Abstract
Background:
Intraoperative identification of pulmonary nodules, particularly small lesions, can be challenging. We hypothesize that folate receptor-targeted intraoperative molecular imagining can be safe and improve localization of pulmonary nodules during resection.
Methods:
Twenty subjects with biopsy proven pulmonary adenocarcinomas were enrolled in a Phase I clinical trial to test the safety and feasibility of OTL38, a novel folate receptor-α (FRα) targeted optical contrast agent. During resection, tumors were imaged in situ and ex vivo and fluorescence was quantified. Resected specimen were analyzed to confirm diagnosis, and immunohistochemistry was utilized to quantify FRα expression. A multivariate analysis using clinical and tumor data was performed to determine variables impacting tumor fluorescence.
Results:
Of the 20 subjects, three Grade I adverse events were observed: all transient nausea/abdominal pain. All symptoms resolved after completing the infusion. Sixteen of 20 (80%) of subjects had tumors with in situ fluorescence with a mean tumor-to-background fluorescence level of 2.9 (IQR, 2.1 to 4.2). The remaining 4 subjects’ tumors fluoresced ex vivo. In situ fluorescence was dependent on depth from the pleural surface. Four subcentimeter nodules not identified on preoperative imaging were detected with intraoperative imaging.
Conclusions:
This Phase I trial provides preliminary evidence suggesting that folate receptor-targeted molecular imaging with OTL38 is safe, with tolerable Grade I toxicity. These data also suggest that OTL38 accumulates in known lung cancers and may improve identification of synchronous malignancies. Our group is initiating a five-center, Phase II study to better understand the clinical implications of intraoperative molecular imaging using OTL38.
Keywords: pulmonary adenocarcinoma, surgery, molecular imaging, folate receptor
Each year, nearly 80,000 patients undergo pulmonary resection for non-small cell lung cancer (NSCLC) in the United States [1]. Of those patients, 10–17% harbor synchronous or metachronous disease that is unidentified preoperatively [2–4]. During minimally invasive (VATS or robotic surgery) pulmonary resection, the surgeon’s ability to inspect the entire lung by palpation is limited, thus intraoperative detection of synchronous lesions may be challenging. Because consequences of an incomplete resection negatively impact survival [5], adjuncts to improve intraoperative disease identification are of importance.
Intraoperative molecular imaging (IMI) is a promising technique that can assist surgeons in localizing nodules during minimally invasive surgery. This approach incorporates systemic delivery of optical contrast agents which accumulate in tissues, thus allowing for real-time imaging using calibrated lighting and camera systems. IMI has been utilized for a variety of malignancies including brain tumors [6], head and neck cancers [7], ovarian cancer [8] and thymoma [9].
Our group has previously investigated several contrast agents for pulmonary adenocarcinomas in human trials [10–13]. Our initial experiences involved systemic delivery of the non-targeted, near-infrared (NIR) agent, indocyanine green (ICG) [14]. This approach was safe and capable of detecting nodules as small as 2mm. Although excellent sensitivity and depth of penetration was appreciated, specificity was lacking. To increase specificity, we explored IMI utilizing a visual-range folate receptor-α (FRα) targeted contrast agent, EC17 [12]. This target was chosen as greater than 85% of pulmonary adenocarcinomas display upregulated FRα expression [15, 16]. We found that EC17 was 100% accurate in identifying pulmonary adenocarcinomas. Unfortunately, IMI with EC17 was limited by autofluorescence and poor depth of penetration (common with visual-range agents).
In this report, we describe our initial experiences involving IMI with a novel optical contrast tracer, OTL38, in 20 patients undergoing pulmonary resection. OTL38 is a NIR agent that selectively targets the FRα [8], thus capitalizing on the advantages of both our previous NIR and targeted experiences. In this study, we sought to (1) evaluate safety and feasibility of IMI with OTL38 and (2) determine if OTL38 reproducibly accumulates in pulmonary adenocarcinomas and generates tumor fluorescence.
Material and Methods
Study Design
A Phase I clinical trial of IMI with OTL38 was approved by the University of Pennsylvania Institutional Review Board. Primary objectives of this study were to assess safety and feasibility of IMI with OTL38. Secondary objectives included determination of OTL38 accumulation within pulmonary adenocarcinomas and assessment of in vivo and ex vivo tumor fluorescence. As part of this study, 20 consecutive subjects were enrolled. Sample size selection was based on our group’s previous experiences with NIR and folate receptor-targeted IMI [10, 12].
Included subjects had a preliminary diagnosis of pulmonary adenocarcinoma based on preoperative endobronchial ultrasound (EBUS) guided fine-needle aspiration or transthoracic needle aspiration (TTNA). Based on limitations in depth of detection with NIR imaging, only patients with lesions within 3cm of the pleural surface on preoperative imaging were enrolled [14]. Complete Inclusion/Exclusion Criteria are listed in Supplemental Table 1.
Subjects provided informed consent and were recruited between July 2015 and February 2016. Subjects underwent preoperative CT and PET-CT,which was reviewed by a thoracic radiologistAll subjects were scheduled for a VATS pulmonary lobectomy.
Study participants received intravenous OTL38 (0.025mg/kg) 3 to 6 hours prior to resection. OTL38 delivery parameters were based on previous human [17] and canine data [18].Surgeons utilized standard white-light thoracoscopy and finger palpation to confirm known nodules and assess for synchronous disease. Next, IMI was used to determine fluorescence of known nodules and determine if additional lesions were present. All lesions were resected. All specimens were imaged ex vivo prior to pathologic examination (See Figure 1 for Overview).
Figure 1:
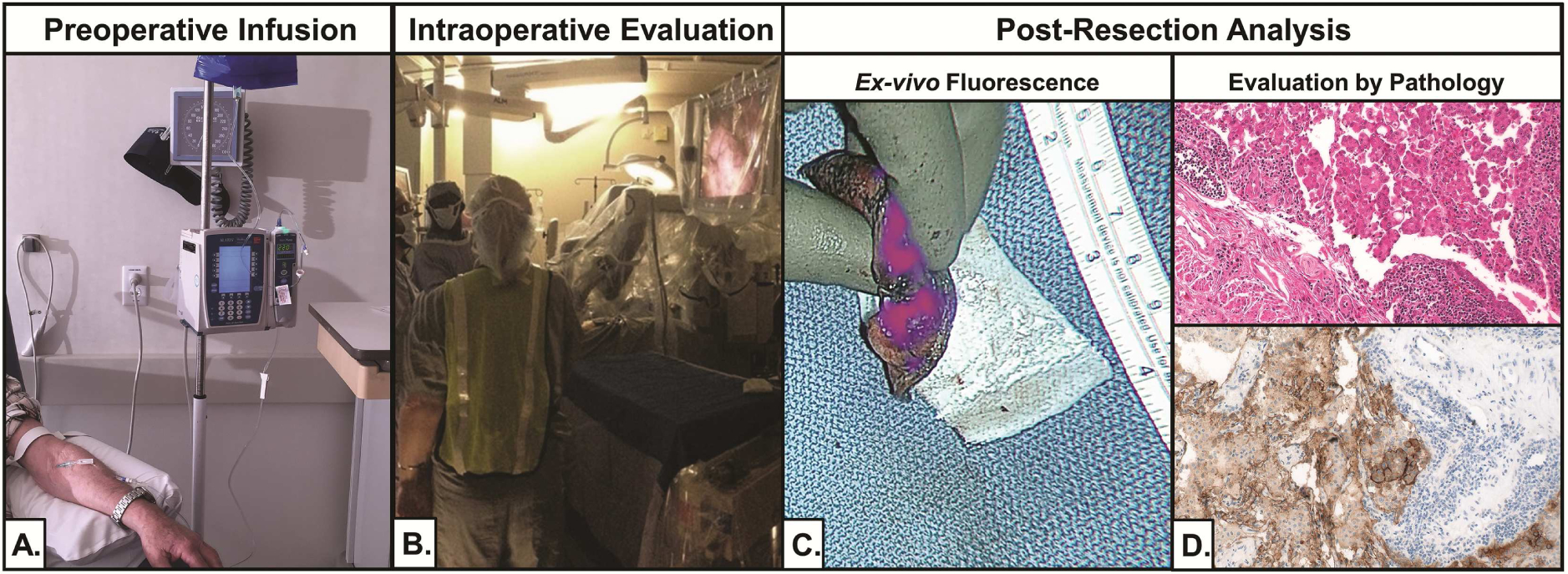
Trial workflow. (A) Subjects were infused with OTL38 4 hours before resection. (B) During resection, real-time IMI was performed using a 5mm thoracoscope. (C) After resection, suspicious nodules were bisected and imaged ex vivo. (D) All specimen were reviewed by a pathologist.
Safety was assessed by periodic subject evaluation from infusion to postoperative follow-up (2–4 weeks following resection). Routine laboratory studies were obtained at post-operative day one or if indicated. Adverse events were described using Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03 [19].
Study Drug
OTL38 (C61H63N9Na4O17S4; molecular weight: 1414.42Da) is a folate analogue conjugated to an NIR fluorescent dye. OTL38 maximally excites at a wavelength of 774–776nm and has a peak emission of 794–796nm [17]. OTL38 (>96% purity) was obtained from On Target Laboratories (West Lafayette, IN, USA). OTL38 was synthesized at Aptuit in compliance with Good Manufacturing Practices. For drug delivery, OTL38 was diluted in 20–220mL of normal saline or 5%-dextrose and infused over 60 minutes.
Imaging Device
In situ, real-time fluorescent imaging was performed using an Iridium® system optimized for detection of OTL38 (Visionsense Corp, Philadelphia, PA) (Supplemental Figure 1a). The Iridium® is a high definition, dual band (white light and NIR) camera system capable of NIR emission and detection. An 785nm excitation source was utilized, and fluorescence was detected using a bandpass filter ranging from 800–835nm. During resection, the Iridium® was equipped with a 5mm, 0-degree thoracoscope (Supplemental Figure 1b). For ex vivo evaluation, a free-standing exoscope was utilized (Supplemental Figure 1c).
Immunofluorescent and Immunohistochemical Microscopy
Excised specimens were examined by a pulmonary pathologist. FRα immunohistochemistry (IHC) staining was performed using the monoclonal antibody, 26B3.F2 (Morphotek, PA). For IHC, human kidney and skeletal muscle were used as positive and negative controls, respectively.
Statistical Analysis
Fluorescence was quantified using region of interest (ROI) software within ImageJ (http://rsb.info.nih.gov/ij). Tumor-to-background fluorescence ratio (TBR) was calculated for all lesions. Data are presented as mean(IQR) unless noted. Given the small sample size, differences were compared by the Mann-Whitney test. Multivariate models were made using linear regression. Comparisons were made use Stata: Release 14 (College Station, TX: StataCorp LP). A p-value of 0.05 was considered significant.
Results
Subject Characteristics and Safety Data
Between July 2015 and February 2016, 20 subjects (n=9 male) with a mean age of 66.8 years (IQR, 59.0 and 75.5 years) were enrolled after meeting inclusion criteria. Except for Subject 20, all subjects had a diagnosis suggestive of pulmonary adenocarcinoma as determined by preoperative tissue biopsy. Subject 20 had two right upper lobe nodules, one with a preoperative diagnosis of suggestive of adenocarcinoma and the other of an unknown histology. Preoperative PET-CT was obtained in all subjects and mean tumor size was 3.6cm (IQR, 2.1 to 4.6cm) with mean standard uptake value (SUV) of 6.9 (IQR, 2.8 to 10.0).
On final pathology, preoperatively identified nodules were confirmed to be invasive pulmonary adenocarcinomas (n=15), invasive mucinous adenocarcinomas (n=3), minimally invasive adenocarcinomas (n=1), adenosquamous carcinomas (n=1) or adenocarcinomas in situ (n=1). Of the 15 invasive pulmonary adenocarcinomas, 6 were poorly differentiated and 9 were moderately differentiated. Pulmonary adenocarcinoma subtypes included: 5 papillary, 4 acinar, 3 solid, 2 mixed and 1 lepidic subtypes. Both minimally invasive adenocarcinomas were well differentiated tumors with lepidic patterns (Table 1). A full summary of patient characteristics and tumor pathology is described in Table 1.
Table 1:
Subject Characteristics
| ID | Age (years) | Gender | Tumor Location | Final Pathology (Grade, Histology) | Maximum Tumor Diameter (cm) | Standard Uptake Value |
|---|---|---|---|---|---|---|
| 1 | 55 | F | RLL | Well Differentiated, Invasive Mucinous AdenoCA | 8.5 | 11.6 |
| 2 | 67 | F | LUL | Poorly Differentiated, Solid predominant AdenoCA | 1.2 | 3.5 |
| 3 | 53 | F | LLL | Poorly Differentiated, Acinar predominant AdenoCA | 3.5 | 2.9 |
| 4 | 65 | M | RUL | Poorly Differentiated, Papillary predominant AdenoCA | 10.5 | 15.3 |
| 5 | 79 | F | LLL | Well-Differentiated, Invasive Mucinous AdenoCA | 1.0 | 10.7 |
| 6 | 77 | F | RLL | Moderately Differentiated, Papillary predominant AdenoCA | 3.0 | 9.4 |
| 7 | 79 | M | RUL | Moderately Differentiated, Papillary predominant AdenoCA | 4.3 | 2.8 |
| 8 | 58 | M | RML | Moderately Differentiated, Mixed Lepidic and Acinar AdenoCA | 1.7 | 2.7 |
| 9 | 65 | M | LUL | Poorly Differentiated, Acinar predominant AdenoCA | 2.5 | 4.1 |
| 10 | 78 | F | RUL | Moderately Differentiated, Acinar predominant AdenoCA | 2.2 | 1.2 |
| 11 | 77 | M | RLL | Well Differentiated, Papillary predominant AdenoCA | 6.5 | 7.5 |
| 12 | 56 | F | LUL | Moderately Differentiated, Papillary predominant AdenoCA | 3.6 | 6.2 |
| 13 | 66 | M | RLL | Moderately Differentiated, Adenosquamous Carcinoma | 9.6 | 15.3 |
| 14 | 64 | F | RUL | Moderately Differentiated, Solid predominant AdenoCA | 2.1 | 3.3 |
| 15 | 58 | M | LUL | Moderately Differentiated, Acinar predominant AdenoCA | 2.3 | 4.2 |
| 16 | 60 | F | LLL | Moderately Differentiated, Lepidic predominant AdenoCA | 2.7 | 18.5 |
| 17 | 64 | M | RUL | Poorly Differentiated, Solid predominant AdenoCA | 2.1 | avid* |
| 18 | 69 | F | RLL | Poorly Differentiated, Mixed Acinar with Solid AdenoCA | 2.8 | 1.0 |
| 19 | 71 | F | LUL | Well Differentiated, Invasive Mucinous AdenoCA | 0.8 | 8.6 |
| 20** | 74 | M | RUL | Well Differentiated, Minimally Invasive AdenoCA with lepidic predominance | 1.2 | 8.6 |
M-Male; F-Female; RUL-Right Upper Lobe; RML-Right Middle Low; RLL-Right Lower Lobe; LUL-Left Upper Lobe; LLL-Left Lower Lobe; AdenoCa-Pulmonary Adenocarcinoma
records from other hospital, unable to quantify
Subject with two RUL nodules. The larger was suspicious for adenocarcinoma based on biopsy. The second nodule was not biopsied.
Subjects received an average of 2.00mg (IQR, 1.71 to 2.25mg) of the study drug 3.6 hours (IQR, 2.8 to 4.5 hours) prior to resection (Table 2). Three Grade I Adverse Events were noted. The three subjects reported transient nausea during drug infusion. Symptoms improved after slowing infusion rate and completely resolved after delivery completed. No laboratory or exam abnormalities were noted after drug infusion or postoperatively. No intraoperative or postoperative drug-related events were noted. Lastly, no long-term sequalae have been observed after 1 year of follow-up.
Table 2:
Toxicology Data
| ID | Weight (kg) | OTL38 given (mg) | Adverse Event* | Time from Infusion to Imaging (hours) |
|---|---|---|---|---|
| 1 | 77.9 | 1.95 | 2.80 | |
| 2 | 72.8 | 1.82 | 5.77 | |
| 3 | 46.0 | 1.15 | 2.28 | |
| 4 | 68.5 | 1.71 | 7.18 | |
| 5 | 62.8 | 1.57 | Nausea** (Grade 1) | 3.00 |
| 6 | 60.3 | 1.51 | 4.53 | |
| 7 | 86.4 | 2.42 | 3.48 | |
| 8 | 113.9 | 2.85 | 4.56 | |
| 9 | 82.6 | 2.07 | 3.81 | |
| 10 | 59.0 | 1.48 | 6.00 | |
| 11 | 89.8 | 2.25 | 3.00 | |
| 12 | 55.8 | 1.40 | Nausea** (Grade 1) | 3.00 |
| 13 | 72.9 | 1.82 | Nausea** (Grade 1) | 6.58 |
| 14 | 83.0 | 2.08 | 3.67 | |
| 15 | 69.4 | 1.74 | 2.68 | |
| 16 | 82.3 | 2.06 | 3.77 | |
| 17 | 131.5 | 3.29 | 4.03 | |
| 18 | 100.2 | 2.55 | 2.38 | |
| 19 | 103.4 | 2.64 | 3.28 | |
| 20 | 74.4 | 1.86 | 2.30 |
Adverse Events graded using the Common Terminology Criteria for Adverse Events (Version 4.03) [19]
Transient nausea during infusion.
OTL38 Accumulates in FRα-High Tissues and Allows for in situ Localization
In situ fluorescence was appreciated through the pleural surface in 16 of 20 subjects (80%). Seventeen of 21 (81%) preoperatively identified nodules were localized (Figure 2) by NIR imaging. The mean TBR of fluorescent nodules was 2.9 (IQR 2.1 to 4.2).
Figure 2:
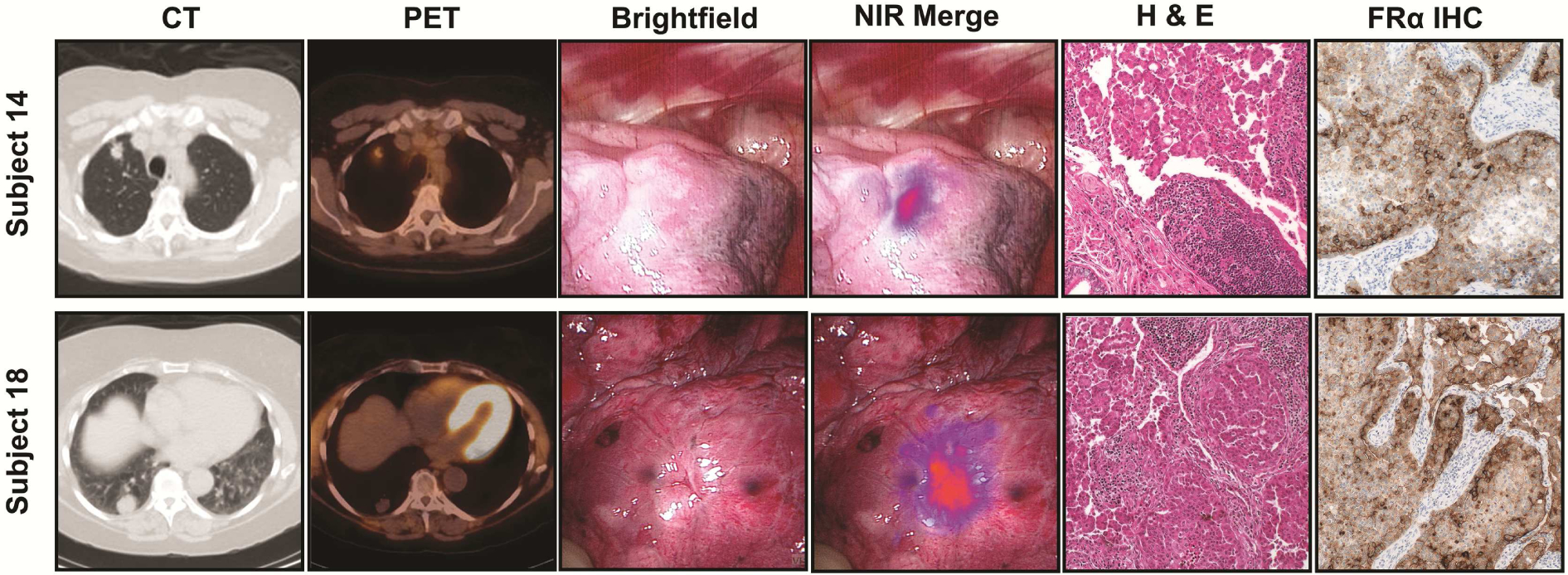
OTL38 accumulates in pulmonary adenocarcinoma and generates fluorescence. Representative Subjects (Subject 14 and 16) displaying in situ fluorescence. Subjects underwent preoperative (A) CT and (B) PET. Intraoperative (C) Brightfield and (D) NIR Merge images. Specimen were reviewed by (E) H&E and (F) FRα immunohistochemistry.
Ex vivo TBR after nodule bisection was 3.7 (IQR 2.7 to 4.4); which was similar to in situ TBR (p>0.05). In all cases, fluorescent specimens were confirmed to be invasive pulmonary adenocarcinoma or adenocarcinoma spectrum lesions on final pathology (Table 1, Figure 2). Lastly, elevated FRα expression within all tumors was confirmed by immunohistochemistry (Figure 2).
To understand factors associated with in situ fluorescence, several variables were evaluated in a multivariate model: tumor size, time from injection to imaging, preoperative SUV, total amount of drug given and depth of tumor (Table 3). Only depth of tumor from pleural surface influenced in situ fluorescence; (p=0.02). Other variables did not significantly influence in situ fluorescence.
Table 3:
Multivariate Model Predicting in situ Fluorescence
| Characteristic | Coefficient | 95% Confidence Interval | p-value |
|---|---|---|---|
| Depth of Nodule (cm) | −0.96 | −1.78 to −0.12 | 0.02 |
| Nodule size (cm) | 0.09 | −0.15 to 0.33 | 0.44 |
| Standard Uptake Value by PET | −0.03 | −0.15 to 0.09 | 0.61 |
| Time to Imaging (hours) | −0.12 | −0.55 to 0.91 | 0.55 |
| Amount of drug given (mg) | 0.58 | −0.69 to 1.82 | 0.35 |
Near-Infrared Localization Is Limited by Depth of Penetration
Four subjects (2, 9, 13 and 17) had limited tumor fluorescence during in situ imaging. Compared to tumors displaying in situ fluorescence, non-fluorescent tumors were deeper (1.86cm versus 0.39cm; p=0.009) (Table 3). In fact, no tumor deeper than 2cm displayed in situ fluorescence. Time to imaging, tumor size, preoperative SUV and total drug administered were similar among those subjects with fluorescent and non-fluorescent in situ tumors (Table 4).
Table 4:
Characteristics of Nodules Displaying in situ Fluorescence versus Nodules Without in situ Fluorescence
| Characteristic | in situ Fluorescent (n=17) | in situ Non-Fluorescent (n=4) | p-value |
|---|---|---|---|
| Tumor Size (cm) | 3.52 | 4.73 | 0.83 |
| SUV | 6.90 | 7.90 | 0.46 |
| Time to Imaging (hours) | 3.77 | 4.80 | 0.12 |
| Distance from pleural surface (cm) | 0.39 | 1.86 | 0.009 |
| Total OTL38 administered (mg) | 1.94 | 2.39 | 0.28 |
To determine if OTL38 accumulated within deeper tumors, ex vivo fluorescence was assessed after tumor bisection (Figure 3). In all four subjects, tumors displayed fluorescence with a mean TBR of 2.8 (range 2.2 to 3.2); this was similar to the ex vivo fluorescence of those tumors that also displayed in situ fluorescence (p=0.64). Three of these tumors were found to be invasive pulmonary adenocarcinomas, while the fourth was an adenosquamous carcinoma. Elevated FRα expression was confirmed in each tumor by immunohistochemistry (Figure 3).
Figure 3:
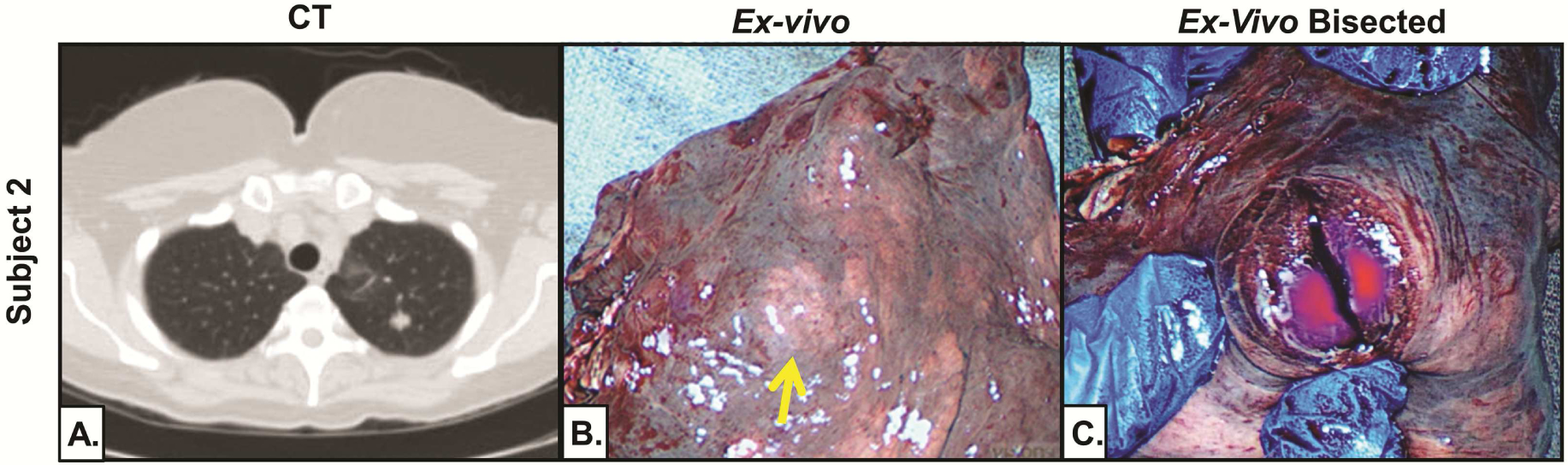
Deep tumors display fluorescence upon bisection. Representative subject (Subject 2) demonstrating ex vivo fluorescence after tumor bisection. (A) Preoperative CT demonstrating tumor 1.6cm from pleural surface. (B) Ex vivo NIR imaging with no tumor fluorescence. (C) Ex vivo NIR imaging with fluorescence after bisection.
These results suggest all preoperatively identified pulmonary adenocarcinomas and adenocarcinoma spectrum lesions accumulate OTL38.
IMI with OTL38 Identifies Otherwise Undetectable Nodules
Two subjects were had additional nodules that were undetectable by preoperative imaging or intraoperative palpation/visualization. First, Subject 8 was a 58-year-old male with a history of papillary thyroid cancer who presented with a right upper lobe adenocarcinoma. During ex vivo fluorescence evaluation, a 0.1cm papillary thyroid metastasis was identified.
Next, Subject 19 presented with a left upper lobe adenocarcinoma. In addition to the known nodule, three left lower lobe nodules (0.8cm, 0.6cm and 0.1cm) were identified during in situ molecular imaging (Figure 4). These 3 nodules were found to be invasive pulmonary adenocarcinomas by frozen-section analysis. (Figure 4). Identification of these nodules upstaged this patient Stage IA(T1N0) to Stage IIIA(T4N0).
Figure 4.
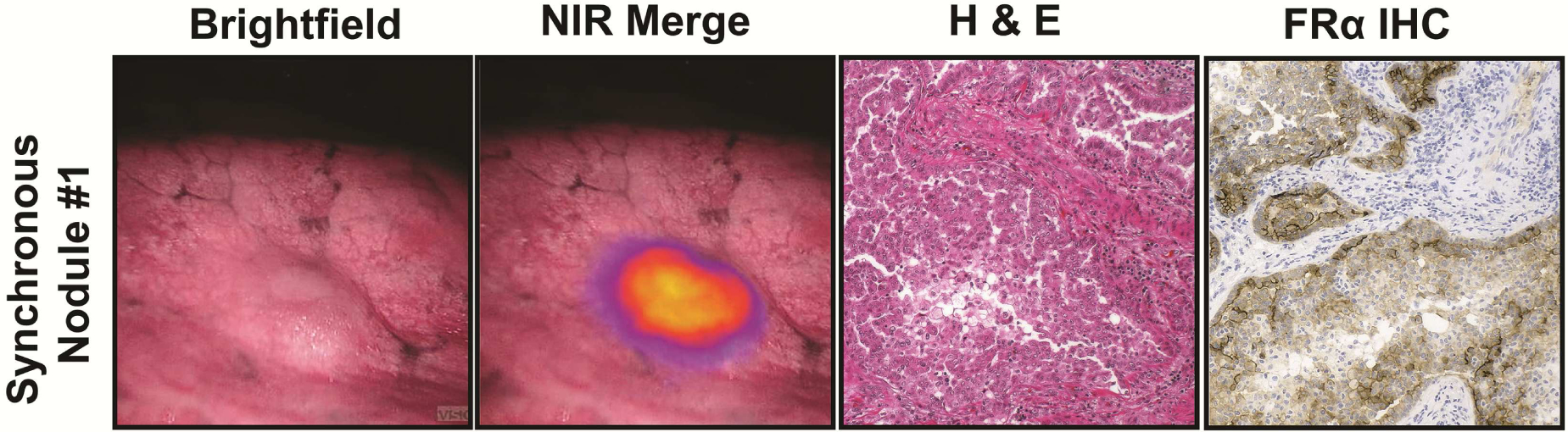
IMI with OTL38 identifies synchronous disease. In Subject 19, synchronous nodules were detected using IMI. A representative nodule is displayed in (A) brightfield and (B) NIR Merge view. The nodule was pulmonary adenocarcinoma by H&E (C) with FRα expression (D).
Comment
Localization of pulmonary nodules during VATS or robotic surgery can be challenging, and utilization of intraoperative molecular imaging may provide a safe and effective approach to assist the thoracic surgeon. In this study, we provide preliminary evidence suggesting that the FRα-targeted, NIR contrast agent, OTL38, (1) is safe, (2) effectively accumulates in pulmonary adenocarcinomas, and (3) identifies subcentimeter pulmonary nodules that may otherwise be challenging to locate. This data is the basis of a several ongoing studies testing this approach in less controlled circumstances.
OTL38 was conceived to maximize benefits of the alternative imaging agents currently under Phase I/II evaluation. OTL38 incorporates highly specific FRα-targeted binding as previously observed with EC17 (folate-FITC) [12]. FRα is an appealing target as its upregulation is found in many malignancies, including pulmonary adenocarcinomas [16]. Unlike EC17, folate is linked to S0456 (a commercially available NIR dye) in OTL38 [23]. NIR imaging agents have advantages over visual-range agents including minimal autofluorescence and improved depth of penetration [10].
In our study, we found IMI with OTL38 to be safe. We observed that only 15% (3 of 20) of subjects experienced an adverse event. All were transient Grade I events of transient nausea occurring during infusion. All symptoms resolved by after completion of the infusion. After modifying our protocol to include empiric diphenhydramine, no additional adverse events were noted during drug infusion. Furthermore, no intraoperative or postoperative toxicity was noted.
OTL38’s low toxicity profile echoes safety observed with other IMI agents being evaluated in Phase I/II trials. For example, 5-aminoleuvolinic acid [20], BLZ-100, Folate-FITC [12], Bevacizumab-IRDye800 [21] and Cetuximab-IRDye800 [7] have displayed almost exclusively Grade I/II toxicities. This provides important patient safety advantages over other localization techniques such as percutaneous wire placement, lymph node mapping and fluoroscopy [22]. One additional advantage of IMI with OTL38 is the ability to deliver the contrast agent a several hours prior to surgery. In contrast, most alternative targeted fluorophores require infusion days prior to surgery which poses obvious logistic hurdles.
In addition to safety, we found that this approach was feasible and required only minor modifications to standard operating room work flow. Subjects presented several hours prior to resection. Once checked-in, subjects received OTL38 within the preoperative holding area. Following delivery, subjects were monitored for toxicity while completing preoperative checklist requirements. During resection, patients were imaged with standard thoracoscopic instruments which were modified to include NIR cameras/light sources. Real-time videos were displayed with fluorescent overlays on standard operating monitors. Overall, imaging added between 5 to 10 minutes to cases as previously noted [12].
100% of preoperatively identified malignancies accumulated OTL38 and displayed fluorescence. In this study, as well as in our previous experiences involving Folate-FITC, we observed signal in greater than 95% of pulmonary adenocarcinomas [12]. Higher than anticipated rates of fluorescence may be due to random error from a small sample size. Alternatively, this may be due to FRβ-expressing tumor associated macrophages, which also bind OTL38 [24]. These results need confirmation in larger studies.
In this study, IMI with OTL38 localized 100% of nodules measuring 1.2cm or less. This trend is encouraging as failure rates of subcentimeter nodule localization during VATS lobectomy approach 60% in some series [25]. These findings also provide preliminary data suggesting that IMI may improve localization of subcentimeter pulmonary nodules or GGOs that harbor malignancy. Further, real-time fluorescent feedback may provide useful information to improve intraoperative margin assessment.
IMI also identified four subcentimeter nodules that were unknown to the surgeon by preoperative imaging and intraoperative assessment. Three invasive pulmonary adenocarcinomas (0.1cm, 0.6cm and 0.8cm) were identified in Subject 19 and one papillary thyroid cancer metastasis (0.1cm) was found in Subject 8. In these subjects, identification of these nodules impacted intraoperative clinical decisions and postoperative therapy. This detection rate echoes previous reports which suggest that 8% of NSCLC patients harbor synchronous disease that is routinely missed during minimally invasive lobectomy [2].
There are several limitations to our study. First, the enrollment population includes only subjects with biopsies suspicious for pulmonary adenocarcinoma. This cohort was chosen to assess the ability of OTL38 to accumulate in FRα expressing tumors. Although encouraging, this data is not applicable to other NSCLC histologies where FRα expression is unknown. Next, given the exploratory nature of this Phase I study, there is no control group to directly assess the impact of IMI with OTL38. With that being stated, we found this technology helpful with both (1) localization of subcentimeter nodules and (2) identification of additional malignancies. More definitive conclusions lie beyond the scope of this Phase I/Feasibility study and will need to be evaluated in larger trials.
More generally, additional hurdles to IMI within the human thorax remain. The first is challenge involves “depth of penetration”. The use of a NIR fluorophore in OTL38 improved our depth of detection as compared to EC17 [12]; however, detection of nodules deeper than 2cm was unreliable. Additional techniques, such as photoacoustic imaging, may provide additional approaches to detect deeper nodules and are currently being investigated in the setting of metastatic melanoma [25]. A second limitation to IMI in the human thorax involves the assessment of nodal disease. Unfortunately, lymph node detection with optical imaging agents is unreliable. This is a result of the macrophage’s to propensity to phagocytose contrast agents and traffic to lymph nodes. Additionally, macrophages upregulate the FRβ which also binds OTL38 [23]. Both factors contribute to high “false positive” rates.
Despite limitations posed by a Phase I study, these data set the stage for additional studies which can better define how IMI with OTL38 improves upon standard treatment protocols. Our group is currently expanding our evaluation of OTL38 in the form a registration trial (NCT02769156) evaluating this drug in patients with ground-glass opacities and solitary pulmonary nodules (patients do not need a preoperative diagnosis of pulmonary adenocarcinoma). Additionally, we are preparing to begin a multi-center Phase II trial (NCT02872701) involving OTL38 which will mark the United States’ first trial of this size involving IMI. These additional investigations with OTL38 will allow us to more accurately assess the value for pulmonary adenocarcinomas and also gauge the utility for other NSCLC histologies in which FRα expression is unknown.
Supplementary Material
Abbreviations:
- AE
adverse event
- BMP
basic metabolic panel
- CBC
complete blood count
- CT
computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- EBUS
endobronchial ultrasound
- FITC
Fluorescein isothiocyanate
- FRα
folate receptor alpha
- FRβ
folate receptor beta
- FNA
fine needle aspiration
- GGO
ground glass opacity
- H&E
hematoxylin and eosin
- ICG
indocyanine green
- IHC
immunohistochemistry
- IMI
intraoperative molecular imaging
- IQR
interquartile range
- LFTs
liver function tests
- NaCl
sodium chloride
- NIR
near infrared
- NSCLC
non-small cell lung cancer
- PET
positron emission tomography
- ROI
region of interest
- SUV
standardized uptake value
- TBR
tumor to background fluorescence ratio
- TTNA
transthoracic needle aspiration
- VATS
video assisted thoracic surgery
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Predina JD, et al. , Clinical implications of positive margins following non-small cell lung cancer surgery. J Surg Oncol, 2016. 113(3): p. 264–9. [DOI] [PubMed] [Google Scholar]
- 2.Cerfolio RJ and Bryant AS, Is palpation of the nonresected pulmonary lobe(s) required for patients with non-small cell lung cancer? A prospective study. J Thorac Cardiovasc Surg, 2008. 135(2): p. 261–8. [DOI] [PubMed] [Google Scholar]
- 3.Ellis MC, et al. , Comparison of pulmonary nodule detection rates between preoperative CT imaging and intraoperative lung palpation. Am J Surg, 2011. 201(5): p. 619–22. [DOI] [PubMed] [Google Scholar]
- 4.Cerfolio RJ, McCarty T, and Bryant AS, Non-imaged pulmonary nodules discovered during thoracotomy for metastasectomy by lung palpation. Eur J Cardiothorac Surg, 2009. 35(5): p. 786–91; discussion 791. [DOI] [PubMed] [Google Scholar]
- 5.Aliperti LA, et al. , Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol, 2011. 18(3): p. 603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marbacher S, et al. , Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus, 2014. 36(2): p. E10. [DOI] [PubMed] [Google Scholar]
- 7.Rosenthal EL, et al. , Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res, 2015. 21(16): p. 3658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoogstins CE, et al. , A Novel Tumor-Specific Agent for Intraoperative Near-Infrared Fluorescence Imaging: A Translational Study in Healthy Volunteers and Patients with Ovarian Cancer. Clin Cancer Res, 2016. 22(12): p. 2929–38. [DOI] [PubMed] [Google Scholar]
- 9.Keating JJ, et al. , Intraoperative imaging identifies thymoma margins following neoadjuvant chemotherapy. Oncotarget, 2016. 7(3): p. 3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keating J, et al. , Near-Infrared Intraoperative Molecular Imaging Can Locate Metastases to the Lung. Ann Thorac Surg, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keating JJ, Kennedy GT, and Singhal S, Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg, 2015. 149(3): p. e51–3. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy GT, et al. , The Optical Biopsy: A Novel Technique for Rapid Intraoperative Diagnosis of Primary Pulmonary Adenocarcinomas. Ann Surg, 2015. 262(4): p. 602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okusanya OT, et al. , Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg, 2015. 150(1): p. 28–35 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okusanya OT, et al. , Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg, 2014. 98(4): p. 1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Shannessy DJ, et al. , Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget, 2012. 3(4): p. 414–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker N, et al. , Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem, 2005. 338(2): p. 284–93. [DOI] [PubMed] [Google Scholar]
- 17.van Dam GM, et al. , Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med, 2011. 17(10): p. 1315–9. [DOI] [PubMed] [Google Scholar]
- 18.Keating JJ, et al. , Intraoperative near-infrared fluorescence imaging targeting folate receptors identifies lung cancer in a large-animal model. Cancer, 2017. 123(6): p. 1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.SERVICES, U.S.D.O.H.A.H., Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2010.
- 20.Stummer W, et al. , Counterbalancing risks and gains from extended resections in malignant glioma surgery: a supplemental analysis from the randomized 5-aminolevulinic acid glioma resection study. Clinical article. J Neurosurg, 2011. 114(3): p. 613–23. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, et al. , Threshold analysis and biodistribution of fluorescently labeled bevacizumab in human breast cancer. Cancer Res, 2016. [DOI] [PubMed] [Google Scholar]
- 22.Keating J and Singhal S, Novel Methods of Intraoperative Localization and Margin Assessment of Pulmonary Nodules. Semin Thorac Cardiovasc Surg, 2016. 28(1): p. 127–36. [DOI] [PubMed] [Google Scholar]
- 23.Mahalingam S K. S; Roy J; Low P, Evaluation of pteroyl-amino acid-NIR dye conjugates for tumor targeted fluorescence guided surgery, in 246th American Chemical Society National Meeting and Exposition. 2013: Indianapolis, IN, USA. [Google Scholar]
- 24.Shen J, et al. , Assessment of folate receptor-beta expression in human neoplastic tissues. Oncotarget, 2015. 6(16): p. 14700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki K, et al. , Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest, 1999. 115(2): p. 563–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


