Abstract
Ten lines of transgenic mice secreting transmissible gastroenteritis coronavirus (TGEV) neutralizing recombinant monoclonal antibodies (rMAbs) into the milk were generated. The rMAb light- and heavy-chain genes were assembled by fusing the genes encoding the variable modules of the murine MAb 6A.C3, which binds an interspecies conserved coronavirus epitope essential for virus infectivity, and a constant module from a porcine myeloma with the immunoglobulin A (IgA) isotype. The chimeric antibody led to dimer formation in the presence of J chain. The neutralization specific activity of the recombinant antibody produced in transiently or stably transformed cells was 50-fold higher than that of a monomeric rMAb with the IgG1 isotype and an identical binding site. This rMAb had titers of up to 104 by radioimmunoassay (RIA) and neutralized virus infectivity up to 104-fold. Of 23 transgenic mice, 17 integrated both light and heavy chains, and at least 10 of them transmitted both genes to the progeny, leading to 100% of animals secreting functional TGEV neutralizing antibody during lactation. Selected mice produced milk with TGEV-specific antibody titers higher than 106 as determined by RIA, neutralized virus infectivity by 106-fold, and produced up to 6 mg of antibody per ml. Antibody expression levels were transgene copy number independent and integration site dependent. Comicroinjection of the genomic β-lactoglobulin gene with rMAb light- and heavy-chain genes led to the generation of transgenic mice carrying the three transgenes. The highest antibody titers were produced by transgenic mice that had integrated the antibody and β-lactoglobulin genes, although the number of transgenic animals generated does not allow a definitive conclusion on the enhancing effect of β-lactoglobulin cointegration. This approach may lead to the generation of transgenic animals providing lactogenic immunity to their progeny against enteric pathogens.
The secretory immunoglobulin A (IgA) provides the initial immunologic barrier against most pathogens that invade the body at mucosal surfaces (46). This is especially true for viruses, since resistance to infection has been strongly correlated with the presence of specific IgA antibody in mucosal secretions (4). At mucosal surfaces, IgA antibodies are particularly stable and, since they are multivalent, might be more protective than IgG (26). The neutralization of viruses by immunoglobulins (Igs) is thought to result from the binding of antibody to virion attachment proteins, preventing their adherence to epithelial cells. In addition, mucosal antibody interacts intracellularly with viruses, preventing their replication, possibly by interfering with virus assembly (34).
Transmissible gastroenteritis coronavirus (TGEV) infects both enteric and respiratory tissues and causes a mortality close to 100% when newborn pigs are infected (41). The major antigenic sites of TGEV involved in the induction of virus neutralizing antibodies are located in the globular portion of the spike (S) protein (13, 15, 20). Investigations by our laboratory into the mechanisms of TGEV neutralization (47) and antigenic and genetic variability (17, 42, 43) have led to the identification of a mouse monoclonal antibody (MAb) which neutralized all the TGEV isolates tested and also neutralized TGEV-related coronaviruses which infect at least three animal species: pigs, dogs, and cats. This MAb, 6A.C3, probably binds to an epitope essential for virus replication, since no neutralization escape mutants appeared when it was used (20).
The immune response to TGEV has been characterized (3, 5, 49), and full protection against TGEV can be provided by lactogenic immunity from immune sows (41). It has also been shown that the passive oral administration of serum elicited by recombinant adenoviruses expressing the spike protein completely protects piglets against virulent-virus challenge (48).
Conventional approaches such as lactogenic immunity and artificial feeding may target the antibody to epithelial surfaces, providing protection against enteric virus infections (41). Alternatively, transgenic animals secreting virus neutralizing antibodies into their milk during lactation should provide immediate protection to piglets against enteric coronavirus infection. The mammary gland expression system is by nature very suitable for the production of proteins that function in the gastrointestinal tract and can be orally administered (31). In this paper, we describe the engineering of a recombinant TGEV neutralizing MAb with a porcine IgA isotype and the comparison of its specific neutralizing activity with a recombinant monomeric antibody having identical variable modules and an IgG1 isotype.
We constructed transgenic mice carrying two expression cassettes containing the cDNA sequences encoding the heavy and light chains of a chimeric IgA and gene expression regulatory sequences derived from the β-lactoglobulin (BLG) gene, to target the recombinant IgA (rIgA) synthesis specifically to the mammary gland. The effect of comicroinjecting the antibody expression cassettes with BLG genomic DNA on expression levels was studied. Transgenic mice that secrete high-titer virus neutralizing rIgA into their milk have been obtained. This strategy may be a general approach to protect against enteric infections of newborns.
MATERIALS AND METHODS
Cells and viruses.
Swine testis (ST) cells (35), simian virus 40 (SV40)-transformed monkey kidney COS-1 cells (ATCC CRL-1650), nonsecreting murine myeloma Sp2/0 cells (ATCC, CRL-1581), and MAb 6A.C3-secreting (14, 23) and S2.1 IgA-secreting porcine hybridoma cells (24) were grown in Dulbecco’s modified Eagle’s medium supplemented with fetal calf serum. TGEV PUR46-MAD (20) was grown, purified, and subjected to titer determination in ST cells as described previously (23).
RIA, virus neutralization, and Western blot analysis.
The rIgA collected from supernatants of stably transformed Sp2/0 cells was purified by anion-exchange high-pressure liquid chromatography and analyzed on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels (linear gradient from 5 to 20% polyacrylamide). The procedures for radioimmunoassay (RIA), virus neutralization, and Western blotting have been described previously (14). The antibody titer as determined by RIA was defined as the reverse of the maximum antibody dilution giving a binding to TGEV threefold higher than the background. The neutralization index was defined as the log of the ratio of the PFU after virus incubation in the presence of medium or the indicated MAb. All the antisera were diluted 1:1,000 in phosphate-buffered saline containing 0.3% bovine serum albumin and 0.1% Tween 20. The antisera used to develop the RIA to detect recombinant mouse-human (rMH) antibodies were rabbit anti-human kappa chain and rabbit anti-human IgG (Cappel). To detect recombinant mouse-swine (rMS) antibodies, rabbit anti-swine IgA (Bethyl Laboratories, Inc.) was used. To detect MAb 6A.C3, rabbit anti-mouse Ig (Cappel) was used.
The rabbit anti-J chain antiserum used to detect the J chain was a gift of R. M. E. Parkhouse (Institute for Animal Health) and has been previously characterized (25, 40).
To compare the neutralizing activity of rMH IgG with that of rMS IgA, the same antiserum [goat anti-mouse F(ab′)2] was used. The neutralizing activities of recombinant MAb dilutions with the same RIA titer were compared.
RNA extraction.
Total cytoplasmic RNA from S2.1 hybridoma cells was prepared by lysing 4 × 106 cells in 200 μl of TSM buffer (0.01 M Tris-hydrochloride [pH 7.6], 0.15 M NaCl, 5 mM MgCl2) with 0.2% Nonidet P-40 and pelleting the nuclei by centrifugation at 13,000 × g for 30 s. RNA was isolated by the addition of 200 μl of urea-SDS lysis buffer (8 M urea, 1.5% SDS, 15 mM EDTA, 0.24 M NaCl, 0.04 M Tris-hydrochloride [pH 7.6]) followed by vigorous vortexing and phenol-chloroform extraction. Poly(A)+ mRNA was isolated with the PolyATtract mRNA Isolation System (Promega).
Synthesis of cDNAs encoding the constant modules of porcine Ig κ and α chains.
To clone the porcine IgA constant (C) module, two cDNAs encoding the constant light (CL) (Fig. 1A) and constant heavy (CH) (Fig. 1B) chains were synthesized from poly(A)+ mRNA isolated from S2.1 hybridoma cells by reverse transcriptase PCR (RT-PCR) with specific primers. The 5′-end oligonucleotide corresponding to the first porcine CL module nucleotides and the 3′-end oligonucleotide complementary to the 3′ untranslated region contained ClaI and BamHI restriction endonuclease sites, respectively. The 5′-end oligonucleotide corresponding to the first porcine CH module nucleotides and the 3′-end oligonucleotide complementary to the 3′ untranslated region contained ApaI and BamHI restriction endonuclease sites, respectively. All the fragments were cloned into Bluescript SK− (Stratagene), and their sequences were verified by direct sequencing (Sequenase 2.0). The primers used in porcine IgA amplification were: flanking 5′ CH, 5′-GAAACGGGCCCCAAAATCTTCCCAC-3′, flanking 3′ CH, 5′-GCGGGATCCTTTATTCGAGGGGCG-3′, flanking 5′ CL, 5′-CCGTATCGATCTTCCCGCCATCG-3′, flanking 3′ CL, 5′-GCAAGGATCCCTTTCACATTTATTC-3′. The primers were designed on the basis of cDNA sequences previously reported for porcine α CH (7) and κ CL (29) modules. Avian myeloblastosis virus RT (Seikagaku America, Inc.) was used at 0.4 U/μl, and Taq polymerase (Perkin Elmer) was used at 0.03 U/μl. Amplifications were performed in a GeneAmp PCR system 9600 apparatus.
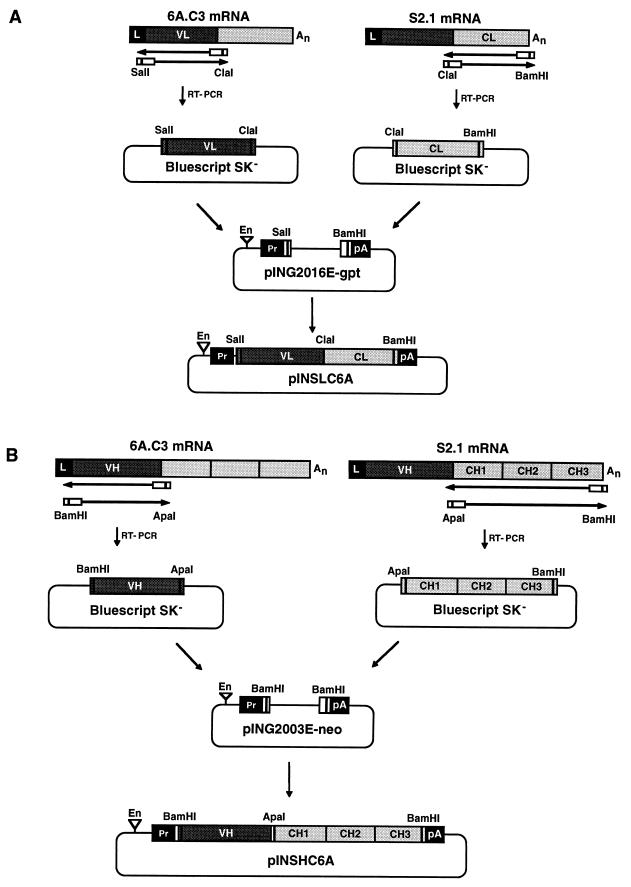
FIG. 1.
Cloning of Ig L and H chain cDNAs into expression vectors. (A) Cloning of the recombinant mouse-porcine L-chain cDNA. Poly(A)+ RNA from hybridoma cells secreting MAb 6A.C3 was used as the template for an RT-PCR to obtain the cDNA encoding the VL module. SalI and ClaI restriction sites were introduced into VL module cDNA at the 5′ and 3′ ends, respectively, to facilitate the cloning of the RT-PCR-derived cDNA into pBluescript SK−. Poly(A)+ RNA from porcine hybridoma cells secreting MAb S2.1 of the IgA isotype was used as the template for an RT-PCR to obtain the cDNA encoding the CL module. ClaI and BamHI restriction sites were introduced into CL module cDNA at the 5′ and 3′ ends, respectively, to facilitate the cloning of the RT-PCR derived cDNA into pBluescript SK−. The resulting VL and CL fragments were joined at the ClaI restriction site and cloned into the expression vector pING2016E-gpt by using the SalI and BamHI sites, yielding plasmid pINSLC6A. (B) Cloning of the recombinant mouse-porcine H-chain cDNA. Poly(A)+ RNA from the hybridoma secreting MAb 6A.C3 was used as the template for an RT-PCR to obtain the cDNA encoding the VH module. BamHI and ApaI restriction sites were introduced into VH module cDNA at the 5′ and 3′ ends, respectively, to facilitate the cloning of the RT-PCR cDNA into pBluescript SK−. Poly(A)+ RNA from porcine hybridoma cells secreting MAb S2.1 of the IgA isotype was used as the template for an RT-PCR to obtain the cDNA encoding the CH module. ApaI and BamHI restriction sites were introduced into CH module cDNA at the 5′ and 3′ ends, respectively, to facilitate the cloning of the RT-PCR-derived cDNA into pBluescript SK−. The resulting VH and CH fragments were joined at the ApaI restriction site and cloned into the BamHI restriction site of plasmid pING2003E-neo, yielding pINSHC6A. En, SV40 enhancer; Pr, SV40 promoter; An, poly(A) sequence; pA, SV40 polyadenylation signal.
Sequencing and characterization of cDNAs encoding porcine IgA α and κ chains.
The cDNA clones encoding the C modules of light (L) and heavy (H) chains from porcine IgA were sequenced by oligodeoxynucleotide primer extension and dideoxynucleotide chain termination procedures (44), using a previously described method (18). To sequence porcine IgA L and H-chain genes, the following primers were used: CL, 5′-CCGTATCGATCTTCCCGCCATCG-3′; CL, 5′-GCAAGGATCCCTTTCACATTTATTC-3′; CL, 5′-CAGGAATGAGTGTGAGGC-3′; CH, 5′-GAAACGGGCCCCAAAATCTTCCCAC-3′, CH, 5′-GCGGGATCCTTTATTCGAGGGGCG-3′; CH, 5′-GTGGCCTGAAAAAATCCG-3′; CH, 5′-CCACCTGCTGCCGCCGCC-3′; CH, 5′-CACGCGTGGGGGACGCC-3′; CH, 5′-CGAGGACTGGAAGCAGGG-3′. The cDNA sequences were compared with other Ig sequences by using Kabat’s database and the computer programs of the Genetics Computer Group (University of Wisconsin).
Construction of Ig expression plasmids.
The chimeric mouse-porcine L-chain expression vector was engineered (Fig. 1A) by ligating the SalI-ClaI VL fragment amplified by RT-PCR from mouse MAb 6A.C3 mRNA (8) to the ClaI-BamHI CL module obtained by RT-PCR from porcine MAb S2.1 mRNA and cloning the resulting cDNA into expression plasmid pING2016E-gpt (33, 55), previously digested with SalI and BamHI. The resulting plasmid was designated pINSLC6A.
The chimeric mouse-porcine H-chain expression vector was engineered (Fig. 1B) by ligating the BamHI-ApaI VH fragment amplified by RT-PCR from mouse MAb 6A.C3 mRNA (8) to the ApaI-BamHI CH module (33, 55) obtained by RT-PCR from porcine MAb S2.1 mRNA and cloning the resulting cDNA into expression plasmid pING2003E-neo previously digested with BamHI. The resulting plasmid was designated pINSHC6A.
Transformation of Sp2/0 myeloma cells with Ig gene expression plasmids.
To express rMS IgA antibody, the L-chain pINSLC6A (5 μg) and H-chain pINSHC6A (5 μg) plasmids were cotransfected. To express rMH IgG1 antibody, L-chain pINLC6A (5 μg) and H-chain pINHC6A (5 μg) plasmids (previously described [8]) were linearized at unique restriction sites after the 3′ ends of Ig genes (BglII for pINSLC6A and pINLC6A and AatII for pINSHC6A and pINHC6A) and were cotransfected into 107 Sp2/0 cells by electroporation (39). The cells were seeded in an M-24 microplate at 4 × 105 per well. Transformants were selected in the presence of the antibiotic Geneticin (G418; 0.8 mg/ml; Boehringer Mannheim). The supernatants from all the wells were positive for TGEV-specific antibodies by RIA. The cells showing the highest expression level were cloned twice by limiting dilution.
Transient expression of Ig genes in COS-1 cells.
COS-1 (8 × 105) cells were transfected by the Lipofectin (GIBCO BRL) method with 5 μg of circular DNA of the same expression vectors used in the stable transformation. Antibody levels were evaluated by RIA and neutralization in supernatants harvested at the indicated times posttransfection.
BLG constructs and plasmids.
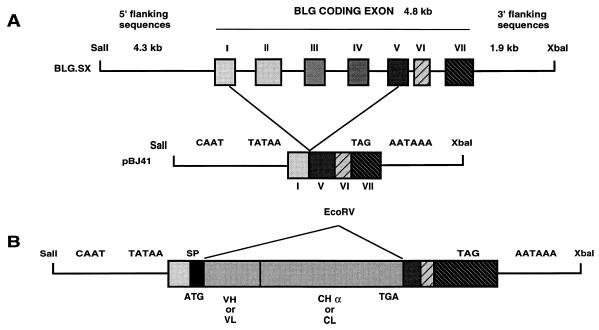
The unmodified BLG construct pSS1tgXS has been described previously (1, 21, 45) and comprises 4.3 kb of 5′-flanking sequences, the 4.9-kb transcription unit, and 1.7 kb of 3′-flanking sequences of the B allele of sheep BLG (2). To generate the expression cassette pBJ41 (Fig. 2), an EcoRV cloning site was created by introducing a linker between the PvuII sites of exons 1 and 5 of the BLG gene. Introns 5 and 6 were removed. This plasmid also includes 4.3 kb of BLG 5′-flanking sequences (from SalI) and 1.7 kb of BLG 3′-flanking sequences (to XbaI) from clone SS1 (1, 21, 45) cloned into pUC19. pBJ41 contains transcription initiation and polyadenylation sites but does not contain a translation initiation site. The cDNAs fragments encoding the chimeric L and H chains of rIgA were cloned separately at the unique EcoRV site of plasmid pBJ41. Constructs to express murine-porcine chimeric L chain (BLG-SLC) and murine-porcine chimeric H chain (BLG-SHC) under the control of BLG regulatory sequences were obtained.
FIG. 2.
Transgene constructs. (A) Structure of the BLG gene (BLG.SX) indicating the introns deleted to generate the pBJ41 plasmid carrying the intronless BLG minigene. The ATG present in the BLG gene was removed in pBJ41, and an EcoRV cloning site was created by introducing a linker between exons I and V. Exon VII does not have an open reading frame. CAAT, TATAA, and AATAAA regulatory signals are indicated. Bold lines indicate 5′- and 3′-flanking sequences and intronic sequences of BLG; boxes indicate BLG exon sequences. (B) Grey boxes, VH- or VL- and CHα- or CL-encoding cDNA segments. All constructs comprise identical 5′- and 3′-regulatory sequences. Relevant restriction enzyme cutting sites are shown. SP, signal peptide.
Generation of transgenic mice.
SalI-XbaI digestion of the plasmid vectors released the 10.5-kb genomic BLG, the 7.7-kb BLG-SLC, and the 8.2-kb BLG-SHC fragments for microinjection. These fragments were purified from a sodium chloride step gradient as described previously (19). BLG− transgenic mice were generated by coinjecting BLG-SLC and BLG-SHC fragments in a 1:1 molar ratio. BLG+ transgenic mice were generated by coinjecting BLG-SLC, BLG-SHC, and 10.5-kb BLG fragments in a 1:1:1 molar ratio. Pronuclear-stage eggs were obtained from superovulated C57BL/6 × CBAF1 females after mating with F1 males. DNA (2 or 3.5 μg/ml) was injected into either pronucleus by standard techniques. The injected eggs were cultured overnight, and two-cell embryos were transferred into the oviducts of pseudopregnant Swiss recipients. Transgenic lines were propagated by mating to (C57BL/6 × CBAF1) F1 hybrids or to B6CBAF1 mice.
DNA analysis.
Genomic DNA was prepared from a tail biopsy specimen, obtained from each mouse at weaning, by proteinase K digestion followed by phenol-chloroform extraction and ethanol precipitation, as described previously (30). Transgenic mice were identified by a PCR assay. To detect the BLG-SLC transgene, we used BLG1 (5′-GGGCTGGCTGGCCTGCATGC-3′) and LKV1 (5′-CCGTCCCAGATCCACTGCC-3′) primers, which hybridize with BLG 5′-regulatory sequences and the VL module of SLC, respectively, defining a 390-bp region present only in transgenic animals. To detect the BLG-SHC transgene, we used BLG1 and HV1 (5′-GGCCTTGCCCTGGAACTTCGGG-3′) primers, which hybridize with BLG 5′-regulatory sequences and the VH module of SHC, respectively, defining a 370-bp region. To detect genomic BLG sequences, we used BLG1 and BLG3 (5′-GAAGCCAGCCCTGCCAACACC-3′) primers, which hybridize with 5′-regulatory sequences and intron 1 sequences, respectively, defining a 400 bp region. Taq polymerase (Perkin Elmer) was used at 0.03 U/μl. Amplifications were performed in a GeneAmp PCR system 9600 apparatus.
EcoRI-cleaved DNA (10 μg) was analyzed by agarose gel electrophoresis, Southern blotting, and hybridization with 32P-labeled DNA probes, using random primers as recommended by the supplier (DECAprimeII DNA-labeling kit; Ambion). The DNA fragment used to probe the blot was specific for the common BLG promoter and therefore detected all three genes simultaneously, allowing the copy numbers of the three genes in each animal to be compared on the same Southern blot. The results were quantified with a Molecular Dynamics PhosphorImager. The transgene copy number was determined by comparison with known amounts of restriction enzyme fragments derived from pSS1tgXS plus BLG-SHC plus BLG-SLC constructs.
Analysis of milk.
Milk was collected daily from 4- to 6-month-old lactating females. The mothers were separated from their pups and 6 h later injected intraperitoneally (i.p.) with 0.5 to 1 IU of oxytocin (SmithKline Beecham). Milk was collected with a vacuum pump. Milk samples were diluted 1/10 in 0.125 M NaCl–25 mM Tris-hydrochloride–5 mM KCl and defatted by centrifugation. rIgA in the milk was detected by RIA and the neutralization assay. The concentration of rIgA in milk was estimated by RIA with internal standards of purified rIgA previously quantified with the bicinchoninic acid protein assay reagent (Pierce, Rockford, Ill.).
RESULTS
Sequence of cDNAs encoding porcine IgA α and κ chains.
To enhance antibody stability in the gastrointestinal tract, the chimeric mouse-human (MH) IgG, with the variable module from MAb 6A.C3 described previously (11), was engineered to substitute the constant modules for those from a porcine IgA. Porcine IgA α and κ genes were cloned by RT-PCR with the mRNA from a porcine hybridoma secreting IgA (24) (Fig. 1). The cDNA sequence obtained for the CH-module of porcine α chain (Fig. 3A) showed no changes from the nucleotide sequence previously reported for a porcine α chain from a Yorkshire gilt (7). The cDNA sequence at the hinge region revealed that this porcine IgA corresponds to the IgAa allelic form described recently (8). The cDNA sequence obtained for the CL module of porcine κ chain showed 15 nucleotide changes (Fig. 3B) in the coding region with respect to the sequence reported previously for a porcine κ chain from an adult Minnesota miniature swine (29), leading to 9 changes in the amino acid sequence. Of the 15 nucleotide changes, 2, at positions 26 and 27 of the C module, have been introduced to create the ClaI restriction endonuclease site, to facilitate the cloning of the L-chain gene. In addition, 11 differences were found in the 3′ untranslated cDNA sequence. Cysteine residues that usually participate in intradomain and inter-H-L chain disulfide bonds were conserved. Similarly, the carboxy-terminal dipeptide after the last cysteine residue, a unique feature among mammalian κ light chains, was also conserved (29). Both cDNAs encoding the porcine IgA CH and CL modules contain polyadenylation signals in the 3′ untranslated region.
FIG. 3.
C domain sequences of porcine κ L-chain and α H-chain cDNAs. The sequences of the C Ig domain starting at nucleotide 1 are shown. (A) The nucleotide sequence of the porcine CHα cDNA domain and the deduced amino acid sequence are shown in the first and second lines, respectively. The ApaI and BamHI cloning sites introduced at the 5′ and 3′ ends, respectively, are underlined. Boundaries between domains are indicated by vertical lines and the name of the domain. (B) The nucleotide sequence of the porcine κ L-chain cDNA cloned in our laboratory and the deduced amino acid sequence are shown in the first and third lines, respectively. In the second and fourth lines, the nucleotide and amino acid substitutions in the sequences previously reported (29) for the porcine κ chain are indicated in boldface type. ClaI and BamHI cloning sites introduced at the 5′ and 3′ ends, respectively, are underlined. Nucleotides are numbered at both sides of each line. ▪, cysteine residues predicted to participate in intradomain disulfide bonds. The polyadenylation signal and the stop codons are shown in boldface type. ▵, absent nucleotides.
Generation of rMAb 6A.C3.
Construction of the recombinant antibody required the fusion of mouse VL and VH modules to porcine κ and α C modules. This was accomplished by introducing ClaI or ApaI restriction endonuclease sites into the Ig genes (Fig. 1). The first 24 nucleotides of the recombinant CL chain corresponds to the MAb 6A.C3 sequence (mouse CL) and is joined in frame to the sequence encoding the C module of the porcine κ light chain. A phenylalanine (encoded by TTC)-to-serine (encoded by TCG) amino acid change at residue 9 of the κ C chain was introduced to create the ClaI site required for the fusion of VL and CL modules. The first 9 nucleotides of the chimeric CH chain corresponds to the mouse MAb 6A.C3 sequence (mouse CH1) and is joined in frame to the constant module sequence of porcine α H chain. The mutagenesis required to create the ApaI restriction site led to a replacement of the serine present in the original sequence (encoded by AGC) by a glycine (encoded by GGC), which corresponds to residue 5 of the CH1 module. cDNAs encoding recombinant antibody L and H chains were subcloned into expression plasmids pINSLC6A and pINSHC6A (Fig. 1), respectively, which carry the SV40 early promoter and a mouse Ig enhancer at the 5′ end of the expression cassettes and the SV40 polyadenylation signals at the 3′ end. Sequencing confirmed that the V and C Ig modules were correctly joined.
The engineering of rIgG1 with the same V modules as those of rIgA has been described previously (8).
Physical characterization of rMAbs.
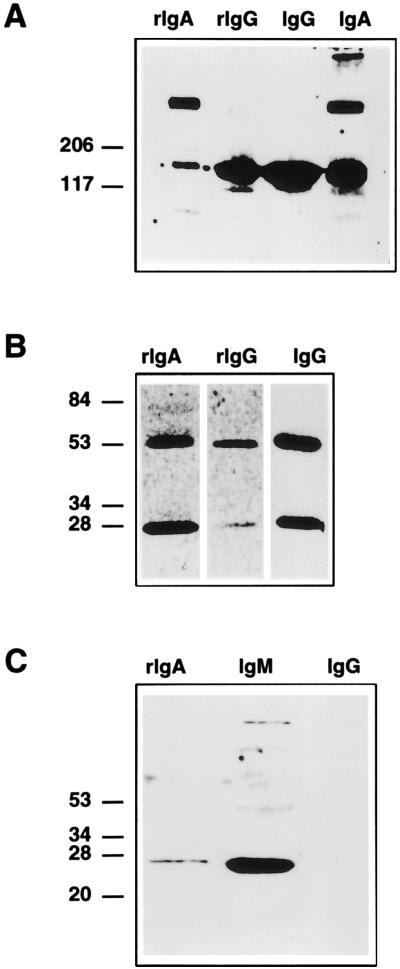
The physical structure of recombinant antibodies, rMH and rMS, secreted by stably transformed Sp2/0 cells was determined by Western blotting (Fig. 4). This analysis, performed under nonreducing conditions to study antibody oligomerization (Fig. 4A), demonstrated that recombinant antibodies with the IgG1 isotype were monomeric (molecular mass, 150 kDa) while rIgA consisted mainly of dimeric forms of about 300 kDa and a smaller amount of monomeric forms. Control IgA secreted by S2.1 porcine hybridoma cells appeared as a mixture of dimeric and monomeric molecules and a minor proportion of higher polymeric forms (Fig. 4A). After reduction of the interchain disulfide bonds (Fig. 4B), recombinant IgA and IgG1 dissociate into the H and L chains, with the expected molecular masses of about 60 and 25 kDa, respectively. Recombinant IgA dimers did not dissociate on treatment with 0.1% SDS and boiling, suggesting that rIgA molecules could be associated through covalent interactions. Nevertheless, the association through noncovalent interactions of a large population of rIgA molecules, in the absence of the Ig J chain, cannot be excluded, since it has been reported previously that IgA dimers associated through noncovalent interactions (36). Western blot analysis under reducing conditions revealed the presence of the Ig J chain in rIgA dimers (Fig. 4C), indicating that this J chain is participating in dimer formation by forming disulfide links to α chains. Although the J chain has a molecular mass of 15 kDa, the denatured form migrates with an apparent molecular mass of 26 kDa, in agreement with reported data (56). The minor bands observed in the IgM lane (Fig. 4C) probably correspond to the J chain associated with polymeric forms of IgM. The murine myeloma cell line Sp2/0 synthesizes J chain and thus is able to assemble and secrete a dimeric rIgA (27).
FIG. 4.
Physical characterization of rMAbs. (A and B) Western blot analysis on 5 to 20% linear gradient SDS-PAGE gels under nonreducing (A) and reducing (B) conditions, developed with rabbit anti-swine IgA and rabbit anti-mouse antisera. (C) Western blot analysis performed under reducing conditions developed with rabbit anti-J-chain antiserum. rIgA, recombinant mouse-swine IgA produced by transformed Sp2/0 cells. rIgG, recombinant mouse-human IgG. IgG, MAb 6A.C3 secreted by the original hybridoma cells. IgM, control polymeric IgM. The positions of the molecular mass markers expressed in kilodaltons are indicated on the left.
Functional analysis of recombinant MAbs with α and γ1 isotypes.
To verify the functionality of rMAb 6A.C3 with IgA or IgG1 isotypes, COS-1 cells were transiently transfected with plasmids encoding the chimeric H and L chains. The secreted chimeric Ig bound TGEV, had RIA titers (i.e., the highest dilution giving a threefold increase above background) up to 103, and neutralized virus infectivity around 104-fold (i.e., neutralization index = 4) (Table 1).
TABLE 1.
Functional characterization of recombinant antibodies
| Antibodya | Titer determined by:
|
|
|---|---|---|
| RIAb | Neutralizationc | |
| MAb-6A.C3 | 104 | >104 |
| rIgG-Sp2/0 cells | 102–103 | 102–103 |
| rIgA-Sp2/0 cells | 102–103 | 104 |
| rIgG-COS cells | 102–103 | 104 |
| rIgA-COS cells | 103–104 | 103 |
| rα | 5 | <0.3 |
Chimeric rIgG1 and rIgA expressed in COS-1 monkey kidney cells and in Sp2/0 myeloma cells were analyzed by RIA and virus neutralization assays. MAb-6A.C3, antibody secreted by the original hybridoma. rIgG-Sp2/0 and rIgA-Sp2/0, recombinant MH and MS antibodies, respectively, secreted by transformed Sp2/0 myeloma cells. rIgG-COS and rIgA-COS, recombinant MH and MS antibodies, respectively, secreted by transformed COS-1 cells. rα, recombinant mouse-porcine α chain.
Similar results were obtained in more than five independent evaluations of the antibodies secreted by the selected cell lines.
The neutralization index is given. Similar results were obtained in the evaluation of more than five independent cultures of transiently transformed COS-1 cells.
Murine Sp2/0 myeloma cells, which did not secrete endogenous Igs, were stably transformed by electroporation with constructs encoding the chimeric H and L chains. Cell transformation frequencies with two genes encoding the H and L Ig chains ranged between 10−3 and 10−4 in different experiments. Binding of the rMAbs to TGEV was determined by RIA with supernatants from clones secreting the highest antibody levels. Titers obtained by RIA ranged between 102 and 103 and were similar to those obtained by transient transfection (Table 1). Sp2/0 myeloma cells that were transformed with the recombinant mouse-porcine α-chain gene produced the corresponding H-chain protein but did not secrete this chain into the medium. Extracts from these cells showed a weak binding to TGEV (Table 1).
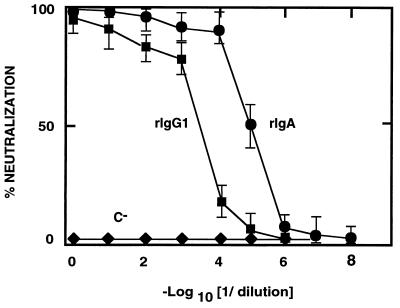
The final aim of this work is to protect newborn piglets against viral enteric infections through lactogenic immunity. IgA-isotype antibodies are known to be more stable in mucosal tissues than are those with an IgG isotype (28). To compare the neutralizing activities of rIgA and rIgG1, supernatants containing recombinant antibodies with the same RIA titer were used in neutralization assays. Recombinant IgA neutralized TGEV 50-fold more efficiently than did rIgG1 when antibody dilutions with the same titer by RIA were compared, as expected for a dimeric Ig with respect to a monomeric one (Fig. 5).
FIG. 5.
Comparison of the specific activity of the recombinant antibodies in TGEV neutralization. The neutralization of TGEV by chimeric rIgG1 and rIgA antibodies with the same RIA titer is shown. C−, supernatant from myeloma Sp2/0 cells. •, rIgA; ▪, rIgG1; ⧫, negative control MAb. The mean and the standard deviation of three experiments is shown.
Generation of transgenic mice.
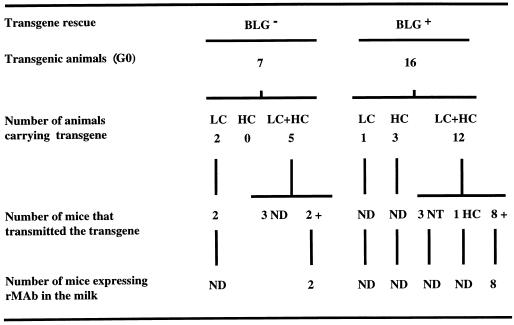
Analysis of DNA prepared from tail biopsy specimens showed that 23 of the 93 generation zero (G0) mice (around 25%) had integrated at least one of the transgenes (Fig. 6).
FIG. 6.
Transmission and expression of transgenes in BLG− and BLG+ transgenic mice. Transgene integration was screened in tail DNA samples from G0 and G1 mice by PCR with primers detecting the constant L (LC), constant H (HC), and BLG transgenes. PCR-positive samples were further analyzed by Southern blotting with BLG-specific probes. In several lines, analysis was extended to the G2 and G3 generation of mice. Transgene expression in milk was determined by RIA with G1 females. ND, not determined; NT, no Ig chain transmission; HC, mice which transmitted only the constant H chain.
Transgene integration in the genome of a modified animal does not guarantee its expression, since it may be integrated in a silent chromosome region. It has been previously reported (11, 12) that it is possible to enhance the efficiency of transgene expression by cointegrating the expression cassette with a genomic clone of BLG. This enhancement in transgene expression (transgene rescue) (12) may be due to the recruitment of transcription factors into the domain of the chromatin where the transgene is cointegrated. To help antibody expression, transgenic mice were produced by coinjection of the BLG gene with BLG-SLC plus BLG-SHC expression cassettes (BLG+ mice). In all (16 of 16) of the BLG+ transgenic mice (Fig. 6) in which one or both Ig genes were integrated, the BLG gene was also integrated (data not shown). The integration of expression cassettes and of genomic BLG was determined by PCR. After screening more than 250 progeny mice, derived from the 16 founder mice, the cosegregation of Ig and BLG genes was observed in a high proportion of transgenic animals (>68%), indicating that in general both Ig and BLG genes had been integrated in the same chromosomal locus. The majority (17 of 23) of the BLG− and BLG+ transgenic mice (Fig. 6) had cointegrated the transgenes encoding the L and H rIgA chains. Twelve of the BLG+ transgenic mice carrying both H and L chains had integrated the BLG gene in approximately a 2:1 ratio in relationship to the rIgA genes (data not shown). A small proportion of transgenic lines (around 25%) had integrated only one of the transgenes. The frequency of integration of only one of the Ig genes was not significantly modified by the comicroinjection of the BLG gene.
At least 10 of 17 transgenic founders carrying both SLC and SHC transmitted both transgenes to their progeny, suggesting that the genes have been cointegrated in a single site in each line. One line of transgenic mice (I70) did inherit the transgenes at a frequency significantly below 50%, which may indicate mosaicism. The comicroinjection of BLG and Ig genes did not affect transgene integration (data not shown).
Expression of rIgA in milk.
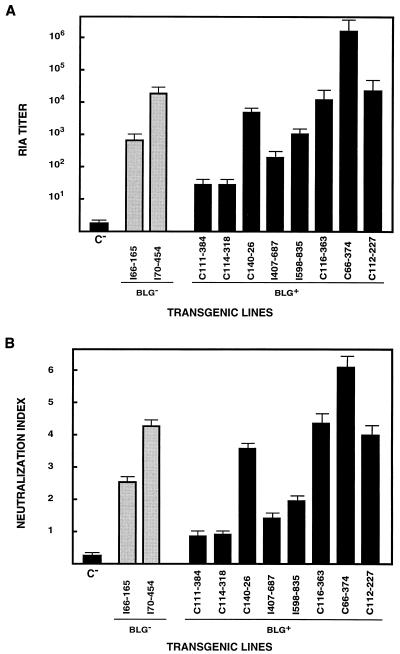
Milk was collected from G0 females or female progeny of mice which transmitted the transgenes for both the H and L genes. rIgA was detected by RIA in the milk of animals of the two BLG− transgenic lines, with titers ranging from 8 × 102 to 3 × 104 (Fig. 7A). Of 12 BLG+ transgenic founders, 8 expressed rIgA in milk (Fig. 7A), with RIA titers ranging from 5 × 101 to 5 × 106, indicating that the expression level could be a function of the integration site.
FIG. 7.
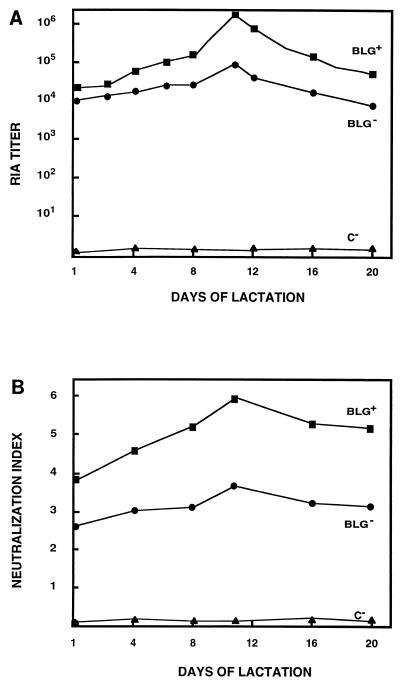
Recombinant IgA expression in the milk of transgenic mice. (A) The RIA titers for rIgA in the milk of BLG− and BLG+ transgenic mice are shown as the mean of the antibody titers in three to six milk samples from the same transgenic mouse, collected at different days through lactation. C−, milk from nontransgenic mice. (B) Neutralization index of rIgA present in the milk of BLG− and BLG+ transgenic lines. The neutralization assays were performed with same milk samples used for the experiment in panel A.
Neutralization assays with milk samples (Fig. 7B) showed that virus infectivity was reduced around 106-fold with the milk with the highest titers (mouse C66-374). These results indicated that the rIgA synthesized in the mammary gland and secreted into the milk of transgenic mice was biologically active in TGEV neutralization.
A significant proportion of the rIgA found in the milk was oligomeric, as determined by Western blot analysis with porcine IgA-specific antisera (results not shown).
No significant differences in transgene expression frequency were observed between BLG− and BLG+ transgenic mice (Fig. 6), nor did the cointegration of BLG lead to a significant increase in the antibody expression levels. Nevertheless, the higher antibody titers (>106) were obtained in mice that had cointegrated Ig and BLG genes (Fig. 7). No detectable levels of rIgA in the serum of transgenic lines were observed, including the transgenic females that were actively secreting the recombinant antibody to the milk with titers higher than 106 (data not shown).
The kinetics of antibody secretion into milk was determined during lactation (Fig. 8). rIgA levels in the milk of transgenic mice producing the highest antibody levels (Fig. 8) were significant from the first day of lactation, by both RIA and the TGEV neutralization assay, compared with antibody levels in wild-type mice (C−). Maximum antibody titers (around 106) were reached at midlactation (around day 10). The higher TGEV neutralizing-antibody titer was around 106 and was also achieved by day 10 of lactation.
FIG. 8.
rIgA expression in the milk from transgenic mice during lactation. (A) The titers of rIgA in the milk of transgenic mice I70-454 and C66-374, generated by microinjecting the Ig chains alone (BLG−) or with the BLG gene (BLG+), during a lactation cycle are represented. These transgenic mice were chosen because their milk had the highest RIA titers. C−, milk from a nontransgenic mouse. (B) Neutralization index for rIgA in the milk of I70-454 (BLG−) and C66-374 (BLG+) transgenic mice during lactation.
The rIgA concentrations in transgenic-mouse milk were measured by RIA with a standard based on purified rIgA; they ranged from 0.01 to 6 mg/ml. No significant differences in the antibody expression pattern in the presence or absence of BLG comicroinjection were observed during lactation (Fig. 8).
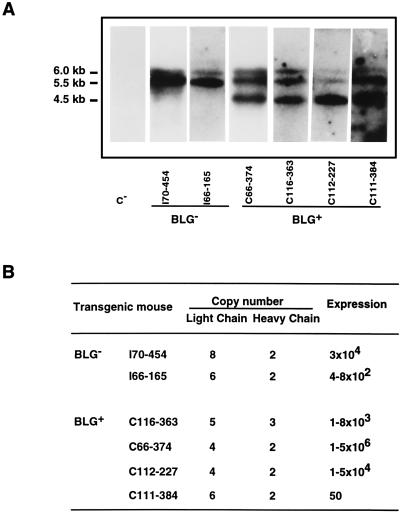
The transgene copy number was determined by Southern blot analysis (Fig. 9). Cleavage of integrated copies with EcoRI released internal fragments of 6, 5.5, and 4.5 kb for SHC, SLC, and BLG transgenes, respectively. These three bands were revealed with a probe specific for the common BLG promoter, and thus all three genes were detected simultaneously, allowing a comparison of the transgene copy number. The results were quantified by comparing band intensities for DNA from tail biopsy specimens with those of known amounts of plasmids pSS1tgXS, BLG-SHC, and BLG-SLC. A discrete variation in the transgene copy number (from 2 to 8) was detected in the transgenic lines analyzed (Fig. 9B), and no correlation was observed between rIgA expression levels and transgene copy number (Fig. 9).
FIG. 9.
Relationship between copy number and rIgA expression in the milk. (A) Southern blot analysis of BLG− and BLG+ transgenic lines. C−, nontransgenic mouse DNA. Tail DNA samples were cleaved with EcoRI and analyzed by electrophoresis and Southern blotting. Filters were probed with 5′-flanking sequences common to the three transgenes. The 6.0-kb BLG-SHC, 5.5-kb BLG-SLC, and 4.5-kb BLG− transgene-specific EcoRI fragments are shown. (B) Comparison of rIgA titers in BLG− and BLG+ transgenic lines carrying different transgene copy numbers. The expression levels are reported as the RIA titers. The RIA titers of rIgA in the milk of BLG− and BLG+ transgenic mice were calculated as the mean of the antibody titers in three to six milk samples from the same mouse.
The genetic stability of the transgenes was studied for three successive generations by evaluating the antibody production in more than 250 transgenic mice. Transgenes segregated as expected for a single-locus Mendelian character (data not shown). The rIgA expression levels in milk were constant through the three generations and were very similar in mice from the same transgenic line (data not shown). No abnormalities were detected during the development of the antibody producer mice. The parameters measured to assess the effect of transgene expression were body weight, progeny number in breedings through three generations, volume of milk collected, offspring survival ratio, anatomical observation, and general behavior (results not shown).
The expression of transgenic IgA in the milk did not significantly affect the levels of the endogenous mouse Igs, as determined by Western blot analysis (results not shown).
DISCUSSION
A recombinant TGEV neutralizing MAb with porcine IgA isotype has been engineered. Transgenic mice were constructed that secreted the rIgA MAb into their milk with titers up to 5 × 106, as determined by RIA, and neutralizing 106-fold virus infectivity, which should be sufficient to protect against enteric infections in animals susceptible to TGEV.
Chimeric IgA antibodies are more efficient in virus neutralization than the recombinant antibodies with identical specificity and IgG isotype, probably because of the tetravalency of dimeric rIgA (16).
The expression levels of functional TGEV-specific rIgA in the milk of several transgenic mice (up to 6 mg/ml) are among the highest expression levels of a complex recombinant protein in any mammalian expression system, including transgenic mice (22).
The rIgA expression levels reported in this paper are of the same order as those found in transgenic mice secreting recombinant IgG1 in the mammary gland of transgenic mice (9), and they clearly fall above the levels of IgA produced in the milk of nontransgenic mice, which are below 1 mg/ml (38).
rIgA expression levels in the supernatant of stably transformed Sp2/0 cells ranged between 20 and 50 μg/ml; these levels were comparable to the antibody levels produced in other cell systems (50). rIgA levels obtained in several transgenic animals are approximately 250-fold higher than in mammalian cell expression systems. This result was particularly interesting since it indicated that the epithelial cells of the mammary gland successfully produced both the H and L Ig chains and provided the adequate environment for the assembly of a functional IgA molecule, which implies the formation of a complex with four protein chains. The dimerization of rIgA molecules by noncovalent interactions in the mammary gland cells, lacking J chain, is anticipated, since previous studies (36) have shown that monomeric IgA can aggregate to form stable IgA dimers, consisting of a complex with eight protein chains, in the absence of J chain. The rIgA produced in the milk of transgenic animals specifically bound and neutralized TGEV, indicating that the mouse mammary gland tissue performs the adequate posttranslational processing required for the correct assembly of antibody molecules.
No detectable levels of rIgA in the serum of transgenic lines were observed, including that of the transgenic females actively secreting the highest antibody titers into milk. In contrast, in transgenic mice secreting into the milk rIgG with identical V modules and titers comparable to those obtained for rIgA, lower but proportional levels of rIgG were detected in the serum (9). The absence of rIgA in the serum could be explained by the fact that IgAs, and not IgGs, are recognized by polymeric Ig receptors and are transported into secretions by epithelial cells via the receptor-mediated transepithelial transport system (37). This mechanism would prevent the rIgA from reaching the systemic circulation, leading to predominant secretion into the milk and mucosal surfaces.
rIgA expression levels in the milk of sows, similar to those produced by the transgenic mice described in this paper, may be high enough to protect piglets against TGEV infection. rIgA contains the V modules of MAb 6A.C3, which very efficiently neutralizes all known TGEV strains and does not lead to the selection of escape mutants, indicating that it binds to an essential viral epitope. This fact and the continuous intake of virus neutralizing recombinant antibodies from the milk of transgenic sows during lactation should provide in vivo protection against TGEV infection (52). High levels of rIgA were detected in the milk of transgenic animals from day 1 of lactation. Furthermore, antibody levels were maintained during the lactation period, with a maximum reached at midlactation. If the same expression pattern is maintained in swine, an effective protection of newborn piglets against TGEV will probably be achieved.
The comicroinjection of BLG sequences with antibody genes has not led to a significant increase in the average antibody expression levels, since in the absence or in the presence of the BLG gene, approximately the same average antibody titers were obtained in the milk of transgenic mice. Nevertheless, it is interesting that maximum antibody expression levels were obtained when BLG and antibody sequences were comicroinjected, although, since a small number of transgenic mice (two without BLG and nine with BLG) were used, the significance of BLG cointegration to the attainment of high antibody expression levels cannot be definitively concluded.
The requirement for introns to achieve an efficient transgene expression, probably due to the presence of cis-acting elements, is well documented (6, 10). However, in our system, cDNAs encoding rIgA H and L chains were inserted into BLG intronless constructs and an efficient expression of rIgA was observed in the milk of the transgenic animals, suggesting that sequences within these cDNAs can also favor expression (53). One possibility is that some sequences present in the V module of MAb 6A.C3 L and H chains enhance the expression, since this MAb has been selected from 2,000 MAb because of its specificity and high expression level (23). In this context, transgene rescue by comicroinjecting the BLG gene with the transgenes (11, 12) may not enhance the efficiency of expression as dramatically as in those cases with a very inefficient expression of the intronless transgenes.
No direct relationship between the transgene copy number and the amount of rIgA protein secreted into the milk was observed, suggesting that the site of integration of the transgene has a greater effect on the transcriptional activity than does gene copy number. Similar results have been obtained by our laboratory in the expression of this rMAb with the IgG1 isotype under the control of the whey acid protein promoter (9) and in the expression of other transgenes (53, 54). As when BLG regulatory sequences were used, antibody expression under whey acid protein control was transgene copy number independent and was maintained throughout the lactation period.
The change in the composition of the milk of transgenic mice was accompanied by no apparent deleterious side effects, either to the lactating transgenic females or to the pups suckling their milk. This was expected, since the synthesis of the rIgA is induced during lactation in the mammary gland and ceases at the end of the lactation period.
Normal development in the mice secreting high-titer coronavirus neutralizing antibodies in the milk was observed, indicating that the production of pathogen-neutralizing antibodies in the milk could be a useful approach to the prevention of enteric infections of the newborn.
Ig expression in transgenic animals has been previously reported. The genes encoding the H and L chains were expressed in lymphoid cells (51). However, the expression was not temporally regulated and association of the endogenous and the Ig chains was observed. Production of chimeric antibodies in other tissues that do not synthesize Ig naturally, such as the mammary gland of transgenic mice, has been reported previously (32), although the antibody expressed by these transgenic animals did not have protective activity against infectious agents and the antibody levels achieved were considerably lower than the ones reported in this paper.
The modular approach to obtain recombinant antibodies (i.e., the fusion of V and C Ig domains) described in this paper could easily be applied to other antibodies with different therapeutic purposes. The secretion of neutralizing MAbs in the milk of transgenic animals could be applied to improve disease resistance in livestock and to prevent neonatal infections by a number of enteric pathogens for which specific MAbs are available.
The cis-acting sequences determining the mammary expression of the BLG gene seem to be correctly interpreted in mice, despite both the absence of an equivalent gene and the species differences in regulation. An equivalent gene does exist in pigs, and the results in the murine system may be taken as an indication that the expression of rIgA under the control of BLG sequences should also work in pigs, the natural host for TGEV.
Transgenic swine expressing TGEV neutralizing rIgA are currently being made by using the same expression cassettes described in this paper. This new system will allow us to directly test whether the lactogenic immunity provided by the transgenic sows to neonates following challenge with TGEV elicits protection. Since MAbs specific for many viruses infecting the enteric tract are available and since the recombinant antibodies have been obtained by a modular approach, this strategy could be the basis of a general procedure to generate animals resistant to viral infections of the enteric tract.
ACKNOWLEDGMENTS
We thank Victor Buckwold for critical reading of the manuscript.
This work has been supported by grants from the Consejo Superior de Investigaciones Científicas, the Comisión Interministerial de Ciencia y Tecnología (CICYT), The Instituto Nacional de Investigación y Tecnología Agraria y Alimentación project SC-GAN94-119, La Consejería de Educación y Cultura de la Comunidad de Madrid, and Laboratorios Fort Dodge from Spain and the European Communities (Projects Science and Biotech). I.S., J.C., and J.M.S.-M. received fellowships from the Consejo Superior de Investigaciones Científicas, the Department of Education, University and Research of the Gobierno Vasco, and the Colegio Oficial de Veterinarios de la Comunidad de Madrid (Spain), respectively. C.B.A.W. and A.J.C. are supported by the BBSRC.
REFERENCES
- 1.Ali S, Clark A J. Characterization of the gene encoding ovine β-lactoglobulin. J Mol Biol. 1988;199:415–426. doi: 10.1016/0022-2836(88)90614-6. [DOI] [PubMed] [Google Scholar]
- 2.Ali S, McClenaghan M, Simons J P, Clark A J. Characterisation of the alleles encoding ovine β-lactoglobulins A and B. Gene. 1990;91:201–207. doi: 10.1016/0378-1119(90)90089-a. [DOI] [PubMed] [Google Scholar]
- 3.Antón I M, Suñé C, Meloen R H, Borrás-Cuesta F, Enjuanes L. A transmissible gastroenteritis coronavirus nucleoprotein epitope elicits T helper cells that collaborate in the in vitro antibody synthesis to the three major structural viral proteins. Virology. 1995;212:746–751. doi: 10.1006/viro.1995.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong S J, Outlaw M C, Dimmock N J. Morphological studies of neutralization of influenza virus by IgM. J Gen Virol. 1990;71:2313–2319. doi: 10.1099/0022-1317-71-10-2313. [DOI] [PubMed] [Google Scholar]
- 5.Brim T A, VanCott J L, Lunney J K, Saif L J. Lymphocyte proliferation responses of pigs inoculated with transmissible gastroenteritis virus or porcine respiratory coronavirus. Am J Vet Res. 1994;55:494–501. [PubMed] [Google Scholar]
- 6.Brinster R L, Allen J M, Behringer R R, Gelinas R E, Palmiter R D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown W R, Butler J E. Characterization of the single C-alpha gene of swine. Mol Immunol. 1994;31:633–642. doi: 10.1016/0161-5890(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 8.Castilla J, Sola I, Enjuanes L. Interference of coronavirus infection by expression of immunoglobulin G (IgG) or IgA virus-neutralizing antibodies. J Virol. 1997;71:5251–5258. doi: 10.1128/jvi.71.7.5251-5258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castilla, J., I. Sola, B. Pintado, J. M. Sánchez-Morgado, and L. Enjuanes. Production of coronavirus neutralizing IgG antibodies in transgenic mouse milk under whey acidic protein promoter. Nat. Biotechnol., in press.
- 10.Choi T, Huang M, Gorman C, Jaenisch R. A generic intron increases gene expression in transgenic mice. Mol Cell Biol. 1991;11:3070–3074. doi: 10.1128/mcb.11.6.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark A J, Archibald A L, McClenaghan M, Simons J P, Wallace R, Whitelaw C B A. Enhancing the efficiency of transgene expression. Transgenic Res Soc London. 1993;339:225–232. doi: 10.1098/rstb.1993.0020. [DOI] [PubMed] [Google Scholar]
- 12.Clark A J, Cowper A, Wallace R, Wright G, Simons J P. Rescuing transgene expression by co-integration. Bio/Technology. 1992;10:1450–1454. doi: 10.1038/nbt1192-1450. [DOI] [PubMed] [Google Scholar]
- 13.Correa I, Gebauer F, Bullido M J, Suñé C, Baay M F D, Zwaagstra K A, Posthumus W P A, Lenstra J A, Enjuanes L. Localization of antigenic sites of the E2 glycoprotein of transmissible gastroenteritis coronavirus. J Gen Virol. 1990;71:271–279. doi: 10.1099/0022-1317-71-2-271. [DOI] [PubMed] [Google Scholar]
- 14.Correa I, Jiménez G, Suñé C, Bullido M J, Enjuanes L. Antigenic structure of the E2 glycoprotein from transmissible gastroenteritis coronavirus. Virus Res. 1988;10:77–94. doi: 10.1016/0168-1702(88)90059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmas B, Laude H. Assembly of coronavirus spike protein into trimers and its role in epitope expression. J Virol. 1990;64:5367–5375. doi: 10.1128/jvi.64.11.5367-5375.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimmock N J. Neutralization of animal viruses. Curr Top Microbiol Immunol. 1993;183:152. doi: 10.1007/978-3-642-77849-0. [DOI] [PubMed] [Google Scholar]
- 17.Enjuanes L, Van der Zeijst B A M. Molecular basis of transmissible gastroenteritis coronavirus epidemiology. In: Siddell S G, editor. The Coronaviridae. New York, N.Y: Plenum Press; 1995. pp. 337–376. [Google Scholar]
- 18.Fichot O, Girard M. An improved method for sequencing of RNA templates. Nucleic Acids Res. 1990;18:6162. doi: 10.1093/nar/18.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fink P S. Using sodium chloride step gradients to fractionate DNA fragments. BioTechniques. 1991;10:447–449. [PubMed] [Google Scholar]
- 20.Gebauer F, Posthumus W A P, Correa I, Suñé C, Sánchez C M, Smerdou C, Lenstra J A, Meloen R, Enjuanes L. Residues involved in the formation of the antigenic sites of the S protein of transmissible gastroenteritis coronavirus. Virology. 1991;183:225–238. doi: 10.1016/0042-6822(91)90135-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris S, McClenaghan M, Simons J P, Ali S, Clark A J. Developmental regulation of the sheep β-lactoglobulin gene in the mammary gland of transgenic mice. Dev Genet. 1991;12:299–307. doi: 10.1002/dvg.1020120407. [DOI] [PubMed] [Google Scholar]
- 22.Hennighausen L, Ruiz L, Wall R. Transgenic animals—production of foreign proteins in milk. Curr Opin Biotechnol. 1990;1:74–78. doi: 10.1016/0958-1669(90)90013-b. [DOI] [PubMed] [Google Scholar]
- 23.Jiménez G, Correa I, Melgosa M P, Bullido M J, Enjuanes L. Critical epitopes in transmissible gastroenteritis virus neutralization. J Virol. 1986;60:131–139. doi: 10.1128/jvi.60.1.131-139.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaeffer B, Bottreau E, Marcon D, Olivier M, Lantier I, Salmon H. Histocompatible miniature pig (d/d haplotype)—generation of hybridomas secreting A or M monoclonal antibody. Hybridoma. 1991;10:731–744. doi: 10.1089/hyb.1991.10.731. [DOI] [PubMed] [Google Scholar]
- 25.Kaji H, Parkhouse R M E. Intracellular J chain in mouse plasmacytomas secreting IgA, IgM and IgG. Nature. 1974;249:45–47. doi: 10.1038/249045a0. [DOI] [PubMed] [Google Scholar]
- 26.Kilian M, Mestecky J, Russell M W. Defence mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koshland M E. The coming of age of the immunoglobulin J chain. Annu Rev Immunol. 1985;3:425–453. doi: 10.1146/annurev.iy.03.040185.002233. [DOI] [PubMed] [Google Scholar]
- 28.Lamm M E, Nedrud J G, Kaetzel C S, Mazanec M B. IgA and mucosal defense. APMIS. 1995;103:241–246. doi: 10.1111/j.1699-0463.1995.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 29.Lammers B M, Beaman K D, Kim Y B. Sequence analysis of porcine immunoglobulin light chain cDNAs. Mol Immunol. 1991;28:877–880. doi: 10.1016/0161-5890(91)90051-k. [DOI] [PubMed] [Google Scholar]
- 30.Lee K F, DeMayo J, Atice S H, Rosen J M. Tissue-specific expression of the rat β-casein gene in transgenic mice. Nucleic Acids Res. 1988;16:1027–1041. doi: 10.1093/nar/16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S H, De Boer H A. Production of biomedical proteins in the milk of transgenic dairy cows: the state of the art. J Controlled Release. 1994;29:213–221. [Google Scholar]
- 32.Limonta J, Pedraza A, Rodríguez A, Freyre F M, Barral A M, Castro F O, Lleonart R, Garcia C A, Gavilondo J V, de la Fuente J. Production of active anti-CD6 mouse-human chimeric antibodies in the milk of transgenic mice. Immunotechnology. 1995;1:107–113. doi: 10.1016/1380-2933(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 33.Liu A Y, Mack P W, Champion C I, Robinson R R. Expression of mouse:human immunoglobulin heavy chain cDNA in lymphoid cells. Gene. 1987;54:33–40. doi: 10.1016/0378-1119(87)90344-1. [DOI] [PubMed] [Google Scholar]
- 34.Mazanec M B, Kaetzel C S, Lamm M E, Fletcher D, Nedrud J G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc Natl Acad Sci USA. 1992;89:6901–6905. doi: 10.1073/pnas.89.15.6901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McClurkin A W, Norman J O. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can J Comp Vet Sci. 1966;30:190–198. [PMC free article] [PubMed] [Google Scholar]
- 36.Morton H C, Atkin J D, Owens R J, Woof J M. Purification and characterization of chimeric human IgA1 and IgA2 expressed in COS and Chinese hamster ovary cells. J Immunol. 1993;151:4743–4752. [PubMed] [Google Scholar]
- 37.Neutra M R, Kraehenbuhl J-P. M cell-mediated antigen transport and monoclonal IgA antibodies for mucosal immune protection. In: Cianci J E, McGhee J R, Keith J M, editors. Genetically engineered vaccines. New York, N.Y: Plenum Press; 1992. pp. 143–150. [DOI] [PubMed] [Google Scholar]
- 38.Parr E L, Bozzola J J, Parr M B. Purification and measurement of secretory IgA in mouse milk. J Immunol Methods. 1995;180:147–157. doi: 10.1016/0022-1759(94)00310-s. [DOI] [PubMed] [Google Scholar]
- 39.Potter H, Weir L, Leder P. Enhancer-dependent expression of human k immunoglobulin genes introduced into mouse pre-B lymphocytes by electroporation. Proc Natl Acad Sci USA. 1984;81:7161–7165. doi: 10.1073/pnas.81.22.7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Randall T D, Parkhouse R M E, Corley R B. J chain synthesis and secretion of hexameric IgM is differentially regulated by lipopolysaccharide and interleukin 5. Proc Natl Acad Sci USA. 1992;89:962–966. doi: 10.1073/pnas.89.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saif L J, Wesley R D. Transmissible gastroenteritis. In: Leman A D, Straw B E, Mengeling W L, D’Allaire S, Taylor D J, editors. Diseases of swine. 7th ed. Ames, Iowa: Wolfe Publishing Ltd; 1992. pp. 362–386. [Google Scholar]
- 42.Sanchez C M, Gebauer F, Suñé C, Méndez A, Dopazo J, Enjuanes L. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology. 1992;190:92–105. doi: 10.1016/0042-6822(92)91195-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez C M, Jiménez G, Laviada M D, Correa I, Suñé C, Bullido M J, Gebauer F, Smerdou C, Callebaut P, Escribano J M, Enjuanes L. Antigenic homology among coronaviruses related to transmissible gastroenteritis virus. Virology. 1990;174:410–417. doi: 10.1016/0042-6822(90)90094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simons J P, McClenaghan M, Clark A J. Alteration of the quality of milk by expression of sheep β-lactoglobulin in transgenic mice. Nature. 1987;328:530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- 46.Staats H F, Jackson R J, Marinaro M, Takahashi I, Kiyono H, McGhee J R. Mucosal immunity to infection with implications for vaccine development. Curr Opin Immunol. 1994;6:572–583. doi: 10.1016/0952-7915(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 47.Suñé C, Jiménez G, Correa I, Bullido M J, Gebauer F, Smerdou C, Enjuanes L. Mechanisms of transmissible gastroenteritis coronavirus neutralization. Virology. 1990;177:559–569. doi: 10.1016/0042-6822(90)90521-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres J M, Sánchez C M, Suñé C, Smerdou C, Prevec L, Graham F, Enjuanes L. Induction of antibodies protecting against transmissible gastroenteritis coronavirus (TGEV) by recombinant adenovirus expressing TGEV spike protein. Virology. 1995;213:503–516. doi: 10.1006/viro.1995.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanCott J L, Brim T A, Simkins R A, Saif L J. Isotype-specific antibody-secreting cells to transmissible gastroenteritis virus and porcine respiratory coronavirus in gut- and bronchus-associated lymphoid tissues of suckling pigs. J Immunol. 1993;150:3990–4000. [PubMed] [Google Scholar]
- 50.Weidle U H, Koch S, Buckel P. Expression of antibody cDNA in murine myeloma cells: possible involvement of additional regulatory elements in transcription of immunoglobulin genes. Gene. 1987;60:205–216. doi: 10.1016/0378-1119(87)90229-0. [DOI] [PubMed] [Google Scholar]
- 51.Weidle U H, Lenz H, Brem G. Genes encoding a mouse monoclonal antibody are expressed in transgenic mice, rabbits and pigs. Gene. 1991;98:185–191. doi: 10.1016/0378-1119(91)90172-8. [DOI] [PubMed] [Google Scholar]
- 52.Wesley R D, Woods R D, Correa I, Enjuanes L. Lack of protection in vivo with neutralizing monoclonal antibodies to transmissible gastroenteritis virus. Vet Microbiol. 1988;18:197–208. doi: 10.1016/0378-1135(88)90087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitelaw C B A, Archibald A L, Harris S, McClenaghan M, Simons L P, Clark A J. Targeting expression to the mammary gland: intronic sequences can enhance the efficiency of gene expression in transgenic mice. Transgenic Res. 1991;1:3–13. doi: 10.1007/BF02512991. [DOI] [PubMed] [Google Scholar]
- 54.Wright G, Carver A, Cottom D, Reeves D, Scott A, Simons P, Wilmut I, Garner I, Colman A. High level expression of active human alpha-1-antitrypsin in the milk of transgenic sheep. Bio/Technology. 1991;9:830–834. doi: 10.1038/nbt0991-830. [DOI] [PubMed] [Google Scholar]
- 55.Xoma Co. May 1987. U.S. Patent WO 87/02671.
- 56.Zikan J, Novotny J, Trapane T L, Koshland M E, Urry D W, Bennett J C, Mestecky J. Secondary structure of the immunoglobulin J chain. Proc Natl Acad Sci USA. 1985;82:5905–5909. doi: 10.1073/pnas.82.17.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]