Abstract
STP-C488 (STP of herpesvirus saimiri [HVS] group C strain 488 [C488]) is the only virus-encoded protein found to associate with cellular ras and activate ras signal transduction pathways. To investigate an important role for ras signal transduction in STP-dependent growth transformation, we constructed recombinant strains of HVS C488 in which the STP-C488 oncogene was replaced with cellular normal ras (c-ras) or viral oncogenic ras (v-ras). Recombinant HVSΔSTP/v-ras immortalized primary common marmoset T lymphocytes to interleukin-2-independent growth as efficiently as wild-type HVS C488 (wt HVS), while recombinant HVSΔSTP/c-ras did so with low efficiency. Whereas wt HVS immortalized CD4− CD8+ single-positive T lymphocytes, HVSΔSTP/c-ras- and HVSΔSTP/v-ras-immortalized cells were principally CD4+ CD8+ double-positive T lymphocytes. In addition, HVSΔSTP/v-ras-immortalized T cells showed a high level of ras expression and exhibited an adherent macrophage-like morphology. These phenotypes were likely caused by the drastic activation of AP-1 transcriptional factor activity. Finally, HVSΔSTP/v-ras and HVSΔSTP/c-ras each induced lymphoma in one of two common marmosets, although onset of disease was more rapid with the v-ras virus. These results demonstrate that ras can substitute for the STP oncogene of HVS C488 to allow immortalized growth of primary lymphoid cells and that an activated form of ras does so more efficiently than the normal cellular form of ras.
Herpesvirus saimiri (HVS) subgroup C strains contain a divergent form of the STP oncogene at the left end of the coding portion of the genome (5, 20). STP of group C strains (STP-C) and STP from group A strains (STP-A) are each sufficient for the transformation of rodent fibroblast cells in vitro, but STP-C is considerably more potent (22, 25). Transgenic mice expressing STP-C488 (STP from group C strain 488 [C488]) developed invasive epithelial cell tumors (32), while STP-A11 (STP from group A strain 11 [A11]) transgenic mice developed peripheral pleomorphic T-cell lymphomas (28). Deletion of either form of STP yields virus no longer capable of immortalizing lymphocytes in vitro or of inducing fatal lymphomas in common marmosets (12–15, 27, 33). Since HVS lacking STP can be repeatedly isolated from peripheral blood of common marmosets for months and years, STP is not required for viral replication or persistence in vivo but it is essential for transformation in cell culture and for lymphoma induction in common marmosets (12, 15).
Transforming proteins of tumor viruses exert their effects in many cases through specific interactions with cellular regulatory proteins (9, 18, 35, 41, 42). We have demonstrated that STP-C488 associates with cellular ras in transformed cells (24). Mutations that disrupt this association with ras disrupt the transforming ability of the STP-C488 oncogene (24). Binding assays show that STP-C488 is capable of competing with raf-1 for binding to ras. Expression of STP-C488 activates the ras signaling pathway, as evidenced by a two- to fourfold increase in the ratio of Ras-GTP to Ras-GDP and by the constitutive activation of mitogen-activated protein kinase. Consistent with an activation of signaling through ras, STP-C488 expression induces ras-dependent neurite outgrowth in PC12 cells (24). Unlike STP-C, STP-A binds to the SH2 domain of Src kinase and is phosphorylated by the associated Src kinase in in vitro kinase assays (29). Mutational analysis of STP-A11 shows that binding to Src kinase requires the tyrosine residue at 115, showing that YAE(V/I) is a binding motif for the SH2 domain of Src. Also, tyrosine phosphorylation of STP-A by Src leads to subsequent binding to Lck and Fyn in vitro (29). These results suggest that STP of subgroup A targets a cellular protein for virus-induced transformation that is different from that used by STP-C.
In this report, we demonstrate that ras is capable of substituting for the HVS STP-C488 function in lymphocyte transformation. Recombinant HVSΔSTP/c-ras and HVSΔSTP/v-ras in which the STP-C488 gene was replaced with a normal cellular ras (c-ras) or an oncogenic viral ras (v-ras) gene were isolated. Recombinant HVSΔSTP/c-ras and HVSΔSTP/v-ras virus immortalized primary T lymphocytes to interleukin-2 (IL-2)- independent growth and induced lymphoma in common marmosets. These results suggest that activation of ras signal transduction pathways is important for T-cell growth transformation by HVS.
MATERIALS AND METHODS
Cell culture and virus propagation.
Owl monkey kidney cells (OMK 637) were cultivated in minimal essential medium supplemented with penicillin, streptomycin, l-glutamine, and 10% (vol/vol) heat-inactivated fetal bovine serum (GIBCO BRL, Grand Island, N.Y.) were used for the propagation of HVS C488. Low-passage OMK cells (<30 passages) were used for the transfections. Primary common marmoset peripheral blood mononuclear cells (PBMCs) were purified by using lymphocyte separation medium (Organon Teknika Corp., Malvern, Pa.). Cultures of common marmoset PBMCs in immortalization assays with HVS recombinants were performed in RPMI 1640 medium supplemented with penicillin, streptomycin, amphotericin B (Fungizone), l-glutamine, 20% (vol/vol) heat-inactivated fetal bovine serum, and 5 mg of β-mercaptoethanol per liter.
Virion DNA isolation.
HVS virion preparations were obtained from culture medium of infected OMK cells after removal of cell debris by low-speed centrifugation, followed by pelleting of the virus at 18,000 rpm for 2 h in an SS-34 rotor. To purify intact virion DNA, the virus was disrupted at 60°C for 2 h in lysis buffer containing 10 mM Tris (pH 8.5), 1 mM EDTA, 1% (vol/vol) Sarkosyl, and 0.1 mg of proteinase K per ml. Extraction of the aqueous solution first with an equal volume of phenol and then twice with chloroform was sufficient to purify the virion DNA for use in transfections. Sterile cut pipette tips were used for manipulating virion DNA without shearing.
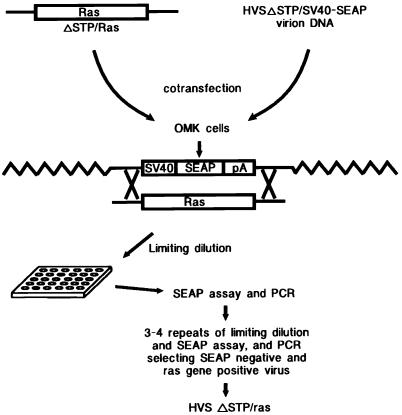
Construction of recombinant HVS.
The complete STP coding sequence was deleted from 3.6 kb of the left end of L-DNA of HVS C488 by PCR, and the multicloning sites were inserted into the STP locus. Human cellular H-ras (2) or oncogenic viral H-ras (2) was cloned into the multicloning sites of 3.1 kb of L-DNA. Linearized plasmid DNA containing 3.5 kb of L-DNA with the ras gene was cotransfected into OMK cells with HVSΔSTP/SV40-SEAP virion DNA by the calcium phosphate protocol. A pure form of recombinant virus with the SEAP reporter replaced with c-ras or v-ras was isolated by limiting dilution and repeated selection of SEAP-negative virus to OMK cell monolayers in 48-well tissue culture plates performed as described previously (16) (Fig. 1). SEAP production was detected by a liquid scintillation counter measurement of the chemiluminescence produced in assays of cell culture medium by using Phospha-Light reagents (Tropix Inc., Bedford, Mass.) according to the manufacturer’s recommendations.
FIG. 1.
Schematic diagram to construct the recombinant HVS containing c-ras or v-ras. The detailed procedure has been described previously (16). pA, polyadenylation site.
In vitro immortalization of common marmoset lymphocytes.
Assays of lymphocyte immortalization in vitro have been described previously (14). PBMCs were isolated from 3-ml heparinized blood specimens from common marmosets (Callithrix jacchus) by centrifugation through lymphocyte separation medium (Organon Teknika) followed by washing in RPMI 1640 culture medium. PBMCs from each animal were individually washed, resuspended in RPMI 1640 medium, and then distributed in 1-ml volumes containing approximately 106 cells into 12-well tissue culture plates. Cells were then infected at a multiplicity of infection ranging from 1 to 5 with 1 ml of purified HVS stocks. Cells were maintained in RPMI 1640, with the growth medium changed every 3 to 4 days. Immortalization or cell death was assessed microscopically.
Experimental infection of common marmosets.
In vivo oncogenicity of the HVS C488 recombinants was assessed by experimental infection of common marmosets (C. jacchus). Marmosets were injected intramuscularly with 105 50% tissue culture infective doses (TCID50) of virus in a volume of 1 ml. Sera and blood cell pellets were collected and frozen at −70°C weekly during the first 4 weeks and every 2 weeks thereafter. Viral loads in PBMC specimens were assessed periodically by duplicate plating of 106 PBMCs and serial threefold dilutions of PBMCs on OMK cells in 24-well tissue culture plates (15). Animals that became moribund were euthanized and received complete necropsies. Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Immunoblotting and antibody.
Cells were harvested and lysed with lysis buffer (0.15 M NaCl, 0.5% Nonidet P-40, 50 mM HEPES buffer [pH 8.0]) containing 1 mM Na2VO3, 1 mM NaF, and protease inhibitors (leupeptin, aprotinin, phenylmethylsulfonyl fluoride, pepstatin, and bestatin). Precleared lysates from 105 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% polyacrylamide gel, transferred onto nitrocellulose membranes, and reacted with antibody. Rabbit polyclonal STP-C488 antibody 109 used in these experiments has been described previously (22). Ras antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.) and Transduction Laboratories (Lexington, Ky.).
Isolation of genomic DNA and PCR analysis.
Genomic DNA was isolated with a Qiagen genomic isolation kit according to the manufacturer’s protocol. Five micrograms of purified genomic DNA was used for PCR amplication using 5′ and 3′ primers, which correspond to the upstream and downstream sequences, respectively, of the STP-C488 gene. Amplified DNA was cloned into the TA cloning vector (Invitrogen, San Diego, Calif.). Both strands of five independent clones were subsequently sequenced by using an ABI PRISM 377 automatic DNA sequencer.
FACS analysis.
For fluorescence-activated cell sorting (FACS) 5 × 105 to 1 × 106 immortalized marmoset lymphocytes were washed twice in phosphate-buffered saline (PBS) and incubated with the appropriate monoclonal antibody for 30 min at 4°C. Thereafter, cells were washed twice with PBS, fixed with 2% paraformaldehyde, and analyzed with a FACscan (Becton Dickinson and Co., Mountain View, Calif.). Antibodies used in this study that react with common marmoset lymphocyte antigens included CD2-phycoerythrin (PE) (Becton Dickinson), CD3-fluorescein isothiocyanate (FITC) (PharMingen, San Diego, Calif.), CD4 (Olympus clone; Chromoprobe, Mountain View, Calif.), CD8-FITC (Coulter, Hialeah, Fla.), CD25-PE, and -FITC (Becton Dickinson), CD56-PE (Becton Dickinson), and HLA-DR PercP (Becton Dickinson).
Reporter assays.
Approximately 107 cells were electroporated at 960 μF and 200 V. All transfections included the construct pGKβgal, which expresses β-galactosidase from a phosphoglucokinase promoter, together with construct OCT-SEAP, NFAT-SEAP, 3X-κB-luc, or TRE-luc. TRE-luc contains the AP-1 binding site upstream of a minimal fos promoter (21). As controls, cells were treated with 10% IL-2 or tetradecanoyl phorbol acetate (TPA). At 24 h posttransfection, cells were washed once in PBS and lysed in 200 μl of reporter lysis buffer (Promega, Madison, Wis.). Assays for luciferase or alkaline phosphatase activity were performed with a luciferase assay (Promega) or with the Phospha-Light chemiluminescence assay (Tropix) in a Luminometer. Values were normalized to β-galactosidase activity.
RESULTS
Isolation of HVSΔSTP/c-ras and HVSΔSTP/v-ras recombinants.
To examine whether ras is capable of substituting for the STP oncogene in lymphocyte transformation, STP-C488 of HVS was replaced with a cellular normal H-ras (c-ras) or an oncogenic viral H-ras (v-ras). We have recently described construction of HVSΔSTP/SV40-SEAP, in which the STP gene was replaced with a gene for simian virus 40 (SV40)-secreted engineered alkaline phosphatase (SEAP) as a reporter for isolation of the recombinant HVS (16). As shown in Fig. 1, nononcogenic HVSΔSTP/SV40-SEAP virion DNA was used for isolating recombinant HVSΔSTP variants containing either the c-ras or v-ras gene via homologous recombination. The complete STP coding sequence was deleted from 3.6 kb of the left end of L-DNA of HVS C488 by PCR, and multicloning sites were inserted into the STP locus. Human cellular H-ras or oncogenic viral H-ras was cloned into the multicloning sites of 3.1 kb of L-DNA. In these constructs, c-ras and v-ras genes were under control of the STP promoter (19). Linearized plasmid DNA containing 3.5 kb of L-DNA with the ras gene insert was cotransfected into OMK cells with HVSΔSTP/SV40-SEAP virion DNA, using calcium phosphate. To isolate recombinant HVSΔSTP/c-ras or HVSΔSTP/v-ras, SEAP-negative virus was recovered from limiting dilutions (Fig. 1). To confirm the correct genetic structure of the recombinant virus, virion DNA from recombinant HVSΔSTP/c-ras or HVSΔSTP/v-ras was used for PCR using 5′ and 3′ primers (see Materials and Methods). Wild-type HVS C488 (wt HVS) virion DNA was used as a control for PCR analysis. Five of five plasmid clones derived from virion DNA of this recombinant virus were shown to contain the presence of c-ras or v-ras, the absence of undesired aberrant mutations, and the absence of wild-type STP sequence.
In vitro immortalization assay.
Common marmoset T lymphocytes are immortalized efficiently to IL-2-independent growth by infection with wt HVS (12, 15). To examine the transforming activity of ras in the context of HVS, assays of in vitro immortalization of primary marmoset T lymphocytes were performed with HVSΔSTP/c-ras and HVSΔSTP/v-ras. wt HVS and HVSΔSTP/SV40-SEAP were used as controls for these assays. HVSΔSTP/c-ras, HVSΔSTP/v-ras, HVSΔSTP/SV40-SEAP, and wt HVS at equivalent viral titers were added to unstimulated PBMCs from 10 common marmoset donors. The recombinant HVSΔSTP/v-ras uniformly immortalized lymphocytes from all 10 of the common marmosets to IL-2-independent growth as was seen with the wt HVS (Table 1). In contrast, the recombinant HVSΔSTP/c-ras immortalized only 2 of the 10 primary cultures of common marmoset lymphocytes (Table 1). These HVSΔSTP/c-ras-immortalized T cells were highly sensitive for growth at low cell density. Additionally, the growth of HVSΔSTP/c-ras-immortalized cells was significantly retarded even after 6 months of culture. As described previously (17), HVSΔSTP/SV40-SEAP did not immortalized any of these primary PBMCs (Table 1). These results demonstrate the transforming capacity of the ras gene within the context of the HVS genome. Additionally, they show that v-ras substitutes for the STP oncogene much more efficiently than c-ras.
TABLE 1.
In vitro and in vivo oncogenicity of HVSΔSTP/c-ras or HVSΔSTP/v-ras
| Virus | No. positive/no. tested
|
Survival (days) | ||
|---|---|---|---|---|
| In vitro immortalizationa | Virus recoveryb | In vivo lymphoma | ||
| None | 0/10 | |||
| HVS C488 | 10/10 | 2/2 | 2/2 | 19 and 20 |
| HVSΔSTP | 2/2 | 0/2 | >300 | |
| HVSΔSTP/SV40-SEAP | 0/10 | 2/2 | 0/2 | >300 |
| HVSΔSTP/c-ras | 2/10 | 2/2 | 1/2 | 38, >300 |
| HVSΔSTP/v-ras | 10/10 | 2/2 | 1/2 | 28, >300 |
Primary T-lymphocyte immortalization.
Virus was recovered from PBMCs of infected marmoset as described previously (15).
In vivo lymphoma induction.
We have shown previously that experimental infection of common marmosets with wt HVS consistently induces lymphomas within 19 to 25 days of infection (12, 15). To further investigate the oncogenic potential of HVSΔSTP/c-ras and HVSΔSTP/v-ras, two common marmosets were injected intramuscularly with 105 TCID50 of each virus in a volume of 1 ml. Two marmosets were inoculated with wt HVS as positive controls, whereas two marmosets were inoculated with each of HVSΔSTP or HVSΔSTP/SV40-SEAP as negative controls. Consistent with prior experiments, infection with wt HVS induced lymphomas in common marmosets. Marmosets infected with wt HVS were sacrificed on days 19 and 20 postinfection, when death appeared imminent (Table 1). One marmoset infected with HVSΔSTP/c-ras and one infected with HVSΔSTP/v-ras also developed lymphoma. The onset of terminal illness with HVSΔSTP/v-ras was slightly delayed compared to wt HVS, while it was further delayed with HVSΔSTP/c-ras (Table 1). The animals infected with HVSΔSTP/v-ras or HVSΔSTP/c-ras were sacrificed on day 28 or 38 postinoculation, respectively. The two marmosets infected with HVSΔSTP/c-ras or HVSΔSTP/v-ras remain alive 10 months postinoculation. Additionally, four of four marmosets infected with HVSΔSTP/SV40-SEAP or HVSΔSTP remained healthy for over 10 months after infection (Table 1). Viruses were isolated from all of the infected animals at week 2 postinoculation and from the remaining live animals after 20 weeks postinoculation (data not shown). To confirm the presence of the ras gene and the absence of the STP gene after infection, virus was isolated from PMBCs of infected animals by cocultivation with OMK cells. One microliter of isolated virus was directly used for PCR to amplify the ras gene. Additionally, genomic DNA of lymph nodes of sacrificed animals was used for PCR and sequence analysis to amplify the ras gene. DNA sequencing confirmed that the amplified fragment contained the c-ras or v-ras gene, with no sign of contamination of wt HVS and with no sequence variation.
Necropsy of animals infected with HVSΔSTP/c-ras, HVSΔSTP/v-ras, and wt HVS revealed multicentric lymphoma consistent with HVS-induced pathology as previously described (12, 15). The most extensive neoplastic infiltrates were observed in the kidneys of wt HVS-, HVSΔSTP/c-ras-, or HVSΔSTP/v-ras-infected marmosets (data not shown). No differences were noted in the nature of lymphomas induced by wt HVS, HVSΔSTP/c-ras, or HVSΔSTP/v-ras. Thus, these results demonstrated that recombinant HVSΔSTP/c-ras and HVSΔSTP/v-ras induced lymphomas in common marmoset.
Surface expression of lymphocyte antigens in transformed cells.
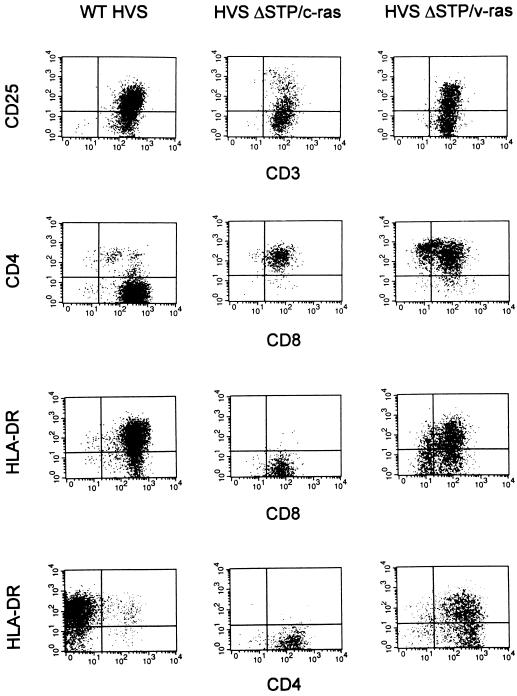
Common marmoset cells immortalized by wt HVS have been shown to be CD3+ CD4− CD8+ CD56+ T cells which are most likely derived from a population of natural killer cells (14). Common marmoset cells transformed by wt HVS, HVSΔSTP/c-ras, or HVSΔSTP/v-ras were used to examine the surface expression of lymphocyte antigens by FACS analysis. We immortalized PBMCs from the same marmoset donor with wt HVS, HVSΔSTP/c-ras, or HVSΔSTP/v-ras for comparative analyses. Common marmoset cells transformed by wt HVS were CD3+ T lymphocytes which were CD4− CD8+ single-positive cells as found previously, whereas common marmoset cells transformed by HVSΔSTP/c-ras or HVSΔSTP/v-ras were CD3+ T lymphocytes which were mainly CD4+ CD8+ double-positive cells (Fig. 2). Additional common marmoset cell lines independently transformed by HVSΔSTP/c-ras or HVSΔSTP/v-ras showed the same phenotypes as those described above (data not shown).
FIG. 2.
Analysis of surface expression of lymphocyte antigens on wt HVS-, HVSΔSTP/c-ras-, or HVSΔSTP/v-ras-transformed cells. Surface phenotypes were determined by flow cytometry. Positive gates were established by using an isotype-matched control antibody.
Surface expression of HLA-DR and CD25, which have been shown to be associated with the activation of T cells (34, 36), was measured on common marmoset T cells immortalized by wt HVS, HVSΔSTP/c-ras, or HVSΔSTP/v-ras. Surface expression of CD25 and HLA-DR on HVSΔSTP/c-ras-transformed T cells was approximately 10- and 100-fold less than that on wt HVS- or HVSΔSTP/v-ras-transformed T cells (Fig. 2). Thus, results demonstrated that transformation of common marmoset PBMCs with HVSΔSTP/v-ras leads to a highly activated, fully transformed phenotype, as seen with wt HVS infection, that is not completely mimicked with HVSΔSTP/c-ras.
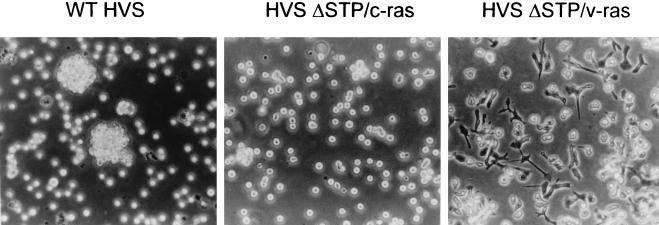
Morphologic changes in HVSΔSTP/v-ras-transformed T cells.
Numerous adherent cells were observed among common marmoset T cells immortalized by HVSΔSTP/v-ras (Fig. 3). Over 60% of the common marmoset T cells immortalized by HVSΔSTP/v-ras exhibited an adherent phenotype with an appearance of the mature macrophages (Fig. 3). In contrast, cells immortalized by wt HVS and HVSΔSTP/c-ras were not adherent (Fig. 3). To investigate the origin of these adherent cells, we used FACS analysis to examine the surface expression of lymphocyte markers. These experiments showed that the adherent cells had the same surface phenotypes as the floating HVSΔSTP/v-ras-transformed T cells, which were CD3+ CD4+ CD8+. Additionally, both adherent and floating HVSΔSTP/v-ras-transformed T cells were negative for surface expression of CD11b, a marker for macrophages (data not shown).
FIG. 3.
Morphological changes in common marmoset T cells transformed by HVSΔSTP/v-ras. Cells were photographed with a phase microscope at a magnification of ×100.
ras expression in transformed cells.
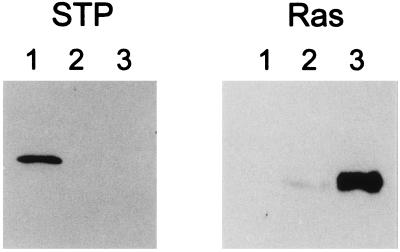
Expression of STP-C488 and ras in common marmoset T cells immortalized by wt HVS, HVSΔSTP/c-ras, or HVSΔSTP/v-ras was examined by immunoblot analyses. STP-C488 expression was not detected in common marmoset T cells immortalized by recombinant HVSΔSTP/c-ras or HVSΔSTP/v-ras, while it was readily detected in common marmoset T cells immortalized by wt HVS (Fig. 4). Surprisingly, ras expression was much higher in common marmoset T cells immortalized by recombinant HVSΔSTP/v-ras than in cells immortalized by HVSΔSTP/c-ras (Fig. 4). Also, cellular ras expression in wt HVS-transformed cells was very low (Fig. 4). Since the tip gene is downstream of the STP gene in a bicistronic transcriptional unit, we also investigated whether insertion of the ras gene at the STP locus affected the expression of tip. Lysates of wt HVS-, HVSΔSTP/c-ras-, or HVSΔSTP/v-ras-immortalized T cells were used for immunoprecipitation with anti-Tip antibody followed by in vitro kinase reaction. This analysis showed the equivalent levels of tyrosine kinase activity associated with tip in different cells (data not shown). Thus, the replacement of STP-C488 with ras did not alter the expression of tip detectably.
FIG. 4.
Immunoblot analysis of HVSΔSTP/c-ras- or HVSΔSTP/v-ras-transformed cells. The same amounts of precleared lysates of 5 × 106 cells were resolved by SDS-PAGE, transferred to nitrocellulose membranes, and reacted with antibodies against STP-C488 or Ras. Reactivity was detected by enhanced chemiluminescence. Lane 1, wt HVS-transformed cells; lane 2, HVSΔSTP/c-ras-transformed cells; lane 3, HVSΔSTP/v-ras-transformed cells.
Activation of AP-1 transcription factor activity in HVSΔSTP/v-ras-transformed cells.
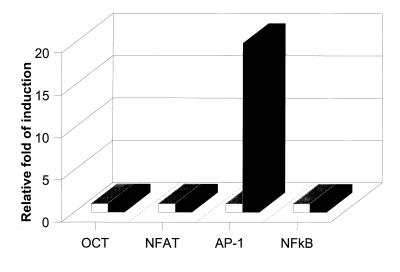
Activation of cellular ras signal transduction leads to activation of AP-1 transcription factor activity, which ultimately induces the expression of a number of cellular genes (21). We measured AP-1 transcriptional factor activity by using a reporter construct. The TRE-luc reporter construct containing the AP-1 binding site upstream of a minimal fos promoter and the pGKβgal construct containing the β-galactosidase gene downstream of a phosphoglucokinase promoter were electroporated into wt HVS- and HVSΔSTP/v-ras-transformed cells. Since the growth of common marmoset T cells immortalized by HVSΔSTP/c-ras was greatly retarded, these cells were not used in these assays. Twenty-four hours after electroporation, luciferase activity was measured and normalized to β-galactosidase activity. We observed that AP-1 transcription factor activity was approximately 20-fold higher in HVSΔSTP/v-ras-transformed cells than in wt HVS-transformed cells (Fig. 5). As controls, wt HVS- or HVSΔSTP/v-ras-transformed cells were treated with TPA or IL-2 for 48 h. This analysis showed dramatically increased AP-1 activity in these cells (data not shown).
FIG. 5.
Activation of AP-1 transcription factor activity in HVSΔSTP/v-ras-transformed cells. A total of 107 cells transformed by wt HVS (□) or HVSΔSTP/v-ras (▪) were electroporated at 960 μF and 200 V with 30 μg of pGKβgal and 30 μg of reporter plasmid TRE-luc, NF-κB-driven 3X-κB-luc, NFAT-SEAP, or OCT-SEAP. At 24 h after transfection, cell supernatants and lysates were used for luciferase, alkaline phosphatase, and β-galactosidase assays. Luciferase and alkaline phosphatase activities were determined and normalized on the basis of β-galactosidase activities. Fold activation represents normalized luciferase and alkaline phosphatase activity relative to that of the wt HVS-transformed cells 24 h after transfection. Values represent averages of three independent experiments.
We similarly measured NF-κB, NFAT, and OCT-1 activity in these cells by using reporter constructs. wt HVS- or HVSΔSTP/v-ras-transformed T cells were transfected with NF-κB-driven reporter plasmid 3X-κB-luc, NFAT-SEAP, or OCT-SEAP and control β-galactosidase plasmid pGKβgal. Unlike AP-1 activity, NF-κB, NFAT, and OCT-1 transcriptional activities were not altered in these cell lines (Fig. 5). Thus, the results demonstrated that HVSΔSTP/v-ras-transformed cells showed a specific increase of AP-1 transcriptional factor activity compared to wt HVS-transformed T cells.
DISCUSSION
The results described here demonstrate that ras is capable of substituting for the STP oncogene of HVS in in vitro T-lymphocyte transformation and in lymphoma induction in vivo. Recombinant HVSΔSTP/v-ras induced transformation much more efficiently than did HVSΔSTP/c-ras. Additionally, the activation of ras signal transduction in HVSΔSTP/v-ras-immortalized T cells resulted in a significant increase in AP-1 transcriptional factor activity as well as an adherent macrophage-like morphology. These data support an important role for ras signal transduction in STP-dependent cell growth transformation of HVS subgroup C strains.
Ras plays a pivotal role in a variety of signal transduction and differentiation processes. Ras is a plasma membrane-associated guanine nucleotide-binding protein that cycles between a GDP-bound form and a GTP-bound form (2, 7). Oncogenic mutant forms of Ras display an increased proportion of Ras in the GTP-bound state relative to the GDP-bound state, which activates the biological functions of Ras. Ras-GTP exerts its biological effects by interacting with cellular effector molecules such as Raf and phosphatidylinositol 3-kinase (26, 31, 37, 39, 40). We have demonstrated that the association of STP-C488 with ras activates ras signaling pathways, as shown by an increase in the ratio of Ras-GTP to Ras-GDP and by the constitutive activation of mitogen-activated protein kinase (24). Additionally, STP-C488 is able to induce PC12 cell differentiation similarly to nerve growth factor, which has been shown to act through the ras signal transduction (24). Thus, the transforming activity of STP-C488 appears to be dependent on the activation of ras signal transduction pathways.
While recombinant ras viruses are capable of inducing in vitro T-lymphocyte transformation, cells immortalized by these viruses are phenotypically different from those immortalized by wt HVS. wt HVS-immortalized cells are CD4− CD8+ single-positive T cells, whereas HVSΔSTP/c-ras- and HVSΔSTP/v-ras-immortalized cells are CD4+ CD8+ double-positive T cells. While human and rhesus T cells immortalized by HVS are often CD4+ CD8+ double positive, common marmoset T cells immortalized by HVS have always been CD4− CD8+ single positive (1, 3–5, 8, 23, 30). Lymphocytes coexpressing CD4 and CD8 antigens have been shown in normal human PBMCs; however, these cells constitute less than 3% of the total T-cell population (6). Lectin treatment in vitro has been shown to stimulate the generation of CD4+ CD8+ cells in human PBMCs, although the functional role of these cells is unclear (6). Thus, it seems likely that HVSΔSTP/c-ras and HVSΔSTP/v-ras can alter the expression profile of lymphocyte surface antigens upon immortalization instead of selectively targeting this cell type for immortalization.
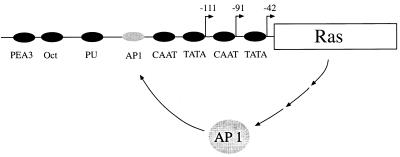
It is intriguing that the level of ras expression is significantly higher in cells transformed by HVSΔSTP/v-ras than in those transformed by HVSΔSTP/c-ras (Fig. 4). Expression of ras in the recombinant HVSΔSTP/v-ras is driven by the STP-C488 promoter. In fact, STP-C488 transcription has been shown to be highly inducible upon phytohemagglutinin or TPA stimulation (19), and the STP promoter has been shown to contain a putative AP-1 transcription factor binding site at position −138 relative to the translational initiation site (19). These features suggest that activation of the cellular AP-1 transcriptional factor by upregulation of the ras signal transduction pathway in HVSΔSTP/v-ras-transformed cells contributes to activation of the STP promoter by positive feedback, which may ultimately lead to the high level of v-ras gene expression (Fig. 6). Thus, the high level of ras expression as well as oncogenic ras activity may enhance the transforming efficiency of HVSΔSTP/v-ras compared to HVSΔSTP/c-ras.
FIG. 6.
Schematic diagram of upregulation of ras expression from STP promoter. The −91 basal transcriptional initiation site and −42 and −111 TPA-inducible transcriptional initiation sites are indicated with arrows as described previously (19).
Over 60% of common marmoset T cells immortalized by HVSΔSTP/v-ras displayed an adherent phenotype. FACS analysis showed that these adherent cells were not derived from macrophage lineage. Several changes in cell morphology take place during lymphocyte activation (11). Spherical resting T cells become polarized during activation, developing a well-defined cytoplasmic projection which has been called the cellular uropod (11). It can be hypothesized that activation of the cellular AP-1 transcriptional factor by upregulation of the ras signal transduction pathway in HVSΔSTP/v-ras-transformed cells induces the surface expression of adhesion molecules, which in turn alters cellular morphology. In fact, expression of cellular adhesion molecules including intercellular cell adhesion molecule 1 and vascular cell adhesion molecule 1 is regulated by the AP-1 transcription factor (10, 38). However, because of the lack of antibodies which cross-react with the surface antigens of New World monkey lymphocytes, expression of these adhesion molecules could not be assessed on the immortalized marmoset cell lines.
In this report, we have shown that an activated form of ras can efficiently substitute for the STP oncogene in the context of HVS to induce lymphocyte transformation. However, only one of two common marmosets infected with recombinant HVSΔSTP/v-ras or HVSΔSTP/c-ras developed lymphoma, while two of two marmosets infected with wt HVS developed lymphoma. Additionally, the onset of disease induction with HVSΔSTP/v-ras was delayed slightly compared to that with wt HVS, whereas it was even further delayed with HVSΔSTP/c-ras. Thus, while activated ras appears sufficient to substitute for the STP oncogene in transformation in the context of viral infection, certain factors may influence the efficiency with which it can do so. For example, the level or timing of ras expression or activation could influence a number of critical variables such as cell survival, susceptibility to immune attack, or per-cell production of virus. Further, additional cellular genes other than ras may be involved in STP function. Recently, we have found that STP binds to tumor necrosis factor receptor-associated factors, and this interaction induces NF-κB activation (unpublished results). Thus, these factors may influence the efficiency of lymphoma induction by the recombinant ras viruses. Nevertheless, results in this report support an important role for ras signal transduction in STP-dependent cell growth transformation by group C strains of HVS. Also, this is the first demonstration at least among gamma herpesviruses that the activated form of a cellular partner can substitute for the activity of a viral gene in vitro and in vivo.
ACKNOWLEDGMENTS
We thank E. Kieff, R. Davis, and G. Crabtree for providing the reporter plasmids. We especially thank R. Desrosiers for discussions and critical reading of manuscript. We also thank J. Newton for manuscript preparation.
This work was supported by Public Health Service grants CA31363, AI38131, and RR00168 and grant RG 2856-A-1 from the National Multiple Sclerosis Society.
REFERENCES
- 1.Alexander L, Du Z, Rosenzweig M, Jung J U, Desrosiers R C. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J Virol. 1997;71:6094–6099. doi: 10.1128/jvi.71.8.6094-6099.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 3.Berend K R, Jung J U, Boyle T J, DiMaio J M, Mungal S A, Desrosiers R C, Lyerly H K. Phenotypic and functional consequences of herpesvirus saimiri infection of human CD8+ cytotoxic T lymphocytes. J Virol. 1993;67:6317–6321. doi: 10.1128/jvi.67.10.6317-6321.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biesinger B, Müller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biesinger B, Trimble J J, Desrosiers R C, Fleckenstein B. The divergence between two oncogenic herpesvirus saimiri strains in a genomic region related to the transforming phenotype. Virology. 1990;176:505–514. doi: 10.1016/0042-6822(90)90020-r. [DOI] [PubMed] [Google Scholar]
- 6.Blue M L, Daley J F, Levine H, Schlossman S F. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985;134:2281–2286. [PubMed] [Google Scholar]
- 7.Bourne H R, Sanders D A, McCormick F. The GTPase superfamily: a conserved switch for diverse cell functions. Nature. 1990;348:125–132. doi: 10.1038/348125a0. [DOI] [PubMed] [Google Scholar]
- 8.Bröker B M, Tsygankov A Y, Müller-Fleckenstein I, Guse A H, Chitaev N A, Biesinger B, Fleckenstein B, Emmrich F. Immortalization of human T cell clones by Herpesvirus saimiri. J Immunol. 1993;151:1184–1192. [PubMed] [Google Scholar]
- 9.Courtneidge S A, Smith A E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983;303:435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 10.Cybulsky M I, Fries J W, Williams A J, Sultan P, Eddy R, Byers M, Shows T, Gimbrone M A J, Collins T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proc Natl Acad Sci USA. 1991;88:7859–7863. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.del Pozo M A, Sanchez-Mateos P, Nieto M, Sanchez-Madrid F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J Cell Biol. 1995;131:495–508. doi: 10.1083/jcb.131.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desrosiers R C, Bakker A, Kamine J, Falk L A, Hunt R D, King N W. A region of the herpesvirus saimiri genome required for oncogenicity. Science. 1985;228:184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- 13.Desrosiers R C, Burghoff R L, Bakker A, Kamine J. Construction of replication-competent herpesvirus saimiri deletion mutants. J Virol. 1984;49:343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desrosiers R C, Silva D, Waldron L M, Letvin N L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986;57:701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duboise S M, Guo J, Czajak S, Desrosiers R C, Jung J U. STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;72:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duboise S M, Guo J, Desrosiers R C, Jung J U. Use of virion DNA as a cloning vector for the construction of mutant and recombinant herpesviruses. Proc Natl Acad Sci USA. 1996;93:11389–11394. doi: 10.1073/pnas.93.21.11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duboise S M, Lee H, Guo J, Choi J-K, Czajak S, Simon M, Desrosiers R C, Jung J U. Mutation of the Lck-binding motif of Tip enhances lymphoid cell activation by herpesvirus saimiri. J Virol. 1998;72:2607–2614. doi: 10.1128/jvi.72.4.2607-2614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dyson N, Howley P M, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989;243:934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 19.Fickenscher H, Biesinger B, Knappe A, Wittmann S, Fleckenstein B. Regulation of the herpesvirus saimiri oncogene stpC, similar to that of T-cell activation genes, in growth-transformed human T lymphocytes. J Virol. 1996;70:6012–6019. doi: 10.1128/jvi.70.9.6012-6019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geck P, Whitaker S, Medveczky M, Medveczky P. Expression of collagen-like sequences by a tumor virus. J Virol. 1990;64:3509–3515. doi: 10.1128/jvi.64.7.3509-3515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Campbell D, Dérijard B, Davis R J. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 22.Jung J U, Desrosiers R C. Identification and characterization of the herpesvirus saimiri oncoprotein, STP-C488. J Virol. 1991;65:6953–6960. doi: 10.1128/jvi.65.12.6953-6960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung J U, Desrosiers R C. Herpesvirus saimiri and ateles. In: Webster R, Granoff A, editors. Encyclopedia of virology. Philadelphia, Pa: Saunders Scientific Publications, Inc.; 1994. pp. 614–622. [Google Scholar]
- 24.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung J U, Trimble J J, King N W, Biesinger B, Fleckenstein B W, Desrosiers R C. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koide H, Satoh T, Nakafuku M, Kaziro Y. GTP-dependent association of Raf-1 with Ha-Ras: identification of Raf as a target downstream of Ras in mammalian cells. Proc Natl Acad Sci USA. 1993;90:8683–8686. doi: 10.1073/pnas.90.18.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koomey J M, Mulder C, Burghoff R L, Fleckenstein B, Desrosiers R C. Deletion of DNA sequences in a nononcogenic variant of herpesvirus saimiri. J Virol. 1984;50:662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kretschmer C, Murphy C, Biesinger B, Beckers J, Fickenscher H, Kirchner T, Fleckenstein B, Rüther U. A Herpes saimiri oncogene causing peripheral T-cell lymphoma in transgenic mice. Oncogene. 1996;12:1609–1616. [PubMed] [Google Scholar]
- 29.Lee H, Trimble J J, Yoon D-W, Regier D, Desrosiers R C, Jung J U. Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular Src. J Virol. 1997;71:3817–3825. doi: 10.1128/jvi.71.5.3817-3825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittrücker H-W, Müller-Fleckenstein I, Fleckenstein B, Fleishcher B. CD2-mediated autocrine growth of herpes virus saimiri-transformed human T lymphocytes. J Exp Med. 1995;176:900–913. doi: 10.1084/jem.176.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moodie S A, Willumsen B M, Weber M J, Wolfman A. Complexes of Ras-GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993;260:1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- 32.Murphy C, Kretschmer C, Biesinger B, Beckers J, Jung J, Desrosiers R C, Müller-Hermelink H K, Fleckenstein B W, Rüther U. Epithelial tumors induced by a herpesvirus oncogene in transgenic mice. Oncogene. 1994;9:221–226. [PubMed] [Google Scholar]
- 33.Murthy S C S, Trimble J J, Desrosiers R C. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J Virol. 1989;63:3307–3314. doi: 10.1128/jvi.63.8.3307-3314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pawelec G, Ziegler A, Wernet P. Dissection of human allostimulatory determinants with cloned T cells: stimulation inhibition by monoclonal antibodies TÜ 22, 34, 36, 37, 39, 43, and 58 against distinct human MHC class II molecules. Hum Immunol. 1985;12:165. doi: 10.1016/0198-8859(85)90333-7. [DOI] [PubMed] [Google Scholar]
- 35.Sarnow P, Ho Y S, Williams J, Levine A J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982;28:387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- 36.Schlossman S, Bloumsell L, Gilks W. White cell differentiation antigens, Leucocyte typing V. New York, N.Y: Oxford University Press; 1995. [Google Scholar]
- 37.VanAelst L, Barr M, Marcus S, Polverino A, Wigler M. Complex formation between Ras and RAF and other protein kinases. Proc Natl Acad Sci USA. 1993;90:6213–6217. doi: 10.1073/pnas.90.13.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.vandeStolpe A, Caldenhoven E, Stade B G, Koenderman L, Raaijmakers J A, Johnson J P, vanderSaag P T. 12-O-tetradecanoylphorbol-13-acetate- and tumor necrosis factor alpha-mediated induction of intercellular adhesion molecule-1 is inhibited by dexamethasone. Functional analysis of the human intercellular adhesion molecular-1 promoter. J Biol Chem. 1994;269:6185–6192. [PubMed] [Google Scholar]
- 39.Vojtek A B, Hollenberg S M, Cooper J A. Mammalian Ras interacts directly with the serine/threonine kinase Raf. Cell. 1993;74:205–214. doi: 10.1016/0092-8674(93)90307-c. [DOI] [PubMed] [Google Scholar]
- 40.Warne P H, Viciana P R, Downward J. Direct interaction of Ras and the amino-terminal region of Raf-1 in vitro. Nature. 1993;364:352–355. doi: 10.1038/364352a0. [DOI] [PubMed] [Google Scholar]
- 41.Werness B A, Levine A J, Howley P M. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 42.Whyte P, Buchkovich K J, Horowitz J M, Friend S H, Raybuck M, Weinberg R A, Harlow E. Association between an oncogene and an antioncogene: the adenovirus E1A protein bind to the retinoblastoma gene product. Nature. 1988;334:124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]