Abstract
Purpose
The cyclin-dependent kinase (CDK) 4/6 inhibitors significantly altered the treatment landscape of hormone-positive (HR+), HER2- metastatic breast cancer (MBC). However, biomarkers predicting long-term benefit and early progression are yet to be defined. Several studies suggested the possibility of diminished efficacy in patients with HER2-low disease. Therefore, we conducted a systematic review and meta-analysis to evaluate the association between low-level HER2 expression and efficacy outcomes (PFS, OS, ORR) with CDK 4/6 inhibitors.
Methods
The Pubmed, Web of Science, and Scopus databases were used to systematically filter the published studies from inception to 08 August 2023 for this systemic review. Studies including MBC patients treated with CDK 4/6 inhibitors and reported survival outcomes according to HER2 expression were included. We performed the meta-analyses with the generic inverse-variance method with a fixed-effects model and used HRs with 95% two-sided CIs as the principal summary measure.
Results
Nine studies encompassing 2705 patients were included in the analyses. In the pooled analysis of nine studies, the risk of progression and/or death was higher in patients with HER2-low tumors compared to HER2-zero (HR: 1.22, 95% CI 1.10–1.35, p < 0.001). In the pooled analysis of five studies, although the median follow-up was short, the risk of death was higher in the HER2-low group compared to the HER2-zero group (HR: 1.22, 95% CI 1.04–1.44, p = 0.010).
Conclusion
The available evidence demonstrates a significantly higher risk of progression or death with CDK 4/6 inhibitors in HER2-low tumors. Further research is needed to improve outcomes in patients with HR+-HER2-low tumors.
Keywords: HER2-low, HER2-zero, Breast cancer, CDK4/6 inhibitors, Prognosis
Introduction
The cyclin-dependent kinase (CDK) 4/6 inhibitors significantly altered the treatment landscape of hormone-positive (HR+), HER2- metastatic breast cancer (MBC) [1–3]. The combination of CDK 4/6 inhibitor plus endocrine therapy became the standard of care option in the first- and second-line settings with improved progression-free (PFS) and overall survival (OS) data [4, 5]. Currently, these agents are being used independent of a biomarker status in clinical scenarios other than visceral crisis, in parallel with pivotal phase III trials [6–8]. However, not all patients uniformly benefit from these treatments, and around 15% of the patients progressed even with first-line use [6, 9]. Therefore, biomarkers predicting long-term benefits and early progression are needed.
The ErbB2 receptor family plays a pivotal role in endocrine treatment resistance, and targeted therapies to this pathway have been used over two decades in HER2+breast cancer [10]. The HER2+tumors are classified as tumors with a 3+IHC or 2+IHC and ISH positivity. Considering the lower levels of HER2 expression in HER2 1 + or HER2 2 + and ISH-negative tumors and the possibility of targeting these tumors with novel anti-HER2 drug antibody conjugates [11, 12], we witnessed the emergence of a new subgroup of breast tumors called “HER2-low breast cancer” [13, 14]. However, the effects of low-level HER2 expression on the survival are yet to be defined [15, 16]. While some studies reported inferior survival in patients with HR+HER2-low tumors, several studies stated similar survival in HER2-low and HER2-zero tumors [17–19]. In addition to the prognosis, the low levels of HER2 expression could affect the efficacy of anti-endocrine agents, including the CDK 4/6 inhibitors, due to the pivotal role of the ErbB2 receptor on endocrine resistance [20]. However, the available studies differed in study designs, patient populations, sample sizes, as well as outcomes. Therefore, we conducted a systematic review and meta-analysis to evaluate the prognostic role of low-level HER2 expression on the outcomes of MBC patients treated with CDK 4/6 inhibitors.
Material and methods
Literature search
We conducted a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidance (PRISMA) [21]. The study protocol was registered with the PROSPERO (CRD42023453557). The Pubmed, Web of Science, and Scopus databases were used to systematically filter the published studies from inception to August 08, 2023, for this systemic review. The selected MeSH search terms were “HER2 low” OR “low HER2” OR “ERBB2 low” OR “low ERBB2” AND “CDK” OR “cyclin-dependent kinase” OR “CDK 4/6” OR “CDK4/6” OR “CDK 4/6 inhibitor.”
Inclusion and exclusion criteria
We included studies that met the following inclusion criteria: (1) prospective or retrospective study to evaluate the potential association of low-level HER2 expression on either progression-free survival (PFS) or overall survival (OS) with CDK 4/6 inhibitors; (2) available hazard ratio and 95% confidence interval for the comparison of HER2-low and HER2-zero groups; and (3) peer-reviewed full-text article or abstract available in English. Exclusion criteria of studies were: (1) duplicated articles; (2) review articles, case reports, case series, editorials, guidelines, dissertations, and opinion papers; (3) animal and cell-line studies; (4) studies including pediatric patients; (5) studies comparing HER2-positive and HER2-negative patients; (6) studies reporting on outcomes other than PFS or OS, and (7) trial protocols.
Study selection and data extraction
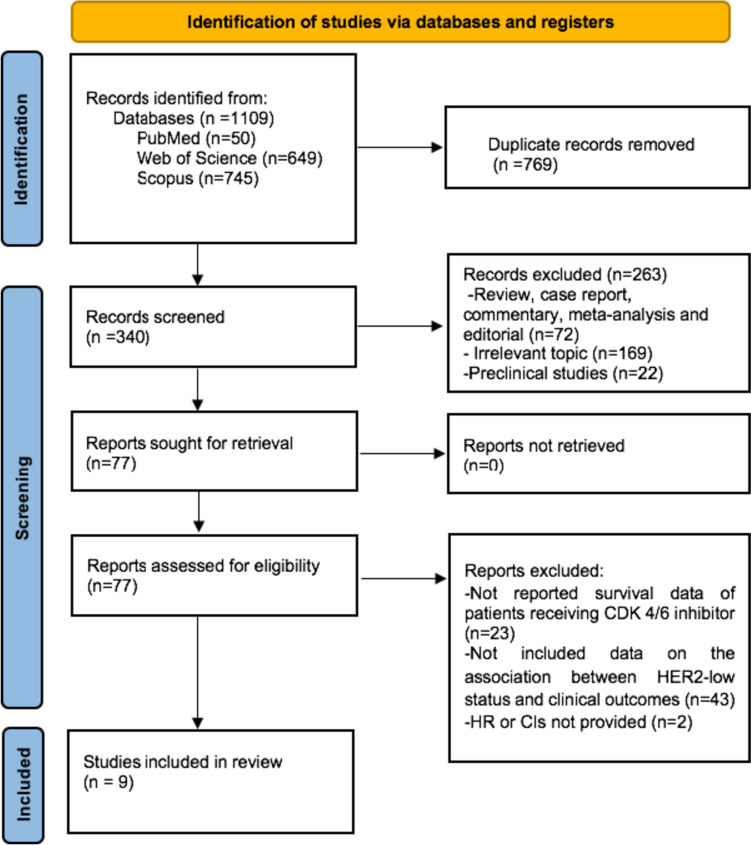
Our systematic search retrieved 1109 records. After removing duplicates (n = 769), we screened the remaining 340 records for inclusion. A total of 263 records were excluded after the screening of titles and abstracts. After evaluation of the full texts of the remaining 77 records, we excluded 67 more records due to no survival data (n = 23), no data on the association between low HER2 status and survival outcomes (n = 43), and no available HR or CI (n = 2); and included nine studies from the systematic search in meta-analyses. The flowchart for article selection is shown in Fig. 1.
Fig. 1.
PRISMA flow diagram
Two authors (DCG, TKS) extracted the data following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines and any discrepancy was resolved by the senior author [22]. The following data were extracted from the available studies: lead author names, year of publication, total number of patients, hazard ratios (HR) with 95% CIs for OS or PFS, and overall response rate (ORR). The individual study qualities and risk of bias were evaluated independently by two authors (DCG and TKS) using the Newcastle–Ottawa Scale.
Meta-analysis
The primary objective of this study was to evaluate the association between PFS and low levels of HER2 expression in patients with HR+breast cancer treated with CDK 4/6 inhibitors. The secondary objective was to evaluate the association between the OS and ORR according to HER2 expression (HER2 low vs. HER2 zero. We conducted further subgroup analyses for PFS according to the treatment line.
We performed the meta-analyses with the generic inverse-variance method with a fixed-effects model, considering the low degree of heterogeneity in the analyses. We used HRs with 95% two-sided CIs as the principal summary measure and reported the heterogeneity within each subgroup with I-square statistics. We conducted the meta-analyses using the Review Manager software, version 5.4 (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) and considered p values below 0.05 statistically significant.
Results
Study characteristics
Nine studies encompassing a total of 2705 patients were included in the analyses. The four studies were conducted in the first line [18, 23–25], while mixed cohorts were present in five studies [17, 19, 26–28]. Five studies were multicenter, and single-center data were reported in four studies. Eight studies were retrospective, while only one included a cohort with prospectively recorded data. All studies included both patients treated with aromatase inhibitors or fulvestrant in combination with CDK 4/6 inhibitors. Sample sizes varied between 84 and 1084, and five of nine studies had sample sizes of less than 200 patients. Four of the studies were from Europe. The PFS and OS were available in five studies, while four studies reported only PFS. The median follow-up time varied between 15 and 36 months across studies (Table 1). Most studies had a low risk of bias, according to the NOS (Table 2).
Table 1.
Characteristics of included studies
| Author, year | Country | Type of Study | Total number of patients | Number of Patients (HER2-Low/Zero) | Median age, year | Line of therapy | Treatment | Median OS (HER2-Low vs HER2-Zero) | Median PFS (HER2-Low vs HER2-Zero) | ORR (HER2-Low vs HER2-Zero) |
Median follow-up, mo |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bao, 2021 [26] | Hong Kong |
Single-center Retrospective |
106 | 82/24 | 58 | Mixed | CDK4/6 inhibitor (Palbociclib/ribociclib)+AI or Fulvestrant | N/A | 8.9 vs. 18.8 mo | N/A | N/A |
| Bortot, 2021 [23] | Italy | Multicenter Retrospective | 84 | N/A | N/A | 1 | CDK4/6 inhibitor+endocrine therapy | N/A | N/A | N/A | N/A |
| Carlino, 2022 [24] | Italy | Multicenter Retrospective | 165 | 71/94 | 64 | 1 | Palbociclib+AI or Fulvestrant | Not reached | 19 vs 23 mo | N/A | 31 mo |
| Douganiotis, 2022 [25] | Greece | Multicenter Retrospective | 191 | 139/52 | 60 | 1 | CDK4/6 inhibitor (Palbociclib/ribociclib/abemaciclib)+AI or Fulvestrant | Not reached |
HER2 +2/ISH-negative: 20.8 mo HER2+1: 26.1 mo HER2-Zero: 40.2 mo |
N/A | 15 mo |
| Lapuchesky,2022 [27] | Argentina |
Single-center Retrospective |
186 | 64/122 | 55 | Mixed | CDK4/6 inhibitor (Palbociclib/ribociclib/abemaciclib)+endocrine therapy | N/A | 15.6 v 19 mo | N/A | N/A |
| Zattarin, 2023 [18] | Italy | Multicenter Retrospective | 428 | 269/159 | N/A | 1 | CDK4/6 inhibitor (Palbociclib/ribociclib/abemaciclib)+endocrine therapy | 48.7 vs 58.3 mo | 23.6 vs 32.3 mo | N/A | 36 mo |
| Yildirim, 2023 [17] | Turkey | Multicenter Retrospective | 204 | 66/138 | 58 | Mixed |
CDK4/6 inhibitor (Palbociclib/ribociclib)+AI (n = 115) CDK4/6 inhibitor (Palbociclib/ribociclib)+Fulvestrant (n = 89) |
Not reached | 19 vs 18 mo | 72.7% vs 66.6% | 22 mo |
| Sharaf, 2023 [19] | Jordan |
Single-center Retrospective |
257 | 143/114 | 49.9 | Mixed | Ribociclib+AI or Fulvestrant | N/A | 17.3 vs 22.2 mo | 39.4% vs 52% | N/A |
| Mouabbi, 2023 [28] | USA |
Single-center Cohort |
1084 | 697/387 | 50 | Mixed | CDK4/6 inhibitor (Palbociclib/ribociclib/abemaciclib)+endocrine therapy |
First-line:32.4 mo vs 31.2 mo Second Line: 31.5 vs 24.9 mo |
First-line:13 vs 11.6 mo Second Line: 7.3 vs 7.1 mo |
N/A | 17.9 mo |
*AI: aromatase inhibitor, mo: months
Table 2.
Newcastle-Ottowa Scores of Included Studies
| First author, publication year | Publication type | Selection | Comparability | Outcome | Total score | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assess-ment of outcome | Was follow-up long enough for outcomes to occur? (1‑year threshold) | Adequacy of follow-up of cohorts | |||
| Bao, 2021 [26] | Full-text article | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Bortot, 2021 [23] | Congress abstract | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Carlino, 2022 [24] | Full-text article | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Douganiotis, 2022 [25] | Full-text article | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Lapuchesky,2022 [27] | Congress abstract | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 6 |
| Zattarin, 2023 [18] | Full-text article | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Yildirim, 2023 [17] | Full-text article | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
| Sharaf, 2023 [19] | Full-text article | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
| Mouabbi, 2023 [28] | Full-text article | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 |
Association between HER2-low status and PFS
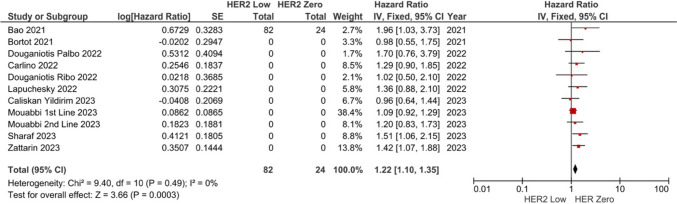
Six of nine studies reported no association between the HER2-low status and PFS with CDK 4/6 inhibitors [17, 23–25, 27, 28]. In the pooled analysis of nine studies, the risk of progression and/or death was higher in patients with the HER2-low tumors compared to HER2 zero (HR: 1.22, 95% CI 1.10–1.35, p < 0.001) (Fig. 2). The included studies had low degree of heterogeneity (I2 = 0%). Sensitivity analyses conducted by the subtraction of the individual studies demonstrated consistent results.
Fig. 2.
Meta-analysis of progression-free survival. Diamond indicates the pooled effect size
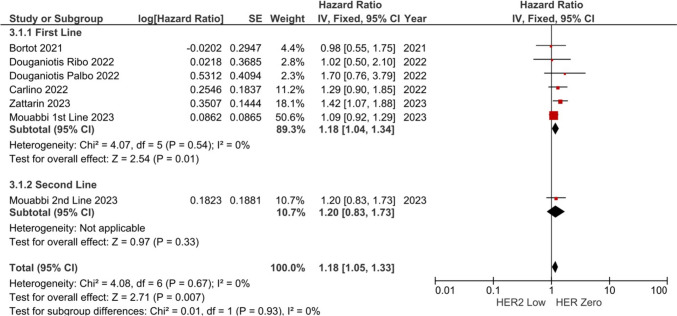
Subgroup analyses were conducted according to the treatment line. The risk of progression and/or death was similar across the lines of treatment (1st-line HR: 1.18, 95% CI 1.04–1.34, p = 0.010, and 2nd-line HR: 1.20, 95% CI 0.83–1.73, p = 0.330, p-value for subgroup differences p = 0.930) (Fig. 3), although four studied did not have separate data for treatment lines and only one study specifically included patients treated in the second line.
Fig. 3.
Subgroup analyses of PFS according to treatment line
Association between HER2-low status and OS/ORR
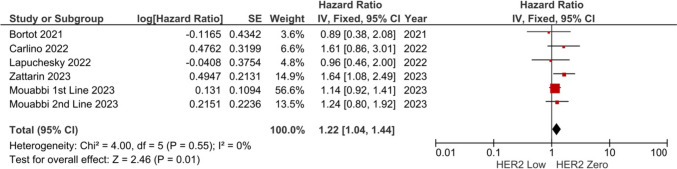
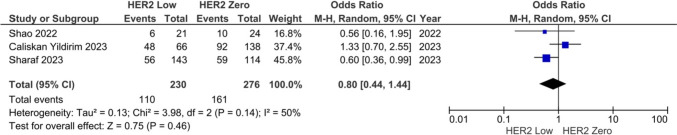
A total of 5 and 3 studies were reported on OS and ORR, respectively. In the pooled analysis of five studies, the risk of death was higher in the HER2-low group compared to the HER2-zero group (HR: 1.22, 95% CI 1.04–1.44, p = 0.010) (Fig. 4). The included studies had low degree of heterogeneity (I2 = 0%), and sensitivity analyses conducted by the subtraction of the individual studies demonstrated consistent results. The pooled ORR with CDK 4/6 inhibitors was 47.8% in the HER2-low group and 58.3% in the HER2-zero group. The ORR was similar independent of the HER2-low status (HR: 0.80, 95% CI 0.44–1.44, p = 0.460) (Fig. 5). The meta-analysis for ORR had a high degree of heterogeneity (I2 = 50%).
Fig. 4.
Meta-analysis of the overall survival. Diamond indicates the pooled effect size
Fig. 5.
Meta-analysis of the overall response rate
Discussion
In this meta-analysis of over 2700 patients, we observed significantly higher progression or death in patients with HR+HER2-low metastatic breast cancer compared to patients with HER2-zero tumors. Although the median follow-up was short, the risk of death was also higher in patients with HER2-low expression. The ORR was similar across the HER2-low and HER2 groups, although the sample size was smaller for this analysis. The PFS analyses were consistent across the treatment line.
The characteristics of patients who had early progression with CDK 4/6 inhibitors is a critical research field. While earlier data suggested several clinical features like visceral metastases and ECOG status, molecular biomarkers like RB1 and CCNE1 were also associated with a higher risk of progression [1, 29–33]. In addition, tumor molecular subtyping via PAM50 (prosigna) was also associated with the efficacy of CDK 4/6 inhibitors [34, 35]. In the study by Prat et al., patients with HER2-enriched HR+breast cancer had early risk progression risk with palbociclib [36]. In contrast, a similar pattern was absent in patients treated with ribociclib. However, the PAM50 (prosigna) assay is not routinely available in daily practice due to financial reasons and primarily licensed for the early breast cancer. Considering the financial limitations of RNA-based profiling for HER2 enrichment, evaluation of HER2-low status by immunohistochemistry could be a surrogate for the activation of the ErbB2 pathway in patients treated with CDK 4/6 inhibitors. Furthermore, it was previously demonstrated that HER2-low tumors had higher ESR1 [37] and AKT expressions [38], features associated with resistance to CDK 4/6 inhibitors. Therefore, using HER2-low status as an efficacy biomarker in patients treated with CDK 4/6 inhibitors could be beneficial due to the strong biological rationale.
Despite the strong interest, the data on the association between HER2-low status and CDK 4/6 inhibitor efficacy are still controversial. A similar problem was present with the survival outcomes with early HER2-low breast cancer, with studies with contrasting results also available [39–42]. One of the main reasons regarding this issue could be the problems and variability with HER2-low case definition. There is significant variability across reading pathologists regarding the HER2-low status [43]. Additionally, it was demonstrated that HER2-low status could vary between the primary tumor and the metastasis [44, 45]. However, the source of the HER2-low definition (primary vs metastasis) was absent in the included studies in the meta-analysis [46]. Further research on the prognostic role of HER2-low status should ideally evaluate interobserver variability for case definition and report on the tissue in which the HER2-low status was evaluated.
The present meta-analysis is subject to several limitations. First, most of the available studies were retrospective and had limited sample sizes. The study cohorts were also heterogeneous regarding the treatment line and endocrine treatment partner limiting the ability to conduct subgroup analyses with adequate power. The follow-up time was short in most studies, limiting the reliability of overall survival results. The adjustments according to additional clinical parameters were absent in most studies. Lastly, due to the retrospective nature of most studies, causality regarding the effects of HER2-low status on survival outcomes could not be assured, and we opted to use the term association instead of effect in our reporting. However, despite these limitations, we observed a negative effect of low-level HER2 expression on survival outcomes in a pooled cohort of over 2700 patients. If our results are supported by prospective studies with longer follow-ups, the patients with advanced HR+HER2-low breast cancer could be candidates for novel combination approaches to improve outcomes with CDK 4/6 inhibitors.
Conclusion
In conclusion, the available evidence demonstrates a significantly higher risk of progression or death with CDK 4/6 inhibitors in HER2-low tumors. While the CDK 4/6 inhibitor plus endocrine therapy is the standard of care independent of the HER2-low status, further research is needed to improve outcomes in patients with HR+HER2-low tumors.
Author contributions
DCG and TKS conceived, designed, and performed the literature search for the review article. DCG and TKS also drafted the article and critically revised the work.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors of this manuscript have no conflict of interest to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 2.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor–positive, human epidermal growth factor receptor 2–negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 4.Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/s1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 5.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/s1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29:1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 7.Giuliano M, Schettini F, Rognoni C, et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: a systematic review and network meta-analysis. Lancet Oncol. 2019;20:1360–1369. doi: 10.1016/s1470-2045(19)30420-6. [DOI] [PubMed] [Google Scholar]
- 8.Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/jco.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 10.Alataki A, Dowsett M. Human epidermal growth factor receptor-2 and endocrine resistance in hormone-dependent breast cancer. Endocr Relat Cancer. 2022;29:R105–r122. doi: 10.1530/erc-21-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosele F, Deluche E, Lusque A, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med. 2023 doi: 10.1038/s41591-023-02478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H, Peng Y. Current biological, pathological and clinical landscape of HER2-low breast cancer. Cancers (Basel) 2022 doi: 10.3390/cancers15010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarantino P, Hamilton E, Tolaney SM, et al. HER2-low breast cancer: pathological and clinical landscape. J Clin Oncol. 2020;38:1951–1962. doi: 10.1200/jco.19.02488. [DOI] [PubMed] [Google Scholar]
- 15.Guven DC, Kaya MB, Fedai B, et al. HER2-low breast cancer could be associated with an increased risk of brain metastasis. Int J Clin Oncol. 2022;27:332–339. doi: 10.1007/s10147-021-02049-w. [DOI] [PubMed] [Google Scholar]
- 16.Yang C, Zhang X, Chen Y, et al. Survival differences between HER2-0 and HER2-low-expressing breast cancer − a meta-analysis of early breast cancer patients. Crit Rev Oncol Hematol. 2023;185:103962. doi: 10.1016/j.critrevonc.2023.103962. [DOI] [PubMed] [Google Scholar]
- 17.Yildirim EC, Atag E, Coban E, et al. The effect of low HER2 expression on treatment outcomes in metastatic hormone receptor positive breast cancer patients treated with a combination of a CDK4/6 inhibitor and endocrine therapy: a multicentric retrospective study. Breast. 2023;70:56–62. doi: 10.1016/j.breast.2023.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zattarin E, Presti D, Mariani L, et al. Prognostic significance of HER2-low status in HR-positive/HER2-negative advanced breast cancer treated with CDK4/6 inhibitors. NPJ Breast Cancer. 2023;9:27. doi: 10.1038/s41523-023-00534-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharaf B, Abu-Fares H, Tamimi F, et al. Differences in treatment outcomes between patients with HER2-low versus HER2-Zero, hormone receptor-positive advanced-stage breast cancer treated with ribociclib. Breast Cancer (Dove Med Press) 2023;15:541–548. doi: 10.2147/bctt.S415432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou FH, Downton T, Freelander A, et al. CDK4/6 inhibitor resistance in estrogen receptor positive breast cancer, a 2023 perspective. Front Cell Dev Biol. 2023;11:1148792. doi: 10.3389/fcell.2023.1148792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooke BS, Schwartz TA, Pawlik TM. MOOSE reporting guidelines for meta-analyses of observational studies. JAMA Surg. 2021;156:787–788. doi: 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- 23.Bortot L, Basile D, Targato G, et al. Clinical characterization and outcome of a HER2-low metastatic breast cancer (mBC) cohort receiving first-line treatment (1L) with ET +/- CDK 4/6 inhibitor (CDKi) Ann Oncol. 2021;32:S493–S493. doi: 10.1016/j.annonc.2021.08.578. [DOI] [Google Scholar]
- 24.Carlino F, Diana A, Ventriglia A, et al. HER2-low status does not affect survival outcomes of patients with metastatic breast cancer (MBC) undergoing first-line treatment with endocrine therapy plus palbociclib: results of a multicenter Retrospective Cohort Study. Cancers (Basel) 2022 doi: 10.3390/cancers14204981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Douganiotis G, Kesisis G, Lalla E, et al. Prognostic significance of low HER2 expression in patients with metastatic hormone receptor-positive breast cancer treated with first line CDK4/6 inhibitors: a Greek multicenter real-world data analysis. Cancer Diagn Progn. 2022;2:585–591. doi: 10.21873/cdp.10146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bao KKH, Sutanto L, Tse SSW, et al. The association of ERBB2-low expression with the efficacy of cyclin-dependent kinase 4/6 inhibitor in hormone receptor-positive, ERBB2-negative metastatic breast cancer. Jama Network Open. 2021 doi: 10.1001/jamanetworkopen.2021.33132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapuchesky LS, Bortz M, Waisberg F, et al. CDK4/6 inhibitors outcomes in patients with advanced breast cancer based on HER2-low expression. J Clin Oncol. 2022;40:1056. doi: 10.1200/JCO.2022.40.16_suppl.1056. [DOI] [Google Scholar]
- 28.Mouabbi JA, Singareeka Raghavendra A, Bassett RL, Jr, et al. Survival outcomes in patients with hormone receptor-positive metastatic breast cancer with low or no ERBB2 expression treated with targeted therapies plus endocrine therapy. JAMA Netw Open. 2023;6:e2313017. doi: 10.1001/jamanetworkopen.2023.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 30.O'Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–1403. doi: 10.1158/2159-8290.Cd-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gombos A, Goncalves A, Curigliano G, et al. How I treat endocrine-dependent metastatic breast cancer. ESMO Open. 2023;8:100882. doi: 10.1016/j.esmoop.2023.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2019;37:1169–1178. doi: 10.1200/jco.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76:2301–2313. doi: 10.1158/0008-5472.Can-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Sullivan CC, Suman VJ, Goetz MP. The emerging role of CDK4/6i in HER2-positive breast cancer. Ther Adv Med Oncol. 2019;11:1758835919887665. doi: 10.1177/1758835919887665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy CG. The role of CDK4/6 inhibitors in breast cancer. Curr Treat Options Oncol. 2019;20:52. doi: 10.1007/s11864-019-0651-4. [DOI] [PubMed] [Google Scholar]
- 36.Prat A, Chaudhury A, Solovieff N, et al. Correlative biomarker analysis of intrinsic subtypes and efficacy across the MONALEESA phase III studies. J Clin Oncol. 2021;39:1458–1467. doi: 10.1200/jco.20.02977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hui T, Li S, Wang H, et al. An analysis of clinical and pathologic features, recurindex genomic profiles, and survival outcomes in HER2-low breast cancer. Oncologist. 2023 doi: 10.1093/oncolo/oyad159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y, Tsang JY, Tam F, et al. Comprehensive characterization of HER2-low breast cancers: implications in prognosis and treatment. EBioMedicine. 2023;91:104571. doi: 10.1016/j.ebiom.2023.104571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hasan S, Neubauer Z, Press RH, et al. Prognostic implications of HER2Neu-low in metastatic breast cancer. American Society of Clinical Oncology. 2022;40:1044. doi: 10.1200/JCO.2022.40.16_suppl.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Abudureheiyimu N, Mo H, et al. In real life, low-level HER2 expression may be associated with better outcome in HER2-negative breast cancer: a study of the National Cancer Center. China Frontiers in oncology. 2022;11:774577. doi: 10.3389/fonc.2021.774577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Almstedt K, Heimes A-S, Kappenberg F, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer. 2022;173:10–19. doi: 10.1016/j.ejca.2022.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Tarantino P, Jin Q, Tayob N, et al. Prognostic and biologic significance of ERBB2-low expression in early-stage breast cancer. JAMA Oncol. 2022;8:1177–1183. doi: 10.1001/jamaoncol.2022.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miglietta F, Griguolo G, Bottosso M, et al. Evolution of HER2-low expression from primary to recurrent breast cancer. npj Breast Cancer. 2021;7:137. doi: 10.1038/s41523-021-00343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tarantino P, Gandini S, Nicolò E, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer. 2022;163:35–43. doi: 10.1016/j.ejca.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Almstedt K, Krauthauser L, Kappenberg F, et al. Discordance of HER2-low between primary tumors and matched distant metastases in breast cancer. Cancers (Basel) 2023 doi: 10.3390/cancers15051413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Molinelli C, Jacobs F, Agostinetto E, et al. Prognostic value of HER2-low status in breast cancer: a systematic review and meta-analysis. ESMO Open. 2023 doi: 10.1016/j.esmoop.2023.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.