Abstract
Background
The aim of the present study is to compare outcomes of the robotic hand-sewn, linear- and circular-stapled techniques performed to create an intrathoracic esophagogastric anastomosis in patients who underwent Ivor-Lewis esophagectomy.
Methods
Patients who underwent a planned Ivor-Lewis esophagectomy were retrospectively analysed from prospectively maintained databases. Only patients who underwent a robotic thoracic approach with the creation of an intrathoracic esophagogastric anastomosis were included in the study. Patients were divided into three groups: hand-sewn-, circular stapled-, and linear-stapled anastomosis group. Demographic information and surgery-related data were extracted. The primary outcome was the rate of anastomotic leakages (AL) in the three groups. Moreover, the rate of grade A, B and C anastomotic leakage were evaluated. In addition, patients of each group were divided in subgroups according to the characteristics of anastomotic fashioning technique.
Results
Two hundred and thirty patients were enrolled in the study. No significant differences were found between the three groups about AL rate (p = 0.137). Considering the management of the AL for each of the three groups, no significant differences were found. Evaluating the correlation between AL rate and the characteristics of anastomotic fashioning technique, no significant differences were found.
Conclusions
No standardized anastomotic fashioning technique has yet been generally accepted. This study could be considered a call to perform ad hoc high-quality studies involving high-volume centers for upper gastrointestinal surgery to evaluate what is the most advantageous anastomotic technique.
Keywords: Esophagogastric, Anastomosis, Robotic, Ivor-Lewis, Esophagectomy
Introduction
Esophageal cancer is the seventh most common cause of cancer morbidity and the sixth leading cause of cancer-related death [1–3]. The milestone for the treatment of esophageal cancer is radical esophagectomy with lymphadenectomy [4]. Totally minimally invasive (laparoscopy and thoracoscopy) Ivor–Lewis (TMIIL) has increased in popularity, providing the well-known advantages on recovery of the minimally invasive procedures. Furthermore, several experiences [5–8] have reported safety and efficacy of minimally invasive esophagectomy, showing similar oncologic results and long-term recurrence rate [9–11].
In the new era of minimally invasive surgery, a robotic approach could offer the chance to overcome technical difficulties of laparoscopic technique due to its three-dimensional view and its EndoWrist® technology. Although robotic surgery could be considered the gold standard only for the treatment of prostate cancer, it has gained popularity in many fields of surgical practice arousing the interest of several surgeons [12–18]. Regarding TMIIL, the creation of an intrathoracic esophagogastric anastomosis is one of the most critical phases and robotic approach seems to facilitate the surgeon especially in this step. On the other hand, no standardized technique has yet been generally accepted. Various approaches have been used and they are basically divided into hand-sewn, mechanical and semi-mechanical, the last two of which involve the use of a stapler, either linear or circular. Circular- (CS) and linear-stapled (LS) are the two most commonly used anastomotic techniques [19]. In LS, the hand-suturing of the anterior aspect of the anastomosis is the most technically difficult phase while in CS this role falls to the complexity of performing the purse-string suture fixing the anvil.
The aim of the present study is to compare outcomes of the robotic hand-sewn-, linear- and circular-stapled techniques and to help robotic surgeons in choosing the best intrathoracic esophagogastric anastomosis technique.
Materials and methods
Patients with middle and lower esophageal cancers or Siewert type 1 or 2 esophagogastric junction carcinoma who underwent a planned Ivor-Lewis esophagectomy at seven high-volume Italian centers for upper gastrointestinal surgery were retrospectively analysed from prospectively maintained databases. Only patients who underwent a robotic thoracic approach with the creation of an intrathoracic esophagogastric anastomosis were included in the study to compare outcomes of the robotic hand-sewn-, linear- and circular-stapled techniques.
A written informed consent was obtained from each patient enrolled in the analysis. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
All operations were performed by senior surgeons experienced in minimally invasive esophagectomy using different techniques to perform the intrathoracic esophagogastric anastomosis (hand-sewn-, circular- or linear-stapled). To minimize the bias related to the presence of different surgeons, only procedures performed by experts of high-volume Italian centers for upper gastrointestinal surgery were considered. In addition, only robotic interventions were included to specifically evaluate the best anastomotic technique in the robotic setting.
Demographic information and surgery-related data were extracted. Demographic information included sex, age, BMI, ASA score and comorbidities (hypertension, diabetes, congestive heart failure and chronic obstructive pulmonary disease COPD). Surgery-related data involved AL rate.
Outcomes
Patients were divided in three groups according to the anastomotic technique used (hand-sewn-, circular- and linear-stapled).
The primary outcome was the rate of AL in the three groups. The diagnosis of AL was based on CT scan and/or upper GI endoscopy which were performed in case of leak suspicion, based on clinical symptoms, routine laboratory tests and/or chest X-ray. Moreover, the rate of grades A, B and C anastomotic leakage in the three groups were evaluated. AL was defined as grade A when it was treated conservatively with no change in patients’ management, it was defined as grade B when it was treated with invasive intervention such as endoscopic or radiological intervention other than repeat surgical intervention and it was defined as grade C when it was treated with surgical intervention.
In addition, patients of each group were divided in subgroups according to the characteristics of anastomotic fashioning technique. In details, patients of the hand-sewn anastomosis group were divided in two subgroups according to the type of suture thread used (barbed, non-braided); patients of the circular-stapled anastomosis group were divided in four subgroups according to the circular-stapled technique (manual, purse-string device) and to the stapler diameter (25 mm, 29 mm); patients of the linear-stapled anastomosis group were divided in nine subgroups according to the stapler type (mechanical, electric), to the stapler length (45 mm, 60 mm), to the layer of defect closing (single, double) and to the type of suture thread used for defect closing (barbed, braided, non-braided).
During postoperative course, patients were evaluated with clinical monitoring and daily blood tests. After the discharge, the patients were submitted to a check after 7, 30, 60 and 90 days.
Surgical technique
All procedures included in the study were performed according to the principles of a radical esophagectomy with the lymphadenectomy [4].
Only patients who underwent a robotic thoracic approach with the creation of an intrathoracic esophagogastric anastomosis were included in the study.
Anastomotic technique
No standardized technique has yet been generally accepted to perform the esophagogastric anastomosis. The approaches used involve hand-sewn-, circular- (CS) and linear-stapled (LS).
The esophagogastric anastomosis is performed above the level of the azygos arch.
In detail, during the robot-sewn esophagogastric anastomotic fashioning technique, once the posterior aspect of the anastomosis is complete, the nasogastric tube is placed under direct vision inside the stomach distally to the anastomosis to accomplish the closure of anterior surface. Particularly, a double-layer closure using a running barbed suture in the first layer for posterior surface and a single layer using a running barbed suture for anterior surface of the anastomosis are performed (Fig. 1).
Fig. 1.
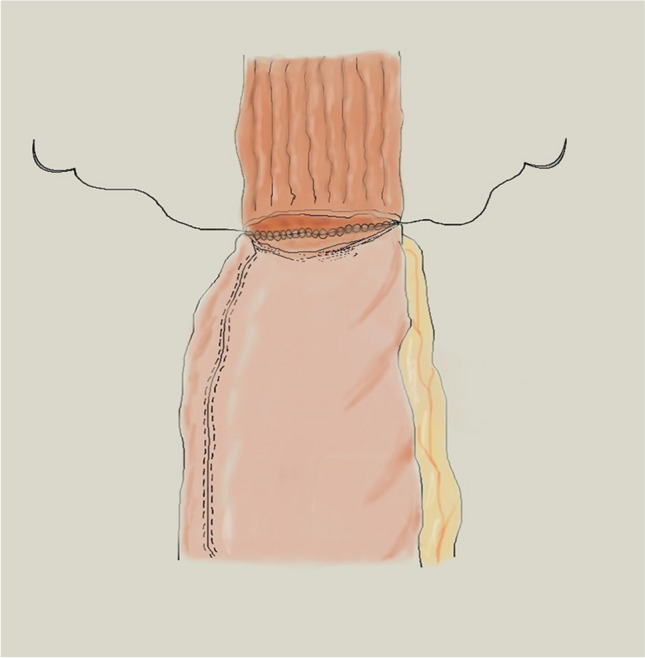
Robotic hand-sewn anastomotic technique
The esophagogastric side-to-side anastomosis is performed using a linear stapler and the enterotomies are closed by hand-sewn sutures, after passing the nasogastric tube in the conduit under direct vision (Fig. 2).
Fig. 2.
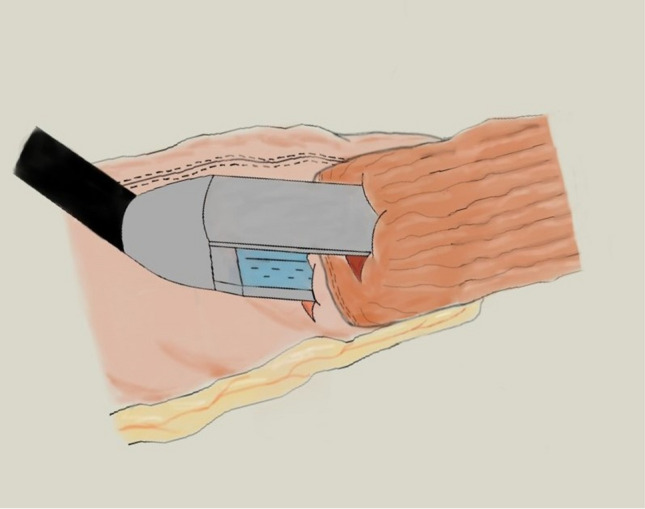
Linear-stapling technique
The esophagogastric end-to-side anastomosis is performed using a circular stapler. In details after esophagus transection, the anvil is secured and the circular stapler is introduced into the gastric tube with the tip of the stapler emerging at the mesenteric side of the gastric conduit, after which the two stapler parts are aligned and the stapler is fred. A linear stapler is then used to close the open end of the gastric tube, and the anastomosis is oversewn with two sutures (Fig. 3).
Fig. 3.
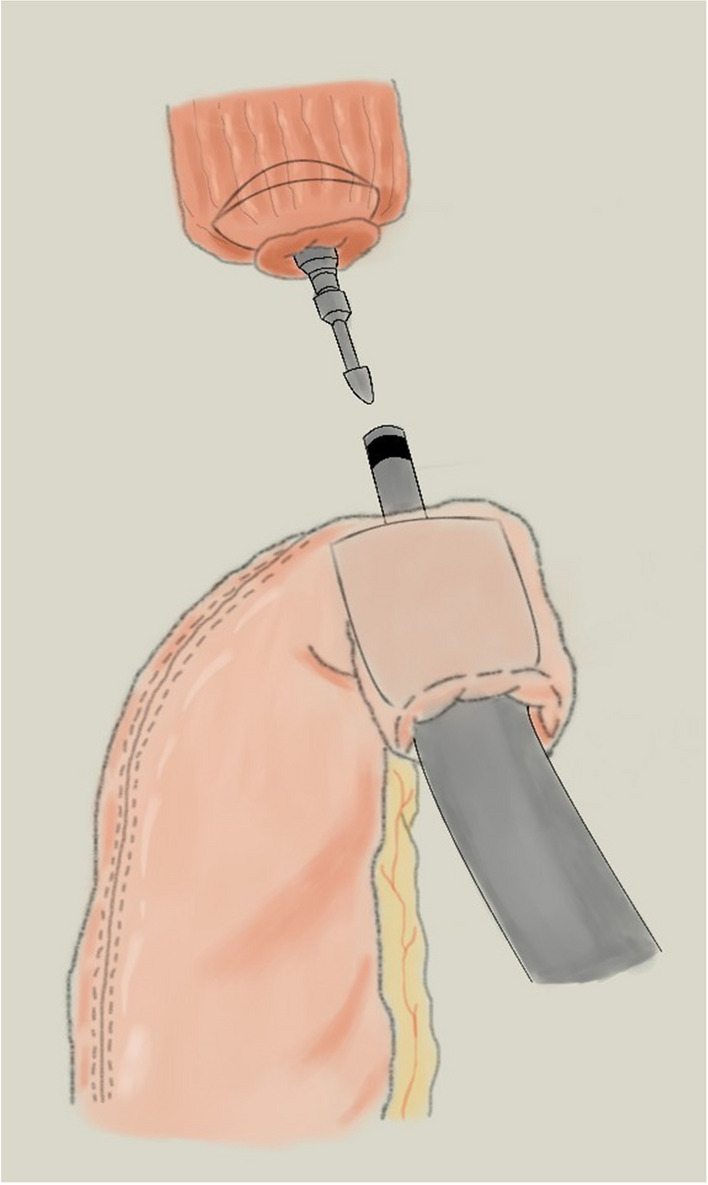
Circular-stapling technique
A leak test is performed with methylene blue.
Statistical analysis
Statistical analysis was performed using the SPSS 28 system (SPSS Inc., Chicago, IL, USA). Continuous data were expressed as mean ± standard deviation, and categorical variables were expressed as percentages. The ANOVA test was performed to compare continuous variables. The chi-square test was employed to analyse categorical data.
Results
Two hundred and ninety-three patients with middle and lower esophageal cancer or Siewert type 1 or 2 esophagogastric junction carcinoma, who underwent a planned Ivor-Lewis esophagectomy at seven high-volume Italian centers for upper gastrointestinal surgery, were eligible for study inclusion but 63 were excluded because two patients underwent an open technique and 61 did not receive an intrathoracic robotic esophagogastric anastomosis. Thus, 230 patients were enrolled in the analysis.
In details, patients were divided into three groups based on the technique used to perform the esophagogastric anastomosis; thus, the hand-sewn anastomosis group involved 64 patients (27.8%), the circular-stapled anastomosis group involved 71 patients (30.9%) and the linear-stapled anastomosis group involved 95 patients (41.3%).
Demographic and tumor characteristics of the three compared groups are showed in Table 1.
Table 1.
Demographic characteristics and tumor characteristics of the three compared groups
| Hand-sewn (n = 64; 27.8%) | Circular-stapler (n = 71; 30.9%) | Liner-stapler (n = 95; 41.3%) | P | |
|---|---|---|---|---|
| Characteristics | ||||
| M/F (%) | 54/10 (84.4/15.6) |
68/3 (95.8/4.2) |
83/12 (87.4/12.6) |
0.081 |
| Age | 66.4 ± 10.6 | 63.6 ± 9.6 | 65.6 ± 9.1 | 0.210 |
| BMI | 25.5 ± 4.1 | 27.1 ± 3.9 | 26.7 ± 3.9 | 0.065 |
|
ASA 1 (%) 2 (%) 3 (%) 4 (%) |
15 (23.4%) 16 (25%) 14 (21.9%) 19 (29.7%) |
13 (18.3%) 24 (33.8%) 18 (25.4%) 16 (22.5%) |
22 (23.2%) 30 (31.6%) 25 (26.3%) 18 (18.9%) |
0.714 |
| Hypertension (%) | 41 (64.1%) | 33 (46.5%) | 46 (48.4%) | 0.079 |
| Diabetes (%) | 11 (17.2%) | 12 (16.9%) | 16 (16.8%) | 0.998 |
| Congestive heart failure (%) | 7 (10.9%) | 9 (12.7%) | 8 (8.4%) | 0.667 |
| COPD (%) | 8 (12.5%) | 10 (14.1%) | 18 (18.9%) | 0.508 |
| Smoking (%) | 25 (39.1%) | 18 (25.3%) | 21 (22.1%) | 0.227 |
|
T Tis (%) T0 (%) T1 (%) T2 (%) T3 (%) T4 (%) |
0 6 (9.4%) 5 (7.8%) 16 (25%) 37 (57.8%) 0 |
1 (1.4%) 16 (22.5%) 6 (8.5%) 17 (24%) 30 (42.2%) 1 (1.4%) |
2 (2.1%) 13 (13.7%) 12 (12.6%) 19 (20%) 46 (48.4%) 3 (3.2%) |
0.254 |
|
N N0 (%) N1 (%) N2 (%) N3 (%) |
29 (45.3%) 14 (21.9%) 8 (12.5%) 13 (20.3%) |
40 (56.3%) 15 (21.1%) 12 (16.9%) 4 (5.6%) |
53 (55.8%) 24 (25.3%) 8 (8.4%) 10 (10.5) |
0.119 |
|
M Mx (%) M0 (%) M1 (%) |
2 (3.1%) 62 (96.9%) 0 |
0 70 (98.6%) 1 (1.4%) |
4 (4.2%) 91 (95.8%) 0 |
0.275 |
| Neoadjuvant therapy (%) | 28 (43.7%) | 38 (53.5%) | 42 (44.2) | 0.411 |
Categorical variables are expressed as numbers and (percentages), while continuous variables are expressed as mean ± SD
M male, F female, BMI body mass index, ASA score American Society of Anesthesiology score, COPD chronic obstructive pulmonary disease
The comparative analysis between the three groups showed no differences about demographic data including sex, age, BMI, ASA score, comorbidities (hypertension, diabetes, congestive heart failure and chronic obstructive pulmonary disease COPD), smoking habit and about pTNM (Table 1). Moreover, 108 out of 230 patients underwent neoadjuvant therapy, of which 28 patients (43.7%) belonged to the hand-sewn anastomosis group, 38 (53.5%) to the circular-stapled group and 42 (44.2%) to the linear-stapled anastomosis group.
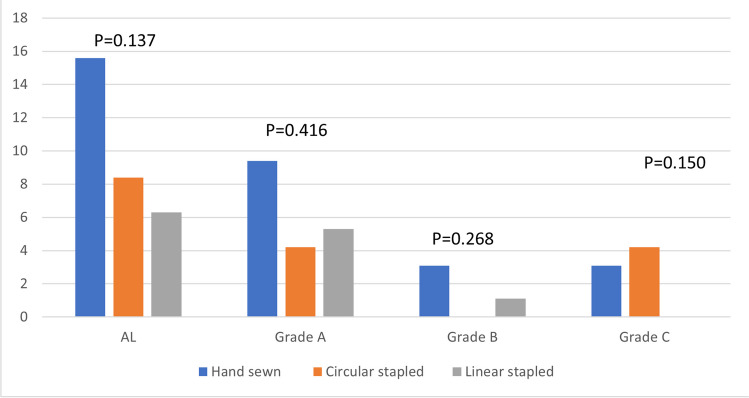
Focusing on the first outcome, no significant differences were found between the three groups about = AL rate (p = 0.137), between the hand-sewn anastomosis group and the circular stapled anastomosis group (p = 0.198), between the circular-stapled anastomosis group and the linear-stapled anastomosis group (p = 0.599) and between the hand-sewn anastomosis group and the linear-stapled anastomosis group (p = 0.06). Moreover, considering the management of the AL (grade A: conservative treatment, grade B: invasive intervention other than repeat surgical intervention, grade C: surgical intervention) for each of the three groups, no significant differences were found. In details of the 14 leakages grade A, six belonged to the hand-sewn anastomosis group, three to the circular-stapled anastomosis group and five to the linear-stapled anastomosis group (p = 0.416), of the three leakages grade B, two belonged to the hand-sewn anastomosis group and one to the linear-stapled group (p = 0.268) and of the five leakages grade C, two belonged to the hand-sewn group and three to the circular-stapled group (p = 0.150) (Fig. 4).
Fig. 4.
Rate of AL grade of the three compared group
Furthermore, the correlation between AL rate and the characteristics of anastomotic fashioning technique was evaluated. Particularly about the hand-sewn anastomotic technique, no significant differences were found between the two subgroups about the type of suture thread used (p = 0.110), regarding the circular-stapled anastomotic technique, no significant differences were found between the four subgroups about the circular-stapled technique (p = 0.763) and the stapler diameter (0.763) and about linear-stapled anastomotic technique, no significant differences were found between the nine subgroups about the stapler type (p = 0.093), the stapler length (p = 0.248), the layer of defect closing (p = 0.544) and the type of suture thread used for defect closing (p = 0.1) (Table 2).
Table 2.
Correlation between the characteristics of each anastomotic technique and the rate of AL
| Suture characteristics | Leak | p-value | |
|---|---|---|---|
| Hand-sewn anastomosis group |
Barbed (n = 40) Non-braided (n = 24) |
4 (10%) 6 (25%) |
0.110 |
| Circular stapled anastomosis group |
Manual (n = 2) Purse string device (n = 69) Stapler diameter (25 mm) (n = 69) Stapler diameter (29 mm) (n = 2) |
0 (0%) 3 (4.3%) 3 (4.3%) 0 (0%) |
0.763 0.763 |
| Linear stapled anastomosis group |
Stapler type (mechanical) (n = 29) Stapler type (electric) (n = 66) Stapler length (45 mm) (n = 37) Stapler length (60 mm) (n = 5 8) Layer of defect closing (single) (n = 43) Layer of defect closing (double) (n = 52) Suture thread for defect closing (barbed) (n = 55) Suture thread for defect closing (braided) (n = 3) Suture thread for defect closing (non-braided) (n = 37) |
0 (0%) 6 (9.1%) 1 (2.7%) 5 (8.6%) 2 (4.6%) 4 (7.7%) 4 (7.3%) 1 (33.3%) 1 (2.7%) |
0.093 0.248 0.544 0.1 |
Discussion
Radical esophagectomy with a complete lymphadenectomy is nowadays considered the standard treatment of the esophageal cancer [20].
Over the last decades, minimally invasive approaches have been gradually gaining favor among surgeons, given that it minimizes surgical trauma and optimizes postoperative outcomes with lower postoperative complication rate and similar oncologic results compared to conventional thoracotomy approaches [21–24].
Recently, robot-assisted minimally invasive esophagectomy (RAMIE) has been introduced as an alternative minimally invasive method which may allow improved the detection of thoracic structures and increased surgical precision [25]. In fact, the three-dimensional view and the EndoWrist® technology of robotic surgery have let to both a better visualization of the operative field and more accurate movements in narrow space [12–17]. Milone et al. has showed that robotic approach for the treatment of esophageal cancer could be considered superior to open approach, being guaranteed less postoperative complications and superior oncologic results, and that it could be considered slightly superior to laparoscopic surgery, providing less postoperative pneumonia and higher number of harvested nodes [18]. Recently, the International Upper Gastrointestinal International Robotic Association (UGIRA) has demonstrated that hybrid laparoscopic RAMIE and full RAMIE were oncologically equivalent with a potential decrease of postoperative complications and shorter (intensive care) stay after full RAMIE [26].
Nevertheless, anastomotic leakage AL remains a severe complication associated with esophagectomy [27]. Occurrence of an AL depends on several factors including nutritional, surgical and anesthesiological factors. Identification of risk factors for AL is of critical importance for prevention and treatment.
Surely, one of the most critical phases during esophagectomy is performing an intrathoracic esophagogastric anastomosis and no standardized anastomotic fashioning technique has yet been generally accepted. The approaches used involve hand-sewn-, circular- (CS) and linear-stapled (LS).
With limited robotic experience, the circular-stapled anastomotic technique might be the preferred approach [28] because of its relative reliability and simplicity [29], while the hand-sewn anastomotic technique or the linear-stapled anastomotic technique are more challenging due to their high technical requirements of suturing ability.
In order to clarify the knowledge about the results of different anastomotic techniques for esophagogastric anastomosis, we retrospectively compared outcomes of the hand-sewn-, linear- and circular-stapled techniques for patients undergoing TMIIL esophagectomy at seven high-volume Italian centers for upper gastrointestinal surgery.
The specific aim of this study is to evaluate the best intrathoracic esophagogastric anastomotic fashioning technique in the robotic setting.
Our results showed no significant differences about AL between the three groups. However, the linear-stapled group presented an incidence of AL (6.3%) lower than that showed by the hand-sewn anastomosis group (15.6%) and the circular-stapled anastomosis group (8.4%). It is interesting to highlight how the linear-stapled group with the highest number of included cases presented the lower incidence of AL (6.3%) according to Kingma BF et al. [30] who found a statistically significant reduction of the AL incidence in the linear-stapled group.
The linear-stapling technique typically results in a functional side-to-side (STS) anastomosis. Although evidences are not available, the SDS configuration could explain the lower incidence of AL due to some rilevant features such as the larger orifice size of the anastomosis and the direction of tension on the anastomosis. In detail, the larger orifice of the anastomosis facilitates intraluminal content passage and decreases circular pressure on the anastomosis. In addition, the weight of the gastric conduit directs the tension on the anastomosis sideways [30, 31].
These technical characterists could also explain the development in the linear-stapled anastomosis gruop of AL easier to trat conservatively. Our results showed indeed no leakages grade C in the linear-stapled anastomosis group (Fig. 4). It makes us to think that the better distribution of tension on the anastomosis could justify leakages which allow a conservative treatment due to their grade and to their less relevant clinical implications.
Moreover, the incidence of AL in the hand-sewn anastomosis group (15.6%) was the highest between the three compared groups.
Thus, our results suggested that the esophagogastric anastomosis performed using a linear stapler was associated to a decrease, although not significant, in AL compared with the levels occurring under other anastomotic techniques [32], and that the linear-stapled technique could be related to AL easier to treat conservatively.
However, being not reached a statistically significance in a multicenter experience, no standardized anastomotic fashioning technique has yet been generally accepted to perform the intrathoracic esophagogastric anastomosis. It remains one of the most technically difficult steps, and no way to perform the anastomosis is considered the best one; but nowadays, the robotic approach could help the surgeon given its 3D-magnified view with better ergonomics and lower physiologic tremor due to EndoWrist instruments.
Conclusion
Our results seem to be in favour of the linear-stapled anastomosis technique but they should not be generalized due to study limitations. These limitations involve retrospective, non-randomized character of the study, the low sample size and the different intrathoracic esophagogastric anastomosis techniques chosen according to the surgeon’s preferences. Moreover, the available scientific evidence regarding both the comparison between the hand-sewn-, circular- (CS) and linear-stapling (LS) approaches and the anastomotic fashioning technique surgical outcomes is limited. Thus, it could be considered a call to perform ad hoc high-quality studies involving high-volume centers for upper gastrointestinal surgery to evaluate what is the most advantageous anastomotic technique and to give definitive conclusions.
Author contributions
Study concept: Marco Milone; study design: Marco Milone; aata acquisition: Paolo Pietro Bianchi, Fabio Cianchi, Andrea Coratti, Anna D’Amore, Giovanni De Manzoni, Carlo Alberto De Pasqual, Giampaolo Formisano, Elio Jovine, Luca Morelli, Mariafortuna Offi, Andrea Peri, Andrea Pietrabissa, Fabio Staderini, Angela Tribuzi, Simone Giacopuzzi; quality control of data and algorithms: Marco Milone, Anna D’Amore; data analysis and interpretation: Marco Milone, Anna D’Amore; statistical analysis: Anna D’Amore; manuscript preparation: Anna D’Amore; manuscript editing: Anna D’Amore; manuscript review: Marco Milone.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Data availability
Data collected for the study are available on request from the corresponding author (Anna D’Amore) upon reasonable request.
Declarations
Ethics approval
The study was conducted according to the guidelines of the 1975 Declaration of Helsinki.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol: WJG. 2013;19(34):5598. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harirchi I, Kolahdoozan S, Hajizadeh S, Safari F, Sedighi Z, Nahvijou A, et al. Esophageal cancer in Iran; a population-based study regarding adequacy of cancer surgery and overall survival. Eur J Surg Oncol (EJSO) 2014;40(3):352–357. doi: 10.1016/j.ejso.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology. 2020;159(1):335–349.e15. doi: 10.1053/j.gastro.2020.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen MF, Yang YH, Lai CH, Chen PC, Chen WC (2013) Outcome of patients with esophageal cancer: a nationwide analysis. Ann Surg Oncol 20(9):3023–3030. 10.1245/s10434-013-2935-4 [DOI] [PubMed]
- 5.Wang B, Zuo Z, Chen H, Qiu B, Du M, Gao Y. The comparison of thoracoscopic-laparoscopic esophagectomy and open esophagectomy: A meta-analysis. Indian J Cancer. 2017;54(1):115–119. doi: 10.4103/ijc.IJC_192_17. [DOI] [PubMed] [Google Scholar]
- 6.Guo W, Ma X, Yang S, Zhu X, Qin W, Xiang J, Lerut T, Li H (2016) Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 30(9):3873–3881. 10.1007/s00464-015-4692-x [DOI] [PubMed]
- 7.Wang K, Zhong J, Liu Q, Lin P, Fu J. A Propensity Score-Matched Analysis of Thoracolaparoscopic vs Open McKeown's Esophagectomy. Ann Thorac Surg. 2022;113(2):473–481. doi: 10.1016/j.athoracsur.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Nuytens F, Dabakuyo-Yonli TS, Meunier B, Gagnière J, Collet D, D’Journo XB, Brigand C, Perniceni T, Carrère N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Markar SR, Piessen G, Fédération de Recherche en Chirurgie (FRENCH) French Eso-Gastric Tumors (FREGAT) working groups (2021) Five-year survival outcomes of hybrid minimally invasive esophagectomy in esophageal cancer: results of the MIRO randomized clinical trial. JAMA Surg 156(4):323–332. 10.1001/jamasurg.2020.7081 [DOI] [PMC free article] [PubMed]
- 9.Mariette C, Markar S, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T, Carrere N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G, FRENCH. FREGAT Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann Surg. 2020;271(6):1023–1029. doi: 10.1097/SLA.0000000000003559. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida N, Yamamoto H, Baba H, Miyata H, Watanabe M, Toh Y, Matsubara H, Kakeji Y, Seto Y. Can Minimally Invasive Esophagectomy Replace Open Esophagectomy for Esophageal Cancer? Latest Analysis of 24,233 Esophagectomies From the Japanese National Clinical Database. Ann Surg. 2020;272(1):118–124. doi: 10.1097/SLA.0000000000003222. [DOI] [PubMed] [Google Scholar]
- 11.Maas KW, Cuesta MA, van Berge Henegouwen MI, Roig J, Bonavina L, Rosman C, Gisbertz SS, Biere SS, van der Peet DL, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ (2015) Quality of life and late complications after minimally invasive compared to open esophagectomy: results of a randomized trial. World J Surg 39(8):1986–1993. 10.1007/s00268-015-3100-y [DOI] [PMC free article] [PubMed]
- 12.van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, Kroese CC, Haj Mohammad N, Mook S, Vleggaar FP, Borel Rinkes IHM, Ruurda JP, van Hillegersberg R. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg. 2019;269(4):621–630. doi: 10.1097/SLA.0000000000003031. [DOI] [PubMed] [Google Scholar]
- 13.Egberts JH, Stein H, Aselmann H, Hendricks A, Becker T. Fully robotic da Vinci Ivor-Lewis esophagectomy in four-arm technique-problems and solutions. Dis Esophagus. 2017;30(12):1–9. doi: 10.1093/dote/dox098. [DOI] [PubMed] [Google Scholar]
- 14.Milone M, Manigrasso M, Velotti N, Torino S, Vozza A, Sarnelli G, et al. (2019) Completeness of total mesorectum excision of laparoscopic versus robotic surgery: a review with a meta-analysis. Int J Colorectal Dis. 2019;34:983–991. doi: 10.1007/s00384-019-03307-0. [DOI] [PubMed] [Google Scholar]
- 15.Ceccarelli G, Andolfi E, Biancafarina A, Rocca A, Amato M, Milone M, Scricciolo M, Frezza B, Miranda E, De Prizio M, Fontani A. Robot-assisted surgery in elderly and very elderly population: our experience in oncologic and general surgery with literature review. Aging Clin Exp Res. 2017;29(Suppl 1):55–63. doi: 10.1007/s40520-016-0676-5. [DOI] [PubMed] [Google Scholar]
- 16.Moon AS, Garofalo J, Koirala P, Vu MLT, Chuang L. Robotic surgery in gynecology. Surg Clin N Am. 2020;82:96–109. doi: 10.1016/j.suc.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Tang AB, Lamaina M, Childers CP, Mak SS, Ruan Q, Begashaw MM, Bergman J, Booth MS, Shekelle PG, Wilson M, Gunnar W, Maggard-Gibbons M, Girgis MD. Perioperative and Long-Term Outcomes of Robot-Assisted Partial Nephrectomy: A Systematic Review. Am Surg. 2021;87(1):21–29. doi: 10.1177/0003134820948912. [DOI] [PubMed] [Google Scholar]
- 18.Manigrasso M, Vertaldi S, Marello A, Antoniou SA, Francis NK, De Palma GD, et al. Robotic esophagectomy. a systematic review with meta-analysis of clinical outcomes. J Pers Med. 2021;11(7):640. doi: 10.3390/jpm11070640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nickel F, Probst P, Studier-Fischer A, et al. Minimally invasive versus open abdominothoracic esophagectomy for esophageal carcinoma (MIVATE) - study protocol for a randomized controlled trial DRKS00016773. Trials. 2021;22:1. doi: 10.1186/s13063-020-04966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gisbertz SS, Hagens ERC, Ruurda JP, Schneider PM, Tan LJ, Domrachev SA, Hoeppner J, van Berge Henegouwen MI. The evolution of surgical approach for esophageal cancer. Ann N Y Acad Sci. 2018;1434(1):149–155. doi: 10.1111/nyas.13957. [DOI] [PubMed] [Google Scholar]
- 21.Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887–1892. doi: 10.1016/S0140-6736(12)60516-9. [DOI] [PubMed] [Google Scholar]
- 22.Lv L, Hu W, Ren Y, Wei X. Minimally invasive esophagectomy versus open esophagectomy for esophageal cancer: a meta-analysis. Onco Targets Ther. 2016;31(9):6751–6762. doi: 10.2147/OTT.S112105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haverkamp L, Seesing MF, Ruurda JP, Boone J, Hillegersberg VR. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus. 2017;30(1):1–7. doi: 10.1111/dote.12480. [DOI] [PubMed] [Google Scholar]
- 24.Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, et al. Minimally invasive versus open esophageal resection: three-year follow-up of the previously reported randomized controlled trial: the TIME trial. Ann Surg. 2017;266(2):232–236. doi: 10.1097/SLA.0000000000002171. [DOI] [PubMed] [Google Scholar]
- 25.Jin D, Yao L, Yu J, Liu R, Guo T, Yang K, et al. Robotic-assisted minimally invasive esophagectomy versus the conventional minimally invasive one: a meta-analysis and systematic review. Int J Med Robot Comput Assist Surg. 2019;15:e1988. doi: 10.1002/rcs.1988. [DOI] [PubMed] [Google Scholar]
- 26.Jung JO, de Groot EM, Kingma BF, Babic B, Ruurda JP, Grimminger PP, et al. Hybrid laparoscopic versus fully robot-assisted minimally invasive esophagectomy: an international propensity-score matched analysis of perioperative outcome. Surg Endosc. 2023;37(6):4466–4477. doi: 10.1007/s00464-023-09911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kassis ES, Kosinski AS, Ross P, Jr, Koppes KE, Donahue JM, Daniel VC. Predictors of anastomotic leak after esophagectomy: an analysis of the society of thoracic surgeons general thoracic database. Ann Thorac Surg. 2013;96(6):1919–1926. doi: 10.1016/j.athoracsur.2013.07.119. [DOI] [PubMed] [Google Scholar]
- 28.Plat VD, Stam WT, Schoonmade LJ, Heineman DJ, van der Peet DL, Daams F. Implementation of robot-assisted Ivor Lewis procedure: robotic hand-sewn, linear or circular technique? Am J Surg. 2020;220(1):62–68. doi: 10.1016/j.amjsurg.2019.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Shen T, Zhang Y, Cao Y, Li C, Li H. Robot-assisted Ivor Lewis Esophagectomy (RAILE): a review of surgical techniques and clinical outcomes. Front Surg. 2022;4(9):998282. doi: 10.3389/fsurg.2022.998282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kingma BF, Grimminger PP, van der Sluis PC, van Det MJ, Kouwenhoven EA, Chao YK, et al. Worldwide techniques and outcomes in robot-assisted minimally invasive esophagectomy (RAMIE): results from the Multicenter International Registry. Ann Surg. 2020;276(5):e386–e392. doi: 10.1097/SLA.0000000000004550. [DOI] [PubMed] [Google Scholar]
- 31.Ben-David K, Tuttle R, Kukar M, et al. Minimally invasive esophagectomy utilizing a stapled side-to-side anastomosis is safe in the western patient population. Ann Surg Oncol. 2016;23(9):3056e3062. doi: 10.1245/s10434-016-5232-1. [DOI] [PubMed] [Google Scholar]
- 32.Price TN, Nichols FC, Harmsen WS, Allen MS, Cassivi SD, Wigle DA, et al. A comprehensive review of anastomotic technique in 432 esophagectomies. Ann Thorac Surg. 2013;95(4):1154–1161. doi: 10.1016/j.athoracsur.2012.11.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data collected for the study are available on request from the corresponding author (Anna D’Amore) upon reasonable request.