Abstract
D-Pantothenic acid, as a momentous vitamin, is extensively applied to feed, medicine, cosmetics and other fields. However, there are still limitations to produce D-pantothenic acid by microbial fermentation at present. In this paper, we constructed a recombinant strain for D-pantothenic acid production by blocking the organic acid pathway, boosting pyruvate biosynthesis, relieving feedback inhibition of acetolactate synthase, improving glucose intake capacity, and modifying essential genes in the metabolic pathway. In addition, a new acetolactate isomeroreductase mutant V412A origin from Escherichia coli (EcAHAIR) encoded by ilvC was obtained to explore its substrate promiscuity. Compared with the wild type, the variant EcAHAIR-V412A has reduced steric hindrance and enhanced intermolecular forces, resulting in a high affinity for 2-acetolactate. Eventually, the fermentation production of the final strain DPAN19/trc-ilvCV412A reached 4.65 g/L, increased by 192.5% compared with strain DPA8 in shake flask cultivation and produced 62.82 g/L D-pantothenic acid in a 5 L bioreactor. The metabolic engineering strategies and enzyme modification approaches described in this paper provide a particular perspective for the bio-manufacturing of D-pantothenic acid, branched-chain amino acids and its derivates.
Keywords: D-pantothenic acid, Enzyme engineering, Glucose intake capacity, Genome integration, Pyruvate substrate pool
Introduction
D-pantothenic acid (D-PA, also called vitamin B5) is an important precursor for the biosynthesis of acetyl-CoA (Leonardi and Jackowski 2007). It is involved in the metabolic process of acyl carriers, which plays a critical role in carbohydrate metabolism, phospholipid biosynthesis, and operation of the tricarboxylic acid (TCA) cycle (Xu et al. 2020). Therefore, it has vital significance in promoting biological growth and maintaining normal physiological function (Hall and Hall 2020). However, researchers found that only plants and microorganisms can synthesize D-PA, which cannot be synthesized by animals (Tigu et al. 2018). Therefore, D-PA has been extensively applied to pharmaceutical, cosmetics, and feed additive industries (Postaru et al. 2015).
At present, D-PA is commercially produced by heating a mixture of D-valerolactone (D-PL) and 3-aminopropionic acid (3-AP) in methanol or ethanol environment (Huang et al. 2010). Both chemical resolution and enzymatic resolution (Rowicki et al. 2006; Honda et al. 2002) require the toxic hydrogen cyanide and sodium cyanide in the reaction, as well as expensive catalysts and strict reaction circumstances (Bonrath and Netscher 2005). Due to the increasing environmental problems and the development of biotechnology, it is a promising choice to use microorganisms to produce D-PA according to metabolic engineering and fermentation engineering technology (Zou et al. 2021).
Developments in synthetic biology offer alternatives to derive biologically active substances that was traditionally produced by chemical synthesis and traditional extraction methods (Grzegorz et al. 2010). At present, Corynebacterium glutamicum and Escherichia coli are frequently utilized to produce sorts of amino acids, for instance, L-valine, L-alanine, and L-leucine (Gao et al. 2021; Liu et al. 2021; Wang et al. 2019a, b), of which the partial overlap of L-valine and D-PA biosynthetic pathways provides theoretical basis and guiding experience for improving D-PA biosynthesis. For example, increasing the copy number of crucial pathway genes, modifying rate-limiting enzymes, weakening competitive pathways, improving redox balance, and strengthening the transport systems. Previous research reported that a new E. coli W3110 derive strain was designed based on metabolic engineering, it produced 32.32 g/L D-PA (Zhang et al. 2021). Moreover, an efficient heterologous D-PA pathway was constructed by screening critical genes involved in D-PA biosynthesis in Saccharomyces cerevisiae, the resultant strain could produce 4.1 g/L D-PA by fed-batch fermentation (Guo et al. 2023).
Acetolactate isomeroreductase (AHAIR, EC: 1.1.1.86), encoded by ilvC, could catalyze different substrates including 2-acetolactate, 3-hydroxy-3-methyl-2-ketobutyrate, 2-aceto-2-hydroxybutanoate and 2-keto acids (Tyagi et al. 2005) and plays an essential role in the biosynthesis of D-PA, branched-chain amino acids (BCAAs) and isobutanol (Bastian et al. 2011). The expression of ilvC is co-induced by 2-acetolactate or 2-aceto-2-hydroxy-butanoate and a positive transcriptional activator encoded by ilvY (Geraskina et al. 2019). The catalytic reaction of AHAIR involves two steps: alkyl migration and reduction, in which the alkyl migration stage exhibits strict Mg2+ dependence (Dumas et al. 1995). At present, the commonly used strategy for D-PA production is a high-level expression, providing sufficient NADPH or transforming cofactor preference of ilvC. Research have shown that mutations in the cofactor binding domain of AHAIR improve the redox balance and increased the production of L-leucine in C. glutamicum (Wang et al. 2019a, b) and L-valine in E. coli (Hao et al. 2020). By screening cofactor regeneration genes and expanding the NADPH pool, the production of D-PA reached 63.58 g/L in a 5 L bioreactor (Zou et al. 2022). However, in view of the low affinity of AHAIR for the critical precursor of D-PA, 2-acetolactate, we focused on exploring its substrate promiscuity with the aim of facilitating the redistribution of carbon flux, which has never been reported for D-PA or BCAAs production.
Although many strategies have been applied to D-PA production on the basis of metabolic engineering in E. coli, the low yield of D-PA by de novo biosynthesis still lacks economic competitiveness. In this study, the D-PA-producing strain DPA8 (Zhang et al. 2019) was used as the chassis for systematic metabolic engineering to enhance the production capacity of D-PA (Fig. 1). We knocked out competitive pathways and improved glucose intake pathways to enrich the pyruvate pool. Then, we relieved feedback inhibition of acetolactate synthase (AHAS) to endow with L-valine resistance and enhanced carbon flux in the D-PA biosynthetic pathway. Most importantly, EcAHAIR was investigated by molecular docking, and crucial sites near the substrate binding pocket were selected for β-alanine scanning to study the influence of mutational sites on D-PA biosynthesis. Finally, the D-PA output of the recombinant strain DPAN19 harboring the mutant plasmid pTrc99a-ilvCV412A was analyzed in a shake flask and 5 L bioreactor. The ultimate strain has a certain potential in D-PA production, which provides a foundation for industrialization. The study of AHAIR mutant in E. coli W3110 shows the potential of genetic engineering to modify and control metabolic pathways for desired outcomes.
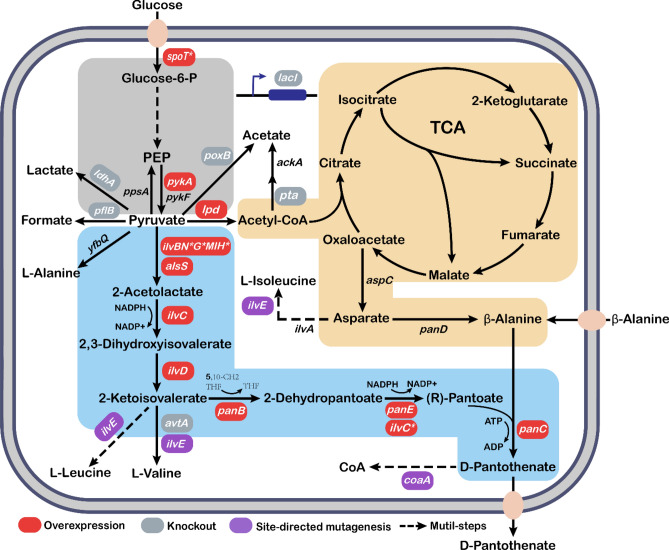
Fig. 1.
The biosynthetic pathway of D-PA in E. coli and partial metabolic engineering strategies involved in this paper. The red boxes indicated gene overexpression, the gray boxes represented gene knockout, the purple boxes and the asterisk meant gene site-directed mutagenesis, and the dotted arrows indicated multi-step reactions. PEP phosphoenolpyruvate, CoA coenzyme A, TCA tricarboxylic acid cycle, THF tetrahydrofolate, 5,10-CH2-THF 5,10-methylenetetrahydrofolate, NADPH nicotinamide adenine dinucleotide phosphate, NADP nicotinamide adenine dinucleotide phosphate, ATP adenosine triphosphate, ADP adenosine diphosphate
Materials and methods
Strains and plasmids
All strains and plasmids involved in this study are exhibited in supplementary Tables S1 and S2. E. coli DH5α (Tsingke, Beijing, China) was used as a universal cloning host, wild-type E. coli W3110, C. glutamicum ATCC 13032, and Bacillus subtilis 168 were purchased from the genetic stock center. The strain DPA8 was constructed previously (Zhang et al. 2019). Plasmids pTarget (Addgene Plasmid #62,226) and pCas9 (Addgene Plasmid #62,225) were donated by researcher Sheng Yang (Shanghai Institute of Plant Physiology and Ecology, Chinese Academy of Sciences). The Plasmid pTrc99a is stored in the laboratory (ampicillin resistance replaced by kanamycin resistance).
Genome editing and plasmid construction
CRISPR-Cas9 system was employed to carry out promoter replacement, gene deletion, and genome integration. Taking poxB knockout as an example, we mutated the first 20 bp of the PAM site on pTarget, which is used as the guiding signal of sgRNA (pTarget-ΔpoxB). Primers (ΔpoxB P1/ΔpoxB P2 & ΔpoxB P3/ΔpoxB P4) were used for polymerase chain reaction (PCR) amplification to obtain donor fragments L and R. Then, homologous arms were connected (donor L + R) through an overlapping polymerase chain reaction. The donor and linearized plasmid pTarget-ΔpoxB were seamlessly ligated by Novizan's ClonExpress® One-Step Cloning Kit. Finally, the recombinant plasmid pTarget-ΔpoxB-donor was electroporated into strain DPA8 harboring plasmid pCas9. Positive colonies were confirmed through sequencing, and plasmids were eliminated after verification. Gene overexpression was achieved through promoter replacement, codon optimization or gene copy number increasement, so we integrated the target segments between the upper and lower homologous arms to form an overexpression plasmid, and the remaining steps are the same as gene knockout. All primers involved in this study are exhibited in Table S3.
Culture conditions
E. coli, C. glutamicum, and B. subtilis were cultured in Luria–Bertani (LB) medium containing (/L): tryptone 10 g, yeast extract 5 g, and NaCl 10 g, incubated at 37 ℃, 200 rpm, 50 μg /mL kanamycin were mixed with media to maintain the plasmid pTrc99a (sterilized by 0.22 μm membrane).
Strains were inoculated into a 500 mL shake flask with 20 mL fermentation culture and maintained at 30 ℃, 200 rpm for 48 h. Shake flask fermentation was carried out in an improved MS medium (/L), which contained glucose 20g, (NH4)2SO4 16 g, yeast extract 2 g, KH2PO4 0.80 g, magnesium sulfate 0.50 g, elementary metal salt solution 1 mL (Zhang et al. 2019), CaCO3 0.30 g, VB1 0.10 mg, VB12 0.04 mg, kanamycin 1 mg, isopropyl-beta-D-thiogalactoside (IPTG) 0.96 mg, BCAAs 0.40 mg (L-valine, L-isoleucine, L-leucine respectively), and 3-AP 40 mg (sterilized by 0.22 μm membrane), sterilized at 115 ℃ for 30 min. Three replicated experiments were set up for flask fermentation.
Fed-batch fermentation process of the 5 L bioreactor is as follows. The single colony was selected in LB solid medium, and inoculated into a 10 mL LB tube. Then, 1 mL seed solution was inoculated into a 500 mL shake flask containing 200 mL LB medium, both were cultured at 37 ℃, 200 rpm for 12 h. The 5 L bioreactor medium (/L) contains glucose 20 g, (NH4)2SO4 16 g, 3-AP 4 g, yeast extract 2 g, KH2PO4 0.8 g, magnesium sulfate 0.5 g, and elementary metal salt solution 2 mL. The supplementary medium (/L) contains glucose 500 g, (NH4)2SO4 10 g, 3-AP 40 g, KH2PO4 14 g, magnesium sulfate 8 g, metal salt solution 1 mL, VB1 5 mg, VB12 2 mg, BCAAs 0.16 g, kanamycin 50 mg, and IPTG 48 mg. Fermentation was carried out with 2 L/min airflow speed, 30 ℃, and 400 rpm/min initial rotation speed. Started feeding and maintained the glucose concentration below 5 g/L when initial glucose was almost depleted, used ammonia (50%) to keep the pH around 6.80. Added 0.4 g BCAAs after 12 h to restore strain growth. Data came from three representative independent experiments.
Tolerance test
The strains tested for tolerance were activated in LB solid medium, then inoculated into LB liquid medium overnight at 37 ℃, 200 rpm to obtain seed liquid. Transferred 1 mL seed solution into 100 mL fermentation medium containing 0 mM, 0.02 mM, 0.05 mM, and 0.1 mM L-valine, respectively, and placed on a shaking table at 30 ℃, 200 rpm. Samples were taken every 12 h, 2 mL each time, using spectrophotometer to determine the OD600 value.
RT-qPCR test for gene expression quantification
To test the effect of promoter substitution on gene expression, D-PA-producing strains DPAN10, DPAN10-1 and DPAN11 were cultured in MS medium at 30 ℃, 200 rpm until the middle of the logarithmic phase (16 h), respectively, collected 2 mL of bacteria, separated at 12,000 rpm for 2 min. Using an RNA extraction kit to extract RNA. Using a reverse transcription kit to reverse-transcribe RNA into cDNA. Then, the mRNA transcribed by ilvH and ilvN was quantified by the quantitative polymerase chain reaction (qPCR) relatively (Genetically Transgenic Biotechnology, Beijing, China), using 16S rRNA as the internal reference gene. Primers for qPCR are listed in Supplementary Table S3.
Analytical methods
Biomass was determined with turbidimetry through measuring OD600 using a D30 spectrophotometer (Eppendorf, Germany).
Residual sugar was determined by 3,5-dinitrosalicylic acid method. Fermentation supernatant was diluted in an appropriate ratio (the concentration of residual sugar was within the standard curve), put 100 μL of sample into a 2 mL EP tube, added 200 μL of DNS reagent, reacted at 100 ℃ for 5 min, cooled and mixed with 1.6 ml of distilled water. Subsequently, recorded the absorbance and calculated the residual sugar based on the standard curve and dilution ratio.
The concentrations of D-PA and 2-ketoisovalerate (2-KIV) were determined by high-performance liquid chromatography (HPLC, Agilent, America). Mobile phase preparation: 93% (950 mL ultra-pure water + 950 μL phosphoric acid) filtrated by 0.22 μm microporous water filtration membrane, 7% acetonitrile filtrated by 0.22 μm microporous organic filtration membrane, and ultrasonic removal of bubbles. Column model: ACQUITYUPLC BEHC18 column (100 mm × 2.1 mm, 1.7 μm, Waters, UK); Parameter setting: injection volume: 10 μL, column temperature: 30 °C, flow velocity: 1 mL/min, detection wavelength: 200 nm. The retention time was 10.7 min.
Molecular modeling and molecular dynamics analysis
Using SWISS-MODEL to generate the homology model of variant EcAHAIR-V412A according to EcAHAIR (PDB entry: 3ULK) and visualized by PyMOL 2.4.0 (DeLano Scientific LLC, San Carlos, CA, USA). Using Autodock Vina with genetic algorithms for molecular docking to research molecular interactions. Molecular dynamics (MD) simulation was performed with the Schrödinger software package (Maestro 10.2) at 303 K for 50 ns to further evaluate the thermostability of wild-type EcAHAIR and its variant.
Results and discussion
Enrichment of the pyruvate pool
The D-PA-producing strain DPA8 (E. coli W3110 ΔavtA-panCpanEpanBilvCilvG*ilvE*coaAR106A) was selected as the chassis in this study (Zhang et al. 2019). Pyruvate (PYR), as the precursor of D-PA biosynthesis, plays a key role in central metabolism and the TCA cycle (Gray et al. 2014) and acts as a precursor for many metabolites, including acetyl-CoA, L-valine, L-alanine, and organic acids (Ma et al. 2019). Reducing the activity of the pyruvate dehydrogenase complex, inhibiting the production of organic acids, or blocking the TCA cycle are possible strategies for enhancing D-PA biosynthesis.
To maximize the pyruvate pool and address the adverse effects of organic acid accumulation as by-products on strain growth (Warnecke and Gill 2005), biosynthetic pathways including acetate, formate, and lactate were blocked. Strains DPAN8-1, DPAN8-2, DPAN8-3, and DPAN9 were constructed, respectively (genotypes are exhibited in Table S1). Results showed that blocking organic acid biosynthesis led to a gradual increase in D-PA production, from 1.59 to 2.09 g/L (Fig. 2a). The inconspicuous alteration in the production of strain DPAN8-2 may be due to the accumulation of acetyl-CoA caused by the deletion of pta (Dittrich et al. 2008), which inhibits the activity of pyruvate dehydrogenase. In addition, acetyl-CoA cannot be converted into acetate, which impedes ATP regeneration and affects D-PA biosynthesis.
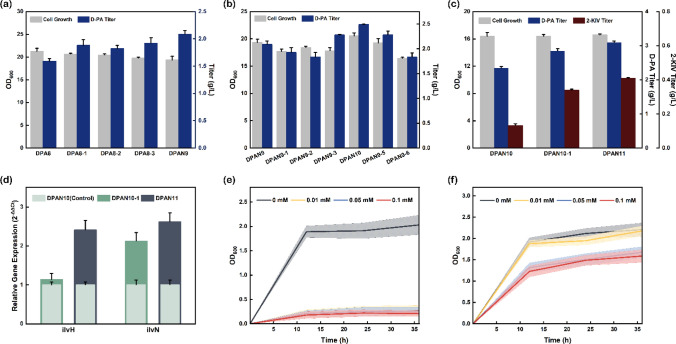
Fig. 2.
Effects of pyruvate enrichment and AHAS modification on D-PA production. a Effects of blocking the organic acid pathway on D-PA production; b Effects of enhancing pyruvate biosynthesis or weakening competitive pathways on D-PA production; c Effects of relieving the negative feedback inhibition of AHAS on cell growth, D-PA biosynthesis, and the accumulation of 2-KIV; d RT-qPCR was performed to verify the transcription levels of ilvN and ilvH; e Tolerance of strain DPAN10 under different concentrations of L-valine; f Tolerance of strain DPAN11 under different concentrations of L-valine. The error bars represent SD (n = 3)
As shown in Fig. 1, aminotransferase (yfbQ gene encoding) catalyzes pyruvate to L-alanine (Kim et al. 2010) and phosphoenolpyruvate synthase (ppsA gene encoding) transforms pyruvate to phosphoenolpyruvate (PEP). To further accumulate pyruvate, we prevented the biosynthesis of L-alanine and PEP (strains DPAN9-1 and DPAN9-2). However, both yield and growth had declined, we speculated that the inactivation of aminotransferase (yfbQ gene coding) might cause the closure of other amino transfer channels, resulting in metabolic disorders and decreased cell growth. Subsequently, we knocked out ppsA and overexpressed pykAF to get strains DPAN9-3, DPAN10, DPAN9-5, DPAN9-6, respectively, of which DPAN10 (DPAN9 derivative, pykA::Ptrc-pykA) had the best effect on D-PA production, increased by 20% (Fig. 2b). Based on metabolic analysis, enhanced pyruvate biosynthesis can provide more precursors for D-PA biosynthesis. However, excessive enrichment of the pyruvate pool may result in a deficiency of PEP, which indirectly leads to the insufficient biosynthetic capacity of aromatic amino acids and the restriction of cell growth (McCloskey et al. 2018), causing the failure to improve D-PA biosynthesis.
Modification of AHAS in the D-PA biosynthetic pathway
AHAS is the first enzyme to introduce pyruvate into the D-PA biosynthetic pathway, which is regulated by negative feedback from BCAAs, and exhibits substrate diversity (Geraskina et al. 2019). We successfully introduced small subunit mutations of AHAS I (G59A, C60T, T62A, A63C, A64T, G66C) and AHAS III (G41A, C50T) into the genome to relieve negative feedback inhibition from BCAAs by enzyme modification (Park et al. 2011), and constructed strains DPAN10-1 and DPAN11. Results showed that DPAN11 accumulated more 2-KIV compared with DPAN10, while having an inapparent effect on biomass, which indicates that mutations of AHAS confer resistance to L-valine feedback inhibition, driving more carbon flux into the D-PA biosynthetic pathway, therefore leading to an increase in D-PA production from 2.35 to 3.09 g/L (Fig. 2c).
In addition, RT-qPCR was performed on ilvN and ilvH of strains DPAN10-1 and DPAN11, respectively. It was found that the transcription levels of ilvN and ilvH were increased by 2.1–2.5 times (Fig. 2d), indicating that feedback inhibition of ilvN and ilvH was successfully relieved. AHAS is mainly feedback inhibited by L-valine (Geraskina et al. 2019). Therefore, DPAN10-1 and DPAN11 were subjected to L-valine tolerance experiment. Results showed that strain DPAN11 could grow normally at higher L-valine concentrations (Fig. 2e, f), further validating the removal of negative feedback inhibition of AHAS.
Improving glucose intake pathway and overexpressing lpd to accumulate pyruvate
Modification of the sugar intake system is indispensable for improving glucose conversion rate, so five potential strategies that can improve the glucose intake ability were screened (Michalowski et al. 2017; Lunin et al. 2004; Deutscher et al. 2006; Ronneau and Hallez 2019). DPAN11 was taken as the chassis, DPAN11-1, DPAN11-2, DPAN11-3, DPAN11-4, and DPAN12 were constructed, respectively. Experimental results exhibited that overexpressed glk (strain DPAN11-1) or introduced spoT mutant at pseudogene position (strain DPAN12) had a positive effect on cell concentration and productivity, among which DPAN12 had the best performance and the production reached 3.32 g/L (Fig. 3a). None of the other potential pathways improved the D-PA production, we proposed it affected the ability of sugar intake, leading to the decline on biomass and D-PA production.
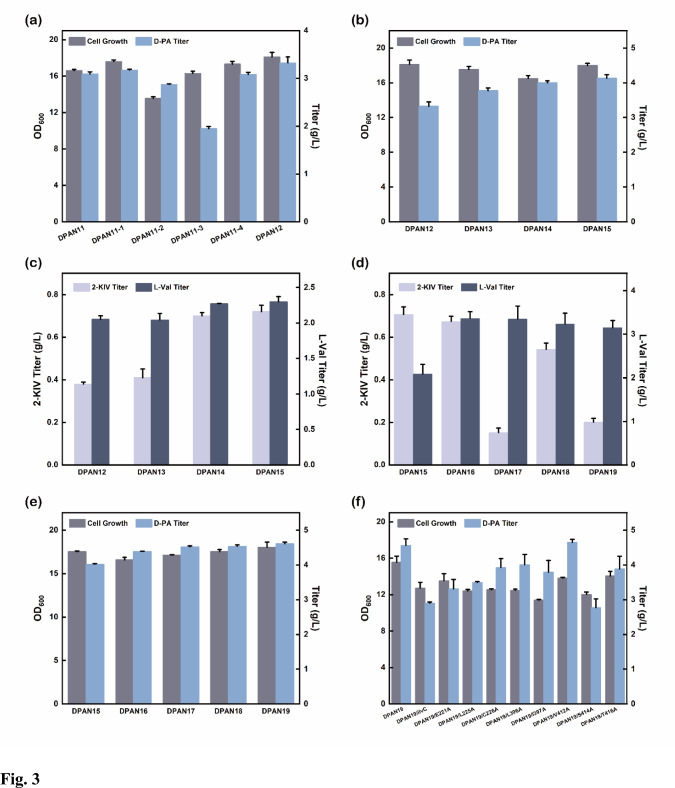
Fig. 3.
Effects of glucose intake capacity improvement and synthetic flux enhancement on D-PA production. a Effects of glucose intake system modification on D-PA production; b Effects of strengthening lpd, ilvD and knocking out lacI on D-PA production; c Accumulation of 2-KIV and L-valine under strengthening lpd, ilvD and knocking out lacI; d Accumulation of 2-KIV and L-valine under enhanced D-PA metabolic pathway; e Effects of enhanced D-PA metabolic pathway on D-PA production; f Effects of overexpression of the wild-type ilvC and its mutant on D-PA production. The error bars represent SD (n = 3)
After upgrading glucose intake capacity and establishing the pyruvate pool, lpd was found to play an essential role in the pyruvate dehydrogenase system, 2-ketoglutarate dehydrogenase system, and glycine lyase system. It may affect the supply of cofactor CH2-MTF by accumulating pyruvate or affecting the glycine lyase system (Frank et al. 2007). Accordingly, we overexpressed lpd based on strain DPAN12 by promoter replacement, resulting in an increase in D-PA production of strain DPAN13 to 3.77 g/L (Fig. 3b).
Upgradation of carbon flux in the D-PA biosynthetic pathway
In rational metabolic engineering, overexpression of vital genes in biosynthesis is one of the most universal strategies for redistributing carbon flux. To construct an engineered bacterium without plasmid and inducer addition in fermentation, we focused on the main pathway of D-PA biosynthesis and further enhanced it. We overexpressed ilvD by promoter replacement to generate strain DPAN14 and knocked out the repressor protein LacI (lacI gene encoding) to obtain a constitutive expression strain DPAN15. Fermentation results reflected that D-PA production was elevated to 4.13 g/L, raised by 9.5% (Fig. 3b).
It was also observed that the enhancement of the D-PA biosynthetic pathway was accompanied by the accumulation of L-valine after overexpressing ilvD, which we inferred was caused by the accumulation of 2-KIV (Fig. 3c), a conjoint precursor of L-valine and D-PA biosynthesis. Besides, the growth of strain DPAN15 was slightly improved after removing lacI, which might be due to the reduced bacterial burden without adding IPTG. Thus, the synthetic capacity of D-PA was also raised.
To introduce more carbon flux into the D-PA generative pathway, alsS (encoding AHAS) controlled by Trc was integrated into ydeU, forming strain DPAN16 with an increase in D-PA output to 4.38 g/L (Fig. 3e), and the accumulation of L-valine elevated from 2.08 to 3.36 g/L (Fig. 3d), indicating that the integration of alsS effectively transferred more carbon flux from pyruvate to 2-acetolactate. Therefore, another copy of ilvD was inserted into pseudogene yjiV to construct strain DPAN17, with a D-PA yield of 4.52 g/L (Fig. 3e).
To further strengthen the metabolic flux of D-PA biosynthesis, the other copy of alsS controlled by Trc was inserted into pseudogene ycdN, resulting in strain DPAN18. Compared with DPAN17, there was no significant change in D-PA production and L-valine accumulation, in contrast, the accumulation of 2-KIV increased (Fig. 3d), which suggests two copies of alsS were sufficient to support D-PA biosynthesis. In our previous study, it was found that panB and panC derived from C. glutamicum could effectively promote D-PA production (data not shown). Accordingly, heterologous panB and panC were tested based on strain DPAN18 to construct strain DPAN19, the D-PA production of DPAN19 reached 4.60 g/L (Fig. 3e), indicating that reinforcing the transformation of the (R)-pantoate pathway could raise D-PA production.
Structural analysis of the EcAHAIR mutant and its effects on D-PA production
To improve the affinity of AHAIR with substrate promiscuity for 2-acetolactate, thereby strengthening the D-PA biosynthetic flux, AHAIR was docked by Autodock Vina software, 8 sites near the enzyme and substrate binding pocket were selected for mutation to increase the specificity of 2-acetolactate by altering the reaction characteristics such as substrate promiscuity, catalytic activity, and selectivity. As shown in Fig. 3f, Results exhibited that D-PA production was promoted in almost all mutants compared with overexpressing wild-type ilvC, the D-PA yield of the mutant EcAHAIR-V412A reached 4.65 g/L, slightly higher than the control strain DPAN19. Furthermore, it was found that the L-valine accumulation of the mutant V412A increased significantly, indicating that mutant V412A was beneficial for introducing carbon flux into the (R)-pantoate pathway.
Further structural analysis of EcAHAIR and its single-site mutant V412A revealed that the valine residue at position 412 located in the active pocket of wild-type EcAHAIR, modification of this site could narrow substrate channel, thus blocking the binding of receptor and ligand (Fig. 4a, b). Nevertheless, the residue alanine in mutant V412A exhibited a minor side-chain, resulting in reduced steric hindrance and improved substrate channel on the cavity.
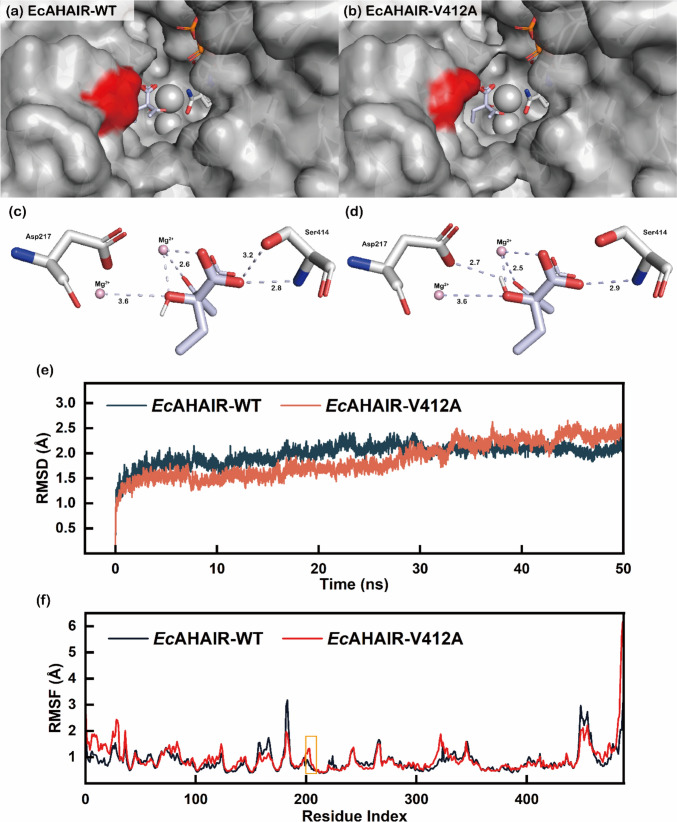
Fig. 4.
Visualization of AHAIR catalytic pocket (a EcAHAIR-WT; b EcAHAIR-V412A, the red area showed the V412 mutation site), hydrogen bond networks between 2-acetolactate and AHAIR (c EcAHAIR-WT; d EcAHAIR-V412A), Backbone Root Mean Square Deviation (in Å) of systems in enzyme–substrate complex forms at 303 K (e) and Backbone Root Mean Square Fluctuations (in Å) of Per-residue in enzyme–substrate complex forms at 303 K (f)
Analysis of the hydrogen bond network around the substrate showed that hydrogen bonds were formed between the substrate and Ser414 with distances of 2.8 Å to 3.2 Å in the wild-type EcAHAIR, while had no interaction with Asp217. Only one hydrogen bond was formed between the substrate and Ser414 in variant V412A, but it formed a new hydrogen bond with Asp217. At the same time, the hydrogen bond distance between 2-acetolactate and Mg2+ was reduced from 2.6 Å to 2.5 Å (Fig. 4c, d), enhancing intermolecular forces thus improving catalytic efficiency, thereby increasing the affinity for 2-acetolactate. Different from the development of NADH-dependent AHAIR, which addressed cofactor imbalance to produce isobutanol (Bastian et al. 2011), as well as from the development of promiscuous tyrosol/tyramine hydroxylase, which improved the biosynthetic efficiency of hydroxytyrosol by rearranging the metabolic flux between multiple-pathway (Chen et al. 2019). Our research is committed to minimizing the negative impact of carbon flux loss caused by substrate promiscuity on production, and the experimental results also demonstrate the practical feasibility of transforming substrate preference.
Molecular system stability and flexibility analysis of the EcAHAIR mutant
To research the structural and geometrical properties of the EcAHAIR variant, we compared the wild-type EcAHAIR with its mutant V412A based on MD simulation by analyzing the root mean square deviation (RMSD) of the backbone over time to investigate the stability of the molecular system. In a short (50 ns) simulation, we observed the RMSD values of the wild-type EcAHAIR fluctuated during the initial 15.5 ns and then maintained a balance around 2 Å at 17 ns, while the mutant EcAHAIR-V412A exhibited lower RMSD values in the form of enzyme–substrate complex with more pronounced fluctuations in the first 30 ns, and reached equilibrium at 33 ns with a slower rate (Fig. 4e), indicating a decrease in molecular system stability of the V412A variant.
Subsequently, we calculated the root mean square fluctuation (RMSF) values of per-residue of the two enzymes to further analyze the flexibility of the backbone. As depicted in Fig. 4f, there was no significant distinction between these two enzymes. Overall, the wild-type EcAHAIR presented relatively lower RMSF values than the mutant V412A, indicating fewer flexible regions. Meanwhile, the residue Asp217 interacted with the substrate, and the residues around it had higher RMSF values, revealing that the residues of the mutant EcAHAIR-V412A located at the active pocket had better flexibility.
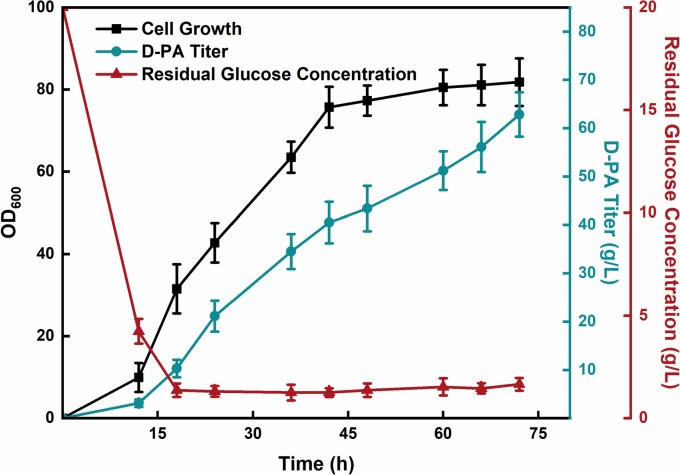
Fed-batch fermentation by DPAN19/trc-ilvCV412A
The continuous and stable supply of substrates and pH control have a significant impact on production capacity, but it is difficult to achieve in shake flasks. Therefore, we used glucose as the primary carbon source, employed a 5 L bioreactor to amplify production and strain DPAN19/trc-ilvCV412A was selected for testing. According to the previous experimental results (Zhang et al. 2019, 2021; Zou et al. 2021), we chose fed-batch fermentation, started feeding after the depletion of initial glucose and employed the pH-feedback feeding strategy at 30 ℃ during fermentation. Results are shown in Fig. 5, the production of D-PA increased synchronously with the growth of the strain, which proliferated in the early stage, and gradually entered the stationary phase after 42 h. The highest OD600 was 81.8 at 72 h, and the production of D-PA reached 62.82 g/L, with a yield of 0.23 g/g glucose, and a productivity of 0.60 g/L/h. Although the production of D-PA was significantly improved, but it was also found that the yield was low and it entered the stationary phase early, which might be an obstacle to further increasing D-PA production.
Fig. 5.
Fed-batch fermentation by strain DPAN19/trc-ilvCV412A in 5 L bioreactors. The error bars represent SD (n = 3)
Conclusions
In this paper, a D-PA-producing strain DPAN19 without plasmid and inducer was constructed by blocking the organic acid pathway, strengthening the enrichment of the pyruvate pool, relieving the feedback inhibition of AHAS, improving glucose intake capacity, and enhancing the expression of D-PA synthetic pathway genes. Besides, we explored the enzyme evolution strategy, and obtained a mutant EcAHAIR-V412A with a high affinity for 2-acetolactate through semi-rational modification, which was further applied to D-PA production for the first time, the experimental results on AHAIR demonstrate the potential for improving and modifying pathway enzymes to obtain desired bio-products, providing a novel perspective for modifications with similar substrate promiscuity. Finally, in fed-batch fermentation, the engineered strain DPAN19/trc-ilvCV412A was constructed by metabolic strategy combined with enzyme engineering, which could produce 62.82 g/L D-PA with 0.23 g/g glucose yield and 0.60 g/L/h productivity. These strategies and the mutant can be applied to further optimize and engineer other microbial strains for the production of various compounds of interest.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0901400).
Abbreviations
- D-PA
D-pantothenic acid
- TCA
Tricarboxylic acid
- D-PL
D-valerolactone
- 3-AP
3-Aminopropionic acid
- AHAS
Acetolactate synthase
- AHAIR
Acetolactate isomeroreductase
- PCR
Polymerase chain reaction
- IPTG
Isopropyl-beta-D-thiogalactoside
- LB
Luria–Bertani
- CRISPR
Clustered Regularly Interspersed Short Palindromic Repeats
- BCAAs
Branched-chain amino acids
- PEP
Phosphoenolpyruvate
- 2-KIV
2-Ketoisovalerate
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NADH
Nicotinamide adenine dinucleotide
Author contributions
BZ: writing-original draft, writing-review, editing. YQZ: designed research, writing-review, data curation. ZLH: investigation, analyzed data. YYX: investigation, methodology. MNT: formal analysis. JPZ: visualization. ZQL: project administration, funding acquisition, supervision, conceptualization. YGZ: project administration, funding acquisition, supervision, conceptualization, funding acquisition, supervision.
Funding
This work was supported by the National Key Research and Development Program of China (2018YFA0901400).
Declarations
Conflict of interest
All authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Leonardi R, Jackowski S (2007) Biosynthesis of pantothenic acid and coenzyme A. EcoSal Plus 2(2). 10.1128/ecosalplus.3.6.3.4 [DOI] [PMC free article] [PubMed]
- Xu JS, Patassini S, Begley P, Church S, Waldvogel HJ, Faull RLM, Unwin RD, Cooper GJS. Cerebral deficiency of vitamin B5 (d-pantothenic acid; pantothenate) as a potentially-reversible cause of neurodegeneration and dementia in sporadic Alzheimer’s disease. Biochem Biophys Res Commun. 2020;527(3):676–681. doi: 10.1016/j.bbrc.2020.05.015. [DOI] [PubMed] [Google Scholar]
- Hall JE, Hall ME (2020) Dietary balances; regulation of feeding; obesity and starvation; vitamins and minerals, in: Hall JE, Hall ME, Guyton and Hall Textbook of Medical Physiology. fourteenth ed Elsevier United Kingdom pp:877–892.
- Tigu F, Zhang JL, Liu GX, Cai Z, Li Y. A highly active pantothenate synthetase from Corynebacterium glutamicum enables the production of D-pantothenic acid with high productivity. Appl Microbiol Biotechnol. 2018;102:6039–6046. doi: 10.1007/s00253-018-9017-2. [DOI] [PubMed] [Google Scholar]
- Postaru M, Cascaval D, Galaction AI. Pantothenic acid-applications, synthesis and biosynthesis. Rev Med Chir Soc Med Nat Iasi. 2015;119(3):938–943. [Google Scholar]
- Huang J, Huang L, Lin JP, Xu ZN, Cen PL. Organic chemicals from bioprocesses in China. Adv Biochem Eng Biotechnol. 2010;122:43–71. doi: 10.1007/10_2010_75. [DOI] [PubMed] [Google Scholar]
- Rowicki T, Synoradzki L, Wlostowski M. Calcium pantothenate, part 1: (R, S)-pantolactone technology improvement at the tonnage scale. Ind Eng Chem Res. 2006;45:1259–1265. doi: 10.1021/ie050774u. [DOI] [Google Scholar]
- Honda K, Kataoka M, Shimizu S. Functional analyses and application of microbial lactonohydrolases. Biotechnol Bioprocess Eng. 2002;7:130–137. doi: 10.1007/BF02932910. [DOI] [Google Scholar]
- Bonrath W, Netscher T. Catalytic processes in vitamins synthesis and production. Appl Catal A-Gen. 2005;280:55–73. doi: 10.1016/j.apcata.2004.08.028. [DOI] [Google Scholar]
- Zou SP, Zhao K, Tang H, Zhang Z, Zhang B, Liu ZQ, Zheng YG. Improved production of D-pantothenic acid in Escherichia coli by integrated strain engineering and fermentation strategies. J Biotechnol. 2021;339:65–72. doi: 10.1016/j.jbiotec.2021.07.014. [DOI] [PubMed] [Google Scholar]
- Grzegorz K, Macko D, Mikulski D (2010) Development of biotechnological methods of biofuels production from renewable sources. Environ Protein Nat Resour 45:118–135. https://www.researchgate.net/publication/234125774
- Gao H, Tuyishime P, Zhang X, Yang TW, Xu MJ, Rao ZM. Engineering of microbial cells for L-valine production: challenges and opportunities. Microb Cell Fact. 2021;20:172. doi: 10.1186/s12934-021-01665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PP, Xu HT, Zhang XL (2021) Metabolic engineering of microorganisms for L-alanine production. J Ind Microbiol Biotechnol 49(2). 10.1093/jimb/kuab057 [DOI] [PMC free article] [PubMed]
- Wang YY, Xu JZ, Zhang WG. Metabolic engineering of l-leucine production in Escherichia coli and Corynebacterium glutamicum: a review. Crit Rev Biotechnol. 2019;39(5):633–647. doi: 10.1080/07388551.2019.1577214. [DOI] [PubMed] [Google Scholar]
- Zhang B, Chen L, Jin JY, Zhong N, Cai X, Zou SP, Zhou HY, Liu ZQ, Zheng YG. Strengthening the (R)-pantoate pathway to produce D-pantothenic acid based on systematic metabolic analysis. Food Biosci. 2021;43:101283. doi: 10.1016/j.fbio.2021.101283. [DOI] [Google Scholar]
- Guo JX, Sun XX, Yuan YJ, Chen QT, Ou ZT, Deng ZX, Ma T, Liu TG. Metabolic Engineering of Saccharomyces cerevisiae for Vitamin B5 Production. J Agric Food Chem. 2023;71(19):7408–7417. doi: 10.1021/acs.jafc.3c01082. [DOI] [PubMed] [Google Scholar]
- Tyagi R, Lee YT, Guddat LW, Duggleby RG. Probing the mechanism of the bifunctional enzyme ketol-acid reductoisomerase by site-directed mutagenesis of the active site. FEBS J. 2005;272(2):593–602. doi: 10.1111/j.1742-4658.2004.04506.x. [DOI] [PubMed] [Google Scholar]
- Bastian S, Liu X, Meyerowitz JT, Snow CD, Chen MMY, Arnold FH. Engineered ketol-acid reductoisomerase and alcohol dehydrogenase enable anaerobic 2-methylpropan-1-ol production at theoretical yield in Escherichia coli. Metab Eng. 2011;13(3):345–352. doi: 10.1016/j.ymben.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Geraskina NV, Sycheva EV, Samsonov VV, Eremina NS, Hook CD, Serebrianyi VA, Stoynova NV. Engineering Escherichia coli for autoinducible production of L-valine: an example of an artificial positive feedback loop in amino acid biosynthesis. PLoS ONE. 2019;14(4):e0215777. doi: 10.1371/journal.pone.0215777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas R, Butikofer MC, Job D, Douce R. Evidence for two catalytically different magnesium-binding sites in acetohydroxy acid isomeroreductase by site-directed mutagenesis. Biochemistry. 1995;34(18):6026. doi: 10.1021/bi00018a004. [DOI] [PubMed] [Google Scholar]
- Wang YY, Zhang F, Xu JZ, Zhang WG, Chen XL, Liu LM. Improvement of L-Leucine production in Corynebacterium glutamicum by altering the redox flux. Int J Mol Sci. 2019;20(8):2020. doi: 10.3390/ijms20082020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao YA, Ma Q, Liu XQ, Fan XG, Men JX, Wu HY, Jiang S, Tian DG, Xiong B, Xie XX. High-yield production of L-valine in engineered Escherichia coli by a novel two-stage fermentation. Metab Eng. 2020;62:198–206. doi: 10.1016/j.ymben.2020.09.007. [DOI] [PubMed] [Google Scholar]
- Zou SP, Zhang Z, Zhao K, Liu ZQ, Zheng YG. Metabolic engineering of Escherichia coli for improved D-pantothenic acid biosynthesis by enhancing NADPH availability. Biochem Eng J. 2022;187:108603. doi: 10.1016/j.bej.2022.108603. [DOI] [Google Scholar]
- Zhang B, Zhang XM, Wang W, Liu ZQ, Zheng YG. Metabolic engineering of Escherichia coli for d-pantothenic acid production. Food Chem. 2019;294:267–275. doi: 10.1016/j.foodchem.2019.05.044. [DOI] [PubMed] [Google Scholar]
- Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71(14):2577–2604. doi: 10.1007/s00018-013-1539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YC, Ma Q, Cui Y, Du LH, Xie XX, Chen N. Transcriptomic and metabolomics analyses reveal metabolic characteristics of L-leucine- and L-valine-producing Corynebacterium glutamicum mutants. Ann Microbiol. 2019;69(5):457–468. doi: 10.1007/s13213-018-1431-2. [DOI] [Google Scholar]
- Warnecke T, Gill RT. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Factories. 2005;4(1):1–8. doi: 10.1186/1475-2859-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich CR, Bennett GN, San KY. Characterization of the acetate-producing pathways in Escherichia coli. Biotechnol Prog. 2008;21(4):1062–1067. doi: 10.1021/bp050073s. [DOI] [PubMed] [Google Scholar]
- Kim SH, Schneider BL, Reitzer L. Genetics and regulation of the major enzymes of alanine synthesis in Escherichia coli. J Bacteriol. 2010;192(20):5304–5311. doi: 10.1128/jb.00738-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloskey D, Xu SB, Sandberg TE, Brunk E, Hefner Y, Szubin R, Feist AM, Palsson BO. Adaptive laboratory evolution resolves energy depletion to maintain high aromatic metabolite phenotypes in Escherichia coli strains lacking the Phosphotransferase System. Metab Eng. 2018;48:233–242. doi: 10.1016/j.ymben.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim TY, Lee KH, Lee SY. Fed-batch culture of Escherichia coli for L-valine production based on in silico flux response analysis. Biotechnol Bioeng. 2011;108(4):934–946. doi: 10.1002/bit.22995. [DOI] [PubMed] [Google Scholar]
- Michalowski A, Siemann-Herzberg M, Takors R. Escherichia coli HGT: Engineered for high glucose throughput even under slowly growing or resting conditions. Metab Eng. 2017;40:93–103. doi: 10.1016/j.ymben.2017.01.005. [DOI] [PubMed] [Google Scholar]
- Lunin VV, Li Y, Schrag JD, Lannuzzi P, Cygler M, Matte A. Crystal structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose. J Bacteriol. 2004;186(20):6915–6927. doi: 10.1128/jb.186.20.6915-6927.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70(4):939–1031. doi: 10.1128/mmbr.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronneau S, Hallez R. Make and break the alarmone: regulation of (p)ppGpp synthetase/hydrolase enzymes in bacteria. FEMS Microbiol Rev. 2019;43(4):389–400. doi: 10.1093/femsre/fuz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RAW, Price AJ, Northrop FD, Perham RN, Luisi BF. Crystal structure of the E1 component of the Escherichia coli 2-oxoglutarate dehydrogenase multienzyme complex. J Mol Biol. 2007;368(3):639–651. doi: 10.1016/j.jmb.2007.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Yao J, Meng J, Han WJ, Tao Y, Chen YH, Guo YX, Shi GZ, He Y, Jin JM, Tang SY. Promiscuous enzymatic activity-aided multiple-pathway network design for metabolic flux rearrangement in hydroxytyrosol biosynthesis. Nat Commun. 2019;10(1):960. doi: 10.1038/s41467-019-08781-2. [DOI] [PMC free article] [PubMed] [Google Scholar]