Abstract
Prior studies in our laboratory have suggested that the CC chemokine macrophage inflammatory protein-1α (MIP-1α) may be an important mediator in the blinding ocular inflammation which develops following herpes simplex virus type 1 (HSV-1) infection of the murine cornea. To directly test this hypothesis, MIP-1α-deficient (−/−) mice and their wild-type (+/+) counterparts were infected topically on the scarified cornea with 2.5 × 105 PFU of HSV-1 strain RE and subsequently graded for corneal opacity. Four weeks postinfection (p.i.), the mean corneal opacity score of −/− mice was 1.1 ± 0.3 while that of the +/+ mice was 3.7 ± 0.5. No detectable infiltrating CD4+ T cells were seen histologically at 14 or 21 days p.i. in −/− animals, whereas the mean CD4+ T-cell count per field (36 fields counted) in +/+ hosts was 26 ± 2 (P < 0.001). In addition, neutrophil counts in the −/− mouse corneas were reduced by >80% in comparison to the wild-type controls. At 2 weeks p.i., no interleukin-2 or gamma interferon could be detected in six of seven −/− mice, whereas both T-cell cytokines were readily demonstrable in +/+ mouse corneas. Also, MIP-2 and monocyte chemoattractant protein-1 protein levels were significantly lower in MIP-1α −/− mouse corneas than in +/+ host corneas, suggesting that MIP-1α directly, or more likely indirectly, influences the expression of other chemokines. Interestingly, despite the paucity of infiltrating cells, HSV-1 clearance from the eyes of −/− mice was not significantly different from that observed in +/+ hosts. We conclude that MIP-1α is not needed to control virus growth in the cornea but is essential for the development of severe stromal keratitis.
Herpes simplex virus type 1 (HSV-1) infection of the murine cornea triggers an intense inflammatory response which persists and can result in blindness. This disease, called herpes stromal keratitis (HSK), is characterized by the extensive infiltration of polymorphonuclear and mononuclear cells (34, 36). While immune CD4+ T cells play a critical role in initiating the immunopathological response, neutrophils are the most prominent infiltrating cell type seen in the cornea.
The events promoting leukocyte infiltration into sites of infection are complex and involve the participation of various factors, including chemokines. Chemokines are a superfamily of more than 30 low-molecular-mass heparin-binding polypeptides which participate in the directed migration of leukocyte subsets (2, 21, 33). They are divided into two main families on the basis of structure. The alpha members have a CXC construction wherein the two initial cysteines are separated by an intervening amino acid, while the initial beta family cysteines are adjacent (CC) to each other. Prior studies in our laboratory have shown that HSV-1 ocular infection in BALB/c mice induces message for at least seven different chemokines (30). Administration of anti-HSV gD monoclonal antibody, which protects against HSK (20), was associated with reduced message for macrophage inflammatory protein-2 (MIP-2), monocyte chemoattractant protein-1 (MCP-1), MIP-1α, and MIP-1β. This finding suggested that one or more of these four chemokines may play an essential role in HSK development. MIP-1α has been reported to be an important chemoattractant for T cells and neutrophils in the mouse (38, 39) and was therefore an attractive choice for further analysis. This chemokine was originally discovered as a protein secreted by the mouse macrophage cell line RAW264.7 following exposure to endotoxin (25, 39). The mature product is 69 amino acids and is produced by several different cell types, including alveolar macrophages, T cells, bronchial epithelial cells, and neutrophils (17, 23, 26). Like other chemokines, MIP-1α binds to a G-protein-coupled seven-transmembrane receptor (7). It also binds to heparin (16), an extracellular matrix glycosaminoglycan.
Recently, mice which have a deletion in almost half of the MIP-1α coding region as well as 300 nucleotides of DNA upstream of the mRNA start site have been generated (5). We have used these gene knockout mice to investigate the function of MIP-1α in HSV-1-induced ocular inflammation. Following HSV-1 corneal infection, HSK was strikingly diminished in MIP-1α-deficient (−/−) hosts although virus replication and clearance did not differ significantly from that seen in infected wild-type (+/+) mice. These results demonstrate that the abrogation of a single chemokine can dramatically affect the outcome of virus infection in the murine eye.
MATERIALS AND METHODS
Animals.
The generation of mice with a targeted disruption of the gene encoding MIP-1α has been previously described (5). Both male and female mutant (−/−) mice and their wild-type (+/+) counterparts were used when 6 to 10 weeks old. The animals were housed in plastic cages in a room with a 12-h light/12-h dark cycle.
Virus infection.
HSV-1 strain RE, a known HSK inducer (20), was used to initiate infection. Virus stocks were grown and titrated on Vero cells as previously described (19). Mice were anesthetized with 1.0 mg of sodium phenobarbital in 0.2 ml of phosphate-buffered saline injected intraperitoneally. The right eye was lightly scarified by three twists of a 2-mm corneal trephine. A 2-μl volume containing 2.5 × 105 to 7 × 105 PFU of virus was then dropped onto the corneal surface and massaged in with the eyelids. Eyes were examined weekly with a dissecting biomicroscope. Corneal opacity was graded on a scale of 0 to +4 as follows: 0, clear cornea; +1, slight corneal haze; +2, moderate corneal opacity; +3, severe corneal opacity with iris visible; +4, severe corneal opacity with iris not visible. Eyes were examined in a coded fashion, with the reader unaware of the treatment given.
Measurement of neutralizing antibody.
To determine whether MIP-1α influences the titers of neutralizing antibody, sera were collected at various times after corneal infection with HSV-1 and titrated for neutralizing antibodies by the plaque reduction method (19). Equal volumes of sera diluted as desired were added to tubes containing 0.2 ml of an HSV-1 suspension (3 × 103 PFU/ml). These virus-serum mixtures were incubated for 30 min at 37°C, and then 0.1-ml aliquots were placed on a Vero cell monolayer. Controls received virus only. After adsorption for 1 h at room temperature, the monolayers were overlaid with 4 ml of 0.5% methylcellulose solution and incubated for 2 days at 37°C in a 5% CO2 atmosphere. The neutralizing titer was taken as the dilution of serum that reduced the mean PFU by 50% in comparison with the controls.
Assay of ocular tissue for infectious HSV-1.
To test whether the absence of MIP-1α affected HSV-1 replication in the cornea, individual whole eyes were excised and placed in 600 μl of 2% fetal bovine serum in Dulbecco modified Eagle medium with antibiotics. Preparations were frozen to −70°C, thawed, and homogenized in a Ten Broeck homogenizer (Bellco, Vineland, N.J.). The homogenates were frozen and thawed again followed by sonication for 15 s in a Sonic 300 Dismembrator (Artek Systems Corporation, Farmingdale, N.Y.). The supernatants were then titrated for infectious virus on Vero cell monolayers in a standard 48-h plaque assay (19).
Histologic examination.
Infected eyes were enucleated, fixed in 10% neutral buffered formalin, and embedded in paraffin, and multiple 5-μm sections were prepared. Sections were stained with hematoxylin-eosin, mounted in Permount, and coverslipped for photomicroscopy.
Cytokine and chemokine quantitation.
To determine the in vivo levels of cytokine or chemokine proteins, and to test whether the absence of MIP-1α influenced the level of these proteins, corneas were removed from MIP-1α +/+ and −/− mice on various days after infection. Corneas were placed in 500 μl of serum-free RPMI 1640 with antibiotic-antimycotic solution (GIBCO BRL). Samples were stored at −70°C until assayed. Samples were thawed, homogenized, minced, sonicated for 30 s, and clarified by centrifugation at 150 × g for 10 min. The clarified cell lysates were assayed for interleukin-2 (IL-2), IL-4, gamma interferon (IFN-γ), MIP-1α, MIP-2, and MCP-1, using commercial enzyme-linked immunosorbent assay (ELISA) kits. The IL-2 kit (assay sensitivity, 3 pg/ml), IL-4 kit (assay sensitivity, 5 pg/ml), and IFN-γ kit (assay sensitivity, 15 pg/ml) were purchased from Endogen, Inc. (Boston, Mass.). The MCP-1 kit (assay sensitivity, 9 pg/ml) was purchased from Biosource International (Camarillo, Calif.). The MIP-2 kit (assay sensitivity, 1.5 pg/ml) and MIP-1α kit (assay sensitivity, 3 pg/ml) were purchased from R & D Systems (Minneapolis, Minn.).
Immunohistochemistry.
Immunohistochemical examination of infected corneas was performed as previously described (12). Infected eyes were enucleated and embedded in O.C.T. (Tissue Tek, Miles, Naperville, Ill.) and snap-frozen in an isopentane dry ice bath, and 6-μm sections were cut at −20°C. The sections were fixed in cold acetone for 10 min and then exposed to the primary antibodies overnight at 4°C. Rat monoclonal antibodies to CD4 T cells (clone GK1.5; American Type Culture Collection, Rockville, Md.) and granulocytes (RB6-8C5; a gift from R. Coffman, DNAX Research Institute, Palo Alto, Calif.) were used as primary antibodies at 2 μg/ml. The sections were then stained by the streptavidin-biotin complex immunoperoxidase staining procedure (Zymed Laboratories, South San Francisco, Calif.). The sections were washed for 10 min and incubated for 30 min with biotinylated rabbit anti-rat immunoglobulin G (heavy and light chains) which had been adsorbed with mouse serum protein and diluted in a mouse skin extract. Following two washes, the sections were exposed to 3% H2O2 in methanol and washed two times for 10 min each to block endogenous peroxidase activity. The sections were then incubated for 10 min with the streptavidin-biotinylated peroxidase complex. Following two washes, sections were incubated for 5 min in AEC (3-amino-9-ethylcarbazole; Sigma Chemical Company) solution, which was made from 1 ml of AEC (4 mg/ml) in N,N-dimethylformamide, 14 ml of 0.1 M acetate buffer (pH 5.5), and 150 μl of 3% H2O2. The slides were then washed in distilled water and counterstained with Mayer’s hematoxylin for 6 min. The microscopic slides containing CD4+ and neutrophil staining were counted in a coded fashion, with the reader unaware of the treatment given.
Statistical analysis.
The Mann-Whitney U test was used to determine significant differences in the corneal opacity scores between control and test groups. Student’s t test was used to determine significant differences in the number of CD4+ T cells and neutrophils in the corneas of MIP-1α +/+ and −/− mice. The level of confidence at which the experiments were judged significant was P < 0.05.
RESULTS
MIP-1α expression in the HSV-1-infected cornea.
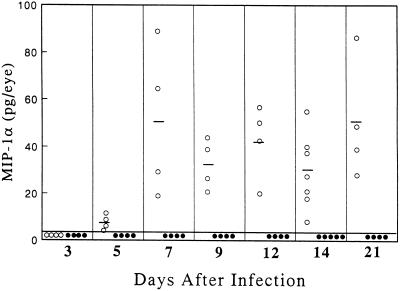
The kinetics of MIP-1α production following HSV-1 corneal infection was determined by ELISA. Figure 1 shows that no protein was detected in +/+ corneas before day 5 postinfection. Histologically examination of the corneas at various times postinfection indicated that the appearance of MIP-1α coincided with the initiation of pronounced leukocyte infiltration into the infected cornea. This CC chemokine continued to be synthesized for 21 days postinfection in all +/+ mice examined. However, at 4 weeks postinfection only 60% of the corneas were positive, and in these the mean concentration had decreased to 6 pg/eye. No MIP-1α protein was detected in any of the 32 mutant hosts examined.
FIG. 1.
Time course of MIP-1α chemokine protein expression in HSV-1-infected corneas. At the indicated times after infection, four to seven eyes from MIP-1α +/+ (○) and −/− (•) mice were removed and individually homogenized, minced, and sonicated, after which the clarified cell lysates were assayed via ELISA. Bars represent mean values for each group on the indicated day after infection.
HSK development in MIP-1α +/+ and −/− mice.
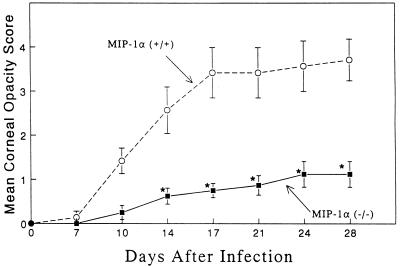
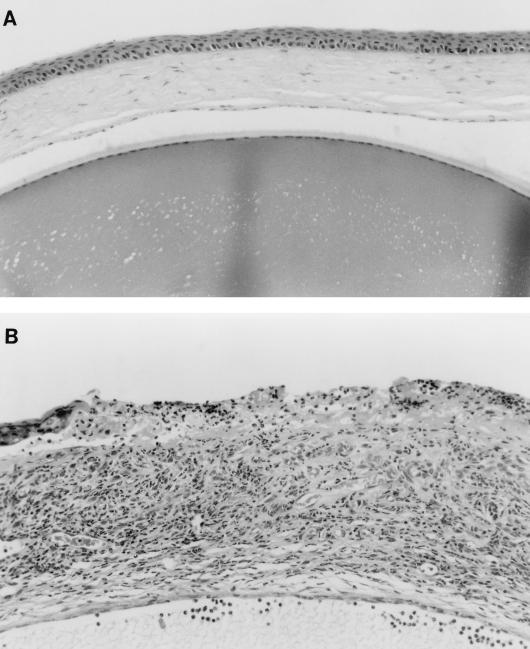
MIP-1α +/+ and −/− mice were evaluated clinically for the development of HSK following HSV-1 corneal infection. Figure 2 shows that 129 × C57BL/6 hosts developed corneal opacity in a time frame quite similar to that previously reported for BALB/c mice (13). In sharp contrast, animals with the disrupted MIP-1α gene were strikingly resistant to the development of severe HSK. These observations were confirmed in three independent experiments. Histologically, the corneas from MIP-1α −/− mice remained avascular and displayed only scattered inflammatory cells (Fig. 3A), whereas the corneas of +/+ hosts were characterized by copious inflammatory cells, neovascularization, and corneal epithelial ulceration (Fig. 3B). To further characterize the cellular infiltrate in the infected corneas, CD4+ T cells and neutrophils were enumerated by immunohistochemical staining with appropriate antisera. Table 1 shows that in contrast to the controls, no CD4+ T cells were detected in the eyes of MIP-1α −/− mice while the neutrophil infiltrate was reduced by >80%. The presence of some neutrophils in the pervascular region of the corneal limbus of MIP-1α −/− mice suggested that a chemokine(s) in addition to MIP-1α participated in promoting neutrophil migration into the HSV-1-infected tissue.
FIG. 2.
Requirement of MIP-1α for the development of HSK. Ten MIP-1α −/− mice along with 10 age-matched +/+ mice were infected topically with 2.5 × 105 PFU of HSV-1 strain RE and then monitored for the development of corneal opacity. *, the treated group was significantly (P < 0.05) different from the control group.
FIG. 3.
Photomicrographs of cross sections of corneas from MIP-1α −/− (A) and MIP-1α +/+ (B) mice removed 28 days after infection. Four corneas per group were examined in two independent experiments. Magnification, ×82.
TABLE 1.
CD4 T-cell and neutrophil numbers in corneas of MIP-1α +/+ and −/− mice
| Day on which corneas were harvesteda | Leukocyte stainingb | No. of cellsc (mean counts ± SE)
|
Pd | |
|---|---|---|---|---|
| MIP-1α +/+ | MIP-1α −/− | |||
| 14 | CD4 T cells | 27 ± 2 | 0 | 0.001 |
| Neutrophils | 112 ± 9 | 19 ± 2 | 0.006 | |
| 21 | CD4 T cells | 24 ± 2 | 0 | 0.001 |
| Neutrophils | 261 ± 14 | 36 ± 2 | 0.002 | |
The scarified right cornea was infected with 2.5 × 105 PFU of the RE strain of HSV-1. Three corneas from each treatment group were obtained on the days indicated, and individual frozen sections were prepared.
CD4 T cells and neutrophils were identified by immunohistochemical staining of frozen sections by using monoclonal antibodies GK1.5 and RB6-8C5, respectively.
The designated cell populations were counted in six different 40× fields in each of three sections from each cornea (total of 36 fields per time point).
Determined by the two-tailed Student t test.
IL-2 and IFN-γ expression in MIP-1α +/+ and −/− mice.
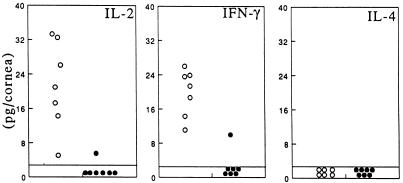
HSK is characterized by elevated levels of the TH1 cytokines IL-2 and IFN-γ, and neutralization of either is reported to suppress HSK development (13, 31). Both cytokines were readily detected in the corneas of +/+ hosts as expected on day 14 (Fig. 4). However, the corneas of MIP-1α −/− mice were virtually devoid of IL-2 and IFN-γ, which is in agreement with the dearth of T cells in this tissue. Assays for IFN-γ at earlier time points, i.e., days 4, 7, and 10 postinfection, revealed that the mean levels in −/− mice were 9-, 3-, and 40-fold lower, respectively, than that seen in +/+ mice. Tests for IL-4, a TH2 cytokine, were negative at all time points in both groups of animals. These results further indicate that endogenous MIP-1α is associated with TH1 cytokine expression in the HSV-1-infected cornea.
FIG. 4.
Reduction of the inflammatory cytokines IFN-γ and IL-2 in MIP-1α −/− mice. Groups of seven MIP-1α −/− mutant mice (•) along with seven age-matched +/+ mice (○) received 2.5 × 105 PFU of HSV-1 strain RE. Fourteen days after infection, the corneas were excised and individually assayed for IL-2, IFN-γ, and IL-4 via ELISA.
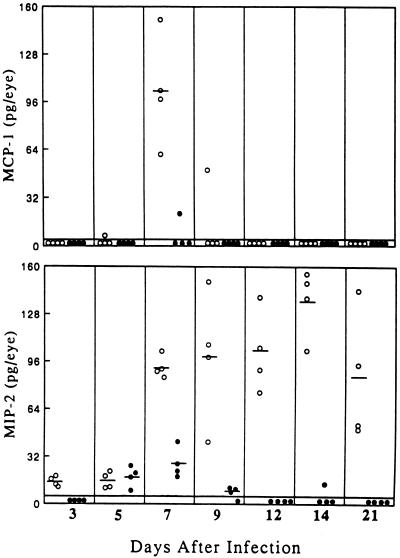
MIP-2 and MCP-1 expression in MIP-1α +/+ and −/− mice.
In addition to MIP-1α, message for the chemokines MIP-2 and MCP-1 is known to be induced in the BALB/c cornea following HSV-1 infection (30). Figure 5 shows that these two chemokines were also produced in 129 × C57BL/6J +/+ mice. MIP-2 was detected at low levels on day 3, increased some fivefold by day 7, peaked at day 14, and persisted for at least 3 weeks. In contrast, synthesis of MCP-1 was quite transient, being present in all hosts only on day 7. Interestingly, the production of both chemokines was sharply reduced in the MIP-1α −/− hosts. This reduction presumedly contributed to the paucity of infiltrating leukocytes in the HSV-1-infected cornea. It appears that MIP-1α synthesis can influence the expression of both MCP-1 and MIP-2, probably by regulating infiltration of the cells that produce them.
FIG. 5.
Reduction of the inflammatory chemokines MCP-1 and MIP-2 in MIP-1α −/− mice. Groups of MIP-1α −/− mice (•) along with age-matched +/+ mice (○) received 2.5 × 105 PFU of HSV-1 strain RE. On the days indicated after infection, corneas were excised and individually assayed for MCP-1 and MIP-2 via ELISA. Bars represent mean values for each group on the indicated day after infection.
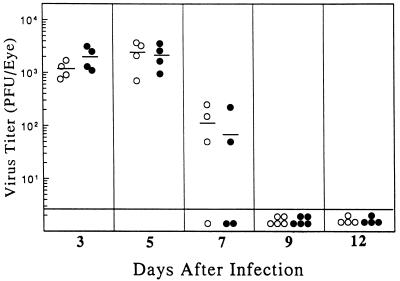
HSV-1 clearance in MIP-1α −/− mice is not impaired.
It was of interest to determine whether the loss of MIP-1α rendered the mutant mice more susceptible to HSV-1 replication. To investigate this question, infectious virus titers in the eyes of mutant mice were compared with that found in the +/+ controls following topical infection of the cornea with 2.5 × 105 PFU. Figure 6 shows that there was no significant difference in virus growth or clearance between the two groups. At a higher challenge dose (7 × 105 PFU) some mortality (20 to 25%) was seen, but it was comparable for both groups. Previous analysis did not reveal any overt hematopoietic abnormalities in MIP-1α −/− mice (5). We found that sera collected from −/− hosts 12 days postinfection had virus neutralizing titers (1:40 to 1:160) which were equivalent to that seen in +/+ mice. Thus, MIP-1α does not appear to influence the production of virus-specific antibody.
FIG. 6.
Infectious virus titers recovered from eyes of MIP-1α +/+ and −/− mice. MIP-1α −/− mice (•) along with their wild-type +/+ counterparts (○) were infected topically on the scarified cornea with HSV-1 at 2.5 × 105 PFU. On the days indicated, four to five animals in each group were sacrificed, and the individual eyes were excised and assayed for infectious virus content. Bars represent mean values for each group on the indicated day after infection.
DISCUSSION
In this study, mice with a disrupted gene for MIP-1α were used to test the hypothesis that this CC chemokine was an essential participant in the development of HSK. We found that animals which did not make MIP-1α protein had markedly reduced corneal opacity compared to the wild-type controls. Histologically, corneas from the gene knockout mice displayed a dramatic reduction in T-cell and neutrophil migration. In addition, the levels of cytokines IL-2 and IFN-γ as well as the chemokines MIP-2 and MCP-1 were significantly lower than those in +/+ mice. The paucity of inflammatory cells and proinflammatory mediators in the corneas of MIP-1α −/− mice provides a logical explanation for the attenuation of ocular disease. Additional evidence supporting MIP-1α participation in HSK development comes from the findings that HSV-1 infection of the corneas of +/+ mice led to the production of MIP-1α and that expression of the chemokine was correlated temporally with the migration of leukocytes into infected ocular tissue and the initiation of clinical corneal disease. Also, higher levels of MIP-1α were found in those corneas which displayed the most severe ocular opacity (data not shown). Recent studies using BALB/c mice in our laboratory have shown that IL-10 treatment, which suppresses HSK development (35), also suppresses MIP-1α synthesis. In addition, passive transfer of specific neutralizing antibody to MIP-1α modestly but significantly reduced the severity of corneal opacity in this mouse strain. Collectively, our results strongly support the premise that MIP-1α plays a critical role in HSK development in the mouse.
MIP-1α is not made constitutively by resident murine corneal cells. In fact, message and protein for this chemokine could not be detected in the cornea until some 5 to 7 days postinfection (30) (Fig. 1). This finding suggests that infiltrating leukocytes are the principal producers. In rodents, neutrophils (23), T cells (17), and macrophages (26, 39) all can make MIP-1α and constitute potential cellular sources.
Our results are in agreement with previous work in which the response of MIP-1α −/− mice to two other virus infections was studied (5). It was observed that MIP-1α null mice were resistant to coxsackievirus-induced myocarditis and had reduced lung inflammation following influenza virus infection. In both virus infections, there was reduced mononuclear cell infiltration. We observed that CD4 T-cell migration into the HSV-1-infected cornea was dramatically reduced in −/− mice. This reduction in T-cell migration conforms with in vitro observation that MIP-1α is chemotactic for T cells (22, 32). In addition, other investigators have observed that administration of neutralizing antibodies to MIP-1α can result in diminished influx of T cells at the site of inflammation (6, 15), although this is not invariably the case (14, 26, 28).
CC chemokines are generally thought to be chemotactic for lymphocytes and cells of the monocyte lineage (2, 21, 33). Our data show that MIP-1α is also needed for maximal recruitment of neutrophils into the cornea. Similarly, other investigators have reported that administration of neutralizing antibody to MIP-1α reduces neutrophil influx into the peritoneal cavity (1) and lungs (14, 24) of different rodent models. Neutralizing MIP-1α antibody treatment also suppressed neutrophil migration into cryptococcal antigen-injected gelatin sponges implanted subcutaneously (6). One explanation for suppressed neutrophil infiltration is that MIP-1α is directly chemotactic for these cells. This possibility exists since mouse neutrophils have recently been reported to express CCRI, a receptor for MIP-1α, and MIP-1α was shown to be chemotactic for mouse neutrophils in vitro (8). Alternatively, or in addition, MIP-1α may act indirectly by inducing cells to make molecules which promote neutrophil chemotaxis. Our observation that MIP-1α −/− mice have decreased expression of MIP-2, a potent murine neutrophil chemoattractant (37, 40), suggests that MIP-1α can influence the expression of CXC chemokines. Further studies are needed to determine whether MIP-1α acts directly or indirectly to reduce neutrophils and T cells and lower cytokine and chemokine levels.
Cook et al. (5) observed that influenza virus was cleared more slowly in MIP-1α −/− mice. On the other hand, coxsackievirus-infected −/− animals were not found to have higher virus titers than the +/+ controls (4). Since T cells appear to be needed for HSV-1 clearance from infected tissue (3, 18, 27, 34), and MIP-1α production in +/+ mice was correlated with the removal of virus, it was anticipated that virus growth would be more exuberant in MIP-1α −/− mice. Surprisingly, there was no difference in clearance of HSV-1 from the eyes (Fig. 6) and trigeminal ganglia (unpublished observations) of −/− hosts compared to +/+ mice. These results are analogous to that seen in coxsackievirus infection wherein −/− animals were not found to have higher virus titers than the +/+ controls (4). It thus appears that MIP-1α participation in virus clearance is variable. The requirement for this chemokine may depend on the nature of the infecting virus and/or the particular tissue that is infected.
The unfolding chemokine story has established that different chemokines can have similar structures, bind to the same receptor, and have overlapping activity in chemotactic assays. For example, MIP-1β is 60% homologous to MIP-1α at the amino acid level, binds to some of the same receptors, and in vitro is a chemoattractant for many of the same cell types as MIP-1α (10, 11). Thus, it was initially considered likely that a similar chemokine would functionally compensate for a missing chemokine. However, in order for one chemokine to fully compensate for another in vivo, it needs to be produced in the same time frame in the target tissue and be as potent. It must also recruit the same leukocyte subsets and, after receptor binding, function as an agonist (as opposed to an antagonist). Given these multiple requirements, it perhaps is not surprising that in an in vivo environment one chemokine may not easily substitute for another.
In summary, we have shown that a single chemokine, MIP-1α, has a dominant role in virus-induced ocular inflammation. MIP-1α has also been implicated in inflammatory cell responses associated with human diseases such as sarcoidosis and idiopathic pulmonary fibrosis (29). It may be possible to develop antagonists of MIP-1α that will be clinically useful (9), especially since our results show that interference with the biological activity of MIP-1α does not necessarily compromise the host’s capacity to clear infectious agents.
ACKNOWLEDGMENTS
We thank Patricia Couling for typing the manuscript and Judy Miller for technical assistance.
This work was supported by National Institute of Health grants EY07564 and EY11493.
REFERENCES
- 1.Appelberg R. Macrophage inflammatory protein MIP-1 and MIP-2 are involved in T cell-mediated neutrophil recruitment. J Leukocyte Biol. 1992;52:303–306. doi: 10.1002/jlb.52.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Bonneau R H, Jennings S R. Modulation of acute and latent herpes simplex virus infection in C57BL/6 mice by adoptive transfer of immune lymphocytes with cytolytic activity. J Virol. 1989;63:1480–1484. doi: 10.1128/jvi.63.3.1480-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook D N. The role of MIP-1α in inflammation and hematopoiesis. J Leukocyte Biol. 1996;59:61–66. doi: 10.1002/jlb.59.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Cook D N, Beck M A, Coffman T M, Kirby S L, Sheridan J F, Pragnell I B, Smithies O. Requirement of MIP-1α for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 6.Doyle H A, Murphy J W. MIP-1α contributes to the anticryptococcal delayed-type hypersensitivity reaction and protection against Cryptococcus neoformans. J Leukocyte Biol. 1997;61:147–155. doi: 10.1002/jlb.61.2.147. [DOI] [PubMed] [Google Scholar]
- 7.Gao J-L, Murphy P M. Cloning and differential tissue-specific expression of three mouse β chemokine receptor-like genes, including the gene for a functional macrophage inflammatory protein-1α receptor. J Biol Chem. 1995;270:17494–17501. doi: 10.1074/jbc.270.29.17494. [DOI] [PubMed] [Google Scholar]
- 8.Gao J-L, Wynn T A, Chang Y, Lee E J, Broxmeyer H E, Cooper S, Tiffany H L, Westphal H, Kwon-Chung J, Murphy P M. Impaired host defense, hematopoiesis, granulomatous inflammation and type 1-type 2 cytokine balance in mice lacking CC chemokine receptor 1. J Exp Med. 1997;185:1959–1968. doi: 10.1084/jem.185.11.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham G J, Mackenzie J, Lowe S. Aggregation of the chemokine MIP-1α is a dynamic and reversible phenomenon—biochemical and biological analyses. J Biol Chem. 1994;269:4947–4978. [PubMed] [Google Scholar]
- 10.Haelens A, Wuyts A, Proost P, Struyf S, Opdenakker G, van Damme J. Leukocyte migration and activation by murine chemokines. Immunobiology. 1996;195:499–521. doi: 10.1016/S0171-2985(96)80019-2. [DOI] [PubMed] [Google Scholar]
- 11.Hedrick J A, Zlotnik A. Chemokines and lymphocyte biology. Curr Opin Immunol. 1996;8:343–347. doi: 10.1016/s0952-7915(96)80123-3. [DOI] [PubMed] [Google Scholar]
- 12.Hendricks R L, Epstein R J, Tumpey T M. The effect of cellular immune tolerance to HSV-1 antigens on the immunopathology of HSV-1 keratitis. Invest Ophthalmol Visual Sci. 1989;30:105–115. [PubMed] [Google Scholar]
- 13.Hendricks R L, Tumpey T M, Finnegan A. IFN-gamma and IL-2 are protective in the skin but pathologic in the corneas of HSV-1 infected mice. J Immunol. 1992;149:3023–3028. [PubMed] [Google Scholar]
- 14.Huffnagle G B, Strieter R M, McNeil L K, McDonald R A, Burdick M D, Kunkel S L, Toews G B. Macrophage inflammatory protein-1α (MIP-1α) is required for the efferent phase of pulmonary cell-mediated immunity to a Cryptococcus neoformans infection. J Immunol. 1997;159:318–327. [PubMed] [Google Scholar]
- 15.Karpus W J, Lukacs N W, McRae C L, Strieter R M, Kunkel S L, Miller S T. An important role for the chemokine macrophage inflammatory protein-1α in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- 16.Koopmann W, Krangel M S. Identification of a glycosaminoglycan-binding site in chemokine macrophage inflammatory protein-1α. J Biol Chem. 1997;272:10103–10109. doi: 10.1074/jbc.272.15.10103. [DOI] [PubMed] [Google Scholar]
- 17.Kwon B S, Kestler D P, Eshhar Z, Oh K O, Wakulchik M. Expression characteristics of two potential T cell mediator genes. Cell Immunol. 1989;121:414–422. doi: 10.1016/0008-8749(89)90040-3. [DOI] [PubMed] [Google Scholar]
- 18.Larsen H S, Russell R G, Rouse B T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983;41:197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lausch R N, Kleinschrodt W R, Monteiro C, Kayes S G, Oakes J E. Resolution of HSV corneal infection in the absence of delayed-type hypersensitivity. Invest Ophthalmol Visual Sci. 1985;26:1509–1515. [PubMed] [Google Scholar]
- 20.Lausch R N, Oakes J E, Metcalf J F, Scimeca J M, Smith L A, Robertson S M. Quantitation of purified monoclonal antibody needed to prevent HSV-1 induced stromal keratitis in mice. Curr Eye Res. 1989;8:499–506. doi: 10.3109/02713688909000030. [DOI] [PubMed] [Google Scholar]
- 21.Oppenheim J J, Zachariae C O C, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine cytokine” family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 22.Schall T J, Bacon K, Camp R D, Kaspari J W, Goeddel D V. Human macrophage inflammatory protein α (MIP-1α) and MIP-1β chemokines attract distinct populations of lymphocytes. J Exp Med. 1993;177:1821–1826. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seebach J, Bartholdi D, Frei K, Spanaus K-S, Ferrero E, Widmer U, Isenmann S, Strieter R M, Schwab M, Pfister H-W, Fontana A. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1 alpha and -2 are produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J Immunol. 1995;155:4367–4375. [PubMed] [Google Scholar]
- 24.Shanley T P, Schmal H, Friedl H P, Jones M L, Ward P A. Role of macrophage inflammatory protein-1α (MIP-1α) in acute lung injury in rats. J Immunol. 1995;154:4793–4802. [PubMed] [Google Scholar]
- 25.Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe S D, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 β. J Exp Med. 1988;168:2251–2259. doi: 10.1084/jem.168.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith R E, Strieter R M, Phan S-H, Lukacs N W, Huffnagle G B, Wilke C A, Burdick M D, Lincoln P, Evanoff H, Kunkel S L. Production and function of murine macrophage inflammatory protein-1 alpha in bleomycin-induced lung injury. J Immunol. 1994;153:4704–4712. [PubMed] [Google Scholar]
- 27.Staats H, Oakes J E, Lausch R N. Anti-glycoprotein D monoclonal antibody protects against herpes simplex virus type 1-induced diseases in mice functionally depleted of selected T-cell subsets or asialo GM1+ cells. J Virol. 1991;65:6008–6014. doi: 10.1128/jvi.65.11.6008-6014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Standiford T J, Kunkel S L, Lukacs N W, Greenberger M J, Danforth J M, Kunkel R G, Strieter R M. Macrophage inflammatory protein-1α mediates lung leukocyte recruitment, lung capillary leak, and early mortality in murine endotoxemia. J Immunol. 1995;155:1515–1524. [PubMed] [Google Scholar]
- 29.Standiford T J, Rolfe M W, Kunkel S L, Lynch III J P, Burdick M D, Gilbert A R, Orringer M B, Whyte R I, Strieter R M. Macrophage inflammatory protein-1 α expression in interstitial lung disease. J Immunol. 1993;151:2852–2863. [PubMed] [Google Scholar]
- 30.Su Y-H, Yan X-T, Oakes J E, Lausch R L. Protective antibody therapy is associated with reduced chemokine transcripts in herpes simplex virus type 1 corneal infection. J Virol. 1996;70:1277–1281. doi: 10.1128/jvi.70.2.1277-1281.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang Q, Hendricks R L. Interferon gamma regulates platelet endothelial cell adhesion molecule 1 expression and neutrophil infiltration into herpes simplex virus-infected mouse corneas. J Exp Med. 1996;184:1435–1447. doi: 10.1084/jem.184.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taub D D, Conlon K, Lloyd A R, Oppenheim J J, Kelvin D J. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 33.Taub D D, Oppenheim J J. Chemokines, inflammation and the immune system. Ther Immunol. 1994;1:229–246. [PubMed] [Google Scholar]
- 34.Tumpey T M, Chen S-H, Oakes J E, Lausch R N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumpey T M, Elner V M, Chen S-H, Oakes J E, Lausch R N. Interleukin-10 treatment can suppress stromal keratitis induced by herpes stromal virus type 1. J Immunol. 1994;153:2258–2265. [PubMed] [Google Scholar]
- 36.Wang H-M, Sheu M M, Stulting R D, Kaplan H J. Immuno-histochemical evaluation of murine HSV-1 keratouveitis. Curr Eye Res. 1989;8:37–46. doi: 10.3109/02713688909013892. [DOI] [PubMed] [Google Scholar]
- 37.Widmer U, Manogue K R, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1α, and MIP-1β, members of the chemokine superfamily of proinflammatory cytokines. J Immunol. 1993;150:4996–5012. [PubMed] [Google Scholar]
- 38.Wolpe S D, Cerami A. Macrophage inflammatory proteins 1 and 2; members of a novel superfamily of cytokines. FASEB J. 1989;3:2565–2573. doi: 10.1096/fasebj.3.14.2687068. [DOI] [PubMed] [Google Scholar]
- 39.Wolpe S D, Davatelis G, Sherry B, Beutler B, Hesse D G, Nguyen H T, Moldawer L L, Nathan C F, Lowery S F, Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988;167:570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolpe S D, Sherry B, Juers D, Davatelis G, Yurt R W, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu X, Dolecki G J, Sherry B, Zagorski J, Lefkowith J B. Chemokines are expressed in a myeloid cell-dependent fashion and mediate distinct functions in immune complex glomerulonephritis in rat. J Immunol. 1997;158:3917–3924. [PubMed] [Google Scholar]