Abstract
BACKGROUND:
Opioid prescribing guidelines recommend reducing or discontinuing opioids for chronic pain if harms of opioid treatment outweigh benefits. As opioid discontinuation becomes more prevalent, it is important to understand whether opioid discontinuation is associated with heroin use. In this study, we sought to assess the association between opioid discontinuation and heroin use documented in the medical record.
METHODS:
A matched nested case-control study was conducted in an integrated health plan and delivery system in Colorado. Patients receiving opioid therapy in the study period (January 2006—June 2018) were included. Opioid discontinuation was defined as ≥45 days with no opioids dispensed after initiating opioid therapy. The heroin use onset date represented the index date. Case patients were matched to up to 20 randomly selected patients without heroin use (control patients) by age, sex, calendar time, and time between initiating opioid therapy and the index date. Conditional logistic regression models estimated matched odds ratios (mOR) for the association between an opioid discontinuation prior to the index date and heroin use.
RESULTS:
Among 22,962 patients prescribed opioid therapy, 125 patients (0.54%) used heroin after initiating opioid therapy, of which 74 met criteria for inclusion in the analysis. The odds of opioid discontinuation were approximately two times higher in case patients (n = 74) than control patients (n = 1045; mOR = 2.19; 95% CI 1.27–3.78).
CONCLUSIONS:
Among patients prescribed chronic opioid therapy, the observed increased risk for heroin use associated with opioid discontinuation should be balanced with potential benefits.
Keywords: Opioid discontinuation, heroin use, prescription opioid use
1. Introduction
In response to the opioid epidemic (Centers for Disease Control and Prevention, 2011), the Centers for Disease Control and Prevention (CDC) issued a guideline that provided clinicians with recommendations on how to improve the safety of opioid prescribing for chronic pain (Dowell et al., 2016). The Guideline encourages clinicians to consider nonopioid medications and nonpharmacologic treatments before initiating opioid therapy. It also recommends that clinicians collaborate with patients to taper or discontinue opioid therapy when the harms of treatment outweigh the benefits. Data suggests that discontinuing opioids can reduce the risk of pharmaceutical opioid overdoses (Glanz et al., 2019).
The CDC Guideline and other educational, policy, and regulatory efforts to improve the safety of opioid prescribing have also been credited with reducing opioid prescribing nationally (Bohnert et al., 2018; Dowell et al., 2016; National Academies of Sciences Engineering and Medicine, 2019; US Department of Health and Human Services, 2018). While reductions in opioid prescribing rates have coincided with reduced or plateaued prescription opioid overdose death rates, heroin and fentanyl overdose death rates have risen significantly since 2010 and 2013, respectively (Gladden, 2016; Hedegaard et al., 2020). Explanations for this epidemiological shift include the increased availability and purity of heroin, lower price, and influx of illicitly-manufactured fentanyl into the heroin supply, in combination with social, psychological and economic factors (Ciccarone, 2017; Dasgupta et al., 2018; Drug Enforcement Administration, 2016). There have also been concerns that efforts to discontinue chronic opioid therapy are having unintended consequences (James et al., 2019; Kroenke et al., 2019), particularly if the discontinuation practices do not adhere to Guideline recommendations. Discontinuing chronic opioid therapy could precipitate pain and withdrawal symptoms, prompting patients to seek illicit opioids. In a publicly insured sample in San Francisco, there was an increased likelihood of heroin and non-prescribed opioid use among participants discontinued from opioid therapy (Coffin et al., 2020). A study of veterans identified a greater risk of death from overdose or suicide after stopping opioids (Oliva et al., 2020) and another study showed that discontinuing opioids was associated with an increased risk of overdose death (James et al., 2019).
To guide sound public health practice and clinical decision-making, additional evidence on the potential benefits and harms of opioid discontinuation on heroin use in broader populations is needed. We therefore sought to evaluate whether discontinuing chronic opioid therapy was associated with heroin use that came to medical attention in a large Colorado health system.
2. Methods
2.1. Study setting, design and data sources
We conducted a matched case-control study nested within a cohort of patients receiving care at Kaiser Permanente Colorado (KPCO), an integrated healthcare delivery system that has commercial, Medicaid, and Medicare plans. A case-control design allowed us to adequately match for age, sex, and follow-up and minimize time-varying confounding.
The primary data source was KPCO’s electronic health record (EHR), which captures social history, laboratory results, and diagnoses and notes from ambulatory, emergency department, and inpatient settings. Demographic information and health plan enrollment were obtained from membership data; death and causes of death were identified from state vital records; and opioid dispensings were extracted from pharmacy databases.
2.2. Study cohort
We identified KPCO Denver-Boulder health plan members ≥18 years of age enrolled in the health plan for ≥90 days between January 1, 2006 and June 30, 2018. In this population, we adapted criteria from other studies of chronic opioid therapy (Dunn et al., 2010; Glanz et al., 2019) to identify a cohort of patients dispensed ≥3 opioid prescriptions in 90 days with ≤10 days without any opioid coverage, excluding sublingual buprenorphine containing products. The date of the third opioid dispensing was considered the initiation of opioid therapy and beginning of each cohort member’s follow-up. We included patients with ≥45 days of health plan enrollment after initiating opioid therapy. Follow-up was censored on the health plan disenrollment date, death date, or June 30, 2018. Across the follow-up, we assessed each patient’s opioid dispensings to identify periods of opioid discontinuation, defined as no opioid therapy for ≥45 days.
2.2.1. Identifying case patients
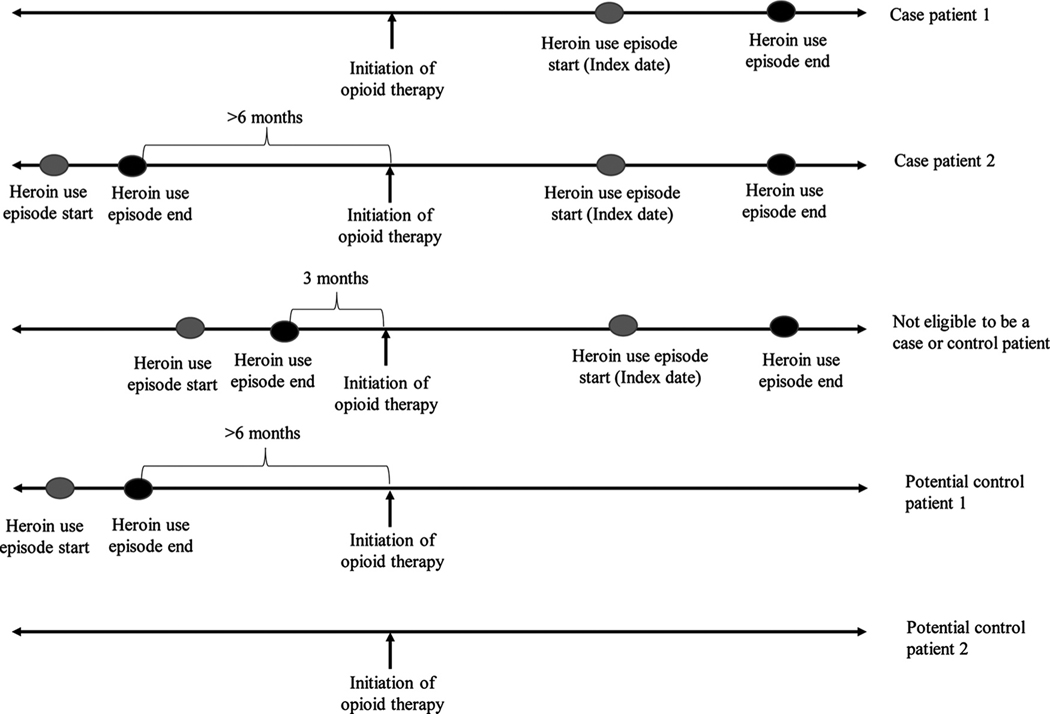
In the opioid therapy cohort, we identified and confirmed case patients with heroin use episodes occurring after initiating opioid therapy in three steps (Fig. 1).
Fig. 1.
Sample eligible case patients and control patients prescribed opioid therapy.
First, we cast a wide net to identify patients with potential heroin use episodes. Among cohort members, we extracted International Classification of Disease – Clinical Modification (ICD-9/10 CM) codes for heroin overdose from the EHR (Safe States Aliance, 2012). We also applied an electronic text string algorithm to identify mentions of heroin use in the EHR. This algorithm identified heroin-related terms (e.g., “heroin use” and “injection drug use”) in medical documentation and heroin metabolites (e.g., “6-monoacetylmorphine”) from urine toxicology results (eTable A in Supplement).
Second, three research assistants followed a medical record review protocol to verify that the mention of heroin use referred to the patient’s use (i.e., not a family member’s use) and did not represent a denial of use. Abstractors examined all encounter notes from primary care, mental health, substance use disorder treatment, emergency department, and inpatient settings that were flagged by the text string search. Abstractors also searched for and examined other encounter notes that commonly had related information, such as patient calls for admission to substance use disorder treatment and new patient behavioral health assessments. Finally, abstractors searched urine toxicology testing results for 6-monoacetylmorphine. There were no restrictions on the earliest encounter examined, as long as the data were present in the medical record as electronic or scanned records. Patients with any uncertainty about heroin use (e.g., if the physician suspected but did not confirm use with the patient) were excluded. Abstractors were supervised by a Master’s level behavioral health research specialist and an addiction and internal medicine trained physician.
Third, abstractors recorded the timing and duration of heroin use episodes, including onset, cessation, resumption, and most recent use dates. We excluded heroin use episodes that did not include dates (e.g., “past heroin use”). If the specified date was a range, we assigned a date as the midpoint of the specified range. For example, a documented heroin use onset date of December 2016 was assigned a date of December 15, 2016. Heroin use onset and last heroin use is a standard component of the initial patient assessment by mental health and substance use disorder treatment therapists, nurses, and physicians. It may be documented during the initial assessment, at follow-up visits, and prior to the initiation of medication treatment. Case patients with heroin use episodes had to meet the following criteria: heroin use (1) started ≥45 days after initiating opioid therapy, (2) occurred during health plan enrollment with at least 45 days of enrollment prior to heroin use, and (3) could not have occurred < 6 months prior to the start of opioid therapy. The first criteria ensured that the patient had the opportunity to be exposed to a 45-day discontinuation (our primary exposure); the second criteria helped ensure that we had accurate data on prescription opioid exposure; and the third criteria reduced the potential that patients were already using heroin when they started opioids. For case patients, the first heroin use date after initiating opioid therapy represented the index date. We also included heroin use that occurred in conjunction with other drugs, such as fentanyl, cocaine, or methamphetamine.
2.2.2. Identifying matched control patients
Potential control patients were identified from the opioid therapy cohort (Fig. 1). To ensure that control patients had not used heroin after initiating opioid therapy, they could not have an ICD code consistent with heroin overdose or a text mention of heroin use. However, patients with a mention of heroin use identified by the text string search could be a control patient if the medical record review did not demonstrate that they had used heroin. Risk-set sampling was used to select control patients (Langholz and Goldstein, 1996). We matched control patients to case patients on the index date (i.e., the exact date of heroin use in the case), age ( ± 5 years), sex, and length of follow-up ( ± 60 days) from the start of opioid therapy to the index date. Among available control patients who met matching criteria, we randomly selected up to 20 as control patients. Similar to case patients, control patients had to be enrolled for at least 45 days prior to the index date.
2.3. Main exposure and covariates
Among case and matched control patients, exposure to opioid discontinuation was assessed retrospectively from the index date. Patients were considered exposed to a discontinuation if they had no opioid therapy for ≥45 days prior to the index date. Given that patients could seek heroin shortly after they run out of opioids, we opted for a shorter time period than prior studies used to define an opioid discontinuation (90 or 182 days) (Glanz et al., 2019; Martin et al., 2011). However, we avoided using a time period less than 45 days to ensure the discontinuation did not just represent a gap in treatment for travel or a missed refill. For a case patient to be considered discontinued (exposed), the date of heroin use onset had to occur while they were not receiving opioid therapy. Cases receiving opioid therapy and using heroin concurrently were considered unexposed.
We assessed baseline covariates that might confound the relationship between opioid discontinuation and heroin use over a one-year period prior to initiating opioid therapy. For patients who entered the study in 2006, we included data from 2005. Covariates assessed included race/ethnicity; insurance; opioid dose in morphine milligram equivalents (MME) (von Korff et al. 2008); medical comorbidity (Modified Charlson comorbidity index (Deyo et al., 1992; Quan et al., 2005)); diagnosis of a mental health condition; tobacco use or use disorder; alcohol use disorder; and the number of ambulatory visits over the follow-up. We did not match on these variables to help ensure there was an adequate number of control patients to match to each case patient (Wacholder et al., 1992).
2.4. Statistical analysis
Assuming 80% statistical power, estimated exposure probability of 20% (Vanderlip et al., 2014), an α = 0.05, and 1–20 control patients per case patient, this study required 50 to 150 heroin use case patients to detect matched odds ratios (mORs) of 2.83 to 1.82, respectively.
Data were analyzed using conditional logistic regression to estimate mORs and 95% confidence intervals (CI). The dependent variable was first verified heroin use episode after initiating opioid therapy (yes/no), and the main exposure variable was opioid discontinuation (yes/no). Variables with a P < 0.20 in the univariate analyses were included in the multivariable models. Missing race/ethnicity values were replaced using multiple imputations based on the available information from the matched sample. Model fit was assessed with the Hosmer-Lemeshow goodness-of-fit test. For continuous variables, the linearity in the logit assumption was evaluated using Box-Tidwell transformations. The mORs were used to calculate percent attributable risks among patients who were discontinued, and attributable risks in the opioid therapy population (Bruzzi et al., 1985).
2.4.1. Secondary analyses
It is possible that patients were exposed to repeated discontinuations. We hypothesized that patients who experienced multiple discontinuation attempts were struggling to remain discontinued from opioid therapy and were therefore at increased risk for heroin use. To address this, we conducted an analysis to evaluate the effect of repeated discontinuations, categorized as 0, 1, 2, and ≥3 discontinuation periods.
To examine the acute effects of opioid discontinuation, we assessed the impact of a shorter discontinuation period of 30 days and the association between discontinuation and heroin use episodes that occurred within 6 months following the opioid discontinuation. To determine if opioid discontinuation increased the risk for first time heroin use, we assessed the association between discontinuation and the first heroin use episode in a subgroup of patients who had never used heroin prior to initiating opioid therapy. To address the potential for reverse causation, we reviewed the medical records of all exposed case patients to determine if the clinician had discontinued opioids due to heroin use. We then conducted an analysis excluding any cases in whom discontinuation appeared to have been due to heroin use.
Despite matching on age, case patients were significantly younger than control patients. We therefore conducted an analysis adjusting for age in addition to matching. Since benzodiazepines are commonly coprescribed with opioids, we also ran a model adjusted for benzodiazepine dispensings over the follow-up period.
Lastly, there were instances where the onset of heroin use was described in the medical record as a date range, and we used the midpoint of the range as the heroin use onset date in our primary analysis. To assess the potential for misclassification of the onset date, we conducted two secondary analyses assuming the heroin use onset dates were at the beginning and end of the date ranges.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). The study was approved by the KPCO Institutional Review Board.
3. Results
The opioid therapy cohort comprised 22,962 patients with an average age of 58.5 (SD = 16.5). The mean baseline opioid dose was 48.2 MME (SD = 79.6), and patients were followed for a mean of 54.1 months (SD = 40.5; Table 1).
Table 1.
Demographic and clinical characteristics of chronic opioid therapy cohort patients, case patients, and control patients and comparisons between case and control patients
| Characteristic | Cohort (N = 22962) | Case patients (n = 74) | Control patients (n = 1045) | P value |
|---|---|---|---|---|
|
| ||||
| Age, mean years (SD)a,b | 58.5 (16.5) | 37.1(12.2) | 42.4(11.2) | < 0.01b |
| Women, No. (%)b | 14065(61.3) | 39(52.7) | 518 (49.6) | 1.00b |
| Race/Ethnicity, No. (%) | 0.37 | |||
| Hispanic | 3098 (13.5) | 18(24.3) | 192 (18.4) | |
| White | 16427(71.5) | 50(67.6) | 704 (67.4) | |
| Black | 1003 (4.4) | 2 (2.7) | 55 (5.3) | |
| All other racial and ethnic groups | 944 (4.1) | 3 (4.1) | 44 (4.2) | |
| Missing | 1490 (6.5) | 1 (1.4) | 50 (4.8) | |
| Insurance, No. (%)a,c | < 0.01 | |||
| Commercial | 10131(44.1) | 42 (56.8) | 728 (69.7) | |
| Medicare | 9306 (40.5) | 3 (4.1) | 73 (7.0) | |
| Medicaid | 2464 (10.7) | 27 (36.5) | 188 (18.0) | |
| Other types of insurance | 1061(4.6) | 2 (2.7) | 56 (5.4) | |
| Modified Charlson Comorbidity Index, mean (SD)d | 1.58 (2.1) | 0.69 (0.9) | 0.75 (1.5) | 0.77 |
| Mental health diagnosis, No. (%)d | 10006(43.6) | 41 (55.4) | 437 (41.8) | 0.04 |
| Tobacco use, No. (%)d | 6315 (27.5) | 51 (68.9) | 356 (34.1) | < 0.01 |
| Alcohol use disorder, No. (%)d | 1045 (4.6) | 7 (9.5) | 68 (6.5) | 0.43 |
| Baseline opioid dose, mean morphine milligram equivalents (SD)e | 48.2 (79.6) | 70.9(107.2) | 46.7(68.3) | < 0.01 |
| Opioid discontinuations for 45 days or more during follow-up, mean (SD) | 1.7 (2.9) | 1.2 (1.7) | 1.0 (1.9) | 0.34 |
| Opioid discontinuation for 45 days or more prior to the index date, No. (%) | N/A | 28 (37.8) | 225 (21.5) | < 0.01 |
| Follow-up time, mean months (SD)b,f,g | 54.1 (40.5) | 39.6(31.1) | 40.1(31.4) | 0.95b |
| Ambulatory visits per month of follow-up, mean (SD) | 0.94 (0.81) | 1.02(0.89) | 0.84(0.84) | 0.22 |
Abbreviations: SD = standard deviation, N/A = not applicable:
Assessed at the initiation of opioid therapy
Cases and controls were matched on age ( ± 5 years), sex, calendar time, and time between the initiation of opioid therapy and the index date; 1 case patient could not be matched to any control patient, for the remaining 74 cases, number of control patients ranged from 1–20 (median = 16.5)
If there was more than one insurance, the following hierarchy was employed: Medicaid, Medicare, commercial, and others
Assessed between 1 year prior to the initiation of opioid therapy and the opioid therapy initiation date
Assessed in the first month after the initiation of opioid therapy
655(2.9%) individuals in the cohort were prescribed opioids for less than 90 days during follow-up
For the case and control patients, this was the time between initiating opioid therapy and the index date
In the cohort, we identified 1728 (7.5%) patients with documentation suggestive of heroin use and 290 (1.3%) were confirmed to have ever used heroin (Fig. 2). Among these, 125 (0.5%) used heroin after initiating opioid therapy. Eighteen patients were excluded because of insufficient documentation of heroin use dates and 75 case patients (0.3% of the cohort) met criteria for inclusion in the analysis. Among case patients, heroin use dates were documented to the day in 74.6%, the month in 14.7% and the year in 10.7%. Among the 75 cases, 2 (2.7%) also had documented fentanyl use. Sixty (80.0%) did not have documented heroin use prior to initiating opioid therapy. In case patients exposed to a discontinuation, the medical record review identified the following reasons for discontinuation, and sometimes multiple reasons: opioid therapy was no longer indicated in the opinion of the prescriber, the patient was using alcohol, the patient missed an appointment, or the patient had suicidal intent or an attempt, early refills, refills from more than one source, jail admission, threatening behavior by family or patient, and inappropriate use of pills. Discontinuation was due to heroin use in one case.
Fig. 2.
Identification of case and control patients in an opioid therapy cohort (N = 22,962).
Abbreviations: ICD-9/10: International Classification of Diseases, Ninth Revision/Tenth Revision
a14 patients had both a heroin ICD 9/10 code positive for heroin overdose and were identified using the electronic text string search algorithm
bHeroin use occurred less than 6 months prior to the start of opioid therapy
Each case patient was matched to up to 20 control patients randomly selected from a pool of 22,735 potential control patients (Fig. 2). One case patient could not be matched to any control patients. Matched case (n = 74) and control (n = 1045) patients differed significantly by age, insurance, tobacco use, mental health diagnosis, and baseline opioid dose (Table 1). Approximately 38% of case patients had discontinued opioids prior to the index date, compared to 21.5% of control patients (P < 0.01). The mean length of time from discontinuing opioids to the index date was 12.9 months (SD=12.8) for the cases. The mean MME preceding the discontinuation was 17.4 (SD 20.0) in the cases and 15.0 (SD=25.7) in the control patients (P=0.65).
In the multivariable model, case patients were more than twice as likely to have been discontinued from opioids than matched control patients (mOR = 2.19; 95% CI 1.27–3.78; Table 2). The model fit was adequate (P = 0.69), and the assumption of linearity for dose was not violated. Among those exposed to a discontinuation, the attributable risk was 54.24% (95% CI 20.94%−73.51%). The population attributable risk was 20.52% (95% CI 3.56%- 34.50%), implying that 15 of the 74 cases of heroin use were attributed to discontinuation.
Table 2.
Univariate and adjusted associations between opioid discontinuation and heroin use in matched case-control study (74 case and 1045 control patients)
| Characteristic | Univariate matched odds ratio (95% CI) | Adjusted matched odds ratio (95% CI) |
|---|---|---|
|
| ||
| Opioid discontinuation for 45 days or more prior to index date | 2.17 (1.29, 3.66) | 2.19 (1.27, 3.78) |
| Race/Ethnicitya | N/A | |
| Hispanic vs. White | 1.52 (0.83, 2.77) | |
| Black vs. White | 0.46 (0.15, 1.42) | |
| Other racial and ethnic groups vs. White | 1.21 (0.46, 3.17) | |
| Insurance | ||
| Medicaid vs. Commercial | 3.05 (1.69, 5.53) | 2.33 (1.27, 4.26) |
| Medicare vs. Commercial | 0.79 (0.23, 2.79) | 0.76 (0.20, 2.83) |
| Other insurance vs. Commercial | 0.71 (0.17, 3.03) | 0.76 (0.17, 3.37) |
| Modified Charlson Comorbidity Indexb | 1.03 (0.86, 1.22) | N/A |
| Mental health diagnosis | 1.67 (1.02, 2.73) | 1.67 (0.99, 2.82) |
| Tobacco use | 4.10 (2.43, 6.92) | 3.64 (2.12, 6.25) |
| Alcohol use disorder | 1.41 (0.60, 3.30) | N/A |
| Baseline opioid dose,c per 10 morphine milligram equivalents increase | 1.03 (1.01, 1.06) | 1.04 (1.01, 1.06) |
| Ambulatory visits | 1.00 (0.99, 1.01) | N/A |
Abbreviations: CI confidence interval; N/A = not applicable
Missing values were imputed using multiple imputation
Per 1 unit increase in the index score
Assessed in the first month after initiating opioid therapy
Compared with no opioid discontinuations, one discontinuation was associated with heroin use (mOR = 2.54; 95% CI 1.33–4.84) but 2 (mOR = 2.07; 95% CI 0.93–4.58) and ≥3 (mOR = 1.82; 95% CI 0.79–4.20) discontinuations were not significantly associated (eTable B in Supplement). Opioid discontinuations of 30 or more days were associated with heroin use (mOR = 2.30; 95% CI 1.35–3.91; eTable C in Supplement). Among case and control patients with less than 6 months between the discontinuation date and the index date, discontinuation remained associated with heroin use (mOR = 2.77; 95% CI 1.37–5.58; eTable D in Supplement).
Among patients without prior heroin use, discontinuation remained associated with heroin use (mOR = 2.07; 95% CI 1.12–3.83; eTable E in Supplement). After excluding the patient who was discontinued due to heroin use from the analysis, discontinuation remained associated with heroin use (mOR = 2.08; 95% CI 1.19–3.61). After matching by and adjusting for age, discontinuation remained associated with heroin use (mOR = 2.10; 95% CI 1.21–3.66). Including benzodiazepines in the model had little effect on association between discontinuation and heroin use (mOR 2.22; 95% CI 1.29–3.84). Finally, assuming heroin started at the beginning and end of the date ranges (when a date was estimated) resulted in no meaningful change in the estimates (mOR 2.17; 95% CI 1.25–3.77 for beginning and mOR 2.43, 95% CI 1.42–4.15 for end, eTable F and eTable G).
4. Discussion
In this population-based case-control study, patients whose opioid therapy had been discontinued were more than twice as likely to have heroin use that came to medical attention than patients who were not discontinued. While the incidence of heroin use after initiating opioid therapy was low (0.5%), we estimated that nearly one fifth of heroin use among patients prescribed chronic opioid therapy could be explained by discontinuation. Based on these results, patients, providers and policy-makers should heed warnings by the Food and Drug Administration and CDC about the potential adverse effects of discontinuing opioid therapy (Dowell et al., 2019; US Food and Drug Administration, 2019).
The CDC issued a statement in 2019 that warned against the misapplication of its opioid prescribing guidelines (Dowell et al., 2019). It stressed that rapid tapers or abrupt discontinuation of opioid therapy among patients with chronic pain could lead to withdrawal, increased pain, or adverse psychological outcomes. Our findings provide empirical evidence to support these concerns. Our findings are also consistent with a study among publicly insured patients in San Francisco (Coffin et al., 2020), in which heroin use was self-reported. Our study identified a mean length of time between opioid discontinuation and heroin use of 12.9 months. Some individuals who have a source for heroin may begin within days, whereas others may not start for months or even years.
In the secondary analysis evaluating the potential for reverse causation, the medical record review confirmed that the heroin use episodes occurred after the discontinuations and that heroin use was not the reason for the discontinuation in 27 of the 28 exposed case patients. However, the medical record review also suggested that many patients were discontinued because of nonadherence and risk factors for substance use. While discontinuing opioid treatment among these patients may be concordant with some guidelines and standard practice, our results suggest that discontinuing such patients could paradoxically increase their risk for heroin use. Clinicians should therefore carefully consider the potential for heroin use when deciding whether to discontinue opioid therapy.
We found that Medicaid coverage, tobacco use, and higher opioid dose at the start of opioid therapy were associated with a higher odds of heroin use. These associations are consistent with findings from nationally representative samples in which Medicaid status and tobacco use were correlates of heroin use and substance use disorders, respectively (Jones et al., 2015; Weinberger et al., 2017).
In addition to considering the risks of heroin use, our findings should be interpreted in the context of the potential benefits of opioid discontinuation. One prior study shows that discontinuation may reduce the risk of opioid overdose, predominantly from pharmaceutical opioids (Glanz et al., 2019), suggesting that discontinuation could prevent more overdoses than it causes. Additionally, after starting opioid therapy, heroin use appeared to be uncommon, occurring in less than one percent of patients. Further, opioid discontinuation may improve pain, function, and quality of life (Darchuk et al., 2010). However, such benefits may not be achieved if the process of discontinuation is not guideline-concordant or exposes patients to other risks, such as high dose variability (i.e. fluctuating doses (Glanz et al., 2019)). To help clinicians weigh the risks and benefits, additional research on the net benefit or harm of discontinuation is needed.
Effective strategies to reduce or discontinue opioids have not been established (Eccleston et al., 2017; Frank et al., 2017). Nonetheless, recommended approaches include ensuring patient collaboration, gradually reducing doses, monitoring for withdrawal symptoms, treating muscle aches, providing cognitive behavioral therapy for pain, screening for opioid use disorder, and providing buprenorphine treatment when appropriate (Berna et al., 2015; Dowell et al., 2019; Sandhu et al., 2018; Sullivan et al., 2017). Additional research on the effectiveness of these approaches is needed.
We identified patients prescribed opioid therapy who had past histories of heroin use. Patients with a history of heroin use may need aggressive pain management due to low pain thresholds (Compton et al., 2012) but may also be susceptible to the harms of opioid therapy, such as overdose and opioid use disorder. However, if clinicians refrain from treating with opioids because of the past heroin use, patients may seek illicit opioids to manage pain. While it is not currently known how to address these dilemmas, patients with a history of heroin use may benefit from education about overdose risk, naloxone, and medications for opioid use disorder.
This study had several strengths. First, we identified and followed a cohort of patients enrolled in an integrated delivery system and health plan with a mix of public and private health insurance. Second, the system has an EHR platform that allowed us to conduct text string searches and detailed medical record reviews to minimize false positive outcomes and identify the timing of heroin use episodes, rather than relying solely on diagnostic codes for heroin overdose, which would yield only a small proportion of true heroin use cases. We also avoided relying on ICD codes for opioid use disorder, which are not specific for heroin use. Our outcome was measured in a population-based cohort, rather than a selected population that is recruited and incentivized to complete surveys or patient interviews, which are susceptible to selection, recall and social desirability biases. Third, in contrast to a clinical trial, conducting an EHR-based observational study within a delivery system allowed us to assess discontinuation as it occurred in clinical practice. Patients willing to participate in a clinical trial may be motivated to reduce their opioid exposure and therefore be at lower risk for using heroin after discontinuation than patients who are discontinued in routine medical practice. Further, a clinical trial may not have been able to recruit an adequate sample size to detect a difference in heroin use, a relatively rare outcome.
4.1. Limitations
A key challenge to assessing the relationship between opioid discontinuation and heroin in an observational study is the potential for reverse causation. We employed several methods to minimize this potential. First, we searched text in medical records and conducted medical record reviews to identify the timing of heroin use episodes. Second, we excluded patients whose previous heroin use overlapped with initiating opioid therapy. Third, we reviewed records to determine whether case patients were exposed to a discontinuation due to heroin use. We conducted sensitivity analyses excluding the one case that appeared to have been discontinued due to heroin use and excluding patients who had any prior heroin use, neither of which had a significant impact on the main results.
Although we employed a medical record review, we excluded 18 heroin use cases due to inadequate documentation of dates and some heroin use episodes may have been misclassified with respect to discontinuation. For example, some patients may have underestimated the duration of their heroin use to their clinicians and some clinicians may not have reliably documented heroin use episodes or dates. In these instances, the onset of some heroin use episodes classified as occurring after an opioid discontinuation may have begun before the discontinuation. This type of misclassification would have led us to overestimate the association between discontinuation and heroin use. However, our secondary analyses varying the onset date did not have a significant impact on the results. While our case validation methods were designed to maximize specificity, we did not capture heroin use among patients who did not obtain related medical care, seek opioid use disorder treatment, or experience an overdose, reducing sensitivity. This implies that some control patients may have used heroin. If discontinuation truly contributes to heroin use, this type of misclassification could have led to an underestimation of the association between discontinuation and heroin use. It is also possible that patients who were discontinued lost their health insurance and started using heroin during a period of disenrollment. This type of loss-to-follow-up could also have led to an underestimation of the association.
While we designed this study to maximize the internal validity of the results, it was conducted in a single health system and the majority of the cohort was white, which may limit its representativeness. Our study primarily occurred before large increases in deaths from fentanyl in our state and before routine toxicologic testing for fentanyl was available in our health system. Further studies should examine fentanyl use as a potential adverse effect of opioid discontinuation.
5. Conclusions
The CDC Guideline (Dowell et al., 2016) recommends that clinicians carefully re-evaluate the harms and benefits of ongoing opioid treatment. Our findings suggest that public health practitioners should consider how to mitigate the potential for heroin use as an adverse effect of opioid discontinuation.
Supplementary Material
Acknowledgements
We wish to acknowledge the valuable data collection assistance of Kris Wain, MS, and J. David Powers, MS, Institute for Health Research, Kaiser Permanente Colorado. We acknowledge the analysis support of Colleen Ross, MS, MPH and Susan Shetterly, MS. We also acknowledge the medical record review assistance of Jennifer Sawyer, Valerie Paolino and Jenn Ellis, MBA, MSPH. Their activities were supported by the grant, and they have given permission to be mentioned here.
Role of Funding Source
This research was supported by award U01CE002791 from the Centers for Disease Control and Prevention. The findings and conclusion of this research are those of the authors and do necessarily represent the official position of the CDC.
Footnotes
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2020.108248.
Declarations of interest
None
References
- Berna C, Kulich RJ, Rathmell JP, 2015. Tapering long-term opioid therapy in chronic noncancer pain: evidence and recommendations for everyday practice. Mayo. Clin. Proc 90, 828–842. [DOI] [PubMed] [Google Scholar]
- Bohnert ASB, Guy GP, Losby JL, 2018. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann. Intern. Med 169, 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C, 1985. Estimating the population attributable risk for multiple risk factors using case-control data. Am. J. Epidemiol 122, 904–914. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2011. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR. Morb. Mortal. Wkly. Rep 60, 1487. [PubMed] [Google Scholar]
- Ciccarone D, 2017. Fentanyl in the US heroin supply: a rapidly changing risk environment. Int. J. Drug Policy 46, 107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Rowe C, Oman N, Sinchek K, Santos GM, Faul M, Bagnulo R, Mohamed D, Vittinghoff E, 2020. Illicit opioid use following changes in opioids prescribed for chronic non-cancer pain. PloS One 15, e0232538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P, Canamar CP, Hillhouse M, Ling W, 2012. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J. Pain 13, 401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darchuk KM, Townsend CO, Rome JD, Bruce BK, Hooten WM, 2010. Longitudinal treatment outcomes for geriatric patients with chronic non-cancer pain at an interdisciplinary pain rehabilitation program. Pain Med. 11, 1352–1364. [DOI] [PubMed] [Google Scholar]
- Dasgupta N, Beletsky L, Ciccarone D, 2018. Opioid crisis: no easy fix to its social and economic determinants. Am. J. Public Health 108, 182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deyo RA, Cherkin DC, Ciol MA, 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol 45, 613–619. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich T, Chou R, 2019. No shortcuts to safer opioid prescribing. N. Engl. J. Med 380, 2285–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R, 2016. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA 315, 1624–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration, 2016. National Heroin Threat Assessment Summary-Updated. Available at https://www.dea.gov/divisions/hq/2016/hq062716_attach.pdf Accessed 9/11/2019. Washington DC: Department of Justice. .
- Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, 2010. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann. Intern. Med 152, 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C, Fisher E, Thomas KH, Hearn L, Derry S, Stannard C, Knaggs R, Moore RA, 2017. Interventions for the reduction of prescribed opioid use in chronic non-cancer pain. Cochrane Database Syst. Rev 11, CD010323. 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JW, Lovejoy TI, Becker WC, Morasco BJ, Koenig CJ, Hoffecker L, Dischinger HR, Dobscha SK, Krebs EE, 2017. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann. Intern. Med 167, 181–191. [DOI] [PubMed] [Google Scholar]
- Gladden RM, 2016. Fentanyl law enforcement submissions and increases in synthetic opioid–involved overdose deaths—27 states, 2013–2014. MMWR. Morb. Mortal. Wkly. Rep 65, 837–843. [DOI] [PubMed] [Google Scholar]
- Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu S, 2019. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Netw. Open 2, e192613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Warner M, 2020. Drug overdose deaths in the United States, 1999–2018, NCHS Data Brief. National Center for Health Statistics, Hyattsville, MD. [Google Scholar]
- James JR, Scott JM, Klein JW, Jackson S, McKinney C, Novack M, Chew L, Merrill JO, 2019. Mortality after discontinuation of primary care–based chronic opioid therapy for pain: a retrospective cohort study. J. Gen. Intern. Med 34, 2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Logan J, Gladden RM, Bohm MK, 2015. Vital signs: demographic and substance use trends among heroin users—United States, 2002–2013. MMWR. Morb. Mortal. Wkly. Rep 64, 719. [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, Haake KJ, Hanling S, Hooten WM, Kertesz SG, 2019. Challenges with implementing the Centers for Disease Control and Prevention opioid guideline: a consensus panel report. Pain Med. 20, 724–735. [DOI] [PubMed] [Google Scholar]
- Langholz B, Goldstein L, 1996. Risk set sampling in epidemiologic cohort studies. Stat. Sci 35–53. [Google Scholar]
- Martin BC, Fan M-Y, Edlund MJ, DeVries A, Braden JB, Sullivan MD, 2011. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J. Gen. Intern. Med 26, 1450–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences Engineering and Medicine, 2019. Pain Management for People with Serious Illness in the Context of the Opioid Use Disorder Epidemic: Proceedings of a Workshop. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Oliva EM, Bowe T, Manhapra A, Kertesz S, Hah JM, Henderson P, Robinson A, Paik M, Sandbrink F, Gordon AJ, 2020. Associations between stopping prescriptions for opioids, length of opioid treatment, and overdose or suicide deaths in US veterans: observational evaluation. BMJ 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi J-C, Saunders LD, Beck CA, Feasby TE, Ghali WA, 2005. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 1130–1139. [DOI] [PubMed] [Google Scholar]
- Safe States Aliance, 2012. Consensus recommendations for national and state poisoning surveillance. The Safe States Atlanta Injury Surveillance Workgroup; 7, Atlanta, GA. [Google Scholar]
- Sandhu H, Underwood M, Furlan A, Noyes J, Eldabe S, 2018. What interventions are effective to taper opioids in patients with chronic pain? BMJ 362, k2990. [DOI] [PubMed] [Google Scholar]
- Sullivan MD, Turner JA, DiLodovico C, D’Appollonio A, Stephens K, Chan Y-F, 2017. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J. Pain 18, 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Department of Health and Human Services, 2018. Strategy to combat opioid abuse. misuse and overdose Accessed April 24, 2020. https://www.hhs.gov/opioids/sites/default/files/2018-09/opioid-fivepoint-strategy-20180917-508compliant.pdf.
- US Food and Drug Administration, 2019. FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Accessed April 24, 2020. https://www.fda.gov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes. [Google Scholar]
- Vanderlip ER, Sullivan MD, Edlund MJ, Martin BC, Fortney J, Austen M, Williams JS, Hudson T, 2014. National study of discontinuation of long-term opioid therapy among veterans. Pain 155, 2673–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Korff M, Saunders K, Ray GT, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter C, Silverberg M, Banta-Green C, 2008. Defacto long-term opioid therapy for non-cancer pain. Clin. J. Pain 24, 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacholder S, Silverman DT, McLaughlin JK, Mandel JS, 1992. Selection of controls in case-control studies: III. Design options. Am. J. Epidemiol 135, 1042–1050. [DOI] [PubMed] [Google Scholar]
- Weinberger AH, Platt J, Esan H, Galea S, Erlich D, Goodwin RD, 2017. Cigarette smoking is associated with increased risk of substance use disorder relapse: a nationally representative, prospective longitudinal investigation. J. Clin. Psychiatry 78, e152–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.