Abstract
Background
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer associated with shorter survival and a higher likelihood of recurrence. In early TNBC, platinum chemotherapy has been shown to improve pathological complete response (pCR); however, its effect on long-term survival outcomes has not been fully elucidated.
Methods
Randomised controlled trials examining neoadjuvant or adjuvant platinum chemotherapy for early TNBC were included. Primary outcomes were disease-free survival (DFS) and overall survival (OS). Secondary outcomes were pCR, treatment adherence, grade III or IV toxicity related to chemotherapy, and quality of life.
Results
From 3972 records, we included 20 published studies. All studies reporting DFS and OS used carboplatin. Inclusion of platinum chemotherapy improved DFS (neoadjuvant: hazard ratio (HR) 0.63, 95% confidence interval (CI) 0.53 to 0.75; adjuvant: HR 0.69, 95% CI 0.54 to 0.88) and OS (neoadjuvant: HR 0.69, 95% CI 0.55 to 0.86; adjuvant: 0.70, 95% CI 0.50 to 0.96). Our analysis confirmed platinum chemotherapy increased pCR rates (risk ratio (RR) 1.44, 95% CI 1.31 to 1.59). There were no differences seen in examined subgroups. Platinum chemotherapy was associated with reduced dose intensity and increased haematological toxicity.
Conclusions
Platinum-based chemotherapy using carboplatin in the adjuvant or neoadjuvant setting improves long-term outcomes of DFS and OS in early TNBC, with no evidence of differences by subgroup. This was at the cost of more frequent chemotherapy delays and dose reductions, and greater haematological toxicity. These findings support the use of platinum-based chemotherapy for people with early TNBC.
Keywords: Carboplatin, Platinum, Triple-negative breast cancer
Highlights
-
•
Carboplatin reduces cancer recurrence in early triple negative breast cancer.
-
•
Carboplatin chemotherapy improves survival in early triple negative breast cancer.
-
•
The benefits are seen if carboplatin is used before or after surgery.
-
•
Platinum chemotherapy is associated with increased side effects.
-
•
Benefit from carboplatin is seen across examined subgroups.
1. Background
Breast cancer is the most common type of cancer in women and the most common cause of cancer death [1]. Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer, which lacks hormone receptors and human epidermal growth factor receptor 2 (HER2) expression. It is associated with shorter survival and a higher likelihood of recurrence, and comprises about 15% of breast cancer diagnoses [[2], [3]]. Early TNBC is defined as cancer that has not spread beyond the breast or axillary lymph nodes, and is potentially curable. Surgery, radiotherapy, and chemotherapy are used to minimise the chance of relapse.
TNBC is more likely to be associated with heritable causes than other breast cancer subtypes. Over 10% of people diagnosed with TNBC under the age of 50 years, without known family history of breast or ovarian cancer, have a heritable mutation in either breast cancer gene 1 or gene 2 (BRCA1 or BRCA2) [4]. Whilst BRCA1 mutation is the most strongly associated, other heritable gene mutations (i.e. BRCA2; partner and localizer of BRCA2 (PALB2); RAD51 paralogue D (RAD51D) and BRCA1 associated RING domain 1 (BARD1)) have also shown associations with TNBC and higher lifetime risks of breast cancer.
The National Comprehensive Cancer Network (NCCN) Guidelines recommend offering chemotherapy to women with TNBC whose cancer is larger than 1 cm, or with involved lymph nodes. Chemotherapy may also be considered for smaller tumours. Standard chemotherapy used in the adjuvant or neoadjuvant setting for TNBC often involves anthracycline and taxane chemotherapy. The role of adjuvant chemotherapy is to treat micrometastatic systemic disease, which is not detectable by standard blood tests and imaging.
In this review, the intervention being studied is platinum chemotherapy (cisplatin, carboplatin or novel agents) alone or in addition to the standard adjuvant or neoadjuvant chemotherapy, to determine whether this improves survival from early TNBC. Our primary outcomes were overall survival (OS) and disease-free survival (DFS). Achieving a pathological complete response (pCR) has strong prognostic value, particularly in the TNBC subtype [5]. Because of the assumed association between survival and pCR, many trials assess pCR while either waiting for data to mature or as their primary endpoint before deciding whether larger trials are feasible. Consequently, we reported pCR, along with OS and DFS.
Potential adverse effects of platinum include an increase in myelosuppression, which can lead to dose omissions, interruptions or dose reduction of platinum chemotherapies, other chemotherapy drugs, or both. There are risks of additional toxicity from myelosuppression, with febrile neutropenia, anaemia or bleeding due to thrombocytopenia. Long-term toxicities from platinum chemotherapy can include peripheral neuropathy, ototoxicity and renal impairment.
Maximising the efficacy of treatment of early breast cancer will reduce rates of metastatic, incurable disease and premature death from this condition. However, given this is a population where the intention is long-term survival, the prevention of permanent toxicity is also a priority.
2. Methods
2.1. Criteria for considering studies for this review
We included randomised controlled trials examining platinum chemotherapy for neoadjuvant or adjuvant treatment for people with early TNBC. This included trials which added a platinum chemotherapy to another standard chemotherapy regimen, or compared a platinum regimen to a non-platinum regimen. To be included, studies must have reported their findings for participants with TNBC separately from other participants.
We included participants aged 18 years or older with early TNBC, defined as breast cancer with disease isolated to the breast and axillary lymph nodes that lack expression of the oestrogen receptor and progesterone receptor (as defined by the trial), and negative for human epidermal receptor 2 (HER2; negative with in situ hybridisation testing; 0 to 1+ with immunohistochemistry (IHC); or 2+ with IHC and negative with fluorescence in situ hybridisation). We included trials with all study locations, and participants of all ethnicities. We excluded trials that did not assess women for HER2 status.
The intervention of interest was any chemotherapy regimen that contained platinum chemotherapy compared to regimens without platinum chemotherapy. Included studies addressed either adjuvant or neoadjuvant delivery of chemotherapy for early TNBC. We recorded and compared the dose and duration of chemotherapy.
Our primary outcomes were.
-
•
Disease-free survival (DFS), defined as time from surgery (in neoadjuvant setting) or randomisation (in adjuvant setting) to first date of a local, regional or distant relapse; diagnosis of a second primary cancer; or death from any cause. We included similar outcomes, such as progression-free survival and time-to-progression in this section.
-
•
Overall survival (OS), defined as the time from randomisation or study entry until death from any cause.
Our secondary outcomes were.
-
•
Pathological complete response (pCR) defined as no invasive carcinoma in the breast or axillary lymph nodes (ypT0/isypN0 TNM (tumour, node, metastasis) staging; [6]) after neoadjuvant therapy.
-
•
Completion of regimens, assessed by absence of delay in treatment or dose reductions, or both, or early cessation of treatment.
-
•
Any grade III/IV toxicity related to chemotherapy
-
•
Quality of life – we aimed to report any quality-of-life data as measured by the many validated tools available to trialists, and at all reported time points
2.2. Data collection and analysis
Detailed search strategies and methods for data extraction and analysis can be accessed via https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014805.pub2/full.
Data was collected on study design; randomisation methods; baseline characteristics of participants; setting; chemotherapy regimens (chemotherapy agent, dose, number of cycles); deliverability of treatment, assessed by dose intensity, dose delays or interruptions; and primary and secondary outcomes. We also collected details regarding type of toxicity for grade III or IV events (according to National Cancer Institute Common Terminology Criteria for Adverse Events [7]), length of follow-up and sources of funding.
For studies with more than one publication, we extracted data from all publications, and considered the most recent full-text version of the study to be the primary reference. RevMan Web 2022 was used for analysis.
We assessed bias using Cochrane's RoB 1 tool [8]. The domains assessed were sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias) and other potential sources of bias. The outcomes were segregated into DFS, OS, pCR, toxicity and treatment adherence, and quality of life.
We used the following effect measures:
-
•
Time-to-event outcomes (DFS, OS): expressed as a hazard ratio (HR) with 95% confidence intervals (CI). For HRs and variances which were not reported in the trial publications, we calculated summary statistics indirectly using the methods outlined in Ref. [9]. HRs less than 1.0 favour regimens with platinum chemotherapy, and HRs greater than 1.0 favour regimens without platinum chemotherapy.
-
•
Dichotomous outcomes (pCR, completion of regimens, toxicity): expressed as risk ratio (RR) with 95% CI. We reported the ratios of treatment effects for pCR (a favourable event) so that RRs greater than 1.0 favour regimens with platinum chemotherapy, and RRs less than 1.0 favour regimens without platinum chemotherapy. For completion of regimens or toxicity outcomes (unfavourable events), RRs greater than 1.0 favour regimens without platinum chemotherapy and RRs less than 1.0 favour regimens with platinum chemotherapy. Data for toxicity were the population included in the study regardless of the proportion of participants with TNBC;
Recommendations in the Cochrane Handbook for Systematic Reviews of Interventions have guided the assessment of heterogeneity [10]. We examined diversity by visually inspecting the forest plots, Chi2 test and I2 statistic. We used a cut-off point of P = 0.10 for the Chi2 test, and considered an I2 > 75% to represent considerable heterogeneity.
We used the following methods to synthesise the data.
-
•
time-to-event data (DFS, OS) – we used a fixed-effect model with an inverse-variance model;
-
•
dichotomous outcomes (pCR, completion of regimens, toxicity) – we used a fixed-effect model. In the case of pCR, one study was an adaptive platform trial and reported results as an estimated rate of complete response with a 95% Bayesian probability interval. To include these data in the meta-analysis, we calculated the discrete number of events in each group by using the adjusted probabilities of pCR.
Planned subgroup analyses included BRCA mutation status, homologous recombination deficiency status, lymph node status, platinum agent used, and characteristics of the chemotherapy protocols used including whether the platinum intervention arm was anthracycline free, and the schedule of the platinum agent.
Planned sensitivity analysis were undertaken based on trials with differences in the definition of triple negative according to hormone receptor IHC cutoffs, potentially confounding extra treatments, a high or unclear risk of bias or considerable heterogeneity.
Certainty of evidence was assessed using the GRADE approach [11].
3. Results
3.1. Results of the search
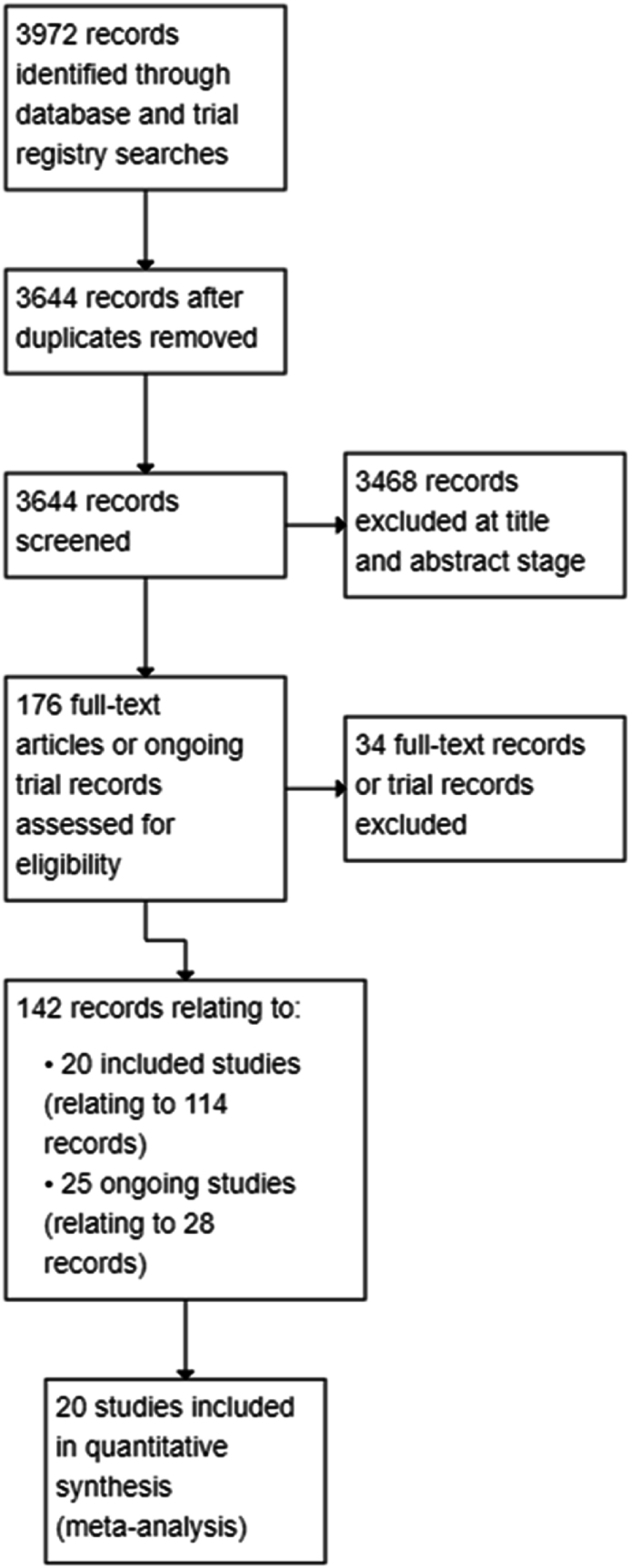
Database and trial registry searches yielded 3972 records, and we screened the titles and abstracts of 3644 records after removing duplicates. We excluded 3468 records at title and abstract screening stage and screened 176 full-text articles or ongoing trial records. Of these, 114 records related to 20 included studies involving 21 treatment comparisons, and 28 records related to 25 ongoing studies. See PRISMA flowchart (Fig. 1).
Fig. 1.
Study flow diagram.
3.2. Included studies
The 20 included studies, involving 4468 participants, contributed to 21 treatment comparisons outlined in Table 1. Notably, the BrighTNess [12] study has more than one intervention that was split into two treatment comparisons (BrighTNess comparison 1; BrighTNess comparison 2), which is why the number of studies and treatment comparisons included in an analysis may differ.
Table 1.
Summary of the included treatment comparisons.
| Trial | Year recruitment started | Intervention (platinum-containing) | Control | Platinum agent | Same backbone? | Adjuvant or neoadjuvant | Hormone receptor IHC cut-off |
|---|---|---|---|---|---|---|---|
| ADAPT-TN[13] | 2013 | Nab-paclitaxel 125 mg/m2 + carboplatin AUC2 days 1 and 8 every 3 weeks for 4 cycles | Nab-paclitaxel 125 mg/m2 + gemcitabine 1000 mg/m2 days 1 and 8 every 3 weeks for 4 cycles | Carboplatin AUC2 every week (days 1 and 8 every 21 days) | No | Neoadjuvant | <1% |
| Ando 2014[14] | 2010 | Carboplatin AUC5 every 3 weeks for 4 cycles + paclitaxel 80 mg/m2 days 1, 8, 15 for 4 cycles, followed by 4 cycles of cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2 and fluorouracil 500 mg/m2 every 3 weeks | Paclitaxel 80 mg/m2 days 1, 8, 15 for 4 cycles followed by 4 cycles of cyclophosphamide 500 mg/m2, epirubicin 100 mg/m2 and fluorouracil 500 mg/m2 every 3 weeks | Carboplatin AUC5 every 3 weeks | Yes | Neoadjuvant | <10% |
| BrighTNess comparison 1 [12] | 2014 | Paclitaxel 80 mg/m2 weekly + carboplatin AUC6 every 3 weeks for 12 weeks + veliparib 50 mg twice a day, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | <1% |
| BrighTNess comparison 2 [12] | 2014 | Paclitaxel 80 mg/m2 weekly + carboplatin AUC6 every 3 weeks for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks, followed by doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | <1% |
| CALGB 40603 [15] | 2009 | Paclitaxel 80 mg/m2 weekly + carboplatin AUC6 every 3 weeks for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles ± bevacizumab 10 mg/kg every 2 weeks for 9 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles ± bevacizumab 10 mg/kg every 2 weeks for 9 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | <10% |
| GEICAM 2006-03[16] | 2007 | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 75 mg/m2 + carboplatin AUC6 every 3 weeks for 4 cycles | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 75 mg/m2 every 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | Yes | Neoadjuvant | Not described |
| GeparOcto[17] | 2014 | Paclitaxel 80 mg/m2 + non-pegylated liposomal doxorubicin 20 mg/m2 + carboplatin AUC1.5 weekly for 18 weeks | Epirubicin 150 mg/m2 + paclitaxel 225 mg/m2 + cyclophosphamide 2000 mg/m2 every 2 weeks for 3 cycles | Carboplatin AUC1.5 every week | No | Neoadjuvant | <1% |
| GeparOLA[18] | 2016 | Paclitaxel 80 mg/m2 + carboplatin AUC2 weekly for 12 weeks followed by epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly + olaparib 100 mg twice a day for 12 weeks followed by epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC2 every week | No | Neoadjuvant | <1% |
| GeparSixto[19] | 2011 | Carboplatin AUC2 or 1.5 + paclitaxel 80 mg/m2 + non-pegylated liposomal doxorubicin 20 mg/m2 + bevacizumab 15 mg/kg every 3 weeks for 18 weeks | Paclitaxel 80 mg/m2 + non-pegylated liposomal doxorubicin 20 mg/m2 weekly + bevacizumab 15 mg/kg every 3 weeks for 18 weeks | Carboplatin AUC1.5 or 2 every week | Yes | Neoadjuvant | <1% |
| Gigolaeva 2019 [20] | NR | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by carboplatin AUC2 weekly + eribulin 1.4 mg/m2 or paclitaxel 175 mg/m2 every 3 weeks for 12 weeks | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by paclitaxel 80 mg/m2 for 12 weeks | Carboplatin AUC2 every week | No | Neoadjuvant | Not described |
| INFORM[21] | 2012 | Cisplatin 75 mg/m2 every 3 weeks for 4 cycles | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2–3 weeks for 4 cycles | Cisplatin 75 mg/m2 every 3 weeks | No | Neoadjuvant | <10% |
| I-SPY2 [22] | 2010 | Paclitaxel 80 mg/m2 weekly + veliparib 50 mg twice daily + carboplatin AUC6 every 3 weeks for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Paclitaxel 80 mg/m2 weekly for 12 weeks followed by doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 or 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | No | Neoadjuvant | <5% |
| Li 2020 [23] | 2011 | Paclitaxel 150 mg/m2 + carboplatin AUC3 every 2 weeks for 8 cycles | Epirubicin 80 mg/m2 and cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles followed by paclitaxel 175 mg/m2 every 2 weeks for 4 cycles | Carboplatin AUC3 every 2 weeks | No | Adjuvant | <1% |
| Nasr 2015[24] | 2008 | 5-fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 every 3 weeks for 3 cycles then docetaxel 80 mg/m2 + carboplatin AUC5 every 3 weeks for 3 cycles, followed by postoperative radiotherapy, followed by cyclophosphamide 50 mg daily and methotrexate 2.5 mg twice daily on days 1, 2 of each week for 1 year | 5-fluorouracil 500 mg/m2 + epirubicin 100 mg/m2 + cyclophosphamide 500 mg/m2 every 3 weeks for 3 cycles then docetaxel 80 mg/m2 every 3 weeks for 3 cycles | Carboplatin AUC5 every 3 weeks | No | Adjuvant | Not described |
| NeoCART[25] | 2016 | Docetaxel 75 mg/m2 + carboplatin AUC6 every 3 weeks for 6 cycles | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 4 cycles | Carboplatin AUC6 every 3 weeks | No | Neoadjuvant | <1% |
| PATTERN[26] | 2011 | Paclitaxel 80 mg/m2 + carboplatin AUC2 days 1, 8, 15, every 28 days for 6 cycles | Cyclophosphamide 500 mg/m2 + epirubicin 100 mg/m2 + fluorouracil 500 mg/m2 every 3 weeks for 3 cycles followed by docetaxel 100 mg/m2 every 3 weeks for 3 cycles | Carboplatin AUC2 every week (days 1, 8, 15 every 28 days) | No | Adjuvant | <1% |
| TBCRC 030 [27] | 2014 | Cisplatin 75 mg/m2 every 3 weeks for 4 cycles | Doxorubicin 60 mg/m2 + cyclophosphamide 600 mg/m2 every 2 weeks for 4 cycles | Cisplatin 75 mg/m2 every 3 weeks | No | Neoadjuvant | <5% |
| Wu 2018[28] | 2014 | Lobaplatin 30 mg/m2 for 4 cycles + epirubicin 80 mg/m2 + docetaxel 75 mg/m2 every 3 weeks presurgery and 2 cycles postsurgery | Epirubicin 80 mg/m2 for 4 cycles + docetaxel 75 mg/m2 every 3 weeks presurgery and 2 cycles postsurgery | Lobaplatin 30 mg/m2 every 3 weeks | Yes | Both | <10% |
| Zhang 2016[29] | 2006 | Paclitaxel 175 mg/m2 + carboplatin AUC5 every 3 weeks for 4–6 cycles | Epirubicin 75 mg/m2 + paclitaxel 175 mg/m2 every 3 weeks for 4–6 cycles | Carboplatin AUC5 every 3 weeks | No | Neoadjuvant | <10% |
| Zhao 2014[30] | Not provided in translation | Paclitaxel 175 mg/m2 day 1, carboplatin AUC5 day 2, every 3 weeks for 2 cycles | Epirubicin 75 mg/m2 day 1, paclitaxel 175 mg/m2 day 2, every 3 weeks for 2 cycles | Carboplatin AUC5 every 3 weeks | No | Neoadjuvant | Not provided in translation |
| Zheng 2022[31] | 2009 | Docetaxel 75 mg/m2 or paclitaxel 175 mg/m2 + carboplatin AUC5 every 3 weeks for 6 cycles | Epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles, followed by docetaxel 75 mg/m2 or paclitaxel 175 mg/m2 every 3 weeks for 4 cycles | Carboplatin AUC5 every 3 weeks | No | Adjuvant | <1% |
AUC: area under the curve; IHC: immunohistochemistry. A single trial reference has been included for each of the included studies; a full list of reviewed study records can be found in the original article https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014805.pub2/full
Table 2 details the number of treatment comparisons by subgroup and efficacy outcome.
-
•
15 studies (16 treatment comparisons) involved neoadjuvant chemotherapy with one study combining neoadjuvant and adjuvant therapy, and four studies involved adjuvant chemotherapy
-
•
17 studies (18 treatment comparisons) used carboplatin, two studies used cisplatin and one study used lobaplatin
-
•
nine studies had an anthracycline-free intervention arm
-
•
six studies stratified results for BRCA mutations, one trial for HRD status, and three by lymph node status
Table 2.
Number of treatment comparisons by subgroup and efficacy outcomes.
| Outcome |
||||
|---|---|---|---|---|
| Treatment comparisons n (%) | DFS n (%) | OS n (%) | pCR n (%) | |
| Overall | 21 | 13 (62%) | 12 (57%) | 16 (76%) |
| Treatment setting | ||||
| Neoadjuvant | 16 (76%) | 8 (38%) | 8 (38%) | 16 (76%) |
| Adjuvant | 4 (19%) | 4 (19%) | 4 (19%) | 0 |
| Both | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| Subgroups | ||||
| BRCA mutation subgroup reported | 6 (29%) | 4 (19%) | 0 | 6 (29%) |
| HRD status subgroup reported | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| Lymph node positive reported | 3 (14%) | 3 (14%) | 0 | 3 (14%) |
| Type of platinum agent | ||||
| Carboplatin | 18 (%) | 12 (57%) | 12 (57%) | 13 (62%) |
| Cisplatin | 2 (10%) | 0 | 0 | 2 (10%) |
| Lobaplatin | 1 (5%) | 1 (5%) | 0 | 1 (5%) |
| Type of regimen | ||||
| Different backbone | 14 (67%) | 7 (33%) | 7 (33%) | 9 (%) |
| Same backbone | 7 (33%) | 6 (29%) | 5 (24%) | 7 (%) |
| Anthracycline content in intervention arm | ||||
| Anthracycline present | 12 (57%) | 7 (33%) | 6 (29%) | 10 (47%) |
| Anthracycline free | 9 (43%) | 6 (29%) | 6 (29%) | 6 (29%) |
| Schedule of platinum agent | ||||
| 3-weekly | 14 (67%) | 9 (43%) | 8 (38%) | 11 (57%) |
| 2-weekly | 1 (5%) | 1 (5%) | 1 (5%) | 0 |
| Weekly | 5 (24%) | 3 (14%) | 3 (%) | 5 (24%) |
| Hormone receptor IHC cut-off | ||||
| >1% or not reported | 11 (57%) | 5 (24%) | 4 (19%) | 9 (43%) |
| <1% | 10 (47%) | 8 (38%) | 8 (38%) | 7 (33%) |
DFS: disease-free survival; HRD: homologous recombination deficiency; IHC: immunohistochemistry; n: number; OS: overall survival; pCR: pathological complete response.
We included studies that examined other subtypes of breast cancer, provided the outcome of DFS, OS or pCR was described for the TNBC subgroup. For such studies, efficacy analyses reported in this analysis are only for the TNBC group (Ando 2014; GEICAM 2006–03; GeparOcto; GeparOLA; GeparSixto; I-SPY2; TBCRC 030). Other outcomes including toxicity and the completion of chemotherapy regimens may be reported for the whole cohort if subgroup data were not published. This is not considered a significant change from the protocol because participants with TNBC are unlikely to have substantially different chemotherapy adverse effects compared to participants with other subtypes of breast cancer.
Notably, there were studies where participants in the intervention group received platinum agents as well as other experimental interventions. Trialists in BrighTNess examined the effects of both carboplatin and veliparib. To compare all participants in this trial receiving platinum chemotherapy, we split this study into two analysis groups, or 'treatment comparisons' (BrighTNess comparison 1 intervention: paclitaxel, veliparib and carboplatin followed by doxorubicin and cyclophosphamide (AC), and BrighTNess comparison 2 intervention: paclitaxel and carboplatin followed by AC). In Nasr 2015, participants randomised to the intervention received platinum chemotherapy as well as a further year of metronomic oral chemotherapy.
Characteristics of excluded and ongoing studies can be found in the original publication (https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014805.pub2/full)
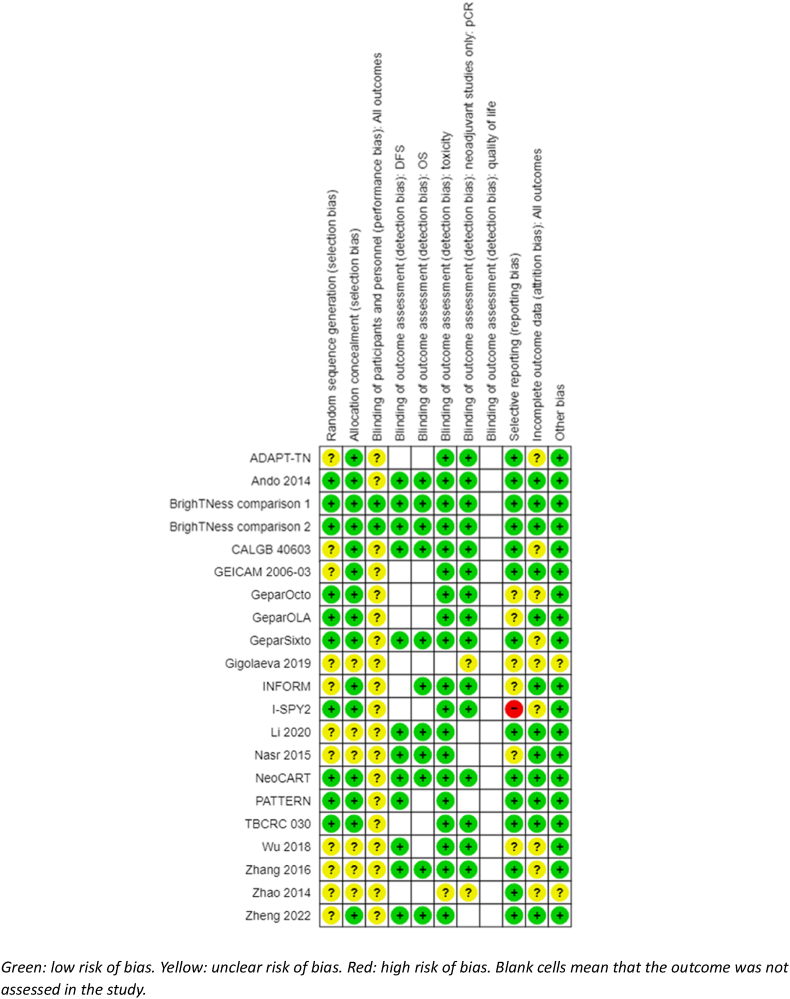
3.3. Risk of bias in included studies
See Fig. 2 for a summary of risk of bias judgements of the included studies.
Fig. 2.
Risk of bias judgements.
Green: low risk of bias. Yellow: unclear risk of bias. Red: high risk of bias. Blank cells mean that the outcome was not assessed in the study.
3.3.1. Allocation
Nine studies (10 treatment comparisons) were at low risk of bias for random sequence generation and 14 studies (15 treatment comparisons) for allocation concealment. Those deemed at unclear risk did not detail procedures for randomisation (ADAPT-TN; CALGB 40603; INFORM; GEICAM 2006–03; Gigolaeva 2019[]; Li 2020; Nasr 2015; Wu 2018, Zhang 2016, Zhao 2014, Zheng 2022]), or whether allocation was performed centrally (Gigolaeva 2019; Li 2020; Nasr 2015; Wu 2018, Zhang 2016, Zhao 2014).
3.3.2. Blinding
Nineteen studies were described as open-label. Performance bias due to lack of blinding of participants and personnel was not considered to be a serious concern given the objective nature of the efficacy outcomes and most toxicity outcomes. As such, these studies were deemed at unclear risk of bias. One study was double blinded throughout the course of the trial (BrighTNess) and judged at low risk of bias for all outcomes.
We assessed detection bias by outcome. For DFS, OS, pCR and toxicity, lack of blinding was perceived as unlikely to have an impact given the nature or method in which each outcome is assessed (i.e. through imaging, biochemical tests, reviewed by independent panels, or a combination of these). All studies reporting DFS or OS were perceived to be at low risk of bias. All studies reporting pCR were deemed to be at low risk of bias except for two studies at unclear risk because the papers did not provide any information on tests used or process to evaluate tumour response (Gigolaeva 2019, Zhao 2014). Similarly, studies reporting toxicities were at low risk of bias except for one study as no information was provided on how toxicity was assessed (Zhao 2014). None of the studies that collected quality of life measures reported data and no risk of bias assessment was possible.
3.3.3. Incomplete outcome data
Most studies did not complete a true intention-to-treat analysis, in that participants who were randomised but did not receive treatment were excluded from the efficacy and safety analysis. Notably, only a very small number of participants were excluded in each study after randomisation. Nine studies were at unclear risk of bias. We judged six studies at unclear risk of bias because the reasons for excluding participants were not detailed (CALGB 40603; GeparOcto; GeparSixto; Wu 2018, Zhang 2016, Zhao 2014). One study was at unclear risk of attrition bias as there were several randomised people with missing pCR data that could not be accounted for (ADAPT-TN). Two studies did not provide a CONSORT diagram or associated information and were classified at unclear risk of bias (Gigolaeva 2019; I-SPY2).
3.3.4. Selective reporting
One study was at high risk of bias as it did not report DFS or OS, despite these outcomes being listed in the trial registry records (I-SPY2). As pCR data were reported in 2016, these important long-term efficacy outcomes would have been expected to be reported by 2022. Four studies with more recent publications which have not yet published results on critical outcomes were at unclear risk (GeparOLA; GeparOcto; INFORM; Wu 2018). Two additional studies did not provide sufficient information for an assessment and were judged at unclear risk of bias (e.g. abstract only; Gigolaeva 2019; Nasr 2015).
Four studies identified quality of life as an outcome in their trial registry records or publications (BrighTNess; GeparOcto; I-SPY2; Zheng 2022); however, there were no published reports of quality-of-life measures from these studies.
3.3.5. Other potential sources of bias
One study was published in abstract form only and did not have an identifiable trial registration record Gigolaeva 2019. As such, the risk of bias assessment was limited and assessed as unclear. Another study required translation (Zhao 2014). While outcome measures were provided in the translation, we did not have sufficient translated information to make risk of bias assessments for this domain and most others.
3.4. Effects of interventions
3.4.1. Neoadjuvant therapy
3.4.1.1. Disease-free survival
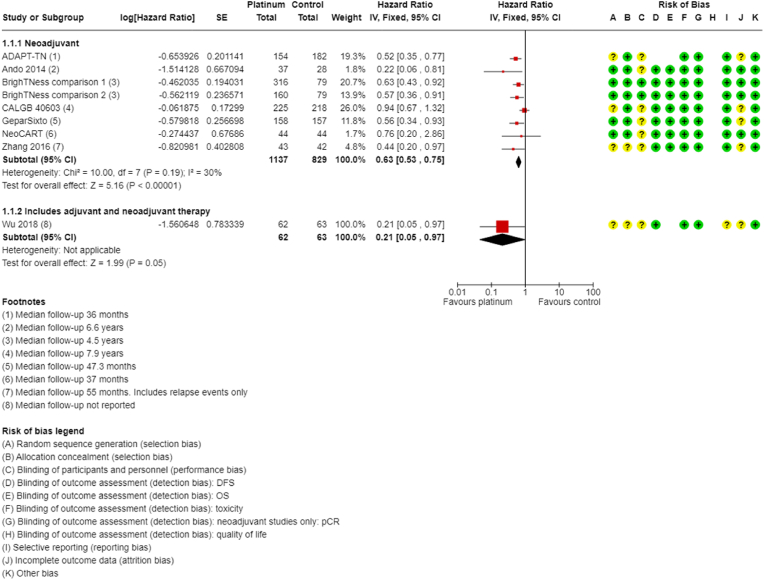
Ten of the 16 neoadjuvant studies collected data on DFS; however, two studies did not report data (GeparOcto; I-SPY2). Median follow-up ranged from 36 to 94.8 months. Platinum-based chemotherapy improved DFS compared to non-platinum-containing chemotherapy (HR 0.63, 95% CI 0.53 to 0.75; P < 0.001, I2 = 30%; 7 studies, 8 treatment comparisons; high-certainty evidence; Fig. 3). A total of 1966 people were included in the analysis with an estimated 500 DFS events.
Fig. 3.
Neoadjuvant therapy disease free survival.
One other study reported on DFS following neoadjuvant and adjuvant treatment, but results could not be separated for neoadjuvant therapy alone (Wu 2018). Based on this one study, the results suggested an improvement in DFS in the platinum-based chemotherapy group (HR 0.21, 95% CI 0.05 to 0.97; 1 study, 125 participants).
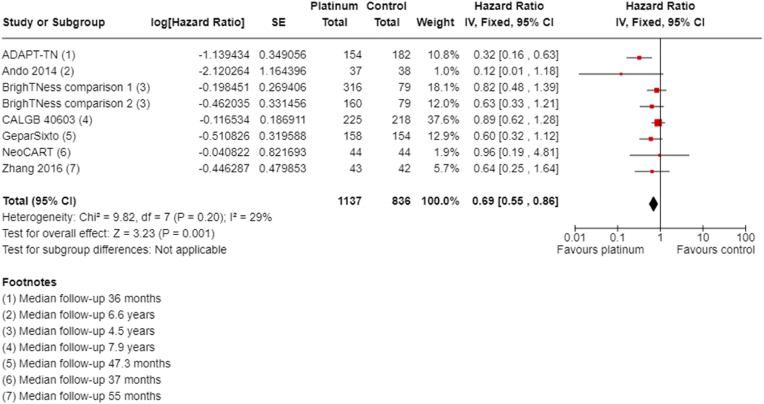
3.4.1.2. Overall survival
Ten of the 16 neoadjuvant studies collected data on OS; however, two studies collected data but did not report them (GeparOcto; I-SPY2). Median follow-up ranged from 36 to 94.8 months. Platinum chemotherapy reduced mortality (HR 0.69, 95% CI 0.55 to 0.86; P = 0.001, I2 = 29%; 7 studies, 8 treatment comparisons; high-certainty evidence; Fig. 4). A total of 1973 participants were involved in these studies, with an estimated 307 deaths.
Fig. 4.
Neoadjuvant therapy overall survival.
One other study collected "all-cause mortality" and reported no deaths in either group and an HR was not provided or estimable (INFORM). Follow-up time statistics for these data are unknown.
3.4.1.3. Pathological complete response
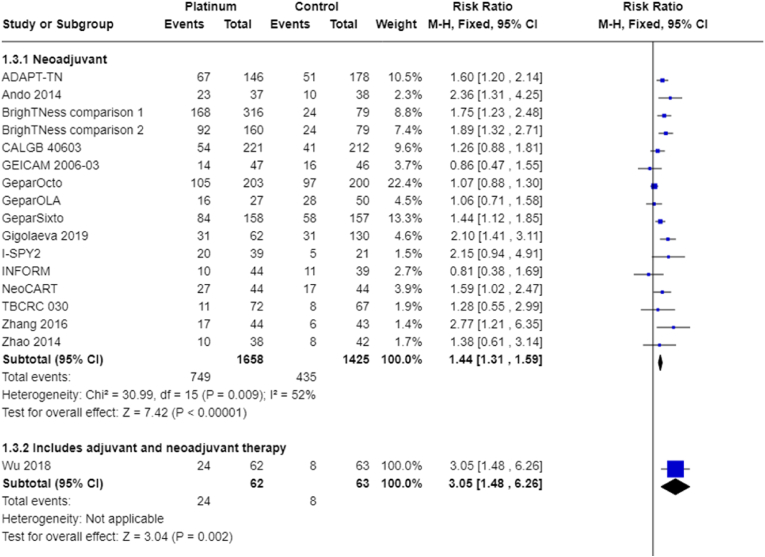
Fifteen trials (16 treatment comparisons) involving only neoadjuvant treatment reported pCR outcome data. Platinum chemotherapy was associated with a large improvement in the rate of pCR (RR 1.44, 95% CI 1.31 to 1.59, P = 0.009, I2 = 52%; 15 studies, 16 treatment comparisons, 3083 participants; high-certainty evidence; Fig. 5). One study reported adjusted probabilities of pCR rather than discrete numbers and a sensitivity analysis (removing the adjusted values) gave a very similar result for pCR (RR 1.43, 95% CI 1.30 to 1.58; 3023 participants) (I-SPY2).
Fig. 5.
Pathological complete response.
One other study that combined neoadjuvant and adjuvant therapy also showed an improvement in tumour response (RR 3.05, 95% CI 1.48 to 6.26; 125 participants) (Wu 2018).
3.4.2. Adjuvant therapy
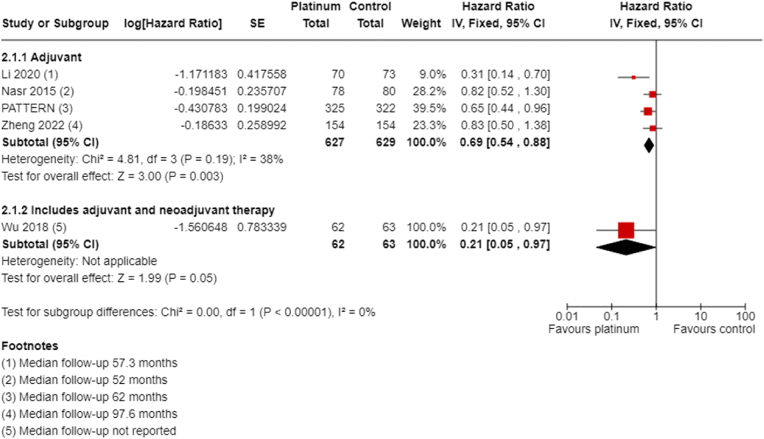
3.4.2.1. Disease-free survival
All four studies of adjuvant chemotherapy collected and reported DFS with median follow-up ranging from 52 to 97.6 months. Platinum chemotherapy improved DFS (HR 0.69, 95% CI 0.54 to 0.88; P = 0.003, I2 = 38%; high-certainty evidence, Fig. 6). These studies included 1256 participants, with an estimated 262 DFS events.
Fig. 6.
Adjuvant disease-free survival.
3.4.2.2. Overall survival
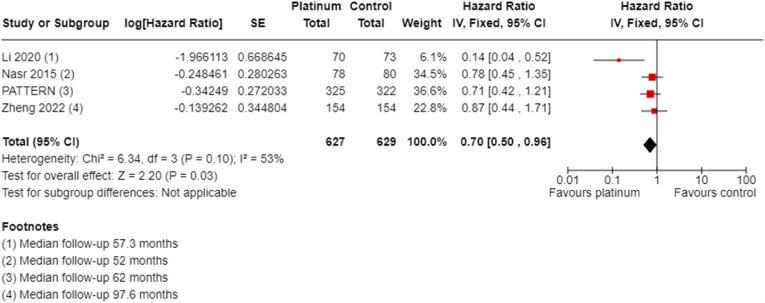
All four studies collected and reported OS with follow-up ranging from 52 to 97.6 months. Adjuvant platinum chemotherapy extended OS (HR 0.70, 95% CI 0.50 to 0.96; P = 0.03, I2 = 53%; high-certainty evidence, Fig. 7). A total of 1256 participants were included in this analysis, with an estimated 153 deaths.
Fig. 7.
Adjuvant overall survival.
3.4.3. Treatment completion and toxicity
To assess the effect of platinum agents on treatment adherence and toxicity overall, we combined data from neoadjuvant and adjuvant studies.
3.4.3.1. Completion of regimens
Participants receiving platinum chemotherapy were more than twice as likely to have delay in starting the next cycle of chemotherapy (RR 2.23, 95% CI 1.70 to 2.94; P < 0.001, I2 = 70%; 4 studies, 5 treatment comparisons; moderate-certainty evidence).
Participants receiving platinum chemotherapy were also more likely to require dose reductions (RR 1.77, 95% CI 1.56 to 2.02; P < 0.001; I2 = 91%; 7 studies, 8 treatment comparisons; moderate-certainty evidence).
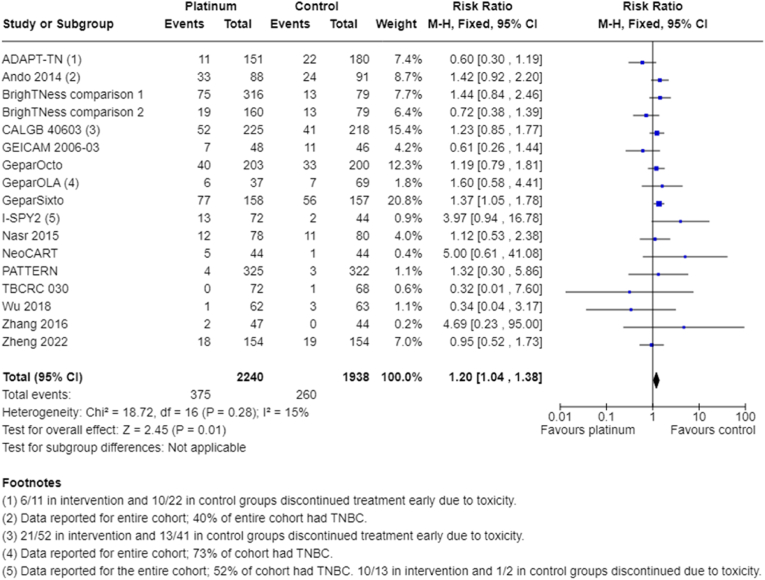
Participants receiving platinum chemotherapy were 20% more likely to require early cessation of treatment (RR 1.20, 95% CI 1.04 to 1.38; P = 0.01; I2 = 15%; 16 studies, 17 treatment comparisons; high-certainty evidence; Fig. 8). This was not always due to toxicity, as indicated by some studies that provided reasons for early cessation (early cessation due to toxicity: ADAPT-TN: 45% in intervention group versus 45% in control group; CALGB 40603: 40% in intervention group versus 32% in control group; I-SPY2: 77% in intervention group versus 50% in control group). Other reasons included progression of disease, withdrawal of consent/refusal of treatment or other/unknown.
Fig. 8.
Early cessation of treatment.
3.4.3.2. Grade III/IV toxicity
We collected data for grade III/IV haematological toxicity, neuropathy, nausea, renal impairment and treatment-related death.
3.4.3.2.1. Haematological toxicity
Participants receiving platinum-based chemotherapy were more likely to have grade III/IV neutropenia (RR 1.53, 95% CI 1.43 to 1.63; P < 0.001; I2 = 97%; 19 studies, 20 treatment comparisons; moderate-certainty evidence; Analysis 3.4). Participants receiving platinum-based chemotherapy were unlikely to have higher rates of grade III/IV febrile neutropenia (RR 1.16, 95% CI 0.89 to 1.49; P = 0.27, I2 = 69%; 11 studies, 12 treatment comparisons; moderate-certainty evidence).
For platinum recipients, there were considerably higher risks of anaemia (RR 8.20, 95% CI 5.66 to 11.89; P < 0.001; I2 = 42%; 18 studies, 19 treatment comparisons; moderate-certainty evidence; Analysis 3.6). There is likely to be a much higher risk of thrombocytopenia in participants receiving platinum chemotherapy (RR 7.59, 95% CI 5.10 to 11.29; P < 0.001, I2 = 44%; 18 studies, 19 treatment comparisons; moderate-certainty evidence).
3.4.3.2.2. Non-haematological toxicity
There is likely little to no difference in rates of grade III/IV neuropathy associated with platinum chemotherapy (RR 1.22, 95% CI 0.95 to 1.57; P = 0.12, I2 = 0; 14 studies, 15 treatment comparisons; moderate-certainty evidence).
Participants receiving platinum chemotherapy had a higher rate of grade III/IV nausea (RR 1.89, 95% CI 1.30 to 2.74; P < 0.001; I2 = 0; 16 studies, 17 treatment comparisons; high-certainty evidence).
Four studies reported data on renal impairment (INFORM; Li 2020; Wu 2018, Zhao 2014). One study reported two events in 60 people in the platinum arm (3%) and no events in 57 people in the non-platinum arm. None of the other studies reported any grade III/IV renal impairment.
3.4.3.3. Treatment-related death
Treatment-related death was a very rare event, with seven events in 3094 participants. This outcome was not different between platinum and non-platinum intervention arms (RR 0.58, 95% CI 0.14 to 2.33; P = 0.44, I2 = 0; 10 studies, 11 treatment comparisons; note 8 studies reported treatment-related deaths but recorded 0 events in both groups. Thus, the RR and CIs were calculated from 3 studies rather than 11; high-certainty evidence).
3.4.4. Quality of life
Although a prespecified outcome of four studies (1198 participants), there were no published quality of life data in the eligible studies available for this review.
3.4.5. Subgroup analysis
The number of participants in these trials with a known BRCA mutation was small, with 222 pathogenic variant carriers, of whom 118 received platinum. Four studies, with 1452 participants, reported DFS outcomes stratified by BRCA mutation status (BrighTNess; GeparSixto; PATTERN; Zheng 2022). There was no evidence of a difference in DFS outcomes based on BRCA mutation status (BRCA wild-type: HR 0.65, 95% CI 0.50 to 0.85; BRCA mutation: HR 0.72, 95% CI 0.41 to 1.25; P = 0.76).
One study, with 521 participants, reported DFS according to HRD status, based on a multigene panel including 12 breast cancer homologous recombination repair (HRR) associated susceptibility genes (ATM, ATR, BARD1, BRCA1, BRCA2, BRIP1, CHEK2, FANCM, PALB2, RAD51C, RAD51D and RECQL) (PATTERN). There was no evidence of a difference in outcomes between HRD-positive and HRD-negative participants (HRD-positive: HR 0.39, 95% CI 0.15 to 1.00; HRD negative: HR 0.70, 95% CI 0.42 to 1.15) with no subgroup difference (P = 0.28). As there was a small number of participants with HRD-positive tumours (120 participants), this analysis may be underpowered.
Three studies, with 1097 participants, reported DFS according to lymph node status (Li 2020; PATTERN; Zheng 2022). Participants were 29% lymph node-positive and 71% lymph node negative in this analysis. There was a trend towards benefit for the addition of platinum in both subgroups (lymph node-positive: HR 0.86, 95% CI 0.54 to 1.37; lymph node-negative: HR 0.82, 95% CI 0.55 to 1.22); there was no subgroup difference (P = 0.85).
No studies reported OS outcomes stratified for BRCA, HRD or lymph node status.
Eleven of 12 studies reporting DFS used carboplatin, demonstrating a benefit (HR 0.65, 95% CI 0.57 to 0.75; Analysis 7.1), and all studies reporting OS used carboplatin. The remaining study reporting DFS, but no OS, assessed a novel platinum compound, lobaplatin, given both before and after surgery. This study also demonstrated DFS benefit albeit with wide CIs (HR 0.21, 95% CI 0.05 to 0.98).
In the 12 studies (13 treatment comparisons) reporting DFS, seven had intervention arms combining platinum chemotherapy with anthracycline chemotherapy (including doxorubicin, epirubicin and non-pegylated liposomal doxorubicin). Six treatment comparisons had anthracycline-free platinum intervention arms. Both subgroups had a similar impact on DFS (anthracycline-free intervention: HR 0.59, 95% CI 0.47 to 0.73; anthracycline-containing intervention: HR 0.69, 95% CI 0.57 to 0.83); there was little evidence of a subgroup difference (P = 0.27).
Eleven studies reported OS, and of these five had intervention arms adding platinum chemotherapy to anthracycline chemotherapy, and six had anthracycline-free intervention arms with a platinum-taxane combination. There was a survival benefit in both subgroups (anthracycline-free studies: HR 0.57, 95% CI 0.41 to 0.78; 1607 participants; anthracycline-containing studies: HR 0.77, 95% CI 0.61 to 0.96; 1622 participants); there was no difference between groups (P = 0.14).
There was benefit across all schedules for DFS: three-weekly (HR 0.71, 95% CI 0.59 to 0.85; 9 treatment comparisons), two-weekly (HR 0.31, 95% CI 0.14 to 0.70; 1 study) and weekly groups (HR 0.58, 95% CI 0.45 to 0.74; 3 studies). This was similar for OS benefits: three-weekly (HR 0.79, 95% CI 0.64 to 0.99; 8 treatment comparisons), two-weekly (HR 0.14, 95% CI 0.04 to 0.52; 1 study) and weekly groups (HR 0.55, 95% CI 0.39 to 0.78; 3 studies) all showing benefit.
Sensitivity analyses addressing different hormone receptor cut-offs, potentially confounding treatments in intervention arm, studies with a high or unclear risk of bias and outcomes with considerable heterogeneity were performed but did not demonstrate any significant variation in the results. Detailed descriptions of these can be found in the original publication (https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD014805.pub2/full)
4. Discussion
Platinum-based chemotherapy using carboplatin in the adjuvant or neoadjuvant setting improved long-term outcomes of DFS and OS in early TNBC, regardless of the examined subgroups. This was at the cost of more frequent chemotherapy delays and dose reductions, and greater haematological toxicity. There was benefit from platinum when platinum agents were used in both anthracycline-containing regimens and in anthracycline-free regimens.
Though there are certainly increased haematological toxicities associated with platinum chemotherapy, permanent toxicity such as grade III/IV neuropathy and treatment-related death were not different between groups.
Attempts in this review to refine subgroups of triple-negative biology, such as those with BRCA mutations or altered HRD status, have higher benefit from platinum therapy found no predictive role. The certainty of this evidence was low since numbers were small. Only one study assessed the role of HRD status on efficacy outcomes in our analysis. It remains unclear if more modern and focused HRD testing may offer better biomarkers for participants who will benefit from platinum chemotherapy. We were also unable to identify if there may be a subgroup of participants who might not benefit, and for whom de-escalation therapy might be appropriate.
These results were generally applicable to people with early TNBC, allowing for the trial to define hormone receptor cut-offs which ranged from 1% to 10%. The range of ages captured in these trials was from 19 to 82 years. Outcomes based on age were not available, and information on participant gender was not collected. While racial background of participants was not captured in our analysis, these trials took place in several countries in Europe, Asia and the US. Black and African participants are likely to be a notable ethnic gap in this meta-analysis given the dearth of trials occurring on the African continent and the low participation rates of Black Americans in cancer clinical trials [32].
Recruitment of the included trials started between six and 16 years ago, and as such the standard therapy arms may not reflect current international standards. This is a shifting target, and the advent of new treatments used in early TNBC such as immunotherapy [33] and poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors [34], as well as the clinical heterogeneity of chemotherapy used in these studies, means the best regimen and timing of platinum chemotherapy remains unclear. Further research into this area is warranted, particularly given the increasing number of drugs used in TNBC and increasing interest in biomarker-directed treatment rationalisation.
No quality-of-life outcomes were reported. This is an important measure particularly when assessing outcomes which are more accurately reported by participants, such as fatigue and effects on cognition. As such, we may be missing important impacts of the addition of platinum chemotherapy on participants of these clinical trials both acutely and in the longer term. This review has highlighted the need for ensuring reporting of the quality-of-life data collected in trials involving early breast cancer. The value of patient-reported outcome measures is being increasingly recognised. Consideration of these outcomes from clinical trials is essential for ensuring person-centred clinical interventions to assess objective disease control as well as more subjective health and well-being.
This systematic review provides evidence from 20 studies, with 4468 participants, and provides high-certainty evidence supporting the addition of platinum chemotherapy in the neoadjuvant and adjuvant settings with an increase in DFS and OS.
Use of platinum chemotherapy is variable, and at the time of writing is still not routinely recommended in NCCN or European Society for Medical Oncology guidelines. A lack of DFS and OS benefit is often cited as a reservation to the routine use of platinum chemotherapy. This review presents relevant, adequately powered outcome data to support the use of platinum chemotherapy in early TNBC, acknowledging the increased rate of haematological toxicity.
5. Conclusions
This review provides high-certainty evidence that platinum-based chemotherapy with carboplatin is associated with improved disease-free survival (DFS), overall survival (OS) and pathological complete response in early triple-negative breast cancer (TNBC). This is at the cost of increased grade III/IV haematological toxicity, though serious adverse events including febrile neutropenia or treatment-related death were not increased.
These findings support the use of carboplatin for people with early TNBC. The optimal dose and regimen are not defined by this analysis, but there is a suggestion that similar relative benefits result from combining carboplatin with anthracycline-free regimens or those containing anthracycline agents. Additionally, our analysis supports a broad rather than focused use of carboplatin based on the benefit seen across the examined subgroups.
Declaration of competing interest
SM: none. MW: none. MW is a member of the Cochrane Breast Cancer editorial team but was not involved in the editorial process for this review. SE: none. JB: none related to this review. Jane has received funding to travel to conferences from Novartis. Jane has served on advisory boards for Pfizer and Lilly for unrelated matters and payment has been made to her institution. RD: none. AG: none related to this review. AG reports consultancy/expert witness or advisory roles for Pfizer (2018, 2019) and AstraZeneca (2018) for matters unrelated to this review topic. There is no competing interest associated with funding of travel to attend an educational meeting, or to provide expertise regarding Cancer Genetic counselling in Australia. AG also receives ongoing funds for editorial support for manuscript writing for the EMBRACA trial (not related to this review). AG is also a member of the Cochrane Breast Cancer editorial team but was not involved in the editorial process for this review.
CRediT authorship contribution statement
Sofia RE. Mason: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. Melina L. Willson: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Writing – review & editing. Sam J. Egger: Formal analysis, Methodology, Validation, Writing – review & editing. Jane Beith: Conceptualization, Supervision, Writing – review & editing. Rachel F. Dear: Supervision, Writing – review & editing. Annabel Goodwin: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – review & editing.
Acknowledgements
We would like to thank the following people at Cochrane Breast Cancer for this review: editorial Nicholas Wilcken (Sign Off Editor), Ava Grace Tan-Koay (Managing Editor), Anne Lawson (Copy Editor). We would like to thank the reviewers of the Cochrane review manuscript: Dr Andrew Redfern, University of Western Australia (clinical/content review), Sara Whiting (consumer review), and Ram Bajpai, Keele University (methods review); and the reviewers for their helpful comments on the protocol: Rebecca Seago-Coyle (Consumer Editor), Bonner Cutting (Alamo Breast Cancer Foundation and Komen Advocate-in-Science; Consumer Reviewer), Alessandra Gennari (MD, PhD, University of Eastern Piedmont, Novara, Italy; Clinical Editor) and the external Clinical Peer-reviewer who wishes to remain anonymous. The Cochrane Methods Support Unit provided methodological feedback on the protocol. We would also like to thank Alexis Lai, who translated the article by Zhao and colleagues, providing results and information related to risk of bias, and Yuan Chi who reviewed two articles published in Chinese journals to advise whether the studies met inclusion criteria.
References
- 1.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al, editor(s). Cancer today (powered by GLOBOCAN 2018) IARC CancerBase No. 15. publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Powered-By-GLOBOCAN-2018--2018 (accessed 17 July 2020).
- 2.Foulkes W.D., Smith I.E., Reis-Filho J.S. Triple-negative breast cancer. N Engl J Med. 2010;363(20):1938–1948. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 3.Lin N.U., Vanderplas A., Hughes M.E., Theriault R.L., Edge S.B., Wong Y.N., et al. Clinicopathological features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer. 2012;118(22):5463–5472. doi: 10.1002/cncr.27581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shimelis H., LaDuca H., Hu C., Hart S.N., Na J., Thomas A., et al. Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing. J Natl Cancer Inst: J Natl Cancer Inst. 2018;110(8):855–862. doi: 10.1093/jnci/djy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cortazar P., Zhang L., Untch M., Mehta K., Costantino J.P., Wolmark N., et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 6.Edge S.B., Compton C.C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 7.Common Terminology Criteria for Adverse Events (CTCAE) v5.0. ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf (accessed 25 March 2021).
- 8.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020) Cochrane; 2020. Available from: training.cochrane.org/handbook/archive/v6.1. [Google Scholar]
- 9.Tierney J., Stewart L.A., Ghersi D., Burdett S., Sydes M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeks J.J., Higgins J.P., Altman D.G. In: Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated september 2020) Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., et al., editors. 2020. Chapter 10: analysing data and undertaking meta-analyses.training.cochrane.org/handbook/archive/v6.1 Cochrane. [Google Scholar]
- 11.Gradepro GDT. Version accessed prior to 4 September 2023. Hamilton (ON): McMaster University (developed by evidence Prime).Available at: gradepro.org..
- 12.Loibl S., O'Shaughnessy J., Untch M., Sikov W.M., Rugo H.S., McKee M.D., et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19(4):497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 13.Gluz O., Nitz U., Christgen M., Grischke E.M., Forstbauer H., Braun M.W., et al. Efficacy of 12 weeks neoadjuvant nab-paclitaxel combined with carboplatinum vs. gemcitabine in triple-negative breast cancer: WSG-ADAPT TN randomized phase II trial. J Clin Oncol. 2015;33(15 Suppl):1032. [Google Scholar]
- 14.Ando M., Yamauchi H., Aogi K., Shimizu S., Iwata H., Masuda N., et al. Randomized phase II study of weekly paclitaxel with and without carboplatin followed by cyclophosphamide/epirubicin/5-fluorouracil as neoadjuvant chemotherapy for stage II/IIIA breast cancer without HER2 overexpression. Breast Cancer Res Treat. 2014;145(2):401–409. doi: 10.1007/s10549-014-2947-1. [DOI] [PubMed] [Google Scholar]
- 15.Sikov W.M., Berry D.A., Perou C.M., Singh B., Cirrincione C., Tolaney S., et al. Impact of the addition of carboplatin (Cb) and/or bevacizumab (B) to neoadjuvant weekly paclitaxel (P) followed by dose-dense AC on pathologic complete response (pCR) rates in triple-negative breast cancer (TNBC): CALGB 40603 (Alliance) Cancer Res. 2013;73(24 Suppl) S5-01. [Google Scholar]
- 16.Alba E., Chacon J.I., Lluch A., Anton A., Estevez L., Cirauqui B., et al. A randomized phase II trial of platinum salts in basal-like breast cancer patients in the neoadjuvant setting. Results from the GEICAM/2006-03, multicenter study. Breast Cancer Res Treat. 2012;136(2):487–493. doi: 10.1007/s10549-012-2100-y. [DOI] [PubMed] [Google Scholar]
- 17.Schneeweiss A., Mobus V., Tesch H., Hanusch C., Denkert C., Lubbe K., et al. Intense dose-dense epirubicin, paclitaxel, cyclophosphamide versus weekly paclitaxel, liposomal doxorubicin (plus carboplatin in triple-negative breast cancer) for neoadjuvant treatment of high-risk early breast cancer (GeparOcto-GBG 84): a randomised phase III trial. Eur J Cancer. 2019;106:181–192. doi: 10.1016/j.ejca.2018.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Fasching P.A., Link T., Hauke J., Seither F., Jackisch C., Klare P., et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study) Ann Oncol. 2021;32(1):49–57. doi: 10.1016/j.annonc.2020.10.471. [DOI] [PubMed] [Google Scholar]
- 19.Loibl S., Weber K.E., Timms K.M., Elkin E.P., Hahnen E., Fasching P.A., et al. Survival analysis of carboplatin added to an anthracycline/taxane-based neoadjuvant chemotherapy and HRD score as predictor of response-final results from GeparSixto. Ann Oncol. 2018;29(12):2341–2347. doi: 10.1093/annonc/mdy460. [DOI] [PubMed] [Google Scholar]
- 20.Gigolaeva L., Krivorotko P., Zhiltsova E., Dashyan G., Chadjimatova S., Pesotckiy R., et al. Neoadjuvant chemotherapy regimens for triple negative breast cancer patients. Breast. 2019;44(Suppl 1):S70. [Google Scholar]
- 21.Tung N., Arun B., Hofstatter E., Hacker M.R., Toppmeyer D.L., Isakoff S.J., et al. Cisplatin versus doxorubicin/cyclophosphamide as neoadjuvant treatment in germline BRCA mutation carriers (BRCA carriers) with HER2-negative breast cancer: results from the INFORM trial (TBCRC 031) Cancer Res. 2019;80(4 Suppl) doi: 10.1200/JCO.19.03292. GS6-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rugo H.S., Olopade O.I., DeMichele A., Yau C., van't Veer L.J., Buxton M.B., et al. Adaptive randomization of veliparib-carboplatin treatment in breast cancer. N Engl J Med. 2016;375(1):23–34. doi: 10.1056/NEJMoa1513749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q., Wang J., Mu Y., Zhang T., Han Y., Luo Y., et al. Dose-dense paclitaxel plus carboplatin vs. epirubicin and cyclophosphamide with paclitaxel as adjuvant chemotherapy for high-risk triple-negative breast cancer. Chin J Cancer Res. 2020;32(4):485–496. doi: 10.21147/j.issn.1000-9604.2020.04.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nasr K.E., Osman M.A., Elkady M.S., Ellithy M.A. Metronomic methotrexate and cyclophosphamide after carboplatin included adjuvant chemotherapy in triple negative breast cancer: a phase III study. Ann Transl Med. 2015;3(19):284. doi: 10.3978/j.issn.2305-5839.2015.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L., Wu Z.Y., Li J., Lin Y., Liu Z., Cao Y., et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): results from a multicenter, randomized controlled, open-label phase II trial. Int J Cancer. 2022;150(4):654–662. doi: 10.1002/ijc.33830. [DOI] [PubMed] [Google Scholar]
- 26.Yu K.D., Ye F.G., He M., Fan L., Ma D., Mo M., et al. Effect of adjuvant paclitaxel and carboplatin on survival in women with triple-negative breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2020;6(9):1390–1396. doi: 10.1001/jamaoncol.2020.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer E.L., Abramson V., Jankowitz R., Falkson C., Marcom P.K., Traina T., et al. TBCRC 030: a phase II study of preoperative cisplatin versus paclitaxel in triple-negative breast cancer: evaluating the homologous recombination deficiency (HRD) biomarker. Ann Oncol. 2020;31(11):1518–1525. doi: 10.1016/j.annonc.2020.08.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X., Tang P., Li S., Wang S., Liang Y., Zhong L., et al. A randomized and open-label phase II trial reports the efficacy of neoadjuvant lobaplatin in breast cancer. Nat Commun. 2018;9(1):832. doi: 10.1038/s41467-018-03210-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P., Yin Y., Mo H., Zhang B., Wang X., Li Q., et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget. 2016;7(37):60647–60656. doi: 10.18632/oncotarget.10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y., Li J.F., Chu G.W. Neoadjuvant chemotherapy regimens for patients with triple-negative breast cancer: TE versus TC. J Pract Oncol. 2014;29(6):576–578. [Google Scholar]
- 31.Zheng F., Du F., Wang W., Wang Y., Li M., Zhao J., et al. Updated efficacy of adjuvant epirubicin plus cyclophosphamide followed by taxanes versus carboplatin plus taxanes in early triple-negative breast cancer in phase 2 trial: 8.1-year median follow-up. Breast Cancer Res Treat. 2022;191(1):97–105. doi: 10.1007/s10549-021-06401-6. [DOI] [PubMed] [Google Scholar]
- 32.Awidi M., Al Hadidi S. Participation of Black Americans in cancer clinical trials: current challenges and proposed solutions. JCO Oncology Practice. 2021;17(5):265–271. doi: 10.1200/OP.21.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid P., Cortes J., Pusztai L., McArthur H., Kümmel S., Bergh J., et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 34.Tutt A.N., Garber J.E., Kaufman B., Viale G., Fumagalli D., Rastogi P., et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med. 2021;384(25):2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]