Abstract
Objectives:
Detection and prediction of disability progression is a significant unmet need in people with progressive multiple sclerosis (PwPMS). Government and health agencies have deemed the use of patient-reported outcomes measures (PROMs) in clinical practice and clinical trials a major strategic priority. Nevertheless, data documenting the clinical utility of PROMs in neurological diseases is scarce. This study evaluates if assessment of PROMs could track progression in PwPMS.
Methods:
EMerging blood BIOmarkers in PROgressive Multiple Sclerosis (EmBioProMS) investigated PROMs (Beck depression inventory-II (BDI-II), multiple sclerosis impact scale-29 (MSIS-29), fatigue scale for motor and cognition (FSMC)) in PwPMS (primary [PPMS] and secondary progressive MS [SPMS]). PROMs were evaluated longitudinally and compared between participants with disability progression (at baseline; retrospective evidence of disability progression (EDP), and during follow up (FU); prospective evidence of confirmed disability progression (CDP)) and those without progression. In an independent cohort of placebo participants of the phase III ORATORIO trial in PPMS, the diagnostic and prognostic value of another PROMs score (36-Item Short Form Survey [SF-36]) regarding CDP was evaluated.
Results:
EmBioProMS participants with EDP in the two years prior to inclusion (n= 136/227), or who suffered from CDP during FU (n of events= 88) had worse BDI-II, MSIS-29, and FSMC scores compared to PwPMS without progression. In addition, baseline MSIS29physical above 70th, 80th, and 90th percentiles predicted future CDP/ progression independent of relapse activity in EmBioProMS PPMS participants (HR of 3.7, 6.9, 6.7, p= 0.002, <0.001, and 0.001, respectively). In the placebo arm of ORATORIO (n= 137), the physical component score (PCS) of SF-36 worsened at week 120 compared to baseline, in cases who experienced progression over the preceding trial period (P= 0.018). Worse PCS at baseline was associated with higher hazard ratios of disability accumulation over the subsequent 120 weeks (HR: 2.01 [30th-], 2.11 [20th-], and 2.8 [10th percentile], P= 0.007, 0.012 and 0.005, respectively).
Conclusions
PROMs could provide additional, practical, cost-efficient, and remotely accessible insight about disability progression in PMS through standardized, structured, and quantifiable patient feedback.
Trial Registration:
EmBioProMS: DRKS (DRKS00020132), ORATORIO: Clinicaltrials.gov (NCT01194570)
Keywords: Progressive Multiple Sclerosis, Patient-reported outcomes, Quality of life, Progression, Fatigue
Introduction:
With the recent introduction of disease-modifying treatments (DMTs) for progressive multiple sclerosis (PMS), the accurate identification of disability progression in people with PMS (PwPMS) has become more critical than ever. Progression independent of relapse activity (PIRA) is now known to occur in all forms of MS, including relapsing-remitting MS (RRMS), and the distinctions between underlying progression mechanisms in relapsing and progressive forms are incompletely understood(Kappos, Wolinsky et al. 2020). In clinical settings, disability progression is mainly identified through detailed medical history and the Expanded Disability Status Scale (EDSS) assessment. Nevertheless, several factors hinder the accurate evaluation of progression. Symptoms such as depression, fatigue, and cognitive deterioration are challenging to be objectively documented, liable to recall bias, and poorly reflected in the EDSS. Furthermore, longitudinal EDSS scores are not always available, and are susceptible to inter-rater variability. In addition, re-evaluation of EDSS based on the recalled walking distance could lead to inaccurate estimation of disability in up to 25% of the cases(Skjerbaek, Boesen et al. 2019).
Patient-reported outcome measurements (PROMs) is increasingly applied in the management of numerous chronic conditions following the recommendations of health authorities like the Food and Drug Administration (FDA) and European medicines agency (EMA)(Venkatesan 2016, Kluzek, Dean et al. 2021, Retzer, Aiyegbusi et al. 2022). In the field of neurology, PROMs are mainly applied as efficacy measures in clinical trials(D’Amico, Haase et al. 2019). PROMs offer a standardized platform to assess symptoms such as fatigue, depression, and deterioration of the overall health(D’Amico, Haase et al. 2019).
This work assessed a battery of PROMs in relation to disability progression in the EmBioProMS study, a prospective multicentric PMS cohort(Abdelhak, Huss et al. 2020). We aimed to evaluate if PROMs assessment can contribute to better differentiation between clinically measurable disability progression cases and those with stable PMS, as well as better prediction of disability. Our battery included three established assessments used in MS: Fatigue Score of Motor and Cognition (FSMC)(Penner, Raselli et al. 2009), Beck Depression Inventory-II (BDI-II)(Sacco, Santangelo et al. 2016), and Multiple Sclerosis Impact Scale-29 (MSIS-29)(Hobart, Lamping et al. 2001). All three PROMs assess specific yet different MS symptoms, have high test-retest reliability, and each takes less than 5 minutes to complete(D’Amico, Haase et al. 2019). As a validation of concept, we evaluated another PROM (short-form health survey, SF-36) collected from participants with PPMS in the placebo arm of the ORATORIO trial(Montalban, Hauser et al. 2017).
Methods:
Study Design
EMerging blood BIOmarkers in PROgressive Multiple Sclerosis (EmBioProMS) is an observational, prospective, multicenter study(Abdelhak, Huss et al. 2020). In summary, 246 PwPMS were recruited in 8 centers in Germany. PPMS was defined according to the 2017 McDonald criteria(Thompson, Banwell et al. 2018), while SPMS was defined as participants with previous RRMS (fulfilling the 2017 McDonald criteria), who developed relapse-independent disability progression for at least one year. Cases with RRMS, a history of relapse in the last three months, other known major inflammatory or non-inflammatory of the nervous system diseases were excluded. Date and symptoms of first manifestation, date of the diagnosis, number of documented relapses, date of the most recent relapse, duration of the progressive phase, and concomitant diseases were recorded at the baseline visit. Medical history, EDSS, Nine-Hole Peg Test (9-HPT) and Timed 25-Foot Walk Test (T25FW) were evaluated at baseline and at each follow-up visits through a certified EDSS rater. Participants who were treated with a immunotherapy/ DMT up to the day of visit or in a predefined period before the visit were assigned to the treated group, depending on the therapy (corticosteroids in the last 30 days; any interferon preparation, glatiramer acetate, natalizumab, dimethyl fumarate, teriflunomide, fingolimod, methotrexate, or azathioprine in the previous three months; rituximab, ocrelizumab, or mitoxantrone in the previous 12 months; or cladribine or alemtuzumab in the previous 24 months). Otherwise, participants were considered untreated. Data management was conducted within the platform of the German MS registry(Ohle, Ellenberger et al. 2021).
The ORATORIO trial(Montalban, Hauser et al. 2017) was an international, multicenter, double-blind, randomized, placebo-controlled, phase 3 trial, in which 732 PwPPMS were randomly assigned in a 2:1 ratio to receive intravenous ocrelizumab or placebo every 24 weeks for at least 120 weeks.
Evaluation of disability progression
The cut-off date for current analysis in EmBioProMS was 2022-09-28. In the EmBioProMS trial, disability progression was defined both pro- and retrospectively using combined outcome parameter. At the baseline visit, retrospective disability progression status (evidence of disability progression (EDP) was determined by the treating physician based on existing, pre-documented, at least for 24-weeks persistent, increase of EDSS beyond established step increase threshold, 9-HPT, or T25FW (both by > 20%) during the two years prior to inclusion. In the prospective part of EmBioProMS, and in ORATORIO study, confirmed disability progression (CDP) was defined using worsening at any of the three combined outcome parameter when confirmed after 24 (EmBioProMS)-, and 12-weeks (ORATORIO). The combined outcome parameter applied the established EDSS step increase threshold, or an increase in the 9-HPT score or the T25FW score by 20% or more(Koch, Cutter et al. 2017, Montalban, Hauser et al. 2017). Both studies applied a roving reference EDSS/T25FW/9-HPT to identify recurrent events. PIRA was defined in case of no evidence of clinical activity (i.e., relapse) between reference EDSS visit and time of diagnosing of the CDP event.
Patient Reported Outcome Measurements (PROMs)
In EmBioProMS, FSMC, BDI-II, and MSIS-29 were evaluated at each of the study visit (baseline and follow-up visit (FU)), on the same day of assessing the remaining clinical variables mentioned above. FSMC has 20 items, ten of which focus on cognitive fatigue and ten on motor fatigue. The minimum value is 20 (no fatigue), and the maximum value is 100(Penner, Raselli et al. 2009). BDI-II is a 21-item multiple-choice inventory. The minimum score is zero, and the maximum score is 63 points(Sacco, Santangelo et al. 2016). MSIS-29 consists of 29 items. Of them, 20 items constitute the physical scale, while nine questions form the psychological scale. Each scale is scored separately and converted to a 0-100 scale(Hobart, Lamping et al. 2001). Higher scores indicate worse performance. For BDI-II and FSMC, missing responses in each score were imputed by the mean value of all other responses when >85% of the corresponding questionnaire was completed. Missing responses in MSIS-29 were treated as recommended before(Hobart, Lamping et al. 2001).
The ORATORIO trial evaluated the 36-Item short-form health survey (SF-36) at the baseline visit, week 48, and week 120(Montalban, Hauser et al. 2017). The SF-36 questionnaire includes 36 questions covering eight sections: vitality, physical functioning, bodily pain, general health perceptions, physical role functioning, emotional role functioning, social role functioning, and mental health. These eight scales can be transformed into two summary scores: the Physical (PCS) and Mental (MCS) Component Summary scores. In contrast to the PROMs in the EmBioProMS, lower SF-36 scores indicate worse disability related qualify of life(Brazier, Harper et al. 1992).
Statistical Analysis
Summary statistics were applied to describe the different variables, i.e., median with interquartile range for continuous variables and frequencies with percentages for categorical variables. Marginal means were used when summary statistics spanned multiple visits. Nonparametric Spearman’s correlation coefficients (rs) were used to assess correlations. Continuous variables were compared between groups by Mann–Whitney U tests. For dichotomous variables chi-squared tests were used instead. The Kaplan-Meier method was used to estimate survival curves. Cox regression was used to model the time from inclusion of the participant to the first CDP. In the EmBioProMS cohort this model adjusted for onset, sex, baseline EDSS, center, disease duration and treatment status and all other models adjusted additionally for disease phenotype. The relationship at the baseline visits between EDP and PROMs scores or their percentiles were analyzed with general linear models. Follow-Up visits were analyzed with general or logistic linear mixed models which in addition adjusted for timing of visit (before vs after the start of the COVID-19 pandemic - cut point 2020-03-25) and individual subject (as random intercept). In the ORATORIO trial all models adjusted for the baseline values of age, sex, disease duration, EDSS and geographical region. In both cohorts, models that analyzed the relationship between CDP and the increase in a PROM score since the baseline visit also adjusted for the baseline value of that score (Figure S1). No adjustment for multiple testing was carried out; descriptive p-values (two-sided) are reported and referred to as statistically significant if smaller 5%. R (version 4.0.2) was used for all computations.
Ethics approval
The Institutional Review Boards at the University Hospital of Ulm (#270/12), and the local ethical committees at all the participating centers approved the EmBioProMS study. The ORATORIO trial was conducted according to the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Results:
PROMs in EmBioProMS:
246 participants had been recruited in the EmBioProMS study with a total of 516 FU visits (median 3 [2-4] per participant). The median FU time is 1.63 years [0.59 – 2.63]. 164 participants had ≥ 3 visits. Only patient-visits with complete PROMs scores were included in the analysis (229 baseline visits, and 464 FU visits). For retrospective evaluation of progression (i.e., EDP), two participants were excluded due to missing data regarding EDP at baseline, thus 227/229 baseline visits were evaluated. The clinical characteristics are reported in Table 1.
Table 1.
Clinical characteristics of the included participants
| SPMS (n=100) | PPMS (n=129) | Total (n=229) | |
|---|---|---|---|
|
| |||
| Age at baseline (in years) | 55.0 (49.4–61.5) | 56.1 (50.0–60.9) | 55.5 (49.7–61.1) |
|
| |||
| Disease duration at baseline (in years) | 19 (13–30) | 8 (4–13) | 12 (6–20) |
|
| |||
| Female (%) | 58 (58.0) | 68 (52.7) | 126 (55.0) |
|
| |||
| EDSS at baseline | 6.0 (4.0–6.0) | 4.5 (3.5–6.0) | 4.5 (3.5–6.0) |
|
| |||
| Immunotherapy at baseline (%) | 49 (49.0) | 71 (55.0) | 120 (52.4) |
|
| |||
| Ocrelizumab | 6 | 51 | 57 |
| 3-monthly methylprednisolone | 19 | 18 | 37 |
| Others | 29 | 3 | 32 |
|
| |||
| EDP at baseline (%) | 52 (53.1) | 84 (65.1) | 136 (59.9) |
|
| |||
| FU duration (years) | 1.54 [0.49 – 2.56] | 1.63 [0.67 – 2.61] | 1.59 [0.51 – 2.59] |
SPMS, secondary progressive multiple sclerosis; PPMS, primary progressive multiple sclerosis; EDSS, Expanded Disability Score Scale; EDP: evidence of disability progression. FU: follow-up visits. Number mentioned are Median (25–75 percentile), or count (percentage)
At baseline, 100 (43.7%) participants had SPMS, and 129 (56.3%) had PPMS. 120 (52.4%) were treated at baseline, with ocrelizumab the most common immunotherapy (n=57, 47.5%), followed by 3 monthly treatment with pulse methylprednisolone (n=37, 30.8%). 64/229 (27.9%) of EmBioProMS participants did not receive any immunotherapy prior to baseline visit (n= 16 and 48 with SPMS and PPMS, respectively). At last available FU visit, 65.2% included participants were receiving a immunotherapy (71.2% of PPMS and 57.8% of SPMS). Clinical relapses were documented in 27/229 EmBioProMS participants (in the two years preceding baseline visit). The scores of the included PROMs at baseline are summarized in Table S1. BDI-II, MSIS-29psychological and FSMC were inversely, but weakly correlated with age at baseline (rs= −0.15, −0.20, and −0.21 p= 0.023, 0.002 and 0.001, respectively). At baseline, we found a moderate correlation between EDSS with MSIS-29physical and FSMC (rs= 0.53 and 0.29, p<0.001 for both), while the correlation between EDSS and BDI-II was weak (rs=0.15, 0.021). All included PROMs showed a weak or moderate correlation with the cerebral functional score (data not shown).
PROMs and disability progression in EmBioProMS:
EDP (retrospective) was diagnosed in 136/227 (59.9%) of baseline visits. Most of the EDP events were confirmed through EDSS increase (n= 122, 89.7%). In 11.8% of the participants (n= 16), all three components for the definition of EDP were fulfilled. Longitudinally, 88 CDP events (including recurrent CDP events per participant) could be diagnosed (88 events /271 FU visit, 32.5%, 56 events in PPMS and 32 in SPMS) in subjects with at least 3 visits, sufficient follow-up time for confirmation, and complete documentation of disease outcome parameters. Most CDP events were diagnosed based on expanded criteria beyond EDSS step-increase with 45 and 31 events of confirmed T25FW and 9HPT increase, respectively. EDSS progression was detected in only 28/88 events of CDP. Clinical relapses were reported preceding CDP in only 3 of the 88 CDP events, rendering almost all events PIRA.
Participants with EDP in the two years before baseline, and those with CDP over the course of FU had worse PROMs scores at baseline and event visit, respectively (Table 2, Figure 1). PROMs association with CDP was independent of severity of depression (Table S2), In addition, CDP events were associated with worsening of PROMs scores (Table S3) in mixed linear regression as described in the statistical section (Figure S2). Moreover, worsening of PROMs scores was associated with higher OR for diagnosing CDP. With 1 point increase in the BDI-II, MSIS-29physical, MSIS-29psychological, and FSMC, OR were 1.09 [1.03 – 1.15, p=0.003], 1.02 [1.00 – 1.05, p= 0.020], 1.02 [1.00 – 1.04, p= 0.049], 1.04 [1.01 – 1.08, p= 0.007], respectively, for diagnosing CDP at this visit. All association remined significant after excluding the three participants with relapses (data not shown).
Table 2.
PROMs scores in relationship to retrospective EDP and prospective CDP
| EDP at baseline visit | CDP in FU visits | |||||||
|---|---|---|---|---|---|---|---|---|
| No EDP Median [25–27 Percentile] (n= 91/227) | EDP Median [25–27 Percentile] (n= 136/227) | Estimate [95% CI]* | p * | No CDP (n=183/271)‡ | CDP (n=88/271)‡ | Estimate of Difference (95% CI) ‡ | p ‡ | |
| BDI-II | 8.0 [5.0 – 13.0] | 11.0 [6.0, – 17.2] | 4.4 [1.8 – 7.0] | 0.001 | 9.6 [7.8 – 11.3] | 12.4 [10.3 – 14.4] | 2.8 [1.2 – 4.5] | <0.001 |
| MSIS physical -29 | 31.2 [17.5 – 41.9] | 47.5 [30.2 – 62.5] | 9.7 [3.8 – 15.5] | 0.001 | 38.3 [34.4 – 42.2] | 43.4 [38.9 – 47.9] | 5.1 [1.3 – 8.9] | 0.009 |
| MSISpsychological-29 | 22.2 [8.9 – 34.7] | 36.1 [16.7 – 52.8] | 11.6 [5.0 – 18.2] | 0.001 | 29.0 [24.4 – 33.6] | 35.5 [30.2 – 40.9] | 6.5 [1.8 – 11.1] | 0.006 |
| FSMC | 59.0 [40.5 – 70.5] | 68.7 [53.0 – 77.0] | 6.9 [1.6 – 12.1] | 0.011 | 58.8 [55.2 – 62.4] | 62.3 [58.3 – 66.3] | 3.5 [0.4 – 6.5] | 0.026 |
EDP: retrospective evidence of disability progression, assessed at baseline visit, CDP: prospectively assessed, confirmed disability progression, FU: follow-up, BDI-II: beck-depression inventory-II, MSIS-29: multiple sclerosis impact scale (physical, and psychological components), FSMC: fatigue scale for motor and cognition.
linear regression as described in the statistical analysis section.
mixed linear model as described in the statistical analysis. Marginal means for the No CDP and the CDP group.
Figure 1. Patient-reported outcome measurements (PROMs) in PwPMS with and without restro- and prospective evidence of disability progression.
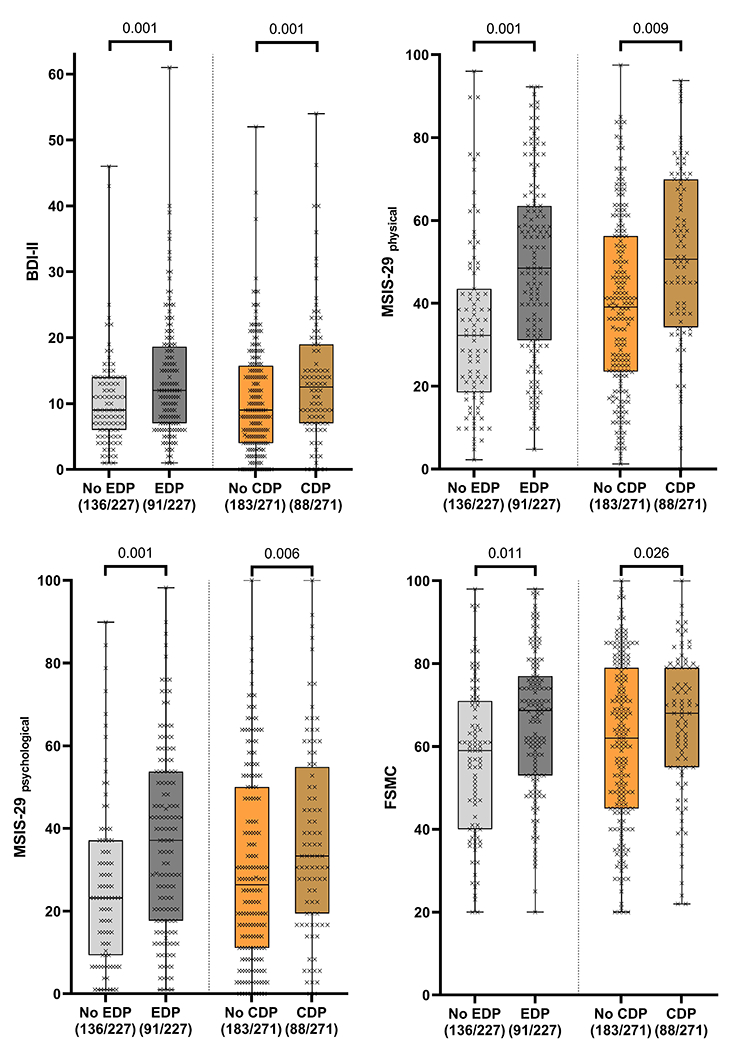
BDI-II, Beck Depression Inventory-II; MSISphysical -29, Multiple Sclerosis Impact Scale-29, physical component; MSISpsychological -29, Multiple Sclerosis Impact Scale, psychological component; FSMC, fatigue score for motor and cognition. EDP: evidence of disability progression (retrospective, CDP: confirmed disability progression (prospective). P-value from linear regression models for EDP and mixed linear models for CDP as defined in the statistical analysis section. The boxplots are overlaid with individual measurements. Higher scores represent worse performance.
Association between baseline PROMs and future CDP:
Of the evaluated PROMs, only baseline MSIS-29physical score cut-off at 70th percentile was significantly associated with future CDP in the whole cohort (HR: 1.8 [1.03 – 3.02], p=0.039) (Table 3). PROMs performed differently between the included PP- and SPMS populations; in PPMS, higher values for MSISphysical-29 were associated to CDP with HR of 3.7 [1.6 – 8.5], 6.9 [2.6 – 18.2], 6.7 [2.1 – 21.1] for ≥70th, ≥80th and ≥90th percentiles (p= 0.002, <0.001, and 0.001, Table 3 and Figure 2). On the other hands, neither the scores, nor the cut-off percentiles were significantly associated with future CDP in SPMS.
Table 3.
Hazard ratio for time to first CDP in EmBioProMS participants
| Baseline PROMS | Whole EmBioProMS population | PPMS | SPMS | |||
|---|---|---|---|---|---|---|
| HR [CI] | p | HR [CI] | p | HR [CI] | p | |
| BDI-II (continuous) | 1.00 [0.97 - 1.03] | 0.783 | 1.01 [0.97 – 1.04] | 0.693 | 0.98 [0.93 – 1.04] | 0.600 |
| BDI-II > 70th percentile | 0.80 [0.45 - 1.42] | 0.447 | 0.74 [0.36 – 1.48] | 0.391 | 0.63 [0.23 – 1.70] | 0.361 |
| BDI-II > 80th percentile | 1.09 [0.60 – 2.00] | 0.776 | 0.94 [0.45 – 1.96] | 0.879 | 0.55 [0.16 – 1.89] | 0.347 |
| BDI-II > 90th percentile | 1.45 [0.69 – 3.05] | 0.328 | 1.34 [0.54 – 3.32] | 0.522 | 0.96 [0.19 – 4.76] | 0.962 |
| MSIS-29 physical component (continuous) | 1.01 [1.00 - 1.02] | 0.066 | 1.03 [1.01 – 1.05] | 0.002 | 1.00 [0.98 – 1.02] | 0.919 |
| MSIS-29physical > 70th percentile | 1.76 [1.03 - 3.02] | 0.039 | 3.72 [1.64 – 8.47] | 0.002 | 1.50 [0.55 – 4.05] | 0.427 |
| MSIS-29physical > 80th percentile | 1.76 [0.96 – 3.21] | 0.066 | 6.86 [2.58 – 18.23] | <0.001 | 0.91 [0.31 – 2.66] | 0.869 |
| MSIS-29physical > 90th percentile | 1.59 [0.78 – 3.21] | 0.200 | 6.69 [2.12 – 21.11] | 0.001 | 0.67 [0.20 – 2.30] | 0.526 |
| MSIS-29 psychological component (continuous) | 1.01 [1.00 - 1.02] | 0.034 | 1.02 [1.00 – 1.03] | 0.009 | 0.99 [0.97 – 1.01] | 0.567 |
| MSIS-29psychological > 70th percentile | 1.39 [0.83 - 2.33] | 0.204 | 1.87 [0.93 – 3.76] | 0.080 | 0.76 [0.28 – 2.02] | 0.578 |
| MSIS-29psychological > 80th percentile | 1.40 [0.80 – 2.44] | 0.238 | 2.51 [1.15– 5.46] | 0.020 | 0.48 [0.15 – 1.52] | 0.213 |
| MSIS-29psychological > 90th percentile | 1.28 [0.60 – 2.72] | 0.524 | 3.38 [1.40 – 8.18] | 0.007 | 0.27 [0.03 – 2.29] | 0.228 |
| FSMC (continuous) | 1.00 [0.99 - 1.02] | 0.962 | 1.01 [0.99 – 1.03] | 0.396 | 1.00 [0.97 – 1.02] | 0.748 |
| FSMC > 70th percentile | 1.13 [0.66 - 1.95] | 0.651 | 1.96 [0.93 – 4.12] | 0.077 | 0.83 [0.32 – 2.14] | 0.703 |
| FSMC > 80th percentile | 1.12 [0.63 – 1.97] | 0.704 | 3.14 [1.45 – 6.82] | 0.004 | 0.51 [0.16 – 1.62] | 0.254 |
| FSMC > 90th percentile | 1.35 [0.67 – 2.73] | 0.406 | 2.98 [1.02 – 8.71] | 0.045 | 0.57 [0.17 – 1.91] | 0.363 |
BDI-II: beck-depression inventory-II, MSIS-29: multiple sclerosis impact scale (physical, and psychological components), FSMC: fatigue scale for motor and cognition. HR: hazard ratio, CI: 95% confidence interval.
Figure 2: Time to first event analysis for the association between the physical component scale of MSIS-29 and disability accumulation in EmBioProMS.
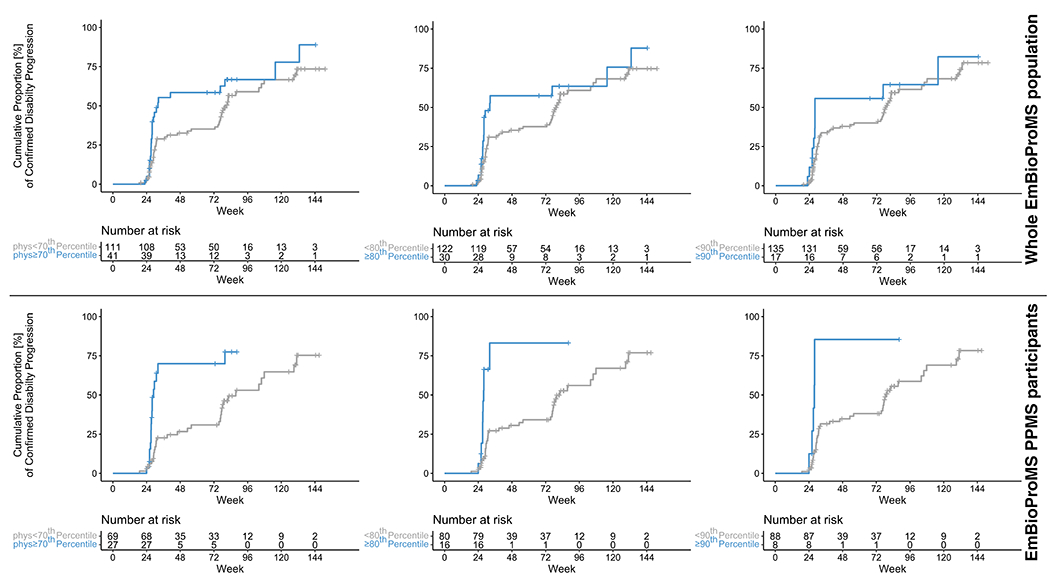
Worse physical component scores (≥70th in blue) at baseline visit in the EmBioProMS whole cohort (upper half) and in PwPMS with primary progressive multiple sclerosis (PPMS, for 70th, 80th, and 90th percentiles) (lower half) were associated with higher hazard rate for suffering from confirmed disability progression. Time is measured in weeks since the baseline visit.
PROMS in the independent cohort (ORATORIO):
From the ORATORIO placebo arm, 137/244 participants with available SF-36 scores at week 120 were included in this analysis. Apart from minimally higher age, the included ORATORIO participants did have similar clinical characteristics as the participants with missing SF-36 scores (Table S4).
All included ORATORIO participants had at least 3 visits (baseline, week 48 and week 120) over the study duration. At the baseline visit, worse (i.e., lower values, in contrary to EmBioProMS scores[higher is worse]) physical component scores (PCS) were associated with higher EDSS (rs= 0.5, p<0.001). There was no statistically significant association between the mental component scores (MCS) and EDSS. Neither of the baseline SF-36 two component scores was significantly correlated with age (data not shown).
In the ORATORIO placebo arm, PwPMS with progression during the FU had worse PCS at week 120 (p= 0.007, Table S5, Figure S3a), while MCS did not differ significantly between cases with CDP and no CDP (p= 0.556, Table S5, Figure S3b). In addition, PCS scores worsened by an estimate of 3.10 points (p= 0.018) in cases with CDP than in cases without CDP (Figure S3c).
Moreover, In ORATORIO, PwPPMS with worse PCS scores at baseline (30th, 20th and 10th percentiles) were more likely to develop CDP over 120 weeks, (Hazard ratio [HR]: 2.01, 2.11, and 2.84, p= 0.007, 0.012, and 0.005 respectively) (Figure 3). Excluding ORATORIO participants with relapse over the FU period did not change the prognostic potential of the PCS score (HR: 2.2 [1.27 – 3.99], 2.47 [1.24 – 4.93], and 2.8 [1.21 – 6.26], p= 0.009, 0.010 and 0.015, respectively).
Figure 3: Survival analysis for the association between the physical component scale of SF-36 and disability accumulation in placebo patients in ORATORIO.
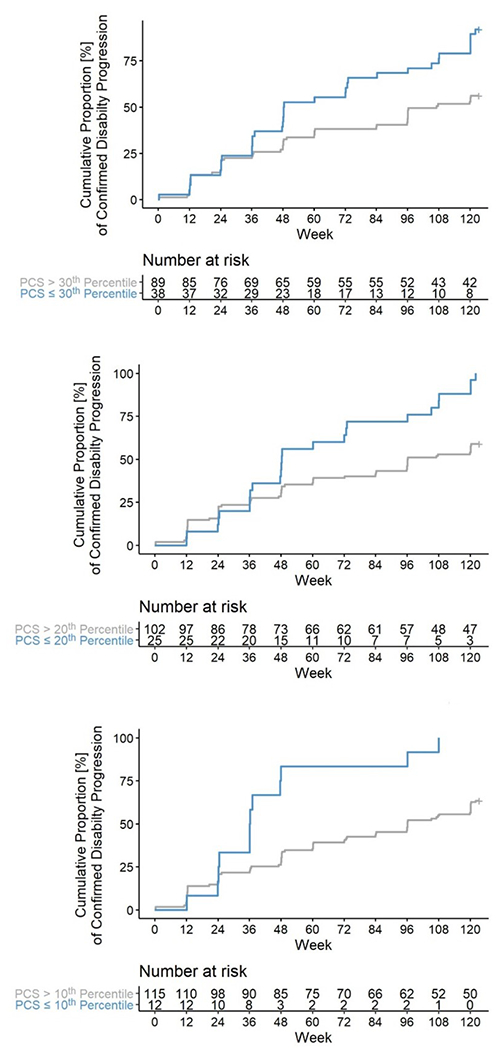
Worse physical component scores (≤30th,20th, and 10th percentiles, in blue) at baseline visit in the placebo arm of the ORATORIO trial were associated with higher hazard rate (HR: 2.01, 2.11, and 2.84, p= 0.007, 0.012, and 0.005, respectively; adjusted as defined in the statistical analysis section) for developing disease progression in the following 120 weeks. Time is measured in weeks since the baseline ORATORIO visit.
Discussion:
In this study, we explored the association of PROMs with disability worsening in PwPMS. We report worse PROMs scores (BDI-II, MSIS-29, FSMC) in EmBioProMS participants who have experienced CDP before inclusion in the study, as well as during follow-up. In addition, baseline PROMs values showed association with future disability progression in PPMS. An independent analysis including SF-36 from the participants of the ORATORIO placebo arm revealed similar findings with another outcome measure (SF-36).
PROMs offer a structured way to quantify various symptoms, including ones that are difficult to identify and measure such as fatigue and depression. Most of those symptoms are highly prevalent among PwPMS (Conway, Thompson et al. 2020, Marchesi, Vizzino et al. 2020). Our results align with evidence from previous studies, which suggested an association between PROMs in RRMS regarding the conversion rate to SPMS(Conway, Thompson et al. 2020, Healy, Glanz et al. 2021, Healy, Zurawski et al. 2021). In another study, higher MSIS-29 scores were associated with reduced survival time in PwMS(Raffel, Wallace et al. 2017).
It is also worth noting that the highest prognostic potential for PROMs was evident in PPMS for MSIS-29 followed by FSMC. Our findings suggest that proper selection of the evaluated PROMs score, and the population is critical in future clinical trials and in the clinical setting. Nevertheless, our results were predominantly driven by findings in PP, not SPMS participants, which is in line with a recent analysis that shows no predictive value of PROMs in SPMS(Strijbis, Repovic et al. 2022). Interestingly, the clinical characteristics of SPMS participants in both studies are similar, especially regarding high baseline EDSS score and long disease duration. Recent studies suggest slower rate of disability accrual in SPMS, which might imply the need for longer FU duration to detect the prognostic value of PROMs in this population(Harding-Forrester, Roos et al. 2023).
Worse PROMs in participants with disability progression can be explained by an increase in the physical and psychological burden with growing mobility limitations at higher EDSS. Therefore, the included PROMs might reflect PwPMS’s perception of added disability. A pathophysiological association between disability ‘progression’s underlying mechanisms and symptoms that contribute to PROMs cannot be excluded. PROMs have been linked to various central nervous system structures typically impacted in PMS (Zorzon, de Masi et al. 2001, Feinstein, Roy et al. 2004, Sepulcre, Sastre-Garriga et al. 2006, Sepulcre, Masdeu et al. 2009, Pellicano, Gallo et al. 2010, Derache, Grassiot et al. 2013, Scalfari, Romualdi et al. 2018, Arm, Ribbons et al. 2019, Singhal, Cicero et al. 2020).
Based on our results, PROMs should be investigated further for potential to be a subsidiary marker for PIRA. This is particularly relevant given the recent finding that PIRA can contribute to up to 90% of progression burden in RMS(Kappos, Wolinsky et al. 2020). Moreover, the PROMs included in our work might have potential to help identifying PMS participants with the highest risk for progression in the short- and medium-term. Therefore, integration of PROMs into decision-making tools might guide the treatment decision and recruitment for clinical trials targeting PMS.
The proportion of patients with EDP events at baseline (60%) in the EmBioProMS cohort is higher than previous reports from the active treatment arms in the PROMISE, OLYMPUS, ORATORIO, and EXPAND trials (all < 40%)(Wolinsky, Narayana et al. 2007, Hawker, O’Connor et al. 2009, Montalban, Hauser et al. 2017, Kappos, Bar-Or et al. 2018). Yet, this percentage was similar to those in the participants of the placebo arm of ORATORIO that were included in our study. A possible cause might be our cohort’s inclusion criteria and settings, as only highly specialized referral MS centers contributed to the study setting, conferring a potential selection bias. Moreover, the recruitment began in parallel to the introduction of DMTs that have demonstrated efficacy in PMS like ocrelizumab(Montalban, Hauser et al. 2017) and siponimod(Kappos, Bar-Or et al. 2018), which might have led to more referrals of PwPMS to initiate treatment. All factors taken together could explain the higher proportion of PPMS patients and patients with clinically evident progression in our cohort compared to previous natural history studies and clinical trials in PMS. Over the follow up period, this rate decreased significantly, which could be attributed to the initiation of DMTs approved for PMS such as ocrelizumab and siponimod. An important observation in our study is the high percentage of CDP events detected due to T25FW test changes compared to EDSS changes. This underpins the importance of combined disability metrics in the clinical setting and render EmBioProMS results to be of high relevance for designing of clinical trials aiming to evaluate PROMs in PMS.
Our analysis has some limitations. First, baseline disability progression in EmBioProMS was assessed in most participants based on documented EDSS scores during the last two years prior to enrolment as the T25FW and 9-HPT still have not been regularly reported in routine clinical settings. Second, PROMs are part of the secondary outcomes and were not necessarily a part of our power calculation(Abdelhak, Huss et al. 2020). In addition, our analysis did not correct for MRI metrics (such as T2 lesion load and atrophy parameters), due to the limited data availability in EmBioProMS. In addition, we restricted our analysis on the placebo arm of ORATORIO, considering the large number of untreated EmBioProMS-PPMS participants. Taken together, our results provide evidence supporting that a more “patient-focused” approach might have potential to contribute to classification, prediction of disability, and be subsequently considered as a valuable addition to the treatment decision algorithm. Standardized, structured, and quantifiable patient reported outcome feedback may be a simple, cost-efficient, and remotely accessible method to suggest (relapse-independent) disability progression.
Supplementary Material
Acknowledgments:
We want to thank all participants, study nurses, technicians, and physicians for their engagement. We want to thank Prof. Martin Kerschensteiner and Prof. Reinhard Hohlfeld for their support. Moreover, we want to thank the German MS Society, Federal Association (DMSG), the German MS trust (Deutsche Multiple Sklerose-Stiftung), and the AMSEL Stiftung Ursula Späth and Bavarian MS Trust (Bayerische MS-Stiftung) for their ongoing generous funding.
References
- Abdelhak A, Huss A, Stahmann A, Senel M, Krumbholz M, Kowarik MC, Havla J, Kumpfel T, Kleiter I, Wustinger I, Zettl UK, Schwartz M, Roesler R, Friede T, Ludolph AC, Ziemann U and Tumani H (2020). “Explorative study of emerging blood biomarkers in progressive multiple sclerosis (EmBioProMS): Design of a prospective observational multicentre pilot study.” Contemp Clin Trials Commun 18: 100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arm J, Ribbons K, Lechner-Scott J and Ramadan S (2019). “Evaluation of MS related central fatigue using MR neuroimaging methods: Scoping review.” J Neurol Sci 400: 52–71. [DOI] [PubMed] [Google Scholar]
- Brazier JE, Harper R, Jones NM, O’Cathain A, Thomas KJ, Usherwood T and Westlake L (1992). “Validating the SF-36 health survey questionnaire: new outcome measure for primary care.” BMJ 305(6846): 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DS, Thompson NR, Meng X, Johnson K and Fox RJ (2020). “Patient reported outcomes and performance metrics at diagnosis of secondary progressive multiple sclerosis.” Mult Scler: 1352458520936214. [DOI] [PubMed] [Google Scholar]
- D’Amico E, Haase R and Ziemssen T (2019). “Review: Patient-reported outcomes in multiple sclerosis care.” Mult Scler Relat Disord 33: 61–66. [DOI] [PubMed] [Google Scholar]
- Derache N, Grassiot B, Mezenge F, Emmanuelle Dugue A, Desgranges B, Constans JM and Defer GL (2013). “Fatigue is associated with metabolic and density alterations of cortical and deep gray matter in Relapsing-Remitting-Multiple Sclerosis patients at the earlier stage of the disease: A PET/MR study.” Mult Scler Relat Disord 2(4): 362–369. [DOI] [PubMed] [Google Scholar]
- Feinstein A, Roy P, Lobaugh N, Feinstein K, O’Connor P and Black S (2004). “Structural brain abnormalities in multiple sclerosis patients with major depression.” Neurology 62(4): 586–590. [DOI] [PubMed] [Google Scholar]
- Harding-Forrester S, Roos I, Nguyen AL, Malpas CB, Diouf I, Moradi N, Sharmin S, Izquierdo G, Eichau S, Patti F, Horakova D, Kubala Havrdova E, Prat A, Girard M, Duquette P, Grand’Maison F, Onofrj M, Lugaresi A, Grammond P, Ozakbas S, Amato MP, Gerlach O, Sola P, Ferraro D, Buzzard K, Skibina O, Lechner-Scott J, Alroughani R, Boz C, Van Pesch V, Cartechini E, Terzi M, Maimone D, Ramo-Tello C, Yamout B, Khoury SJ, La Spitaleri D, Sa MJ, Blanco Y, Granella F, Slee M, Butler E, Sidhom Y, Gouider R, Bergamaschi R, Karabudak R, Ampapa R, Sanchez-Menoyo JL, Prevost J, Castillo-Trivino T, McCombe PA, Macdonell R, Laureys G, Van Hijfte L, Oh J, Altintas A, de Gans K, Turkoglu R, van der Walt A, Butzkueven H, Vucic S, Barnett M, Cristiano E, Hodgkinson S, Iuliano G, Kappos L, Kuhle J, Shaygannejad V, Soysal A, Weinstock-Guttman B, Van Wijmeersch B, Kalincik T and investigators MS (2023). “Disability accrual in primary and secondary progressive multiple sclerosis.” J Neurol Neurosurg Psychiatry. [DOI] [PubMed] [Google Scholar]
- Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, Hauser S, Waubant E, Vollmer T, Panitch H, Zhang J, Chin P, Smith CH and group O. t. (2009). “Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial.” Ann Neurol 66(4): 460–471. [DOI] [PubMed] [Google Scholar]
- Healy BC, Glanz BI, Swallow E, Signorovitch J, Hagan K, Silva D, Pelletier C, Chitnis T and Weiner H (2021). “Confirmed disability progression provides limited predictive information regarding future disease progression in multiple sclerosis.” Mult Scler J Exp Transl Clin 7(2): 2055217321999070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy BC, Zurawski J, Chitnis T, Weiner HL and Glanz BI (2021). “Patient-reported outcomes associated with transition to secondary progressive multiple sclerosis.” Qual Life Res. [DOI] [PubMed] [Google Scholar]
- Hobart J, Lamping D, Fitzpatrick R, Riazi A and Thompson A (2001). “The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure.” Brain 124(Pt 5): 962–973. [DOI] [PubMed] [Google Scholar]
- Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, Vermersch P, Arnold DL, Arnould S, Scherz T, Wolf C, Wallstrom E, Dahlke F and Investigators EC (2018). “Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study.” Lancet 391(10127): 1263–1273. [DOI] [PubMed] [Google Scholar]
- Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, Model F, Koendgen H, Manfrini M, Belachew S and Hauser SL (2020). “Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials.” JAMA Neurol 77(9): 1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluzek S, Dean B and Wartolowska KA (2021). “Patient-reported outcome measures (PROMs) as proof of treatment efficacy.” BMJ Evid Based Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MW, Cutter GR, Giovannoni G, Uitdehaag BMJ, Wolinsky JS, Davis MD, Steinerman JR and Knappertz V (2017). “Comparative utility of disability progression measures in PPMS: Analysis of the PROMiSe data set.” Neurol Neuroimmunol Neuroinflamm 4(4): e358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi O, Vizzino C, Meani A, Conti L, Riccitelli GC, Preziosa P, Filippi M and Rocca MA (2020). “Fatigue in multiple sclerosis patients with different clinical phenotypes: a clinical and mri study.” Eur J Neurol. [DOI] [PubMed] [Google Scholar]
- Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS and Investigators OC (2017). “Ocrelizumab versus Placebo in Primary Progressive Multiple Sclerosis.” N Engl J Med 376(3): 209–220. [DOI] [PubMed] [Google Scholar]
- Ohle LM, Ellenberger D, Flachenecker P, Friede T, Haas J, Hellwig K, Parciak T, Warnke C, Paul F, Zettl UK and Stahmann A (2021). “Chances and challenges of a long-term data repository in multiple sclerosis: 20th birthday of the German MS registry.” Sci Rep 11(1): 13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano C, Gallo A, Li X, Ikonomidou VN, Evangelou IE, Ohayon JM, Stern SK, Ehrmantraut M, Cantor F, McFarland HF and Bagnato F (2010). “Relationship of cortical atrophy to fatigue in patients with multiple sclerosis.” Arch Neurol 67(4): 447–453. [DOI] [PubMed] [Google Scholar]
- Penner IK, Raselli C, Stocklin M, Opwis K, Kappos L and Calabrese P (2009). “The Fatigue Scale for Motor and Cognitive Functions (FSMC): validation of a new instrument to assess multiple sclerosis-related fatigue.” Mult Scler 15(12): 1509–1517. [DOI] [PubMed] [Google Scholar]
- Raffel J, Wallace A, Gveric D, Reynolds R, Friede T and Nicholas R (2017). “Patient-reported outcomes and survival in multiple sclerosis: A 10-year retrospective cohort study using the Multiple Sclerosis Impact Scale-29.” PLoS Med 14(7): e1002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzer A, Aiyegbusi OL, Rowe A, Newsome PN, Douglas-Pugh J, Khan S, Mittal S, Wilson R, O’Connor D, Campbell L, Mitchell SA and Calvert M (2022). “The value of patient-reported outcomes in early-phase clinical trials.” Nat Med 28(1): 18–20. [DOI] [PubMed] [Google Scholar]
- Sacco R, Santangelo G, Stamenova S, Bisecco A, Bonavita S, Lavorgna L, Trojano L, D’Ambrosio A, Tedeschi G and Gallo A (2016). “Psychometric properties and validity of Beck Depression Inventory II in multiple sclerosis.” Eur J Neurol 23(4): 744–750. [DOI] [PubMed] [Google Scholar]
- Scalfari A, Romualdi C, Nicholas RS, Mattoscio M, Magliozzi R, Morra A, Monaco S, Muraro PA and Calabrese M (2018). “The cortical damage, early relapses, and onset of the progressive phase in multiple sclerosis.” Neurology 90(24): e2107–e2118. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Masdeu JC, Goni J, Arrondo G, Velez de Mendizabal N, Bejarano B and Villoslada P (2009). “Fatigue in multiple sclerosis is associated with the disruption of frontal and parietal pathways.” Mult Scler 15(3): 337–344. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Sastre-Garriga J, Cercignani M, Ingle GT, Miller DH and Thompson AJ (2006). “Regional gray matter atrophy in early primary progressive multiple sclerosis: a voxel-based morphometry study.” Arch Neurol 63(8): 1175–1180. [DOI] [PubMed] [Google Scholar]
- Singhal T, Cicero S, Pan H, Carter K, Dubey S, Chu R, Glanz B, Hurwitz S, Tauhid S, Park MA, Kijewski M, Stern E, Bakshi R, Silbersweig D and Weiner HL (2020). “Regional microglial activation in the substantia nigra is linked with fatigue in MS.” Neurol Neuroimmunol Neuroinflamm 7(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjerbaek AG, Boesen F, Petersen T, Rasmussen PV, Stenager E, Norgaard M, Feys P, Kjeldgaard-Jorgensen ML, Hvid LG and Dalgas U (2019). “Can we trust self-reported walking distance when determining EDSS scores in patients with multiple sclerosis? The Danish MS hospitals rehabilitation study.” Mult Scler 25(12): 1653–1660. [DOI] [PubMed] [Google Scholar]
- Strijbis EM, Repovic P, Mostert J, Bowen JD, Uitdehaag BM, Cutter G and Koch MW (2022). “The MSIS-29 and SF-36 as outcomes in secondary progressive MS trials.” Mult Scler 28(10): 1606–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintore M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC and Cohen JA (2018). “Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria.” Lancet Neurol 17(2): 162–173. [DOI] [PubMed] [Google Scholar]
- Venkatesan P (2016). “New European guidance on patient-reported outcomes.” Lancet Oncol 17(6): e226. [DOI] [PubMed] [Google Scholar]
- Wolinsky JS, Narayana PA, O’Connor P, Coyle PK, Ford C, Johnson K, Miller A, Pardo L, Kadosh S, Ladkani D and Group PRTS (2007). “Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial.” Ann Neurol 61(1): 14–24. [DOI] [PubMed] [Google Scholar]
- Zorzon M, de Masi R, Nasuelli D, Ukmar M, Mucelli RP, Cazzato G, Bratina A and Zivadinov R (2001). “Depression and anxiety in multiple sclerosis. A clinical and MRI study in 95 subjects.” J Neurol 248(5): 416–421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


