Summary
Background
The multifactorial nature of inflammatory bowel disease (IBD), which manifests differently in individuals creates a need for a better understanding of the behaviour and pattern of the disease due to environmental factors. The current study aimed to study the changes in IBD behaviour, presentation, and characteristics in patients over the past two decades with a goal of improving patients’ diagnosis, management and outcomes.
Methods
During a 6-month period (1/02/2022–30/07/2022), the information of patients with IBD who attended IBD outpatient clinics of 11 referral centre's in six countries was collected, and based on the first time of diagnosis with IBD, they were allocated as group A (those who were diagnosed more than 15 years ago), group B (those who were diagnosed with IBD between 5 and 15 years ago) and group C (IBD cases who diagnosed in recent 5 years). Then the most prevalent subtypes and characters of the disease are evaluated and compared to make clear if the presenting pattern and behaviour of the disease has changed in the last 2 decades.
Findings
Overall 1430 patients with IBD including 1207 patients with ulcerative colitis (UC) (84.5%) and 205 patients with Crohn's disease (CD; 14.3%) included. Mean age of participants at the first time of diagnosis with IBD was 30 years. The extra-intestinal involvement of IBD in groups A and B was more prevalent in comparison with group C. Most of those in groups A & B had academic education but in group C, the most prevalent educational status was high school or diploma (P = 0.012). In contrast to groups A and B, the relative prevalence of medium socioeconomic level in group C had decreased (65%). Relative prevalence of UC subtypes was similar among groups A and B (extensive colitis as most prevalent) but in group C, the most prevalent subtype is left side colitis (38.17%). The most prevalent subtype of CD in groups A and B was ileocolic involvement while in group C, upper GI involvement is significantly increased. The rate of food sensitivity among groups A and B was more than group C (P = 0.00001). The relative prevalence of patients with no flare has increased with a steady slope (P < 0.00001). Relative prevalence of presenting symptoms among patients with UC in group C differs and nowadays the rate abdominal pain (70.7%) and bloating (43.9%) have increased and frequency of diarrhoea (67.4%) has decreased.
Interpretation
In the recent 5 years, the pattern of UC presentation has changed. The rate of upper GI involvement in CD and relative prevalence of patients with no disease flare increased and the rate of extra intestinal involvement decreased.
Funding
None.
Keywords: IBD, UC, CD, Flare, Behavior
Research in context.
Evidence before this study
The authors searched PubMed and google scholar on November 1st 2021 until April 30 2023; the Montreal classification criteria was used to include or exclude studies and the search terms were related to inflammatory bowel disease (IBD), ulcerative colitis (UC) and Crohn's disease (CD), disease behaviour and environment in English language, considering the impact of climate changes on the behaviour of multifactorial disorders including IBD.
Added value of this study
We found that there is lack of any multinational chronological study in the field of IBD and this article can help understanding of IBD as a multifactorial disease.
Implications of all the available evidence
Findings of the current work provide evidence about changing of IBD behavior in line with the changing environment and climate.
Introduction
Inflammatory Bowel disease (IBD) is a collective term for chronic gastrointestinal inflammatory diseases including, but not limited to, Crohn's disease and ulcerative colitis.1,2 Usually characterised by prolonged inflammation of the gastrointestinal (GI) mucosa, these subsets of diseases could be present with a variety of symptoms such as abdominal pain, diarrhea, fatigue, joint pain, night sweats, vomiting, and bleeding.3 Because of its ability to turn into a life-threatening condition with disease progression, it is vital to diagnose and provide treatment in a timely manner.2
Due to the heterogeneity of this disease, mechanisms involved in IBD are poorly understood and morbidity rates are rapidly increasing, making it not only an important concern for healthcare practitioners but also for healthcare facilities around the globe.4 This is even more concerning given the fact that the relative risk of death related to IBD has not decreased despite several advances in the field and the development of new drugs for its treatment.5 Therefore, it is vital to understand various risk factors that affects the course of these diseases, which will in turn inform us to formulate better treatment plan for the patients.
It has been reported that environmental factors play an important role in the development of IBD by altering intestinal permeability and disrupting native microbiota.6 Although smoking, public hygiene, air pollution, and rapidly changing environmental factors have been isolated as the common aggravating factors in IBD, it is unclear how these factors affect the progression and change of the course of the disease in patients during the last several decades.7, 8, 9 On the other hand, the phenomena of global warming and associated and predictable climate changes in the last decades have been characterised by extreme weather events such as the increase in temperature, rainfall, the length of the warmest season, and droughts and subsequently epidemiological disease cycles and patterns have changed.10 The multifactorial nature of IBD manifesting differently in individuals creates a need for a better understanding of the behavior and pattern of the disease due to such environmental factors. Given the rise of cases and death rates concerning IBD, this study aimed to isolate and study the changes in IBD behavior, presentation, and characteristics in patients over the last two decades with a goal of improving patients’ diagnosis, management and outcomes.
Methods
Study design and participants
During a 6-month period (1/02/2022–30/07/2022), the information of patients with IBD who attend IBD outpatient clinics of 11 referral centres in 6 countries was collected, and based on the first time of diagnosis with IBD, they were allocated as group A (those who were diagnosed more than 15 years ago), group B (those who involved with IBD between 5 and 15 years ago) and group C (IBD cases who diagnosed in recent 5 years). Different IBD centres joined the study via social media announcing. The diagnosis and type of IBD (ulcerative colitis (UC) vs Crohn's disease (CD)) allocation confirmed by academic gastroenterologist based on patients history, previous documents and endoscopic and pathologic reports.
Procedures and outcomes
After obtaining a consent form, the participants were requested to fill out the study questionnaire which was designed by 3 expert gastroenterologists. Then the most prevalent subtypes and characters of the disease are evaluated and compared to make clear if the presenting pattern and behavior of the disease have changed in the last 2 decades.
Statistical analysis
We used Statistical Package for the Social Sciences (SPSS) software version 16.0 for data analysis. Data was checked for normality using Kolmogorov–Smirnov and Shapiro–Wilk tests. Mann Whitney U test and independent two-sample t-test were employed for comparing two quantitative variables. The chi-square test was used to evaluate the association between two qualitative variables. Analyses were declared significant for P value <0.05.
Ethics information
This study followed the Declaration of Helsinki, and all participants signed written informed consent forms before data collection began. The study was approved by the ethical committee of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1400.269).
Role of the funding source
This research performed under supervision and approval of Alimentary Tract Research Centre (ATRC) in Ahvaz Jundishapur University of medical Sciences without any financial support and there was no funding source for this study. All of the authors had full access to all of the data in the study and accept responsibility for the decision to submit for publication.
Results
During this multinational observational study, overall information of 1430 patients with IBD including 1207 patients with UC (84.5%), 205 patients with CD (14.3%), and 18 cases with indeterminate colitis (1.3%) from 11 referral centres of 6 countries (Egypt, India, Iran, Pakistan, Poland, Vietnam) were included. The participants were originally citizens of 9 countries (Fig. 1) and 48.1% of them (687 cases) were male. The mean age was 37.2 years (range 3–83) (Table 1) and 45.8% of them were Caucasian in descent (Table 2). Mean number of family members per living area was 5 persons (range 1–18). The mean age of participants at the first time of diagnosis with UC among groups A, B, and C were 31, 31.5, and 33.6 y respectively (P > 0.05), and about patients with CD were 27, 28.6, and 26.7 y respectively which was also similar in 3 groups (P > 0.05). The extra-intestinal involvement of IBD in groups A and B was more prevalent in comparison with group C (19.6% and 19.2% vs 13.7%) (P = 0.08 & 0.009 respectively) (Table 1).
Fig. 1.
Original country of participants.
Table 1.
Demographic characteristics of participants; group A: those who diagnosed with IBD more than 15 years ago, group B: those who diagnosed with IBD between 5 and 15 years ago, group C: IBD cases who diagnosed in recent 5 years; BMI, body Mass Index.
| Character | Group A | Group B | Group C |
|---|---|---|---|
| Male | 54 | 236 | 397 |
| Female | 80 | 241 | 422 |
| Male/Female ratio | 54/80 | 236/241 | 397/422 |
| Mean Age (range) | 49.5 (24–77) | 40.1 (3–83) | 33.4 (6–75) |
| BMI (mean) | 26.22 | 26.28 | 24.63 |
| Food sensitivity | 25% | 22.30% | 9.40% |
| Family History of IBD | 11.30% | 13.30% | 7.20% |
| Smoking | 9% | 11.20% | 9.20% |
| Alcohol drinking | 4.50% | 4.60% | 3.60% |
| extra intestinal involvement | 19.60% | 19.20% | 13.70% |
Table 2.
Race of participants (this racial categorization was taken from Fig. 2 in Irritable Bowel Syndrome Demographics; https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9489318/).
| Race | number | Percent |
|---|---|---|
| Caucasian | 655 | 45.8% |
| Arab | 489 | 34.50% |
| Indian | 111 | 7.83% |
| Asian | 80 | 5.64% |
| Turk | 60 | 4.19% |
| African | 26 | 1.83% |
| Afghan | 8 | 0.56% |
| Spanish | 1 | 0.07% |
Educational status
Most of those who were diagnosed more than 5 years ago (groups A & B) had academic education (41.6% and 42.3% respectively) but among those whose disease began in 5 most recent years, the most prevalent educational status was high school or diploma (45.07%, P = 0.012) (Fig. 2).
Fig. 2.
Educational condition of participants.
Economic status
Among groups A and B, most of the patients belong to the medium socioeconomic class (83% & 74% respectively) but in group C (those who were involved during the last 5 years), the relative prevalence of medium socioeconomic level decreased (65%). Subsequently, in recent years (group C) more people with high or low monthly income diagnosed as IBD in comparison with group A (15% vs 6%, P = 0.004 and 20% vs 11%, P = 0.015 respectively) (Fig. 3).
Fig. 3.
Relative proportion of IBD among different socioeconomic condition (IBD, inflammatory bowel disease).
Subtypes of IBD
-
a.
Ulcerative colitis
Relative prevalence of UC subtypes based on anatomic involvement was almost similar among groups A and B with E3 (extensive colitis) as the most prevalent subtype (48.73% and 48.47% respectively) but among those who involved in recent 5 years (group C), the most prevalent subtype is left side colitis (E2, 38.17% vs 24.55% as E1 and 37.27% as E3; Montreal Classification) (Fig. 4).
-
b.
Crohn's Disease
Fig. 4.
Relative proportion of UC subtypes (UC, ulcerative colitis; Montreal Classification; E1, ulcerative Proctitis; E2, left sided UC; E3, Extensive UC).
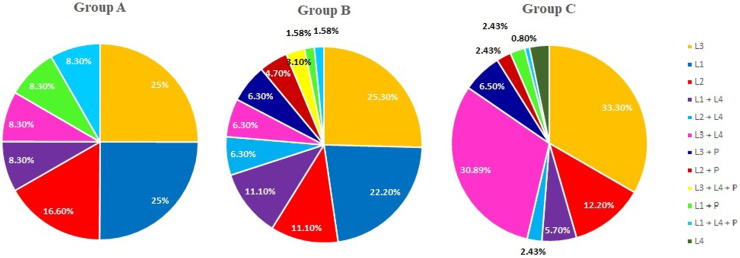
The most prevalent subtype of CD in groups A and B was ileocolic involvement (L3, 25%, and 25.3% respectively; Montreal Classification) while among those who involved during the last 5 years (group C), upper GI involvement (L3 + L4) is significantly increased (30.89% vs 8.3% in group A and 6.3% in group B; P = 0.09 & 0.0009 respectively) (Fig. 5).
Fig. 5.
Relative proportion of CD subtypes (CD, Crohn's disease; GI, gastrointestinal; Montreal Classification; L1, Terminal Ilium; L2, Colon; L3, Ileocolon; L4, upper GI; L1 + L4, Terminal Ilium + Upper GI; L2 + L4, Colon + Upper GI; L3 + L4, Ileocolon + Upper GI).
Food sensitivity
The rate of food sensitivity among groups A and B (25% and 22.3% respectively) was more than group C (9.4%) (P = 0.00001, Table 1).
Habitual behavior
The rate of recreational behavior such as smoking and drinking alcohol was similar between the 3 groups (P > 0.05) (Table 1).
Disease flare
The relative prevalence of patients who have not experienced disease flare raised among study groups with a steady slope (group A 15.9%, group B 22.9%, and group C 42.38%, P < 0.00001; Fig. 6).
Fig. 6.
The rate of disease flare per year.
Presenting symptoms
Regardless of bleeding, the relative prevalence of the majority of presenting symptoms among patients with UC in recent 5 years (group C) differs from those who were diagnosed more than 15 years ago (group A) (Table 3) and nowadays the rate of symptoms such as abdominal pain (70.7% vs 54.1%, P = 0.0003), bloating (43.9% vs 20.8%, P = 0.00001) and fatigue (28.9% vs 13.3%, P = 0.0003) have increased and frequency of diarrhea (67.4% vs 80.8%, P = 0.003) has decreased.
Table 3.
Relative proportion of clinical symptoms during the first presentation of UC (UC, ulcerative colitis; total percentage is more than 100% because most of participants presented with more than one symptom).
| Symptom | Group A | Group B | Group C | P value |
|---|---|---|---|---|
| Bleeding | 90% | 90.07% | 84.76% | 0.132 |
| Diarrhea | 80.80% | 78.41% | 67.45% | 0.003 |
| Abdominal pain | 54.10% | 57.56% | 70.71% | 0.0003 |
| Weight loss | 30% | 23.07% | 19.08% | 0.0065 |
| Bloating | 20.80% | 29.52% | 43.93% | 0.00001 |
| Fever | 18.30% | 8.68% | 4.28% | 0.00001 |
| Fatigue | 13.30% | 23.57% | 28.99% | 0.0003 |
| Articular pain | 12.50% | 11.66% | 9.02% | 0.232 |
| Mucus discharge | 12.50% | 7.69% | 2.66% | 0.00001 |
| Nausea & Vomiting | 10.83% | 10.66% | 4.88% | 0.01 |
| Hair loss | 5.83% | 3.47% | 5.32% | 0.820 |
| Anorexia | 5.83% | 5.95% | 3.10% | 0.135 |
| Constipation | 4.16% | 5.45% | 1.62% | 0.067 |
| Skin involvement | 1.66% | 2.23% | 0.88% | 0.430 |
| Oral ulcer | 1.66% | 3.72% | 1.33% | 0.771 |
| Blurred vision | 0.83% | 0.74% | 0.73% | 0.912 |
| Miscellaneous | 5.83% | 4.96% | 1.92% | 0.011 |
Bold indicates significance level <0.05.
Among patients with CD, the relative prevalence of presenting symptoms is almost similar between new cases in recent years (group C) and those who were diagnosed earlier (Table 4).
Table 4.
Relative proportion of clinical symptoms during the first presentation of CD (CD, Crohn's disease; total percentile is more than 100% because most of participants presented with more than one symptom).
| Symptom | Group A | Group B | Group C | P value |
|---|---|---|---|---|
| Diarrhea | 75% | 73.84% | 71.20% | 0.780 |
| Abdominal pain | 75% | 86.15% | 84.60% | 0.377 |
| Fatigue | 75% | 55.38% | 37.60% | 0.018 |
| Bleeding | 41.60% | 43.07% | 24.80% | 0.204 |
| Weight loss | 41.60% | 66.15% | 50.40% | 0.398 |
| Bloating | 33.30% | 49.23% | 32.80% | 0.970 |
| Anorexia | 33.30% | 32.30% | 15.20% | 0.108 |
| articular pain | 25% | 26.15% | 23.20% | 0.888 |
| Nausea & Vomiting | 25% | 36.92% | 17.60% | 0.526 |
| Fever | 16.60% | 36.92% | 16% | 0.952 |
| Skin involvement | 16.60% | 15.38% | 6.40% | 0.191 |
| Hair loss | 8.30% | 20% | 15.20% | 0.519 |
| Constipation | 8.30% | 1.53% | 2.40% | 0.243 |
| Oral ulcer | 8.30% | 23.07% | 18.40% | 0.380 |
| Blurred vision | 8.30% | 4.61% | 1.60% | 0.127 |
Bold indicates significance level <0.05.
Discussion
IBD is an increasing health-care problem all over the world and environmental factors have a considerable role in the pathophysiology of these disorders.11 In the Recent decades, the world encountered with phenomena of global warming and its predictable impact on the behavior of not only vital species but also epidemiology and presentation of different disorders including IBD.10,12,13 So, it is expected that the clinical presentation and behavior of various subtypes of IBD be modified and this item highlights the necessity of following the behavior of these disorders in a chronological way for better understanding and management.
Based on the findings of the current study, while the average age of the first presentation of IBD has not changed during the last 2 decades, the rate of extra intestinal involvement has decreased during the last 5 years (19.6% and 19.2% vs 13.7%; P = 0.08 & 0.009 respectively). Regardless of etiology, extra-intestinal manifestation (EIM) could affect quality of life and may require additional healthcare resources.14 So the decreased prevalence of EIM with considering the relative stability of its risk factors such as female gender, too high or too low body mass index, moderate-to-severe disease activity, extensive Crohn's disease, lower hemoglobin level, lower serum albumin, and higher C-reactive protein level, could be a harvest of earlier diagnosis and more effective management of IBD in recent years15 while it may present later on the disease course.
Previously, IBD was more prevalent among those with academic education (groups A & B 41.6% and 42.3% respectively) and related to more tension in life,16 but in recent years the relative prevalence of those who were diagnosed as IBD without any higher level education has raised (45.07%, P = 0.012) and could illustrate the role of social media and broadcasting in creating not only awareness but also anxiety, depression and stress especially among the younger generation.17,18 On the other hand, recently more people with low or high socioeconomic levels have been diagnosed with IBD and this item could also be because of changes in the epidemiology of social stress.19,20
In recent years the most prevalent subtype of ulcerative colitis has been left-side colitis (E2, 38.17%) and could be a reflection of better awareness, more rapid diagnosis, and development of more potent and effective medication.21,22 Mutually the relative prevalence of upper GI involvement by CD has increased (31% vs 8% in group A and 6% in group B; P = 0.09 & 0.0009 respectively). This is in concordance with the findings of the Greuter et al. study which reported male sex and young age as main risk factors.23 In this regard, another study in 2019 found the female sex as a protective factor and advised more aggressive initial therapy for L4 phenotype.24 The authors of the current study believe that more research is necessary to identify the potential causes of increase the incidence upper GIT involvement in cases of CD which we think may be related to better health care awareness and screening of upper GIT in such cases.
The rate of food sensitivity has decreased among recent cases (9.4% vs 25% and 22.3% in groups A & B respectively; P = 0.00001). The potential reason could be a better understanding of the anti-inflammatory properties of foods and providing patients with IBD with better evidence on which foods they should eat or avoid to reduce flares.25,26 The rate of recreational behavior such as smoking and drinking alcohol saw no changes in the last 2 decades and necessitates further focus and public education regarding awareness about their hazards. Multiple lifestyle factors may significantly impact natural history and clinical outcomes in patients with established IBD. So, a conscious and deliberate assessment of these lifestyle factors is warranted for every patient with IBD and serves as a key aspect in engaging patients in adjunctive self-management of their disease.27
The relative prevalence of those patients who have not experienced disease flares has risen in the last 2 decades with a steady slope and again could reflect more effective management and availability of more potent medications.28, 29, 30 On the other hand, the relative prevalence of most of the presenting symptoms, regardless of rectal bleeding, has changed among patients with UC and could be a warning sign to avoid misdiagnosis of the disease. Nowadays more patients present with previously considered nonspecific symptoms such as bloating and fatigue and the frequency of diarrhea has decreased. These findings are in concordance with the study of Song et al. about different behaviors and course of the disease.31 In contrast to UC, the presenting symptoms and clinical pattern of CD have not changed in the last 2 decades.
The current study focused on the changes in IBD behavior in the last decades but have also some limitations. We have no data from territories of Europe or America and performing similar studies with the participation of more countries and more cases could help in better understanding and management of IBD.
While in recent 5 years, the pattern of UC presentation has changed and the rate of upper GI involvement by CD increased but also the relative prevalence of patients with IBD who have not experienced disease flare increased and the rate of extra intestinal involvement decreased which means better understanding and management of disease.
Contributors
PA as corresponding author and main colleague in case collection, clinical supervisor, final draft writing and verified the underlying DATA; SJH as main colleague in case collection; NB as main colleague in case collection; AH as main colleague in case collection; AE as main colleague in case collection; NED as main colleague in case collection; MPM as main colleague in case collection; MA as main colleague in case collection; SP as main colleague in case collection; DVH as main colleague in case collection and final draft writing; AMM as main colleague in case collection; AP as main colleague in case collection; HJ as main colleague in case collection; AR as main colleague in case collection; KMP as main colleague in case collection; MA as main colleague in case collection; MHA as main colleague in case collection; FF as main colleague in case collection; MA as main colleague in case collection; AQ as main colleague in case collection; QTT as main colleague in case collection and figures designing; FA as main colleague in case collection; SMAA as main colleague in case collection; BC as statistician and epidemiologist for DATA analysis; ER as statistician for DATA analysis; MFG as main colleague in case collection; BS as main colleague in final draft writing; AAQ as main colleague in case collection; RH as main colleague in final draft writing and editing; MM as main colleague in case collection; EG as main colleague in case collection, clinical supervisor, final draft writing and verified the underlying DATA.
All of the authors had full access to all of the data in the study and accept responsibility for the decision to submit for publication.
Data sharing statement
The study DATA could be shared based on direct personal request to the corresponding author.
Declaration of interests
The authors have none to declare.
Acknowledgements
This study was approved and endorsed by the alimentary tract research centre of Ahvaz Jundishapur University of Medical Sciences without any financial support.
The authors would like to acknowledge Professor Ibrahim Mostafa for his endless efforts and continued support in creating and promoting the WEO emerging star group as a global network of young researchers. We also would like to appreciate Professor Rupa Banerjee for her effort in the field of IBD especially creating the IBD-ENC consortium to unify the eastern Asian territory for better management of IBD.
References
- 1.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019 doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colombel J.-F., D’haens G., Lee W.-J., Petersson J., Panaccione R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J Crohns Colitis. 2020;14:254–266. doi: 10.1093/ecco-jcc/jjz131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh S., Blanchard A., Walker J.R., Graff L.A., Miller N., Bernstein C.N. Common symptoms and stressors among individuals with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2011;9(9):769–775. doi: 10.1016/j.cgh.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 4.Ye Y., Pang Z., Chen W., Ju S., Zhou C. The epidemiology and risk factors of inflammatory bowel disease. Int J Clin Exp Med. 2015;8(12) [PMC free article] [PubMed] [Google Scholar]
- 5.Amarapurkar A.D., Amarapurkar D.N., Rathi P., et al. Risk factors for inflammatory bowel disease: a prospective multi-centre study. Indian J Gastroenterol. 2018;37:189–195. doi: 10.1007/s12664-018-0850-0. [DOI] [PubMed] [Google Scholar]
- 6.Dam A.N., Berg A.M., Farraye F.A. Environmental influences on the onset and clinical course of Crohn's disease—Part 1: an overview of external risk factors. Gastroenterol Hepatol. 2013;9(11):711. [PMC free article] [PubMed] [Google Scholar]
- 7.Ananthakrishnan A.N. Environmental triggers for inflammatory bowel disease. Curr Gastroenterol Rep. 2013;15(1):1–7. doi: 10.1007/s11894-012-0302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nejad P.A., Mard S.A., Larki S., et al. Role of air pollution in inflammatory bowel disease flares: a retrospective study. Govaresh. 2016;20(4):261–267. [Google Scholar]
- 9.Piovani D., Danese S., Peyrin-Biroulet L., Nikolopoulos G.K., Lytras T., Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology. 2019;157(3):647–659. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 10.El-Sayed A., Kamel M. Climatic changes and their role in emergence and re-emergence of diseases. Environ Sci Pollut Control Ser. 2020;27(18):22336–22352. doi: 10.1007/s11356-020-08896-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ananthakrishnan A.N., Bernstein C.N., Iliopoulos D., et al. Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 2018;15(1):39–49. doi: 10.1038/nrgastro.2017.136. [DOI] [PubMed] [Google Scholar]
- 12.Giorgini P., Di Giosia P., Petrarca M., Lattanzio F., Stamerra C.A., Ferri C. Climate changes and human health: a review of the effect of environmental stressors on cardiovascular diseases across epidemiology and biological mechanisms. Curr Pharm Des. 2017;23(22):3247–3261. doi: 10.2174/1381612823666170317143248. [DOI] [PubMed] [Google Scholar]
- 13.Ananthakrishnan A.N., Kaplan G.G., Ng S.C. Changing global epidemiology of inflammatory bowel diseases: sustaining health care delivery into the 21st century. Clin Gastroenterol Hepatol. 2020;18(6):1252–1260. doi: 10.1016/j.cgh.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Sîngeap A.M., Girleanu I., Diculescu M., et al. Risk factors for extraintestinal manifestations in inflammatory bowel diseases-data from the Romanian National Registry. J Gastrointestin Liver Dis. 2021;30(3):346–357. doi: 10.15403/jgld-3818. [DOI] [PubMed] [Google Scholar]
- 15.Cohen S., Padlipsky J., Yerushalmy-Feler A. Risk factors associated with extraintestinal manifestations in children with inflammatory bowel disease. Eur J Clin Nutr. 2020;74:691–697. doi: 10.1038/s41430-019-0490-1. [DOI] [PubMed] [Google Scholar]
- 16.Jiang L., Xia B., Li J., et al. Retrospective survey of 452 patients with inflammatory bowel disease in Wuhan city, central China. Inflamm Bowel Dis. 2006;12(3):212–217. doi: 10.1097/01.MIB.0000201098.26450.ae. [DOI] [PubMed] [Google Scholar]
- 17.Alavinejad P., Ghanavati P.M., Alboraie M., et al. Irritable bowel syndrome demographics: a middle eastern multinational cross-sectional study. Middle East J Dig Dis. 2022;14(2):222. doi: 10.34172/mejdd.2022.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iftikhar R., Abaalkhail B. Health-seeking influence reflected by online health-related messages received on social media: cross-sectional survey. J Med Internet Res. 2017;19(11):e382. doi: 10.2196/jmir.5989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner R.J., Wheaton B., Lloyd D.A. The epidemiology of social stress. Am Socio Rev. 1995;60(1):104–125. [Google Scholar]
- 20.Hossain M.M., Tasnim S., Sultana A., et al. Epidemiology of mental health problems in COVID-19: a review. F1000Res. 2020;9:636. doi: 10.12688/f1000research.24457.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanauer S.B., Present D.H. The state of the art in the management of inflammatory bowel disease. Rev Gastroenterol Disord. 2003;3(2):81–92. [PubMed] [Google Scholar]
- 22.Chudy-Onwugaje K.O., Christian K.E., Farraye F.A., Cross R.K. A state-of-the-art review of new and emerging therapies for the treatment of IBD. Inflamm Bowel Dis. 2019;25(5):820–830. doi: 10.1093/ibd/izy327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greuter T., Piller A., Fournier N., et al. Upper gastrointestinal tract involvement in Crohn's disease: frequency, risk factors, and disease course. J Crohns Colitis. 2018;12(12):1399–1409. doi: 10.1093/ecco-jcc/jjy121. [DOI] [PubMed] [Google Scholar]
- 24.Sun X.W., Wei J., Yang Z., et al. Clinical features and prognosis of Crohn's disease with upper gastrointestinal tract phenotype in Chinese patients. Dig Dis Sci. 2019;64:3291–3299. doi: 10.1007/s10620-019-05651-1. [DOI] [PubMed] [Google Scholar]
- 25.Campmans-Kuijpers M.J., Dijkstra G. Food and food groups in inflammatory bowel disease (IBD): the design of the groningen anti-inflammatory diet (GrAID) Nutrients. 2021;13(4):1067. doi: 10.3390/nu13041067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alavinejad P., Nayebi M., Parsi A., et al. Is dairy foods restriction mandatory for inflammatory bowel disease patients: a multinational cross-sectional study. Arq Gastroenterol. 2022;59:358–364. doi: 10.1590/S0004-2803.202203000-65. [DOI] [PubMed] [Google Scholar]
- 27.Rozich J.J., Holmer A., Singh S. Effect of lifestyle factors on outcomes in patients with inflammatory bowel diseases. Am J Gastroenterol. 2020;115(6):832. doi: 10.14309/ajg.0000000000000608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berg D.R., Colombel J.F., Ungaro R. The role of early biologic therapy in inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(12):1896–1905. doi: 10.1093/ibd/izz059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman T.P., Gomes C.F., Louis E., Colombel J.F., Satsangi J. De-escalation of immunomodulator and biological therapy in inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2020;5(1):63–79. doi: 10.1016/S2468-1253(19)30186-4. [DOI] [PubMed] [Google Scholar]
- 30.Ng S.C. Changing epidemiology and future challenges of inflammatory bowel disease in Asia. Intest Res. 2010;8(1):1–9. [Google Scholar]
- 31.Song E.M., Yang S.K. Natural history of inflammatory bowel disease: a comparison between the East and the West. Intest Res. 2022;20(4):418–430. doi: 10.5217/ir.2021.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]