Abstract
Primary mucinous ovarian neoplasms, gastrointestinal-type (GI-type), are composed of mucin-producing tumor cells resembling intestinal goblet cells or gastric foveolar epithelium. In contrast to seromucinous tumors, which exhibit endocervical-type mucinous differentiation and are thought to be derived from endometriosis, the cell/tissue-of-origin of most GI-type mucinous ovarian tumors is unknown. We identified 8 GI-type mucinous ovarian tumors (cystadenomas, n=4; borderline tumor/carcinoma, n=4) with spatially distinct areas that showed morphologic features of Mullerian-type epithelial differentiation (ciliated cells or endometrioid-type glands). Immunohistochemistry for cell lineage markers and Alcian Blue (pH 2.5)/Periodic Acid-Schiff (AB/PAS) staining were performed. Morphologically distinct components were isolated by microdissection, from which extracted DNA was analyzed by targeted next generation sequencing. In all cases, immunohistochemistry demonstrated mucin-producing cells to be positive for at least one GI marker (CK20 or CDX2), while areas with morphologic features of Mullerian differentiation were positive for PAX8, ER and/or PR, and lacked expression of CK20 and CDX2; CK7 was strongly and diffusely positive in all tumor cells. Tumor cells with a gastric-type phenotype produced neutral mucin, while acidic mucin was present within intestinal-type goblet cells. Targeted sequencing revealed ARID1A mutations in all mixed borderline tumors/carcinomas (n=4); other recurrent genetic alterations included KRAS (n=2) and TP53 mutations (n=2). Shared mutations were present in paired Mullerian and GI-type mucinous tumor components in 4 mixed borderline tumors/carcinomas, with more shared mutations between components than private mutations specific to each component. All mixed borderline tumors/carcinomas were associated with endometriosis (n=3) or Mullerian inclusion cysts (n=1); mutation or loss of ARID1A expression was seen in these putative precursor lesions in 2 cases. Hence, ovarian neoplasms composed of clonally related GI-type mucinous and Mullerian-type epithelial components harbor ARID1A mutations and are frequently associated with endometriosis. The existence of a Mullerian stem/progenitor cell with the capacity to differentiate towards cell lineages within the GI-tract may be involved in the pathogenesis of at least a subset of GI-type mucinous ovarian neoplasms.
Keywords: Gastro-intestinal type mucinous ovarian neoplasm, Mullerian carcinoma, Mixed ovarian tumor, transdifferentiation
Introduction
Primary mucinous ovarian tumors of gastrointestinal (GI)-type include cystadenoma, borderline tumor, and carcinoma, representing a stepwise sequence of ovarian mucinous carcinogenesis(1). These tumors are composed of mucin-producing epithelial cells lining the GI tract, including intestinal goblet cells, gastric foveolar-type epithelium, and occasional Paneth-like or neuroendocrine cells. By immunohistochemistry, they are often diffusely positive for CK7 with variable expression of CK20, and are negative for ER and PR. Those morphologically resembling lower GI tract neoplasms show more prominent expression of CK20 and CDX2, which are known markers of intestinal differentiation. Distinction from a metastatic mucinous carcinoma originating from the GI tract is a common diagnostic dilemma, given the overlapping morphologic and immunophenotypic features. Recent molecular genetic profiling studies(2, 3) have identified KRAS mutation and CDKN2A mutation/deletion, as the most common somatic alterations in mucinous borderline tumors and carcinomas, followed by ERBB2 amplification and TP53 mutations, the latter being enriched in carcinomas compared to borderline tumors; ARID1A mutations have also been reported in a minority of cases.
Ovarian epithelial tumors with endocervical-type mucinous differentiation represent a pathogenically distinct group from the GI-type(4). In addition to endocervical-type mucinous cells, they typically exhibit a variable admixture of cell types resident within the gynecologic tract, including serous-like, endometrioid, and non-specific cells with abundant eosinophilic cytoplasm. By immunohistochemistry, these tumors are diffusely positive for CK7, ER, PR and PAX8, while negative for CK20 and CDX2. They are thought to arise from endometriosis and frequently harbor ARID1A mutations(5). Formerly categorized as mucinous ovarian neoplasms of endocervical-type, the term “seromucinous” is currently used to describe the borderline tumors in this category, while the malignant tumors are now classified as a variant of endometrioid carcinomas(4). “Mullerian mucinous” is another proposed descriptor for this group of neoplasms(6).
Despite the controversy over diagnostic terminology, the Mullerian origin of seromucinous/endometrioid tumors, specifically from endometriosis, is undisputed(4, 5). In contrast, the cell/tissue-of-origin of most GI-type ovarian mucinous neoplasms remains elusive. In the present study, we report rare examples of GI-type mucinous ovarian tumors with co-existing Mullerian epithelial elements. Our morphologic, phenotypic and molecular analyses support the classification of a novel variant of mixed ovarian mucinous neoplasm and provides evidence which may be generalizable towards inferring the origin of at least a subset of GI-type mucinous ovarian tumors.
Materials and Methods
Case Selection
To identify mixed ovarian borderline tumors or carcinomas with a GI-type mucinous component co-existing with another epithelial ovarian tumor subtype, a database search was conducted using the search terms “endometrioid,” “serous,” “clear cell” or “Mullerian” and “mucinous” located anywhere within the diagnosis section of the pathology report. Slides were retrieved and reviewed by two gynecologic pathologists (MHC, LHE) following criteria set by the WHO 2020 Classification of Female Genital Tumors(7). Cases lacking morphologic evidence of GI-type mucinous differentiation (i.e. intestinal goblet cells or gastric-type foveolar epithelium) were excluded from further analysis.
Slide review identified 5 cases of mucinous borderline tumor or mucinous carcinoma of GI-type associated with a distinct cellular population showing Mullerian-type differentiation. One of these was subsequently excluded after molecular analyses revealed discordant mutational profiles between the morphologically distinct lesions. This was a mucinous borderline tumor associated with a benign seromucinous cystadenoma, but the lesions were spatially separated by ovarian stroma, without interconnection of their respective epithelium. The seromucinous cystadenoma was found to harbor a KRASG12D mutation, while the mucinous borderline tumor had a KRASG12V mutation, consistent with these being independent neoplastic processes, rather than components of a mixed tumor.
Four ovarian mucinous cystadenomas with morphologically distinct areas showing tubal-like differentiation, which were recently encountered during routine sign-out, were also included in the study. The final cohort was comprised of 8 cases (cystadenomas, n=4, borderline tumors or carcinomas, n=4).
Histochemistry and immunohistochemistry
Alcian blue (pH 2.5)/Periodic Acid-Schiff (AB/PAS) histochemical staining was performed using standard methods, to highlight intracellular mucin and to distinguish neutral (magenta) from acidic (blue) mucin by light microscopy.
The following primary antibodies were used for immunohistochemistry: CK7 (OV-TL-12/30, Dako, 1:1600), CK20 (KS20.8; Dako, 1:800), PAX8 (BC12; Cell Signaling, 1:50), CDX2 (CDX2–88; Biogenex, 1:100), ER (SP1; Ventana, pre-diluted), PR (1E2; Ventana, pre-diluted), MUC6 (CLH5; Novocastra, 1:100), CD10 (56C6; Vector, 1:50), WT1 (WT49; Leica, pre-diluted), ARID1A (HPA005456; Sigma, 1:400, 30’). All immunohistochemical stains were performed on the BOND RX platform (Leica), using the standard protocol, with BOND Epitope Retrieval Solution ER2 (Leica) for 30 minutes, incubation of primary antibody for 30 minutes at room temperature and BOND Polymer Refine Detection (Leica).
Next-generation sequencing and analysis
DNA was extracted from formalin-fixed paraffin embedded tissue sections of tumor and matched normal tissue for molecular analysis. For the borderline tumors and carcinomas (n=5), the spatially distinct mucinous and Mullerian areas were microdissected separately. For one cystadenoma (n=1), a single sample of epithelial lining composed of a mixture of cell types was analyzed. Targeted panel sequencing of matched tumor and normal DNA was performed using MSK-IMPACT, a hybridization capture-based next-generation sequencing assay targeting all exons and selected intronic regions of 505 cancer-related genes(8). Single nucleotide variants, insertions and deletions were detected using MuTect, Pindel and VarDict, as previously described (for more details, see https://github.com/mskcc/roslin-variant/wiki/Roslin-Methods-v2.5). Variants were annotated by OncoKB(9). Total and allele-specific copy number was estimated using FACETS(10).
Results
Mixed GI-type mucinous / Mullerian cystadenomas
We have recently encountered mucinous cystadenomas which focally exhibited stretches of non-mucin-producing, attenuated or columnar epithelium, which included ciliated cells (Cases Cy-1 to Cy-4; Figure 1). The mucin-producing cells, which were the predominant cell type, were typically columnar with foamy clear or slightly eosinophilic cytoplasm and distinct cell borders (Figure 1C). AB/PAS histochemical staining highlighted intracellular neutral mucin (magenta) within these cells, which also expressed the gastric mucin, MUC6 by immunohistochemistry. Previous studies have shown that gastric-type glands can be distinguished from normal endocervical glands by their production of neutral mucin (as opposed to acidic endocervical mucin) and expression of the gastric mucin, MUC6, which is typically absent in endocervical mucosa(11). In Cy-4, some glands were composed of intestinal-type goblet cells. In all 4 cases, the mucinous components were either negative for ER and PR (n=2) or only focally and weakly positive (n=2); focal expression was also observed for PAX8 (n=2), CDX2 (n=2) and CK20 (n=2), (Figure 1D-K). In contrast, in all cystadenomas, the ciliated epithelium expressed PAX8, ER and PR, but not CDX2 or CK20. Despite the presence of ciliated cells, WT-1 was negative. The morphologic and immunophenotypic features were consistent with mixed mucinous cystadenomas, predominantly GI-type, with areas exhibiting Mullerian epithelial differentiation. These lesions are distinguished from the more common seromucinous cystadenoma, which show endocervical mucinous differentiation. Notably, however, 1 case (Cy-4) appeared to exhibit both endocervical and GI-type mucinous components (Figure 1K).
Figure 1:
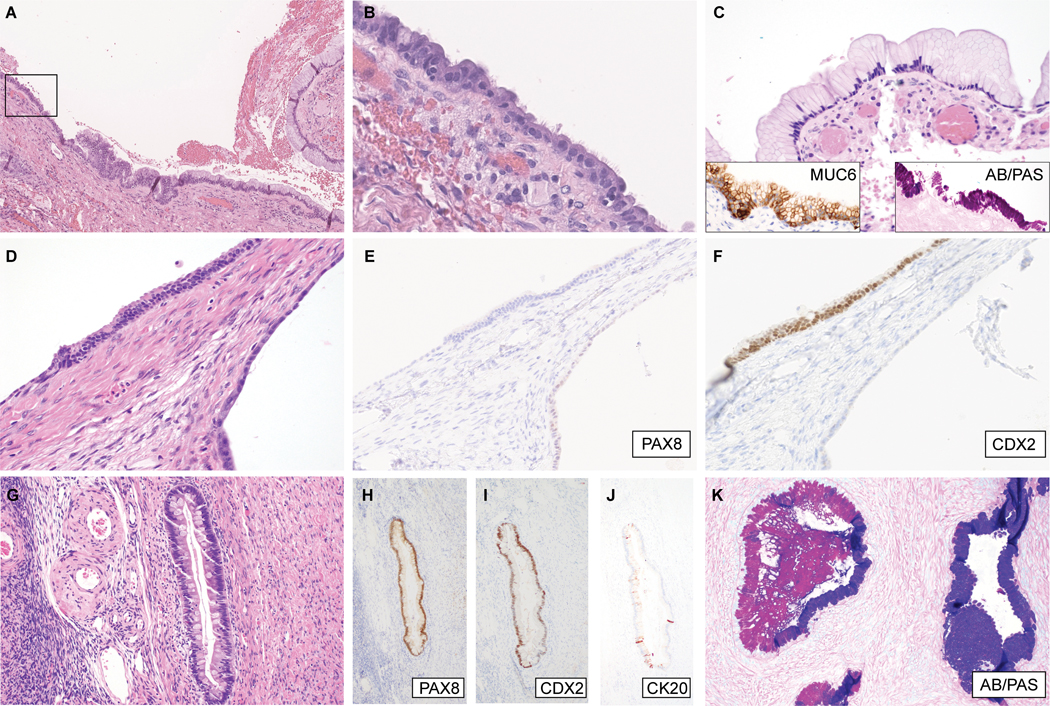
Mixed gastrointestinal-type mucinous / Mullerian cystadenomas. (A-C) Cy-1, (A) low-magnification image showing the spatial relationship of Mullerian and mucinous components; (B) High magnification of boxed area in (A), illustrating cuboidal/columnar cells with cilia; (C) another area showing gastric foveolar differentiation, with diffuse MUC6 expression (inset, left) and neutral mucin, staining magenta by AB/PAS histochemistry (inset, right). (D-F) Cy-3; (D) Cystic structure (right) lined by attenuated simple epithelium and an overlying stretch of columnar epithelium with Paneth-like cells at the surface. These foci show differential expression of (E) PAX8, and (F) CDX2, indicative of Mullerian and gastrointestinal differentiation, respectively. (G-K) Cy-4; (G) Within this cystadenoma, there are intestinal-type glands containing goblet cells and scattered neuroendocrine cells. The intestinal-type glandular epithelium is positive for (H) PAX8, (I) CDX2, and (J) CK20 (focal). (K) Other areas show mucinous glands exhibiting a hybrid gastric/endocervical phenotype, containing cells producing neutral and acidic mucin, staining magenta and blue, respectively, by AB/PAS histochemistry.
Immunohistochemistry for ARID1A showed intact expression in Cy-1, Cy-3, and Cy-4 but was uninterpretable in Cy-2 (lack of staining in stromal cells which served as internal control). Targeted sequencing was therefore performed on Cy-2, which did not reveal any pathogenic somatic alterations in ARID1A or other cancer genes.
Mixed ovarian borderline tumors and carcinomas with GI-type mucinous and Mullerian components
Clinicopathologic features
The mixed ovarian neoplasms (MO-1 to MO-4) were at least at the level of borderline tumor for both GI-type mucinous and Mullerian epithelial components (Table 1). The median age was 56; all tumors were unilateral and confined to the ovary, with only MO-4 showing ovarian surface involvement. The median length of clinical follow-up was 45 months (range: 6–54 months) and all patients were alive with no evidence of disease recurrence at last follow-up.
Table 1:
Clinicopathologic features of mixed ovarian borderline tumors and carcinomas with gastrointestinal-type mucinous and Mullerian epithelial components
| Case # | Age | Diagnosis | Stage | Endometriosis | Adjuvant Chemo | Clinical follow-up |
|---|---|---|---|---|---|---|
| MO-1 | 53 | Mixed GI-type mucinous / endometrioid carcinoma | IA | Not identified | Yes | NED (54 months) |
| MO-2 | 60 | Mixed GI-type mucinous / endometrioid borderline tumor | IA | Present | No | NED (36 months) |
| MO-3 | 74 | GI-type mucinous borderline tumor with intraepithelial carcinoma of Mullerian-type | IA | Present | No | NED (6 months) |
| MO-4 | 33 | High grade carcinoma with GI-type mucinous and Mullerian components | IC | Present | Yes | NED (54 months) |
While the pattern of expression for any given immunohistochemical marker was variable, in all cases, the mucinous component was positive for at least one GI marker (CK20 or CDX2), while the Mullerian component was positive for at least one Mullerian marker (PAX8, ER or PR) and lacked expression of CK20 and CDX2 (Table 2). Mucinous components showed either complete absence or decreased expression of PAX8, ER and PR relative to their corresponding Mullerian components.
Table 2:
Immunophenotypic features and mucin histochemical characterization of mixed ovarian borderline tumors and carcinomas with gastrointestinal-type mucinous and Mullerian epithelial components
| Case # | Component (predominant cell type) | CK7 | CK20 | ER | PR | Pax8 | CDX2 | MUC6 | WT1 | ARID1A | AB/PAS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MO-1 | Mucinous (intestinal>gastric) | +++ | ++ | − | − | − | ++ | + | − | Loss | neutral/acidic |
| Mullerian (endometrioid) | +++ | − | +++ | +++ | ++ | − | − | + | Loss | No intracellular mucin | |
| MO-2 | Mucinous (gastric) | +++ | + | − | − | + | − | − | − | Loss | Neutral |
| Mullerian (endometrioid) | +++ | − | + | +++ | ++ | − | − | − | Subclonal loss | No intracellular mucin | |
| MO-3 | Mucinous (gastric>intestinal) | +++ | ++ | − | − | ++ | ++ | − | − | Intact | Mixture (neutral>acidic) |
| Mullerian (endometrioid/clear cell) | +++ | − | ++ | ++ | +++ | − | − | − | Intact | Only rare cells with mucin (acidic) | |
| MO-4 | Mucinous (gastric>intestinal) | +++ | + | + | − | + | + | ++ | − | Loss | Mixture (neutral>acidic) |
| Mullerian (endometrioid/clear cell) | +++ | − | ++ | − | ++ | − | − | − | Loss | No intracellular mucin |
Immunohistochemistry results reported as follows:
, negative;
, focal/weak positive;
moderate/patchy positive;
, strong/diffuse positive.
MO-1 was a mixed GI-type mucinous and endometrioid carcinoma (Figure 2A-F). The spatially distinct components were separated by an intervening adenofibromatous area containing morphologically benign Mullerian/cortical inclusion cysts. The endometrioid carcinoma component was comprised of confluent glands with foci of solid growth. The mucinous component was composed of borderline tumor-like areas, characterized by papillary and cystic structures with intraluminal necrotic debris, with invasive glands associated with desmoplastic stroma or floating in pools of extracellular mucin. The mucin-producing cells were predominantly goblet cells with acidic mucin, while the endometrioid glands lacked mucin. By immunohistochemistry, the endometrioid carcinoma component and cortical inclusion cysts were positive for ER, PR and PAX8, and focally positive for WT1, but were negative for CK20, CDX2 and MUC6, while the converse (CK20, CDX2 positive, and MUC6 focally positive; PAX8, ER, PR, and WT1 negative) was observed in the mucinous component.
Figure 2:
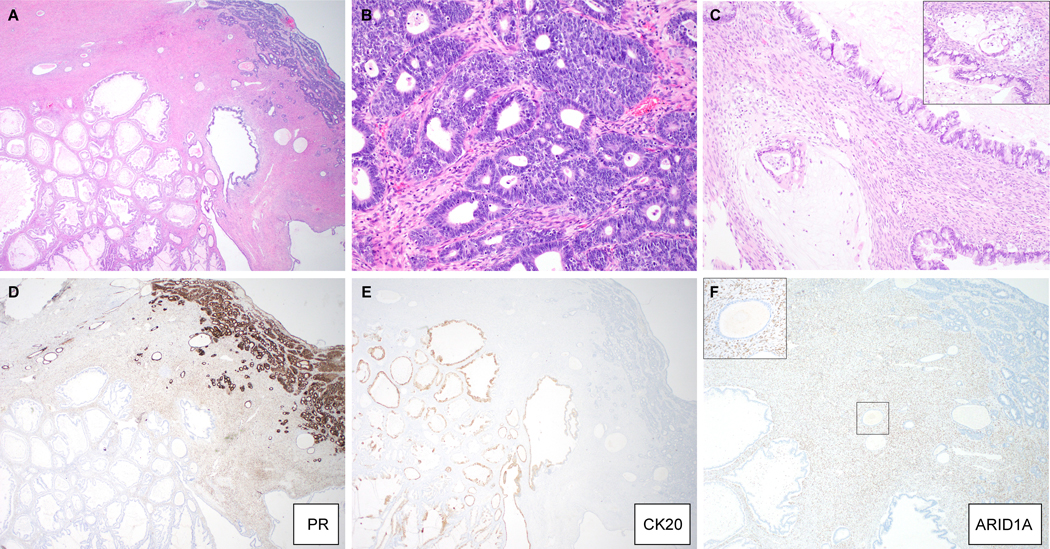
Mixed gastro-intestinal type mucinous and endometrioid carcinoma (MO-1). (A) Low-magnification image showing spatial relationship of both components, with scattered benign cortical inclusion cysts; (B) endometrioid carcinoma component, (C) invasive glands floating in pools of mucin in a background of mucinous borderline tumor (inset: another invasive focus). Immunohistochemical stains for (D) PR, (E) CK20 and (F) ARID1A (inset: high magnification of boxed area highlights loss of ARID1A expression in the cortical inclusion cyst epithelium).
MO-2 was a mixed GI-type mucinous and endometrioid borderline tumor associated with endometriosis (Figure 3A-E). Foci of endometriosis merged with more cellular areas of packed endometrioid glands, which architecturally reached the level of borderline tumor. The closely associated mucinous borderline tumor component was composed of gastric-type glands producing neutral mucin, with areas of stratification but lacked confluent or invasive growth. Immunophenotypically, the endometrioid component expressed Mullerian markers (weak ER, strong PR, moderate PAX8) exclusively, while the mucinous component exhibited focal weak expression of CK20 and PAX8 and was negative for all other markers.
Figure 3:
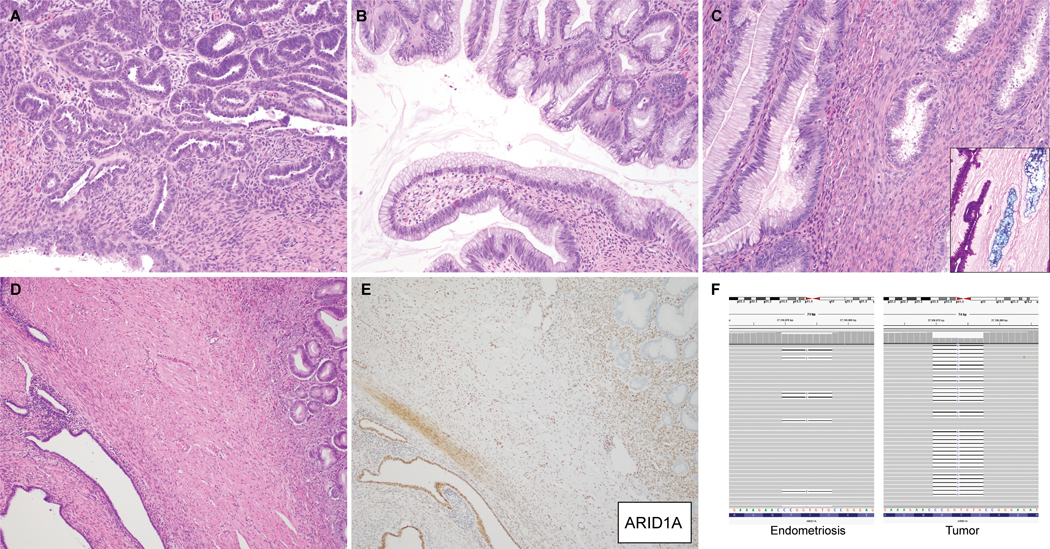
Mixed gastrointestinal-type mucinous and endometrioid borderline tumor associated with endometriosis (MO-2). (A-D) Sections, illustrating (A) endometrioid component; (B) mucinous component; (C) admixture of endometrioid and mucinous glands, with neutral mucin exclusive to the mucinous glands (inset, AB/PAS histochemical stain); (D) adjacent endometriosis, with corresponding ARID1A immunohistochemistry (E), showing loss of expression exclusive to neoplastic glands. (F) Sequencing reads of the ARID1A locus, showing a frameshift mutation, due to small deletion, present in endometriosis, with loss-of-heterozygosity in tumor (endometrioid component shown).
MO-3 was a GI-type mucinous borderline tumor with a focus of intraepithelial carcinoma of Mullerian-type (Figure 4A-G). The intraepithelial carcinoma exhibited marked nuclear pleomorphism and clear cytoplasm and appeared to arise abruptly from a cystic structure lined by bland cuboidal cells with occasional ciliated cells. By immunohistochemistry, the epithelial lining was positive for PAX8, ER, and PR. There were associated CD10-positive stromal cells, suggestive of an endometriotic origin. In contrast, the mucinous component, which made up the bulk of the neoplasm, expressed CDX and CK20, and showed weak, patchy PAX8 expression. AB/PAS staining revealed a mixture of neutral and acidic mucin-producing cells, consistent with a mixture of intestinal-type and gastric-type mucinous tumor cells, with the latter predominating.
Figure 4:
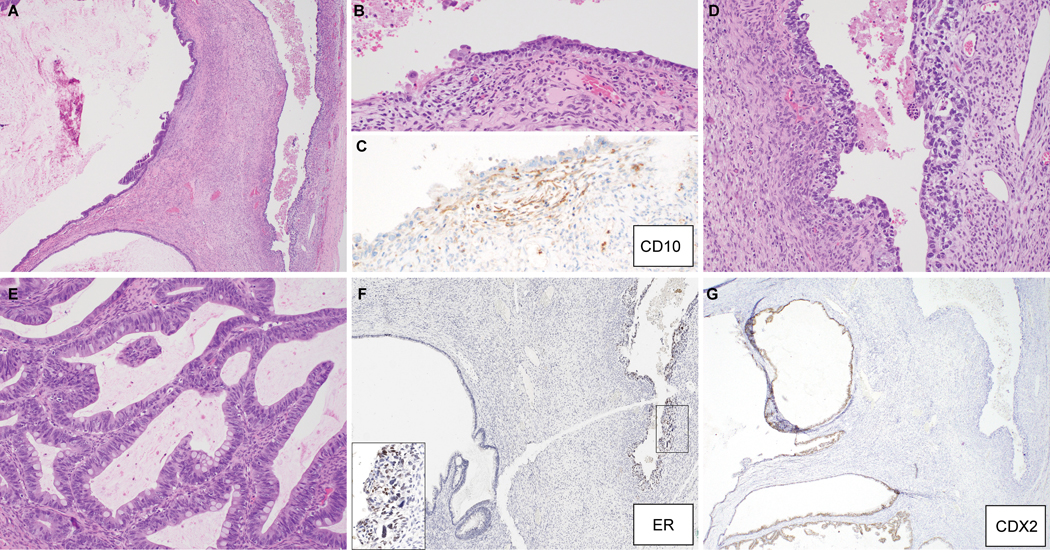
Gastrointestinal-type mucinous borderline tumor with intraepithelial carcinoma of Mullerian-type (MO-3). (A) Low-magnification image showing both components; (B) area morphologically suggestive of endometriosis, supported by CD10 immunoreactivity in stromal cells (C), which is contiguous with a focus with marked nuclear atypia and cytoplasmic clearing (D). (E) Another area of mucinous borderline tumor, exhibiting goblet cells. Immunohistochemical stains for (F) ER and (G) CDX2, shows differential expression in morphologically distinct areas, in line with mixed Mullerian and gastrointestinal-type mucinous differentiation.
MO-4 was a high-grade carcinoma with Mullerian and mucinous components. The Mullerian component showed papillary growth, with overlapping endometrioid and clear cell cytologic features, and patchy expression of PAX8 and ER. The mucinous component showed heterogeneous expression of PAX8, CDX2, and CK20 and was composed of confluent glands and foci of tumor cells floating within mucin pools. Mucinous tumor cells with a gastric phenotype predominated, which were highlighted by strong MUC6 expression and intracytoplasmic neutral mucin. Focal endometriosis was identified.
Molecular genetic features
Shared mutations were identified in paired mucinous and Mullerian components for MO-1 to MO-4, indicative a clonal relationship (Figure 5A). All mixed borderline tumors and carcinomas harbored ARID1A mutations. Other recurrent driver genetic alterations included KRAS (n=3), ASXL1 (n=2), PIK3CA (n=2), TP53 (n=2), and U2AF1 (n=2). The majority of mutations were conserved between mucinous and Mullerian components (median 84% of total mutations per case, range 62–97%), as reflected in phylogenetic trees with long trunks and short branches (Figure 5B). In MO-1, MO-2 and MO-3, private (i.e. non-shared) mutations were only present in the mucinous component. In MO-4, both components had private mutations; notably, ERBB2 amplification was present only in the mucinous component.
Figure 5:
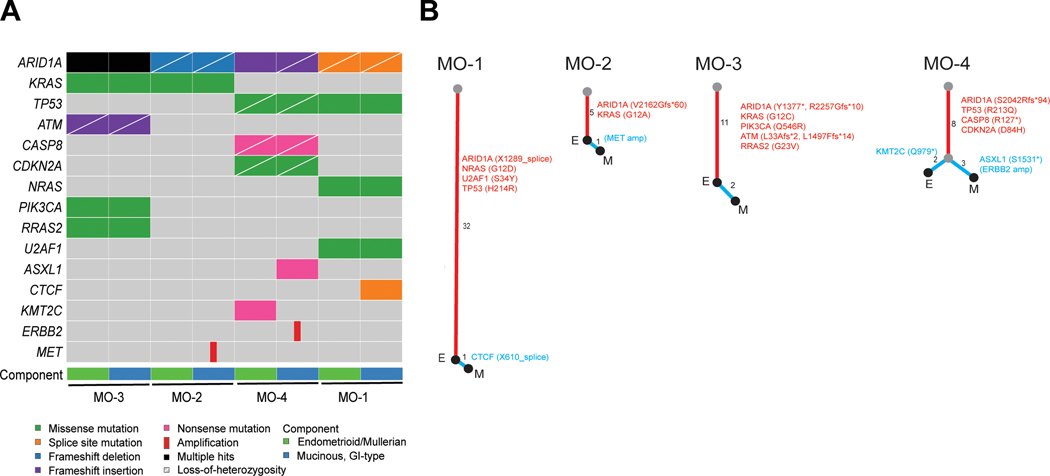
Somatic genetic alterations of mixed gastrointestinal-type mucinous and Mullerian ovarian borderline tumors and carcinomas. (A) Oncoplot showing pathogenic alterations only in each component, isolated by microdissection and analyzed by targeted next-generation sequencing. (B) Phylogenetic trees constructed based on all identified somatic genetic variants, illustrating the evolutionary relationships between the endometrioid/Mullerian (E) and gastrointestinal-type mucinous (M) components. For each case, the trunk (red) represents variants common to both components, while the branches (blue) represent variants exclusive to each respective component. Trunk/branch lengths are proportional to the number of genetic variants, as indicated next to each segment. Pathogenic alterations, including relevant gene amplifications (italicized, in brackets) are also listed. Note that copy number alterations were not considered in determining trunk/branch lengths.
Association of ARID1A genetic alterations with immunohistochemical expression
Immunohistochemical analysis of ARID1A revealed loss of expression in both mucinous and Mullerian components in MO-1 (Figure 2F) and MO-2 (Figure 3E), which in both cases, harbored ARID1A truncating mutations with loss-of-heterozygosity of the remaining allele. In MO-1, loss of ARID1A expression was also observed in adjacent morphologically benign cortical inclusion cysts, suggesting that they may represent the common precursor lesion for both tumor components. In contrast, in MO-2, adjacent foci of endometriosis showed intact ARID1A expression. Genetic analysis revealed these foci to harbor a heterozygous ARID1A mutation, identical to that within the tumor, along with an intact wildtype allele (which accounts for the retained immunohistochemical expression; Figure 3F). Despite harboring 2 truncating ARID1A mutations, MO-3 showed retained ARID1A expression throughout, suggesting the possibility that both mutations were present on the same allele. In MO-4, immunohistochemical loss of ARID1A was observed only in the mucinous component, corresponding with genetic inactivation of both alleles restricted only to this component. Intact ARID1A expression was observed in the Mullerian component, which harbored a heterozygous mutation only, as well as in endometriosis, which, given the limited size of the lesion, was not amenable to genetic analysis.
Discussion
Histomophologic and molecular studies have implicated an extra-ovarian origin for most types of epithelial ovarian tumors (12). The tubal precursor of high-grade serous carcinoma and the origin of endometrioid and clear cell carcinomas from endometriosis are prototypic examples used to support the proposal that ovarian carcinoma is an “imported disease(13).” While these tumor subtypes exhibit at least some degree of resemblance to cell types native to the fallopian tube or endometrium, ovarian mucinous tumors of GI-type are characterized by a line of differentiation that is not normally found in the gynecologic tract. The cell/tissue-of-origin and histogenesis of this group of tumors has therefore been enigmatic. It is well established that rare GI-type mucinous tumors arise from mature teratomas (1, 14). Most cases, however, are non-germ cell-derived. Recent work has suggested that they may be derived from the mucinous epithelium of Brenner tumors or mucinous metaplasia of transitional type epithelium at the tubo-peritoneal junction (15, 16). While mucinous metaplasia of ovarian surface epithelium or cortical inclusion cysts has been proposed, to date, there is no evidence to support this speculation (12, 17). Furthermore, implicit to the separation of GI-type mucinous and endocervical-type mucinous ovarian tumors is the widely held assumption that only the latter originates from Mullerian epithelium. Nevertheless, invoking a Mullerian epithelial origin for ovarian GI-type mucinous tumors is not unfounded, considering the existence of gastric-type and intestinal-type adenocarcinomas of the endocervix/endometrium(11, 18).
We acknowledge that what constitutes “GI-type” mucinous differentiation is not well-established. While subclassification of mucinous epithelium as GI-type or Mullerian-type implies a binary distinction, in actuality, it is likely that these phenotypes lie on a continuum. Indeed, CDX2, CK20, and MUC6 expression have been reported in endometrial and ovarian endometrioid carcinomas, often in a focal or patchy distribution(19–21), which may represent an incomplete form of GI-type mucinous metaplasia, particularly when gastric or intestinal morphologic features are lacking. As our primary aim was demonstrate, as proof-of-concept, a Mullerian origin for the pathogenesis of GI-type mucinous tumors, we restricted our cases to those in which the mucinous component could be diagnosed morphologically as a GI-type mucinous ovarian neoplasm if considered in isolation, with corroborating evidence from immunohistochemical and mucin staining characteristics.
To our knowledge, we describe for the first time, non-mucin-producing, ciliated cells residing within ovarian GI-type mucinous cystadenomas. It should first be re-iterated that these were not seromucinous cystadenomas, otherwise known as Mullerian mucinous cystadenomas, which are defined by the presence of ciliated or endometrioid cells admixed with endocervical-type mucinous epithelium (4, 6). In contrast to the acidic mucin produced by endocervical glandular epithelium, intracytoplasmic neutral mucin, observed in the mucinous cystadenomas in our cohort was suggestive of a gastric phenotype. Expression of GI markers, including the gastric mucin, MUC6, and intestinal markers, CK20 and CDX2, further support GI-type mucinous differentiation. It is likely, however, that some mucinous cystadenomas exhibit both endocervical and GI-type mucinous epithelium, as exemplified in Case Cy-4, which contained endocervical, gastric and intestinal-type mucinous cells.
Our description of cystadenomas with GI-type mucinous and Mullerian components has implications for understanding the pathogenesis of mucinous ovarian neoplasms. These “mixed” cystadenomas may represent a transitional state of a Mullerian-to-GI-type metaplastic conversion, that may evolve into pure mucinous cystadenomas. This sets the stage for potential progression to mucinous borderline tumors and carcinomas, through the accumulation of oncogenic genetic alterations, such as KRAS mutations (Figure 6). It is unknown what proportion of pure GI-type mucinous ovarian neoplasms develop in this manner. However, the expression of PAX8 in up to 40% of mucinous borderline tumors/carcinomas suggest that a significant proportion may have arisen from a Mullerian epithelial precursor(22, 23).
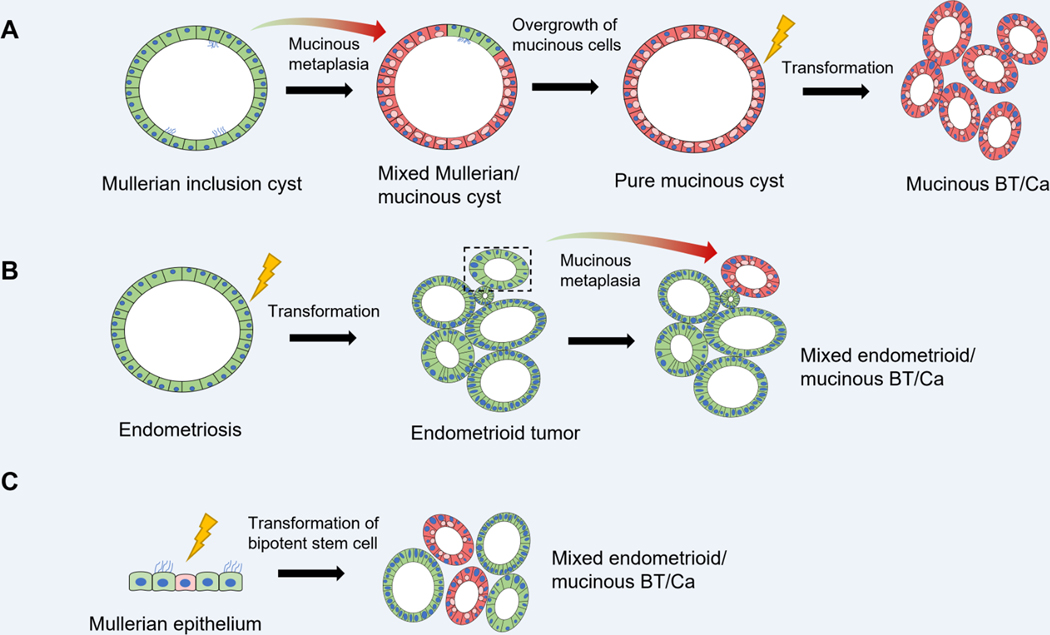
Figure 6:
Proposed models of pathogenesis for pure and mixed gastro-intestinal type mucinous ovarian tumors originating from Mullerian epithelial precursors. (A). Transdifferentiation of a subset of cells within a benign Mullerian-type cyst, resulting in a mixed gastrointestinal-type mucinous / Mullerian cyst. Eventual replacement of the entire cyst lining by mucinous cells could potentially result in a pure mucinous cystadenoma, which may serve as the substrate for development of mucinous borderline tumors or carcinomas. (B) Endometriosis gives rise to an endometrioid borderline tumor or carcinoma, within which a subpopulation undergoes mucinous metaplasia, resulting in a mixed endometrioid / gastrointestinal-type mucinous tumor. (C) Mixed tumors can also potentially result from direct transformation of a putative bipotent stem cell within Mullerian epithelium (e.g. endometriosis, cortical inclusion cyst epithelium, etc) capable of gastrointestinal and Mullerian lineage differentiation.
In contrast to the sequential progression from cystadenoma to borderline tumor and carcinoma established for pure mucinous ovarian neoplasms, it is unlikely that the mixed GI-type mucinous/Mullerian borderline tumors and carcinomas originate from mixed GI-type mucinous/Mullerian cystadenomas. In our cohort, the borderline tumors and carcinomas (MO-1 to MO-4) all harbored ARID1A mutations, and the Mullerian components were predominantly endometrioid, co-existing with endometriosis, while these features were lacking in the cystadenomas. Furthermore, the Mullerian-to-GI-type mucinous transdifferentiation appears to occur later, after neoplastic transformation, in the borderline tumors and carcinomas.
Molecular analyses of spatially distinct morphologic components in these tumors demonstrated a high degree of overlap in their mutational profiles, in line with descent from a common ancestral clone, with late evolutionary divergence. The use of a targeted sequencing panel covering only 500 genes, however, precludes definitive conclusions to be drawn regarding the degree of similarity between matched pairs of tumor samples and the directionality of tumor progression. There is no doubt that other passenger mutations across the genome were not analyzed in this selected genetic analysis. Nevertheless, there are several lines of evidence suggesting that the mucinous component arose later in progression.
First, in MO-1, MO-2 and MO-3, only the mucinous component harbored additional private mutations, suggesting a linear model of tumor progression(24), in which the Mullerian tumor component preceded the mucinous component. For MO-4, both components harbored a similar number of private mutations, consistent with branching evolution. Notably, heterozygous ARID1A mutation was present in the Mullerian component, with LOH in the mucinous component, which again suggests that the latter arose later in tumor progression. Second, putative Mullerian precursor lesions, which were morphologically benign, in the form of endometriosis or cortical inclusion cysts were identified, with ARID1A mutation or loss of expression identified in lesions from MO-2 and MO-1, respectively. In MO-2, foci of endometriosis harbored the same ARID1A mutation as that found in the borderline tumor components. The retained expression of ARID1A can be explained by an intact wild-type allele, which was subsequently lost upon neoplastic transformation.
Given the high degree of overlap in the genetic alterations in the Mullerian and mucinous components, it is likely that transdifferentiation to GI cell lineages was mediated by epigenetic mechanisms, possibly in response to regional differences in the tumor microenvironment. In this context, it is particularly intriguing that all the borderline tumors and carcinomas in our cohort harbored truncal mutations in ARID1A, a chromatin-remodeling gene within the SWI/SNF family, involved in the epigenetic regulation of a multitude of cellular processes, including differentiation (25). However, whether loss of ARID1A is directly involved in GI lineage conversion is unknown. Given that this genetic alteration is frequently observed in endometriosis and endometriosis-associated ovarian tumors, namely, seromucinous borderline tumors, and endometrioid and clear cell carcinomas, the presence of ARID1A mutation in mixed GI-type mucinous/Mullerian tumors may simply reflect an origin from endometrial tissue.
Given the fact that all the mixed tumors were Stage I at resection and the small sample size, we are unable to make any definitive conclusions about their clinical behavior relative to pure mucinous ovarian tumors. However, it is notable that all patients remained disease-free at last follow-up (up to 54 months). The prognosis of mixed ovarian tumors with a mucinous component compared to pure mucinous ovarian tumors, as well as the clinical significance of subclassifying mucinous tumors based on their putative pathogenic origin (teratoma, Brenner tumor, Mullerian), warrant further investigation. Furthermore, given our current data supporting a Mullerian origin for at least a subset of primary ovarian GI-type mucinous tumors, future studies are needed to understand the molecular mechanisms that underlie GI-type mucinous transdifferentiation of Mullerian epithelium and the how this process interacts with genetic alterations to drive neoplastic transformation.
source of funding:
Research reported in this publication was supported in part by a Cancer Center Support Grant of the NIH/NCI (Grant No. P30CA008748).
Footnotes
Conflicts of interest
The authors have no competing financial interests.
References
- 1.Vang R, Khunamornpong S, Kobel M, et al. Mucinous cystadenoma and adenofibroma; Mucinous borderline tumor; Mucinous carcinoma of the ovary. WHO Classification of Tumours 5th Edition. Lyon, France: IARC Press; 2020:48–54. [Google Scholar]
- 2.Cheasley D, Nigam A, Zethoven M, et al. Genomic analysis of low-grade serous ovarian carcinoma to identify key drivers and therapeutic vulnerabilities. J Pathol. 2021;253:41–54. [DOI] [PubMed] [Google Scholar]
- 3.Ryland GL, Hunter SM, Doyle MA, et al. Mutational landscape of mucinous ovarian carcinoma and its neoplastic precursors. Genome Med. 2015;7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobel M, Kim K-R, McCluggage WG, et al. Seromucinous cystadenoma and adenofibroma; Seromucinous borderline tumour; Seromucinous carcinoma. WHO Classification of Tumours 5th Edition - Female Genital Tumours. Lyon, France: IARC Press; 2020:68–70. [Google Scholar]
- 5.Kurman RJ, Shih Ie M. Seromucinous Tumors of the Ovary. What’s in a Name? Int J Gynecol Pathol. 2016;35:78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins JC, Young RH. Müllerian Mucinous Cystadenomas of the Ovary: A Report of 25 Cases of an Unheralded Benign Ovarian Neoplasm Often Associated With Endometriosis and a Brief Consideration of Neoplasms Arising From the Latter. Int J Gynecol Pathol. 2022;41:68–75. [DOI] [PubMed] [Google Scholar]
- 7.Cheung AN, Ellenson LH, Gilks CB, et al. Tumours of the ovary. WHO Classification of Tumours 5th Edition - Female Genital Tumours. Lyon, France: IARC Press; 2020:33–167. [Google Scholar]
- 8.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarty D, Gao J, Phillips SM, et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017;10.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen R, Seshan VE. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 2016;44:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talia KL, McCluggage WG. The developing spectrum of gastric-type cervical glandular lesions. Pathology. 2018;50:122–133. [DOI] [PubMed] [Google Scholar]
- 12.Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186:733–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuhn E, Kurman RJ, Shih IM. Ovarian Cancer Is an Imported Disease: Fact or Fiction? Curr Obstet Gynecol Rep. 2012;1:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Schwartz LE, Anderson D, et al. Molecular analysis of ovarian mucinous carcinoma reveals different cell of origins. Oncotarget. 2015;6:22949–22958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Wu RC, Shwartz LE, et al. Clonality analysis of combined Brenner and mucinous tumours of the ovary reveals their monoclonal origin. J Pathol. 2015;237:146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidman JD, Yemelyanova A, Zaino RJ, et al. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30:4–11. [DOI] [PubMed] [Google Scholar]
- 17.Feeley KM, Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology. 2001;38:87–95. [DOI] [PubMed] [Google Scholar]
- 18.Wong RW, Ralte A, Grondin K, et al. Endometrial Gastric (Gastrointestinal)-type Mucinous Lesions: Report of a Series Illustrating the Spectrum of Benign and Malignant Lesions. Am J Surg Pathol. 2020;44:406–419. [DOI] [PubMed] [Google Scholar]
- 19.Park KJ, Bramlage MP, Ellenson LH, et al. Immunoprofile of adenocarcinomas of the endometrium, endocervix, and ovary with mucinous differentiation. Appl Immunohistochem Mol Morphol. 2009;17:8–11. [DOI] [PubMed] [Google Scholar]
- 20.Hodgson A, Parra-Herran C, Mirkovic J. Immunohistochemical expression of HIK1083 and MUC6 in endometrial carcinomas. Histopathology. 2019;75:552–558. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Rambau PF, Kelemen LE, et al. Nuclear β-catenin and CDX2 expression in ovarian endometrioid carcinoma identify patients with favourable outcome. Histopathology. 2019;74:452–462. [DOI] [PubMed] [Google Scholar]
- 22.Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35:816–826. [DOI] [PubMed] [Google Scholar]
- 23.Aldaoud N, Erashdi M, AlKhatib S, et al. The utility of PAX8 and SATB2 immunohistochemical stains in distinguishing ovarian mucinous neoplasms from colonic and appendiceal mucinous neoplasm. BMC Res Notes. 2019;12:770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis A, Gao R, Navin N. Tumor evolution: Linear, branching, neutral or punctuated? Biochim Biophys Acta Rev Cancer. 2017;1867:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu JN, Roberts CW. ARID1A mutations in cancer: another epigenetic tumor suppressor? Cancer Discov. 2013;3:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]