Graphical abstract
Keywords: Pulmonary regurgitation, Tricuspid regurgitation, Right heart dilatation, Three-dimensional transthoracic echocardiography, Computed tomography
Highlights
-
•
Severe progressive PR and TR in adults without histories of cardiac surgery is rare.
-
•
Carcinoid heart disease is an important differential in isolated PR and TR.
-
•
Three-dimensional TTE is helpful in identifying morphologic abnormalities of the PV.
-
•
CCT and cardiovascular magnetic resonance also help evaluate structures near the PV.
Introduction
Pulmonary regurgitation (PR) is rarely life threatening because, even in adult patients, it is usually mild. Nevertheless, severe PR can occur and typically results in right ventricular (RV) dilation, secondary tricuspid regurgitation (TR), and right-sided heart failure, leading to life-threatening conditions.1 Severe symptomatic PR in adult patients usually follows childhood cardiac surgery for tetralogy of Fallot, and only a few cases of noncongenital PR have been reported.2 Because of their extreme rarity, the outcome and prognosis of surgical treatment is not fully understood.
Here, we report the case of an elderly woman with severe PR and TR who underwent successful surgical pulmonary valve replacement (PVR) and tricuspid valve (TV) repair.
Case Presentation
A 79-year-old woman presented to our hospital with shortness of breath on exertion of 6 months’ duration and leg edema refractory to diuretic treatment. The patient had a history of hypertension and diabetes, as well as a right internal carotid artery aneurysm that had been treated with coil embolization when the patient was 68 years old. The patient had no history of flushing, episodic high heart rate or blood pressure, or refractory diarrhea. The patient was taking the following medications: olmesartan 20 mg/d and nifedipine 20 mg/d for hypertension, sitagliptin 25 mg/d for diabetes, aspirin 100 mg/d for intracerebral aneurysm, and furosemide 20 mg/d for right heart failure symptoms.
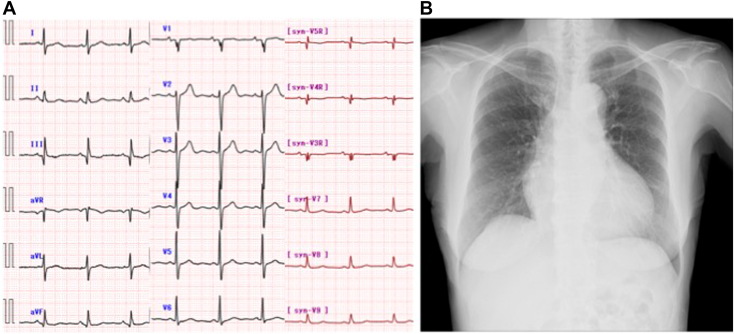
Physical examination revealed a normal body mass index of 20.9 kg/m2 (height 146 cm, weight 44.5 kg), blood pressure of 161/92 mm Hg, and a heart rate of 64 beats/min. Cardiac auscultation revealed a systolic murmur at the third left sternal border, and lung sounds were clear. The patient had jugular venous distention and leg edema. Electrocardiography revealed normal sinus rhythm without prolongation of the QRS interval or rSr morphology in the right precordial leads (Figure 1A), and chest radiography revealed cardiac dilation with a 63% cardiothoracic ratio without pulmonary edema or pleural effusion (Figure 1B). Laboratory tests revealed a serum creatinine level of 0.80 mg/dL, a direct bilirubin level of 0.3 mg/dL, a brain natriuretic peptide level of 136.2 pg/mL, and a C-reactive protein level of 0.1 mg/dL. Results of a treponema pallidum antibody test were negative.
Figure 1.
(A) Electrocardiography on admission revealed normal sinus rhythm without signs of volume overload of the right ventricle. (B) Chest radiography on admission revealed cardiac dilation with a 63% cardiothoracic ratio without pulmonary edema or pleural effusion.
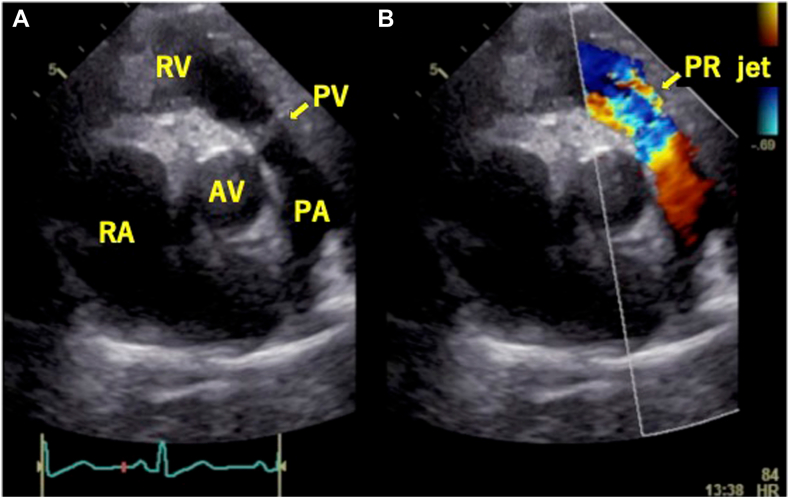
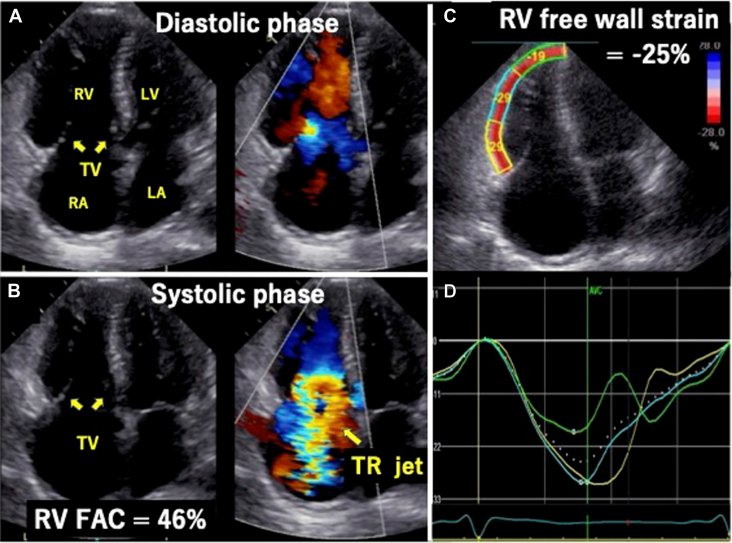
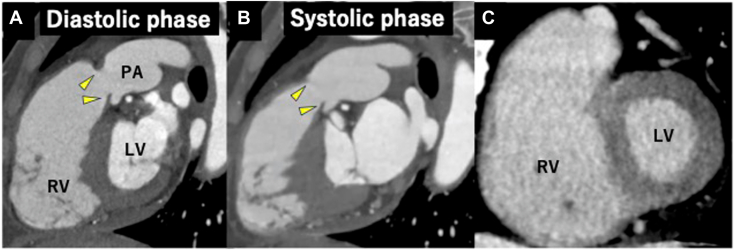
Transthoracic echocardiography (TTE) demonstrated severe PR (vena contracta 0.72, pressure half-time 71 msec) and a suspected unicuspid pulmonary valve (PV; Figure 2, Video 1). Three-dimensional (3D) TTE and multiplane view revealed that the PV had three leaflets, and all leaflets were thickened, shortened, and immobile at the opening position (Figures 3 and 4, Videos 2 and 3). Both PV area and anatomic regurgitant orifice area measured using 3D TTE were 1.27 cm2. The right ventricle and right atrium were dilated, and RV function was preserved, with a fractional area change ratio of 46% and RV free wall strain of −25%. TR was torrential because of tethering with a dilated right ventricle (Figure 5, Video 4); TR vena contracta width (using biplane imaging) was 22 mm, and effective regurgitant orifice area using a proximal isovelocity surface area was 1.09 cm2. The annular diameter of the TV was 36 mm, and systolic pulmonary arterial pressure was estimated at 43 mm Hg. Even though the left ventricle was compressed by the right ventricle during the diastolic phase, left ventricular (LV) function and size were normal, and LV ejection fraction was 64%. The left-sided valves were intact. Cardiac computed tomography (CCT) also revealed abnormally thickened and immobile PV leaflets and no abnormal findings such as anomalous structures around the PV (Figure 6A and B). Even though the patient had a history of right internal carotid artery aneurysm, there were no abnormal findings in systemic artery and pulmonary artery (PA) on CCT suggestive of inflammatory disease. Pulmonary embolism and abnormal late enhancement of LV myocardium were not detected on CCT (Figure 6C). Whole-body computed tomography revealed no evidence of mass lesion. In respiratory function testing, percentage vital capacity was 108%, and the forced ratio of expiratory volume to forced vital capacity was 103%. Right heart catheterization revealed no evidence of pulmonary hypertension; systolic PA and RV pressures were 18 and 27 mm Hg, resulting in a pressure gradient between the PA and right ventricle of 9 mm Hg. Coronary artery stenosis was not detected on coronary angiography.
Figure 2.
Two-dimensional TTE, basal parasternal short-axis view, diastolic phase, without (A) and with (B) color-flow Doppler, demonstrates severe PR (arrow). AV, Aortic valve; PA, pulmonary artery; PV, pulmonary valve; RA, right atrium; RV, right ventricle.
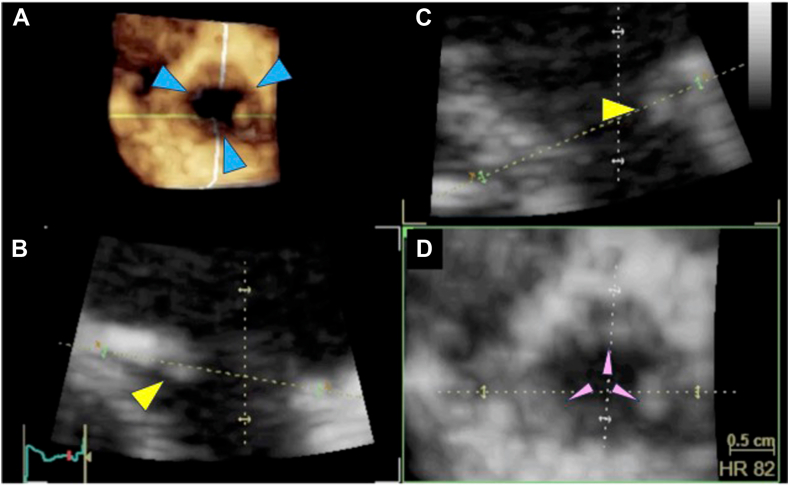
Figure 3.
Three-dimensional TEE, volume-rendered reconstruction display of the PV from the RV outflow tract perspective (A), diastolic phase, and two orthogonal reconstructed long-axis views of the PV (B,C) demonstrate the thickened and shortened PV leaflets (yellow arrowheads). Three commissures (blue arrowheads) and three leaflets (pink arrowheads) were confirmed from the 3D image and the reconstructed short-axis view of the PV (D).
Figure 4.
Two-dimensional TTE, multiplane orthogonal long-axis views of the PV, diastolic phase (A) and systolic phase (B), demonstrates thickened, immobile PV leaflets (arrowheads).
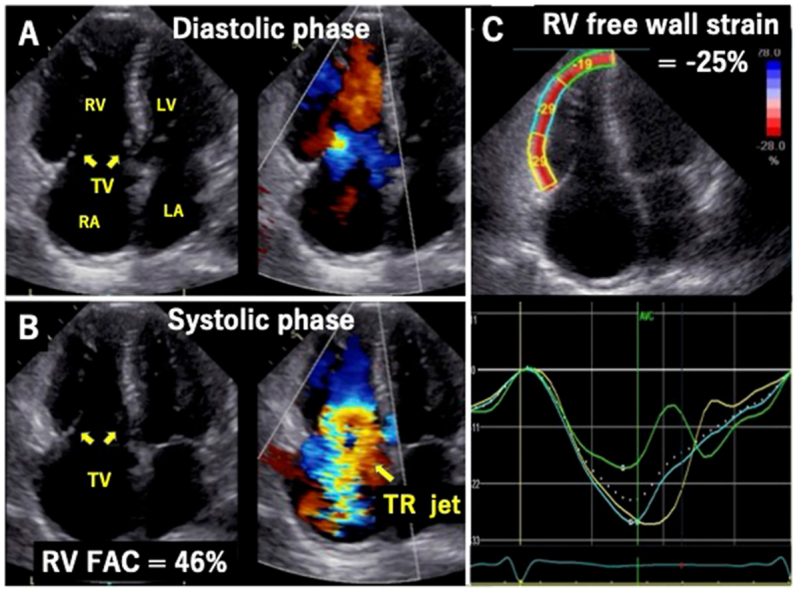
Figure 5.
Two-dimensional TTE, RV–focused apical four-chamber view, diastolic phase (A) and systolic phase (B), without (left) and with (right) color-flow Doppler, demonstrates the dilated right atrium (RA) and RV with preserved RV systolic function (RV FAC 46%) and torrential TR due to fixed leaflets in the open position. Longitudinal free wall strain analysis also demonstrates a normal value of −25% (C) and normal segmental curve display (D). FAC, Fractional area change; LA, left atrium; LV, left ventricle.
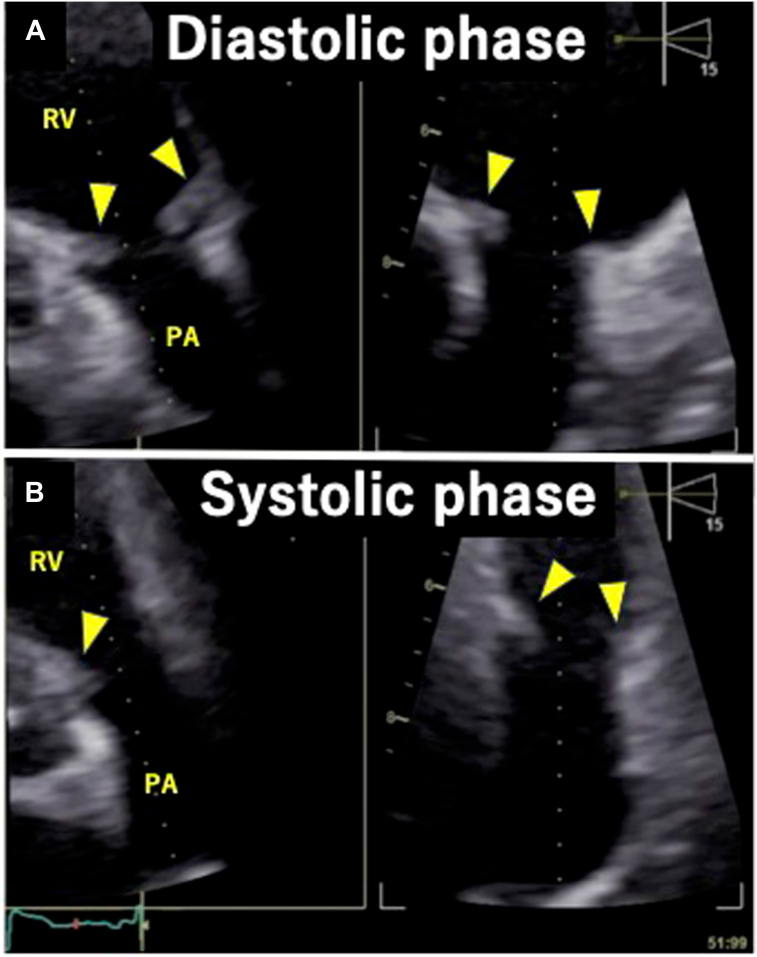
Figure 6.
CCT, oblique sagittal long axis view of the RV outflow tract and PV, diastolic phase (A) and systolic phase (B), demonstrates thickened and shortened PV leaflets (yellow arrowheads), which are essentially fixed in the open position. The short-axis view of the LV demonstrates normal myocardium without late enhancement (C).
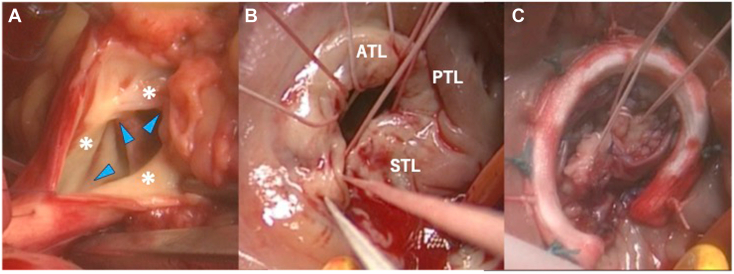
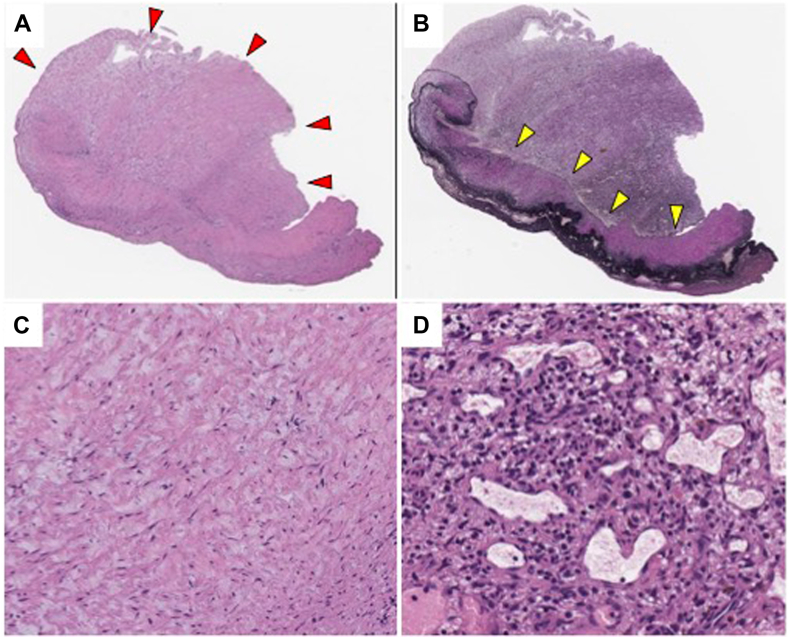
Because these symptoms of right heart failure were not treatable with drugs, surgical PVR and TV repair were performed. Intraoperative transesophageal echocardiography allowed a more detailed TV evaluation and revealed the abnormally shortened leaflets and thick chordae. Intraoperative findings showed that the three PV leaflets were abnormally thickened and shortened and also that the TV leaflets were abnormally shortened (Figure 7A and B). PVR with a 25-mm bioprosthetic valve and PV annular enlargement with bovine pericardium patch were performed. TV repair was performed with autologous pericardium patch augmentation of an anterior and a septal leaflet by annuloplasty using a 26-mm prosthetic annuloplasty ring (Figure 7C). Postoperative evaluation by intraoperative transesophageal echocardiography showed moderate residual TR with preserved RV function. Histologically, fibrous plaque was found on the atrial aspect of the PV. The plaque was intermixed with myofibroblasts, collagen, elastin, extracellular myxoid matrix, neovascularization, and infiltration of lymphocytes (Figure 8). The underlying valvular structure was preserved, thus presenting a “stuck-on” appearance.
Figure 7.
Intraoperative findings demonstrate all three PV leaflets are abnormally thickened and shortened (asterisks), and the commissures (blue arrowheads) are fused (A). The TV leaflets are also abnormally thickened and retracted (B). TV repair using autologous pericardial patch augmentation was performed (C). ATL, Anterior tricuspid leaflet; PTL, posterior tricuspid leaflet; STL, septal tricuspid leaflet.
Figure 8.
Histological findings of the PV. Fibrous plaque (red arrowheads) is observed on the atrial aspect of the valve. The underlying valvar structure is preserved, corresponding to “stuck-on” appearance (A). The endothelium (yellow arrowheads) of the fibrosa remains smooth and the plaque does not destroy the underlying structure (B). The plaque comprises the myofibroblasts and extracellular matrix (fibrous band and myxoid ground substance; C). An area of neovascularization and lymphocyte infiltration is also observed (D). In (A), (C), and (D), hematoxylin-1 eosin; in (B) Elastica van Gieson.
Postoperative TTE revealed normal prosthetic valve function, moderate residual TR, and reduced RV function, with a fractional area change ratio of 30% and RV free wall strain of −16%. Routine follow-up on TTE after discharge showed no improvement in right heart function or residual moderate TR. One year after surgery, the patient required hospitalization because of right heart failure. Two years after surgery, the patient developed refractory pleural effusion and was in and out of the hospital repeatedly. After careful reevaluation of the echocardiographic and pathologic findings retrospectively, carcinoid heart disease was suspected, and the evaluation of the urinary 5-hydroxy-indole acetic acid (5-HIAA) level was considered. However, the patient died of respiratory failure before further assessment was obtained.
Discussion
Severe symptomatic PR in the adult population is rare. Causes in adults include (1) congenital conditions, including uni-, bi-, and quadricuspid valve; (2) postoperative conditions after surgical or catheter treatment for pulmonary stenosis of tetralogy of Fallot; and (3) acquired conditions, such as endocarditis, carcinoid heart disease, rheumatic heart disease, Behçet disease, pulmonary hypertension, systemic lupus erythematosus, and syphilis.3 Furthermore, valvular heart disease due to valve degeneration as a side effect of dopamine receptor agonists has been reported.4 In our present case, both PV and TV showed degenerative lesions, and carcinoid heart disease was suspected.5 In carcinoid heart disease, serotonin and other bioactive substances secreted by carcinoid tumors damage the valve leaflets, mainly in the right heart system. The fibrous plaque of the valvular leaflet characterizes the histopathology of carcinoid heart disease. The plaque comprises myofibroblasts, extracellular matrix (collagen, myxoid ground matrix, and elastin), lymphocytes, mast cells, and neovascularization. The plaque appears “stuck on” in the leaflet, without destroying the valvar structure.6,7 The diagnosis of carcinoid heart disease requires proof of the presence of a high urinary concentration of 5-HIAA, the major metabolite of serotonin, because the pathologic finding alone is not specific. In this case, echocardiographic and pathologic findings led us to strongly suspect carcinoid heart disease, but a final diagnosis of carcinoid heart disease was not made, because urinalysis to check the 5-HIAA level was not performed. However, the fact that the pathology was accompanied by findings consistent with carcinoid heart disease and the fact that this patient died 2 years after surgery with refractory effusions further supports the possibility of a missed diagnosis of carcinoid heart disease. In this case, whole-body computed tomography did not show any mass lesion suspicious for a carcinoid tumor, but if carcinoid heart disease had been kept in mind at an early stage, a more detailed tumor search could have been performed using scintigraphy. Once the diagnosis of carcinoid disease is made, tumor removal or treatment with somatostatin analogs may be possible, and survival may be prolonged. In the case of drug-induced carcinoid syndrome caused by dopamine receptor agonists, discontinuation of the causative drug should be considered. The finding of “short, thick restricted leaflets” is characteristic of carcinoid disease, and early suspicion of carcinoid disease is necessary when this finding is seen. We should be careful not to miss this deadly diagnosis, which forecloses the opportunity to treat with somatostatin analogs.
Chronic severe PR has a detrimental effect on RV function and increases the risk for right heart failure, arrhythmia, and sudden cardiac death.1 To maintain RV function, practice guidelines on valvular heart disease recommend PVR for severe PR with RV dilation or RV dysfunction or progressive RV dilation or RV dysfunction, which both improves quality of life and reduces arrhythmia risk.8 However, severe PR is less likely to cause symptoms; indeed, in one study, 6% of patients with severe PR developed symptoms within 20 years, and 29% developed symptoms within 40 years.9 When RV enlargement and RV dysfunction are detected on TTE, it is important to evaluate PR as a possible cause. If timely surgical treatment can be performed for PR, significant improvement in RV dilation and RV function can be expected. Conversely, if surgical treatment for PR is delayed, such recovery cannot be adequately anticipated. Surgical treatment for PR should therefore be performed before RV functional decline or the manifestation of symptoms.1
RV free wall strain measured using TTE is a significant indicator of RV function in patients with severe PR10 and severe TR11 despite other RV functional parameters being overestimated and requiring careful evaluation in cases with severe TR.12 In our case, although preoperative TTE revealed RV enlargement, right heart function parameters, including RV free wall strain, were preserved, and we accordingly considered that it was not too late to conduct surgery. However, the patient experienced right heart failure, and the postoperative course was poor. Although moderate TR remained after surgery, it has been reported that residual TR of moderate or less after torrential TR treatment does not affect prognosis and is unlikely to be the cause of recurrent right heart failure after surgery.13 There was no other evidence of abnormalities that could have caused the postoperative right heart failure, such as prosthetic valve abnormality or constrictive pericarditis, which suggested that the patient had myocardial damage undetectable by preoperative imaging evaluation.
Recent improvement in the diagnostic performance of ultrasound equipment has allowed detailed morphologic evaluation of the PV. In this case, 3D TTE was used to evaluate detailed PV morphology, which was useful for preoperative diagnosis. Even though a unicuspid valve was suspected on TTE, three leaflets were detected on 3D TTE. As the PV is located on the anterior surface of the body, the distance from the echocardiographic probe to the PV is small, and good-quality images can be obtained. Our case illustrates that evaluation using 3D TTE for PR in adulthood is valuable. In some cases, the PV may be difficult to assess on TTE because of the retrosternal location; CCT or cardiovascular magnetic resonance imaging analysis helps evaluate PV morphology in such cases.14 Because of their wide field of view, these modalities are also valuable in detecting anomalous structures around the PV and intra- and extracardiac comorbidities. These features also make them suitable for searching for underlying diseases in PR, such as connective tissue disorders. In fact, the PV was clearly detected on CCT in this case. In addition, these examinations can also detect myocardial abnormalities as late enhancements. In cases of concomitant pulmonary hypertension, late enhancement in the RV attachment of the interventricular septum is often observed, and its presence can serve as evidence for pulmonary hypertension, which is prone to hemodynamic changes.15 Pulmonary hypertension was suspected in the initial TTE in this case but was not found on right heart catheterization. As a result, there were no characteristic findings of late enhancement of the LV myocardium on CCT, and we therefore considered that the initially suspected pulmonary hypertension was either largely absent or, if present, only mild. Additionally, the analysis of late enhancement on CCT is also helpful in the differential diagnosis of underlying cardiac disease of PR.
The dilation and stretching of the right ventricle impede interventricular conduction and establish a mechanoelectrical substrate for reentry circuits, increasing the likelihood of sustained ventricular tachycardia. Therefore, prolongation of the QRS interval and rSr morphology in the right precordial leads reflects volume overload of the right ventricle and are important predictors of malignant arrhythmia and sudden cardiac death in patients with PR.1 Our patient had no treatment-associated arrhythmic events, and the absence of QRS interval prolongation or rSr morphology on the patient’s electrocardiography is compatible with this absence of significant arrhythmic events.
Conclusion
We reported the case of an elderly woman with severe degenerative PR and TR. Three-dimensional TTE was helpful in the preoperative assessment of morphologic abnormalities of the PV and helped guide subsequent management.
Ethics Statement
The authors declare that the work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Consent Statement
The authors declare that since this was a non-interventional, retrospective, observational study utilizing de-identified data, informed consent was not required from the patient under an IRB exemption status.
Funding Statement
This work was partially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grant 22K20498.
Disclosure Statement
The authors report no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2023.12.034.
Supplementary Data
Two-dimensional TTE, basal parasternal short-axis view without (left) and with (right) color-flow Doppler, demonstrates severe PR.
Three-dimensional TEE, volume-rendered reconstruction display of the PV from the RV outflow tract perspective (top left) and two orthogonal reconstructed long-axis views of the PV (top right, bottom left), demonstrate the thickened, shortened, and immobile PV leaflets. Three commissures and three leaflets were confirmed from the 3D image and the reconstructed short-axis view of the PV (bottom right).
Two-dimensional TTE, multiplane orthogonal long-axis views of the PV, demonstrates the thickened, immobile PV leaflets.
Two-dimensional TTE, RV-focused apical four-chamber view, without (left) and with (right) color-flow Doppler, demonstrates the dilated right atrium and right ventricle with preserved RV function and torrential TR due to fixed leaflets in the open position.
References
- 1.Bouzas B., Kilner P.J., Gatzoulis M.A. Pulmonary regurgitation: not a benign lesion. Eur Heart J. 2005;26:433–439. doi: 10.1093/eurheartj/ehi091. [DOI] [PubMed] [Google Scholar]
- 2.Hufton A., Newman J.S., Pupovac S.S., Mattia A., Hartman A.R. Degenerative pulmonary valve insufficiency in a patient with a prior Bentall procedure. Ann Thorac Surg. 2021;111:e333–e334. doi: 10.1016/j.athoracsur.2020.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Waller B.F., Howard J., Fess S. Pathology of pulmonic valve stenosis and pure regurgitation. Clin Cardiol. 1995;18:45–50. doi: 10.1002/clc.4960180112. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen V.G., Østergaard K., Dupont E., Poulsen S.H. The risk of valvular regurgitation in patients with Parkinson's disease treated with dopamine receptor agonists. Mov Disord. 2011;26:801–806. doi: 10.1002/mds.23470. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi H., Okada K., Asano M., Matsumori M., Morimoto Y., Okita Y. Bioprosthetic pulmonary and tricuspid valve replacement in carcinoid heart disease from ovarian primary cancer. Circ J. 2009;73:1554–1556. doi: 10.1253/circj.cj-08-0561. [DOI] [PubMed] [Google Scholar]
- 6.Simula D.V., Edwards W.D., Tazelaar H.D., Connolly H.M., Schaff H.V. Surgical pathology of carcinoid heart disease: a study of 139 valves from 75 patients spanning 20 years. Mayo Clin Proc. 2002;77:139–147. doi: 10.4065/77.2.139. [DOI] [PubMed] [Google Scholar]
- 7.Jin C., Sharma A.N., Thevakumar B., Majid M., Al Chalaby S., Takahashi N., et al. Carcinoid heart disease: pathophysiology, pathology, clinical manifestations, and management. Cardiology. 2021;146:65–73. doi: 10.1159/000507847. [DOI] [PubMed] [Google Scholar]
- 8.Nishimura R.A., Otto C.M., Bonow R.O., Carabello B.A., Erwin J.P., III, Guyton R.A., et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 9.Shimazaki Y., Blackstone E.H., Kirklin J.W. The natural history of isolated congenital pulmonary valve incompetence: surgical implications. Thorac Cardiovasc Surg. 1984;32:257–259. doi: 10.1055/s-2007-1023399. [DOI] [PubMed] [Google Scholar]
- 10.Bidviene J., Muraru D., Kovacs A., Lakatos B., Ereminiene E., Liptai C., et al. Global and regional right ventricular mechanics in repaired tetralogy of Fallot with chronic severe pulmonary regurgitation: a three-dimensional echocardiography study. Cardiovasc Ultrasound. 2021;19:28. doi: 10.1186/s12947-021-00260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ancona F., Melillo F., Calvo F., Attalla El Halabieh N., Stella S., Capogrosso C., et al. Right ventricular systolic function in severe tricuspid regurgitation: prognostic relevance of longitudinal strain. Eur Heart J Cardiovasc Imaging. 2021;22:868–875. doi: 10.1093/ehjci/jeab030. [DOI] [PubMed] [Google Scholar]
- 12.Prihadi E.A., van der Bijl P., Dietz M., Abou R., Vollema E.M., Marsan N.A., et al. Prognostic implications of right ventricular free wall longitudinal strain in patients with significant functional tricuspid regurgitation. Circ Cardiovasc Imaging. 2019;12:e008666. doi: 10.1161/CIRCIMAGING.118.008666. [DOI] [PubMed] [Google Scholar]
- 13.Lurz P., Stephan von Bardeleben R., Weber M., Sitges M., Sorajja P., Hausleiter J., et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. 2021;77:229–239. doi: 10.1016/j.jacc.2020.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Saremi F., Gera A., Ho S.Y., Hijazi Z.M., Sánchez-Quintana D. CT and MR imaging of the pulmonary valve. Radiographics. 2014;34:51–71. doi: 10.1148/rg.341135026. [DOI] [PubMed] [Google Scholar]
- 15.Patel A.R., Addetia K. Prediction of prognosis in pulmonary hypertension using CMR: what happens where the right and left ventricles meet? JACC: Cardiovasc Imag. 2014;7:1218–1220. doi: 10.1016/j.jcmg.2014.09.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two-dimensional TTE, basal parasternal short-axis view without (left) and with (right) color-flow Doppler, demonstrates severe PR.
Three-dimensional TEE, volume-rendered reconstruction display of the PV from the RV outflow tract perspective (top left) and two orthogonal reconstructed long-axis views of the PV (top right, bottom left), demonstrate the thickened, shortened, and immobile PV leaflets. Three commissures and three leaflets were confirmed from the 3D image and the reconstructed short-axis view of the PV (bottom right).
Two-dimensional TTE, multiplane orthogonal long-axis views of the PV, demonstrates the thickened, immobile PV leaflets.
Two-dimensional TTE, RV-focused apical four-chamber view, without (left) and with (right) color-flow Doppler, demonstrates the dilated right atrium and right ventricle with preserved RV function and torrential TR due to fixed leaflets in the open position.