Abstract
Purpose of review
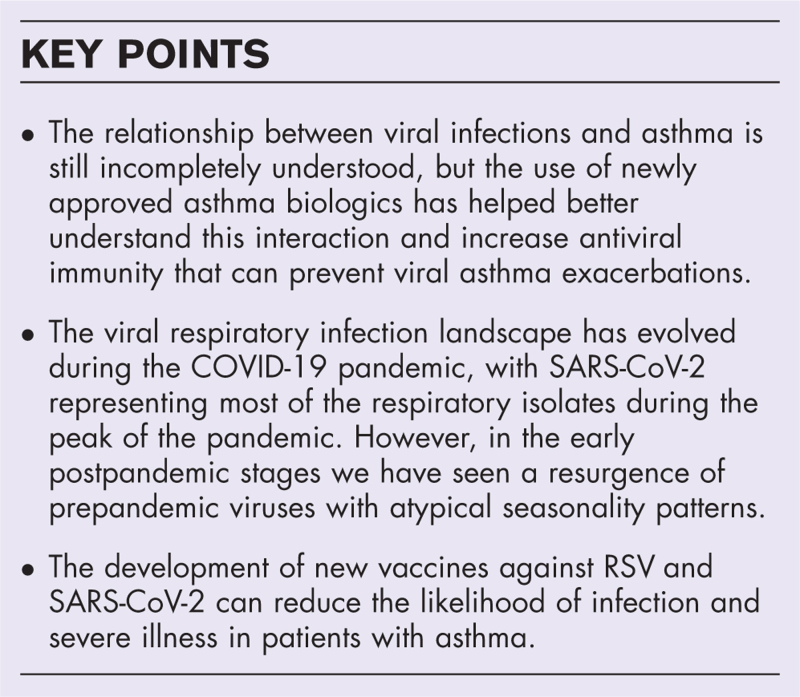
Asthma exacerbations are associated with substantial symptom burden and healthcare costs. Viral infections are the most common identified cause of asthma exacerbations. The epidemiology of viral respiratory infections has undergone a significant evolution during the COVID-19 pandemic. The relationship between viruses and asthmatic hosts has long been recognized but it is still incompletely understood. The use of newly approved asthma biologics has helped us understand this interaction better.
Recent findings
We review recent updates on the interaction between asthma and respiratory viruses, and we address how biologics and immunotherapies could affect this relationship by altering the respiratory mucosa cytokine milieu. By exploring the evolving epidemiological landscape of viral infections during the different phases of the COVID-19 pandemic, we emphasize the early post-pandemic stage, where a resurgence of pre-pandemic viruses with atypical seasonality patterns occurred. Finally, we discuss the newly developed RSV and SARS-CoV-2 vaccines and how they reduce respiratory infections.
Summary
Characterizing how respiratory viruses interact with asthmatic hosts will allow us to identify tailored therapies to reduce the burden of asthma exacerbations. New vaccination strategies are likely to shape the future viral asthma exacerbation landscape.
Keywords: asthma biologics, asthma exacerbations, COVID-19 pandemic, mRNA vaccines, RSV vaccines, viral respiratory infections
INTRODUCTION
Asthma exacerbations are episodes of worsening symptoms in patients suffering from asthma. Asthma exacerbations not only lead to worsening symptoms that impact patients’ quality of life, but are also associated with decrease in lung function over time, substantial healthcare costs and missed workdays. Systemic corticosteroids are the main treatment for asthma exacerbations potentially exposing patients to their numerous side effects. The majority of the asthma-related deaths occur in the setting of an exacerbation, and severe exacerbations can also happen in patients who have mild asthma [1].
Asthma exacerbations often follow an infectious or allergen trigger. Viral infections are the leading identified culprit, especially in the setting of increasing use of highly sensitive PCR assays. Viral respiratory infection-related asthma exacerbations (VAE) tend to have higher severity than exacerbations caused by other triggers. The pathogenic interactions between asthma and a viral infection leading to the exacerbation of symptoms have long been recognized [2]. In this article, we will review recent developments in the understanding of this complex interaction and how new asthma biologic therapies could affect it. We will also explore the epidemiological changes in the viral infection landscape that occurred during the COVID-19 pandemic and in the current early postpandemic phase. Finally, we will discuss the implications of the new respiratory syncytial virus (RSV) and severe-acute-respiratory-syndrome-related coronavirus-2 (SARS-CoV-2) vaccines in preventing VAE.
Box 1.
no caption available
HOW ASTHMA FACILITATES VIRAL INFECTIONS AND HOW BIOLOGIC THERAPIES COULD REGULATE THIS INTERACTION
Individuals with asthma, especially those with Th2 endotypes, have impaired antiviral responses making them more susceptible to respiratory viral infections [3,4▪]. Moreover, concomitant exposure to allergens and respiratory viruses has a deleterious synergistic effect in clinical outcomes [5]. At least two mechanisms for this synergy have been identified: allergen-bound immunoglobulin E (IgE) inhibits the type I interferon production by the plasmacytoid dendritic cell and suppressor of cytokine signaling 1 (SOCS-1) produced by type 2 T helper (Th2) and innate lymphoid cells (ILC-2) inhibits the production of antiviral interferons by type 1 T helper (Th1) cells and epithelial cells [6]. Porsbjerg et al.[7▪] recently showed that atopic vs. nonatopic asthma vary in how bronchial epithelial immune cells respond to viral infections. The fact that allergen immunotherapy improves antiviral responses at the mucosal site further enhances the evidence that Th2 inflammation is associated with lower antiviral activity [8▪].
Following respiratory viral infections, people with asthma have a delayed but prolonged upregulation of Th2 cytokines. Liu et al.[9▪▪] showed that interferons peak at day 3, whereas the Th2 cytokines interleukins (IL) 4 and 5 peak at day 8 post symptom onset. This could explain the lingering postinfection airway symptoms commonly seen in patients with asthma. There is also a reciprocal pathogenic mechanism perpetuating the cycle of viral infection and airway inflammation. In the postinfectious period, patients are at an increased risk for noninfectious asthma exacerbations since viruses damage the airway epithelial barrier, thereby opening the door for allergens and irritants to trigger subsequent exacerbations [10].
The pathogenesis of VAE is molecularly distinct from asthma exacerbations from other causes. Using a systems-scale network analysis, Altman et al. described different cellular transcriptional pathways in VAE versus nonviral asthma exacerbations. They also showed that the use of systemic corticosteroid corrected only certain pathways altered during the asthma exacerbations [11]. It would be interesting to see how different asthma biologics affect these pathways.
The use of asthma biologics can not only control Th2 inflammation and stabilize the integrity of the respiratory epithelium in patients with asthma, but also indirectly increase antiviral immune responses, as recently reviewed by Efthimiou et al.[12]. Specifically, biologics inhibiting the IL-5 cytokine pathway (benralizumab, mepolizumab, and reslizumab) increase expression of toll-like receptor-7 (TLR-7) that is essential to recognize viral single-stranded RNA in endosomes. Dupilumab, through inhibition of the IL-4 cytokine pathway, reduces expression of SOCS-1 and enhances Th1/antiviral interferon responses. Omalizumab (through direct blocking of IgE) and dupilumab (by indirectly inhibiting IgE plasma cell differentiation) reduce the IgE-mediated inhibition of interferon secretion by plasmacytoid dendritic cells (pDCs). See Table 1.
Table 1.
Proposed mechanisms of how asthma biologics can increase antiviral responses
| Biologic | Target | Proposed mechanism of boosting antiviral responses |
| Omalizumab | IgE | Allergen-bound immunoglobulin E (IgE) inhibits antiviral type I interferons produced by plasmacytoid dendritic cell (pDC). Blocking IgE prevents inhibition of pDC and enhances type I interferon secretion. |
| Mepolizumab (IL-5), reslizumab (IL-5), benralizumab (IL-5Ra) | IL-5/IL-5Ra | Toll-like receptor-7 (TLR-7) is crucial in recognizing viral single stranded RNA in endosomes. Interleukin-5 (IL-5) reduces expression of toll-like recptor-7 (TLR-7). Blocking IL-5 or its receptor (IL-5Ra) restores TLR-7 expression. |
| Dupilumab | IL-4Ra | Suppressor of cytokine signaling-1 (SOCS-1) inhibits Th1 responses and is stimulated by Th2 cytokines in particular interleukin 4 (IL-4). By blocking IL-4 pathway there is a reduces production of SOCS-1 and therefore a stronger antiviral Th1 response. Additionally, IL-4 is necessary for IgE class switching of B cells and IgE plasma cell differentiation. By inhibiting IL-4, dupilumab reduces IgE levels and boosts type I interferon production by pDC. |
| Tezepelumab | TSLP | Thymic stromal lymphopoietin (TSLP) is an upstream alarmin secreted with the respiratory epithelial cells in the presence of infection or allergen. Inhibiting TSLP reduces downstream Th2 inflammation and might help stabilize the respiratory epithelium but does not change interferon levels. Unlike the other asthma biologics that increase interferon levels and therefore antiviral immunity, it seems that tezepelumab may have a more neutral effect towards viral infections. |
Benralizumab is anti-IL-5 receptor alpha (IL-5Ra) mAb with antibody-dependent cellular cytotoxic properties that induces natural killer cell and macrophage activation [13▪]. It rapidly depletes eosinophils, making it a good candidate to use in the acute setting of asthma exacerbations. This drug has been applied successfully in cases of acute asthma exacerbations, but further studies would be needed to evaluate the safety and efficacy of this off-label use [14].
Dupilumab, an anti-IL4 receptor alpha (IL-4Ra) mAb that blocks signaling of the IL-4 and IL-13 pathways, increases antiviral interferons and has been recently associated with improved clinical outcomes in SARS-CoV-2 infection [15▪▪]. Moreover, dupilumab can reduce herpes simplex virus-1 (HSV-1) infection in patients with atopic dermatitis by decreasing HSV-1-specific IgE levels and increasing numbers of HSV-1-specific cytotoxic T cells with enhanced interferon gamma production [16].
Although asthma biologics targeting IgE, IL-4, and IL-5 pathways likely increase innate antiviral mediators in the setting of viral infections, we have shown that patients on these asthma biologics have decreased adaptive immune responses to SAR-CoV-2 mRNA vaccines [17▪].
Tezepelumab is the newest asthma biologic approved for clinical use. It is a monoclonal antibody against thymic stromal lymphopoietin (TSLP), an upstream alarmin implicated in the asthma pathogenesis that is secreted by the airway epithelial cells. Sverrild et al.[18▪▪] showed that tezepelumab reduced secretion of IL-33, IL-4, IL-13, and IL-17A by the bronchial epithelial cells, while the antiviral type-I and type-III interferons remained unchanged. Furthermore, in an in-vitro RSV infection, the viral loads remained unchanged. Therefore, unlike the other asthma biologics that increase interferon levels and therefore antiviral immunity, it seems that tezepelumab may have a more neutral effect towards viral infections. However, IL-33 has recently been found to drive the neutrophilic inflammation and NETosis in the early phases of the VAE [19], and so whether tezepelumab affects this pathogenic mechanism is still unclear.
EPIDEMIOLOGY OF RESPIRATORY VIRAL INFECTIONS: FROM PRE TO POST COVID-19 PANDEMIC
The epidemiology of viral isolates in asthma exacerbations has been less well studied than the overall epidemiology of respiratory viral infections during the pandemic years. In this section, we will discuss viral infections during the different phases of the COVID-19 pandemic, with an emphasis on the infection patterns emerging in the early postpandemic phase.
Rhinovirus has classically been identified as the most frequent culprit of viral asthma exacerbations during the prepandemic phase [20]. However, the epidemiology of viral infections has drastically been impacted by the COVID-19 pandemic. At the end of 2019, SARS-COV-2 (the virus causing COVID-19 disease) had made its way to the United States, and by 2021, it was classified as a pandemic causing severe respiratory disease around the world. The high infectivity of the SARS-CoV-2, along with lack of prior immune exposure during the early stages of the pandemic, created a perfect storm for the rapid spread of this virus. In 2020, it rapidly took over most of the respiratory infections identified, and there was a significant drop in non-SARS-CoV-2 viral respiratory infections [21▪▪]. Overall rates of asthma exacerbations during 2020 were lower than prepandemic levels, likely due to social distancing measures [22▪]. SARS-CoV-2 infection worsens asthma symptoms [23▪], and prolonged airway symptoms have also been identified as part of the post-COVID conditions (also known as Long COVID) [24,25,26▪].
As the SARS-CoV-2 virus spread and vaccines became available worldwide, individuals developed natural and/or vaccination-induced immunity. This increasing immunity along with viral evolution that allowed for more transmissible but less virulent variants to emerge (i.e., variants from the Omicron lineage) were associated with a decreased COVID-19 disease severity [27–29]. As a result, many precautionary measures were lifted, allowing for a rapid resurgence of other viral illnesses, including RSV, rhinovirus, and influenza virus. A large proportion of children and adults were hospitalized in the months following the pandemic for viral illness, namely RSV infections [21▪▪,30]. While RSV infection does not induce life-long immunity, there is temporary protective immunity following RSV infection that creates a refractory period during which a repeat infection is unlikely [31]. Because there were very few RSV infections during the pandemic, once pandemic restrictions were relaxed, the number of people susceptible to a new RSV infection was much higher.
There has been a shift in seasonality of respiratory viral infections since the COVID-19 pandemic. Daniels et al.[32] showed an increased RSV incidence in New York City after lifting pandemic precautionary measures, and a change in the RSV season onset from the winter months in prepandemic years to the fall months during 2021 and 2022. Another retrospective analysis found an increased postpandemic incidence of influenza, metapneumovirus, RSV, and rhinovirus. Not only did the number of cases increase overall, but peak incidence shifted drastically for certain viruses [33▪]. Shifts in viral seasonality and peak infectivity were noted, highlighting that viral competition may be playing a role. A meta-analysis that examined the prevalence of several respiratory viruses found that the normal seasonal peaks for these viruses were either shifted, less prevalent, or absent entirely during 2021 compared to prior years [21▪▪].
Overall, the above changes in viral incidence and seasonality were probably due to lifting precautionary measures along with the lack of immune exposure to other viruses during the SARS-COV-2 pandemic.
VIRAL INFECTION-RELATED ASTHMA EXACERBATIONS: PREVENTION VIA VACCINATION
A crucial way of preventing VAE is by avoiding viral infections altogether via vaccination. Vaccines confer the immunity necessary to prevent infection or to reduce the severity of viral illness, and therefore the frequency and severity the asthma exacerbations. Moreover, vaccination often reduces the likelihood that an infected patient will spread the disease, having public health benefits in addition to the individual advantages of being immunized [34▪]. In this section, we discuss the technology and the impact in viral illness of the recently developed and FDA-approved SARS-CoV-2 and RSV vaccines. Table 2 summarizes the advantages and disadvantages of each vaccine technology described here.
Table 2.
Summary of vaccine subtypes and their relative benefits and drawbacks
| Type of vaccine | Mechanism | Pro | Con |
| Protein Subunit | Presents stabilized antigen to the patient along with viral-like particles (VLP), nanoparticles or adjuvants to enhance immune response | Lack viral genome renders the vaccine incapable of replicating within the patient. Less biohazard concerns during vaccine development. Good temperature stability. |
Requires adjuvants for optimal immunogenicity. May need multiple doses for optimal immunogenicity. |
| mRNA Vaccine | Uses mRNA to instruct cells on how to produce antigen specific antibodies. | No biohazard concerns during vaccine development. Fast development. Produces high vaccine titers. |
Requires cold temperatures for optimal storage. Gradual decline in vaccine efficacy. |
| Mucosal Vaccine | Uses inactivated adenovirus vector or other virus vector to stimulate immunogenicity and present protein subunits. May also be a live attenuated or inactivated virus presented to mucosal cells as well. |
Stimulates both mucosal and systemic immunity. Potential to decrease viral shedding, severity of disease and progression of upper respiratory diseases to lower respiratory diseases. |
Adjuvants needed to break through immune tolerance. Research still needed focusing on effective antigen delivery, immunogenicity, and stimulation of both mucosal and T-cell immune responses. Durability and combination efforts with other vaccine subtypes is not fully understood either. |
In the past few years, we have witnessed the rapid development of the mRNA vaccine technology in its implementation in the setting of the SARS-CoV-2 with impressive results [35]. Vaccine efficacy in both mRNA vaccines was reported up to 90% [36,37]. The widespread implementation of SARS-CoV-2 vaccines led to the decline in death toll and severe disease from COVID-19, thus emphazising that mRNA vaccines are an effective option in pandemic scenarios. Resource-limited countries, however, could not safely use mRNA vaccines due to refrigeration requirements for storage purposes. Nanoparticle vaccines using Spike protein and adjuvants to stimulate immune response have a similar efficacy to mRNA vaccines, but also require refrigeration for storage [38▪]. Inactivated SARS-COV-2 vaccines proved to be an effective alternative vaccination strategy, preventing infection in more than 90% of cases [39], though they had much slower production times than mRNA vaccines [40]. Depite the success of vaccination against SARS-COV-2, the protective immunity generated by the vaccines is temporary [41▪]. Questions remain whether the short-lived immunity is due to the mRNA technology, the strutural nature of the SARS-CoV-2 Spike antigen [42], or both.
RSV vaccine efforts date back to the 1960 s, with initial vaccination development mimicking that of the Polio vaccines using a formalin-inactivated adjuvanted vaccine approach. The original RSV vaccine candidate was unfortunately associated with higher disease severity and death upon subsequent natural infection with RSV, despite inducing a four-fold or greater rise in serum neutralizing antibodies [43,44]. Since then, five decades have been devoted to RSV research in vaccination. Throughout this time, it has been ascertained that the two surface proteins F and G are responsible for creating neutralizing antibodies. The focus for vaccine development was placed on Protein F because this protein has less genetic variation [45]. Since then, studies with protein subunit vaccines specifically containing stabilized prefusion Protein F showed 10-fold boosts in neutralizing antibodies compared to baseline in animal models [46]. These developments eventually opened the door once again for human trials with RSV subunit vaccines. In 2023, two RSV vaccines were approved, a bivalent recombinant subunit vaccine and an adjuvanted subunit vaccine to the prefusion Protein F, both of which were up to 90% effective against RSV infection [47,48▪▪]. Additionally, in late 2023, an mRNA vaccine in phase II-III clinical trials had a promising preliminary efficacy of up to 83% [49▪▪]. These protein subunit vaccines are currently approved for adults 60 years and older and preganant women and will hopefully get approved for patients with cardiopulmonary disease, which would include patients with asthma. Nevertheless, it will take years to understand the full beneficial impact of RSV vaccines on asthma exacerbation rates.
With systemic vaccines, there are questions whether they actually induce sterilizing immunity. Since most respiratory pathogens gain infectivity by breaking the mucosal barrier, providing mucosal immunity would be an early line of defense in preventing infection and therefore transmission to others [50]. Benefits include increasing IgA production and local mucosal immunity to inhibit viral shedding and preventing the spread from upper respiratory infection to lower respiratory infection [51]. Recombinant SARS-CoV-2 viral based mucosal vaccines have shown clinical efficacy and are currently being used outside the United States [52,53]. One study combined a mucosal vaccine with attenuated viral intramuscular vaccines and found neutralizing antibodies up to six times higher than when using attenuate vaccine by itself [52]. Although additional studies are needed, perhaps mucosal vaccines in combination with systemically administered vaccines will provide more complete protection by stimulating both systemic and mucosal immunity.
There is evidence that influenza vaccination reduces both rates of infection and asthma exacerbations [54]. More studies will be needed to see wheather these newer SARS-CoV-2 and the RSV vaccines can also reduce rates of asthma exacerbations.
CONCLUSION AND FUTURE DIRECTIONS
Our understanding of the complex molecular interactions between respiratory viruses and hosts with asthma is constantly evolving. Asthma biologics have revolutionized the treatment of asthma in the past 15 years. All FDA-approved asthma biologics have shown to significantly reduce rates of exacerbations. However, it is not clear if all asthma biologics reduce incidence of VAE or if they preferentially reduce the rates of asthma exacerbations due to noninfectious causes. It is possible that biologics improve antiviral immunity and increase infection clearance, but most clinical studies that evaluated the efficacy of biologics did not classify exacerbations by cause and the trial with omalizumab did not find differences in virus identification between groups [55]. In our clinical practice, we have experienced that our well controlled patients on asthma biologics can occasionally exacerbate, and when they do, it is frequently in the context of a viral infection. However, detailed clinical studies should be designed to address this question. At the same time, identification of the molecular mechanisms of how different biologics impact the interaction of asthma and viral infections at the mucosal site will help clinicians tailor biological therapies to individual patient endotypes.The viral respiratory infection epidemiology has significantly changed over the past few years, with SARS-CoV-2 accounting for most respiratory infections during the COVID-19 pandemic. However, in the current early postpandemic stages, we have started to see other viruses re-emerge with high rates of infection and atypical seasonal patterns. It would be interesting to see how and when the postpandemic viral epidemiology settles. The increasing global interconnectivity and climate change are likely to play a
role in the viral infections and allergen exposure in future decades. Finally, the design of new vaccine technologies and the implementation of intelligent immunization strategies will also dictate the future landscape of viral infections.
Acknowledgements
None.
Financial support and sponsorship
NIH/National Institute of Allergy and Infectious Diseases: P01AI125180, U54CA260563, R01AI172254, U01AI141993, and NIH/National Heart, Lung, and Blood Institute: T32HL116271
NIH Department of Health and Human Services/Public Health Services: 5T32AI74492-14
Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases.
Conflicts of interest
P.A.L. is supported by NIH/NHLBI T32HL116271.
V.C. is supported by NIH/NIAID 5T32AI74492.
F.E.L. is supported by NIH/NIAID grants P01AI125180, U54CA260563, R01AI172254, U01AI141993, research grants from Genentech and Gates Foundation. Received royalties from BLI INC for Plasma cell survival media. Scientific advisory board of Be Bio Pharma. Has patents on plasma cell survival media and MENSA (media of elaborated newly synthesized antibodies). Is the founder and owner of MicroB-plex, Inc.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Bourdin A, Bjermer L, Brightling C, et al. ERS/EAACI statement on severe exacerbations in asthma in adults: facts, priorities and key research questions. [DOI] [PubMed] [Google Scholar]
- 2.Gern JE. Viral and bacterial infections in the development and progression of asthma. J Allergy Clin Immunol 2000; 105:S497–S502. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DJ, Gern JE. Rhinovirus infections and their roles in asthma: etiology and exacerbations. J Allergy Clin Immunol Pract 2022; 10:673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Wirz OF, Jansen K, Satitsuksanoa P, et al. Experimental rhinovirus infection induces an antiviral response in circulating B cells which is dysregulated in patients with asthma. Allergy 2022; 77:130–142. [DOI] [PMC free article] [PubMed] [Google Scholar]; Decreased interferon responses against rhinovirus have been extensively described in patients with asthma but this study shows that antiviral B cells responses are also decreased.
- 5.Bakakos A, Sotiropoulou Z, Vontetsianos A, et al. Epidemiology and immunopathogenesis of virus associated asthma exacerbations. J Asthma Allergy 2023; 16:1025–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagome K, Nagata M. Innate immune responses by respiratory viruses, including rhinovirus, during asthma exacerbation. Front Immunol 2022; 13:865973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7▪.Porsbjerg C, Nieto-Fontarigo JJ, Cerps S, et al. Phenotype and severity of asthma determines bronchial epithelial immune responses to a viral mimic. Eur Respir J 2022; 60:2102333. [DOI] [PubMed] [Google Scholar]; Study that classifies the antiviral cytokine profiles by asthma type and severity.
- 8▪.Woehlk C, Ramu S, Sverrild A, et al. Allergen immunotherapy enhances airway epithelial antiviral immunity in patients with allergic asthma (VITAL Study): a double-blind randomized controlled trial. Am J Respir Crit Care Med 2023; 207:1161–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]; This randomized clinical trial showed that sublingual dust mite immunotherapy increased antiviral type I and type III interferons in patients with allergic asthma.
- 9▪▪.Liu M, Zhang Y, Hu Y, et al. The upregulation of peripheral CD3(−)CD56(+)CD16(+) natural killer cells correlates with Th1/Th2 imbalance in asthma patients during acute upper respiratory viral infections. BMC Immunol 2023; 24:40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Translational observational study that showed the timing of Th1 versus Th2 cytokine elevation during a viral asthma exacerbation. Showed that CD3-CD56+CD16+ natural killer cells coorelate with the Th1 response timing. Most importantly for this review, this study showed that patients with asthma have a delayed but prolonged Th2 response after a viral infection.
- 10.Ojanguren I, Satia I, Usmani OS. The role of viral infections on severe asthma exacerbations: present and future. Arch Bronconeumol 2022; 58:632–634. [DOI] [PubMed] [Google Scholar]
- 11.Altman MC, Gill MA, Whalen E, et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat Immunol 2019; 20:637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efthimiou J, Poll C, Barnes PJ. Dual mechanism of action of T2 inhibitor therapies in virally induced exacerbations of asthma: evidence for a beneficial counter-regulation. Eur Respir J 2019; 54:1802390. [DOI] [PubMed] [Google Scholar]
- 13▪.Dagher R, Kumar V, Copenhaver AM, et al. Novel mechanisms of action contributing to benralizumab's potent antieosinophilic activity. Eur Respir J 2022; 59:2004306. [DOI] [PMC free article] [PubMed] [Google Scholar]; Translational study that uses confocal microscopy to describe in detail the steps involed in how benralizumab induces macrophage phagocytosis of eosinophils.
- 14.Ramakrishnan S, Camp JR, Vijayakumar B, et al. The use of benralizumab in the treatment of near-fatal asthma: a new approach. Am J Respir Crit Care Med 2020; 201:1441–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪▪.Donlan AN, Mallawaarachchi I, Sasson JM, et al. Dupilumab use is associated with protection from Coronavirus Disease 2019 mortality: a retrospective analysis. Clin Infect Dis 2023; 76:148–151. [DOI] [PMC free article] [PubMed] [Google Scholar]; Clinical observational study that using healthcare electronical record databases found that patients on dupilumab had lower mortality associated to COVID-19. This shows that dupilumab can have antiviral effects in clinically-relevant scenerio.
- 16.Traidl S, Harries L, Kienlin P, et al. Dupilumab strengthens herpes simplex virus type 1-specific immune responses in atopic dermatitis. J Allergy Clin Immunol 2023; 152:1460–1469. e5. [DOI] [PubMed] [Google Scholar]
- 17▪.Runnstrom MC, Morrison-Porter A, Ravindran M, et al. Reduced COVID-19 vaccine response in patients treated with biologic therapies for asthma. Am J Respir Crit Care Med 2022; 205:1243–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]; Prospective observational study that shows that mRNA SARS-CoV-2 vaccine-induced immune responses were dimishied in patients on benralizumab, mepolizumab, or dupilumab.
- 18▪▪.Sverrild A, Cerps S, Nieto-Fontarigo JJ, et al. Tezepelumab decreases airway epithelial IL-33 and T2-inflammation in response to viral stimulation in patients with asthma. Allergy 2023; 00:1–11. [DOI] [PubMed] [Google Scholar]; Study that looked at bronchioalveolar lavage of patients with asthma and found that those receiving tezepelumab had lower Th2 cytokines, but there was no difference in the antiviral interferon levels or viral loads in an in-vitro Rhinovirus infection model.
- 19.Curren B, Ahmed T, Howard DR, et al. IL-33-induced neutrophilic inflammation and NETosis underlie rhinovirus-triggered exacerbations of asthma. Mucosal Immunol 2023; 16:671–684. [DOI] [PubMed] [Google Scholar]
- 20.Denlinger LC, Sorkness RL, Lee WM, et al. Lower airway rhinovirus burden and the seasonal risk of asthma exacerbation. Am J Respir Crit Care Med 2011; 184:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪▪.Schuz ML, Dallmeyer L, Fragkou PC, et al. Global prevalence of respiratory virus infections in adults and adolescents during the COVID-19 pandemic: a systematic review and meta-analysis. Int J Infect Dis 2023; 137:16–24. [DOI] [PubMed] [Google Scholar]; Epidemiological study of the respiratory viral infections during the COVID-19 pandemic showing. It showed resurfacing of non-SARS-CoV-2 respiratrory viruses in the second part of the pandemic.
- 22▪.Karcher H, Schoenberger M, Rayban T, et al. Impact of COVID-19 measures on exacerbation rates and healthcare visits in US asthma patients. Allergy Asthma Proc 2023; 44:422–428. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that the overall rates of asthma exacerbations were lower during the pandemic that previous years. Findings are likely explained by implementation of public health measures during the pandemic.
- 23▪.Agondi RC, Menechino N, Marinho A, et al. Worsening of asthma control after COVID-19. Front Med (Lausanne) 2022; 9:882665. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study found that patients can have prolonged asthma symmtoms after with mild or moderate COVID-19.
- 24.Eggert LE, He Z, Collins W, et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy 2022; 77:173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwok WC, Tam TCC, Lam DCL, et al. Worsening of asthma control after recovery from mild to moderate COVID-19 in patients from Hong Kong. Respir Res 2023; 24:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Philip KEJ, Buttery S, Williams P, et al. Impact of COVID-19 on people with asthma: a mixed methods analysis from a UK wide survey. BMJ Open Respir Res 2022; 9:e001056. [DOI] [PMC free article] [PubMed] [Google Scholar]; Study showed that it is common to have persistent asthma symptoms after COVID-19 illness.
- 27.Mella-Torres A, Escobar A, Barrera-Avalos C, et al. Epidemiological characteristics of Omicron and Delta SARS-CoV-2 variant infection in Santiago, Chile. Front Public Health 2022; 10:984433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharyya RP, Hanage WP. Challenges in inferring intrinsic severity of the SARS-CoV-2 Omicron variant. N Engl J Med 2022; 386:e14. [DOI] [PubMed] [Google Scholar]
- 29.Radhakrishnan N, Liu M, Idowu B, et al. Comparison of the clinical characteristics of SARS-CoV-2 Delta (B.1.617. 2) and Omicron (B. 1. 1. 529) infected patients from a single hospitalist service. BMC Infect Dis 2023; 23:747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice E, Oakes DB, Holland C, et al. Respiratory syncytial virus in children: epidemiology and clinical impact post-COVID-19. Curr Opin Infect Dis 2023; 36:522–528. [DOI] [PubMed] [Google Scholar]
- 31.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis 1991; 163:693–698. [DOI] [PubMed] [Google Scholar]
- 32.Daniels D, Wang D, Suryadevara M, et al. Epidemiology of RSV bronchiolitis among young children in Central New York before and after the onset of the COVID-19 pandemic. Pediatr Infect Dis J 2023; 42:1056–1062. [DOI] [PubMed] [Google Scholar]
- 33▪.Presti S, Manti S, Gambilonghi F, et al. Comparative analysis of pediatric hospitalizations during two consecutive influenza and respiratory virus seasons post-pandemic. Viruses 2023; 15:1825. [DOI] [PMC free article] [PubMed] [Google Scholar]; Epidemiological study showing that timing of onset and peaks of incidence of respiratory virus differ from prepandeimic to postpandemic years.
- 34▪.Poria R, Kala D, Nagraik R, et al. Vaccine development: current trends and technologies. Life Sci 2024; 336:122331. [DOI] [PubMed] [Google Scholar]; Recent review on vaccine technologies.
- 35.Jain S, Venkataraman A, Wechsler ME, Peppas NA. Messenger RNA-based vaccines: past, present, and future directions in the context of the COVID-19 pandemic. Adv Drug Deliv Rev 2021; 179:114000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020; 384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas SJ, Moreira ED, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med 2021; 385:1761–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Dunkle LM, Kotloff KL, Gay CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med 2022; 386:531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]; Efficacy trial using SARS-CoV-2 vaccine with nanoparticle technology.
- 39.Ella R, Reddy S, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis 2021; 21:950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed TI, Rishi S, Irshad S, et al. Inactivated vaccine Covaxin/BBV152: a systematic review. Front Immunol 2022; 13:863162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41▪.Nguyen DC, Lamothe PA, Woodruff MC, et al. COVID-19 and plasma cells: is there long-lived protection? Immunol Rev 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]; Recent review on challenges in generating long-term protective immunity to SARS-CoV-2.
- 42.Bachmann MF, Mohsen MO, Zha L, et al. SARS-CoV-2 structural features may explain limited neutralizing-antibody responses. NPJ Vaccines 2021; 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim HW, Canchola JG, Brandt CD, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 1969; 89:422–434. [DOI] [PubMed] [Google Scholar]
- 44.Graham BS. The journey to RSV vaccines — heralding an era of structure-based design. N Engl J Med 2023; 388:579–581. [DOI] [PubMed] [Google Scholar]
- 45.Ngwuta JO, Chen M, Modjarrad K, et al. Prefusion F-specific antibodies determine the magnitude of RSV neutralizing activity in human sera. Sci Transl Med 2015; 7:309ra162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crank MC, Ruckwardt TJ, Chen M, et al. A proof of concept for structure-based vaccine design targeting RSV in humans. Science 2019; 365:505–509. [DOI] [PubMed] [Google Scholar]
- 47.Papi A, Ison MG, Langley JM, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med 2023; 388:595–608. [DOI] [PubMed] [Google Scholar]
- 48▪▪.Walsh EE, Perez Marc G, Zareba AM, et al. Efficacy and safety of a bivalent RSV prefusion F vaccine in older adults. N Engl J Med 2023; 388:1465–1477. [DOI] [PubMed] [Google Scholar]; Phase 3 clinical trial showing efficay and safety of bivalent RSV A and RSV B vaccine showing a 67% efficacy in preventing lower respiratory ilness in adults 60 years and older.
- 49▪▪.Wilson E, Goswami J, Baqui AH, et al. Efficacy and safety of an mRNA-based RSV PreF vaccine in older adults. N Engl J Med 2023; 389:2233–2244. [DOI] [PubMed] [Google Scholar]; Phase 3 clinical trial of monovalent RSV (RSV A only) vaccine that showed a well tolerated profile and protective immunity and against both RSV A and RSV B.
- 50.Lavelle EC, Ward RW. Mucosal vaccines - fortifying the frontiers. Nat Rev Immunol 2022; 22:236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dotiwala F, Upadhyay AK. Next generation mucosal vaccine strategy for respiratory pathogens. Vaccines (Basel) 2023; 11:1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sunagar R, Prasad SD, Ella R, Vadrevu KM. Preclinical evaluation of safety and immunogenicity of a primary series intranasal COVID-19 vaccine candidate (BBV154) and humoral immunogenicity evaluation of a heterologous prime-boost strategy with COVAXIN (BBV152). Front Immunol 2022; 13:1063679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao J. Vaccination with CanSinoBIO's inhaled COVID-19 vaccine has begun in China. J Biosaf Biosecur 2022; 4:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vasileiou E, Sheikh A, Butler C, et al. Effectiveness of influenza vaccines in asthma: a systematic review and meta-analysis. Clin Infect Dis 2017; 65:1388–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med 2011; 364:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]