Abstract
Adrenal leiomyomas are rare and often reported as Epstein-Barr virus (EBV)-associated smooth muscle tumor (SMT) in association with EBV infection in immunocompromised patients. We experienced a case of right adrenal leiomyoma that was incidentally found in a man in his 70s. Computed Tomography (CT) showed a well-circumscribed mass of 3.1 cm in diameter in the right adrenal gland, which increased to 4.9 cm in diameter over 1 year. Preoperative diagnosis was difficult due to the lack of specific imaging findings. He had a history of diffuse large B-cell lymphoma (DLBCL) 8 years ago, and EBV had been detected in his blood. EBV-encoded small RNA(EBER) in situ hybridization (EBER-ISH) of the right adrenal leiomyoma was positive, and the final diagnosis was EBV-associated leiomyoma.
Keywords: Adrenal gland, Leiomyoma, Smooth muscle tumor, SMT, Epstein-Barr virus, EBV
Introduction
Adrenal mesenchymal tumors are extremely rare and mostly benign. WHO 2017 listed only myelolipoma and schwannoma as adrenal mesenchymal tumors [1]. Except for benign lipid tumors, schwannoma and leiomyoma may occur, but preoperative diagnosis is difficult due to the absence of specific radiological and clinical features [[2], [3]].
Leiomyomas are benign tumors consisting of neoplastic smooth muscle proliferation. Adrenal leiomyoma is very rare, and often reported as Epstein-Barr virus (EBV)-associated smooth muscle tumor (SMT) in immunosuppressed patients [[2], [4]].
We herein report a case of right adrenal leiomyoma found as an incidentaloma in patient with a history of DLBCL and EBV infection, and postoperatively proven to be EBV-associated SMT.
Case report
A man in his 70s underwent an abdominal ultrasonography (US) for follow-up of chronic hepatitis B, and a right adrenal tumor was pointed out incidentally. He had a history of DLBCL 8 years ago. Blood tests showed no obvious abnormalities except for mild liver function abnormalities.
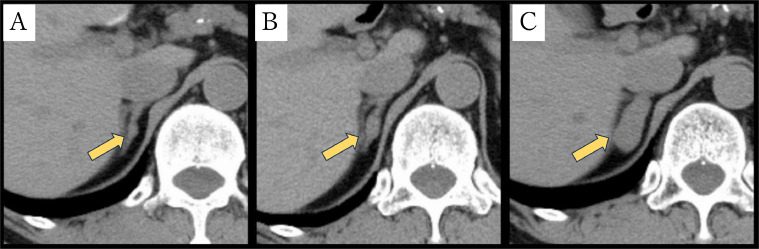
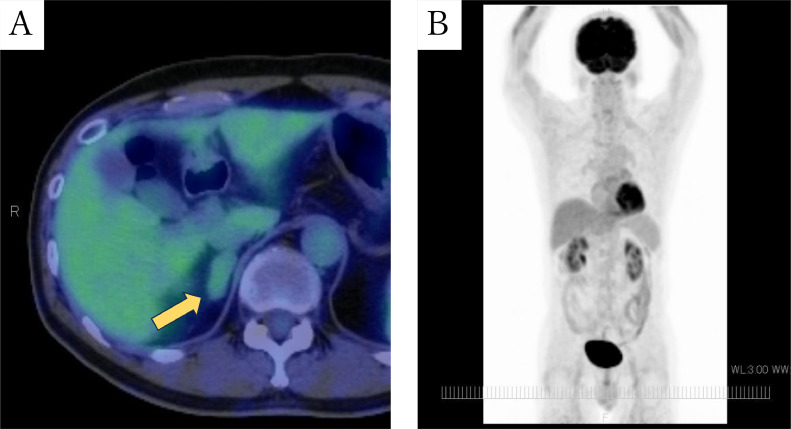
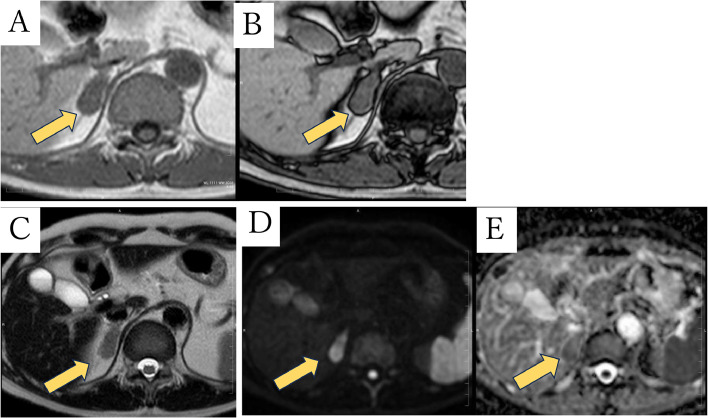
Abdominal CT revealed a well-circumscribed right adrenal mass measuring about 3.1 cm without calcification or fat. Retrospectively, CT 4 years ago showed a nodule about 0.5 cm in diameter in the right adrenal gland, and the nodule increased in size over time (Fig. 1). 18-fluoro-2-deoxyglucose-positron emission tomography/computed tomography (18-FDG PET/CT) images showed the mass with a maximum standardized uptake value (SUVmax) of 2.4, which is equivalent to background (Fig. 2). Magnetic Resonance Imaging (MRI) showed lower signal on T1-weighted image (T1WI) and higher signal on T2-weighted image (T2WI) than the liver (Fig. 3). No signal drop occurred in the in-phase/opposed-phase sequence (Fig. 3).
Fig. 1.
CT changes in the right adrenal gland over time. A 0.5-cm nodule was demonstrated in the right adrenal gland on CT taken 4 years ago (A). The nodule increased in size to 1.5 cm on CT taken 2 years ago (B). The 3.1-cm right adrenal mass is shown on the current CT, and calculated average CT value in the mass is about 30 HU.(C).
Fig. 2.
FDG-PET/CT (A: fusion, B: maximum intensity projection(MIP)). The fusion image shows mild FDG uptake (SUVmax of 2.4) in the right adrenal mass (A), and MIP shows no abnormal FDG uptake suggesting malignant lesion (B).
Fig. 3.
MRI images. The right adrenal mass shows low intensity on T1WI gradient-echo in-phase image (A) and no signal drop on opposed-phase image (B). The mass shows slightly higher intensity than that of liver on T2WI (C), high intensity on DWI (b value of 1000sec/mm2: D) and equivalent value on ADC map (ADC of 1.8 mm2/sec: E).
Endocrinological laboratory tests including cortisol, aldosterone, dehydroepiandrosterone sulfate (DHEA-S), adrenaline, noradrenaline, and dopamine were all within normal limits. Thus, the tumor was considered nonfunctioning and was followed up.
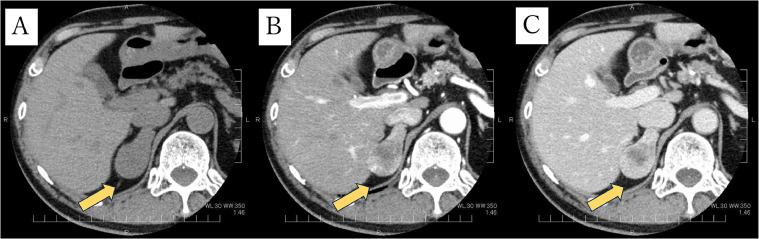
Contrast-enhanced CT 1 year later revealed that the adrenal mass had increased to 4.9 cm in diameter, and the mass showed inhomogeneous peripheral early and gradual centripetal delayed enhancement (Fig. 4).
Fig. 4.
CT images after 1 year follow-up. The right adrenal mass has increased to 4.9 cm in diameter (A), and the mass shows inhomogeneous peripheral early enhancement (B) and it shows gradual centripetal enhancement(C).
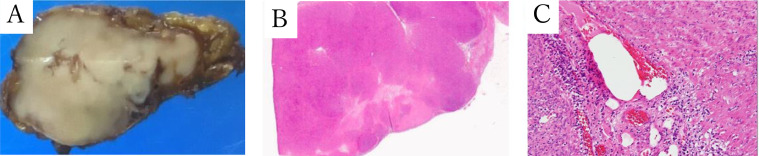
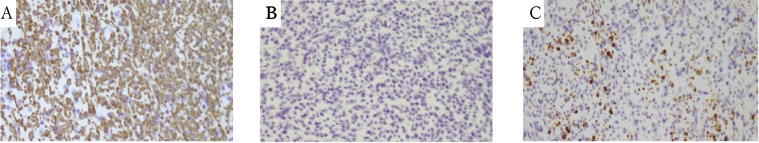
According to the American College of Radiology (ACR) guidelines [[5], [6]], right adrenalectomy was performed laparoscopically. A well-circumscribed pale brown mass, measuring 4.7 × 3.0 cm occupied the right adrenal gland. Histologically, the tumor was composed of fascicles of spindled cells with eosinophilic cytoplasm and elongated nuclei with some enlarged ones. The cellularity was moderate, mitotic activity was low, and neither hemorrhage nor tumor necrosis was observed (Fig. 5). A diagnosis of leiomyoma was supported by immunohistochemical studies; positive for smooth muscle actin (SMA) and partly for caldesmon, and negative for desmin, S-100, CD34, c-kit, and AE1/AE3. Ki-67 labeling index of tumor cells was about 10% (Fig. 6).
Fig. 5.
Macroscopic and microscopic appearance of adrenal leiomyoma. The right adrenalectomy specimen is a light brown mass with a clear boundary of 4.7 × 3.0 cm. (A) Histologically, tumor cells with acidophilic cytoplasm and spindle-shaped nuclei proliferate in bundles from the adrenal medulla to the cortex. They have low pleomorphism but a few of them have enlarged nuclei. The cellularity are moderate, no bleeding or necrosis is noted. (B,C) (B: Roupe, C: Optical magnification × 10).
Fig. 6.
Immunohistochemical staining of the adrenal leiomyoma. The results of immunohistochemical staining are as follows; smooth muscle actin (SMA)(A), positive; CD34(B), negative; caldesmon(C), occasionally positive (A-C: Optical magnification × 10).
Discussion
In this case, the adrenal incidentaloma was found in a patient with a history of DLBCL, and it was necessary to diagnose whether it was a relapse of DLBCL. Blake et al. reported that malignant adrenal lesion showed higher FDG uptake than liver, and mean lesion-liver SUV ratio was about 4 [7]. Metser et al. reported that when malignant lesions were compared with adrenal adenomas, SUV cutoff of 3.1 yielded a sensitivity, specificity of 98.5%, 92%, respectively [8]. FDG uptake (SUVmax=2.4) of the tumor was similarly to that of liver, thus, we considered this tumor was not a relapse of DLBCL or not likely other malignancies such as adrenal carcinoma or adrenal metastasis.
Adenomas are the most frequent adrenal incidental tumor [9], [10], [11]. On unenhanced CT, HU of <10 is the most widely used threshold attenuation value for the diagnosis of adrenal adenoma. On enhanced CT, adenomas take up CT contrast rapidly, but also have a rapid loss of contrast [11]. The CT value of this tumor was 30HU and there was no rapid enhancement nor washout. On MRI, no signal drop was observed in the in-phase/opposed-phase sequence. Thus, we considered this tumor was not likely as adrenal adenoma.
Myelolipomas are the second frequent adrenal incidentaloma, but it would be excluded, since there was no obvious fat density on CT [10].
In this case, the adrenal tumor did not show any characteristic imaging findings of malignant or typical benign features, as mentioned above. According to the recent guidelines, surgical resection was recommended for the indeterminate adrenal mass of more than 4cm in diameter [[3], [5], [6], [10]], laparoscopic right adrenalectomy was performed since the tumor size increased to 4.9 cm.
Although rare, other mesenchymal tumors such as schwannoma and leiomyoma have been reported [[12], [13], [14], [15], [16]]. Zhang et al. reported that all 8 cases of adrenal schwannoma were well-circumscribed, rounded or oval, heterogeneous enhancement with cystic components, with 2 cases exhibiting calcification, and 3 cases with septa on CT [12]. Adrenal leiomyoma is also rare, with 28 cases reported in English. literatures by 2020, according to a review by Sakellariou et al [4]. They described the imaging findings of leiomyomas as well-circumscribed, round, or lobulated tumors, which may show poor internal enhancement, T2WI hyperintensity, internal necrosis and calcification in larger tumors [[4], [14], [15], [16]]. Furthermore, Sakellariou et al reported that about half of the adrenal leiomyoma cases were immunocompromised, 5 of which were EBV-positive, and 2 of the immunocompetent cases were also EBV-positive.
In the WHO classification fifth edition, EBV-associated leiomyoma is described as EBV-associated SMT [2]. EBV-associated SMT is a smooth muscle tumor associated with EBV infection, usually in the setting of immunosuppression such as AIDS [[17], [18]], post-organ transplantation [19], and congenital immunodeficiency. EBV-associated SMT can occur anywhere in the body, including sites unusual for sporadic leiomyomas, sometimes multicentric. Pathological diagnosis is usually made by demonstration of EBV in the tumor cells by EBER-ISH [2].
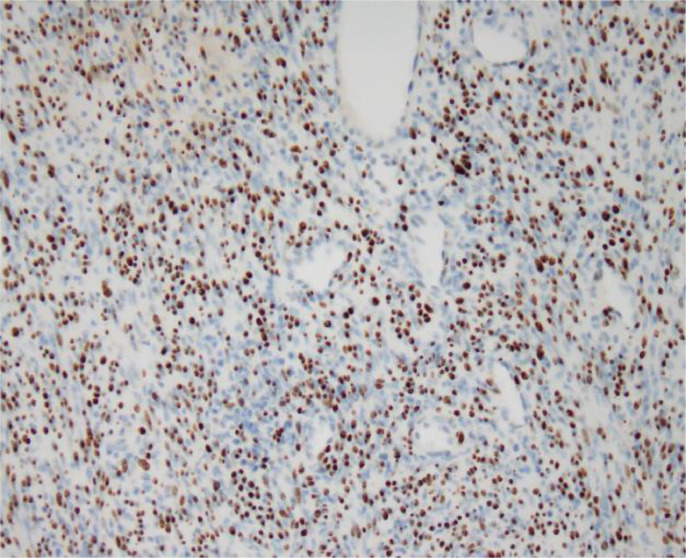
This patient had a history of oropharyngeal DLBCL, but when the adrenal tumor was pointed out, DLBCL was in complete response and he was considered in immunocompetent state. However, because the adrenal gland is an uncommon site for leiomyoma, we reviewed medical records during treatment for lymphoma, and it was revealed that EBV had been detected in his blood. We therefore requested an EBER-ISH of the adrenal leiomyoma for the pathology department, which was positive (Fig. 7). The final diagnosis was EBV-associated adrenal leiomyoma.
Fig. 7.
EBER-ISH of the adrenal leiomyoma. The nuclei of the tumor cells are positive with EBER-ISH.
Patient consent
Informed consent for the publication of this case report was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Lam AKY, Chuah KL, de Pinieux G, Fisher C, Lack E. Endocrine organs. 4th ed. IRAC; Lyon, France: 2017. Mesenchymal and stromal tumours. WHO Classification of Tumours Editional Board; pp. 175–176. [Google Scholar]
- 2.Watanabe R, Schafernak KT, Soares FA. EBV-associated smooth muscle tumour. WHO Classification of Tumours Editional Board. Soft tissue and bone tumours (5th ed). IRAC; 2020:190-192
- 3.Clinical practice guideline for non-functioning adrenal tumor 2022 [in Japanese]. The japanese urological association, Japan Association of Endocrine Surgery, Medical Review, Inc. Yodoyabashi MI Building, 3-2-8 Hiranomachi, Chuo-ku, Osaka Japan.
- 4.Sakellariou M, Dellaportas D, Peppa M, Schizas D, Pikoulis E, Nastos K. Review of the literature on leiomyoma and leiomyosarcoma of the adrenal gland: a systematic analysis of case reports. In Vivo. 2020;34(5):2233–2248. doi: 10.21873/invivo.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berland LL, Silverman SG, Gore RM, Mayo-Smith WW, Megibow AJ, Yee J, et al. Managing incidental findings on abdominal CT: white paper of the. ACR incidental findings committee. J Am Coll Radiol. 2010;7(10):754–773. doi: 10.1016/j.jacr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Mayo-Smith WW, Song JH, Boland GL, Francis IR, Israel GM, Mazzaglia PJ, et al. Management of incidental adrenal masses: a white paper of the acr incidental findings committee. J Am Coll Radiol. 2017;14(8):1038–1044. doi: 10.1016/j.jacr.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Blake MA, Slattery JMA, Kalra MK, Halpern EF, Fischman AJ, Mueller PR, et al. Adrenal lesions: characterization with fused PET/CT image in patients with proved or suspected malignancy initial experience. Radiology. 2006;238(3):970–977. doi: 10.1148/radiol.2383042164. [DOI] [PubMed] [Google Scholar]
- 8.Metser U, Miller E, Lerman H, Lievshitz G, Avital S, Even-Sapir E. 18F-FDG PET/CT in the evaluation of adrenal masses. J Nucl Med. 2006;47(1):32–37. [PubMed] [Google Scholar]
- 9.Ichijo T, Ueshiba H, Nawata H, Yanase T. A nationwide survey of adrenal incidentalomas in Japan: the first report of clinical and epidemiological features. Endocr J. 2020;67(2):141–152. doi: 10.1507/endocrj.EJ18-0486. [DOI] [PubMed] [Google Scholar]
- 10.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190(5):1163–1168. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 11.Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collabolation with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. doi: 10.1530/EJE-16-0467. [DOI] [PubMed] [Google Scholar]
- 12.Zhang YM, Lei PF, Chen MN, Lv XF, Ling YH, Cai PQ, et al. CT findings of adrenal schwannoma. Clin Radiol. May 2016;71(5):464–470. doi: 10.1016/j.crad.2016.01.010. 10.1016. [DOI] [PubMed] [Google Scholar]
- 13.Timilsina S, Joshi SP, Sharma S, Kharel S, Karki S, Tiwari SB, et al. Adrenal schwannoma: a case report of an unusual incidentaloma. Int J Surg Case Rep. 2021;83 doi: 10.1016/j.ijscr.2021.106018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma S, Timilsina S, Joshi SP, Bist A, Shrestha S, Tiwari SB. Adrenal Leiomyoma: a case report. Int J Surg Case Rep. 2021;85 doi: 10.1016/j.ijscr.2021.106249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Wasco MJ, Korobkin M, Doherty G, Giordano TJ. Leiomyoma of the adrenal gland presenting as a non-functioning adrenal incidentaloma: case report and review of the literature. Endocr Pathol. 2007;18(4):239–243. doi: 10.1007/s12022-008-9013-7. [DOI] [PubMed] [Google Scholar]
- 16.Pramod SV, Siregar S, Safriadi F, Hernowo BS, Firdaus GI. The largest adrenal leiomyoma: a case report and literature review. Urol Case Rep, 2020;29 doi: 10.1016/j.eucr.2019.101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purgina B, Rao UNM, Miettinen M, Pantanowitz L. AIDS-Related EBV-associated smooth muscle tumors: a review of 64 published cases. Patholog Res Int. 2011;2011 doi: 10.4061/2011/561548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McClain KL, Leach CT, Jenson HB, Joshi VV, Pollock BH, Parmley RT, et al. Association of Epstein-Barr virus with leiomyosarcomas in young people with AIDS. N Engl J Med. 1995;332(1):12–18. doi: 10.1056/NEJM199501053320103. [DOI] [PubMed] [Google Scholar]
- 19.Lee ES, Locker J, Nalesnik M, Reyes J, Jaffe R, Alashari M, et al. The association of Epstein-Barr virus with smooth-muscle tumors occurring after organ transplantation. N Engl J Med. 1995;332(1):19–25. doi: 10.1056/NEJM199501053320104. [DOI] [PubMed] [Google Scholar]