Abstract
Background
Renal interstitial fibrosis is the pathophysiological basis of type 2 diabetes mellitus (T2DM). Exercise appears to improve kidney interstitial fibrosis in T2DM, in which silent information regulator factor 2-related enzyme 1 (Sirt1) is a critical regulator. However, the role of Sirt1 in mediating exercise on renal tissue as well as its mechanism remains unknown.
Methods
T2DM mouse models were created using a high-fat diet mixed with streptozotocin, followed by 8 weeks of treadmill exercise and niacinamide (Sirt1 inhibitor) intervention. Kits for detecting biochemical indices of renal function were used. The pathological appearance and severity of renal tissue were examined using hematoxylin and eosin, Masson and immunohistochemical staining. The mRNA and protein expression of relevant signaling pathway factors were determined to use real-time reverse transcriptase-polymerase chain reaction and western blotting.
Results
T2DM can promote renal interstitial fibrosis, increase kidney index, serum creatinine, blood urea nitrogen and 24 h urinary total protein and cause pathological changes in renal tissue and affect renal function. After 8 weeks of exercise intervention, the biochemical indicators in the kidney of T2DM mice were decreased, Sirt1 expression was increased, the expression of TGF-β1, Smad3, collagen type I (COL1) and collagen type III (COL3) were decreased, and the renal interstitial fibrosis, renal tissue structural lesions and renal function were improved. However, after the nicotinamide intervention, renal interstitial fibrosis of T2DM mice was aggravated, and the improvement effect of exercise on renal interstitial fibrosis of T2DM mice was abolished.
Conclusion
The upregulation of Sirt1 expression by exercise can inhibit the transforming growth factor β1/Smad3 pathway, thereby inhibiting the expression and deposition of COL1 and COL3 in renal interstitium, thereby improving renal interstitial fibrosis in T2DM.
Keywords: exercise, renal interstitial fibrosis, sirtuin 1, transforming growth factor-β1/Smad3, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic disorder of metabolism, which is often accompanied by hyperglycemia, insulin resistance and other symptoms. Renal interstitial fibrosis, characterized by the pathological changes where normal renal tubules and renal interstitial structures are replaced by a large amount of deposited extracellular matrix (ECM), is both the typical symptom presented at the terminal stage of T2DM and nephropathy, and the main cause of the patient’s pathological progression to death. Meanwhile, its fibrosis degree affects renal function (1, 2).
Silent information regulator factor 2-related enzyme 1 (Sirt1), as a key factor in regulating energy metabolism, exerts a nephroprotective effect by mediating renal anti-fibrosis and inflammatory response through the protein effect of nicotinamide adenine dinucleotide+ (NAD+) coenzyme (3, 4). Some studies found that the downregulation of Sirt1 expression in T2DM kidney led to renal interstitial fibrosis, accompanied by tubular atrophy or expansion, degeneration and interstitial connective tissue hyperplasia, capillary loss, inflammation, and other pathological manifestations, causing impaired renal function (5, 6). Simic et al. (7) have also confirmed that the diminished expression of Sirt1 would contribute to the higher occurrence of renal interstitial fibrosis in T2DM. That is related to the fact that the downregulation of Sirt1 expression can activate the transforming growth factor β1 (TGF-β1); TGF-β1 binds its transmembrane type II receptor and leads to phosphorylation of type I receptors (p-I receptors) (8). Next, p-I receptors can stimulate the SSXS motif at the C-terminus of R-Smads and start the p3TP-Lux promoter reporter and phosphorylate downstream Smad3, and translocate into the nucleus, which promotes the transformation of renal tubular epithelial cells into mesenchymal cells and the activation of collagen type I (COL1) and collagen type III (COL3)α1/α2 promoters, as well as their massive expression and deposition in the renal tubulointerstitium, causing excessive accumulation of ECM in the renal interstitium and resulting in fibrosis (9, 10, 11).
Exercise is an important means to improve renal interstitial fibrosis in T2DM (12). The study showed that treadmill exercise could upregulate Sirt1 expression in T2DM kidneys (13). The 8-week treadmill exercise improved T2DM renal interstitial fibrosis by inhibiting the TGF-β1/Smad3 pathway and the protein expression of COL1 and COL3 (6, 14). Although studies showed that the Sirt1-mediated TGF-β1/Smad3 pathway regulates the occurrence and development of renal interstitial fibrosis in T2DM (9, 11), and treadmill exercise could upregulate Sirt1 and inhibit the TGF-β1/Smad3 pathway in kidney tissue of T2DM mice (5, 6), there are few studies published on its mechanism.
In this case, the present study should conduct a preliminary exploration in such a field by modeling T2DM mice with high-fat diets and a streptozotocin (STZ) injection. Then, the mice were subjected to the 8-week downhill running exercise and Sirt1 inhibitor intervention. After that, the changes of renal interstitial fibrosis among T2DM mice and the action mechanism of the Sirt1-mediated TGF-β1/Smad3 pathway in improvement of renal interstitial fibrosis associated with exercise were analyzed at multiple levels, such as renal tissue structure, degree of renal tissue fibrosis and gene expression using hematoxylin and eosin (HE) staining, Masson staining, reverse transcription polymerase chain reaction, western blotting, immunohistochemistry and other technologies and methods.
Materials and methods
Laboratory animals
Fifty-five 4-week-old male C57BL/6 mice with an initial body weight of 16.42 ± 0.63 g were purchased from the Laboratory Animal Center of Yangzhou University (SCXK (Jiangsu) 2017-0007). Mice were maintained in a room with a temperature of 25 ± 3°C, a relative humidity of 60 ± 10%, and a 1:1 circadian ratio. All animal experiments were approved by the Institutional Animal Care and Use Committee of Yangzhou University. The code is No. YZU-TYXY-31. After 1 week of adaptive feeding, the mice were randomly divided into normal group (ZC, n = 12) and T2DM model group (n = 43). Then T2DM mice were treated with nicotinamide and downhill running for 8 weeks. In this study, 40 T2DM mice were successfully cultivated and the rate of model development was 93.02%, and they were randomly divided into four groups: T2DM control group (TC, ten mice), T2DM + Sirt1 inhibitor group (TY, ten mice), T2DM exercise group (TP, ten mice) and T2DM exercise + Sirt1 inhibitor group (TH, ten mice).
Animal model
To establish T2DM mice model, mice were adaptively fed with a normal chow diet for 1 week and then fed with a high-fat diet (15). After 8 weeks of high-fat diet feeding, the mice were intraperitoneally injected with a single dose of STZ, 110 mg/kg (572201, Sigma-Aldrich) (16), and non-model mice were injected with the same dose of sodium citrate buffer. The fasting blood glucose (FBG) of mice was measured on the third day after injection, and if it was higher than 16.7 mmol/L, the model was successfully established (12).
Inhibitor injection protocol
The mice in the TY and TH groups were intraperitoneally injected with nicotinamide (Sirt1 inhibitor) (S1899, Selleck Chemicals, Houston, TX, USA). The dose was 500 mg/kg one day apart for 8 weeks (17). Then, the mice in the TC and TP groups were intraperitoneally injected with the same dose of 0.9% saline (18, 19).
Exercise training protocol
The mice were trained at a −9° tilt angle for 8 weeks (6 days/week). In the first week of adaptive training, the training time was gradually raised from 30 to 50 min, and the training time was increased by 10 min every 2 days, and the training speed was 0.8 km/h. Then, the formal treadmill training was conducted from the second to eighth weeks, and the training speed was 0.8 km/h for 50 min per day, 6 days a week.
Measurements of body weight, fasting blood glucose and insulin levels
Body weight and blood glucose were measured from week 1 and monitored weekly thereafter until the end of the experimental period. FBG levels were determined using fresh blood samples collected from the tail vein of mice using an Abbott Medisense Optium glucose meter (Optium Xceed, Abbott Diabetes Care Ltd, Shanghai, China). Fasting serum insulin levels were measured using the Mouse Insulin Assay kit (SEKM-0141, Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) according to the manufacturer’'s instructions (20).
Oral glucose tolerance test and HOMA-IR
After fasting for 12 h, the mice were gavaged with glucose (2 mg/g), glucose concentrations were measured before administration and 15, 30, 60 and 120 min after glucose treatment (21). The area under the curve (AUC) value of blood glucose was calculated using GraphPad Prism software. Insulin resistance was calculated using the following formula: HOMA-IR = fasting insulin (mIU/L) × FBG (mmol/L) / 22.5 (22).
Biochemical analysis
After the 8-week exercise, the weight of mice in each group was measured by an electronic scale. The bilateral kidneys of the mice in each group were removed and the outer envelope and adipose tissue around the kidneys were cleared. The weight of the bilateral kidneys of each mouse was recorded, and the kidney index (KI) = kidney weight (mg)/body weight (kg) was calculated. The content of urinary total protein (UTP) was detected according to the standard procedure of pyrogallol red molybdenum method, and the 24 h UTP was calculated using the formula (UTP × 24 h urine volume = 24 h UTP) (23). Serum creatinine (SCr) was measured using the standard procedures of oxidase test, and blood urea nitrogen (BUN) was measured carefully using the diacetyl monoxime method (24).
Histopathological staining
Renal tissues fixed in 4% PFA at 4°C were dehydrated, permeabilized and embedded in paraffin to obtain 4 μm kidney tissue sections. Hematoxylin–eosin staining (G1120, Beijing Solarbio Science & Technology Co., Ltd.) was used to observe the structure and morphology of renal tissue, and Masson trichrome staining (G1346, Beijing Solarbio Science & Technology Co., Ltd.) was used to examine the renal interstitial fibrosis changes. The staining results were observed using a fluorescence (IX73, Olympus) inverted microscope (25).
Immunohistochemical staining
The expression of COL1 (1:100, PA1-26204, ThermoFisher Scientific) protein in renal interstitium was detected according to the standard steps of immunohistochemical staining of renal tissue sections (26), and the positive areas on the 4 μm paraffin-embedded sections of kidney tissue were quantitatively detected by an Olympus fluorescence inverted microscope and ImageJ software.
RT-qPCR analysis
Total RNA was extracted from 100 mg of the right kidney using the TRIzol method after grinding. The concentration and purity of RNA were detected, and the extracted RNA was reverse transcribed into cDNA by Takara reverse transcription kit (RR037A, Takara Bio). Then, the mRNA expression was quantitatively detected using the Takara quantitative kit (RR014A, Takara Bio). Moreover, we selected β-actin as the endogenous control, and used the 2–△△Ct method to calculate the relative gene expression compared with the reference gene was calculated using the 2− △△Ct method. Primer sequences of the related genes were designed and analyzed using Primer premier software (Table 1) and synthesized by Shanghai Sangon Bioengineering Co., Ltd. (Shanghai, China).
Table 1.
Primer sequences of related factors.
| Gene | Primer | Sequence |
|---|---|---|
| Sirt1 | Forward | 5′-CTACGGAACTCAGCGAAGG-3′ |
| Reverse | 5′-GTAACTTCTGGAACACCAACTC-3′ | |
| TGF-β1 | Forward | 5′-TGCGCTTGCAGAGATTAAAA-3′ |
| Reverse | 5′-AGCCCTGTATTCCGTCTCCGT-3′ | |
| Smad3 | Forward | 5′-CATTCCATTCCCGAGAACACTAA-3′ |
| Reverse | 5′-GCTGTGGTTCATCTGGTGGT-3′ | |
| COL1 | Forward | 5′-CACGTTTGGCTCAGAGTGAG-3′ |
| Reverse | 5′-CAAGGTCAAGAGTTGTGTGC-3′ | |
| COL3 | Forward | 5′-CATTATGGTAGCGCAAATTCG-3′ |
| Reverse | 5′-CGGGCCTACCTTAGAGTTGGG-3′ | |
| β-actin | Forward | 5′-ACCCAGAAGACTGTGGATGG-3′ |
| Reverse | 5′-TTCAGCTCAGGGATGACCTT-3′ |
Western blotting analysis
To obtain the total protein, mouse kidney tissues were lysed and ground in lysate. The supernatant was centrifuged and the protein concentration was determined using the BCA assay. The total protein was then separated, transferred and blocked using gel electrophoresis. The membranes were subsequently treated with primary antibodies overnight at 4°C. After washing, the membranes were treated with the appropriate secondary antibody. Following another round of washing, electrochemiluminescence solution was added drop by drop to develop the images. The pictures were developed using the ODYSSEY far-infrared dual-color fluorescence imaging system, and the relevant data were analyzed using Image software. The antibodies used are listed in Table 2.
Table 2.
Antibodies used for western blot analysis.
| Antibody | Catalog number | Company | Dilution ratio |
|---|---|---|---|
| Sirt1 | mAb#8469 | CST | 1:1000 |
| TGF-β1 | mAb#70667 | CST | 1:1000 |
| p-Smad3 | mAb#9520 | CST | 1:1000 |
| COL1 | mAb#72026 | CST | 1:1000 |
| COL3 | mAb#66887 | CST | 1:1000 |
| β-actin | mAb#4970 | CST | 1:1000 |
Statistical analysis
In this study, SPSS® 22.0 software was used to analyze the relevant data and show the mean ± s.d. Independent sample t-test and two-way analysis of variance were used to compare the differences between groups. P < 0.05 was regarded as a significant difference, and if P < 0.01, it means the difference was quite significant.
Results
Exercise improves body weight, hyperglycemia and insulin resistance in T2DM mice
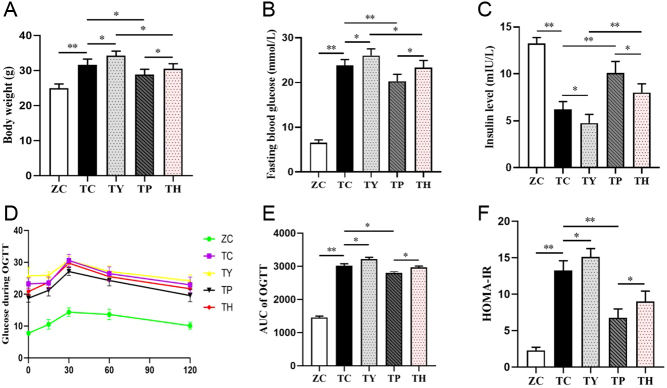
Body weight and FBG levels were significantly higher in mice with T2DM induced by a high-fat diet and STZ than in normal control mice (Fig. 1A and B). Blood glucose levels in all mice began to rise after the oral administration of glucose, peaked within 15 min, and then gradually returned to initial levels (Fig. 1D), and the oral glucose tolerance test AUC results showed a significant decrease in glucose tolerance in normal control mice (Fig. 1E). The level of insulin in the T2DM group was significantly lower than that in the normal control group (Fig. 1C), while the level of insulin resistance was significantly higher (Fig. 1F). The findings demonstrated that insulin resistance and overt hyperglycemia were prevalent in T2DM mice. However, the T2DM symptoms deteriorated after nicotinamide injection intervention. Compared with the TC group, the body weight, FBG level and insulin resistance level of the TY group were increased, and the insulin level was decreased. High fat, hyperglycemia, and insulin resistance are pathological symptoms of T2DM, which can be greatly alleviated by an 8-week exercise intervention. The body weight, FBG level and insulin resistance of the TP group were lower than those of the TC group, and the insulin level was higher than that of the TC group. Compared with the TY group, the body weight, FBG level and insulin resistance level of the TH group rats were decreased, and the insulin level was increased. However, niacinamide, as a blocking drug targeting Sirt1, may counteract the beneficial effects of exercise after injection. Body weight, FBG level and insulin resistance were increased in the TH group, while the insulin level was decreased in the TP group (Fig. 1A, B, C and F).
Figure 1.
Exercise reduces body weight and hyperglycemia and improves insulin resistance. (A) Body weight. (B) Fasting blood glucose. (C) Insulin levels. (D) Results of the OGTT. (E) Area under the curve of the OGTT. (F) Result of HOMA-IR. *P < 0.05, **P < 0.01. AUC, are under the curve; OGTT, oral glucose tolerance test.
Exercise improves the morphological and functional damage of renal tissue in T2DM but alleviated by nicotinamide intervention
The morphology of renal tissue revealed by HE staining was used to assess the changes in renal structure in T2DM, in addition to the effects of nicotinamide and exercise intervention. In the TC group, the renal pelvis was dilated, the renal parenchyma was narrowed, the number of renal tubules was reduced, the boundary was unclear as opposed to the ZC group. Moreover, the interstitium’s connective tissues multiplied, the glomeruli were crowded and dilated and some renal tubular epithelial cells were vacuolated, necrotic, atrophic, and exfoliated. In T2DM, nicotinamide treatment worsens renal interstitial fibrosis. The degenerative abnormalities in the kidney were more severe in the TY group than those in the TC group. Conversely, exercise can ameliorate the renal impairment associated with type 2 diabetes. The TP group had less renal pathology than the TC group, and the inflammatory lesions of the renal tubules, interstitium and perivascular were milder, as was the glomerular congestion. In contrast to the TY group, the renal inflammatory lesions were made better in the TH group, as well as the glomerular congestion. Nevertheless, niacinamide intervention diminished the positive effects of exercise. Compared with the TP group, the expression of KI, SCr, BUN and 24 h UTP was declined in the TH group (Fig. 2A).
Figure 2.
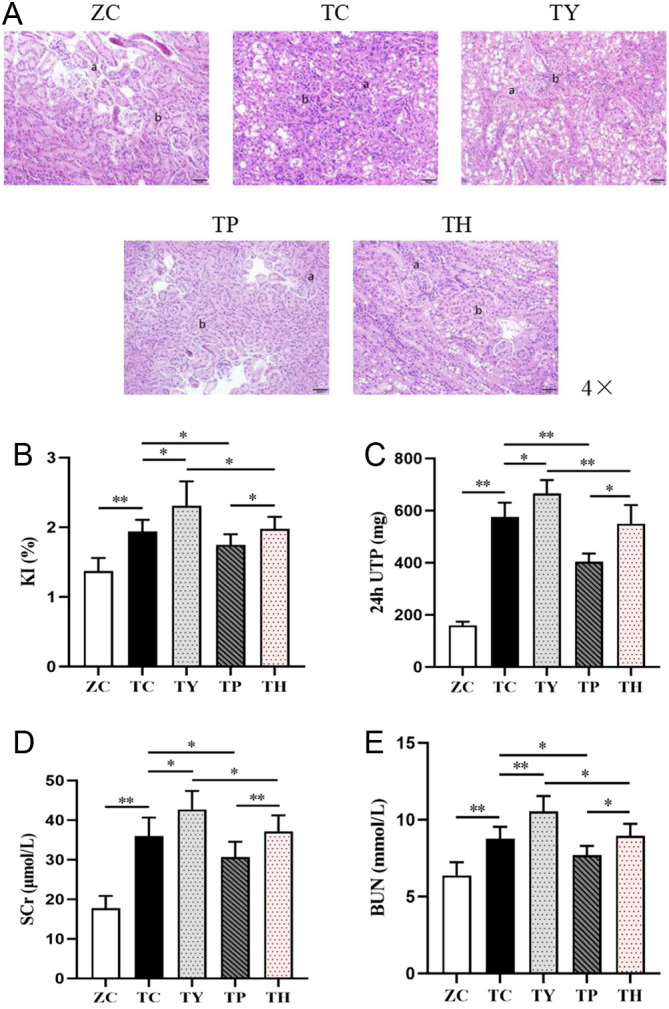
Renal injury in each group. (A) Representative images of H&E staining. Bar: 50 μm. (B, C, D, E) Expression levels of KI, 24UTP, SCr and BUN. (a) Glomerulus. (b) Renal interstitium. *P < 0.05, **P < 0.01.
Then, we examined biochemical markers in kidney tissue and discovered that KI, SCr, BUN and 24 h UTP in the TC group were considerably higher than those in the ZC group. Furthermore, niacinamide treatment may reduce Sirt1 expression, which substantially compromises renal function in T2DM. KI, SCr, BUN and 24 h UTP in the TY group were considerably greater than those in the TC group. The findings were consistent with those of the TP group when compared to the TH group. Exercise can improve renal tissue function in T2DM mice. Nevertheless, there was a decrease in the expression of KI, SCr, BUN and 24 h UTP in the TC group compared with the TP group. As the TY group was compared to the TH group, the results were consistent (Fig. 2B, C, D and E).
Exercise alleviates renal interstitial fibrosis induced by T2DM
The proportion of the interstitial glial fiber area was measured using Masson staining to detect renal interstitial fibrosis. Compared to the ZC group, the renal interstitial area in the TC group was greatly enhanced, and a considerable number of blue-purple collagen fibers were deposited in the renal tubular interstitium; the percentage of collagen area was also greatly enhanced. Nicotinamide can induce severe renal inflammation in T2DM mice, which can be improved by an 8-week exercise intervention. When compared to the TC group, the blue-purple collagen deposition in the renal tubulointerstitium was dramatically enhanced in the TY group, while it was significantly decreased in the TP group. The favorable effect of exercise improvement was reduced after concurrent nicotinamide and exercise intervention. In contrast to the TY group, the TH group had much less blue-purple collagen deposition in the renal tubulointerstitium. The proportion of the blue-purple collagen deposition region in the renal tubule interstitium in the TH group was substantially higher than in the TP group (Fig. 3A and B).
Figure 3.
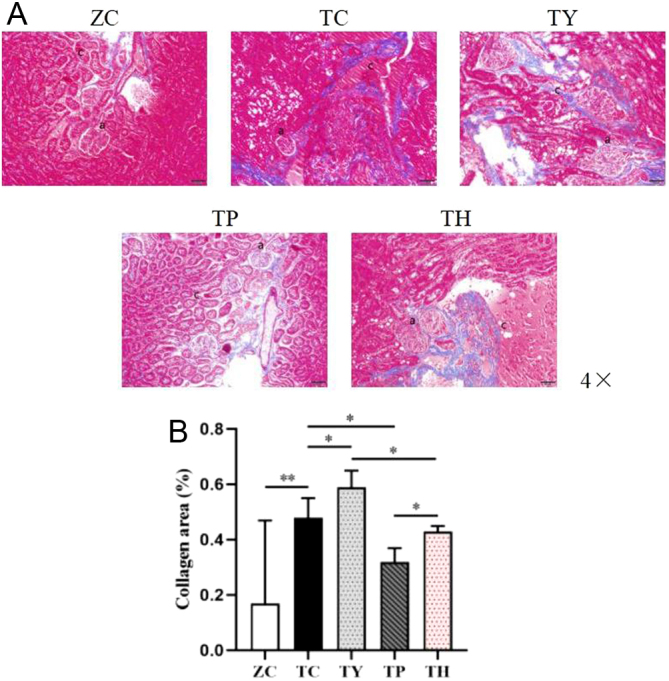
Renal interstitial fibrosis in each group. (A) Representative images of Masson staining. Bar: 50 μm. (B) The percentage of renal tubulointerstitial collagen area. (a) Glomerulus. (c) Collagen. *P < 0.05, **P < 0.01.
Sirt1 expression in renal tissue is regulated by exercise and nicotinamide
We investigated Sirt1 secretion levels in renal tissues in order to find out its contribution to the exercise-induced improvement of T2DM nephropathy. The mRNA and protein expression levels of Sirt1 in the TC group were lower than those in the ZC group. Following an 8-week exercise intervention, Sirt1 expression in the TP group was greater than that in the TC group. The results of the TH group were consistent with those of the TY group. It is well known that nicotinamide is the precursor of nicotinamide adenine dinucleotide, and Sirt1 is an NAD-dependent class III histone deacetylase, so nicotinamide is often used as an inhibitor of Sirt1 (17). After nicotinamide injection, the TY group experienced a decrease in Sirt1 mRNA and protein expression in contrast to the TC group. According to these findings, Sirt1 expression in the kidney declines with the onset of T2DM but can be restored by exercise intervention. Sirt1 is believed to regulate energy metabolism and may be implicated in the improvement of renal function impairment in T2DM by exercise. Nicotinamide obstructed the ameliorative effect of exercise in both treatments, and the expression of Sirt1 in the TH group was higher than that in the TY group but lower than that in the TP group (Fig. 4A, B and C).
Figure 4.
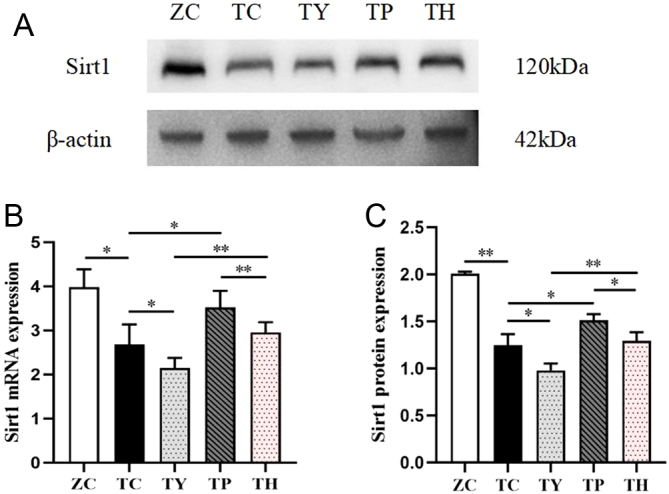
Sirt1 expression in kidney tissue of each group. (A) Sirt1 protein expression. (B) Sirt1 mRNA expression. (C) Quantification of Sirt1 protein expression. *P < 0.05, **P < 0.01.
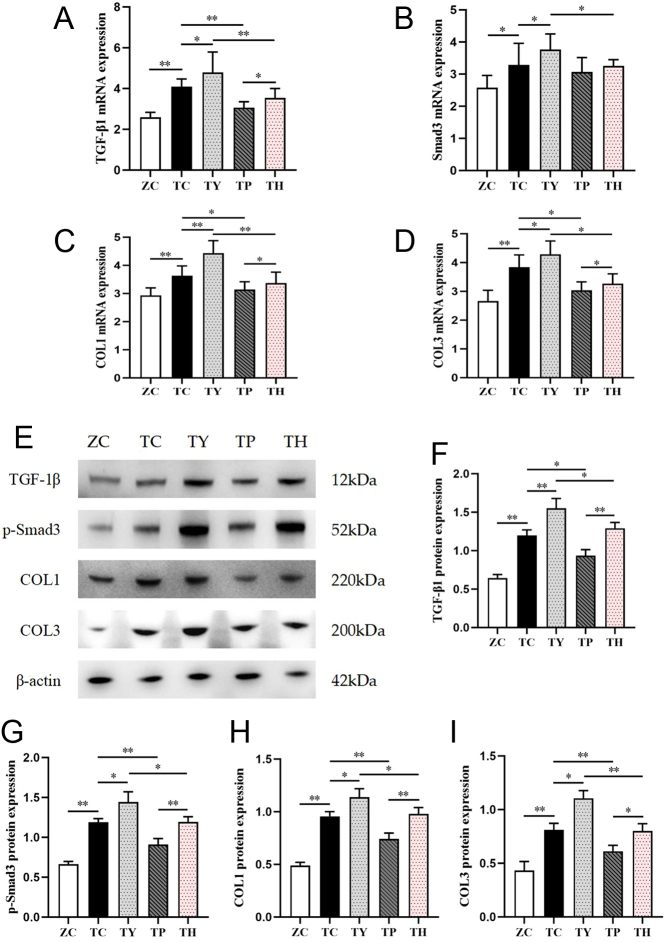
Exercise alleviates renal interstitial fibrosis in T2DM mice through the Sirt1-mediated TGF-β1/Smad3 pathway
TGF-β1 may initiate the downstream Smad protein transduction pathway, which impacts renal fibrosis and inflammation. We hypothesized that exercise could improve renal interstitial fibrosis by inhibiting the TGF-β1/Smad3 pathway. Compared to the ZC group, the mRNA and protein expression of TGF-β1, COL1 and COL3 were significantly upregulated in the TC group. The results were consistent with the expression of Smad3 mRNA and p-Smad3 protein. Sirt1 is a regulator of energy metabolism and can regulate the TGF-β1/Smad3 signaling pathway. Therefore, we blocked Sirt1 signaling by nicotinamide. Compared with the TC group, the mRNA and protein expression of TGF-β1, COL1 and COL3 were significantly upregulated in the TY group. The same was true for Smad3. Exercise intervention reversed the activation of this pathway. The mRNA and protein expression of TGF-β1, COL1 and COL3 were significantly downregulated in the TP group; however, Smad3 mRNA showed a downregulation trend, but the difference was not significant. Compared with the TY group, the mRNA and protein expression of TGF-β1, COL1 and COL3 were significantly downregulated in the TH group. Nevertheless, nicotinamide attenuated the effect of exercise intervention. Compared to the TP group, the mRNA and protein expression of TGF-β1, COL1 and COL3 were significantly upregulated in the TH group. The difference of Smad3 protein expression was more significant than that of mRNA expression (Fig. 5).
Figure 5.
Changes in mRNA and protein expression of TGF-β1/Smad3 pathway factors in the kidneys of each group. (A, B, C, D) mRNA expression of TGF-β1, Smad3, COL1 and COL3. (E, F, G, H, I) Protein expression and quantitative analysis of TGF-β1, Smad3, COL1 and COL3. *P < 0.05, **P < 0.01.
Immunohistochemistry results revealed that the percentage of a positive area of COL1 expression in the renal tissue in the TC group was considerably higher than that in the ZC group. Compared with the TC group, the percentage of a positive area of COL1 expression significantly increased in the TY group, and significantly decreased in the TP group. The percentage of positive area of COL1 expression markedly decreased in the TH group compared to the TY group. Moreover, the percentage of COL1 expression positive area in the TH group was significantly lower than that in the TY group, and significantly higher than that in the TP group (Fig. 6A and B).
Figure 6.
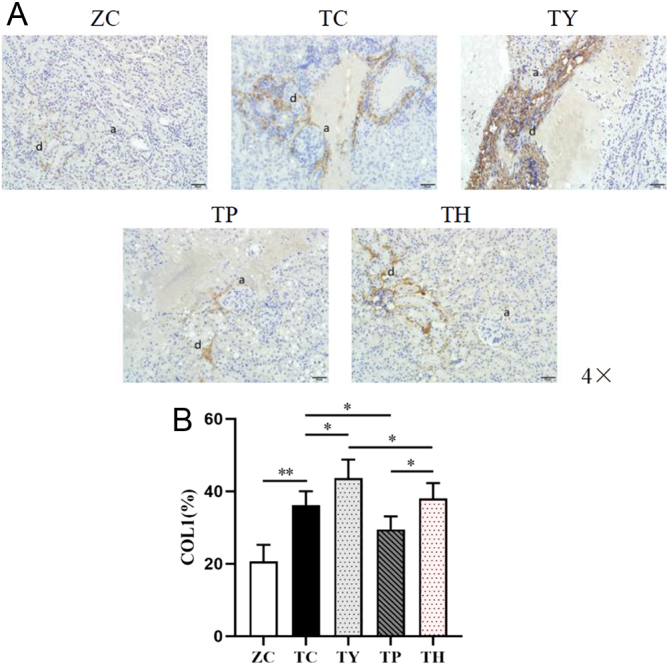
COL1 localization and expression in the kidney of each group. (A) Representative images of immunohistochemical staining. Bar: 50 μm. (B) The percentage variation of positive area of COL1 expression. (a) Glomerulus. (d) COL1. *P < 0.05, **P < 0.01.
Discussion
High-fat diet combined with STZ method was used to build T2DM model, and niacinamide (Sirt1 inhibitor) and exercise were added to intervene in T2DM mice. The key findings were as follows: (1) renal fibrosis in T2DM mice was closely related to the activation of the TGF-β1/Smad3 pathway after the downregulation of Sirt1 expression, which promoted the expression of COL1 and COL3 in renal tissue. (ii) Exercise significantly improved renal interstitial fibrosis among T2DM mice, and the main mechanism was that exercise upregulated Sirt1 expression and inhibited the TGF-β1/Smad3 pathway, consequently reducing the protein expression and deposition of COL1 and COL3 in the renal interstitium, thus improving renal interstitial fibrosis (Fig. 7).
Figure 7.
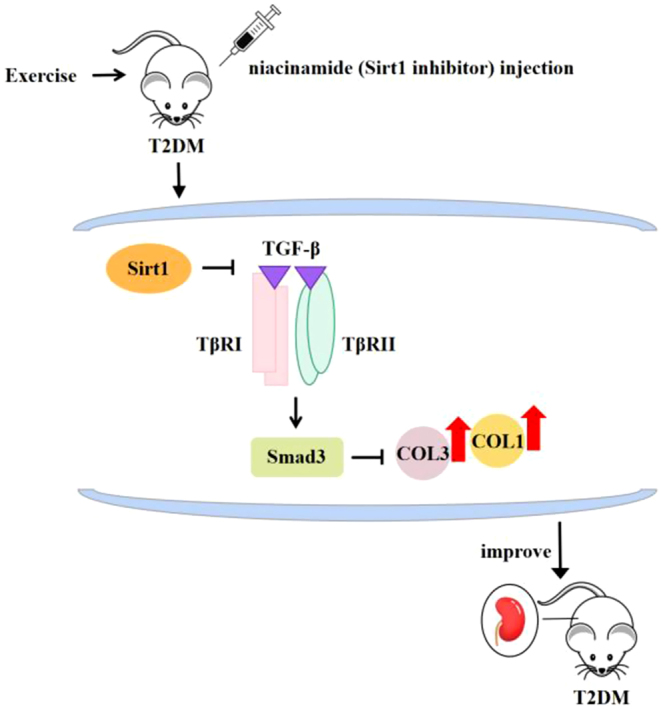
Schematic depicting the mechanism of the Sirt1-mediated TGF-β1/Smad3 pathway in exercise to improve renal interstitial fibrosis in T2DM.
Renal interstitial fibrosis is the pathological underpinning for the progressive development of T2DM-related renal lesions, and the KI index and biochemical indicators (SCr, BUN, 24 h UTP) in the blood and urine of T2DM mice were increased following renal dysfunction (27, 28). The present investigation found that KI, SCr, BUN and 24 h UTP were higher in T2DM mice. These findings showed that the renal function of T2DM mice was impaired because pathological changes in hemodynamics and hemorheology caused by T2DM hyperglycemia led to glomerular hyperperfusion and hyperfiltration, resulting in fine damage to renal tubules and impairments of large vessels (29). However, glomerular permeability increased while tubular reabsorption decreased, as did SCr, BUN, 24 h UTP and KI, and renal function was reduced (30, 31). Nogueira et al. (32) found that renal tissue microstructure lesions were caused by renal interstitial fibrosis. HE staining of the renal tissue showed that the renal pelvis was dilated; the renal parenchyma was narrowed; the number of renal tubules was reduced; the boundary was unclear; a large number of connective tissue hyperplasia in the interstitium; the glomerulus was congested and dilated; some renal tubular epithelial cells were vacuolated, necrotic, atrophic and shed; and inflammatory cell infiltration was seen in the renal tubulointerstitium and blood vessels. The number of renal tubules was reduced, the boundary was unclear and inflammatory cell infiltration was observed in the interstitium of renal tubules and around blood vessels. The fraction of collagen area in renal tissue in the ZC group was greater than that in the TC group, according to Masson staining. This study discovered a significant level of collagen expression and deposition in the renal interstitium of T2DM mice, as well as a severe degree of renal interstitial fibrosis, supporting previous findings (31).
Sirt1 is implicated in regulating renal interstitial fibrosis as a crucial element in regulating energy metabolism (33, 34). It was discovered that when Sirt1 expression was reduced, the TGF-β1/Smad3 pathway was activated, which exacerbated the deposition of ECM of COL1 and COL3 in the renal interstitium, leading to renal interstitial fibrosis (5, 35). The findings of the study were that the expression of Sirt1 mRNA and protein was remarkably reduced, while the mRNA and protein expression of TGF-β1, COL1, COL3 and Smad3 mRNA expression and p-Smad3 protein expression were rising in the TC group. The percentage of COL1 protein-positive area in the kidney was increased, and the excessive expression of collagen protein would aggravate renal interstitial fibrosis (9). After nicotinamide treatment, the mRNA and protein expressions of Sirt1, TGF-β1, COL1 and COL3 in the TY group were consistent with those in the TC group, and the differences were more significant, showing that the renal interstitial fibrosis in T2DM is more serious. Among them, Smad3 mRNA expression was not significant, the expression of p-Smad3 protein was downregulated, as it has been confirmed that Smad3 played a certain role in its own differential phosphorylation (36). In this study, it also found that the area percentage of mice’s renal interstitial collagen was increased in the TY group by Masson staining of kidney tissue, which led to significant structural and functional degeneration of the renal pelvis, glomerulus and tubule presented by HE staining. Meanwhile, impaired renal function led to significant increases in KI, SCr, BUN and 24 h UTP in the TY group. We could see that the Sirt1-mediated TGF-β1/Smad3 pathway regulates the occurrence and development of renal interstitial fibrosis in T2DM mice. Studies have found that with the development of T2DM, renal tissue will appear obvious fibrosis in the later stage, while hyperglycemia leads to internal environmental inflammation, oxidative stress and apoptosis, which increases the risk of developing or aggravating diabetic nephropathy. In contrast, dysfunction of the glomerular capillary barrier to circulating proteins leads to protein overload into the tubular epithelial cells, which damages the tubulointerstitium (30). In addition, previous studies have shown that hyperglycemia can induce the expression of TGF-β1 in mesangial cells, which leads to the accumulation of an ECM. The imbalance of Smad3, a signal transduction factor of TGF-β1 family, is a key mechanism of renal fibrosis (37).
Exercise could improve renal interstitial fibrosis in T2DM (38, 39). After the 8-week treadmill exercise, KI, SCr, BUN and 24 h UTP in the TP group declined. And HE staining showed that the tubular structure, interstitial and perivascular inflammatory lesions were significantly eased, and glomerular congestion was improved in the TP group. Masson staining showed reduced collagen deposition in the mesenchyme. All of these suggested that 8-week exercise could improve renal interstitial fibrosis in T2DM, and then improve the pathological structure of renal tissue and renal function, which was related to the upregulation of the TGF-β1/p38 mitogen-activated protein kinase (p38 MAPK) pathway and epithelial–mesenchymal transition and the inhibition of TGF-β1/connective tissue growth factor pathway mediated by interleukin 6 in the kidney of T2DM mice. Activation or inhibition of these signaling pathways reduced renal interstitial ECM deposition and improved renal interstitial fibrosis (12). To investigate the function of Sirt1-mediated exercise in alleviating renal interstitial fibrosis in T2DM patients, Sirt1 mRNA and protein expression were enhanced in the TP group, but TGF-β1, COL1 and COL3 mRNA and protein expressions were diminished. After phosphorylation, Smad3 plays a role in the pathway. Furthermore, immunohistochemical staining observation also confirmed that the positive percentage of COL1 expression in renal tissue was also significantly downregulated. In addition, Sirt1 secretion was increased in the TH group, and secretion of TGF-β1, COL1 and COL3 was decreased in renal tissue, further confirming the role of the Sirt1-mediated TGF-β1/Smad3 pathway in exercise in improving T2DM renal interstitial fibrosis. Immunohistochemistry labeling revealed that COL1 protein expression in renal tissue was reduced. Exercise intervention executed after nicotinamide injection could downregulate the Sirt1 expression in the kidney of T2DM mice, which activated the TGF-β1/Smad3 pathway and reduced renal interstitial collagen fiber deposition. In the TP group, KI, SCr, BUN and 24 h UTP were decreased, and the renal tubular structural damage, interstitial and perivascular inflammatory lesions were eased. It may be that exercise improves renal interstitial fibrosis, renal disease and renal function damage in T2DM, while any exercise-related improvement in the TH group was inhibited after nicotinamide injection. An analysis on the mechanism showed that the augmented levels of Sirt1 by exercise inhibited the activation of TGF-β1, resulting in the dephosphorylation of Smad3 into the nucleus and acting on the SBE region of 353 bp fragment of COL1 and COL3 A2 promoters, which would inhibit their expression and deposition in the kidney and lead to the improvement of renal interstitial fibrosis, renal tissue microstructure lesions and renal function impairment among T2DM mice (40).
In conclusion, this study revealed that the development of present exercise upregulation of Sirt1 expression inhibits the TGF-β1/Smad3 pathway, which inhibits renal mesangial COL1/COL3 expression and deposition and improves renal mesangial fibrosis in T2DM. Although great efforts have been made, the present research is far from satisfactory. In this study, we only injected nicotinamide to inhibit the expression of Sirt1 in the study of the Sirt1-mediated TGF-β1/Smad3 pathway affecting renal fibrosis in T2DM mice. In subsequent studies, the Sirt1 gene can be knocked out in mice by gene knockout technology. The mechanism of Sirt1 affecting renal fibrosis in T2DM mice was confirmed. In addition, this study explored the effects of two different exercise intensities on renal fibrosis in T2DM mice, but considering that the two exercise methods are classified as running platform exercise, the exercise intervention can be performed in mice by choosing to change the form, time, frequency, etc. in subsequent studies to better explore the effects of different exercise on renal fibrosis in T2DM mice. Because of its deposition in the renal interstitium, COL3 is one of the primary causes of renal interstitial fibrosis. The primary disadvantage of this study is that it only used the western blotting test to identify the expression of COL3 protein but failed to discover the percentage of positive area and expression of COL3 with immunohistochemical staining. In addition, the upregulation and downregulation of Sirt1 expression have significant effects on the TGF-β1/Smad3 pathway mediating renal fibrosis in T2DM mice. In the fields of biology and medicine, the development of related drugs based on Sirt1 can treat T2DM and its complications.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding
This work was supported by The China Postdoctoral Science Foundation (grant numbers 2019M661957), The Special grants from China Postdoctoral Science Foundation (grant numbers 2021T140580), 2020 Yangzhou University ‘High-end Talent Support Program’ and 2021 Yangzhou University ‘Qinglan Project.’
Author contribution statement
XC and KY conceived the project; XC and KY designed the experiments; XYZ, XQ, CL and PL performed all experiments, data collection and data analysis; XXZ and ZS prepared the reagents and collected the samples; XC and KY supervised the study; XC, KY, XYZ and XQ wrote the manuscript.
References
- 1.Kakuta K, Dohi K, Miyoshi M, Yamanaka T, Kawamura M, Masuda J, Kurita T, Ogura T, Yamada N, Sumida Y, et al.Impact of renal function on the underlying pathophysiology of coronary plaque composition in patients with type 2 diabetes mellitus. Cardiovascular Diabetology 201716131. ( 10.1186/s12933-017-0618-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loeffler I & Wolf G. Epithelial-to-mesenchymal transition in diabetic nephropathy: fact or fiction? Cells 4631–652. ( 10.3390/cells4040631) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morigi M Perico L & Benigni A. Sirtuins in renal health and disease. Journal of the American Society of Nephrology 2018291799–1809. ( 10.1681/ASN.2017111218) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wakino S Hasegawa K & Itoh H. Sirtuin and metabolic kidney disease. Kidney International 201588691–698. ( 10.1038/ki.2015.157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo CS, Shi Y, Chenier I, Ghosh A, Wu CH, Cailhier JF, Ethier J, Lattouf JB, Filep JG, Ingelfinger JR, et al.Heterogeneous nuclear ribonucleoprotein F stimulates Sirtuin-1 gene expression and attenuates nephropathy progression in diabetic mice. Diabetes 2017661964–1978. ( 10.2337/db16-1588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi H Huang C Shi Y Cao Q Chen J Chen XM & Pollock CA. Metformin attenuates renal fibrosis in a mouse model of adenine-induced renal injury through inhibiting TGF-β1 signaling pathways. Frontiers in Cell and Developmental Biology 20219603802. ( 10.3389/fcell.2021.603802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng L Qin R Rao Z & Xiao W. High-intensity interval training induces renal injury and fibrosis in type 2 diabetic mice. Life Sciences 2023324121740. ( 10.1016/j.lfs.2023.121740) [DOI] [PubMed] [Google Scholar]
- 8.Kumari A Sodum N Ravichandiran V & Kumar N. Role of SIRT-1 as a target for treatment and prevention of diabetic nephropathy: a review. Current Molecular Pharmacology 202316811–831. ( 10.2174/1874467216666230109140134) [DOI] [PubMed] [Google Scholar]
- 9.Mao Q Chen C Liang H Zhong S Cheng X & Li L. Astragaloside IV inhibits excessive mesangial cell proliferation and renal fibrosis caused by diabetic nephropathy via modulation of the TGF-β1/Smad/miR-192 signaling pathway. Experimental and Therapeutic Medicine 2019183053–3061. ( 10.3892/etm.2019.7887) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponnusamy M Zhou X Yan Y Tang J Tolbert E Zhao TC Gong R & Zhuang S. Blocking sirtuin 1 and 2 inhibits renal interstitial fibroblast activation and attenuates renal interstitial fibrosis in obstructive nephropathy. Journal of Pharmacology and Experimental Therapeutics 2014350243–256. ( 10.1124/jpet.113.212076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren Y Du C Yan L Wei J Wu H Shi Y & Duan H. CTGF siRNA ameliorates tubular cell apoptosis and tubulointerstitial fibrosis in obstructed mouse kidneys in a Sirt1-independent manner. Drug Design, Development and Therapy 201594155–4171. ( 10.2147/DDDT.S86748) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C Matavelli LC Akhtar S & Siragy HM. (Pro)renin receptor contributes to renal mitochondria dysfunction, apoptosis and fibrosis in diabetic mice. Scientific Reports 2019911667. ( 10.1038/s41598-019-47055-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HW Kao HH & Wu CH. Exercise training upregulates SIRT1 to attenuate inflammation and metabolic dysfunction in kidney and liver of diabetic db/db mice. Nutrition and Metabolism 20191622. ( 10.1186/s12986-019-0349-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen N, Li X, Zhou T, Bilal MU, Du N, Hu Y, Qin W, Xie Y, Wang H, Wu J, et al.Shensong Yangxin Capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling. Journal of Ethnopharmacology 2014157161–170. ( 10.1016/j.jep.2014.09.035) [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Yang K, Jin X, Meng Z, Liu B, Yu H, Lu P, Wang K, Fan Z, Tang Z, et al.Bone autophagy: a potential way of exercise-mediated Meg3/P62/Runx2 pathway to regulate bone formation in T2DM mice. Diabetes, Metabolic Syndrome and Obesity 2021142753–2764. ( 10.2147/DMSO.S299744) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng C, Zhou W, Wang T, You P, Zhao Y, Yang Y, Wang X, Luo J, Chen Y, Liu M, et al.A novel TGR5 activator WB403 promotes GLP-1 secretion and preserves pancreatic β-cells in type 2 diabetic mice. PLoS One 201510e0134051. ( 10.1371/journal.pone.0134051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang JG Zhao G Qin Q Wang B Liu L Liu Y Deng SC Tian K & Wang CY. Nicotinamide prohibit s proliferation and enhances chemosensitivity of pancreatic cancer cells through deregulating SIRT1 and Ras/Akt pathways. Pancreatology 201313140–146. ( 10.1016/j.pan.2013.01.001) [DOI] [PubMed] [Google Scholar]
- 18.Ren MT Gu ML Zhou XX Yu MS Pan HH Ji F & Ding CY. Sirtuin 1 alleviates endoplasmic reticulum stress-mediated apoptosis of intestinal epithelial cells in ulcerative colitis. World Journal of Gastroenterology 2019255800–5813. ( 10.3748/wjg.v25.i38.5800) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y Zhou B Wen M Hu M Peng JG Wang Y Fan LL & Tang L. ZG02 improved hepatic glucose metabolism and insulin sensitivity via activation of AMPK/Sirt1 signaling pathways in a high-fat diet/streptozotocin-induced type 2 diabetes model. Diabetes, Metabolic Syndrome and Obesity 2020134333–4339. ( 10.2147/DMSO.S275145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X Yang K Sun P Zhao R Liu B & Lu P. Exercise improves bone formation by upregulating the Wnt3a/β-catenin signalling pathway in type 2 diabetic mice. Diabetology and Metabolic Syndrome 202113116. ( 10.1186/s13098-021-00732-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lertpatipanpong P Lee J Kim I Eling T Oh SY Seong JK & Baek SJ. The anti-diabetic effects of NAG-1/GDF15 on HFD/STZ-induced mice. Scientific Reports 20211115027. ( 10.1038/s41598-021-94581-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin L Wang Y Xu W Huang C Hu J Chen X Lv X Qin Y Zhao X & Li H. Aerobic exercise improves type 2 diabetes mellitus-related cognitive impairment by inhibiting JAK2/STAT3 and enhancing AMPK/SIRT1 pathways in mice. Disease Markers 202220226010504. ( 10.1155/2022/6010504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan SB Liu GL Wang YH & Zhang JJ. Epithelial-to-mesenchymal transdifferentiation of renal tubular epithelial cell mediated by oxidative stress and intervention effect of Probucol in diabetic nephropathy rats. Renal Failure 2012341244–1251. ( 10.3109/0886022X.2012.718711) [DOI] [PubMed] [Google Scholar]
- 24.Du N Xu Z Gao M Liu P Sun B & Cao X. Combination of ginsenoside Rg1 and Astragaloside IV reduces oxidative stress and inhibits TGF-β1/Smads signaling cascade on renal fibrosis in rats with diabetic nephropathy. Drug Design, Development and Therapy 2018123517–3524. ( 10.2147/DDDT.S171286) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou HC Wen LL Chang CC Lin CY Jin L & Juan SH. From the cover: l-carnitine via PPARγ- and Sirt1-dependent mechanisms attenuates epithelial-mesenchymal transition and renal fibrosis caused by perfluorooctanesulfonate. Toxicological Sciences 2017160217–229. ( 10.1093/toxsci/kfx183) [DOI] [PubMed] [Google Scholar]
- 26.Duan YC Shi L Jin Z Hu M Huang H Yan T & Zhang KR. Swimming exercise ameliorates hypertension-induced kidney dysfunction via alleviating renal interstitial fibrosis and apoptosis. Kidney and Blood Pressure Research 202146219–228. ( 10.1159/000514680) [DOI] [PubMed] [Google Scholar]
- 27.Gutta S Grobe N Kumbaji M Osman H Saklayen M Li G & Elased KM. Increased urinary angiotensin converting enzyme 2 and neprilysin in patients with type 2 diabetes. American Journal of Physiology-Renal Physiology 2018315F263–F274. ( 10.1152/ajprenal.00565.2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karsiyakali N Sarikaya S & Karatas OF. Anatomic basis and clinical effect of selective dorsal neurectomy for patients with lifelong premature ejaculation: a randomized controlled trial. Journal of Sexual Medicine 2019161122. ( 10.1016/j.jsxm.2019.05.002) [DOI] [PubMed] [Google Scholar]
- 29.Zhang R Yu Y Deng J Zhang C Zhang J Cheng Y Luo X Han B & Yang H. Sesamin ameliorates high-fat diet-induced dyslipidemia and kidney injury by reducing oxidative stress. Nutrients 20168276. ( 10.3390/nu8050276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kang YH Park SH Sim YE Oh MS Suh HW Lee JY & Lim SS. Highly water-soluble diacetyl chrysin ameliorates diabetes-associated renal fibrosis and retinal microvascular abnormality in db/db mice. Nutrition Research and Practice 202317421–437. ( 10.4162/nrp.2023.17.3.421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin S Yu L Ni Y He L Weng X Lu X & Zhang C. Fibroblast growth factor 21 attenuates diabetes-induced renal fibrosis by negatively regulating TGF-β-p53-Smad2/3-mediated epithelial-to-mesenchymal transition via activation of AKT. Diabetes and Metabolism Journal 202044158–172. ( 10.4093/dmj.2018.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueira A Pires MJ & Oliveira PA. Pathophysiological mechanisms of renal fibrosis: a review of animal models and therapeutic strategies. In Vivo 2017311–22. ( 10.21873/invivo.11019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J Qu X Ricardo SD Bertram JF & Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. American Journal of Pathology 20101771065–1071. ( 10.2353/ajpath.2010.090923) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y Connelly KA Thai K Wu X Kapus A Kepecs D & Gilbert RE. Sirtuin 1 activation reduces transforming growth factor-β1-induced fibrogenesis and affords organ protection in a model of progressive, experimental kidney and associated cardiac disease. American Journal of Pathology 201718780–90. ( 10.1016/j.ajpath.2016.09.016) [DOI] [PubMed] [Google Scholar]
- 35.Wang X Xue N Zhao S Shi Y Ding X & Fang Y. Upregulation of miR-382 contributes to renal fibrosis secondary to aristolochic acid-induced kidney injury via PTEN signaling pathway. Cell Death and Disease 202011620. ( 10.1038/s41419-020-02876-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J Zhang L Zha D & Wu X. Inhibition of miRNA135a5p ameliorates TGFβ1induced human renal fibrosis by targeting SIRT1 in diabetic nephropathy. International Journal of Molecular Medicine 2020461063–1073. ( 10.3892/ijmm.2020.4647) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue S Li YX Lu XX & Tang W. Dapagliflozin can alleviate renal fibrosis in rats with streptozotocin‑induced type 2 diabetes mellitus. Experimental and Therapeutic Medicine 202326572. ( 10.3892/etm.2023.12271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang LX Wang B & Wu ZK. Aerobic exercise training alleviates renal injury by interfering with mitochondrial function in Type-1 diabetic mice. Medical Science Monitor 2018249081–9089. ( 10.12659/MSM.912877) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong L Li J Lian Y Tang ZX Zen Z Yu P & Li Y. Long-term intensive lifestyle intervention promotes improvement of Stage III diabetic nephropathy. Medical Science Monitor 2019253061–3068. ( 10.12659/MSM.913512) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu A Pallero MA Owusu BY Borovjagin AV Lei W Sanders PW & Murphy-Ullrich JE. Calreticulin is important for the development of renal fibrosis and dysfunction in diabetic nephropathy. Matrix Biology Plus 202010003. ( 10.1016/j.mbplus.2020.100034) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a