Abstract
Background and Objectives
B-cell–depleting therapies increase the risk of infections and hypogammaglobulinemia. These relationships are poorly understood. The objectives of these analyses were to estimate how much of this rituximab-associated infection risk is mediated by hypogammaglobulinemia and to identify other modifiable risk factors in persons with multiple sclerosis (pwMS).
Methods
We conducted a retrospective cohort study of rituximab-treated pwMS from January 1, 2008, to December 31, 2020, in Kaiser Permanente Southern California. Cumulative rituximab dose was defined as ≤2, >2 and ≤4, or >4 g. Serious infections were defined as infections requiring or prolonging hospitalizations, and recurrent outpatient infections as seeking care for ≥3 within 12 months. Exposures, outcomes, and covariates were collected from the electronic health record. Adjusted hazard ratios (aHRs) were estimated using Andersen-Gill hazards models, and generalized estimating equations were used to examine correlates of IgG values. Cross-sectional causal mediation analyses of rituximab and hypogammaglobulinemia were conducted.
Results
We identified 2,482 pwMS who were treated with rituximab for a median of 2.4 years (interquartile range = 1.3–3.9). The average age at rituximab initiation was 43.0 years, 71.9% were female, 49.7% were White, non-Hispanic patients, and 29.6% had advanced disability (requiring walker or worse). Seven hundred patients (28.2%) developed recurrent outpatient infections, 155 (6.2%) developed serious infections, and only 248 (10.0%) had immunoglobulin G (IgG) < 700 mg/dL. Higher cumulative rituximab dose (>4 g) was correlated with lower IgG levels (Beta = −58.8, p < 0.0001, ref ≤2 g) and, in models mutually adjusted for hypogammaglobulinemia, both were independently associated with an increased risk of serious (>4 g, aHR = 1.56, 95% CI 1.09–2.24; IgG < 500, aHR = 2.98, 95% CI 1.56–5.72) and outpatient infections (>4 g, aHR = 1.73, 95% CI 1.44–2.06; IgG < 500 aHR = 2.06, 95% CI 1.52–2.80; ref = IgG ≥ 700). Hypogammaglobulinemia explained at most 17.9% (95% CI −47.2–119%) of serious infection risk associated with higher cumulative rituximab exposure but was not significant for outpatient infections. Other independent modifiable risk factors were advanced physical disability for serious (aHR = 5.51, 95% CI 3.71–8.18) and outpatient infections (aHR = 1.24, 95% CI 1.06–1.44) and COPD (aHR = 1.68, 95% CI 1.34–2.11) and obesity (aHR = 1.25, 95% CI 1.09–1.45) for outpatient infections.
Discussion
Higher cumulative rituximab doses increase the risk of infections even in this population where 90% of patients maintained normal IgG levels. Clinicians should strive to use minimally effective doses of rituximab and other B-cell–depleting therapies and consider important comorbidities to minimize risks of infections.
Introduction
Anti-CD20 therapies including rituximab, its biosimilars, and ocrelizumab are rapidly becoming the most used treatments for persons with multiple sclerosis (pwMS) worldwide. Although highly effective at controlling inflammatory disease activity,1 anti-CD20 therapies are associated with a clinically significant increased risk of serious2-4 and outpatient2 infections along with drug-induced hypogammaglobulinemia.4-10
Strategies to minimize risk of infections with anti-CD20 therapies are lacking, in large part because the relationship between prolonged use, cumulative dose, drug-induced hypogammaglobulinemia, and other potential modifiable risk factors of infections are poorly understood. Several studies have observed that hypogammaglobulinemia increases with longer duration of anti-CD20 therapy4-10 and that hypogammaglobulinemia inadequately accounts for the increased risk of serious infections.4-11 Yet no published studies have quantified the effects of increasing cumulative dose exposure or assessed the influence of comorbidities on risk of infections or hypogammaglobulinemia.
Because hypogammaglobulinemia is a well-known risk factor of infections, our group, among others,8 monitors immunoglobulin (IgG) levels before infusions and commonly reduce rituximab dose or extend dosing intervals to avoid drug-induced hypogammaglobulinemia. However, to what extent this approach can minimize risk of serious infections or clinically significant recurrent outpatient infections is unknown.
The aims of these analyses were to estimate to what extent the increased risk of infections associated with higher cumulative rituximab doses is mediated by hypogammaglobulinemia and to identify other modifiable factors that influence infection risk or IgG levels in a large, diverse, population-based cohort of pwMS.
Methods
We conducted a retrospective cohort study using Kaiser Permanente Southern California’s (KPSC) complete electronic health record (EHR). The sociodemographic characteristics of KPSC's >4.8 million members, representing ∼20% of the Southern California population, are representative of the underlying population.12,13
The EHR was electronically searched between January 1, 2008, and December 31, 2020, to identify the following: pwMS, rituximab infusion dates and doses, IgG levels, clinical and demographic characteristics, and serious and outpatient infections. The EHR of rituximab-treated persons were reviewed to confirm MS diagnosis.14,15 Figure 1 depicts the cohort assembly. Inclusion criteria were as follows: (1) at least 1 rituximab infusion for MS and (2) ≥6-month continuous membership. Self-identified race and ethnicity obtained from the EHR was classified as White, non-Hispanic (referred to as White), Hispanic, Black (regardless of ethnicity), Asian/Pacific Islander, Native American/Alaskans, or mixed-race individuals, as a surrogate measure for structural racism and purported variations in humoral immunity.16 Due to small sample, individuals with mixed (n = 1) and Native American/Alaskan (n = 4) race and ethnicity were excluded.
Figure 1. Cohort Assembly for Main Effects and Mediation Analyses.
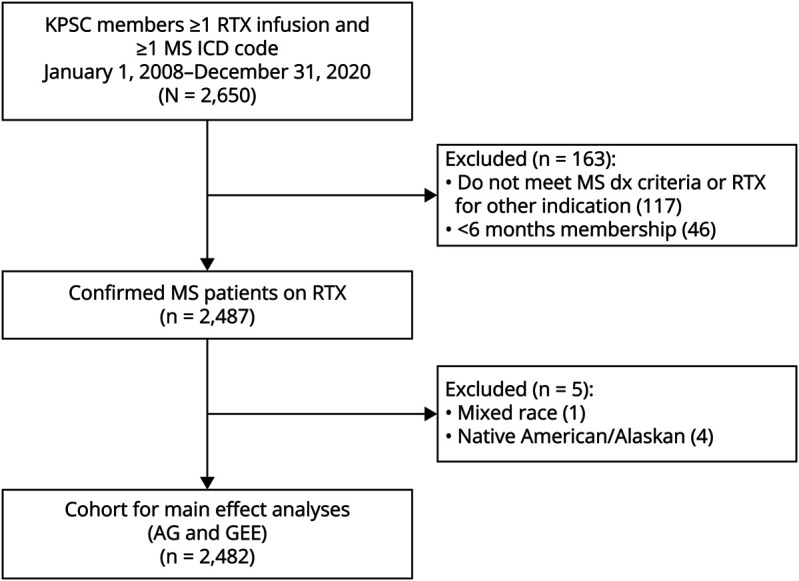
Kaiser Permanente Southern California (KPSC) members who met multiple sclerosis (MS) diagnostic (dx) criteria and received at least 1 dose of rituximab (RTX) for MS were included in main analyses. To be able to assess the effect of race and ethnicity on immunoglobulin levels and infection risk, patients with mixed or native American/Alaskan native race were excluded due to small sample. AG = Andersen-Gill; ICD = International Classification of Diseases; GEEs = generalized estimation equations.
ICD9/10 codes (eTable 1) were used to identify serious and outpatient infections, other than novel corona virus 2019, as previously described.2 In brief, for outpatient infections, as previously validated,17 if the same 3-digit ICD codes were entered within 3 months it was considered as 1 episode. Recurrent outpatient infection was defined as 3 or more infections within a 12-month period because seeking care for 1 or 2 infections annually was similar between rituximab-treated pwMS and non-MS controls2 (eFigure 1). For serious infections, we manually reviewed the electronic health records or hospitalizations with primary and secondary infectious discharge ICD codes to assure that infections met criteria in accordance with the Common Terminology Criteria for Adverse Events (CTCAE)18: an infection leading to hospitalization (including hospitalization for pseudorelapses due to infections) or an infectious complication leading to prolonged hospital course. The CTCAE is the standard used to define infection severity in MS randomized controlled trials (RCTs).
Statistical Analyses
Serious or recurrent outpatient infectious outcomes were analyzed in separate models. We conducted 3 analyses to investigate the 2 outcomes. First, we assessed the association of rituximab exposure and other factors with IgG levels using generalized estimation equations (GEEs) where longitudinal IgG level was considered as a continuous variable and rituximab exposure was considered as a time-varying independent variable. Second, we examined the independent association of rituximab exposure and hypogammaglobulinemia with infections using multivariable Anderson-Gill (AG) models19 where longitudinal infection data were modeled. Last, we conducted cross-sectional causal mediation analyses accounting for identified factors associated with IgG levels, rituximab exposure, or infections.20 Rituximab was considered discontinued if the last infusion was >2 years ago.
For AG models of recurrent infectious outcomes, each rituximab infusion defined the start of a treatment episode, and the end was defined as either: (1) the day before a subsequent rituximab infusion; (2) 2 years after last rituximab dose; (3) end of study period; (4) membership termination; or (5) death, whichever came first. Covariates in AG and GEE models were defined at the start of each treatment episode or IgG measurement, respectively. Cumulative rituximab exposure (time varying) was defined as <2 (g, reference), ≥2–4, or >4 g. These thresholds were chosen based on the distribution of the variable and its relationship to infection risk. Hypogammaglobulinemia (AG models, time varying) was defined as <300, ≥300 to <500, ≥500 to <700, or ≥700 mg/dL (normal reference value) based on values obtained within 3 months before infusion. In the 3 patients who started IV immunoglobulin (IvIg), only IgG values before IvIg initiation were used. We used multiple imputation to impute missing IgG levels (n = 339, 13.7% of pwMS with missing values at last rituximab infusion and of a total of 10,722 rituximab infusions, n = 3,932, 35.8% infusions with missing values) because values were not missing at random (eTable 2). Primary analyses used observed and imputed IgG values.
Covariates were hypothesis driven, defined a priori, and included sex (female/male), prior use of highly effective (HET, natalizumab, fingolimod, cyclophosphamide, mitoxantrone, and siponimod) or modestly effective disease-modifying therapy (meDMT, interferon beta, glatiramer acetate, dimethyl fumarate, teriflunomide, mycophenolic acid, azathioprine, and methotrexate) in the 12 months before rituximab initiation (yes/no), race and ethnicity, and the time-dependent covariates, age (continuous), diabetes, chronic obstructive pulmonary disease (COPD, eTable 3), obesity (BMI ≥30 kg/m2), and advanced ambulatory disability (defined as requiring walker, wheelchair, Hoyer lift, and/or hospital bed; Expanded Disability Status Scale, EDSS, equivalent of 6.5 or higher; yes/no). Factors with p < 0.20 were included in the final adjusted model.
The crude incidence rates (IRs) and 95% confidence intervals (95% CIs) were calculated after tabulating all infection counts and person-year (PY) contributions in each group. Person-year contributions began at rituximab start during the study period and ended at membership termination, 2 years after last rituximab dose, or death, whichever came first.
The following sensitivity analyses were conducted: (1) defining recurrent outpatient infections as 4 or more per 12-month period; (2) modeling decline in IgG values among patients with IgG values >1,000 mg/dL or between 700 and 1,000 mg/dL were conducted to assess whether percent decline in IgG confers increased risk of infections in patients within the normal range; and (3) restricting the analyses to the first 2 years on treatment and comparing only those patients treated with the highest dosing protocol (highest-dose group, >1,500 mg induction dose followed by 1 g q6month) with patients on the lowest dosing protocol (lowest-dose group, 500 mg or less induction dose followed by 500 mg or less every 12 months) to assess whether higher cumulative doses given over the same time frame increase the risk of infections.
Causal Mediation Analyses
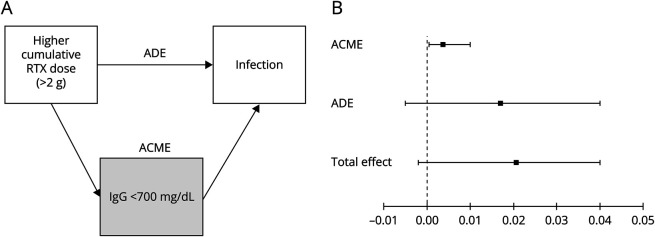
To assess how much of the total effect of higher cumulative rituximab doses (>2 g) on risk of serious or recurrent outpatient infections is mediated through hypogammaglobulinemia (<700 mg/dL), we conducted cross-sectional analyses using the cumulative rituximab dose and last IgG value before infection or, for patients without infections, the end of the study period. The hypothetical model is shown in Figure 2A. The average direct effect (ADE, not mediated by hypogammaglobulinemia), average causal mediations effects (ACME, indirect effect mediated by hypogammaglobulinemia), total effect (direct + indirect), and proportion mediated are reported.21 PwMS were excluded from the cross-sectional mediation model if: (1) they received only 1 rituximab infusion and did not have an IgG measurement after this (n = 441) and (2) they had their first infection before the second rituximab infusion and did not have an IgG measurement within 30 days of the outcome (n = 21 serious infections, n = 73 recurrent outpatient infections). To satisfy assumptions of causal mediation models,21 the models were adjusted for all variables identified in prior models as confounding the relationship between rituximab or IgG and infection (advanced disability, obesity, COPD, and hospitalization for infections in the previous 5 years2) or rituximab and IgG (age, race and ethnicity, diabetes, and prior DMT use). Rituximab dose (>2 g) and hypogammaglobulinemia (<700 mg/dL, the lower limit of normal) were dichotomized because the relationship between these variables and infection risk is ordinal rather than continuous, and mediation models require modeling average or dichotomized values.
Figure 2. Infection Risk in Rituximab-Treated Persons With MS Mediated by Serum IgG Levels.
Depicted is the hypothetical model of causal pathway in rituximab-treated persons with MS (pwMS) and serious or recurrent outpatient infections (A). The average direct effect (ADE) is the effect of higher cumulative rituximab (RTX) doses (>2 g) on infection risk not mediated by hypogammaglobulinemia (<700 mg/dL). The average causal mediation effect (ACME) is the estimated indirect effect of rituximab mediated through hypogammaglobulinemia, and the total effect represents the direct and indirect effects. Serious infections and recurrent outpatient infections were modeled separately. Depicted in (B) are the results of the mediation analysis in rituximab-treated pwMS and the risk of serious infections. While the ACME at 0.0037 (95% CI 0.0005–0.0100) was statistically significant (p = 0.022), indicating that a proportion of the increased risk of serious infections with higher rituximab doses (greater than 2 g) is mediated by drug-induced hypogammaglobulinemia, it accounts for very little of the total effect (0.021, 95% CI −0.002 to 0.040). By contrast, the ADE at 0.017 (95% CI −0.005 to 0.040) was quite similar to the total effect indicating that most of the effect of rituximab on serious infection risk is not mediated by hypogammaglobulinemia. This is consistent with a relatively small estimated proportion mediated of 17.9% (95% CI −47.2 to 119). IgG = immunoglobulin G.
Statistical significance was set at p < 0.05. No adjustment was made for multiple comparisons. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC). For causal mediation analyses consistent with reporting guidelines,20 interpretation of results focused on ACME and ADE rather than proportion mediated. R mediation package version 4.5.0 was used.
Sankey Diagrams were generated using R ggalluvial 0.12.5 package. For rituximab dosing, induction dose was defined as total dose given within 1 month of each other and annual maintenance defined by the dose and frequency given during that year. For IgG levels, if more than 1 level was checked during that year, the average value during that year was plotted. The year was calculated based on time since the first rituximab infusion.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the KPSC Institutional Review Board (IRB) with a waiver of informed consent because data used were already collected in the EHR and the no more than minimal risk to the members.
Data Availability
Due to the KPSC IRB, data are available on reasonable request.
Results
Demographic and clinical characteristics are summarized in Table 1. Of the 2,482 pwMS included in the main analyses, the median rituximab treatment duration was 2.4 years, average age at rituximab initiation was 43.0 years, 71.9% were female, 29.6% had advanced disability, 16.5% were Black, 31.0% were Hispanic, and 2.8% were Asian/Pacific Islander. 593 patients were treated for more than 4 years. 700 patients (28.2%) developed recurrent outpatient infections and 155 (6.2%) serious infections; yet only 10.0% (n = 248) of patients had IgG <700 mg/dL at the end of follow-up (or before infectious events). Rituximab-treated pwMS who developed serious and/or recurrent outpatient infections were older, had more advanced disability, used other DMTs before rituximab, received more rituximab infusions and higher cumulative dose, and had lower IgG levels at last follow-up compared with those who did not have infections. Female patients were more likely to experience recurrent outpatient infections but less likely to develop serious infections than male patients. Selected comorbidities were more common in those who experienced recurrent infections compared with those who did not. Patients with recurrent outpatient infections were more likely to have serious infections and vice versa. Black, White, and Hispanic race and ethnicity did not differ significantly between patients with or without infectious outcomes.
Table 1.
Demographic and Clinical Characteristics of Rituximab-Treated Persons With Multiple Sclerosis
| Total (n = 2,482) | Serious infections | Recurrent outpatient infections | |||||
| Yes (n = 155) | No (n = 2,327) | p Value | Yes (n = 700) | No (n = 1782) | p Value | ||
| Age, mean (SD), y | 43.0 (12.3) | 47.9 (12.5) | 42.7 (12.2) | <0.0001 | 44.4 (12.7) | 42.5 (12.1) | 0.0010 |
| Female, n (%) | 1785 (71.9) | 98 (63.2) | 1,687 (72.5) | 0.0129 | 571 (81.6) | 1,214 (68.1) | <0.0001 |
| Race and ethnicity, n (%) | 0.1821 | 0.0264 | |||||
| Asian/Pacific Islander | 70 (2.8) | 4 (2.6) | 66 (2.8) | 9 (1.3) | 61 (3.4) | ||
| Black | 409 (16.5) | 28 (18.1) | 381 (16.4) | 121 (17.3) | 288 (16.2) | ||
| Hispanic | 769 (31.0) | 36 (23.2) | 733 (31.5) | 211 (30.1) | 558 (31.3) | ||
| White | 1,234 (49.7) | 87 (56.1) | 1,147 (49.3) | 359 (51.3) | 875 (49.1) | ||
| Prior use of other DMTa, n (%) | 0.0273 | <0.0001 | |||||
| Highly effective | 448 (18.1) | 37 (23.9) | 411 (17.7) | 156 (22.3) | 292 (16.4) | ||
| Modestly effective | 930 (37.5) | 64 (41.3) | 866 (37.2) | 292 (41.7) | 638 (35.8) | ||
| None | 1,104 (44.5) | 54 (34.8) | 1,050 (45.1) | 252 (36.0) | 852 (47.8) | ||
| Advanced disability, n (%) | 735 (29.6) | 123 (79.4) | 612 (26.3) | <0.0001 | 302 (43.1) | 433 (24.3) | <0.0001 |
| Comorbidities, n (%) | |||||||
| Obesity | 1,149 (46.3) | 70 (45.2) | 1,079 (46.4) | 0.7704 | 358 (51.1) | 791 (44.4) | 0.0024 |
| Diabetes | 212 (8.5) | 18 (11.6) | 194 (8.3) | 0.1577 | 69 (9.9) | 143 (8.0) | 0.1416 |
| COPD | 216 (8.7) | 10 (6.5) | 206 (8.9) | 0.3045 | 76 (10.9) | 140 (7.9) | 0.0170 |
| Serious infection, n (%) | 155 (6.2) | — | — | 109 (15.6) | 46 (2.6) | <0.0001 | |
| Recurrent outpatient infections, n (%) | 700 (28.2) | 109 (70.3) | 591 (25.4) | <0.0001 | — | — | |
| Rituximab use characteristics | |||||||
| Cumulative dose, n (%), g | <0.0001 | <0.0001 | |||||
| ≤2 | 1,232 (49.6) | 24 (15.5) | 1,208 (51.9) | 170 (24.3) | 1,062 (59.6) | ||
| >2 and ≤4 | 691 (27.8) | 47 (30.3) | 644 (27.7) | 253 (36.1) | 438 (24.6) | ||
| >4 | 559 (22.5) | 84 (54.2) | 475 (20.4) | 277 (39.6) | 282 (15.8) | ||
| No. of infusions, median (IQR) | 4.0 (2.0, 6.0) | 6.0 (4.0, 8.0) | 3.0 (2.0, 6.0) | <0.0001 | 6.0 (4.0, 8.0) | 3.0 (2.0, 5.0) | <0.0001 |
| Duration of use, median (IQR), y | 2.4 (1.3, 3.9) | 4.1 (3.1, 5.8) | 2.3 (1.2, 3.7) | <0.0001 | 3.6 (2.5, 5.2) | 1.9 (1.0, 3.2) | <0.0001 |
| Average annual dose, g | 0.8205 | <0.0001 | |||||
| Mean (SD) | 1.8 (9.2) | 1.2 (0.6) | 1.8 (9.5) | 1.1 (0.6) | 2.1 (10.9) | ||
| Median (IQR) | 1.1 (0.8, 1.4) | 1.1 (0.9, 1.3) | 1.1 (0.8, 1.4) | 1.0 (0.8, 1.3) | 1.1 (0.9, 1.5) | ||
| IgG levels before infection/end of follow-up, n (%) | <0.0001 | <0.0001 | |||||
| ≥700 mg/dL | 2,234 (90.0) | 124 (80.0) | 2,110 (90.7) | 597 (85.3) | 1,637 (91.9) | ||
| ≥500 and <700 mg/dL | 221 (8.9) | 23 (14.8) | 198 (8.5) | 85 (12.1) | 136 (7.6) | ||
| ≥300 and <500 mg/dL | 24 (1.0) | 5 (3.2) | 19 (0.8) | 15 (2.1) | 9 (0.5) | ||
| <300 mg/dL | 3 (0.1) | 3 (1.9) | 0 (0.0) | 3 (0.4) | 0 (0.0) | ||
| Median (IQR) | 1,000.0 (842.0, 1,170.0) | 894.0 (743.0, 1,100.0) | 1,005.0 (850.0, 1,170.0) | <0.0001 | 940.6 (794.0, 1,140.0) | 1,020.0 (865.0, 1,189.0) | <0.0001 |
| Total follow-up time, n (%), y | <0.0001 | <0.0001 | |||||
| ≤2 | 1,016 (40.9) | 14 (9.0) | 1,002 (43.1) | 101 (14.4) | 915 (51.3) | ||
| >2 and ≤4 | 873 (35.2) | 57 (36.8) | 816 (35.1) | 294 (42.0) | 579 (32.5) | ||
| >4 | 593 (23.9) | 84 (54.2) | 509 (21.9) | 305 (43.6) | 288 (16.2) | ||
| Median (IQR), mo | 28.8 (15.6, 46.8) | 49.8 (36.8, 69.1) | 27.1 (14.9, 44.7) | <0.0001 | 43.5 (29.7, 62.3) | 23.0 (12.2, 38.7) | <0.0001 |
Abbreviations: COPD = chronic obstructive pulmonary disease; DMT = disease-modifying therapy; IgG = immunoglobulin G; IQR = interquartile range; RTX = rituximab.
Prior use is defined as within 12 mo before rituximab initiation.
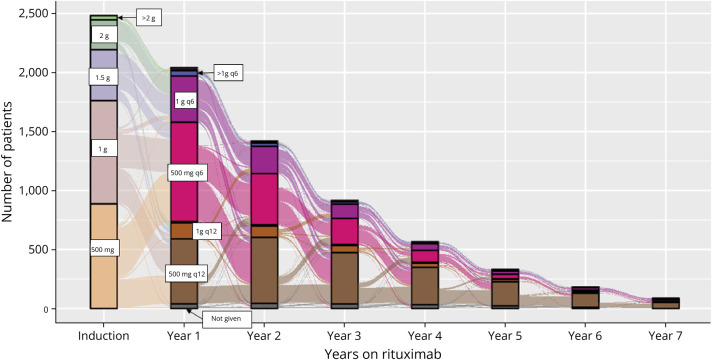
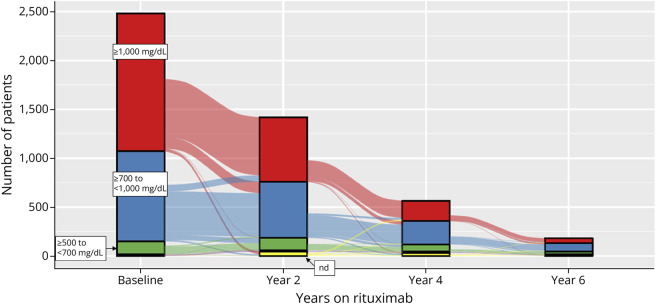
Annual rituximab dose declined as patients remained on treatment over time (Figure 3, eTable 4). Choice of induction dose varied widely. The most common initial maintenance dose was 500 mg every 6 months (q6mo) followed by reduction to 500 mg q12mo, particularly after the third year of treatment. IgG levels began to decline during the first year following rituximab initiation (Figure 4, eTable 5) and continued to decline over time; yet even for patients who completed 4 or 6 years of treatment, most of them had normal IgG levels (>700 mg/dL, 84.8% and 79.7%, respectively).
Figure 3. Patient Flow Through Rituximab Dosing Schedules After Treatment Initiation.
Depicted is the total number of patients on various rituximab dosing schedules throughout the study period. Induction doses and maintenance doses and frequencies are indicated by color and labeled in the diagram. The most common induction doses were 500 mg (n = 887, 35.7%) and 1,000 mg (n = 875, 35.3%). The most common maintenance dose during the first year after first rituximab infusion was 500 mg every 6 months (q6mo in hot pink; n = 842, 41.3% of the 2,040 patients who received at least 1 infusion that year). During year 2, use of 500 mg q12 months (q12mo in brown, n = 559, 39.3%) was slightly higher than 500 mg q6mo (n = 424, 30.6%), and over time, 500 mg q12mo was the most common dosing schedule used (65.4% by year 6). The total number of patients receiving at least 1 dose of rituximab in a particular year declined with each year of follow-up (n = 565 in year 4, n = 182 in year 6) reflecting primarily year of treatment initiation and follow-up time rather than treatment discontinuation.
Figure 4. IgG Levels per Patient at Baseline and After Rituximab Initiation.
Depicted is the total number of patients with normal IgG levels ≥1,000 mg/dL, in red and ≥700 and <1,000 mg/dL in blue) and low levels (<700 and ≥500 in green, <500 not visible) or a value was not obtained (not done, nd) because no infusion was planned during that year at baseline (before rituximab initiation) and after completing years 2, 4, and 6 of treatment. Decline in IgG levels began after treatment initiation and continued decline with longer duration of rituximab use, but even in patients who completed 4 years (n = 565) and 6 years (n = 182) of treatment, most of them had normal IgG levels (84.8% year 4 and 79.7% year 6). IgG = immunoglobulin G.
The crude incidence rate of serious or recurrent outpatient infections was 3.0 per 100-PY (95% CI 2.6–3.4) and 21.3/100-PY (95% CI 20.4–22.3) in the entire cohort. In the subgroup with advanced disability (n = 735, 29.6%, EDSS >6.5), the crude incidence rates were 6.4/100-PY (95% CI 5.4–7.3) and 26.2/100-PY (95% CI 24.5–27.8) for serious and outpatient infections, respectively. By contrast, in those with no or moderate disability (n = 1747, 70.4%, EDSS ≤6.0), the crude incidence rates were significantly lower at 0.8/100-PY (95% CI 0.5–1.1) and 18.1/100-PY (95% CI 17.0–19.3) for serious and outpatient infections, respectively.
Modifiable and Fixed Factors Associated With IgG Levels
Table 2 summarizes the results of crude and multivariable GEE analyses estimating the independent relationships between cumulative rituximab dose, demographic characteristics, and clinical characteristics with IgG levels in RTX-treated pwMS throughout the study period. Higher cumulative RTX dose was associated with significantly lower IgG levels in a dose-dependent fashion (adjusted β = −41.00 and −58.76, >2–4 g and >4 g, respectively). Diabetes and prior HET but not meDMT use were other modifiable factors associated with lower IgG levels. Among nonmodifiable factors, Black (β = 132.96) or Asian (β = 151.67) and to a lesser extent Hispanic pwMS (β = 21.10) had significantly higher IgG levels, whereas older age and prior DMT use corresponded to lower levels and female sex, advanced disability, COPD, and obesity had no independent association with IgG levels. Not surprisingly, baseline IgG levels before rituximab initiation were strongly associated with subsequent IgG levels.
Table 2.
Determinants of Serum IgG Levels
| Crude GEE | Adjusted GEE | |||||
| β | SE | p Value | β | SE | p Value | |
| Intercept | — | — | — | 1,235.51 | 15.53 | <0.0001 |
| Cumulative dose (ref ≤2 g) | ||||||
| >2 and ≤4 g | −45.08 | 3.12 | <0.0001 | −41.00 | 3.15 | <0.0001 |
| >4 g | −66.88 | 4.80 | <0.0001 | −58.76 | 4.92 | <0.0001 |
| Age per 10 y | −69.74 | 4.53 | <0.0001 | −12.12 | 2.97 | <0.0001 |
| Race and ethnicity (ref = White) | ||||||
| Asian/Pacific Islander | 282.90 | 34.55 | <0.0001 | 151.67 | 25.28 | <0.0001 |
| Black | 269.02 | 15.53 | <0.0001 | 132.96 | 12.52 | <0.0001 |
| Hispanic | 92.29 | 10.58 | <0.0001 | 21.10 | 7.23 | 0.0035 |
| Female sex | 27.73 | 11.62 | 0.0170 | 1.51 | 7.30 | 0.8357 |
| Advanced disability | −13.84 | 5.61 | 0.0141 | −2.83 | 4.92 | 0.5651 |
| Prior DMT use (ref = none) | −46.87 | 10.63 | <0.0001 | |||
| Highly effective | −104.82 | 13.80 | <0.0001 | −32.74 | 8.24 | <0.0001 |
| Modestly effective | −23.80 | 11.65 | 0.0410 | −5.41 | 7.48 | 0.4691 |
| Diabetes | −36.99 | 18.94 | 0.0508 | −19.51 | 11.13 | 0.0797 |
| COPD | −26.95 | 19.86 | 0.1748 | −15.71 | 12.62 | 0.2131 |
| Obesity | −1.66 | 4.72 | 0.7259 | — | — | — |
| Baseline IgG levels (ref ≥1,000 mg/dL) | ||||||
| ≥700 to <1,000 mg/dL | −341.91 | 6.54 | <0.0001 | −303.54 | 6.27 | <0.0001 |
| ≥500 to <700 mg/dL | −582.13 | 8.16 | <0.0001 | −525.78 | 8.47 | <0.0001 |
| <500 mg/dL | −764.75 | 25.18 | <0.0001 | −697.39 | 26.76 | <0.0001 |
Abbreviations: COPD = chronic obstructive pulmonary disease; DMT = disease-modifying therapy; GEE = generalized estimation equation; IgG = immunoglobulin G; SE = standard error.
Association of Rituximab and Hypogammaglobulinemia With Infections
Table 3 summarizes the results of crude and multivariable adjusted AG models estimating the independent relationship between higher rituximab cumulative dose, lower IgG levels, demographic characteristics, and clinical characteristics with serious or recurrent outpatient infections. Both higher rituximab cumulative dose and lower IgG levels were independently associated with increased risks of serious and outpatient infections. Obesity and COPD were the other modifiable factors identified that increased the risk of outpatient infections independent of IgG levels and cumulative rituximab dose. Advanced disability was associated with a significantly increased risk of serious (aHR = 5.51, 95% CI 3.71–8.18) and outpatient infections (aHR = 1.24, 95% CI 1.06–1.44). Female sex was associated with a decreased risk of serious but increased risk of recurrent outpatient infections, whereas older age, race or ethnicity, and diabetes showed no independent associations with infection risk after accounting for IgG levels and cumulative rituximab dose.
Table 3.
Association of Rituximab and Hypogammaglobulinemia With Infections
| Serious infections | Recurrent outpatient infections | |||||||
| Crude HR | Adjusted HR | Crude HR | Adjusted HR | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Cumulative dose (ref ≤2 g) | ||||||||
| >2 and ≤4 g | 1.48 (1.01–2.16) | 0.0432 | 1.27 (0.87–1.85) | 0.2249 | 1.54 (1.34–1.76) | <0.0001 | 1.55 (1.35–1.77) | <0.0001 |
| >4 g | 2.36 (1.66–3.36) | <0.0001 | 1.56 (1.09–2.24) | 0.0162 | 1.82 (1.54–2.14) | <0.0001 | 1.73 (1.44–2.06) | <0.0001 |
| Serum IgG level (ref ≥700 mg/dL) | ||||||||
| ≥500 and <700 mg/dL | 1.93 (1.23–3.02) | 0.0042 | 1.60 (1.05–2.42) | 0.0279 | 1.31 (1.02–1.67) | 0.0328 | 1.19 (0.93–1.53) | 0.1611 |
| <500 mg/dL | 4.01 (2.06–7.82) | <0.0001 | 2.98 (1.56–5.72) | 0.0010 | 2.50 (1.80–3.46) | <0.0001 | 2.06 (1.52–2.80) | <0.0001 |
| Age per 10 y | 1.32 (1.16–1.51) | <0.0001 | 1.06 (0.92–1.22) | 0.4506 | 1.05 (0.99–1.12) | 0.1277 | 1.01 (0.94–1.08) | 0.8380 |
| Race and ethnicity (ref = White) | ||||||||
| Black, API, Hispanic, or Other/Unk | 0.81 (0.57–1.14) | 0.2319 | — | — | 1.04 (0.89–1.22) | 0.6283 | — | — |
| Female sex | 0.57 (0.40–0.82) | 0.0020 | 0.53 (0.38–0.75) | 0.0003 | 1.91 (1.53–2.37) | <0.0001 | 1.90 (1.53–2.35) | <0.0001 |
| Advanced disability | 6.12 (4.31–8.71) | <0.0001 | 5.51 (3.71–8.18) | <0.0001 | 1.40 (1.20–1.62) | <0.0001 | 1.24 (1.06–1.44) | 0.0062 |
| Prior DMT use (ref = none) | ||||||||
| Highly effective | 0.91 (0.57–1.44) | 0.6843 | — | — | 1.38 (1.12–1.71) | 0.0024 | 1.17 (0.95–1.44) | 0.1435 |
| Modestly effective | 0.97 (0.65–1.47) | 0.8984 | — | — | 1.15 (0.96–1.38) | 0.1206 | 1.03 (0.86–1.22) | 0.7613 |
| Diabetes | 1.46 (0.88–2.43) | 0.1425 | 1.15 (0.72–1.84) | 0.5588 | 1.16 (0.92–1.47) | 0.2197 | — | — |
| COPD | 0.94 (0.47–1.90) | 0.8684 | — | — | 1.58 (1.26–1.97) | <0.0001 | 1.68 (1.34–2.11) | <0.0001 |
| Obesity | 0.80 (0.55–1.15) | 0.2213 | — | — | 1.28 (1.10–1.48) | 0.0010 | 1.25 (1.09–1.45) | 0.0021 |
Abbreviations: API = Asian/Pacific Islander; COPD = chronic obstructive pulmonary disease; DMT = disease-modifying therapy; HR = hazard ratio; IgG = immunoglobulin G; Unk = unknown.
Sensitivity Analyses
Defining recurrent outpatient infections as 4 or more per 12-month period yielded similar results to the main analyses. Percentage decline in IgG levels among patients with normal levels showed no association with either serious (aHR = 1.02, 95% CI 0.98–1.06; p = 0.38; aHR = 1.00, 95% CI 0.97–1.03, p = 0.95 ≥ 1,000 mg/dL and ≥700 and <1,000 mg/dL, respectively) or recurrent outpatient infection risks (aHR = 1.00, 95% CI 0.99–1.02; p = 0.85; aHR = 0.99, 95% CI 0.98–1.01, p = 0.35 ≥ 1,000 mg/dL and ≥700 and <1,000 mg/dL, respectively).
Of the 107 patients in the highest-dose group, the adjusted HR for serious infections during the first 2 years was 6.26 (95% CI 0.90–43.4, p = 0.06) higher compared with the lowest-dose group. The risk of recurrent outpatient infections was also higher (aHR = 3.17, 95% CI 0.73–13.73, p = 0.12), although neither finding reached statistical significance because both subgroups are small.
Causal Mediation Models
Of the 2,482 rituximab-treated pwMS included in the main models, 2020 (81.4%) were included in the cross-sectional mediation model of serious infections and 1968 (79.3%) for recurrent outpatient infections. IgG levels below 700 mg/dL while being treated with rituximab only partially mediated the association between treatment with greater than 2 cumulative grams of rituximab and serious infections (Figure 2B). While the ACME was statistically significant (p = 0.022), it was small (0.0037, 95% CI 0.0005–0.0100) compared with the average direct effect and total effect, which were quite similar. This is consistent with the estimated proportion mediated of 17.9% (95% CI −47.2%–119%, p = 0.09). Results of mediation models for recurrent outpatient infections were not significant (neither ACME nor proportion mediated).
Discussion
In this large, diverse, real-world cohort of rituximab-treated pwMS, we found that higher cumulative rituximab doses increased the risk of infections, even though most of the patients had normal IgG levels both at rituximab initiation and at last follow-up. As expected, increasing cumulative rituximab doses were associated with lower IgG levels, and hypogammaglobulinemia was a significant independent risk factor of infections. Independent of cumulative rituximab dose and hypogammaglobulinemia, advanced physical disability was a key driver of infection risk and was associated with a 5-fold increased risk of serious infections and a 24% increase in recurrent outpatient infections. By contrast, in pwMS with no or moderate physical disability, the rate of serious infections was quite low, 0.8 per 100-PY particularly, compared with nondisabled ocrelizumab-treated pwMS (∼2.1/100-PY).22 Comorbidities increased the risk of infections either directly (obesity, COPD, and recurrent outpatient infections) or indirectly through lower IgG levels (diabetes) providing additional evidence that prevention and treatment of these comorbidities should be part of routine MS care. Taken together with the existing literature, our findings highlight the need to identify the lowest effective dosing regimen of B-cell–depleting therapies and provide further evidence that the risk-benefit profile is unfavorable in pwMS with advanced disability.
Our finding that higher cumulative rituximab doses increase the risk of infections primarily through mechanisms other than hypogammaglobulinemia is novel, although not surprising. In addition to maturing into plasma cells and producing antibodies, B cells enable T-cell responses important for fighting infections including T-cell stimulation, differentiation, and formation of long-lived memory T cells through antigen presentation, costimulation, and cytokine production.23 It is also consistent with the COVID-19 pandemic experience where B-cell–depleting agents increased the risk of severe COVID-19 in nonvaccinated individuals regardless of serum IgG levels.24-26 That, at most, a small proportion of serious infection risk associated with higher rituximab exposure is mediated by hypogammaglobulinemia also likely reconciles why some studies in pwMS found no association between rituximab-induced or ocrelizumab-induced hypogammaglobulinemia and increased risk of infections, while other studies, including those reported herein, reported an association.
Our findings that higher cumulative rituximab dose is associated with lower IgG levels is consistent with reports of increasing prevalence of hypogammaglobulinemia with longer duration4-10 of ocrelizumab4,6,9,10 or rituximab5,7,8,27 use and declining mean IgG values during the RCTs of ocrelizumab in pwMS.11 Whether the doses of rituximab we use lead to lower rates of hypogammaglobulinemia compared with standard dose ocrelizumab (600 mg every 6 months) cannot be ascertained due to nebulous reporting of IgG values and rates of decline in ocrelizumab populations and significant heterogeneity in patient populations. As our findings show herein, direct comparison of drug-induced hypogammaglobulinemia across studies would require not only reporting specific IgG cutoff values but also factors associated with lower IgG values including older age, White race, prior HET use, and comorbid diabetes.
Advanced disability was the most important risk factor of serious infections, larger in magnitude than either higher rituximab doses or hypogammaglobulinemia in our cohort. Reflecting real-world practices in the United States,28 ∼30% of our population were dependent on a walker, wheelchair, or worse, though there is only weak evidence of the benefit of B-cell–depleting therapies in walker-dependent pwMS and no evidence of benefit in those with higher levels of disability. Not surprisingly, the crude rates of serious and recurrent infections were remarkably high in this population, consistent with findings from a real-world US-based ocrelizumab cohort.28 These findings call into question the wisdom of off-label prescribing of B-cell–depleting therapies in pwMS with advanced disability. At the very least, prescribers should strongly consider stopping B-cell–depleting therapies in those with declining IgG values, outpatient infections, or advancing disability.
By contrast, the rates of serious infections we observed in those pwMS without advanced disability (EDSS = 0–6.0, 0.8/100-PY) seems to be substantially lower than those reported in ocrelizumab-treated relapsing MS studies, despite heterogeneity in patient populations that would be expected to produce lower rates in the ocrelizumab-treated populations. The open-label study of ocrelizumab treatment in new-onset, relapsing MS with minimal or no disability (EDSS = 0–3.0) reported 8% with serious infections over 4 years for an estimated ∼2.1/100-PY.22 This was quite similar to the subgroup analyses from the relapsing MS ocrelizumab RCTs of early treatment initiation of 2.13/100-PY.29 The most likely explanation for this large difference in serious infection risk is that we routinely extend rituximab dosing intervals in stable patients, whereas the ocrelizumab-treated pwMS were persistently treated with 600 mg every 6 months.
The contributions of comorbidities to infection risk or hypogammaglobulinemia in pwMS treated with B-cell–depleted therapies have not been explicitly studied.3-11,22,28 We previously reported that COPD and obesity increased the risk of seeking care for at least 1 outpatient infection in DMT-treated pwMS.2 In this study, we show that COPD and obesity significantly increase the risk of recurrent outpatient infections in rituximab-treated pwMS independent of rituximab dose or IgG levels. We also report for the first time that comorbid diabetes is associated with lower IgG levels in rituximab-treated pwMS. Whether this is true for persons without MS is uncertain, although 1 large cross-sectional study reported lower IgG levels in persons with diabetes.30 Taken together with the association of specific comorbidities with adverse MS outcomes, our findings provide additional evidence that prevention and treatment of these comorbidities, including smoking cessation, weight management, dietary and exercise counseling, and blood glucose monitoring, should be part of routine MS counseling and care.
That Black, Asian, and, to a lesser extent, Hispanic rituximab-treated pwMS had higher serum IgG levels, yet no increased or decreased infection risks compared with White pwMS are novel findings that are challenging to interpret. Our findings are consistent with previous reports of higher serum IgG levels in Black and Asian individuals in large studies of healthy individuals31 and higher CSF antibody–producing cells and IgG index in small cross-sectional studies of Black, White, and Hispanic pwMS.16 Yet these higher IgG levels had no protective effect on infection risk in our large diverse cohort, even in crude analyses. Rather than being genetically inherent to a person's race or ethnicity, we speculate our findings reflect that the higher serum IgG levels are downstream consequences of structural racism (e.g., chronic psychosocial stress and increased pathogen exposures at younger ages). Future studies with measures of experienced discrimination or other aspects of embodied racism are needed to clarify this. Practically speaking, these findings indicate that clinicians should focus on disability level, cumulative rituximab exposure, IgG levels, and comorbidities when judging an individual patient's infection risk rather than their race or ethnicity.
The main limitations of this study, inherent to observational studies, are residual confounding by indication because rituximab dosing intervals and doses were lowered over the course of the study period in patients with clinical and radiographic stability, particularly if IgG values were declining or they were experiencing infections. This would explain why so few patients had very low IgG values (<500), even those treated for more than 4 years, and relatively few patients with no or moderate disability experienced serious infections, particularly when compared with standard dosing of ocrelizumab. In addition, the doses we use, particularly over time, are lower than other groups8,27; thus, it is possible that with higher cumulative doses, hypogammaglobulinemia may mediate a larger proportion of infectious risk than seen with our protocol. Cumulative dose was so closely tied to duration of use that we could not completely disentangle the relative contributions of these 2 factors to infection risk. Although our findings that those treated with the highest dosing protocol had much greater infection risk than those treated with the lowest dosing protocol over the first 2 years indicate that cumulative dose is more important than duration of use. Another limitation is that we did not measure hypertension,32 metabolic syndrome,33 and nonalcoholic fatty liver disease,34 all of which have been associated with lower IgG levels in at least one large study of non–MS patients. We also did not account for corticosteroid use, which is a known risk factor for infections. These limitations, in addition to the limitations inherent to current mediation modeling methods, methods that cannot incorporate available longitudinal data or allow for examining RTX and IgG values as ordinal variables, warrant caution in interpreting the point estimate of percentage of effect mediated by hypogammaglobulinemia. The inability for current mediation methods to incorporate the time-varying relationship between rituximab dosing, IgG levels, and infections, or model rituximab as an ordinal variable is the most likely explanation for why the ADE did not reach statistical significance in these models. Our findings should be confirmed in larger patient populations with higher cumulative doses, longer duration of B-cell–depleting therapy use, and more complete measures of comorbidities.
The strengths of this study include the large, diverse real-world cohort of pwMS and rigorous methods used. By carefully examining the relative contributions and relationships between higher cumulative doses of rituximab, hypogammaglobulinemia, disability, comorbidities, and infection risk, these analyses highlight that using lower cumulative doses decreases the risk of serious infections, recurrent outpatient infections, and hypogammaglobulinemia. Our findings also confirm that declining IgG values in patients with normal levels (>700 mg/dL) do not increase the risk of infections. Our large real-world cohort unmasked the strong effects of disability and nuanced contributions of specific comorbidities and race on infection risk and IgG levels, something that is not addressed in RCTs because patients with advanced disability and significant comorbidities are excluded from participation and minority populations are vastly underrepresented.16 In addition, recurrent outpatient infections, frequent enough to be bothersome to patients and warrant consideration of drug discontinuation, have not been considered either in RCTs or prior observational studies. The findings provided herein suggest potential strategies to reduce risk, specifically prevention of COPD and obesity. We used rigorous methods to identify serious infections in line with the CTCAE definitions allowing for direct comparison with Pharma-sponsored RCTs, rather than relying on ICD codes,2,3 a method that slightly overestimates the risk.2 Last, rituximab-associated infection risk is known to vary by the underlying disease being treated.35 Thus, including only pwMS, among the other strengths mentioned earlier, optimizes the generalizability of these findings to other populations with MS.
Advanced disability is the most important risk factor of serious infections. Given the lack of evidence of efficacy in this population, it seems highly plausible that the risks of B-cell–depleting therapies outweigh the benefits.11 Patients should be informed accordingly, and clinicians should strongly reconsider prescribing rituximab or ocrelizumab in these patients.
In pwMS without advanced disability, on the contrary, the rituximab dosing protocol we use seems quite safe and resulted in far fewer serious infections compared with standard dosing of ocrelizumab.22,29 While monitoring for declining IgG levels before each infusion and extending dosing intervals and/or reducing dose should continue to be part of standard B-cell–depleting therapy practice, clinicians and patients should be aware that the risk of infection increases with increasing use even when IgG levels are normal (i.e., >700 mg/dL). Given mounting evidence that continuous B-cell depletion is not required to maintain efficacy in relapsing MS36 and demonstration herein that there is a cumulative, dose-dependent increased risk of serious and recurrent outpatient infections, studies to identify the lowest effective dosing regimen of B-cell–depleting therapies are urgently needed.
Glossary
- ACME
average causal mediation effects
- ADE
average direct effect
- aHR
adjusted hazard ratio
- CTCAE
Common Terminology Criteria for Adverse Events
- DMT
disease-modifying therapy
- EHR
electronic health record
- GEE
generalized estimation equation
- IgG
immunoglobulin G
- IR
incidence rate
- IvIg
IV immunoglobulin
- KPSC
Kaiser Permanente Southern California
- pwMS
persons with multiple sclerosis
- PY
person-year
- RCTs
randomized controlled trials
Appendix. Authors
| Name | Location | Contribution |
| Annette Langer-Gould, MD, PhD | Department of Neurology, Los Angeles Medical Center, Southern California Permanente Medical Group; Department of Clinical Science, Kaiser Permanente Bernard J. Tyson School of Medicine | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; study concept or design; and analysis or interpretation of data |
| Bonnie H. Li, MS | Department of Research and Evaluation, Southern California Permanente Medical Group | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Jessica B. Smith, MPH | Department of Research and Evaluation, Southern California Permanente Medical Group | Drafting/revision of the article for content, including medical writing for content; major role in the acquisition of data; and analysis or interpretation of data |
| Stanley Xu, MS, PhD | Department of Research and Evaluation, Southern California Permanente Medical Group; Department of Health Systems Science, Kaiser Permanente Bernard J. Tyson School of Medicine | Drafting/revision of the article for content, including medical writing for content; analysis or interpretation of data |
Study Funding
This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Program Award (MS-1511-33-196). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosure
A. Langer-Gould has received grant support and awards from the Patient-Centered Outcomes Research Institute, the National MS Society, and Atara Biotherapeutics. She currently serves as a voting member on the California Technology Assessment Forum, a core program of the Institute for Clinical and Economic Review (ICER). She has received sponsored and reimbursed travel from ICER. B.H. Li reports no disclosures relevant to the manuscript. J.B. Smith reports no disclosures relevant to the manuscript. S. Xu reports no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Institute for Clinical and Economic Review (ICER). Oral and Monoclonal Antibody Treatments for Relapsing Forms of Multiple Sclerosis: Effectiveness and Value. Final Evidence Report; 2023. Accessed May 1, 2023. icer.org/wp-content/uploads/2022/04/ICER_MS_Final_Evidence_Report_022123.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langer-Gould AM, Smith JB, Gonzales EG, Piehl F, Li BH. Multiple sclerosis, disease-modifying therapies, and infections. Neurol Neuroimmunol Neuroinflamm. 2023;10(6):10. doi: 10.1212/nxi.0000000000200164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luna G, Alping P, Burman J, et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 2020;77(2):184-191. doi: 10.1001/jamaneurol.2019.3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mears V, Jakubecz C, Seeco C, Woodson S, Serra A, Abboud H. Predictors of hypogammaglobulinemia and serious infections among patients receiving ocrelizumab or rituximab for treatment of MS and NMOSD. J Neuroimmunol. 2023;377:578066. doi: 10.1016/j.jneuroim.2023.578066 [DOI] [PubMed] [Google Scholar]
- 5.Avouac A, Maarouf A, Stellmann JP, et al. Rituximab-induced hypogammaglobulinemia and infections in AQP4 and MOG antibody-associated diseases. Neurol Neuroimmunol Neuroinflamm. 2021;8(3):e977. doi: 10.1212/NXI.0000000000000977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Capasso N, Palladino R, Cerbone V, et al. Ocrelizumab effect on humoral and cellular immunity in multiple sclerosis and its clinical correlates: a 3-year observational study. J Neurol. 2023;270(1):272-282. doi: 10.1007/s00415-022-11350-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McAtee CL, Lubega J, Underbrink K, et al. Association of rituximab use with adverse events in children, adolescents, and young adults. JAMA Netw Open. 2021;4(2):e2036321. doi: 10.1001/jamanetworkopen.2020.36321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perriguey M, Maarouf A, Stellmann JP, et al. Hypogammaglobulinemia and infections in patients with multiple sclerosis treated with rituximab. Neurol Neuroimmunol Neuroinflamm. 2022;9(1):e1115. doi: 10.1212/NXI.0000000000001115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saidha S, Bell J, Harold S, et al. Systematic literature review of immunoglobulin trends for anti-CD20 monoclonal antibodies in multiple sclerosis. Neurol Sci. 2023;44(5):1515-1532. doi: 10.1007/s10072-022-06582-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seery N, Sharmin S, Li V, et al. Predicting infection risk in multiple sclerosis patients treated with ocrelizumab: a retrospective cohort study. CNS Drugs. 2021;35(8):907-918. doi: 10.1007/s40263-021-00810-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration and The Center for Drug Evaluation and Research. APPLICATION NUMBER: 761053Orig1s000 MEDICAL REVIEW(S): Ocrelizumab (Ocrevus); 2016. Accessed May 1, 2023. accessdata.fda.gov/drugsatfda_docs/nda/2017/761053orig1s000medr.pdf [Google Scholar]
- 12.Davis AC, Voelkel JL, Remmers CL, Adams JL, McGlynn EA. Comparing Kaiser permanente members to the general population: implications for generalizability of research. Perm J. 2023;27(2):87-98. doi: 10.7812/TPP/22.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koebnick C, Langer-Gould AM, Gould MK, et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37-41. doi: 10.7812/TPP/12-031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162-173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 15.Langer-Gould A, Lucas R, Xiang AH, et al. MS Sunshine Study: sun exposure but not vitamin D is associated with multiple sclerosis risk in blacks and hispanics. Nutrients. 2018;10(3):268. doi: 10.3390/nu10030268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Telesford KM, Amezcua L, Tardo L, et al. Understanding humoral immunity and multiple sclerosis severity in Black, and Latinx patients. Front Immunol. 2023;14:1172993. doi: 10.3389/fimmu.2023.1172993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith JB, Li BH, Gonzales EG, Langer-Gould A. Validation of algorithms for identifying outpatient infections in MS patients using electronic medical records. Mult Scler Relat Disord. 2022;57:103449. doi: 10.1016/j.msard.2021.103449 [DOI] [PubMed] [Google Scholar]
- 18.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0 [PDF]; 2017. Accessed May 1, 2023. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf [Google Scholar]
- 19.Amorim LD, Cai J. Modelling recurrent events: a tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324-333. doi: 10.1093/ije/dyu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H, Cashin AG, Lamb SE, et al. A guideline for reporting mediation analyses of randomized trials and observational studies: the AGReMA statement. JAMA. 2021;326(11):1045-1056. doi: 10.1001/jama.2021.14075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R package for causal mediation analysis. J Stat Softw. 2014;59(5):1-38. doi: 10.18637/jss.v059.i0526917999 [DOI] [Google Scholar]
- 22.Bermel R, Hartung HP, Brochet B, et al. Low Disease Activity over 4 Years of Ocrelizumab Therapy in Treatment-Naive Patients with Early-Stage Relapsing-Remitting Multiple Sclerosis: The Phase IIIb ENSEMBLE Study. P004.S46. Presented at the 75th Annual Meeting of the American Academy of Neurology, April 22-27, 2023. Boston, MA.
- 23.Cyster JG, Allen CDC. B cell responses: cell interaction dynamics and decisions. Cell. 2019;177(3):524-540. doi: 10.1016/j.cell.2019.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.REDONE.br – Neuroimmunology Brazilian Study Group Focused on COVID-19 and MS. Incidence and clinical outcome of Coronavirus disease 2019 in a cohort of 11,560 Brazilian patients with multiple sclerosis. Mult Scler. 2021;27(10):1615-1619. doi: 10.1177/1352458520978354 [DOI] [PubMed] [Google Scholar]
- 25.Smith JB, Gonzales EG, Li BH, Langer-Gould A. Analysis of rituximab use, time between rituximab and SARS-CoV-2 vaccination, and COVID-19 hospitalization or death in patients with multiple sclerosis. JAMA Netw Open. 2022;5(12):e2248664. doi: 10.1001/jamanetworkopen.2022.48664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sormani MP, De Rossi N, Schiavetti I, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol 2021;89(4):780-789. doi: 10.1002/ana.26028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vollmer BL, Wallach AI, Corboy JR, Dubovskaya K, Alvarez E, Kister I. Serious safety events in rituximab-treated multiple sclerosis and related disorders. Ann Clin Transl Neurol. 2020;7(9):1477-1487. doi: 10.1002/acn3.51136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smoot K, Chen C, Stuchiner T, Lucas L, Grote L, Cohan S. Clinical outcomes of patients with multiple sclerosis treated with ocrelizumab in a US community MS center: an observational study. BMJ Neurol Open. 2021;3(2):e000108. doi: 10.1136/bmjno-2020-000108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cerqueira JJ, Berthele A, Filippi M, et al. Long-Term Treatment with First-Line Ocrelizumab in Patients with Early RMS: 9-Year Follow-Up Data from the OPERA Trial. Oral Presentation 002.S46. Presented at the 75th Annual Meeting of the American Academy of Neurology, April 22-27, 2023. Boston, MA.
- 30.Guo X, Meng G, Liu F, et al. Serum levels of immunoglobulins in an adult population and their relationship with type 2 diabetes. Diabetes Res Clin Pract. 2016;115:76-82. doi: 10.1016/j.diabres.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Khan SR, van der Burgh AC, Peeters RP, van Hagen PM, Dalm V, Chaker L. Determinants of serum immunoglobulin levels: a systematic review and meta-analysis. Front Immunol. 2021;12:664526. doi: 10.3389/fimmu.2021.664526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang X, Li Y, Li H, et al. Relationship of serum immunoglobulin levels to blood pressure and hypertension in an adult population. J Hum Hypertens. 2018;32(3):212-218. doi: 10.1038/s41371-018-0029-2 [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Fu J, Gu Y, et al. Relationship between serum levels of immunoglobulins and metabolic syndrome in an adult population: a population study from the TCLSIH cohort study. Nutr Metab Cardiovasc Dis. 2019;29(9):916-922. doi: 10.1016/j.numecd.2019.05.053 [DOI] [PubMed] [Google Scholar]
- 34.Sun SM, Wang YY, Zhang Q, et al. Serum levels of immunoglobulins in an adult population and their relationship with nonalcoholic fatty liver disease. J Dig Dis. 2018;19(8):498-507. doi: 10.1111/1751-2980.12646 [DOI] [PubMed] [Google Scholar]
- 35.Tony HP, Burmester G, Schulze-Koops H, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res Ther. 2011;13(3):R75. doi: 10.1186/ar3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starvaggi Cucuzza C, Longinetti E, Ruffin N, et al. Sustained low relapse rate with highly variable b-cell repopulation dynamics with extended rituximab dosing intervals in multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2023;10(1):10. doi: 10.1212/nxi.0000000000200056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to the KPSC IRB, data are available on reasonable request.