Abstract
Mycobacterium tuberculosis complex (MTBC) is a group of bacteria responsible for causing tuberculosis in animals and humans. In South Africa (S.A), slaughterhouses are registered by the government and closely inspected and audited for hygienic slaughter practices. Meat inspection to detect lesions has been used for passive surveillance, monitoring, and diagnosis of the disease status. Information on the current status of bovine tuberculosis (bTB) in livestock in the country is limited. Hence, we investigated the occurrence of Mycobacterium spp. in the tissues of slaughtered livestock and environmental samples in abattoirs in Gauteng province of South Africa (S.A). The cross-sectional study employing random sampling from cattle, pigs, and sheep (with the collection of liver, lung, spleen, and different lymph nodes) irrespective of lesions was carried out in 19 red meat abattoirs. Five hundred animals were sampled, comprising cattle (n = 369), pigs (n = 90), and sheep (n = 41). Additionally, 19 environmental samples were collected from feedlots, or where animals drink water while awaiting slaughter, to identify mycobacterial species using culture, acid-fast bacteria staining, and polymerase chain reaction (PCR). The Chi-square and Fisher's Exact tests were used to detect statistically significant differences in the frequency of detection of Mycobacterium spp. according to the variables investigated (types of tissues, livestock, abattoirs, etc.). The PCR assays detected no MTBC complex species DNA in the bacterial isolates from cattle (n = 32). Sequence analysis (16S rDNA) of the isolates from eight cattle confirmed only two species, namely Mycobacterium colombiense (99.81% identity) and Mycobacterium simiae (99.42% identity). The remaining isolates were identified as members of the Actinomadura species. From the environmental samples, bacterial isolation was made from three samples, and two could only be identified up to the genus level (Mycobacterium species) while the remaining isolate was identified as Mycobacterium senuense (99.22% identity). The study revealed the absence of bovine tuberculosis-causing pathogens in red meat abattoirs of the Gauteng province. Although non-tuberculous Mycobacteria have been implicated as potentially causing tuberculosis-like diseases in livestock, their occurrence in the current study was found to be low, but the potential to cause disease cannot be ignored.
1. Introduction
Bovine tuberculosis (bTB), caused mainly by Mycobacterium bovis, is a zoonotic disease with economic losses estimated at billions of dollars annually worldwide, yet the economic impact of the disease in African countries remains unquantified [1]. In South Africa, TB in cattle is mostly known to be caused by M. bovis and it is classified as a controlled disease according to the national Animal Diseases Act, Act 35 of 1984. Control strategies have been put in place since 1969, and reduced the disease prevalence in communal cattle herds to less than 1%, although sporadic outbreaks still occur [2, 3]. The true disease prevalence in different provinces of the country including Gauteng province is however, currently unknown, more especially in communal cattle populations. This is mostly due to the fact that control of the disease has become less prioritised by the government over the years due to lack of funding [4].
In S.A, slaughtering of livestock is officially conducted at abattoirs, although some back yard slaughtering also occur. Official abattoirs are registered by government and closely inspected and audited for hygienic slaughter practices [4]. Meat inspection in slaughterhouses to detect lesions has been used for passive surveillance, monitoring, and diagnosis of the disease [5]. However, this method only detects lesions in advanced stages of infection when visible lesions are observed [6]. Furthermore, it should be noted that lesions observed are not only due to M. bovis or other members of the Mycobacterium tuberculosis complex (MTBC), but some members of the Mycobacterium avium complex (MAC) and other non-tuberculous mycobacteria (NTM) are also both pathogenic and opportunistic and may produce lesions in the infected animals [7]. Hence, confirmation of the causative agent is of outmost importance, as NTM were previously also found to interfere with cell mediated immunity (CMI) response diagnostic tests for bTB [8, 9]. Studies have suggested that meat inspection should be done together with routine culturing and followed by molecular methods for characterizing different mycobacterial species [10] especially in regions with a high TB burden and where NTMs remain undiagnosed [11]. It has been reported that approximately one-third of the NTMs are responsible for human disease [12]. In the last three decades, there has been a recorded increase in NTM laboratory isolation [13]. In a study by Oloya [14] in Uganda, 19 M. bovis and 11 NTM were isolated from 61 tissue samples from slaughter livestock, confirming the widespread prevalence of bTB in this country. In another study, Awah-Ndukum et al. [15] confirmed the presence of bTB by isolating M. bovis from samples collected at abattoirs. In a recent study, from Rwanda, both M. bovis and Mycobacterium tuberculosis were isolated from slaughtered cattle, indicating that bTB is present in this country, although at low prevalence [16]. Molecular epidemiology of bTB is useful in the determination of risk factors of bTB transmissions, identification of the sources of contamination, tracking of the geographic distribution, and the spread of Mycobacterial species.
Information on the current status of bTB in livestock in the country is limited. Hence, we investigated the occurrence of Mycobacterium spp. in the tissues of slaughtered livestock and environmental samples in abattoirs in Gauteng province of S.A and determined the possible food safety risks posed to meat consumers.
2. Materials and Methods
2.1. Description of the Study Area, Abattoirs, Sample Type, and Size Determination
This study was conducted in 19 red meat abattoirs across the Gauteng province of South Africa and was done in parallel with our previous study [17]. Briefly, the investigators obtained a list of functional red meat abattoirs (mono- and multi-species) located across Gauteng province from the Gauteng Department of Agriculture and Rural Development (GDARD). Based on the information provided for each abattoir which included the type and number of livestock slaughtered daily, location, and their facilities, a total of 19 abattoirs were randomly selected, comprising 16 high-throughput (HT) and three low-throughput (LT) abattoirs for the study. The study was conducted in all six districts of Gauteng province, namely, the City of Johannesburg, the City of Tshwane, Ekurhuleni, Metsweding, Sedibeng, and West Rand (Figure 1). At each selected abattoir, slaughtered cattle, pigs, and sheep were sampled on a single day using a systematic random sampling method, and the abattoir setting determined this. Approximately 30 animals were sampled during each sampling visit to the abattoir, and the number sampled per livestock species was proportional to the expected number to be slaughtered on that day, as provided by the abattoir workers. The prevalence of Mycobacterium spp. in tissues collected from slaughtered livestock in South Africa is currently unknown. The prevalence was therefore estimated to be 50% based on the standard practice used for unknown prevalence, and sample size determination was calculated using the formula no = 1.962 × Pexp × (1 − Pexp)/d2 recommended by Thrusfield [18], where no represented the minimum sample size, Pexp represented the expected prevalence and d2 represented the desired precision value of 5%. Although the minimum estimated sample size was 384, a total of 500 animals were sampled, and the distribution per livestock species (cattle, sheep, and pigs) was proportional to the number of animals slaughtered per species during abattoir visits.
Figure 1.
Map showing the six districts where red meat abattoirs sampled are located within the Gauteng province of South Africa.
2.2. Pre- and Post-Slaughter Inspection
The professional meat inspectors assigned to each abattoir conducted pre- and post-slaughter inspections according to a standard procedure, which included detecting clinical TB lesions as previously described [17]. Biographical information about the slaughtered livestock, including gender, breed, district, and municipality origin of animals, was recorded [17].
2.3. Sample Collection
While the inspectors were conducting physical examinations of the carcasses, the following organs were collected, i.e., lymph nodes (retropharyngeal, abdominal, mesenteric), liver, lung, and spleen for the Mycobacterium species culture test. A total of 500 animals were sampled, i.e., cattle (n = 369), pigs (n = 90), and sheep (n = 41). Overall, 2000 tissue samples were collected from different organs of each animal. Tissue samples were labelled and placed in sterile Ziploc bags or specimen containers. All the tissues were transported to the Tuberculosis laboratory at the Agricultural Research Council-Onderstepoort Veterinary Research (ARC-OVR) in ice-cooled boxes. Environmental samples (water; n = 19), one from each abattoir, were collected from feedlots or where animals drink water while waiting for slaughter. All the collected environmental and tissue samples were cultured according to established standard laboratory protocols and monitored weekly for bacterial growth.
2.4. Isolation of Mycobacterium Species from Tissue Samples
To culture tissue samples for Mycobacterium spp., standard methods available in the ARC-OVR Tuberculosis laboratory were used [8]. Approximately 944 samples from 236 animals were individually processed. Representatives from the same animals were processed as a pool for the remaining 1056 samples (264). Briefly, the collected tissue samples were cut into approximately 5 g pieces, and all the fat was removed. Using the Ultra-Turrax® homogenizer (Separation Scientific, SA), the samples were then homogenized. The homogenates were poured into two 50 ml tubes in preparation for the decontamination. Approximately 7 ml of each homogenized sample was transferred into two 15 ml centrifuge tubes each and then decontaminated in the first tube with HCL to a final concentration of 1.0% and in the second tube with NaOH to a final concentration of 2.0% for 10 minutes, followed by centrifugation (3500 rpm). The Lowenstein-Jensen (LJ) media supplemented with pyruvate (4 slopes) and glycerol (2 slopes) was inoculated with the sample pellets following neutralization with sterile distilled water and centrifugation (3500 rpm). For each batch of samples tested, positive and negative controls were included. The positive control was a sample collected from either skin test positive/suspect reactor or slaughter cattle with suspect tuberculous lesions and confirmed by culture and PCR, while the negative control sample was collected from a carcass declared fit for human consumption and underwent microbiological testing for confirmation. The inoculated media slopes were incubated at 37°C for 10 weeks and examined weekly for bacterial growth (colonies).
2.5. Isolation of Mycobacterium Species from Water Samples
For the isolation of Mycobacterium species, water samples were collected from the selected abattoirs and cultured according to the standard laboratory protocol at the Tuberculosis laboratory. Positive (water sample spiked with a colony of an in-house Mycobacterium bovis, TB 9854A) and negative (unspiked sterile distilled water) controls were included for each batch of samples tested. Briefly, 2% NaOH was added to the samples and centrifuged, thereafter 5.0% oxalic acid was added. After centrifugation, Lowenstein-Jensen (LJ) slopes (3x) were inoculated with the sample and incubated at 27°C. Also, another set was inoculated and incubated at 37°C and monitored for up to 10 weeks for colonies typical of Mycobacterium species. Thereafter, colonies were selected for Ziehl-Neelsen staining and PCR identification.
2.6. Ziehl-Neelsen Staining and Microscopy
The presence of colonies following the detection of growth on the selective media after incubation was suggestive of the presence of Mycobacteria, and bacterial smears were prepared on microscopic slides for Ziehl-Neelsen (ZN) staining to confirm acid-fastness. The ZN-stained smears were then observed under a microscope.
2.7. DNA Extraction in Preparation for Polymerase Chain Reaction
DNA extraction was conducted as previously described [19]. DNA templates were prepared from colonies typical of Mycobacterium species, and individual colonies were picked up from the L-J media and mixed with 100 μl ultra-pure water. DNA was extracted from isolates by heat-treating the suspension at 100°C for 25 minutes and allowed to cool down at room temperature. DNA templates were stored at −20°C until PCR analysis [19].
2.8. Identification of Mycobacterium tuberculosis Complex Species
To identify Mycobacterium tuberculosis complex species (MTBC), a modified PCR assay was conducted using primers targeting the region encoding MPB 70 antigen belonging to MTBC (forward primer: 5′ GAACAATCCGGAGTTGACAA 3′ and reverse primer: 5′ AGCACGCTGTCAATCATGTA 3′) [20]. Briefly: a 50 μl reaction consisting of 25 μl ultra-pure water, 5 μ of 10x buffer, 3 μl of 25 mM MgCl2, 2.5 μl of dNTP mix (1 mM), 2 μl of each TB 1A (20 pmol/μl) and TB 1B (20 pmol/μl) primers, 10 μl of the acid-fast bacterial lysate (DNA template). The enzyme 0.5 μl of Taq polymerase (supertherm) was added, and the reaction mixture was placed in a thermocycler under appropriate PCR conditions. PCR cycling conditions were as follows: Initial denaturation at 94°C for 5 minutes, denaturation at 94°C for 30 seconds, annealing at 64°C for 30 seconds, and extension at 72°C for 2 minutes for 40 cycles [19, 20].
2.9. Gel Electrophoresis
The PCR products were visualized on a 1.5% agarose gel stained with 20 μl ethidium bromide (10 μg/ml) and run at 80 V for 3 h. A 100 bp ladder (Inqaba Biotechnical Industries) was included and used to estimate the size of the resulting PCR products. Positive and negative controls (previously tested DNA templates) were included for quality control. In addition, distilled water was included as a blank control.
2.10. Identification of Non-Tuberculous Mycobacterium spp. by PCR and Sequence Analysis
Non-tuberculous mycobacteria (NTM) were identified by PCR and sequence analysis of the 577 bp of the Mycobacterium 16S rDNA gene using the following primers: 16S rDNA forward 5′ AGA GTT TGA TCC TGG CTC AG 3′ and 16S rRNA reverse 5′ GCG ACA AAC CAC CTA CGA G 3′ as previously described [9]. The mycobacterial cell lysate was used as a DNA template in a 25 μl PCR mixture containing 12.4 μl deionized water, 2.5 μl of 10x PCR buffer (160 mM) (Tris Cl, KCl, (NH4)2SO4), 2 μl MgCl2 (25 mM), 1 μl dNTPs (10 mM), 0.1 μl Taq polymerase (Qiagen Hotstar Taq, Whitehead Scientific, South Africa), 5 μl of 5x Q-solution, 1 μl of each forward and reverse primers (50 pmol) and 1-2 μl DNA template. The PCR cycling parameters were as follows: initial denaturation at 95°C for 15 minutes, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 30 seconds and a final extension at 72°C for 10 minutes. The amplicons were sent to Inqaba Biotechnical Industries, Ltd, SA, for the forward 16S rRNA gene sequencing using an ABI sequencer. Sequences were edited manually, and pairwise alignments were undertaken using the BioEdit Sequence alignment editor (version 7.1.9). The sequences were analyzed on the NCBI BLAST platform for species identification by the mega blast [9].
2.11. Data Analysis
The data obtained was entered into Microsoft Excel (Microsoft, United States) database and only descriptive analysis was conducted. Analysis of the data was based on the prevalence of Mycobacterium spp. based on individual positivity, regardless of the tissues (lymph nodes, liver, lungs, and spleen). For the study, the dependent variable was the isolation of Mycobacterium spp., and the independent variables were the type, gender, and breed of animal species, livestock origin, district, municipality, and abattoirs (HT and LT). Chi-square and Fisher's Exact analysis were conducted to determine whether there were statistically significant differences in the frequency of isolation of Mycobacterium spp. among the livestock tissues tested according to the independent variables mentioned above. The level of significance was determined at an alpha level of 0.05.
3. Results
3.1. Sample Collection
A total of 500 animals were sampled, comprising 369 adult cattle (Bonsmara, n = 277; Jersey, n = 39; Nguni, n = 51; Brahman, n = 1; Holstein, n = 1), 90 pigs and 41 sheep (Dorper) of any gender were collected (Tables 1–3).
Table 1.
Characteristics of cattle sampled and the estimated prevalence of Mycobacterium species in Gauteng abattoirs.
| Variable | Level | N | Prevalence (%) |
|---|---|---|---|
| Species | Bovine | 369 | 0.54 |
|
| |||
| Gender | Female | 92 | 0 |
| Male | 277 | 0.54 | |
|
| |||
| Breed | Bonsmara | 275 | 0.54 |
| Nguni | 51 | 0 | |
| Jersey | 43 | 0 | |
| Brahman | 0 | 0 | |
| Holstein | 0 | 0 | |
|
| |||
| District | Tshwane | 156 | 0.27 |
| Sedibeng | 112 | 0 | |
| Metsweding | 14 | 0 | |
| West Rand | 30 | 0 | |
| Ekurhuleni | 57 | 0.27 | |
|
| |||
| Municipality | City of Tshwane | 156 | 0.27 |
| Ekurhuleni Metro | 57 | 0.27 | |
| Emfuleni | 52 | 0 | |
| Kungwini | 14 | 0 | |
| Lesedi | 60 | 0 | |
| Mogale City | 30 | 0 | |
|
| |||
| Abattoirs | Abattoir A | 30 | 0 |
| Abattoir B | 30 | 0 | |
| Abattoir C | 30 | 0 | |
| Abattoir D | 22 | 0 | |
| Abattoir E | 30 | 0 | |
| Abattoir P | 27 | 0.27 | |
| Abattoir G | 30 | 0 | |
| Abattoir H | 30 | 0 | |
| Abattoir I | 30 | 0 | |
| Abattoir J | 30 | 0 | |
| Abattoir K | 28 | 0.27 | |
| Abattoir M | 30 | 0 | |
| Abattoir N | 14 | 0 | |
| Abattoir O | 8 | 0 | |
|
| |||
| Origin of animals | Auctions | 58 | 0.27 |
| Farm/feedlots | 311 | 0.27 | |
|
| |||
| Abattoir type | HT-multi | 347 | 0.54 |
| LT-multi | 22 | 0 | |
|
| |||
| Total | 369 | 0.54 | |
N: indicates number of cattle sampled.
Table 2.
Characteristics of sheep sampled in the study and the estimated prevalence of Mycobacterium species in Gauteng abattoirs.
| Variable | Level | N | Prevalence (%) |
|---|---|---|---|
| Species | Ovine | 41 | 0 |
|
| |||
| Gender | Female | 14 | 0 |
| Male | 27 | 0 | |
|
| |||
| Breed | Dorper | 41 | 0 |
|
| |||
| District | Tshwane | 34 | 0 |
| Metsweding | 7 | 0 | |
|
| |||
| Municipality | City of Tshwane | 4 | 0 |
| Kungwini | 37 | 0 | |
|
| |||
| Abattoir | Abattoir L | 30 | 0 |
| Abattoir N | 7 | 0 | |
| Abattoir K | 4 | 0 | |
|
| |||
| Origin of animals | Auctions | 4 | 0 |
| Farm/feedlots | 37 | 0 | |
|
| |||
| Abattoir type | HT-multi | 34 | 0 |
| LT-multi | 7 | 0 | |
|
| |||
| Total | 41 | 0 | |
N: indicates the number of sheep sampled.
Table 3.
Characteristics of pigs sampled in the study and the estimated prevalence of Mycobacterium species in Gauteng abattoirs.
| Variable | Level | N | Prevalence (%) |
|---|---|---|---|
| Species | Porcine | 90 | 0 |
|
| |||
| Gender | Female | 40 | 0 |
| Male | 50 | 0 | |
|
| |||
| Breed | Large white | 90 | 0 |
|
| |||
| District | Sedibeng | 50 | 0 |
| West Rand | 20 | 0 | |
| Ekurhuleni | 20 | 0 | |
|
| |||
| Municipality | City of Johannesburg | 20 | 0 |
| Ekurhuleni Metro | 20 | 0 | |
| Midvaal | 20 | 0 | |
| Lesedi | 30 | 0 | |
|
| |||
| Abattoirs | Abattoir F | 30 | 0 |
| Abattoir Q | 20 | 0 | |
| Abattoir R | 20 | 0 | |
| Abattoir S | 20 | 0 | |
|
| |||
| Origin of animals | Auctions | 30 | 0 |
| Farm/feedlots | 60 | 0 | |
|
| |||
| Abattoir type | HT-multi | 50 | 0 |
| LT-multi | 40 | 0 | |
|
| |||
| Total | 90 | 0 | |
N: indicates the number of pigs samples.
3.2. Bacterial Isolation from Tissue Samples and Ziehl Neelsen Staining
Tissue samples originating from 19 different red meat abattoirs located in the Gauteng province (Figure 1) were collected and tested at the Tuberculosis Laboratory at the ARC-OVR for Mycobacterium spp. isolation. Colonies of bacterial growth typical of mycobacteria were observed. Bacterial isolation was made from 32 samples representing individual cattle from 12 different red meat abattoirs. Because of the single colony growth from most of the samples, only a few could be subjected to Ziehl Neelsen staining to confirm acid fastness. No isolation was made from pig and sheep samples.
3.3. Identification of Mycobacterium tuberculosis Complex Species by PCR
Out of the 32 isolates, no MTBC complex species was detected.
3.4. Characterization of Mycobacterium Isolates from Tissue Samples by 16S rRNA PCR and Gene Sequence Analysis
Out of the 32 isolates, 8 (25%) from individual animals originating from 4 abattoirs displayed a 577 bp PCR product by 16S rRNA PCR analysis (Figure 2). The 16S rRNA gene sequence analysis following NCBI blasting identified only two of the isolates as Mycobacteria, i.e., Mycobacterium colombiense (99.81% identity) isolated from cattle from animal # 18, which originated from abattoir P; and Mycobacterium simiae (99.42%) isolated from cattle from animal # 27 sampled at abattoir K. The remaining 6 isolates were identified as members of the Actinomadura species, hence the overall prevalence of Mycobacterium spp. in cattle slaughtered at Gauteng abattoirs was estimated to be 0.5%.
Figure 2.
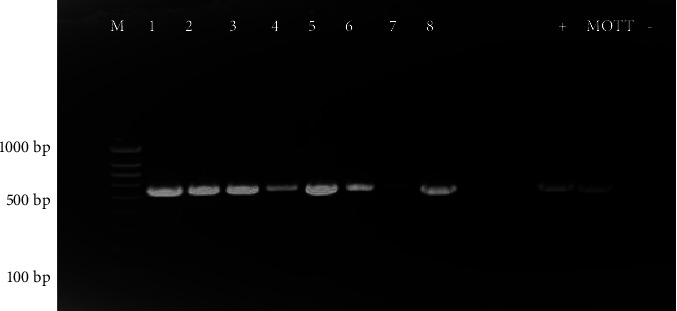
Gel electrophoresis results obtained from 16S rRNA PCR analysis. Lane M is a 100 bp DNA marker; lane 1 represents an isolate from abattoir N, lanes 2–4 represent isolates from abattoir P, lanes 5–7 represent isolates from abattoir B while lane 8 represents an isolate from abattoir K, lane + represent positive control (TB 9845A), lane MOTT represents a Mycobacteria positive control (TB9801B) and lane-represents the negative control which was distilled water.
3.5. Isolation of Mycobacteria from Environmental Samples
Bacterial isolation was made from water samples from five abattoirs: L, P, O, G, and D. All isolates were confirmed as acid-fast by Ziehl-Neelsen staining.
3.6. Characterization of Mycobacterium spp. from Environmental Samples by 16S rRNA PCR and Gene Sequence Analysis
Bacterial isolation was made from five of the 19 (26.3%) environmental samples. Specific PCR amplification was obtained from four of the five (15.8%) acid-fast isolates, and the remaining isolate was negative. The expected size of the PCR product was approximately 577 bp indicative of a mycobacterium species or closely related species. A weak PCR product was however observed for an isolate from abattoir P (results not shown), hence it could not be submitted for sequencing. Sequence data analysis following NCBI blasting identified one of the isolates up to genus level (Mycobacterium species at 98.89% identity). This isolate was from a sample collected at the abattoir G. Mycobacterium avium (99.61% identity) was isolated from a sample collected at abattoir L, and Mycobacterium senuense (99.02% identity) from abattoir D. The overall prevalence of Mycobacteria isolated from environmental samples was 15.8%.
4. Discussion
In this study, no MTBC species were isolated from tissues of slaughtered livestock and environmental samples in abattoirs in the Gauteng province of S.A, and no possible food safety risks were posed to meat consumers. This is not a surprise because no lesions resembling M. bovis infection were visible on any of the samples processed. Tuberculosis-like lesions are an indicator of the presence of tuberculosis [21, 22]. According to Botha et al. [23], tuberculosis is a slow and progressive disease that remains asymptomatic for an extended period until the advanced stages of infection when lesions appear. This may have been the case in this study that when livestock are taken to the abattoirs for slaughter, the animals could still be in the early stages of infection and the bacterial concentrations are too low to be detected by culture. In South Africa, implementing the national bovine tuberculosis programme, especially in commercial cattle, decreased the national prevalence of bovine tuberculosis to 0.4% over 25 years ago, from 11.85% over half-century ago [3] with sporadic outbreaks still occurring [2]. In communal cattle farming, the disease prevalence is largely still unknown, with a few isolated studies reporting varying prevalence ranging from less than 0.5% to more than 15% [24, 25]. It should be noted that animals sampled in the current study were from auctions and feedlots originating from different provinces of the country. The prevalence of bovine tuberculosis in SA is however, still low contrary to other African countries such as Zambia, Uganda, and Ethiopia, where herd prevalence of up to 50% have been reported in cattle [14, 26, 27]. No isolation was made from pigs and sheep sampled in the current study. Tuberculosis in sheep is generally rare and there are conflicting opinions regarding the susceptibility of sheep to MTBC species. Considering the chronic nature of the disease, potentially infected sheep get slaughtered before the disease develops [28, 29]. With that said, a few studies have however, reported cases of M. bovis infection in sheep [29–31]. Although bovine tuberculosis in sheep has never been reported in South Africa [17], known zoonotic mycobacteria, including Mycobacterium avium subsp. hominisuis, Mycobacterium avium subsp. avium, Mycobacterium intracellulare, Mycobacterium bovis and Mycobacterium tuberculosis were previously isolated from slaughtered pigs during the years 1991–2002, hence a potential public health concern [32].
In our previous study, conducted in parallel with the current research, Mareledwane [17] reported an estimated bTB seroprevalence of 4.4% in cattle, indicating past exposure or detection of early infection, an advantage of the cell-mediated immune response-based tests. Although a 0.0% prevalence (absence) for MTBC species was recorded in the current study, NTM were isolated, with the overall prevalence of Mycobacterium spp. estimated to be 0.5% for cattle and 15.8% for environmental sample. Non-tuberculous mycobacteria isolated include Mycobacterium colombiense, Mycobacterium simiae, Mycobacterium avium, and Mycobacterium senuense. It is well known that non-tuberculous mycobacteria interfere with the most widely used tests for the diagnosis of bovine tuberculosis such as the intradermal tuberculin test as well as the gamma interferon test, hence of outmost importance to identify such interferences [9, 33]. The NTMs identified in the current study were previously found to occur in the environment and animals during a country wide study conducted [9].
One of the NTM isolated in our study, M. simiae, has been shown to cause pathological conditions, especially in humans, and has been isolated more especially in immunocompromised patients [34]. It has been reported that infection by this NTM leads to extrapulmonary infections [35].
This is the first study to investigate the occurrence of Mycobacteria and to isolate NTMs from slaughtered cattle in red meat abattoirs in the Gauteng province of SA. In a previous report, Hlokwe and co-workers isolated Mycobacterium nonchromogenicum and an NTM species closely related to Mycobacterium moriokaense from cattle on two farms in the Eastern Cape province of South Africa. Similar to the outcome of the current study, these NTM species were detected from samples without visible lesions, hence their clinical significance could not be determined but the potential to cause disease cannot be ignored [8].
5. Conclusion
The current study demonstrated a very low prevalence of Mycobacterium species in Gauteng red meat abattoirs as confirmed by culture and molecular assays, hence posing a low risk of infection to meat consumers. Importantly, the study demonstrated the absence (0.0% prevalence) of MTBC species in all the abattoir samples tested. However, future studies should pay attention to NTM isolated from abattoirs, as their role in causing mycobacteriosis both in humans and animals still needs to be better understood.
Acknowledgments
The authors thank Dr. Charles Katsande, meat inspectors, abattoir managers, and abattoir personnel for their assistance with sampling and sampling arrangements. The authors also thank Dr. Nomakorinte Gcebe for reviewing the manuscript. This research was funded by the Gauteng Department of Agriculture and Rural Development (GDARD), the Health and Welfare Sector Education and Training Authority (HWSETA), and the Red Meat Research and Development South Africa (RMRD-SA; project no. P10000142). The authors thank the ARC-Onderstepoort Veterinary Research for publication funding. Open Access funding enabled and organized by SANLiC Gold.
Data Availability
All data are available from the author upon reasonable request.
Ethical Approval
The Animal Ethics Committees of the Agricultural Research Council-Onderstepoort Veterinary Research (AEC 12.16) and the University of Pretoria (V104–17) approved the study. Authorization to carry out the study at the abattoir was granted by the Department of Agriculture, Land Reforms and Rural Development (DALRRD) through section 20 approval (reference 12/11/1/1/6).
Disclosure
The work was presented in the form of a poster at the 2024 ARC-DALRRD conference. The funders played no role in the views expressed in the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Asebe G. Molecular characterization of Mycobacterium bovis and its significance: role for control of zoonotic tuberculosis in Africa. Journal of Medical Diagnostic Methods . 2017;6(3):1–10. doi: 10.4172/2168-9784.1000250. [DOI] [Google Scholar]
- 2.Hlokwe T. M., Van Helden P., Michel A. L. Evidence of increasing intra and inter-species transmission of Mycobacterium bovis in South Africa: are we losing the battle? Preventive Veterinary Medicine . 2014;115(1-2):10–17. doi: 10.1016/j.prevetmed.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Michel A. L. Mycobacterium fortuitum infection interference with Mycobacterium bovis diagnostics: natural infection cases and a pilot experimental infection. Journal of Veterinary Diagnostic Investigation . 2008;20(4):501–503. doi: 10.1177/104063870802000415. [DOI] [PubMed] [Google Scholar]
- 4.Davey S. Challenges to the control of Mycobacterium bovis in livestock and wildlife populations in the South African context. Irish Veterinary Journal . 2023;76(1):p. 14. doi: 10.1186/s13620-023-00246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woldemariam T., Pal M., Zewude A., Mamo G., Gutama G. A. K. P. A study on the prevalence of tuberculosis in cattle at selected abattoirs in Ethiopia. Journal in Research Microbiology . 2021;2(2):9–13. [Google Scholar]
- 6.Shitaye J. E., Getahum B., Alemayehu T., et al. A prevalence study of bovine tuberculosis in cattle in different farming sytems in the Eastern zone of Tanzania Preventative. Veterinary Medicine . 2006;57:162–172. [Google Scholar]
- 7.Dvorska L., Matlova L., Bartos M., et al. Study of Mycobacterium avium complex strains isolated from cattle in the Czech Republic between 1996 and 2000. Veterinary Microbiology . 2004;99(3-4):239–250. doi: 10.1016/j.vetmic.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Hlokwe T. M., Said H., Gcebe N. Mycobacterium tuberculosis infection in cattle from the eastern Cape province of South Africa. BMC Veterinary Research . 2017;13(1):299–9. doi: 10.1186/s12917-017-1220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gcebe N., Rutten V., Gey van Pittius N. C., Michel A. Prevalence and distribution of non-tuberculous mycobacteria (NTM) in cattle, african buffaloes (Syncerus caffer) and their environments in South Africa. Transboundary and Emerging Diseases . 2013;60:74–84. doi: 10.1111/tbed.12133. [DOI] [PubMed] [Google Scholar]
- 10.Marfil M. J., Garbaccio S. G., Barandiaran S., et al. Isolation of NontuberculousMycobacteriafrom bovine raw lungs bought in butchers’ shops. Foodborne Pathogens and Disease . 2021;18(11):805–811. doi: 10.1089/fpd.2021.0026. [DOI] [PubMed] [Google Scholar]
- 11.Gopinath K., Singh S. Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLoS Neglected Tropical Diseases . 2010;4(4):p. e615. doi: 10.1371/journal.pntd.0000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoch V. M. Infections due to non-tuberculous mycobacteria (NTM) Indian Journal of Medical Research . 2004;120(4):290–304. [PubMed] [Google Scholar]
- 13.Johnson M. M., Odell J. A. Nontuberculous mycobacterial pulmonary infections. Journal of Thoracic Disease . 2014;6(3):210–220. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oloya J., Muma J. B., Opuda-Asibo J., Djønne B., Kazwala R., Skjerve E. Risk factors for herd-level bovine-tuberculosis seropositivity in transhumant cattle in Uganda. Preventive Veterinary Medicine . 2007;80(4):318–329. doi: 10.1016/j.prevetmed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Awah-Ndukum J., Kudi A. C., Bradley G., et al. Prevalence of bovine tuberculosis in cattle in the highlands of Cameroon based on the detection of lesions in slaughtered cattle and tuberculin skin tests of live cattle. Veterinarni Medicina . 2012;57(2):59–76. doi: 10.17221/5252-vetmed. [DOI] [Google Scholar]
- 16.Ntivuguruzwa J. B., Michel A. L., Kolo F. B., Mwikarago I. E., Ngabonziza J. C. S., van Heerden H. Prevalence of bovine tuberculosis and characterization of the members of the Mycobacterium tuberculosis complex from slaughtered cattle in Rwanda. PLoS Neglected Tropical Diseases . 2022;16(8):p. 9964. doi: 10.1371/journal.pntd.0009964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mareledwane V. E., Adesiyun A. A., Thompson P. N., Hlokwe T. M. Application of the gamma‐interferon assay to determine the prevalence of bovine tuberculosis in slaughter livestock at abattoirs in Gauteng, South Africa. Veterinary Medicine and Science . 2021;8(6):2568–2575. doi: 10.1002/vms3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thrusfield M. Presenting Numerical Data Veterinary Epidemiology . 4th. Oxford, UK: Blackwell Science Ltd; 2013. [Google Scholar]
- 19.Hlokwe T. M., Jenkins A. O., Streicher E. M., et al. Molecular characterisation of <i>Mycobacterium bovis</i> isolated from African buffaloes (<i>Syncerus caffer</i>) in Hluhluwe-iMfolozi Park in KwaZulu-Natal, South Africa. Onderstepoort Journal of Veterinary Research . 2011;78:1–6. doi: 10.4102/ojvr.v78i1.232. [DOI] [PubMed] [Google Scholar]
- 20.Cousins D. V., Wilton S. D., Francis B. R., Gow B. L. Use of polymerase chain reaction for rapid diagnosis of tuberculosis. Journal of Clinical Microbiology . 1992;30(1):255–258. doi: 10.1128/jcm.30.1.255-258.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biet F., Boschiroli M. L., Thorel M. F., Guilloteau L. A. Zoonotic aspects of Mycobacterium bovis and Mycobacterium avium-intracellulare complex (MAC) Veterinary Research . 2005;36(3):411–436. doi: 10.1051/vetres:2005001. [DOI] [PubMed] [Google Scholar]
- 22.Kriek N. P., Areda D. B., Dibaba A. B. Tuberculosis in Animals: An African Perspective . Cham, Germany: Springer; 2019. The diagnosis of bovine tuberculosis; pp. 171–235. [Google Scholar]
- 23.Botha L., Gey van Pittius N. C., Van Helden P. D. Mycobacteria and disease in southern Africa. Transboundary and Emerging Diseases . 2013;60:147–156. doi: 10.1111/tbed.12159. [DOI] [PubMed] [Google Scholar]
- 24.Musoke J., Hlokwe T., Marcotty T., du Plessis B. J. A., Michel A. L. Spillover ofMycobacterium bovisfrom Wildlife to Livestock, South Africa. Emerging Infectious Diseases . 2015;21(3):448–451. doi: 10.3201/eid2103.131690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sichewo P. R., Etter E. M., Michel A. L. Prevalence of Mycobacterium bovis infection in traditionally managed cattle at the wildlife-livestock interface in South Africa in the absence of control measures. Veterinary Research Communications . 2019;43(3):155–164. doi: 10.1007/s11259-019-09756-w. [DOI] [PubMed] [Google Scholar]
- 26.Munyeme M., Muma J. B., Samui K. L., et al. Prevalence of bovine tuberculosis and animal level risk factors for indigenous cattle under different grazing strategies in the livestock/wildlife interface areas of Zambia. Tropical Animal Health and Production . 2009;41(3):345–352. doi: 10.1007/s11250-008-9195-5. [DOI] [PubMed] [Google Scholar]
- 27.Dejene S. W., Heitkönig I. M., Prins H. H., et al. Risk factors for bovine tuberculosis (bTB) in cattle in Ethiopia. PLoS One . 2016;11(7) doi: 10.1371/journal.pone.0159083.159083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gelalcha B. D., Zewude A., Ameni G. Tuberculosis caused by Mycobacterium bovis in a sheep flock colocated with a tuberculous dairy cattle herd in Central Ethiopia. Journal of Veterinary Medicine A . 2019;2019:6. doi: 10.1155/2019/8315137.8315137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marianelli C., Cifani N., Capucchio M. T., et al. A case of generalized bovine tuberculosis in a sheep. Journal of Veterinary Diagnostic Investigation . 2010;22(3):445–448. doi: 10.1177/104063871002200319. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz‐Mendoza M., Romero B., Del Cerro A., et al. Sheep as a potential source of bovine TB: epidemiology, pathology and evaluation of diagnostic techniques. Transboundary and emerging diseases . 2016;63(6):635–646. doi: 10.1111/tbed.12325. [DOI] [PubMed] [Google Scholar]
- 31.Konold T., Dale J., Spiropoulos J., Simmons H., Godinho A. Case of TB in a sheep caused by Mycobacterium bovis with transmission to another sheep and a steer in the same building. Veterinary Record Case Reports . 2020;8(4):p. 1151. doi: 10.1136/vetreccr-2020-001151. [DOI] [Google Scholar]
- 32.Gcebe N., Pierneef R. E., Michel A. L., Hlokwe M. T. Mycobacteriosis in slaughter pigs from South Africa from 1991 to 2002: Mycobacterium spp. diversity and Mycobacterium avium complex genotypes. Frontiers in Microbiology . 2023;14 doi: 10.3389/fmicb.2023.1284906.1284906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamieh A., Tayyar R., Tabaja H., et al. Emergence of Mycobacterium simiae: a retrospective study from a tertiary care center in Lebanon. PLoS One . 2018;13(4) doi: 10.1371/journal.pone.0195390.195390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampaio J. L. M., Artiles N., Pereira R. M. G., Souza J. R., Leite J. P. G. Mycobacterium simiae infection in a patient with Acquired Immunodeficiency Syndrome. Brazilian Journal of Infectious Diseases . 2001;5(6):352–355. doi: 10.1590/s1413-86702001000600010. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Buendia A., Alvarez J., Bezos J., et al. Non-tuberculous mycobacteria: occurrence in skin test cattle reactors from official tuberculosis-free herds. Frontiers in Veterinary Science . 2024;11 doi: 10.3389/fvets.2024.1361788.1361788 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available from the author upon reasonable request.


