Abstract
Key Clinical Message
Spinal cord compression from non‐Hodgkin lymphoma (NHL) should be considered as a potential diagnosis in cases of acute signs of myelopathy in pediatric patients.
Abstract
Spinal cord compression in pediatric non‐Hodgkin lymphoma (NHL) is a rare presentation with potential diagnostic challenges. We report on two pediatric patients with NHL who exhibited myelopathy signs as initial presentation. Considering NHL as a differential diagnosis in pediatric patients presenting with spinal cord compression is crucial for optimizing the outcome of these patients.
Keywords: chemotherapy, myelopathies, NHL, non‐Hodgkin lymphoma, pediatric, spinal cord compression
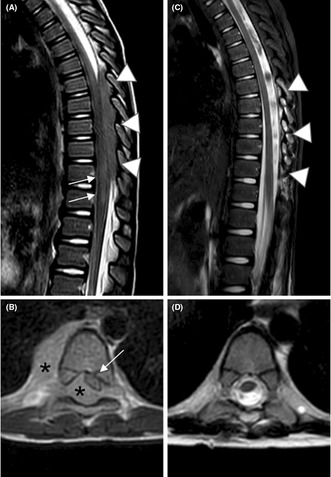
Pre‐operative scan reveals epidural tissue from D7 to D11, which compresses and dislocates the dural sac. Post‐ operative scan shows surgical removal of the neoplastic intacanal component, with complete resolution of the mass effect.
1. INTRODUCTION
Pediatric Non‐Hodgkin lymphoma (NHL) presenting with epidural spinal cord compression at diagnosis is an uncommon disease presentation. Due to its rarity, it may not always be considered among the differential diagnoses by pediatricians when faced with a suspected spinal lesion. In this report, our aim is to describe the clinical presentation of two cases of pediatric NHL with spinal cord compression, focusing specifically on the clinical features observed at the time of presentation. Our intention is to provide valuable insights to pediatricians who may encounter similar scenarios, helping them promptly recognize and manage this challenging diagnosis in order to prevent sequelae and improve patient outcomes.
2. CASE 1
Patient 1 is a 6‐year‐old boy with no previous medical history. He came for intermittent cramp‐like abdominal pain in the last 48 h, with no fever, vomiting or diarrhea. Since some signs of inflammation in the appendix were found at an initial abdominal ultrasound scan, Mattia was sent to pediatric emergency room (PER). Physical examination showed signs of acute abdomen. Therefore, he underwent a video‐assisted trans‐umbilical appendectomy with a successive histological confirmation of appendicitis' inflammation. After first clinical improvement, on postoperative day four he started to autonomously walk. At that point, we observed a progressive exacerbation of abdominal pain associated with difficulty in maintaining the sitting position.
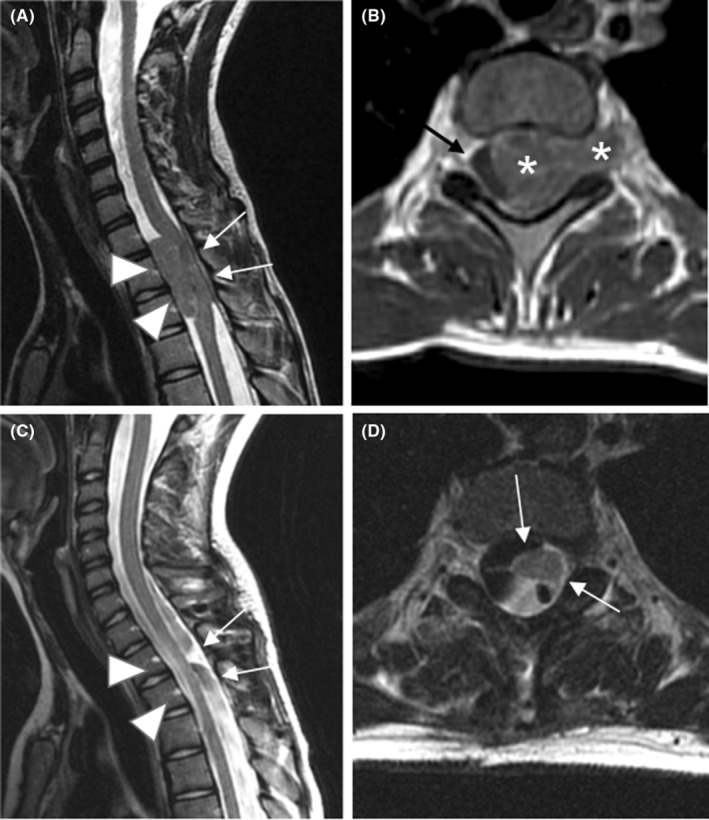
The next day we also noticed an intermittent tenderness in the lower limbs, inability to keep the upright position, and nocturnal enuresis. Throughout a neuropsychiatric evaluation, the patient reported that he did not feel the urge to urinate and refused to leave autonomously the wheelchair. Neurological evaluation highlighted the presence of hypotonia and hyposthenia of the lower limbs, abolished patellar osteotendinous reflexes, and abnormal plantar reflex in bilateral toe extension (Babinski sign). He underwent an electroneurography, a lumbar puncture and magnetic resonance imaging (MRI) to complete the diagnosis. The neurographic study showed only an asymmetry between the left and right limbs. Lumbar puncture showed elevated protein level (>700 mg/dL) in the cerebrospinal fluid, which supported the suspect of Guillain‐Barre syndrome (GBS). Therefore, prompt treatment with intravenous immunoglobulin (dose 0.5 g/kg/day) was started. The following day the clinical conditions of the patient appeared unchanged. An encephalic and spinal MRI was executed, showing an epidural intracanal tissue located from D7 to D11, associated with blurred signs of medullary suffering (Figure 1A,B).
FIGURE 1.
Sagittal (A) and axial (B) T2‐weighted images. Pre‐operative scan reveals epidural tissue (arrowheads in A and asterisks in B), located within the spinal canal, from D7 to D11, slightly hyperintense to the spinal cord. The lesion compresses and dislocates the dural sac antero‐laterally, towards the left (white arrow in B), and it extends through the right conjugation foramen, into the adjacent cost‐vertebral space (asterisk in B). An area of spinal cord signal abnormality is seen at D11‐D12 (arrows in A). Sagittal STIR (C) and axial (D) T2 weighted images. Post‐operative scan shows surgical removal of the neoplastic intracanal component, with complete resolution of the mass effect (arrowheads in C) as well as the spinal cord signal abnormality described. The component seen in the right right costovertebral space showed a prompt response to chemotherapy.
An urgent surgical decompressive laminotomy on 5 levels was performed, with the removal of most of pathological tissue (Figure 1C,D). The histological examination reported a pre‐B lymphoblastic lymphoma. The patient has been subsequently transferred to the Onco‐hematology department to undertake a specific chemotherapy treatment for the underlying disease. He started a high‐dose of methylprednisolone succinate.
After surgery, patient 1 showed persistent severe hypotonia in the lower limbs, absence of spontaneous movements with weak patellar and Achilles' osteotendinous reflexes, and recovery of tactile sensitivity.
During the hospitalization, the initial paraparesis gradually improved thanks to a targeted rehabilitation program. The distal motor deficiency was associated with intestinal dysmotility, anal sphincter dysfunction and spastic neurogenic bladder. The initial difficulty in perceiving the stimulus to defecate and the anal sphincter atony has progressively improved, until resolution. Neurogenic bladder, characterized by an increase in daily urinations with a small amount of urine and mintional urge, was treated by oxybutynin. Intermittent catheterizations were performed six times a day as prophylaxis for urinary tract infections. Thanks to catheter urinary sample cultures, the patient was found to have 3 asymptomatic bacteriuria, all treated according to antibiogram. Currently, the number of daily urinations has partially reduced and the mintional urge has improved.
3. CASE 2
Patient 2 is a 13‐year‐old boy admitted to the pediatric emergency room, because of a hypoesthesia of the lower limbs, with increasing difficulty in walking, during the last 7 days.
About 25 days before, the boy reported difficulty in bending the head after physical activity (weight throw). Due to persistent pain, he underwent an orthopedic examination with a cervical spine x‐ray. The imaging showed vertebral instability characterized by moderate retrolisthesis of C3‐C4 and C4‐C5.
After a while, the patient began to complain of paresthesiae of the lower limbs associated with walking difficulties. Taping was removed, with immediate benefit. After a few hours, however, paresthesia and difficulties in walking appeared again. Then, a child neuropsychiatric evaluation was performed, but the neurological objective examination was negative.
After 48 h the boy returned to the PER, complaining inability to walk, with no pain in the lower limbs. At physical examination he was not able to keep the standing position. Lower limb muscle contractions were present intermittently for short intervals of time. These muscles had normal consistency with no tenderness to the palpation. The deep tendon reflex was present and symmetrical. No sphincter, nor sensitive deficits were detected. After confirming a worsening of the motor deficit, we decided to hospitalize the patient.
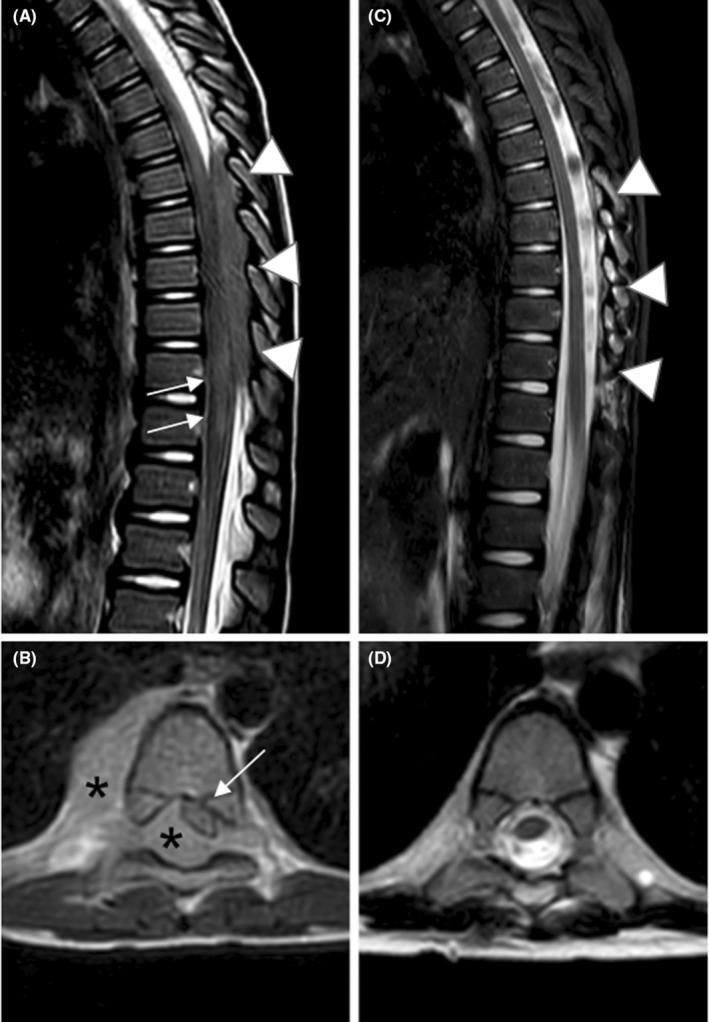
On the following day, brain and spinal MRI were performed, with evidence of an expansive intravertebral epidural lesion in the thoracic area, extending from D1 to D3, which involves and widens the conjugation foramina of the left side (Figure 2A,B). Additionally, there was also another polylobed formation with similar characteristics in the right mid‐thoracic area along the costal margin line.
FIGURE 2.
Sagittal T2‐weighted (A) and T1‐weighted images after gadolinium chelates administration (B). Presurgical MRI scan reveals an epidural tissue, located within the spinal canal at D1‐D3 (arrowheads in A and asterisks in B), characterized by a signal intensity comparable to that of the spinal cord, due to high cellularity, as well as by faint contrast enhancement (asterisks in B). The lesion fills almost completely the spinal canal, with a severe compression to the dural sac and its contents (white arrows in A), displaced laterally to the right (black arrow in B). Sagittal (C) and axial (D) T2‐weighted images. Postoperative MRI scan shows the removal of the lesion (arrowheads in C), with an almost complete resolution of the associated mass effect. During early post‐operative weeks the spinal cord early appears distorted, due to previous compression (arrows in C and D), however, without relevant signal abnormality.
Thus, the patient underwent an urgent neurosurgical intervention of laminotomy and removal of the mass compressing the medulla (Figure 2C,D).
Histological examination showed an anaplastic large‐cell ALK‐positive lymphoma. After surgery, the patient presented incomplete paraplegia. A specific chemotherapy protocol was undertaken, followed by rehabilitative management, with the execution of daily exercise sessions.
After the first discharge from the Department of Pediatric Oncohematology, following about 2 months of physiotherapy, patient showed an initial motility of the lower limbs with the possibility of loading. Over time there has been a progressive improvement of the motor deficit, until complete resolution. At present, he usually plays tennis.
4. DISCUSSION
We report the unusual cases of two children with symptoms suggestive of acute myelopathy, who resulted both being affected by epidural NHL, followed in the Bologna hospital. 1 Although spinal cord compression represents a rare clinical presentation, NHL should always be included in the differential diagnosis of acute myelopathy in the pediatric patient, because any delay in the diagnosis and treatment could significantly affect the prognosis of this disease and the onset of long‐term sequelae.
In fact, NHL is included among the main oncological causes of compressive myelopathy in the pediatric population (Table 4). NHL represents a heterogeneous group of malignant neoplasms of lymph node tissue derived from the progenitors or mature B‐lymphocyte cells or, less frequently, T‐ lymphocyte cells. While low‐grade, clinically indolent lymphomas are predominant in adults, pediatric cases are primarily characterized by high‐grade lymphoma subtypes with aggressive behavior. 2 The extranodal presentation of NHL in the child‐adolescent population, in contrast to that in adults, is more common and often involves sites such as the mediastinum, abdomen, head–neck region, bone marrow, or central nervous system, with symptoms and signs specific of the anatomic site involved. 3 Symptoms often develop rapidly, over a period of 1–3 weeks (see Table 1 for signs and symptoms). The onset of lymphadenopathy is commonly indolent, with slow growth in size and diameter of the lymph nodes. This is true also for the extranodal tissue, which eventually leads to compression symptoms of the surrounding structures.
TABLE 4.
Main tumors that can cause spinal cord compression in childhood.
| Neoplasms of the spinal cord | ||||
|---|---|---|---|---|
| Extradural | Intradural | |||
| Epidural | Osteo‐cartilaginous | Peri‐vertebral | Intramedullary | Extramedullary |
|
|
|
|
|
TABLE 1.
Main clinical manifestations of the LNH.
|
Our two reports emphasize how the initial presentation of LNH frequently constitutes a challenge for the pediatrician because of the variety of possible clinical manifestations at disease onset. For example, chronic dull abdominal pain that is deep in nature and lacks specific localization can serve as a non‐specific indicator of myelopathy. This occurs due to the stimulation of nerve terminations in the bowel wall by the neoplastic growth (refer to Table 2 for red flags).
TABLE 2.
Red Flags of warning signs and symptoms for LNH with primarily medullary localization.
|
|
|
|
|
|
|
|
However, acute abdominal pain is frequently a diagnostic challenge in children due to the nonspecific nature of the symptom, especially in younger patients. Compression of the spinal cord can lead also to impaired motor function, with a rapid progression to paraplegia and paralysis. The four main etiological groups (see Table 3) of motor paralysis and/or functional deficit of the lower limbs in pediatric age consist of 4 , 5 :
trauma (e.g., from falls or road accidents);
vascular pathologies, including epidural spinal hematoma, caused by the rupture of epidural veins in correspondence of a locus minoris resistentiae following a sudden increase in intrathoracic or intra‐abdominal pressure, due to efforts (even when of low intensity such as cough or defecation);
inflammatory diseases (including primary infections, abscesses, polyradiculoneuropathy and infection‐associated processes, such as transverse myelitis and encephalomyelitis);
compressions (tumors, syringomyelia).
TABLE 3.
Main causes of myelopathy in pediatric patients.
| Congenital | Genetic disorders | Acquired |
|---|---|---|
|
|
|
According to an Australian case study, the most common cause of acute flaccid paralysis of the lower limbs (up to 47% of cases) is Guillain—Barré syndrome (GBS), and this differential diagnosis needs to be ruled out. 6 Malignant compression of the spinal cord (MCSC), whether it is caused by a primary or metastatic tumor localization, can be further classified into two subtypes: extradural (the most frequent in adults, extending from the vertebral bodies or from structures external to the dura mater) and intramedullary. 7 Despite their impact on morbidity and mortality, only a small amount of data on the incidence of the disease are available in the pediatric population. Acute spinal cord compression can occur in a not negligible percentage of children with cancer, often at the time of diagnosis. 8 Tumors associated with medullary compression in childhood are shown in Table 4.
An Italian case study in pediatric oncology shows that the onset symptom of MCSC in all patients is the motor deficit, while only 60% of patients experienced pain and only 43% sphincteric deficiency. 9 When spinal cord compression is suspected, MRI is the diagnostic technique of choice. 10 This exam should be performed as soon as possible because the neurological prognosis is strongly related to the time from symptoms onset to spinal cord decompression surgery. 4 Complications of spinal cord compression, such as urinary dysfunction, fecal incontinence, spasticity, painful syndromes and psychological sequelae severely affect the overall outcome of children and adolescents treated for NHL. 10 According to the latest 2013 guidelines of the American Association of Neurological Surgeons and the Congress of Neurological Surgeons, the use of glucocorticoids in acute traumatic spinal cord injury is no longer recommended. 11 The American Academy of Emergency Medicine states that glucocorticoid treatment remains an acceptable option. Many experts affirm that there are compelling and undeniable data justifying the clinical use of glucocorticoids, particularly in patients with partial compression. The molecule to be used should be methylprednisolone, administered intravenously. The therapeutic scheme is:
30 mg/kg in bolus in 15 min
After 45′, 5.4 mg/kg infused every hour for 23 h. 12
Bladder dysfunction can lead to difficulties in urination associated with changes in intravesical pressure, an increased risk of infection and kidney damage as well as a source of social distress. Urinary symptoms may vary, ranging from increased urinary frequency to complete urinary retention. It is therefore mandatory to perform a proper neurological examination (complete with an evaluation of sphincter function and reflexes), a mintional diary, to measure residual post‐mintional volumes, and to execute urodynamic studies. Similarly, the presence of neurogenic bowel can be a source of serious social distress and skin impairment. Laxatives or anti‐diarrheal drugs with pelvic floor rehabilitation can improve sphincteric control. 12
Several studies show how neurological recovery in childhood is better than in adulthood 6 thanks to the greater plasticity of the immature spinal cord. 13 , 14 , 15 In a recent work, the presence of residual muscle activity in children, found in electromyography analysis of the motor sites located below the level of injury, documents the existence of a residual descending influence from the spinal motor circuits. This observation, independently of its immediate functional relevance, can represent an objective indicator of the potential recovery of both intentional and postural motor function. 16 Proper physiotherapy following damage to the spinal cord, remains a key intervention for the rehabilitation of these patients. 17 , 18 Age at the time of diagnosis, site and extension of the lesion of the spinal cord injury are the main prognostic factors for the recovery of deambulation. Children under 5 years of age, with incomplete injuries, located in the thoracic or lumbar spine, show the highest chance of functional recovery thanks to physiotherapy. 16
5. CONCLUSIONS
A child presenting recent gait or motor impairment, whether or not associated with pain and sphincter deficit, must be promptly evaluated by a physician, especially if rapidly progressive. Root pain and motor impairment may be the first signs of spinal compression. The rapid onset and evolution of symptoms have been an important warning signal.
It is essential to know the possible clinical manifestations of medullary compression in order to be able to recognize them and avoid unsuitable treatments for patients.
AUTHOR CONTRIBUTIONS
Daniele Zama: Conceptualization; formal analysis; writing – review and editing. Egidio Candela: Conceptualization; data curation; formal analysis; writing – original draft. Gennaro Pagano: Conceptualization; writing – original draft. Francesco Venturelli: Conceptualization; writing – original draft. Fraia Melchionda: Writing – review and editing. Francesco Toni: Data curation; resources. Mino Zucchelli: Supervision; writing – review and editing. Andrea Pession: Validation; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
ETHICS STATEMENT
Written informed consent was obtained from the patient for the publication of these case reports and any accompanying images. Ethics committee approval was not obtained because we report a clinical case of only two patients, we have not included any identifiable information, and we obtained written consent for publication from the patient's parents according to the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective review of patient data did not require ethical approval in accordance with local guidelines.
ACKNOWLEDGMENTS
This research received no external funding and support.
Zama D, Candela E, Pagano G, et al. Pediatric non‐Hodgkin lymphoma as a rare cause of spinal cord injury: When lymphoma hides in the canal. Clin Case Rep. 2024;12:e7789. doi: 10.1002/ccr3.7789
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Candela E, Zagariello M, Di Natale V, et al. Cystathionine Beta‐synthase deficiency: three consecutive cases detected in 40 days by newborn screening in Emilia Romagna (Italy) and a comprehensive review of the literature. Children (Basel). 2023;10(2):396. doi: 10.3390/children10020396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chun GYC, Sample J, Hubbard AK, Spector LG, Williams LA. Trends in pediatric lymphoma incidence by global region, age and sex from 1988‐2012. Cancer Epidemiol. 2021;73:101965. doi: 10.1016/j.canep.2021.101965 [DOI] [PubMed] [Google Scholar]
- 3. Principi N, Rubino A. Pediatria generale e specialistica. 2nd ed. Casa Editrice Ambrosiana; 2017. [Google Scholar]
- 4. New PW. A narrative review of pediatric nontraumatic spinal cord dysfunction. Top Spinal Cord Inj Rehabil. 2019;25(2):112‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vogel LC, Betz RR, Mulcahey MJ. Spinal cord injuries in children and adolescents. Handb of Clin Neurol. 2012;109:131 e48. [DOI] [PubMed] [Google Scholar]
- 6. Ide W, Melicosta M, Trovato MK. Acute Flaccid Myelitis. Phys Med Rehabil Clin N Am. 2021;32(3):477‐491. doi: 10.1016/j.pmr.2021.02.004 [DOI] [PubMed] [Google Scholar]
- 7. Parent S, Mac‐Thiong JM, Roy‐Beaudry M, Sosa JF, Labelle H. Spinal cord injury in the pediatric population: a systematic review of the literature. J Neurotrauma. 2011;28(8):1515‐1524. doi: 10.1089/neu.2009.1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atkinson DA, Mendez L, Goodrich N, Aslan SC, Ugiliweneza B, Behrman AL. Muscle activation patterns during movement attempts in children with acquired spinal cord injury: neurophysiological assessment of residual motor function below the level of lesion. Front Neurol. 2019;20(10):1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Martino L, Spennato P, Vetrella S, et al. Symptomatic malignant spinal cord compression in children: a single‐center experience. Ital J Pediatr. 2019;45:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ruppert LM. Malignant spinal cord compression: adapting conventional rehabilitation approaches. Phys Med Rehabil Clin N Am. 2017;28(1):101‐114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walters BC, Hadley MN, Hurlbert RJ, et al. Guidelines for the management of acute cervical spine and spinal cord injuries: 2013 update. Neurosurgery. 2013;60(CN_suppl_1):82‐91. [DOI] [PubMed] [Google Scholar]
- 12. Karsy M, Hawryluk G. Modern medical Management of Spinal Cord Injury. Curr Neurol Neurosci Rep. 2019;19(9):65. doi: 10.1007/s11910-019-0984-1 [DOI] [PubMed] [Google Scholar]
- 13. Kirshblum S, Snider B, Eren F, Guest J. Characterizing Natural Recovery after Traumatic Spinal Cord Injury. J Neurotrauma. 2021;38(9):1267‐1284. doi: 10.1089/neu.2020.7473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci. 2004;27:145‐167. [DOI] [PubMed] [Google Scholar]
- 15. Martin JH. Neuroplasticity of spinal cord injury and repair. Handb Clin Neurol. 2022;184:317‐330. doi: 10.1016/B978-0-12-819410-2.00017-5 [DOI] [PubMed] [Google Scholar]
- 16. Donenberg JG, Fetters L, Johnson R. The effects of locomotor training in children with spinal cord injury: a systematic review. Dev Neurorehabil. 2019;22(4):272‐287. [DOI] [PubMed] [Google Scholar]
- 17. Calhoun CL, Schottler J, Vogel LC. Recommendations for mobility in children with spinal cord injury. Top Spinal Cord Inj Rehabil. 2013;19(2):142‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Benmelouka A, Shamseldin LS, Nourelden AZ, Negida A. A review on the etiology and management of pediatric traumatic spinal cord injuries. Adv J Emerg Med. 2019;4(2):e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.



 Radicular pain (cervical‐brachial, abdominal, lumbar‐cruralgia, lumbar‐sciatalgia)
Radicular pain (cervical‐brachial, abdominal, lumbar‐cruralgia, lumbar‐sciatalgia)