid can form important hydrogen bonds with alanine 161, lysine 118, lysine 162, tyrosine 120, threonine 19, histidine 17, arginine 15, glutamic acid 16, and glutamic acid 20 (Fig. S2). Further, to verify whether the suppression of SB extract on the RhoA/ROCK1 pathway, the RhoA/ROCK1 pathway-related markers were examined. TNBC cells were subjected to 500 μg/mL SB extract for 24 h and then treated with or without 50 ng/mL EGF (RhoA/ROCK1 pathway activator) for 24 h. As presented in Fig. 2A and B, SB extract notably attenuated the phosphorylation levels of RhoA and ROCK1 in MDA-MB-231 and BT-20 cells. However, EGF treatment partially reversed the effect of SB extract on the phosphorylation levels of RhoA and ROCK1. Moreover, we also reconfirmed that EGF significantly promoted RhoA and ROCK1 phosphorylation, and found EGF enhanced Ezrin expression in TNBC cells (Fig. S3). Taken together, SB extract could indeed restrain the RhoA/ROCK1 pathway.
Fig. 2.
SB extract restrained the activation of the RhoA/ROCK1 pathway. A and B) After TNBC cells were subjected to 500 μg/mL SB extract for 24 h, and then treated with or without 50 ng/mL RhoA/ROCK1 pathway activator, EGF for 24 h, Western blot was selected to examine the levels of RhoA, p-RhoA, ROCK1, and p-ROCK1 in MDA-MB-231 and BT20 cells. n = 3. Data were presented as mean ± SD. *P < 0.05, **P < 0.01.
SB extract regulated TNBC cell function by weakening the RhoA/ROCK1 pathway
As shown in Fig. 3A and B, EGF partially offset the low cell survival rate and small number of clones caused by SB extract. The results of transwell elucidated that the suppression of SB extract on TNBC cell migration and invasion is reversed by EGF (Fig. 3C). The further flow cytometry assay confirmed that the apoptosis of TNBC cells induced by SB extract is greatly inhibited by EGF (Fig. 3D). Then, we detected apoptosis-related markers using western blot. SB extract largely up-regulated the expressions of Bax and C-Capase3 but down-regulated the level of Bcl-2 in TNBC cells, which was counteracted after EGF intervention (Fig. 3E). Altogether, the suppression of SB extract on the progression of TNBC was influenced by the RhoA/ROCK1 pathway activator EGF.
Fig. 3.
SB extract regulated TNBC cell function by weakening the RhoA/ROCK1 pathway. TNBC cells were subjected to 500 μg/mL SB extract for 24 h and then treated with or without 50 ng/mL RhoA/ROCK1 pathway activator, EGF for 24 h. A) CCK-8 detected the cell viability of TNBC cells. B) The colony formation assay determined the clone formation of TNBC cells. C) Transwell assay analyzed the numbers of cell migration and invasion. D) The flow cytometric was used to assess the apoptosis of TNBC cells. E) The protein levels of Bax, Bcl-2 and C-Capase3 were measured by western blot. n = 3. Data were presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
RhoA/ROCK1 pathway regulated Ezrin-mediated function of TNBC cells
To validate whether the RhoA/ROCK1 pathway regulated Ezrin-mediated TNBC cell function, TNBC cells transfected with or without oe-NC/oe-Ezrin were intervened with or without 1 μM RhoA/ROCK1 pathway inhibitor, GSK429286A for 24 h. As shown in Fig. 4A–C, GSK obviously weakened TNBC cell proliferation, clone formation, migration, and invasion, while oe-Ezrin treatment reversed the effect of GSK on TNBC cells. Furthermore, GSK obviously promoted TNBC cell apoptosis, while oe-Ezrin reversed this trend (Fig. 4D). The expression of Bcl-2 protein decreased, and the expressions of Bax and C-Capase3 enhanced after GSK treatment, while this trend was partially offset by oe-Ezrin (Fig. 4E). To further understand the mechanism of the RhoA/ROCK1 pathway regulated Ezrin-mediated function of TNBC cells, the western blot results indicated that the expressions of ROCK1, p-ROCK1, and Ezrin decrease in the GSK group than the control group, while the expressions of RhoA and p-RhoA remain unchanged (Fig. 4F). However, the expression of Ezrin protein increased in the GSK + oe-Ezrin group than the GSK + oe-NC group, while the expression of RhoA, p-RhoA, ROCK1, and p-ROCK1 protein remained unchanged (Fig. 4F). The above data unveiled that the RhoA/ROCK1 pathway regulated Ezrin-mediated TNBC cell function.
Fig. 4.
RhoA/ROCK1 pathway regulated Ezrin-mediated function of TNBC cells. After TNBC cells transfected with or without oe-NC/oe-Ezrin were intervened with or without 1 μM RhoA/ROCK1 pathway inhibitor, GSK429286A for 24 h. A) The cell viability was evaluated by CCK-8. B) Clone formation was measured by the colony formation assay. C) The numbers of cell migration and invasion were tested utilizing transwell assay. D) The numbers of apoptotic cells were tested with flow cytometric. E) The protein levels of Bax, Bcl-2 and C-Capase3 were tested with western blot. F) The levels of RhoA, p-RhoA, ROCK1, p-ROCK1, and Ezrin were tested with western blot. n = 3. Data were manifested as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
SB extract regulated Ezrin-mediated function of TNBC cells by impeding the RhoA/ROCK1 pathway
As shown in Fig. 5A and B, oe-Ezrin reversed the low cell survival rate and small number of clones caused by SB extract. Transwell results clarified that oe-Ezrin prominently neutralizes the inhibition of SB extract on TNBC cell migration and invasion (Fig. 5C). Furthermore, SB extract could induce cell apoptosis but oe-Ezrin introduction could reverse this trend (Fig. 5D). Western blot results demonstrated that SB extract attenuates Bcl-2 protein expression, and advances the expressions of Bax and C-Capase3, while the above effect was reversed by Ezrin overexpression (Fig. 5E). Moreover, SB extract alone largely alleviated the phosphorylation levels of RhoA and ROCK1 and the protein level of Ezrin in TNBC cells (Fig. 5F). However, relative to the SB + oe-NC group, the expression of Ezrin protein increased in the SB + oe-Ezrin group, while the expressions of RhoA, p-RhoA, ROCK1, and p-ROCK1 protein remained unchanged (Fig. 5F). Altogether, SB extract could modulate Ezrin-mediated TNBC cell function by suppressing the RhoA/ROCK1 signaling.
Fig. 5.
SB extract regulated Ezrin-mediated function of TNBC cells by impeding the RhoA/ROCK1 pathway. After TNBC cells transfected with or without oe-NC/oe-Ezrin were treated with or without 500 μg/mL SB extract for 24 h. A) The cell viability was determined utilizing CCK-8. B) Clone formation was measured using the colony formation assay. C) The migration and invasion numbers of TNBC cells were tested with transwell assay. D) The numbers of apoptotic cells were tested with flow cytometric. E) The levels of Bax, Bcl-2, and C-Capase3 were tested with western blot. F) The levels of RhoA, p-RhoA, ROCK1, p-ROCK1, and Ezrin were assessed with western blot. n = 3. Data were exhibited as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
TNBC has the characteristics of high invasion, high malignancy, easy metastasis, and poor prognosis, with approximately 30% of patients experiencing recurrence and metastasis.18,19 Therefore, anti-TNBC recurrence and metastasis are the focus of treatment. The major findings from this experiment were: SB extract regulated Ezrin-mediated function of TNBC cells by impeding the RhoA/ROCK1 pathway. This finding supported the latent capacity to develop a range of SB-based drug therapies for TNBC.
In clinical practice, SB is widely used in the treatment of tumors, such as colon tumor and BC.20,21 Some scholars have manifested that SB can impede the generation and metastasis of bone-metastatic BC cells through a variety of signal pathways, so as to achieve the therapeutic effect of bone metastasis of BC.22 Zheng et al. found that total flavonoids from SB can weaken the proliferation, migration, and invasion of breast carcinoma cells through the down-regulation of the PTHrP pathway, thus impeding human breast carcinoma bone metastasis.23 The main components of BC in this study are long-chain fatty acids and terpenoids. This study has found that SB extract can restrain the proliferation, migration, and invasion of TNBC cells but promote apoptosis. The results of this study are consistent with the report of Jiang et al., namely, Breast Defend (BD), a natural dietary supplement containing SB, has a significant inhibitory effect on the proliferation and invasive behavior of MDA-MB-231 cells.24 Apoptosis is an active and programmed process of cell death that is precisely regulated by multiple genes under certain physiological or pathological conditions, which is an important self-stabilizing regulatory mechanism in the body.25 We discovered that the expression of anti-apoptotic Bcl-2 protein in TNBC cells treated with SB extract decreased, and the expressions of pro-apoptotic Bax and C-Capase3 protein increased.
RhoA/ROCK1 signaling exhibits a crucial role in cancer development and progression, which induces cytoskeleton reorganization, cell migration, apoptosis, and stress fiber formation.26 The activation of RhoA/ROCK1 pathway can accelerate the proliferation and migration of BC.27 The active ingredient of SB, ursolic acid was reported to participate in the regulation of the RhoA/ROCK signaling, which could reverse liver fibrosis by suppressing the NOX4/ROS pathway in Hepatic Stellate Cells and down-regulating the expression of Rac1 (a member of the RhoA Homeotic gene family).17 Based on the inspiration of previous studies, this study explored whether SB extract attenuated the malignant phenotype of TNBC cells by inhibiting the RhoA/ROCK1 signaling. Excitingly, SB extract weakened TNBC cell malignant phenotypes by weakening the RhoA/ROCK1 pathway.
Ezrin is an important member of the ERM family of cytoskeleton-related proteins and is a transport protein between membrane proteins and actin fibers.28 The study suggests that Ezrin may play a role in invasion and metastasis by regulating a variety of cell processes, including the formation of microvillus, the maintenance of cell morphology, the connection between cells, and the promotion of cell movement in cervical cancer.29 Clinical data shows that the abnormal localization and expression of Ezrin are positively correlated with poor prognosis in several cancer tissues including breast cancer.30 The overexpression of Ezrin protein also showed the role of promoting BC metastasis in animal model experiments.31 The RhoA/ROCK1 signaling pathway can increase NHERF1 reverse transcription, leading to an increase in Ezrin protein-dependent secretion.32,33 Consistent with the research findings of Ma et al.,34 we also found that EGF alone largely promoted RhoA and ROCK1 phosphorylation, and enhanced Ezrin expression in TNBC cells. This study further validated the effect of Ezrin on TNBC cells using GSK429286A (RhoA/ROCK1 inhibitor). By detecting cell biological behaviors and RhoA/ROCK1 pathway-related markers, this study found that the RhoA/ROCK1 pathway can indeed regulate Ezrin-mediated TNBC cell function. Previous studies have shown that RhoA and ROCK1 mainly regulate Ezrin protein in the form of phosphorylation, affecting the invasion and metastasis of BC cells.35 It is believed that p-RhoA and p-ROCK1 may regulate the invasion and metastasis of BC cells as upstream signals of Ezrin. Our innovation is that the petroleum ether extract of SB modulates Ezrin-mediated TNBC cell function by suppressing the RhoA/ROCK1 signaling.
Ezrin overexpression can significantly enhance Ezrin expression. However, some findings on cell viability, invasion, migration, and apoptosis could not be restored to control levels because cell viability, invasion, migration, and apoptosis are not entirely dependent on the effect of Ezrin. Because the regulation of cellular processes is quite complex, it is not completely regulated by a single molecule or pathway. Extracts of SB may also affect other proteins and pathways. In summary, in the RhoA/ROCK1 signal transduction pathway, SB extract can impede the expressions of p-RhoA, p-ROCK1, and Ezrin protein, thereby inhibiting the proliferation, metastasis, and differentiation of TNBC cells. Due to the complexity of traditional Chinese medicine ingredients and the diversity of disease target protein molecular pathways, this study is only based on the preliminary exploration of the RhoA/ROCK1 signal and Ezrin protein in the treatment of TNBC with SB extract, and further detailed research is needed in the future. In the future, we will separate the different components of SB extract and study the effects of each component on breast cancer cells to find the most effective one or several components for drug development and clinical research.
Conclusions
The findings in the present study indicated that SB extract regulates Ezrin-mediated TNBC cell proliferation, migration, invasion, and apoptosis via suppressing the RhoA/ROCK1 signaling, but further detailed research is needed in the future.
Supplementary Material
Acknowledgments
We would like to thank the anonymous reviewers who have helped to improve the paper.
Contributor Information
Junjie Niu, Department of Medical Oncology, Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine, No. 58, Lushan Road, Yuelu District, Changsha, Hunan Province 410000, P. R. China.
Jinyang Hu, Department of Medical Oncology, Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine, No. 58, Lushan Road, Yuelu District, Changsha, Hunan Province 410000, P. R. China.
Zhu Wang, Department of Medical Oncology, Affiliated Hospital of Hunan Academy of Traditional Chinese Medicine, No. 58, Lushan Road, Yuelu District, Changsha, Hunan Province 410000, P. R. China.
Author contributions
Jinyang Hu guaranteed the integrity of the entire study; Junjie Niu designed the study and literature research; Jinyang Hu defined the intellectual content; Junjie Niu performed experiment; Zhu Wang collected the data; Zhu Wang analyzed the data; Jinyang Hu wrote the main manuscript and prepared figures. All authors reviewed the manuscript.
Funding
Not applicable. This study did not receive any funding in any form.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not Applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1. Li H, Liu RB, Long CM, Teng Y, Cheng L, Liu Y. Development and validation of a new multiparametric random survival Forest predictive model for breast cancer recurrence with a potential benefit to individual outcomes. Cancer Manag Res. 2022:14:909–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheng J, Li C, Dong M, Jiang K. Identification by comprehensive bioinformatics analysis of KIF15 as a candidate risk gene for triple-negative breast cancer. Cancer Manag Res. 2020:12:12337–12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shi H, Wang XH, Gu JW, Guo GL. Development and validation of nomograms for predicting the prognosis of triple-negative breast cancer patients based on 379 Chinese patients. Cancer Manag Res. 2019:11:10827–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lv X, He M, Zhao Y, Zhang L, Zhu W, Jiang L, Yan Y, Fan Y, Zhao H, Zhou S, et al. Identification of potential key genes and pathways predicting pathogenesis and prognosis for triple-negative breast cancer. Cancer Cell Int. 2019:19(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Qian W, Yang L, Ni Y, Yin F, Qin L, Yang Y. LncRNA LINC01857 reduces metastasis and angiogenesis in breast cancer cells via regulating miR-2052/CENPQ axis. Open Med (Wars). 2022:17(1):1357–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lin Y, Jin X, Nie Q, Chen M, Guo W, Chen L, Li Y, Chen X, Zhang W, Chen H, et al. YTHDF3 facilitates triple-negative breast cancer progression and metastasis by stabilizing ZEB1 mRNA in an m(6)A-dependent manner. Ann Transl Med. 2022:10(2):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li L, Xu X, Wu L, Zhu H, He Z, Zhang B, Chi Y, Song G. Scutellaria barbata polysaccharides inhibit tumor growth and affect the serum proteomic profiling of hepatoma H22-bearing mice. Mol Med Rep. 2019:19(3):2254–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kan X, Zhang W, You R, Niu Y, Guo J, Xue J. Scutellaria barbata D. Don extract inhibits the tumor growth through down-regulating of Treg cells and manipulating Th1/Th17 immune response in hepatoma H22-bearing mice. BMC Complement Altern Med. 2017:17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xue S, Geng A, Lian T, Liu Y. Scutellaria barbata D. Doninhibits cervical cancer cell proliferation, migration, and invasion via miR-195-5p/LOXL2 axis. Toxicol Res (Camb). 2022:11(5):804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu L, Liu T, Tao W, Liao N, Yan Q, Li L, Tan J, Shen W, Cheng H, Sun D. Flavonoids from Scutellaria barbata D. Don exert antitumor activity in colorectal cancer through inhibited autophagy and promoted apoptosis via ATF4/sestrin2 pathway. Phytomedicine. 2022:99:154007. [DOI] [PubMed] [Google Scholar]
- 11. Kim GD. Ursolic acid decreases the proliferation of MCF-7 cell-derived breast cancer stem-like cells by modulating the ERK and PI3K/AKT Signaling pathways. Prev Nutr Food Sci. 2021:26(4):434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esposito D, Pant I, Shen Y, Qiao RF, Yang X, Bai Y, Jin J, Poulikakos PI, Aaronson SA. ROCK1 mechano-signaling dependency of human malignancies driven by TEAD/YAP activation. Nat Commun. 2022:13(1):703. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Gu M, Ni H, Sheng X, Pauciullo A, Liu Y, Guo Y. RhoA phosphorylation mediated by Rho/RhoA-associated kinase pathway improves the anti-freezing potentiality of murine hatched and diapaused blastocysts. Sci Rep. 2017:7(1):6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Privat M, Cavard A, Zekri Y, Ponelle-Chachuat F, Molnar I, Sonnier N, Bignon YJ. A high expression ratio of RhoA/RhoB is associated with the migratory and invasive properties of basal-like breast Tumors. Int J Med Sci. 2020:17(17):2799–2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009:1796(2):91–98. [DOI] [PubMed] [Google Scholar]
- 16. Aleskandarany MA, Sonbul S, Surridge R, Mukherjee A, Caldas C, Diez-Rodriguez M, Ashankyty I, Albrahim KI, Elmouna AM, Aneja R, et al. Rho-GTPase activating-protein 18: a biomarker associated with good prognosis in invasive breast cancer. Br J Cancer. 2017:117(8):1176–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang C, Gan D, Luo F, Wan S, Chen J, Wang A, Li B, Zhu X. Interaction mechanisms between the NOX4/ROS and RhoA/ROCK1 Signaling pathways as new anti- fibrosis targets of Ursolic acid in hepatic stellate cells. Front Pharmacol. 2019:10:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gong S, Song Z, Spezia-Lindner D, Meng F, Ruan T, Ying G, Lai C, Wu Q, Liang Y. Novel insights into triple-negative breast cancer prognosis by comprehensive characterization of aberrant alternative splicing. Front Genet. 2020:11:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Xue B, Zhang Y, Guo G, Duan X, Dou D. Up-regulated circBACH2 contributes to cell proliferation, invasion, and migration of triple-negative breast cancer. Cell Death Dis. 2021:12(5):412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yue GG, Chan YY, Liu W, Gao S, Wong CW, Lee JK, Lau KM, Lau CB. Effectiveness of Scutellaria barbata water extract on inhibiting colon tumor growth and metastasis in tumor-bearing mice. Phytother Res. 2021:35(1):361–373. [DOI] [PubMed] [Google Scholar]
- 21. Li Z, Li J, Liu X, Sun Z, Sun X. Ethyl acetate fraction from Hedyotis Diffusa plus Scutellaria Barbata inhibits the progression of breast cancer via targeting LMO1 and AKT/Mtor Signaling pathway. Comb Chem High Throughput Screen. 2023:26:1–10. [DOI] [PubMed] [Google Scholar]
- 22. Fang T, Yan YX, Yang Y, Lv YX, Chang QQ, Zhang DD. Ethyl acetate fraction from Hedyotis diffusa plus Scutellaria barbata suppresses migration of bone-metastatic breast cancer cells via OPN-FAK/ERK/NF-κB Axis. Evid Based Complement Alternat Med. 2020:2020:3573240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zheng X, Kang W, Liu H, Guo S. Inhibition effects of total flavonoids from Sculellaria barbata D. Don on human breast carcinoma bone metastasis via downregulating PTHrP pathway. Int J Mol Med. 2018:41(6):3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiahua Jiang, Wojnowski R, Jedinak A, Sliva D. Suppression of proliferation and invasive behavior of human metastatic breast cancer cells by dietary supplement BreastDefend. Integr Cancer Ther. 2011:10(2):192–200. [DOI] [PubMed] [Google Scholar]
- 25. Bui-Xuan NH, Tang PM, Wong CK, Fung KP. Photo-activated pheophorbide-a, an active component of Scutellaria barbata, enhances apoptosis via the suppression of ERK-mediated autophagy in the estrogen receptor-negative human breast adenocarcinoma cells MDA-MB-231. J Ethnopharmacol. 2010:131(1):95–103. [DOI] [PubMed] [Google Scholar]
- 26. Gkretsi V, Louca M, Stylianou A, Minadakis G, Spyrou GM, Stylianopoulos T. Inhibition of breast cancer cell invasion by Ras Suppressor-1 (RSU-1) silencing is reversed by growth differentiation Factor-15 (GDF-15). Int J Mol Sci. 2019:20(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan L, Li H, An W, Wei W, Zhang X, Wang L. Mex-3 RNA binding MEX3A promotes the proliferation and migration of breast cancer cells via regulating RhoA/ROCK1/LIMK1 signaling pathway. Bioengineered. 2021:12(1):5850–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bretscher A, Reczek D, Berryman M. Ezrin: a protein requiring conformational activation to link microfilaments to the plasma membrane in the assembly of cell surface structures. J Cell Sci. 1997:110(24):3011–3018. [DOI] [PubMed] [Google Scholar]
- 29. Kong J, Di C, Piao J, Sun J, Han L, Chen L, Yan G, Lin Z. Ezrin contributes to cervical cancer progression through induction of epithelial-mesenchymal transition. Oncotarget. 2016:7(15):19631–19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan K, Long B, Deshpande GM, Fox PL. Bidirectional tumor-promoting activities of macrophage Ezrin. Int J Mol Sci. 2020:21(20):7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li N, Kong J, Lin Z, Yang Y, Jin T, Xu M, Sun J, Chen L. Ezrin promotes breast cancer progression by modulating AKT signals. Br J Cancer. 2019:120(7):703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuang YQ, Pang W, Zheng YT, Dupré DJ. NHERF1 regulates gp120-induced internalization and signaling by CCR5, and HIV-1 production. Eur J Immunol. 2012:42(2):299–310. [DOI] [PubMed] [Google Scholar]
- 33. Zhou Q, Jiang J, Chen G, Qian C, Sun G. Inflammatory immune cytokine TNF-α modulates Ezrin protein activation via FAK/RhoA Signaling pathway in PMVECs Hyperpermeability. Front Pharmacol. 2021:12:676817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ma L, Liu YP, Zhang XH, Xing LX, Wang JL, Geng CZ. Effect of RhoA signaling transduction on expression of Ezrin in breast cancer cell lines. Ai Zheng. 2009:28(2):108–111. [PubMed] [Google Scholar]
- 35. Ma L, Liu YP, Zhang XH, Geng CZ, Li ZH. Relationship of RhoA signaling activity with ezrin expression and its significance in the prognosis for breast cancer patients. Chin Med J. 2013:126(2):242–247. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.