Abstract
Circadian rhythm is an internal timing system and harmonizes a variety of cellular, behavioral, and physiological processes to daily environment. Circadian disturbance caused by altered life style or disrupted sleep patterns inevitably contributes to various disorders. As the rapidly increased cancer occurrences and subsequent tremendous financial burdens, more researches focus on reducing the morbidity rather than treating it. Recently, many epidemiologic studies demonstrated that circadian disturbance was tightly related to the occurrence and development of cancers. For urinary system, numerous clinical researches observed the incidence and progress of prostate cancer were influenced by nightshift work, sleep duration, chronotypes, light exposure, and meal timing, this was also proved by many genetic and fundamental findings. Although the epidemiological studies regarding the relationship between circadian disturbance and kidney/bladder cancers were relative limited, some basic researches still claimed circadian disruption was closely correlated to these two cancers. The role of circadian chemotherapy on cancers of prostate, kidney, and bladder were also explored, however, it has not been regularly recommended considering the limited evidence and poor standard protocols. Finally, the researches for the impacts of circadian disturbance on cancers of adrenal gland, penis, testis were not found at present. In general, a better understanding the relationship between circadian disturbance and urological cancers might help to provide more scientific work schedules and rational lifestyles which finally saving health resource by reducing urological tumorigenesis, however, the underlying mechanisms are complex which need further exploration.
Keywords: Circadian rhythm, Circadian disturbance, Circadian sleep disorder, Epidemiological and fundamental evidence, Urological cancer
Introduction
Alternation of day and night is the most predictable environmental changes on our rotating planet, which accompanied with daily variations in environmental temperature, humidity, illumination, and food availability [1]. In order to adapt the Earth’s temporal rhythms, all organisms have evolved and internalized universally circadian clock that synchronize individual physiology and behavior to a solar day [2–4]. Before sunrise or sunset of the day, endogenous and self-sustaining circadian (circa = about, dies = day) clock has been precisely set to a 24-h oscillating rhythm [3]. The well-known mammalian circadian clock rhythm is the body temperature, heart rate, and blood pressure which rise in the morning, but fall at night [5]. Others include sleep−wake cycle [6, 7], homeostasis [6, 8, 9], diet [6, 10], and hormone secretion (e.g., melatonin and cortisol) [6, 8, 9]. Thus, circadian rhythm plays essential roles in the orchestration of daily life, and most physiological functions are strictly presented with regular activity and rest rhythm to guarantee the optimal performance [1].
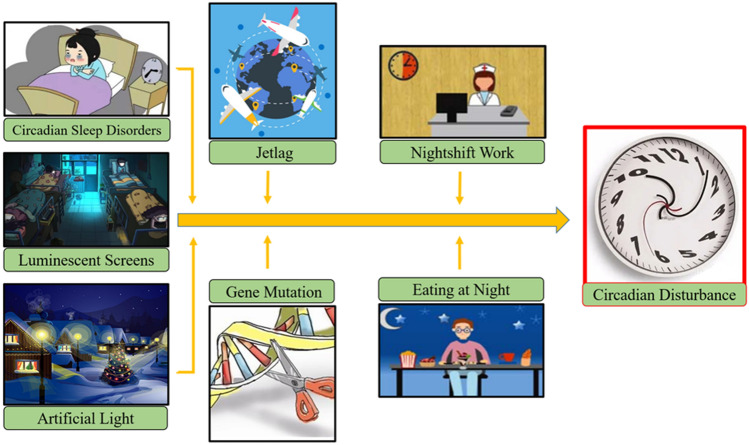
During the past history before modern society, human habits are more closely to natural environment and evolve under distinct day and night alterations [3]. However, the technological advance and global industrialization within the past century dramatically promote modern medical science and improve human health, which coincide with rising rates of numerous disease [11]. Nowadays, artificial lights or luminescent screens have been extensively popularized due to elevated work program, increased social pressure, and gradually personal habits with entertainment technological communication platforms [11]. Meanwhile, prolonged nightshift work, jetlag, circadian sleep disorders, and long-distance travel across multiple time zones are more frequent [11]. All these factors inevitably alter the daily sleep/wake cycle and slowly disturb internal circadian rhythm (Fig. 1) [5, 11], which finally damage homeostasis, promote oxidative stress, induce inflammatory responses, and accelerate coagulatory process [6, 8, 9, 12]; while such individuals are also more vulnerable to type 2 diabetes mellitus (T2DM), hypertension, hyperlipemia, obesity, atherosclerosis, and cancers (Fig. 2) [5, 6, 11, 13, 14].
Fig. 1.
Misaligned environmental factors contribute to diseases through disturbing circadian clock. As the global industrialization and technological advances, rising rates of numerous diseases have coincided with altered lifestyles and work patterns. Nowadays, artificial lights or luminescent screens have been extensively popularized due to elevated work program, increased social pressure, and gradually personal habits with entertainment technological communication platforms. The prolonged nightshift work, jetlag, circadian sleep disorders, and long distance travel across multiple time zones are more frequent. All these factors sharply alter the daily sleep/wake cycle and slowly disturb internal circadian clock
Fig. 2.
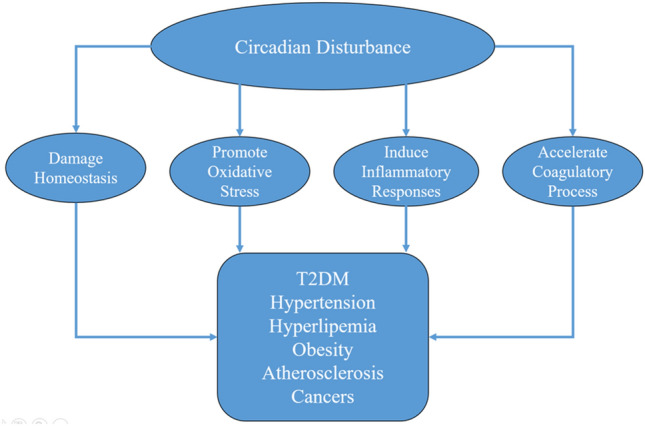
Circadian disturbance can damage homeostasis, promote oxidative stress, induce inflammatory responses, and accelerate coagulatory process, which contribute to numerous diseases including. T2DM, hypertension, hyperlipemia, obesity, atherosclerosis, and cancers.
The World Health Organization (WHO) has ranked cancers as the most common cause of death which annually take away 7 million lives worldwide [15]. Updated data in 2019 reveals that about 24.6 million people suffer from cancer, with an estimated 16 million new diagnoses and 10 million cancer death happen in 2020 [15]. The latest CA Cancer J Clin based on American Cancer Society also estimates that approximately 1,898,160 new cancer cases (about 5200 cases/day) and 608,570 cancer death (> 1600 cases/day) will occur in US in 2021 [16], while urological malignancy (mainly cancers of prostate, kidney, and bladder) accounts for 12% of worldwide cancer death [17]. When considering the tremendous financial burdens and great damages to human health, it is essential to clarify the mechanistic risks of urological cancer to reduce or avoid the tumor occurrence.
It has been demonstrated that some critical processes in tumorigenesis are tightly involved with circadian clock rhythm, like cell division cycle [3]. Firstly, circadian rhythm regulates cell cycle via the relationship between molecular circadian clock elements [3, 18] and cyclin−cyclin-dependent kinase (CDK) complexes [2, 3] or the cell cycle inhibitors at transcriptional or protein level, which finally manipulates gating of unidirectional progress through cell cycle stages [3, 19]. Moreover, circadian rhythm shares common enzymatic regulators in the phosphorylation and ubiquitination pathways with cellular cycle [3]. Thus, it is not surprising to establish interactions between circadian rhythm and tumorigenesis [3], considering the circadian disturbance is closely related to biological property of cancer cells like DNA repair, proliferation, apoptosis, metabolism, and stemness [20].
Tremendous researches have proved that circadian disturbance or circadian sleep disorders contribute to cancers of lung, breast, liver, pancreas, ovary, colon [6, 11, 13, 14], multiple lymphomas, and leukemias [3, 21]. However, its impact on urological cancer is scattered and unsystematic. Herein, we comprehensively review the epidemiological researches on the relationship between circadian rhythm and urological cancer, we also discuss the underlying mechanism of how circadian disturbance contribute to urological carcinoma.
What is circadian clock and circadian rhythm
In 1971, Konopka and Benzer firstly reported the mutant clock gene in Drosophila [1, 22] while the affected Period was subsequently cloned [1, 23], and veil of circadian rhythm was then gradually uncovered. The 2017 Nobel Prize in Physiology or Medicine was awarded to Michael W. Young, Jeffrey C. Hall, and Michael Rosbash, just in order to reward their excellent achievements to discovery and clarify molecular regulatory mechanisms in circadian clock rhythm. Conceptually, there are three basic elements for an intact circadian clock system in whole animals: a master oscillator, an input pathway, and a humoral/neural output approach [1].
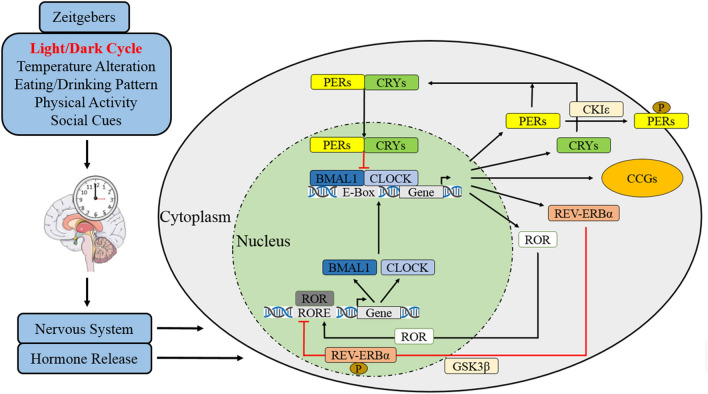
Suprachiasmatic nuclei (SCN) in anterior hypothalamus is the master oscillator of circadian clock for mammals [1, 3, 24]. It is comprised by ~ 20,000 densely packed small neurons in rodents and ~ 50,000 neurons in human [3, 25–27], its cellular component is also diverse and contains a variety of neurotransmitters and peptides [3, 28, 29]. Beside the central oscillator in SCN, circadian clock exists in virtually almost every peripheral tissue of multicellular organisms [1, 3, 30], this is proved by a robust oscillation of circadian gene being found with cell-autonomous and self-sustained manners in cultured cells in vitro [1, 3, 31, 32]. The input pathway is passage which synchronizes the non-24 h rhythm of circadian clock to 24-h and adapts the phase to environment or local time [1, 33, 34]. For instance, the retina perceives photic cues and transmits environmental light–dark cycles to SCN as electrical signal [9], others include the serotonin input to SCN from Raphe nuclei or neuropeptide Y from intergeniculate leaflet through geniculohypothalamic tract [1, 35–37]. The perceived or transferred cues to SCN are known as Zeitgeber or time givers, while the fundamental one is light/dark cycles, others cover temperature alterations, eating/drinking patterns, physical activities, and social cues [9, 38, 39]. The final element is humoral/neural pathways which transfer central circadian information to peripheral effector organs of dominant rhythm [1]. The major neural pathways are subparaventricular zone, paraventricular nucleus of thalamus, as well as dorsomedial nucleus and medial preoptic area of hypothalamus [1, 35, 37, 40–42]. Moreover, almost all hormones are rhythmically secreted with the daily cycle [1, 43, 44], this is also believed to be controlled by SCN rhythm (Fig. 3) [1, 45].
Fig. 3.
Molecular mechanism of circadian clock. After Zeitgebers of light, temperature, eating/drinking, physical activity, and social cues are perceived and transmitted to SCN, the central circadian clock system synchronizes with geophysical time and feedbacks to the downstream brain regions and peripheral organs by nervous system and hormone release. Briefly, CLOCK and BMAL1 form positive transcription factor of a heterodimer complex to promote rhythmic transcriptions of PER, CRY, and clock-controlled genes (CCGs) by integrating with E-box enhancers. PER and CRY accumulate in cytoplasm through the day and then translocate back to nucleus to reduce CLOCK/BMAL1 activity by forming a heterodimer complex. PER/CRY complex is also subsequently disassembled and resolved after CLOCK/BMAL1 concentration is declined. The remained free PER in cytoplasm is phosphorylated by casein kinase Iε (CKIε), and subsequently being ubiquitinated and degraded. Moreover, nuclear receptors of orphan nuclear receptor (REV-ERBα) and retinoic acid related orphan receptor alpha (RORα) can also regulate CLOCK/BMAL1 complex, while REV-ERBα is phosphorylated by glycogen synthase kinase-3β (GSK3β) to transcriptionally repress BMAL1 but RORα induces it
Circadian clock regulates the fluctuant rhythms by positive and negative feedback loops [6, 9, 11, 46]. Briefly, protein of circadian locomotor output cycles kaput (CLOCK) and brain/muscle arnt-like protein 1 (BMAL1) form the positive transcription factor of a heterodimer complex to promote rhythmic transcriptions of Period (PER1, PER2, and PER3) and Cryptochrome (CRY1 and CRY2) by integrating with E-box enhancers [1, 3, 6, 11]. PER and CRY accumulate in cytoplasm through the day and then translocate back to nucleus to reduce CLOCK/BMAL1 activity by forming a heterodimer complex [1, 3, 47, 48]. PER/CRY complex is also subsequently disassembled and resolved after CLOCK/BMAL1 concentration is declined [6, 11]. This feedback loop requires about 24-h to accomplish a full cycle [1, 3]. The remained free PER in cytoplasm is phosphorylated by casein kinase Iε (CKIε), and subsequently being ubiquitinated and degraded [49]. Moreover, the nuclear receptor REV-ERBα (also known as nuclear receptor subfamily 1 group D member 1, Nr1d1) and retinoic acid-related orphan receptor alpha (RORα) can also regulate CLOCK/BMAL1 complex, while REV-ERBα is phosphorylated by glycogen synthase kinase-3β (GSK3β) to transcriptionally repress BMAL1 but RORα induces it [6, 50].
Circadian disturbance and cancer
A reciprocal relationship has been found between circadian rhythm and cell cycles [2, 3, 51]. Circadian signals in different tumor types are always disturbed typically by DNA hypermethylation [52, 53], histone modification, or alterations in chromatin conformation and interactions [53, 54], which finally induce tumor cells to cycle at different rates [53]. As the cell cycle exit is always impaired in cancer, disturbed circadian singles probably accelerate such cycle exit impairment to facilitate cell cycle progression, and thereby promote tumor initiation, growth, and spread [53]. Misaligned circadian signal has been clarified during occurrence or progression in variable carcinoma hallmarks [53, 55] like maintaining proliferative signals, avoiding growth suppressors, resisting cell death, achieving replicative immortality, activating or sustaining angiogenesis, inducing invasion or metastasis, leading instability or mutation in genome, deregulating cell energetics, promoting tumor inflammation, and evading immune destruction [53].
In 1960s, the impact of circadian asynchrony on tumorigenesis was firstly reported [4, 56]. In 1980s, studies proved that disturbed endocrine rhythms promoted mammary tumor growth in rats [4, 57, 58]. In 2007, the International Agency for Research on Cancer Monograph classified circadian disturbance resulting from nightshift work or jetlag as probably carcinogenic to humans (Group 2A), however, this was only supported by sufficient experimental animal evidence, but lacking enough clinical researches [59–61]. Since then, scientific communities proved that circadian disturbance contributed to initiation or development of various cancers such as lung, breast, pancreatic, colorectal, endometrial, leukemias, and lymphomas [1, 3, 15, 17, 21, 62–64]. The Nurses’ Health Study revealed that women with rotating nightshift work ≥ 15 years showed approximately 28% increased risk [multivariate relative risk (MVRR) = 1.28, 95% confidence interval (CI) 1.07–1.53; p = 0.03] of lung cancer [3, 65]. Females with predominantly (> 60%) nightshift work > 1.5 years had higher risk [odds ratio (OR) = 1.5; 95% CI 1.2–1.7] to develop breast cancer, while OR increased with the duration of nighttime employment [3, 66]. Female nurses receiving rotating shiftwork > 15 years experienced increased risk of colorectal cancer (MVRR = 1.35; 95% CI 1.03–1.77; p = 0.04) [3, 65]. A US prospective cohort found the nightshift workers with duration > 20 years were more likely to suffer endometrial cancer (MVRR = 1.47; 95% CI 1.03–1.14) [3, 67].
Moreover, as advantages of circadian rhythm in physiological process, chronotherapy which termed as using timed dosing to obtain optimal therapeutic effects has been raised and shown good efficacy in cancer [68]. For instance, the chrono-chemotherapy was explored 50 years ago [69], while Walton et al. recently recruited 92 colorectal cancer patients which found that individuals received 5-Fluorouracil at the cycle peak (0400 h) experienced increased tolerability and better median survival rate than those with constant rate infusions [68, 70]. Patients with head and neck cancers who received chrono-radiotherapy demonstrated lower radiotherapy toxicity [68, 71], while the higher radiotoxicity incidence in dark months also suggested the seasonal effects of radiotherapy [68, 72]. In addition, other chronotherapies in cancers including immunotherapy, tyrosine kinase inhibitors, as well as antiangiogenic and hormone therapy are all exploring [68].
However, the impact of circadian disturbance on urological cancer has been investigated but is far from being clarified. We thus comprehensively review the relationship between circadian disturbance and urological cancer from clinical data, while discuss related experimental evidence and chronotherapy. We finally expect to provide more sensible advices on sleeping, eating, working, and resting rhythm for public.
Circadian disturbance and prostate cancer
Being the most common urological cancer and the second most prevalent male malignancy, prostate cancer (PCA) accounts for 15% of all malignancies with 1.1 million new cases diagnosed worldwide in 2012 [73]. A latest paper in CA Cancer J Clin even estimated PCA as the most common male cancer in US in 2021, which accounts 26% (248,530 cases) of all male malignancies [16]. Thanks to the PSA testing and advanced treatments like Da Vinci system or second-line endocrine drugs, the mortality of PCA has constantly declined by 4% per year during 1990s to 2000s [16]. The American Cancer Society also estimates that PCA shares the highest 5-year relative survival rate (98%) among all cancers; however, it still contributes the second leading cancer death that predicts to take away 34,130 lives in US in 2021 [16]. Apart from some established risk factors such as age, ethnicity, and family history, researchers try to clarify the impacts of circadian disturbance on PCA [59, 61].
It was in 1996 that a retrospective Canadian study found pilots, an occupational representative for shiftwork or insufficient sleep, suffered increased risk of PCA [74, 75]. A subsequent Nordic study in 2002 proved pilots with trans-meridian flights > 10,000 h had higher PCA incidences [75, 76]. Such risks were also observed on public safety workers and waiters who always worked at night or experienced short sleep [75, 77]. Since then, more relevant clinical and fundamental researches were performed to explore whether and how circadian disturbance impacts PCA.
Nightshift work and PCA
Nightshift work means working at night but sleeping at day [78]. It comprises more than 15% of the workforce [14, 79–81] and up to 50% of some professions have nightshift work, including police, firefighters, hospital workers, transport drivers, and manufacturing employees [78]. Altered sleep−wake cycle in nightshift work inevitably impairs internal circadian rhythm to lead human disease [5, 82], including PCA.
The Canadian population-based case−control study has shown that night workers suffered from threefold increased risk of PCA (OR = 2.77; 95% CI 1.96–3.92) [60, 83]. A Japan Collaborative Cohort (JACC) study enrolled 20,363 males and found that rotating nightshift work was associated with higher incidence of PCA (HR = 1.42; 95% CI 0.95–2.12) after a follow-up for 14.2 years [84]. The MCC-Spain case−control study randomly analyzed the difference between 1095 PCA cases and 1388 controls which revealed that nightshift work ≥ 1 year slightly enhanced PCA incidence (OR = 1.14; 95% CI 0.94–1.37) [85, 86]. This risk was elevated with longer duration of nightshift exposure (≥ 28 years; OR = 1.37; 95% CI 1.05–1.81; p = 0.047) and was particularly obvious for morning chronotype (OR = 1.79; 95% CI 1.16–2.76) [85, 86]. The CAPLIFE study also absorbed 456 PCA patients and 410 controls which observed that the percentage of ever-night workers was higher in PCA group (20.9%) than control arm (16.1%), while nightshift work was tightly correlated to PCA (OR = 1.47; 95% CI 1.02–2.11) especially for rotating nightshift work (OR = 1.73; 95% CI 1.09–2.75) [87].
On the contrary, a German retrospective cohort study found that rotating nightshift work was not related to PCA incidence (HR = 0.93; 95% CI 0.71–1.21) [60, 88]. A Swedish prospective cohort study enrolled 12,322 male twins and reported the cumulative incidences of PCA was similar between night workers (3.3%) and non-night workers (3.9%) (p = 0.16) after following up for 8.7 years [89]. Cox regression analysis showed that night work did not impact PCA risk after adjustment for covariates, nor association was observed regarding the duration of night work [89]. The Prostate Cancer and Environment Study (PROtEuS) also included 1,904 PCA case and 1,965 controls but found no relation between nightshift work and overall PCA incidence [90].
Considering the discrepant results, reviews and meta-analyses were performed to better explore the association between nightshift work and PCA. In 2015, the first meta-analysis with 2,459,845 participants and 9669 PCA cases found that nightshift work was significantly correlated to elevated risk of PCA (RR = 1.24, 95% CI 1.05–1.46; p = 0.011) [59]. Another meta with 2,546,822 individuals and 10,715 PCA patients showed that ever exposure to nightshift work increased PCA risk (RR = 1.23; 95% CI 1.08–1.41; p < 0.001) [91]. Although a nonlinear association was observed between the duration of nightshift work and PCA risk (p = 0.001); subgroup analysis further demonstrated a positive correlation with PCA incidence in rotating shiftwork group (RR = 1.10; 95% CI 1.00–1.26; p = 0.156) but not other shiftwork models [91]. Such conclusion was similar to another paper which reported that rotating nightshift work (RR = 1.06; 95% CI 1.01–1.12; I2 = 50%) rather than fixed nightshift work (RR = 1.01; 95% CI 0.81–1.26; I2 = 33%) significantly increased risk of PCA, while the RR was 20% higher in rotating group [92]. Moreover, although a meta-analysis showed that nightshift work was not related to PCA risks (RR = 1.05; 95% CI 1.00–1.11; p = 0.06; I2 = 24.00%), subgroup analysis still found that shiftwork in Asian countries (RR = 2.45; 95% CI 1.19–5.04; p 0.02; I2 = 0.00%) rather than Western countries (RR = 1.05; 95% CI 0.99–1.11; p 0.09; I2 = 0.00%) promoted PCA risks [93]. This result was also insisted by another meta research which proved that Asian populations with nightshift work suffered slightly higher PCA incidence (RR = 1.98; 95% CI 1.34–2.93; p = 0.618) [91]. On the contrary, the latest meta with 18 studies claimed that rotating nightshift work did not impact PCA risk (OR = 1.07; 95% CI 0.99–1.15; I2 = 45.7%; p = 0.016) [61]. Thus, the original and secondary researches did not provide consistent conclusion on whether nightshift work promoted PCA risk, authors speculated this discrepancy as the result of different definitions on nightshift work and other confounders [90].
In addition, the impact of circadian disturbance on prostate specific antigen (PSA) level is also inconsistent. The National Health and Nutrition Examination Survey (NHANES) study found that tPSA level in nightshift workers (1.32 ± 2.06 ng/mL) was significantly higher than nonshift workers (1.18 ± 1.34 ng/mL) [75]. The nightshift workers have higher risk of tPSA ≥ 4.0 ng/mL after age (OR = 2.48; 95% CI 1.08–5.70; p = 0.03) and confounders (OR = 2.62; 95% CI 1.16–5.95; p = 0.02) adjustment, while more nightshift workers also suffered from f/t ≤ 25% (OR: 3.13; 95% CI 1.38–7.09; p = 0.01) when tPSA ≥ 4.0 ng/mL [75]. In contrast, another research claimed that tPSA concentration in nightshift workers was slightly lower than the day workers, however, the unknown confounders were not excluded and analyzed [94].
Sleep duration/chronotypes and PCA
Although the National Sleep Foundation suggests a sleep duration of 7–9 h for population aged 18–64 years and 7–8 h for aged > 65 years [95, 96], the National Health Interview Survey observed that 30% of workers (approximately 40.6 million) slept less than 6 h in US [97]. Defined as midpoint between sleep onset and awakening when alarm clock is not used, chronotype is a marker of circadian rhythm which represent timing of sleep−wake cycle [98]. The impact of sleep duration or chronotype on PCA is also complicated.
A prospective study enrolled 22,320 Japanese men revealed that sleep duration was inversely associated to PCA risk, that males sleeping ≥ 9 h/night was less likely to develop PCA (RR = 0.48; 95% CI 0.29–0.79; p = 0.02) [99]. The US Health Professionals Follow-Up Study (HPFS) revealed that men slept ≥ 10 h/night (RR = 0.70; 95% CI 0.50–0.99) had a 30% reduced risk of PCA than slept 8 h/night, while those never feeling rest when they woke up were more likely (RR = 3.05; 95% CI 1.15–8.10) to develop fatal PCA than with feeling rest [100]. The AGES-Reykjavik prospective cohort study recruited 2102 men and diagnosed 135 PCA (6.4%) after following-up for 5 years [101]. Meanwhile, those experienced severe (HR = 1.7; 95% CI 1.0–2.9) or very severe (HR = 2.2; 95% CI 1.2–3.9) sleep problems suffered more PCA incidence, this association was stronger (HR = 2.1; 95% CI 0.7–6.2; HR = 3.2; 95% CI 1.1–9.7) when restricted to advanced PCA (≥ T3 or lethal stage) [101]. A US prospective cohort study observed that rotating shift workers (RR = 1.08; 95% CI 0.95–1.22) or fixed night workers (RR = 0.72; 95% CI 0.44–1.18) did not associate to fatal PCA than fixed day workers [102]. During the first follow-up of 8 years, sleep duration for 3–5 h/night (RR = 1.64; 95% CI 1.06–2.54) and 6 h/night (RR = 1.28; 95% CI 0.98–1.67) significantly increased PCA risk compared with sleeping for 7 h/night, however, this association was not observed during the later follow-up [102].
Moreover, the EPICAP study analyzed 818 PCA cases and 875 controls which found that night work did not correlated to PCA risk, but the incidence of PCA was increased in evening chronotype (OR = 1.83, 95% CI 1.05–3.19) and elevated as night work duration was lengthened (p = 0.01) [103]. While permanent night work duration > 20 years promoted aggressive PCA occurrence (OR = 1.76, 95% CI 1.13–2.75), and the risk was more pronounced when combined with ≥ 6 consecutive nights (OR = 2.43, 95% CI 1.32–4.47) or shift length > 10 h/night (OR = 4.64, 95% CI 1.78–12.13) [103]. Although the CAPLIFE study reported that duration of nightshift work was not related to PCA incidence (OR = 1.08; 95% CI 0.51–2.29), rotating shift intensity (> 74 nights/ year) enhanced PCA risk (OR = 2.60; 95% CI 1.34–5.02) [87]. In addition, the evening chronotype suffered more PCA risk (OR = 3.14; 95% CI 0.91–10.76) than morning type (OR = 1.25; 95% CI 0.78–2.00), while the highest risk was found when evening chronotype was combined to permanent night work (OR = 3.53; 95% CI 0.76–16.34) or rotating nightshift work (OR = 2.72; 95% CI 0.54–13.62) [87].
On the contrary, a Swedish National March Cohort study identified 785 PCA cases after a following-up for 13 years, however, neither sleep duration nor disruption (sleep quality, restorative power of sleep, and difficulty in falling asleep or maintaining sleep) was correlated to PCA risk, whether for overall or advanced/lethal cases [104].
Generally speaking, the current studies reveal that insufficient sleep promotes PCA occurrence and prolonged sleep duration reduces it, however, how much does sleep duration works remain to be better investigated. Moreover, evening chronotype increases PCA incidence particularly when combined to other circadian disorders like nightshift work, but how does other chronotypes act is also indistinct [105].
Other disturbed circadian rhythms
Light is the primary Zeitgeber (or time synchronizer). As known, more than 80% worldwide population and one-fifth terrain in our planet suffer from light pollution as the widespread of artificial lights, which dramatically changes internal circadian rhythm and contributes tremendous diseases [106]. The MCC-Spain Study which explored impact of artificial light-at-night (ALAN) exposure found that sleeping with more illuminated bedrooms (indoor ALAN) increased PCA risk than sleeping with total darkness (OR = 2.79; 95% CI 1.55–5.04) [106]. Although the higher tertile of blue light spectrum (outdoor ALAN-MSI) was associated with more PCA incidence (OR = 2.05; 95% CI 1.38–3.03) than the lowest tertile, however, the highest tertile of visual light (outdoor ALAN) decreased PCA risk (OR = 0.56; 95% CI 0.38–0.84) [106]. Kim et al. observed that ALAN (OR = 1.02; p = 0.0369) and urbanization (OR = 1.06; p = 0.0055) significantly promoted PCA incidence, while a relatively more PCA risk (72.6%) was found with higher ALAN level (25% vs. 75%) [107]. Another study also observed that ALAN was a highly significant predictor of PCA after age-standardized (p < 0.01) [108].
Defined as difference between social and biological time or between work and free days [109], the jetlag can also induce circadian disturbance which induces obesity, metabolic disorders, and cardiovascular risk [86]. The Alberta’s Tomorrow Project collected 7455 cancer-free men and followed-up for about 10 years which found that individuals with social jetlag for 1–2 h (HR = 1.52; 95% CI 1.10–2.01) or > 2 h (HR = 1.69, 95% CI 1.15–2.46) significantly increased PCA risk than those without (p = 0.004), such interaction remained even sleep duration was adjusted (p = 0.006) [86].
Meal timing is also synchronized with peripheral circadian rhythm and the MCC-Spain found that sleeping two or more hours after supper decreased incidence of PCA than those sleeping immediately after supper (OR = 0.74; 95% CI 0.55–0.99), while the protection was more pronounced when supper-sleep interval was longer (OR = 0.65; 95% CI 0.44–0.97) [110].
In a word, the current reports suggest tightly interaction between other forms of disturbed circadian rhythm (like increased light exposure, jetlag, and meal timing) and PCA risk, however, the published studies are still relatively small.
Genetic findings
Apart from the epidemiologic studies, genetic investigations have also explored whether and how circadian clock was involved to PCA.
For gene polymorphisms among nightshift workers, the EPICAP population-based study found that core-circadian pathway (p = 0.0006) was significantly correlated to PCA, both for low (p = 0.002) and high (p = 0.01) grade tumors [111]. This connection was also significant among nightshift workers with aggressive PCA (p = 0.004), and especially predominant for those working at night < 20 years (p = 0.0002) or receiving long nightshift > 10 h/night (p = 0.001) [112]. Although ARNTL, NPAS2, and RORA were all significantly related to aggressive PCA for nightshift workers at gene level [111, 112]. A population-based study which analyzed 41 tagging single nucleotide polymorphisms (SNPs) in 10 circadian core genes among Caucasian men (1308 PCA and 1266 controls) [113]. It found that more than one SNP in nine circadian genes (PER1/2/3, CSNK1E, CRY1/2, ARNTL, CLOCK, NPAS2) were significantly correlated to PCA (both for overall and aggressive risks), while four SNPs in three genes (PER1/3, CLOCK) were varied by disease aggressiveness [113]. A Chinese population-based study found that CRY2-variant C allele indicated about 1.7-fold increased PCA risk (95% CI 1.1–2.7) than GG genotype [114]. Meanwhile, the Prostate Cancer Prevention Trial (PCPT) found that NPAS2 variation was related to PCA risk in finasteride group, and one SNP remained statistically significant (rs746924; OR: 1.5; p = 9.6 × 10−5) even after Bonferroni correction [115]. Another research proved a SNP of NPAS2 (rs6542993 A > T) was significantly connected to higher risk of PCA progression, both for localized (p = 0.001) and advanced (p = 0.039) cases [116]. Moreover, a gene- and pathway-based study found that aggregate circadian genetic variation and melatonin pathway were closely correlated to PCA risk after Bonferroni correction (ppathway < 0.00625), while the top two significant genes were NPAS2 (pgene < 0.0062) and AANAT (pgene < 0.00078) after Bonferroni correction [117]. In addition, the Consortium meta-analysis of six GWASs (14,160 PCA and 12,724 controls) also proved that circadian pathway genetic variation was significantly correlated to PCA (p = 4.1 × 10–6; top gene ARNTL, gene p = 0.0002); subgroup analysis revealed that seven circadian pathway variation (PER1/2, TIMELESS, NPAS2, ARNTL, RORα/β) were significantly related to aggressive PCA risk [118]. Taken together, genetic variations in circadian clock genes have potential roles in carcinogenesis and progression of PCA [113, 116, 117], although functional and fundamental studies are warranted to better explore its underlying biological mechanisms [117] (Table 1).
Table 1.
Summary for the association between PCA and circadian clock genes
| Author | Refs | Study design | Study size | Function | Main result |
|---|---|---|---|---|---|
| Wendeu-Foyet | [111, 112] | Case–control study | 732 cases and 783 controls | Genotyping prediction |
The core-circadian pathway (CLOCK, BMAL1, CRY1/2, PER1/2/3, CSNK1E, NPAS2) (p = 0.0006) was correlated to PCA, both for low (p = 0.002) and high (p = 0.01) grade tumors These pathway was also significant for nightshift workers with aggressive PCA (p = 0.004), and especially predominant for those working at night < 20 years (p = 0.0002) or receiving long nightshift > 10 h/night (p = 0.001) ARNTL, NPAS2, and RORA were significantly related to aggressive PCA for nightshift workers at gene level |
| Zhu | [113] | Case–control study | 1308 cases and 1266 controls | Genotyping prediction | One SNP in nine circadian genes (PER1/2/3, CSNK1E, CRY1/2, ARNTL, CLOCK, NPAS2) were significantly correlated to PCA (both for overall and aggressive risks), while four SNPs in three genes (PER1/3, CLOCK) were varied by disease aggressiveness |
| Chu | [114] | Case–control study | 187 cases and 242 controls | Genotyping prediction | CRY2-variant C allele indicated about 1.7-fold increased PCA risk (95% CI 1.1–2.7) than GG genotype |
| Chu | [115] | Case–control study | 450 cases and 422 controls | Genotyping prediction | NPAS2 variation was related to PCA risk, with one SNP remained statistically significant (rs746924) after Bonferroni correction |
| Yu | [116] | Case–control study | 458 localized and 324 advanced PCA | Genotyping prediction | A SNP of NPAS2 (rs6542993 A > T) was significantly connected to PCA progression, both for localized (p = 0.001) and advanced (p = 0.039) cases |
| Gu | [117] | Bioinformatics tool | 14,818 cases and 14,227 controls | Genotyping prediction | The top two significant genes related to PCA were NPAS2 (pgene < 0.0062) and AANAT (pgene < 0.00078) after Bonferroni correction |
| Mocellin | [118] | Consortium meta-analysis | 14,160 cases and 12,724 controls | Genotyping prediction |
Circadian pathway genetic variation was significantly correlated to PCA (p = 4.1*10–6; top gene ARNTL, gene p = 0.0002) Seven circadian pathway variation (PER1/2, TIMELESS, NPAS2, ARNTL, RORα/β) were significantly related to aggressive PCA |
| Markt | [119] | Kernel machine test | 24, 40, and 105 fatal cases respectively | Genotyping prediction |
None of the 96 SNPs in 12 circadian clocks was individually consistent involved to fatal PCA Even CRY1 variation was just nominally involved to fatal PCA (p = 0.01, 0.05, 0.01 for AGES-Reykjavik, PHS, and HPFS, respectively) |
| Cao | [120] | – | Animal and/or cells | Suppressor-Per1 |
Per1 could interact with AR to inhibit its transcriptional activity in LNCaP Overexpressed Per1 significantly reduced tumor growth and induced apoptosis for PCA cells |
| Jung-Hynes | [121] | – | Animal and/or cells |
Suppressor-Per2, Clock Promoter-Bmal1 |
Bmal1 was increased but Clock and Per2 were dramatically decreased in PCA cells Upregulated Per2 could inhibit tumor growth and viability Melatonin preserved Per2 and Clock while decreased Bmal1 to manage PCA |
| Li | [122] | – | Animal and/or cells |
Suppressor-Per3 Promoter-Bmal1 |
PER3 was downregulated both in human PCA tissue and ALDHhiCD44+ (DP) PCA cells, PER3 concentration was associated to better patient survival Low PER3 level induced expression of BMAL1 to lead phosphorylation of β-catenin and activate WNT/β-catenin pathway in TME |
| Cai | [123] | – | Animal and/or cells | Suppressor-Per3 |
PER3 in paclitaxel-resistant PCA tissue was significantly lower than nonresistant group, upregulation of PER3 induced paclitaxel-resistant PCA being sensitive to paclitaxel Overexpressed PER3 significantly reduced IC-50, arrested cell cycle, and increased apoptosis Overexpressed PER3 attenuated this paclitaxel-resistance by inhibiting Notch1 |
On the contrary, after reviewing 3 studies (AGES-Reykjavik, Physicians’ Health Study (PHS), and Health Professionals Follow-Up Study (HPFS)), Chu et al. found that none of the 96 SNPs in 12 circadian clocks was individually consistent involved to fatal PCA [119]. Although even the CRY1 variation was just nominally involved to fatal PCA (p = 0.01, 0.05, 0.01 for AGES-Reykjavik, PHS, and HPFS, respectively) in gene-based analyses [119] (Table 1). Thus, the relationship between circadian disturbance and PCA risk is still controversial at gene level [111, 115].
Fundamental researches
A meta-analysis from microarray expression has shown that PER1/2 were downregulated in human PCA sample [120]. Per1 could interact with AR to inhibit its transcriptional activity in LNCaP, and overexpressed Per1 significantly reduced tumor growth and induced apoptosis for PCA cells [120]. At gene and protein levels, another study found that Bmal1 was increased but Clock and Per2 were dramatically decreased in PCA cells, while upregulated Per2 could inhibit tumor growth and viability [121]. Furthermore, as the regulator of circadian clock rhythm, melatonin preserved Per2 and Clock while decreased Bmal1 to manage PCA; it also resynchronized the circadian oscillatory rhythm of tumor cells (Per2 and Dbp) [121]. In the meantime, PER3 was downregulated both in human PCA tissue and ALDHhiCD44+ (DP) PCA cells, while its concentration was associated to better patient survival [122]. The clonogenicity and tumorigenicity in DP cells were significantly inhibited with the overexpressed PER3, while the colony-forming and tumor-initiating abilities in DN cells were enhanced with PER3 knockdown; this was mechanistically explained by that low level of PER3 induced the expression of BMAL1 to lead phosphorylation of β-catenin and activate WNT/β-catenin pathway in TME [122]. Moreover, PER3 in paclitaxel-resistant PCA tissue was significantly lower than nonresistant group, while upregulation of PER3 induced paclitaxel-resistant PCA being sensitive to paclitaxel [123]. Subsequent research proved that overexpressed PER3 significantly reduced IC-50, arrested cell cycle, and increased apoptosis; meanwhile, it also attenuated this paclitaxel-resistance by inhibiting Notch1 [123]. All these suggest a key role of PER in PCA transformation [49, 120], however, studies about the other circadian clock genes are still limited (Table 1).
The impact of circadian disturbance was also performed on CRPC rat model, that continuous light decreased melatonin level to stimulated PCA growth while melatonin supplement inhibited tumor growth and reversed enzalutamide resistance [124]. Further analysis clarified that melatonin regulated epigenetic modification of carboxylesterase 1 (CES1) by promoting SIRT1-mediated DNA methyltransferase 1 (DNMT1) deacetylation, while the restored carboxylesterase 1 (CES1) then reduced lipid droplet accumulation to induce endoplasmic reticulum (ER) stress-related apoptosis and block intratumoral androgen synthesis, which finally inhibited CRPC progression and reversed enzalutamide resistance [124].
Circadian radiotherapy and PCA
Circadian radiotherapy strategy has been performed to improve the therapeutic effects or to reduce toxicities. In 2016, a retrospective study separated localized PCA cases with radiotherapy into daytime (n = 267; before 5 PM) and evening (n = 142; after 5 PM) groups [125]. As was shown, the evening group showed significantly increased acute gastrointestinal (HR = 0.79; 95% CI 1.18–2.72; p = 0.01) and genitourinary toxicities (HR = 2.41; 95% CI 1.49–3.88; p < 0.001), it also had more late gastrointestinal toxicities (≥ grate 2) (HR: 2.96; 95% CI 1.63–5.37; p < 0.001) especially for patients aged > 70 years [125]. Considering the circadian variation of DNA synthesis revealed an acrophase in the morning and a bathyphase in the evening, while S-phase cells were more tolerated to radiation-induced DNA damage, authors suggested that rectal mucosa with less DNA synthesis in the evening might be more susceptible to radiation damages [125]. For advanced PCA (≥ T2b), the evening group (69%) demonstrated poorer biochemical failure-free survival (BFFS) than daytime patients (81%) (p = 0.04), although after matching cohort for age, tumor stage, GS score, initial PSA, and ADT (HR = 1.95; 95% CI 1.00–3.81; p = 0.05) [125, 126]. This was explained by that the more advanced tumor stage had more circadian variation in prolactin, melatonin, and thyroid-stimulating hormone [125]. Even though, the exact mechanism is unclear, and whether circadian treatment really works still need more clinical and basic evidence.
Circadian disturbance and kidney cancer
Renal cell carcinoma (RCC) represents approximately 90% of all kidney cancer and accounts for 3% of all human malignancies [127]. The annual incidence of RCC increases about 2% in Europe and worldwide during the past twenty years, which contributes to nearly 99,200 new cases of RCC and 39,100 kidney cancer-associated death in European Union in 2018 [127], as well as more than 400,000 new cases and 175,000 cancer-related death across the world [128]. Since 1990s, the overall mortality of RCC is generally stabilized or decreased in Europe, but is increased in other regions (like Greece, Croatia, Ireland, Estonia, and Slovakia) [127]. The risk factors vary for kidney cancer, including obesity, age, hypertension, and cigarette smoking [17]; while association between circadian disturbance and RCC has also been explored.
Epidemiological finding
The Montreal Multisite Case−Control study found nightshift work slightly increased the risk of kidney cancer (OR = 1.42, 95% CI 0.86–2.35) [83]. However, the NIH-AARP Health and Diet Study claimed that neither short nor long sleep duration impacts the kidney cancer incidence [129]. Hence, the current epidemiological researches on this issue are relatively small and the results are controversial. However, some fundamental studies still try to explore whether circadian disturbance is involved in the onset or progression of kidney cancer from molecular level.
Genetic and fundamental research
In 2012, Mazzoccoli evaluated clock gene machinery on 11 kidney cancer patients which found that PER2, TIPIN, and TIMELESS were significantly decreased but SERPINE1 was increased [130]. Commonly treated as a tumor suppressor in kidney cancer, Per2 revealed a circadian rhythmicity in Caki-2 cells; while HIF-1α could induce Per2 transcription and promote amplitude of Per2 oscillation by directly binding to its promoter [131]. Although HIF-1α-related gene pathway was the major risk factor for tumor growth in kidney cancer, this study suggested that HIF-1α might induce Per2 transcriptional activity to inhibit tumor proliferation [131]. Considering the fundamental evidence on association between circadian disturbance and kidney cancer is also limited and result is inconsistent, authors try to explore this issue from other angles.
In 2021, a Mendelian randomization (MR) study based on the UK Biobank observed that genetically predicted long-sleep duration decreased the odds of kidney cancer (OR = 0.44; 95% CI 0.21–0.90; p = 0.025) while genetic liability to continuous sleep period had lower risk of kidney cancer (OR = 0.50; 95% CI 0.25–0.99; p = 0.046), indicating a protective role of long-sleep duration on kidney malignancy [132]. A multi-omics analysis with bioinformatics tools investigated the impact of nine core circadian clock genes (CLOCK, BMAL1, CRY1/2, PER1/2/3, RORA, and NR1D1) on kidney renal clear cell carcinoma (KIRC) [128]. It was found that patients with highly expressed CLOCK, CRY1/2, PER2/3, and RORA had better overall survival (OS); those with increased CLOCK, CRY1/2, PER1/2/3, and RORA enjoyed better disease-free survival (DFS) [128]. A analysis from Cancer Genome Atlas (TCGA) using R and Perl programming languages proved that high levels of CLOCK, PER1/2/3, NR1D2, and RORA experienced longer OS for kidney cancer patients, however, those with high Timeless and NPAS2 levels suffered from poor survival [133]. These suggested that most of the low expressed circadian clock genes led to poor prognosis in kidney cancer patients; while the authors speculated the decreased clock genes might contribute to downregulation of cell-cycle genes and impair cell cycle in various stages, such alteration could change the malignant cell periodicity and induce abnormal uncontrolled cell proliferation which finally deteriorated OS [133]. In addition, an integrated analysis with genomic, transcriptomic, and clinical data clarified 32 circadian clock genes as putative loss of-function (ClockLoss, recurrently deleted and downregulated genes including CLOCK, CRY2, FBXL3, FBXW11, NR1D2, PER1, PER2, PER3, PRKAA2, RORA, and RORB) and gain-of-function (ClockGain, recurrently amplified and upregulated genes including BMAL2/ARNTL2 and NR1D1) players [4]. They found that downregulation of ClockLoss genes revealed significantly higher mortality rates in pan-kidney (p = 0.011) and ccRCC (p < 0.0001) cancers, while high upregulated ClockGain genes indicated poorer survival in pan-kidney (p = 0.0034) and ccRCC (p = 0.014) cancers [4]. This declared that ClockLoss and ClockGain were correlated with roles of tumor-suppressing and tumor-promoting for kidney cancer, respectively [4]; but the underlying mechanism is far from being understood (Table 2).
Table 2.
Summary for the association between kidney cancer and circadian clock genes
| Author | Refs | Study design | Study size | Function | Main result |
|---|---|---|---|---|---|
| Zhou | [128] | Bioinformatics tool | 533 KIRC samples | Genotyping prediction |
Highly expressed CLOCK, CRY1/2, PER2/3, and RORA had better OS Increased CLOCK, CRY1/2, PER1/2/3, and RORA enjoyed better DFS BMAL1, PER1/2, RORA, and NR1D1 were significantly upregulated but CLOCK and CRY1/2 were downregulated |
| Qiu | [134] | Bioinformatics tool | 538 cases and 72 adjacent normal tissues | Genotyping prediction |
High levels of CLOCK, PER1/2/3, NR1D2, and RORA experienced longer OS Increased Timeless and NPAS2 levels indicated poor survival CLOCK, PER1/2/3, CRY2, NPAS2, NR1D2, and RORA were highly expressed in kidney cancer patients with TNM stage (I-II) PER1/2/3, CRY2, Timeless, NPAS2, NR1D2, and RORA were highly expressed in well differentiated carcinoma type (G1, G2) |
The multi-omics analysis found that all circadian genes in KIRC tissues were fluctuant expressed, except RORA; while BMAL1, PER1/2, RORA, and NR1D1 (p < 0.001) were significantly upregulated but CLOCK and CRY1/2 (p < 0.001) were downregulated [128]. Analysis from TCGA showed that high expression rate of CLOCK, PER1/2/3, CRY2, NPAS2, NR1D2, and RORA were observed in kidney cancer patients with TNM stage (I-II), and high level of PER1/2/3, CRY2, Timeless, NPAS2, NR1D2, and RORA were found in well differentiated carcinoma type (G1, G2) [133] (Table 2). Thus circadian rhythm was tightly related to the stage of kidney cancer, while authors inferred that circadian genes promoted abnormal expression of oncogenes, tumor-suppressor genes, anti-apoptosis and pro-apoptotic genes during the initiation and progress of carcinoma [133]. Meanwhile, circadian rhythm was involved to several KIRC-related hallmark pathways, including cell cycle, apoptosis, DNA damage response; it also regulated DNA binding and gene expression in enrichment analysis [128]. Moreover, circadian rhythm was strongly related to immune cells like B cell, CD4/8 cell, macrophage, neutrophil, and dendritic cell [128]. These proved that circadian rhythm regulated tumor microenvironment and immune system in kidney cancer [128, 134], however, the specific mechanism remained to be clarified.
Circadian chemotherapy and kidney cancer
Circadian chemotherapy strategy has explored to reduce the therapeutic toxicity of kidney cancer for decades [135]. In 1990s, Hrushesky et al. found that continuous fluorodeoxyuridine (FUDR) infusion with a circadian-modified schedule (68% of the daily dose was given between 15:00 and 21:00) was effective for metastatic RCC compared with a constant schedule, however, the permitted safety was higher and toxicity is markedly reduced in the circadian model [135, 136]. Moreover, another multicenter study proved that metastatic RCC patients receiving FUDR infusion in circadian strategy (65% of the daily dose was administrated between 20:00 and 02:00) had less diarrhea and dose-limiting toxicity than the flat schedule, meanwhile more dose escalation was given but less dose reduction was found in circadian arm [135, 137]. Although chemotherapy is currently invalid for kidney cancer, whether the circadian strategy improves therapeutic effect or reduces toxicity for other treatments (like targeted drug or PD-1) remains to be well studied.
Although the research is limited and evidence is weak, the current epidemiological studies reveals that nightshift work potentially promote kidney cancer occurrence while genetic and fundamental analyses demonstrate that circadian clock genes are altered in tumor tissues and involved with patients’ prognosis. Whether other forms of disturbed circadian rhythms like light/dark alteration, temperature cycles, eating/drinking patterns, physical activity, or social cues [1, 35, 43, 138] affect kidney cancer remains to be explored, how circadian clock genes involve in kidney cancer at molecular level also need further investigation.
Circadian disturbance and bladder cancer
Once as the most usual urological malignancy, bladder cancer is now the 7th most commonly diagnosed cancer for men and 11th for women [139]. The worldwide age-standardized incidence of bladder cancer (per 100,000 person/year) is 9.0 for males and 2.2 for females while the mortality rate is 3.2 for males and 0.9 for females [139], with an estimated 430,000 new cases and 165,100 deaths being found in 2012 [140]. When considering the tremendous incidence and mortality rates, bladder cancer has led huge burdens to patients and families [140]. According to the data from Occupational Cancer Research Centre (OCRC), the estimated total cost for 199 new-diagnosed bladder cancer was $131 million, with an average per-case charge of $658,055 [141]. Bladder cancer has been proved being tightly related to factors of tobacco consumption, chemical exposure, industrialization and urbanization, and unhealthy lifestyles [140]. Recently, the association between circadian disturbance and bladder cancer is also been concerned.
Epidemiological finding
The NIH-AARP Health and Diet cohort study found that sleep less than 7–8 h potentially increased bladder cancer incidence (HR = 1.10; 95% CI 1.00–1.20; p = 0.07) [129, 142]. The Montreal Multisite Case − Control Cancer study observed that long-term night work (> 10 years duration) significantly promoted occurrence of bladder cancer (OR = 1.74; 95% CI 1.22–2.49) [83]. Meanwhile, an integrated analysis proved the down-regulation of ClockLoss genes was significantly related to higher mortality incidence (p = 0.027) and poorer OS (HR = 1.776; p = 0.043) for bladder cancer [4]. However, the MR study based on UK Biobank claimed that neither the genetic predicted short (OR = 1.15; 95% CI 0.79–1.66; p = 0.459) nor long (OR = 0.96; 95% CI 0.51–1.81; p = 0.892) sleep duration was correlated to bladder cancer [132]. Although the current study on the association is small and conclusion is controversial, some researches are performed to explore this issue on fundamental level.
Genetic and fundamental research
The mRNA levels of BMAL1, CLOCK, and PER1/2/3 in bladder tumor were significantly decreased than neighboring benign mucosa, while CRY1 was dramatically upregulated and CRY2 was downregulated in both tumor and neighboring tissue when compared with normal donor tissue [143]. A data-mining analysis found that TP53 (HUB node) was tightly correlated to CSNK1ε (circadian node), while some other circadian modes was related to other HUB nodes after passing one or two nodes [144]. Senescence is an important tumor suppressive mechanism, while the senescence induced by radio- or chemotherapy prevents the occurrence and development of cancer by restricting unlimited cell proliferation [145–147]. The CRY1 was accumulated in quiescent cisplatin-resistant bladder cells while CRY1 knockdown increased PTX-induced senescence [147]. Further research proved that elevated CRY1 in cisplatin-resistant bladder cells induced p53 degradation by promoting FOXO1 binding to its ubiquitin E3 ligase MDM2, which finally preventing PTX-induced senescence [147]. A genome-wide open chromatin analysis observed that overexpressed NPAS2 inhibited trans-well migration in SCABER cell probably via repressing a subset basal maker genes like KRT5, KRT6A, and TFAP2C, while TCGA data also proved that higher NPAS2 promoted OS for patients with bladder cancer [148]. These finally inferred that circadian disturbance might contribute additional mechanisms to progression and behavior for bladder cancer [143, 144], even the detail is blurry [148] (Table 3).
Table 3.
Summary for the association between bladder cancer and circadian clock genes
| Author | Refs | Study design | Study size | Function | Main result |
|---|---|---|---|---|---|
| Litlekalsoy | [144] | Case–control study | 27 cases and benign tissues, and 15 normal bladder tissue |
Suppressor-Per2, Clock Promoter-Bmal1 |
BMAL1, CLOCK, and PER1/2/3 in bladder tumor were significantly decreased than neighbouring benign mucosa CRY1 was dramatically upregulated and CRY2 was downregulated in both tumor and neighbouring tissue |
| Polo | [145] | Bioinformatics tool | no | Suppressor-CSNK1ε | TP53 (HUB node) was tightly correlated to CSNK1ε (circadian node), some other circadian modes was related to other HUB nodes after passing one or two nodes |
| Jia | [148] | – | Animal and/or cells | Promoter-CRY1 |
CRY1 was accumulated in quiescent cisplatin-resistant bladder cells while CRY1 knockdown increased PTX-induced senescence Elevated CRY1 in cisplatin-resistant bladder cells induced p53 degradation by promoting FOXO1 binding to its ubiquitin E3 ligase MDM2, which finally preventing PTX-induced senescence |
| Iyyanki | [149] | – | Animal and/or cells | Suppressor-NPAS2 |
Overexpressed NPAS2 inhibited trans-well migration in SCABER cell probably via repressing a subset basal maker genes like KRT5, KRT6A, and TFAP2C Higher NPAS2 promoted OS for patients with bladder cancer |
Circadian chemotherapy and bladder cancer
Hrushesky et al. has explored the monthly circadian-timed doxorubicin (morning)–cisplatin (evening) chemotherapy immediately after cystectomy which proved that circadian-timed regimen with full doses for nine courses might delayed and prevented local or distant recurrence for locally advanced bladder cancer [135, 149]. Such circadian based adjuvant chemotherapy is more tolerated than other combination regimens [135, 149]. For metastatic bladder cancer, circadian-timed combination chemotherapy achieved excellent quality of life with limited toxicity, which finally promoted patients’ experience [135, 150]. However, a well-designed control group is necessary before any conclusion on circadian chemotherapy is made for bladder cancer.
Thus, the limited clinical studies found nightshift work or disturbed sleep rhythms potentially promoted risk of bladder cancer, while genetic and fundamental researches observed that circadian clock genes were involved to its tumorigenesis and progression. Just like the situation on kidney cancer, researches on association between circadian disturbance and bladder cancer is small and the evidence is also weak. More epidemiological studies on whether circadian disturbance impacts bladder cancer are warrant, while fundamental studies about how circadian disturbance regulates bladder cancer are also being expected.
Conclusion
The altered life style or elevated work pressure in modern society has gradually disturbed internal circadian rhythm, which finally contributes to various disease including cancer. More studies are continuous clarifying whether and how circadian disturbance are involved with occurrence and progression of various malignancies. Regarding the impact of circadian disturbance on urological cancers, some researches have proved a potential connection with PCA, while investigations for kidney and bladder cancers are relatively small, but no studies focus on other urological malignancies. These associations are generally controversial and evidence is relatively weak, whether from epidemiological or fundamental results. The impact of circadian disturbance on urological cancers is worth to be expected. However, it is difficult to clarify which specific circadian disorder contribute to urological cancer as the Zeitgeber is various and circadian clock is very vulnerable to be affected; moreover, circadian clock cycle is consisted by 32 genes (about 7 core genes), which specific circadian gene is responsible for some cancer is also hard to illuminate.
Author contributions
All authors contributed to the study conception and design. TL, YJ, and YB wrote the first draft of the manuscript; KJ, GD, and PC edited the English language; CL and LL searched and reviewed the referenced papers; JQ and JS reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This manuscript was funded by the Science and Technology Foundation Project of Guizhou Provincial Health Commission (gzwkj2024-150), the National Nature Science Foundation of China (No. 82060276 and 82360295), the Science and Technology Department of Guizhou Province (QianKeHeJiChu-ZK[2021]YiBan382), the Sichuan Province Science and Technology Innovation Seedling Project (2021039), and the Doctor Start-up Fund of Affiliated Hospital of Guizhou Medical University (gyfybsky-2023-03).
Data availability
Data sharing is not applicable to this article as no datasets were used during the current review.
Declarations
Conflict of interest
Ethical approval is not applicable for this review, the authors have no relevant financial or nonfinancial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tao Li, Yiting Jiang and Yunjin Bai contributed equally to this work.
Contributor Information
Jun Qiao, Email: 80373496@qq.com.
Jun Shen, Email: shenjun@gmc.edu.cn.
References
- 1.Noh JY, Han DH, Yoon JA, Kim MH, Kim SE, Ko IG, Kim KH, Kim CJ, Cho S. Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J. 2011;15:64–73. doi: 10.5213/inj.2011.15.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shostak A. Circadian clock, cell division, and cancer: from molecules to organism. Int J Mol Sci. 2017;18:873. doi: 10.3390/ijms18040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker WH, 2nd, Bumgarner JR. Light pollution and cancer. Int J Mol Sci. 2020;21:9360. doi: 10.3390/ijms21249360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang WH, Lai AG. Timing gone awry: distinct tumour suppressive and oncogenic roles of the circadian clock and crosstalk with hypoxia signalling in diverse malignancies. J Transl Med. 2019;17:132. doi: 10.1186/s12967-019-1880-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fatima N, Rana S. Metabolic implications of circadian disruption. Pflugers Arch. 2020;472:513–526. doi: 10.1007/s00424-020-02381-6. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Huang L, Zhao J, Chen S, Liu J, Li G. The circadian clock and inflammation: a new insight. Clin Chim Acta. 2020;512:12–17. doi: 10.1016/j.cca.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013;119:155–190. doi: 10.1016/B978-0-12-396971-2.00007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–1015. doi: 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan MC, Spieth PM, Quinn K, Parotto M, Zhang H, Slutsky AS. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med. 2012;40:246–253. doi: 10.1097/CCM.0b013e31822f0abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manoogian ENC, Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 2017;39:59–67. doi: 10.1016/j.arr.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McAlpine CS, Swirski FK. Circadian influence on metabolism and inflammation in atherosclerosis. Circ Res. 2016;119:131–141. doi: 10.1161/CIRCRESAHA.116.308034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobaldini E, Costantino G, Solbiati M, Cogliati C, Kara T, Nobili L, Montano N. Sleep, sleep deprivation, autonomic nervous system and cardiovascular diseases. Neurosci Biobehav Rev. 2017;74:321–329. doi: 10.1016/j.neubiorev.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Masri S, Sassone-Corsi P. The emerging link between cancer, metabolism, and circadian rhythms. Nat Med. 2018;24:1795–1803. doi: 10.1038/s41591-018-0271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vignozzi L, Maggi M. Circadian rhythm and erectile function: is there a penile clock? Nat Rev Urol. 2020;17:603–604. doi: 10.1038/s41585-020-00376-7. [DOI] [PubMed] [Google Scholar]
- 15.Mogavero M, DelRosso L, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: state of the art and future perspectives. Sleep Med Rev. 2020;56:101409. doi: 10.1016/j.smrv.2020.101409. [DOI] [PubMed] [Google Scholar]
- 16.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021 doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 17.Mehrzadi MH, Hosseinzadeh A, Juybari KB, Mehrzadi S. Melatonin and urological cancers: a new therapeutic approach. Cancer Cell Int. 2020;20:444. doi: 10.1186/s12935-020-01531-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soták M, Sumová A, Pácha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med. 2014;46:221–232. doi: 10.3109/07853890.2014.892296. [DOI] [PubMed] [Google Scholar]
- 20.Xuan W, Khan F, James CD, Heimberger AB, Lesniak MS, Chen P. Circadian regulation of cancer cell and tumor microenvironment crosstalk. Trends Cell Biol. 2021 doi: 10.1016/j.tcb.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 2010;9:1097–1103. doi: 10.4161/cc.9.6.11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy P, Zehring WA, Wheeler DA, Pirrotta V, Hadfield C, Hall JC, Rosbash M. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984;38:701–710. doi: 10.1016/0092-8674(84)90265-4. [DOI] [PubMed] [Google Scholar]
- 24.Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- 25.Lydic R, Albers HE, Tepper B, Moore-Ede MC. Three-dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. J Comp Neurol. 1982;204:225–237. doi: 10.1002/cne.902040303. [DOI] [PubMed] [Google Scholar]
- 26.Lydic R, Schoene WC, Czeisler CA, Moore-Ede MC. Suprachiasmatic region of the human hypothalamus: homolog to the primate circadian pacemaker? Sleep. 1980;2:355–361. doi: 10.1093/sleep/2.3.355. [DOI] [PubMed] [Google Scholar]
- 27.Van den Pol AN. The hypothalamic suprachiasmatic nucleus of rat: intrinsic anatomy. J Comp Neurol. 1980;191:661–702. doi: 10.1002/cne.901910410. [DOI] [PubMed] [Google Scholar]
- 28.Kriegsfeld LJ, LeSauter J, Silver R. Targeted microlesions reveal novel organization of the hamster suprachiasmatic nucleus. J Neurosci. 2004;24:2449–2457. doi: 10.1523/JNEUROSCI.5323-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- 30.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 32.Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 34.Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giebultowicz J. Chronobiology: biological timekeeping. Integr Comp Biol. 2004;44:266. doi: 10.1093/icb/44.3.266. [DOI] [PubMed] [Google Scholar]
- 36.Hay-Schmidt A, Vrang N, Larsen PJ, Mikkelsen JD. Projections from the raphe nuclei to the suprachiasmatic nucleus of the rat. J Chem Neuroanat. 2003;25:293–310. doi: 10.1016/s0891-0618(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 37.Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 38.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 39.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90:1063–1102. doi: 10.1152/physrev.00009.2009. [DOI] [PubMed] [Google Scholar]
- 40.Deurveilher S, Burns J, Semba K. Indirect projections from the suprachiasmatic nucleus to the ventrolateral preoptic nucleus: a dual tract-tracing study in rat. Eur J Neurosci. 2002;16:1195–1213. doi: 10.1046/j.1460-9568.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 41.Deurveilher S, Semba K. Indirect projections from the suprachiasmatic nucleus to major arousal-promoting cell groups in rat: implications for the circadian control of behavioural state. Neuroscience. 2005;130:165–183. doi: 10.1016/j.neuroscience.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz MD, Urbanski HF, Nunez AA, Smale L. Projections of the suprachiasmatic nucleus and ventral subparaventricular zone in the Nile grass rat (Arvicanthis niloticus) Brain Res. 2011;1367:146–161. doi: 10.1016/j.brainres.2010.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Challet E. Minireview: Entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. doi: 10.1210/en.2007-0804. [DOI] [PubMed] [Google Scholar]
- 44.Hastings MH, Maywood ES, Reddy AB. Two decades of circadian time. J Neuroendocrinol. 2008;20:812–819. doi: 10.1111/j.1365-2826.2008.01715.x. [DOI] [PubMed] [Google Scholar]
- 45.Kalsbeek A, Perreau-Lenz S, Buijs RM. A network of (autonomic) clock outputs. Chronobiol Int. 2006;23:521–535. doi: 10.1080/07420520600651073. [DOI] [PubMed] [Google Scholar]
- 46.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302:255–259. doi: 10.1126/science.1086271. [DOI] [PubMed] [Google Scholar]
- 47.Griffin EA, Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286:768–771. doi: 10.1126/science.286.5440.768. [DOI] [PubMed] [Google Scholar]
- 48.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 49.Kiss Z, Ghosh PM. Women in cancer thematic review: circadian rhythmicity and the influence of 'clock' genes on prostate cancer. Endocr Relat Cancer. 2016;23:T123–T134. doi: 10.1530/ERC-16-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hergenhan S, Holtkamp S, Scheiermann C. Molecular interactions between components of the circadian clock and the immune system. J Mol Biol. 2020;432:3700–3713. doi: 10.1016/j.jmb.2019.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feillet C, Krusche P, Tamanini F, Janssens RC, Downey MJ, Martin P, Teboul M, Saito S, Lévi FA, Bretschneider T, van der Horst GT, Delaunay F, Rand DA. Phase locking and multiple oscillating attractors for the coupled mammalian clock and cell cycle. Proc Natl Acad Sci USA. 2014;111:9828–9833. doi: 10.1073/pnas.1320474111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Z, Yu K, Zheng J, Lin H, Zhao Q, Zhang X, Feng W, Wang L, Xu J, Xie D, Zuo ZX, Liu ZX. Dysregulation, functional implications, and prognostic ability of the circadian clock across cancers. Cancer Med. 2019;8:1710–1720. doi: 10.1002/cam4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cash E, Sephton S, Woolley C, Elbehi AM, Anu RI, Ekine-Afolabi B, Kok VC. The role of the circadian clock in cancer hallmark acquisition and immune-based cancer therapeutics. J Exp Clin Cancer Res. 2021;40:119. doi: 10.1186/s13046-021-01919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med. 2019;11:82. doi: 10.1186/s13073-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton T. Influence of environmental light and melatonin upon mammary tumour induction. Br J Surg. 1969;56:764–766. doi: 10.1002/bjs.1800561018. [DOI] [PubMed] [Google Scholar]
- 57.Aubert C, Janiaud P, Lecalvez J. Effect of pinealectomy and melatonin on mammary tumor growth in Sprague-Dawley rats under different conditions of lighting. J Neural Transm. 1980;47:121–130. doi: 10.1007/BF01670163. [DOI] [PubMed] [Google Scholar]
- 58.Mhatre MC, Shah PN, Juneja HS. Effect of varying photoperiods on mammary morphology, DNA synthesis, and hormone profile in female rats. J Natl Cancer Inst. 1984;72:1411–1416. [PubMed] [Google Scholar]
- 59.Rao D, Yu H, Bai Y, Zheng X, Xie L. Does night-shift work increase the risk of prostate cancer? A systematic review and meta-analysis. Onco Targets Ther. 2015;8:2817–2826. doi: 10.2147/OTT.S89769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wendeu-Foyet MG, Menegaux F. Circadian disruption and prostate cancer risk: an updated review of epidemiological evidences. Cancer Epidemiol Biomark Prev. 2017;26:985–991. doi: 10.1158/1055-9965.EPI-16-1030. [DOI] [PubMed] [Google Scholar]
- 61.Rivera-Izquierdo M. Shift work and prostate cancer: an updated systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17:1345. doi: 10.3390/ijerph17041345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood PA, Yang X, Hrushesky WJ. Clock genes and cancer. Integr Cancer Ther. 2009;8:303–308. doi: 10.1177/1534735409355292. [DOI] [PubMed] [Google Scholar]
- 63.Canaple L, Kakizawa T, Laudet V. The days and nights of cancer cells. Cancer Res. 2003;63:7545–7552. [PubMed] [Google Scholar]
- 64.Lewis P, Hellmich M. Perinatal photoperiod and childhood cancer: pooled results from 182,856 individuals in the international childhood cancer cohort consortium (I4C) Chronobiol Int. 2020;37:1034–1047. doi: 10.1080/07420528.2020.1740724. [DOI] [PubMed] [Google Scholar]
- 65.Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst. 2003;95:825–828. doi: 10.1093/jnci/95.11.825. [DOI] [PubMed] [Google Scholar]
- 66.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12:74–77. doi: 10.1097/00001648-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Viswanathan AN, Hankinson SE, Schernhammer ES. Night shift work and the risk of endometrial cancer. Cancer Res. 2007;67:10618–10622. doi: 10.1158/0008-5472.CAN-07-2485. [DOI] [PubMed] [Google Scholar]
- 68.Kisamore C, Elliott B, DeVries A, Nelson R, Walker W. Chronotherapeutics for solid tumors. Pharmaceutics. 2023 doi: 10.3390/pharmaceutics15082023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhu W, He Q, Feng D, Wei Q, Yang L. Circadian rhythm in prostate cancer: time to take notice of the clock. Asian J Androl. 2023;25:184–191. doi: 10.4103/aja202255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walton J, Walker W, Bumgarner J, Meléndez-Fernández O, Liu J, Hughes H, Kaper A, Nelson R. Circadian variation in efficacy of medications. Clin Pharmacol Ther. 2021;109:1457–1488. doi: 10.1002/cpt.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abu-Samak A-A, Abu-Samak M, Al-Waeli H, Cai W, Al-Tamimi M, Tamimi F, Nicolau B. Chronotherapy in head and neck cancer (HNC): a systematic review. J Clin Oncol. 2023;41:e18016-e. [Google Scholar]
- 72.Bermúdez-Guzmán L, Blanco-Saborío A, Ramírez-Zamora J, Lovo E. The time for chronotherapy in radiation oncology. Front Oncol. 2021;11:687672. doi: 10.3389/fonc.2021.687672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 74.Band PR, Le ND, Fang R, Deschamps M, Coldman AJ, Gallagher RP, Moody J. Cohort study of Air Canada pilots: mortality, cancer incidence, and leukemia risk. Am J Epidemiol. 1996;143:137–143. doi: 10.1093/oxfordjournals.aje.a008722. [DOI] [PubMed] [Google Scholar]
- 75.Flynn-Evans EE, Mucci L, Stevens RG, Lockley SW. Shiftwork and prostate-specific antigen in the national health and nutrition examination survey. J Natl Cancer Inst. 2013;105:1292–1297. doi: 10.1093/jnci/djt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pukkala E, Aspholm R, Auvinen A, Eliasch H, Gundestrup M, Haldorsen T, Hammar N, Hrafnkelsson J, Kyyrönen P, Linnersjö A, Rafnsson V, Storm H, Tveten U. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ. 2002;325:567. doi: 10.1136/bmj.325.7364.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pukkala E, Martinsen JI, Lynge E, Gunnarsdottir HK, Sparén P, Tryggvadottir L, Weiderpass E, Kjaerheim K. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncol. 2009;48:646–790. doi: 10.1080/02841860902913546. [DOI] [PubMed] [Google Scholar]
- 78.Man AWC, Li H, Xia N. Circadian rhythm: potential therapeutic target for atherosclerosis and thrombosis. Int J Mol Sci. 2021 doi: 10.3390/ijms22020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lue TF. Erectile dysfunction. N Engl J Med. 2000;342:1802–1813. doi: 10.1056/NEJM200006153422407. [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez KM, Kohn TP, Kohn JR, Sigalos JT, Kirby EW, Pickett SM, Pastuszak AW, Lipshultz LI. Shift work sleep disorder and night shift work significantly impair erectile function. J Sex Med. 2020;17:1687–1693. doi: 10.1016/j.jsxm.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cho JW, Duffy JF. Sleep, sleep disorders, and sexual dysfunction. World J Mens Health. 2019;37:261–275. doi: 10.5534/wjmh.180045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stenvers DJ, Scheer F, Schrauwen P, la Fleur SE, Kalsbeek A. Circadian clocks and insulin resistance. Nat Rev Endocrinol. 2019;15:75–89. doi: 10.1038/s41574-018-0122-1. [DOI] [PubMed] [Google Scholar]
- 83.Parent M-É, El-Zein M, Rousseau M-C, Pintos J, Siemiatycki J. Night work and the risk of cancer among men. Am J Epidemiol. 2012;176:751–759. doi: 10.1093/aje/kws318. [DOI] [PubMed] [Google Scholar]
- 84.Arafa A, Eshak ES, Iso H, Muraki I, Tamakoshi A. Night work, rotating shift work and the risk of cancer in japanese men and women: the JACC study. J Epidemiol. 2020;31:585–592. doi: 10.2188/jea.JE20200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Papantoniou K, Castaño-Vinyals G, Espinosa A, Aragonés N, Pérez-Gómez B, Burgos J, Gómez-Acebo I, Llorca J, Peiró R, Jimenez-Moleón JJ, Arredondo F, Tardón A, Pollan M, Kogevinas M. Night shift work, chronotype and prostate cancer risk in the MCC-Spain case-control study. Int J Cancer. 2015;137:1147–1157. doi: 10.1002/ijc.29400. [DOI] [PubMed] [Google Scholar]
- 86.Hu L, Harper A, Heer E, McNeil J, Cao C. Social jetlag and prostate cancer incidence in alberta's tomorrow project: a prospective cohort study. Cancers (Basel). 2020;12:3873. doi: 10.3390/cancers12123873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lozano-Lorca M, Olmedo-Requena R, Vega-Galindo MV, Vázquez-Alonso F, Jiménez-Pacheco A, Salcedo-Bellido I, Sánchez MJ, Jiménez-Moleón JJ. Night shift work, chronotype, sleep duration, and prostate cancer risk: CAPLIFE study. Int J Environ Res Public Health. 2020;17:6300. doi: 10.3390/ijerph17176300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yong M, Blettner M, Emrich K, Nasterlack M, Oberlinner C, Hammer GP. A retrospective cohort study of shift work and risk of incident cancer among German male chemical workers. Scand J Work Environ Health. 2014;40:502–510. doi: 10.5271/sjweh.3438. [DOI] [PubMed] [Google Scholar]
- 89.Åkerstedt T, Narusyte J, Svedberg P, Kecklund G, Alexanderson K. Night work and prostate cancer in men: a Swedish prospective cohort study. BMJ Open. 2017;7:e015751. doi: 10.1136/bmjopen-2016-015751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barul C, Richard H, Parent ME. Night-shift work and risk of prostate cancer: results from a Canadian case-control study, the prostate cancer and environment study. Am J Epidemiol. 2019;188:1801–1811. doi: 10.1093/aje/kwz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gan Y, Li L, Zhang L, Yan S, Gao C, Hu S, Qiao Y, Tang S, Wang C, Lu Z. Association between shift work and risk of prostate cancer: a systematic review and meta-analysis of observational studies. Carcinogenesis. 2018;39:87–97. doi: 10.1093/carcin/bgx129. [DOI] [PubMed] [Google Scholar]
- 92.Mancio J, Leal C. Does the association of prostate cancer with night-shift work differ according to rotating vs. fixed schedule? A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2018;21:337–344. doi: 10.1038/s41391-018-0040-2. [DOI] [PubMed] [Google Scholar]
- 93.Du H-B, Bin K-Y, Liu W-H, Yang F-S. Shift work, night work, and the risk of prostate cancer: a meta-analysis based on 9 cohort studies. Medicine (Baltimore) 2017;96:e8537. doi: 10.1097/MD.0000000000008537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho S, Park WJ. Night shiftwork and prostate-specific antigen level in a tire manufacturing factory. Ann Occup Environ Med. 2019;31:e19. doi: 10.35371/aoem.2019.31.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Katz ES, Kheirandish-Gozal L, Neubauer DN, O'Donnell AE, Ohayon M, Peever J, Rawding R, Sachdeva RC, Setters B, Vitiello MV, Ware JC, Adams Hillard PJ. National sleep foundation's sleep time duration recommendations: methodology and results summary. Sleep Health. 2015;1:40–43. doi: 10.1016/j.sleh.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 96.Liu PY. A clinical perspective of sleep and andrological health: assessment, treatment considerations and future research. J Clin Endocrinol Metab. 2019;104:4398–4417. doi: 10.1210/jc.2019-00683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kling JM, Manson JE, Naughton MJ, Temkit M, Sullivan SD, Gower EW, Hale L, Weitlauf JC, Nowakowski S, Crandall CJ. Association of sleep disturbance and sexual function in postmenopausal women. Menopause. 2017;24:604–612. doi: 10.1097/GME.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Starreveld DEJ, Habers GEA, Valdimarsdottir HB, Kessels R, Daniëls LA, van Leeuwen FE, Bleiker EMA. Cancer-related fatigue in relation to chronotype and sleep quality in (non-)hodgkin lymphoma survivors. J Biol Rhythms. 2021;36:71–83. doi: 10.1177/0748730420987327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kakizaki M, Inoue K, Kuriyama S, Sone T, Matsuda-Ohmori K, Nakaya N, Fukudo S, Tsuji I, Study OC Sleep duration and the risk of prostate cancer: the Ohsaki cohort study. Br J Cancer. 2008;99:176–178. doi: 10.1038/sj.bjc.6604425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Markt SC, Flynn-Evans EE, Valdimarsdottir UA, Sigurdardottir LG, Tamimi RM, Batista JL, Haneuse S, Lockley SW, Stampfer M, Wilson KM, Czeisler CA, Rider JR, Mucci LA. Sleep duration and disruption and prostate cancer risk: a 23-year prospective study. Cancer Epidemiol Biomarkers Prev. 2016;25:302–308. doi: 10.1158/1055-9965.EPI-14-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sigurdardottir LG, Valdimarsdottir UA, Mucci LA, Fall K, Rider JR, Schernhammer E, Czeisler CA, Launer L, Harris T, Stampfer MJ, Gudnason V, Lockley SW. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol Biomark Prev. 2013;22:872–879. doi: 10.1158/1055-9965.EPI-12-1227-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gapstur SM, Diver WR, Stevens VL, Carter BD, Teras LR, Jacobs EJ. Work schedule, sleep duration, insomnia, and risk of fatal prostate cancer. Am J Prev Med. 2014;46:S26–33. doi: 10.1016/j.amepre.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 103.Wendeu-Foyet MG, Bayon V, Cénée S, Trétarre B, Rébillard X, Cancel-Tassin G, Cussenot O, Lamy P-J, Faraut B, Ben Khedher S, Léger D, Menegaux F. Night work and prostate cancer risk: results from the EPICAP Study. Occup Environ Med. 2018;75:573–581. doi: 10.1136/oemed-2018-105009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Markt SC, Grotta A, Nyren O, Adami H-O, Mucci LA, Valdimarsdottir UA, Stattin P, Bellocco R, Lagerros YT. Insufficient sleep and risk of prostate cancer in a large swedish cohort. Sleep. 2015;38:1405–1410. doi: 10.5665/sleep.4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sun X, Ye D, Jiang M, Qian Y, Mao Y. Genetically proxied morning chronotype was associated with a reduced risk of prostate cancer. Sleep. 2021 doi: 10.1093/sleep/zsab104. [DOI] [PubMed] [Google Scholar]
- 106.Garcia-Saenz A, de Miguel AS, Espinosa A, Valentin A, Aragonés N, Llorca J, Amiano P, Martín Sánchez V, Guevara M, Capelo R, Tardón A, Peiró-Perez R, Jiménez-Moleón JJ, Roca-Barceló A, Pérez-Gómez B, Dierssen-Sotos T, Fernández-Villa T, Moreno-Iribas C, Moreno V, García-Pérez J, Castaño-Vinyals G, Pollán M, Aubé M, Kogevinas M. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain Study) Environ Health Perspect. 2018;126:047011. doi: 10.1289/EHP1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim KY, Lee E, Kim YJ, Kim J. The association between artificial light at night and prostate cancer in Gwangju City and South Jeolla Province of South Korea. Chronobiol Int. 2017;34:203–211. doi: 10.1080/07420528.2016.1259241. [DOI] [PubMed] [Google Scholar]
- 108.Rybnikova NA, Haim A, Portnov BA. Is prostate cancer incidence worldwide linked to artificial light at night exposures? Review of earlier findings and analysis of current trends. Arch Environ Occup Health. 2017;72:111–122. doi: 10.1080/19338244.2016.1169980. [DOI] [PubMed] [Google Scholar]
- 109.Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23:497–509. doi: 10.1080/07420520500545979. [DOI] [PubMed] [Google Scholar]
- 110.Kogevinas M, Espinosa A, Castelló A. Effect of mistimed eating patterns on breast and prostate cancer risk (MCC-Spain Study) Int J Cancer. 2018;143:2380–2389. doi: 10.1002/ijc.31649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wendeu-Foyet MG, Koudou Y, Cénée S, Trétarre B, Rébillard X, Cancel-Tassin G, Cussenot O, Boland A, Bacq D, Deleuze JF, Lamy PJ, Mulot C, Laurent-Puig P, Truong T, Menegaux F. Circadian genes and risk of prostate cancer: findings from the EPICAP study. Int J Cancer. 2019;145:1745–1753. doi: 10.1002/ijc.32149. [DOI] [PubMed] [Google Scholar]
- 112.Wendeu-Foyet MG, Cénée S, Koudou Y, Trétarre B, Rébillard X, Cancel-Tassin G, Cussenot O, Boland A, Olaso R, Deleuze JF, Blanché H, Lamy PJ, Mulot C, Laurent-Puig P, Truong T, Menegaux F. Circadian genes polymorphisms, night work and prostate cancer risk: findings from the EPICAP study. Int J Cancer. 2020;147:3119–3129. doi: 10.1002/ijc.33139. [DOI] [PubMed] [Google Scholar]
- 113.Zhu Y, Stevens RG, Hoffman AE, Fitzgerald LM, Kwon EM, Ostrander EA, Davis S, Zheng T, Stanford JL. Testing the circadian gene hypothesis in prostate cancer: a population-based case-control study. Cancer Res. 2009;69:9315–9322. doi: 10.1158/0008-5472.CAN-09-0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chu LW, Zhu Y, Yu K, Zheng T, Yu H, Zhang Y, Sesterhenn I, Chokkalingam AP, Danforth KN, Shen MC, Stanczyk FZ, Gao YT, Hsing AW. Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–348. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 115.Chu LW, Till C, Yang B. Circadian genes and risk of prostate cancer in the prostate cancer prevention trial. Mol Carcinog. 2018;57:462–466. doi: 10.1002/mc.22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu CC, Chen LC, Chiou CY, Chang YJ, Lin VC, Huang CY, Lin IL, Chang TY, Lu TL, Lee CH, Huang SP, Bao BY. Genetic variants in the circadian rhythm pathway as indicators of prostate cancer progression. Cancer Cell Int. 2019;19:87. doi: 10.1186/s12935-019-0811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gu F, Zhang H, Hyland PL, Berndt S, Gapstur SM, Wheeler W, Ellipse Consortium T. Amos CI, Bezieau S, Bickeböller H, Brenner H, Brennan P, Chang-Claude J, Conti DV, Doherty JA, Gruber SB, Harrison TA, Hayes RB, Hoffmeister M, Houlston RS, Hung RJ, Jenkins MA, Kraft P, Lawrenson K, McKay J, Markt S, Mucci L, Phelan CM, Qu C, Risch A, Rossing MA, Wichmann HE, Shi J, Schernhammer E, Yu K, Landi MT, Caporaso NE. Inherited variation in circadian rhythm genes and risks of prostate cancer and three other cancer sites in combined cancer consortia. Int J Cancer. 2017;141:1794–1802. doi: 10.1002/ijc.30883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mocellin S, Tropea S, Benna C, Rossi CR. Circadian pathway genetic variation and cancer risk: evidence from genome-wide association studies. BMC Med. 2018;16:20. doi: 10.1186/s12916-018-1010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Markt SC, Valdimarsdottir UA, Shui IM, Sigurdardottir LG, Rider JR, Tamimi RM, Batista JL, Haneuse S, Flynn-Evans E, Lockley SW, Czeisler CA, Stampfer MJ, Launer L, Harris T, Smith AV, Gudnason V, Lindstrom S, Kraft P, Mucci LA. Circadian clock genes and risk of fatal prostate cancer. Cancer Causes Control. 2015;26:25–33. doi: 10.1007/s10552-014-0478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao Q, Gery S, Dashti A, Yin D, Zhou Y, Gu J, Koeffler HP. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69:7619–7625. doi: 10.1158/0008-5472.CAN-08-4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jung-Hynes B, Huang W, Reiter RJ, Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J Pineal Res. 2010;49:60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li Q, Xia D, Wang Z, Liu B, Zhang J, Peng P, Tang Q, Dong J, Guo J, Kuang D, Chen W, Mao J, Li Q, Chen X. Circadian rhythm gene PER3 negatively regulates stemness of prostate cancer stem cells via WNT/β-catenin signaling in tumor microenvironment. Front Cell Dev Biol. 2021;9:656981. doi: 10.3389/fcell.2021.656981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cai DW, Chen D, Sun SP, Liu ZJ, Liu F, Xian SZ, Wu PS, Kong GQ. Overexpression of PER3 reverses paclitaxel resistance of prostate cancer cells by inhibiting the Notch pathway. Eur Rev Med Pharmacol Sci. 2018;22:2572–2579. doi: 10.26355/eurrev_201805_14950. [DOI] [PubMed] [Google Scholar]
- 124.Zhou L, Zhang C, Yang X, Liu L, Hu J, Hou Y, Tao H, Sugimura H, Chen Z, Wang L. Melatonin inhibits lipid accumulation to repress prostate cancer progression by mediating the epigenetic modification of CES1. Clin Transl Med. 2021;11:e449. doi: 10.1002/ctm2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hsu FM, Hou WH, Huang CY, Wang CC, Tsai CL, Tsai YC, Yu HJ, Pu YS, Cheng JC. Differences in toxicity and outcome associated with circadian variations between patients undergoing daytime and evening radiotherapy for prostate adenocarcinoma. Chronobiol Int. 2016;33:210–219. doi: 10.3109/07420528.2015.1130049. [DOI] [PubMed] [Google Scholar]
- 126.Chan S, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Danjoux C, Barnes E, Popovic M, Lam H, DeAngelis C, Chow E. Does the time of radiotherapy affect treatment outcomes? A review of the literature. Clin Oncol (R Coll Radiol) 2017;29:231–238. doi: 10.1016/j.clon.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 127.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 128.Zhou L, Luo Z, Li Z, Huang Q. Circadian clock is associated with tumor microenvironment in kidney renal clear cell carcinoma. Aging (Albany NY) 2020;12:14620–14632. doi: 10.18632/aging.103509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gu F, Xiao Q, Chu LW, Yu K, Matthews CE, Hsing AW, Caporaso NE. Sleep duration and cancer in the NIH-AARP diet and health study cohort. PLoS ONE. 2016;11:e0161561. doi: 10.1371/journal.pone.0161561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mazzoccoli G, Piepoli A, Carella M, Panza A, Pazienza V, Benegiamo G, Palumbo O, Ranieri E. Altered expression of the clock gene machinery in kidney cancer patients. Biomed Pharmacother. 2012;66:175–179. doi: 10.1016/j.biopha.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 131.Okabe T, Kumagai M, Nakajima Y, Shirotake S, Kodaira K, Oyama M, Ueno M, Ikeda M. The impact of HIF1α on the Per2 circadian rhythm in renal cancer cell lines. PLoS ONE. 2014;9:e109693. doi: 10.1371/journal.pone.0109693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.English DR, Hopper JL, Southey MC, Giles GG, Milne RL, Titova OE. Sleep duration and risk of overall and 22 site-specific cancers: a Mendelian randomization study. Int J Cancer. 2021;148:914–920. doi: 10.1002/ijc.33286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Qiu MJ, Liu LP, Jin S, Fang XF, He XX, Xiong ZF, Yang SL. Research on circadian clock genes in common abdominal malignant tumors. Chronobiol Int. 2019;36:906–918. doi: 10.1080/07420528.2018.1477792. [DOI] [PubMed] [Google Scholar]
- 134.Yang Y, Yuan G, Xie H, Wei T, Zhu D, Cui J, Liu X, Shen R, Zhu Y, Yang X. Circadian clock associates with tumor microenvironment in thoracic cancers. Aging (Albany NY) 2019;11:11814–11828. doi: 10.18632/aging.102450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kobayashi M, Wood PA, Hrushesky WJ. Circadian chemotherapy for gynecological and genitourinary cancers. Chronobiol Int. 2002;19:237–251. doi: 10.1081/cbi-120002600. [DOI] [PubMed] [Google Scholar]
- 136.Hrushesky WJ, von Roemeling R, Lanning RM, Rabatin JT. Circadian-shaped infusions of floxuridine for progressive metastatic renal cell carcinoma. J Clin Oncol. 1990;8:1504–1513. doi: 10.1200/JCO.1990.8.9.1504. [DOI] [PubMed] [Google Scholar]
- 137.Hrushesky W, Roemeling R, Sothem RB. Circadian chronotherapy: From animal experiments to human cancer chemotherapy. Chronopharmacol Cell Biochem Interact Cell Clocks. 1989;3:439–473. [Google Scholar]
- 138.Mistlberger RE, Skene DJ. Social influences on mammalian circadian rhythms: animal and human studies. Biol Rev Camb Philos Soc. 2004;79:533–556. doi: 10.1017/s1464793103006353. [DOI] [PubMed] [Google Scholar]
- 139.Chavan S, Bray F, Lortet-Tieulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66:59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 140.Yang Y, Cheng Z, Jia X, Shi N, Xia Z, Zhang W, Shi X. Mortality trends of bladder cancer in China from 1991 to 2015: an age-period-cohort analysis. Cancer Manag Res. 2019;11:3043–3051. doi: 10.2147/CMAR.S189220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jung YL, Tompa E, Longo C, Kalcevich C, Kim J, Song C, Demers P. The economic burden of bladder cancer due to occupational exposure. J Occup Environ Med. 2018;60:217–225. doi: 10.1097/JOM.0000000000001242. [DOI] [PubMed] [Google Scholar]
- 142.Fankhauser CD, Mostafid H. Prevention of bladder cancer incidence and recurrence: nutrition and lifestyle. Curr Opin Urol. 2018;28:88–92. doi: 10.1097/MOU.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 143.Litlekalsoy J, Rostad K, Kalland K-H, Hostmark JG, Laerum OD. Expression of circadian clock genes and proteins in urothelial cancer is related to cancer-associated genes. BMC Cancer. 2016;16:549. doi: 10.1186/s12885-016-2580-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Polo A, Crispo A, Cerino P, Falzone L, Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon A, Costantini S. Environment and bladder cancer: molecular analysis by interaction networks. Oncotarget. 2017;8:65240–65252. doi: 10.18632/oncotarget.18222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer. 2010;10:51–57. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee S, Lee JS. Cellular senescence: a promising strategy for cancer therapy. BMB Rep. 2019;52:35–41. doi: 10.5483/BMBRep.2019.52.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jia M, Su B, Mo L, Qiu W, Ying J, Lin P, Yang B, Li D, Wang D, Xu L, Li H, Zhou Z, Li X, Li J. Circadian clock protein CRY1 prevents paclitaxel-induced senescence of bladder cancer cells by promoting p53 degradation. Oncol Rep. 2021;45:1033–1043. doi: 10.3892/or.2020.7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iyyanki T, Zhang B, Wang Q, Hou Y, Jin Q, Xu J, Yang H, Liu T, Wang X, Song F, Luan Y, Yamashita H, Chien R, Lyu H, Zhang L, Wang L, Warrick J, Raman JD, Meeks JJ, DeGraff DJ, Yue F. Subtype-associated epigenomic landscape and 3D genome structure in bladder cancer. Genome Biol. 2021;22:105. doi: 10.1186/s13059-021-02325-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hrushesky WJ, Roemeling RV, Wood PA, Langevin TR, Lange P, Fraley E. High-dose intensity, circadian-timed doxorubicin and cisplatin adjuvant chemotherapy for bladder cancer. Cancer Treat Rep. 1987;71:915–919. [PubMed] [Google Scholar]
- 150.Hrushesky WJ, Roemeling RV, Wood PA, Langevin TR, Lange P, Farley E. High-dose intensity systemic therapy of metastatic bladder cancer. J Clin Oncol. 1987;5:450–455. doi: 10.1200/JCO.1987.5.3.450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were used during the current review.