Abstract
Restless legs syndrome (RLS) and periodic limb movements of sleep (PLMS) have been variably implicated in risk for cardiovascular disease (CVD), but there is lack of consensus on these relationships. We sought to assess subclinical CVD measures and RLS/PLMS in a large cohort to further evaluate these associations. The Emory Center for Health Discovery and Well Being cohort is composed of employed adults, with subclinical CVD measures including endothelial function (flow-mediated vasodilation), microvascular function (reactive hyperemia index, RHI), arterial stiffness (pulse wave velocity and augmentation index), and carotid intima-media thickness (cIMT). Participants were grouped based on presence (N = 50) or absence (N = 376) of RLS and subclinical CVD measures compared between groups. A subset of participants (n = 40) underwent ambulatory monitoring for PLMS and obstructive sleep apnea. PLMS association with subclinical CVD measures was assessed. RLS status was significantly associated with flow-mediated dilation in univariate analyses but not after controlling for potential confounders; RLS was not associated with other subclinical CVD measures. PLMS were significantly correlated with the RHI, augmentation index, and cIMT in univariate analyses; only the association between PLMS and cIMT remained significant (p = 0.04) after controlling for RLS status, age, apnea–hypopnea index, hyperlipidemia, and hypertension. The observed association between higher PLMS and greater cIMT suggests that PLMS may be a marker of subclinical CVD. Further work is needed to determine the relationship between PLMS and CVD risk.
Supplementary Information
The online version contains supplementary material available at 10.1007/s41105-023-00497-7.
Keywords: Periodic limb movements of sleep, Restless legs syndrome, Cardiovascular disease, Carotid artery, Intima-media thickness
Introduction
Cardiovascular disease (CVD) is the leading cause of death globally, and accurately assessing CVD risk is imperative [1]. Numerous studies have implicated insufficient sleep and sleep disorders as important risk factors for cardiovascular morbidity and mortality. Historically, longitudinal observational and cross-sectional studies have focused on the connection between obstructive sleep apnea (OSA) and CVD [2, 3]. Fewer studies have probed the associations of other sleep disorders, such as restless leg syndrome (RLS) or periodic limb movements of sleep (PLMS), with CVD. RLS is a sensorimotor disorder defined by an urge to move the legs that is worst at rest, is relieved by movement, and peaks at night or in the evening. It is one of the most common sleep disorders, with a prevalence of 10–15% [4]. PLMS are recurrent movements of the legs, most commonly ankle flexion via the anterior tibialis that occur every 5–90 s during sleep. Most people with RLS have PLMS, but PLMS are also common in people without RLS, occurring in approximately 25% of adults [5, 6]. RLS and PLMS share some genetic susceptibility. [5]
As disorders that are associated with sleep disruption, it is plausible that RLS or PLMS may lead to increased CVD risk and their treatment might provide additional opportunities for CVD risk factor reduction [7]. Epidemiologic studies of RLS and CVD have shown mixed results, with some showing no association with CVD while others show increased risk in patients with RLS [8]. Fewer studies have explored PLMS and CVD, but available evidence more consistently points to an increased risk of CVD in those with PLMS [8]. More firmly establishing the relationship between RLS and/or PLMS with subclinical CVD measures such as arterial stiffness, endothelial function, and arterial wall thickness might help elucidate the pathophysiology underlying these associations.
In 2017, Gottlieb et al. published a roadmap for exploring the mechanistic underpinnings underlying the association between RLS and CVD [9]. The authors described several potential neural, metabolic, and vascular mechanisms drawn from the OSA literature that have yet to be extensively explored in RLS. The targets they proposed were modeled after the mechanisms currently believed to increase the incidence of CVD in patients with OSA, including arterial stiffness, compliance and pro-inflammatory markers. Using data from the Emory Predictive Health Institute’s Center for Health Discovery and Well Being (CHDWB), we conducted a cross-sectional analysis to evaluate relationships between RLS and PLMS and subclinical CVD.
Materials and methods
Participants
Data were obtained from the CHDWB. In depth descriptions of the inclusion and exclusion criteria for this cohort have been covered elsewhere [10, 11]. In brief, this cohort began with approximately 750 randomly selected employees of Emory University who have been followed annually for 5 years since 2008. At the time of enrollment, participants were 18 years of age or older, had no hospitalizations or newly diagnosed chronic diseases within the previous year, and had no acute illnesses in the previous two weeks. In 2009, the CHDWB cohort was expanded to include additional questionnaires for RLS diagnosis [12], RLS severity as measured by the International RLS Study Group severity scale (IRLS) [13], and symptoms of other sleep disorders. A subgroup of the cohort was recruited to complete monitoring at home to assess for obstructive sleep apnea and to measure PLMS.
Clinical and demographic features
Clinical and demographic information was obtained for the CHDWB participants via questionnaires and included past medical history, age, race, and gender. Participants selected race from 18 options, of which the majority selected (in descending order) White (n = 402), Black (n = 109), Asian (n = 26), American Indian (n = 5), and Alaskan native (n = 1). Because of small numbers of participants other than White and Black, particularly within the PLMS subgroup, race was dichotomized to White vs racialized minority for subsequent analyses. Sleep-specific questionnaires included measures of sleep quality (the Pittsburgh Sleep Quality Index, PSQI, in which scores > 5 indicate poor sleep quality) [14] and daytime sleepiness (the Epworth sleepiness scale, in which higher scores indicate worse sleepiness) [15]. Presence of hypertension, hyperlipidemia, and diabetes were determined based on questionnaire, which asked if participants had a history of each of these disorders. Use of tobacco and alcohol was also collected via questionnaire. The complete list of surveys administered can be found elsewhere [10]. Body mass index was measured with a Tanita body composition analyzer (Tanita, Tokyo, Japan) [16].
RLS identification
The Cambridge Hopkins RLS questionnaire (CH-RLSq), a validated questionnaire for diagnosing RLS [12], was used to ascertain RLS status. This seven-question scale assesses the four core RLS criteria and two common RLS mimics, positional discomfort and leg cramps. Participants were considered to have RLS if they met all four core RLS criteria, without either mimic. Participants were considered to be RLS negative if they answered “no” to the second question in the CH-RLSq that asks if the participant has ever experienced the recurrent need or urge to move their legs while sitting or lying down. All other participants, including participants who endorsed an urge to move but did not meet all four core RLS criteria or who endorsed an RLS mimic, were considered to have indeterminant RLS status and were excluded from further analyses. RLS status was determined based on the first visit in which each participant completed the CH-RLSq. The IRLS, a 40-point scale with higher numbers indicating more severe RLS [13], was administered to all participants who were diagnosed with RLS based on the CH-RLSq. We applied standard IRLS cutoffs for severity, i.e., 0–10 mild, 11–20 moderate, 21–30 severe, and 31–40 very severe.
PLMS measurement
CHDWB participants were asked if they would be willing to wear monitors at home to assess additional features of sleep. A subgroup of those who answered affirmatively were contacted, without respect to RLS status, and were assessed for PLMS via ankle accelerometers. The PAM-RL tri-axial accelerometer (Respironics, Murrysville, PA) was worn on the same ankle for five consecutive nights. PLMS were quantified for each night of monitoring by a single scorer, with review of raw data and application of the software algorithm that accompanies the PAM-RL. The PAM-RL provides an accurate estimate of polysomnographically derived PLMS (Pearson’s correlation r = 0.87, p < 0.0001) [17] and discriminates between PLMs and normal nocturnal motor activity [18]. The mean number of PLMs per hour, or PLM Index (PLMI), was averaged across recorded nights.
Sleep apnea risk assessment
Risk for sleep apnea was classified using the STOP assessment [19], with individual elements collected elsewhere in the CHDWB, including presence or absence of snoring, tiredness/sleepiness (defined as Epworth > 10), observed apneas, and diagnosed hypertension. Scores of 2 or higher were considered high risk for obstructive sleep apnea.
The subgroup of participants who underwent PLMS measurement also wore a WatchPAT home sleep apnea test (Itamar Medical, Caesarea, Israel) for a single night, during the period they wore ankle accelerometry [20, 21]. The WatchPAT-derived apnea hypopnea index (pAHI) was used as an objective measure of OSA for this subgroup.
Laboratory tests
Participants underwent blood tests relevant to RLS, including measures of peripheral iron stores (ferritin), anemia (hemoglobin, hematocrit), renal function (creatinine), and lipid panel (low density lipoprotein, LDL, and high density lipoprotein, HDL). Serologic testing was carried out by Quest Diagnostics.
Subclinical CVD measurements
Subclinical CVD measurements were obtained from the CHDWB study, including measures of microvascular function, endothelial dysfunction, arterial stiffness, and carotid arterial wall thickness. Microvascular function was measured via pulse amplitude tonometry, which measures pulse amplitude at the fingertip at rest and following reactive hyperemia; a lower reactive hyperemia response, as reflected in the reactive hyperemia index, indicates poorer microvascular function. Endothelial function was assessed via flow-mediated dilation (FMD) ultrasound of the brachial artery, measured at rest and following occlusion. FMD was calculated as the percent change in diameter between baseline and reactive hyperemia conditions. Allometric scaling of FMD was performed by taking the natural log of each measure and including baseline measures as a covariate. Arterial stiffness was measured by Sphygmocor device (Atcor Medical, Australia), which measures pressure waveforms at peripheral pulse sites via high-fidelity tonometer. Two complementary measures of arterial stiffness were collected, the augmentation index and the pulse wave velocity. The augmentation index, a composite measure of the magnitude of arterial wave reflections, is normalized to a heart rate of 75 beats/min; analyses of augmentation index were controlled for height. The pulse wave velocity was measured between carotid and femoral arteries, providing an estimate of the speed of the pressure wave travelling along the aorta and reflecting large artery stiffness. Common carotid artery intima-media thickness (cIMT) was measured via ultrasound and averaged between right and left carotid arteries. Full details of collection of these vascular measures within the CHDWB have been described elsewhere [22, 23].
Statistical analysis
The relationships between RLS status (yes/no) and continuous demographic and clinical features were assessed via t tests, adjusted for unequal variances when appropriate. For categorical demographic and clinical variables, relationship with RLS status was evaluated via Chi-square or Fisher exact tests. For the subgroup of participants in whom PLMS were monitored, clinical and demographic features were compared to PLMS, treating PLMS as a continuous variable. PLMS measurements were compared to other continuous variables via univariate linear regression. In cases where regression residuals were not normally distributed, continuous variables were log-transformed to ensure this statistical assumption was met. For categorical clinical and demographic features, number of PLMS was compared between dichotomous variables via Wilcoxon rank sum test.
Similar analyses were performed to assess for relationships between RLS status or PLMI and subclinical CVD measures. In the case of significant associations (p < 0.05) observed between individual measures of subclinical CVD and either RLS or PLMS, multiple linear regression analyses were performed to control for potential confounders. Covariates were included in these multivariable models if they were significantly or nearly significantly (p < 0.10) associated with RLS or PLMS in univariate analyses. Because age, diabetes, and hypertension may be particularly relevant covariates for both RLS/PLMS and subclinical CVD, these were also included in the RLS model; age and hypertension were included in the PLMS models but diabetes was excluded because only a single person in the PLMS sample had this condition.
Results
RLS group
Of the 543 participants who underwent RLS assessment (including participants that did not meet criteria for positive or negative RLS), 9.2% had RLS (50/543). For the remaining analyses, only those positive (n = 50) or negative (n = 376), but not indeterminant (n = 117), for RLS were included. Among those with RLS, average IRLS severity score was 10.2 (± standard deviation 4.4), with 23 participants having mild symptoms, 25 moderate symptoms, 2 severe, and 0 very severe. Participants with RLS were significantly older and were more likely to have poor self-reported sleep quality than those without RLS (Table 1). Serum ferritin and creatinine levels did not differ between those with and without RLS, while hemoglobin and hematocrit were modestly and significantly lower in the RLS group.
Table 1.
Demographic and clinical characteristics by RLS status
| RLS negative (n = 376) | RLS positive (n = 50) | p value | |
|---|---|---|---|
| Age (n = 426) | 49.5 (11.1) | 53.5 (12.3) | 0.02 |
| Female gender (n = 426) | 234 (62.2%) | 38 (76.0%) | 0.06 |
| White (n = 426) | 281 (74.7%) | 40 (80%) | 0.42 |
| PSQI > 5 (n = 423) | 136 (36.5%) | 31 (62.0%) | 0.0005 |
| ESS > 10 (n = 424) | 35 (9.4%) | 7 (14.0%) | 0.31 |
| High risk for OSA (n = 424) | 37 (9.9%) | 6 (12.0%) | 0.64 |
| IRLS severity score (n = 50) | – | 10.2 (4.4) | – |
| Hypertension (n = 426) | 64 (17.0%) | 7 (14.0%) | 0.59 |
| Diabetes (n = 426) | 17 (4.5%) | 5 (10.0%) | 0.16 |
| Hyperlipidemia (n = 426) | 86 (22.9%) | 13 (26.0%) | 0.62 |
| Current or past smoking (n = 424) | 65 (17.4%) | 14 (28.0%) | 0.07 |
| Current alcohol use (n = 423) | 310 (83.1%) | 40 (80.0%) | 0.58 |
| BMI (n = 389) | 27.1 (6.1) | 26.7 (6.3) | 0.72 |
| Serum ferritin (n = 374) | 86.3 (83.2) | 75.0 (69.2) | 0.39 |
| Hemoglobin (n = 372) | 14.0 (2.1) | 13.4 (1.4) | 0.02 |
| Hematocrit (n = 372) | 41.3 (4.7) | 39.8 (3.9) | 0.049 |
| Creatinine (n = 374) | 1.1 (4.5) | 0.8 (0.2) | 0.33 |
| LDL, mg/dL (n = 373) | 108.1 (30.0) | 106.8 (31.0) | 0.79 |
| HDL, mg/dL (n = 372) | 61.3 (18.0) | 64.0 (20.9) | 0.34 |
Bold entries indicate statistical significance
Data are presented as mean (standard deviation) or number (percent)
BMI body mass index, BUN blood urea nitrogen, Cr creatinine, ESS Epworth Sleepiness Scale, HDL high density lipoprotein, IRLS International RLS study group severity scale, LDL low density lipoprotein, OSA obstructive sleep apnea, PSQI Pittsburgh Sleep Quality Index, RLS restless legs syndrome
RLS and CVD risk factors
The presence of RLS was not associated with traditional CVD risk factors, including hypertension, diabetes, lipid profile, smoking, or BMI (see Table 1).
RLS and subclinical CVD
Univariate associations between RLS status and subclinical CVD measures are presented in Table 2. In univariate analyses, only flow-mediated dilation was significantly different between those with and without RLS (5.40 ± 0.47 in those with RLS and 6.45 ± 0.18 in those without, p = 0.04, indicating poorer endothelial function in those with RLS). However, the relationship between FMD and RLS was not significant in the adjusted model. Carotid intima-media thickness trended to be higher in those with compared to without RLS (0.66 mm with RLS, 0.63 mm without RLS, p = 0.07).
Table 2.
Subclinical CVD measures by RLS status
| RLS negative (n = 376) | RLS positive (n = 50) | Univariate p value* | Covariate adjusted p value** | |
|---|---|---|---|---|
| Reactive hyperemia index (n = 368) | 2.05 (0.63) | 2.17 (1.01) | 0.43 | |
| Flow-mediated dilation, controlling for baseline diameter (%) (n = 368) | 6.45 (0.18) | 5.40 (0.47) | 0.04 | 0.18 |
| Augmentation Index, controlled for height and heart rate (n = 372) | 20.1 (0.5) | 21.6 (1.4) | 0.32 | |
| Pulse wave velocity, m/s (n = 368) | 6.95 (1.20) | 6.80 (1.03) | 0.40 | |
| Mean carotid IMT, mm (n = 369) | 0.63 (0.12) | 0.66 (0.13) | 0.07 |
Bold entries indicate statistical significance
Data are presented as mean (standard deviation), except augmentation index and FMD, which are LS means and standard error
RLS restless legs syndrome, IMT intima-media thickness, PSQI Pittsburgh Sleep Quality Index
*Univariate p values reflect t test comparison of RLS positive vs RLS negative participants, for all variables except augmentation index and flow-mediated dilation, which reflect multivariate linear regression. **Covariate adjusted analyses were performed in the case of significant univariate associations, and were additionally controlled for age, gender, poor sleep by PSQI, smoking, hemoglobin, diabetes, and hypertension. p value reflects the relationship between RLS status and variable of interest
PLMS subgroup
A total of 40 participants completed ambulatory monitoring for PLMS and had known RLS status. Ten of these forty participants (25%) had RLS as defined by CH-RLSq scores; the remaining 30 did not have RLS. The median (interquartile range) PLMI was 15.1 (3.6, 38.5) in those with RLS and 4.1 (2.4, 8.2) in those without (p = 0.06). PLMI was not significantly associated with demographic or clinical features other than hyperlipidemia and log pAHI (Tables 3 and 4).
Table 3.
Relationship of PLMI and demographic and clinical characteristics (categorical characteristics)
| PLMI in participants WITH demographic/clinical characteristic | PLMI in participants WITHOUT demographic/clinical characteristic | Wilcoxon p value | |
|---|---|---|---|
| Female gender (n = 40) | 5.6 (2.2, 13.8) | 5.3 (2.7, 15.6) | 0.99 |
| White (n = 40) | 5.0 (2.1, 16.1) | 6.8 (3.8, 9.6) | 0.81 |
| RLS status (n = 40) | 15.1 (3.6, 38.5) | 4.1 (2.4, 8.2) | 0.06 |
| PSQI > 5 (n = 39) | 5.6 (3.0, 12.8) | 4.5 (1.2, 15.6) | 0.42 |
| ESS > 10 (n = 39) | 16.1 (0.1, 23.0) | 4.8 (2.5, 10.8) | 0.81 |
| Hypertension (n = 40) | 3.2 (2.1, 20.6) | 6.2 (2.7, 12.1) | 0.70 |
| Hyperlipidemia (n = 40) | 23.0 (4.5, 45.1) | 3.8 (2.1, 9.6) | 0.04 |
| Current or past smoking (n = 40) | 10.8 (9.6, 12.1) | 4.8 (2.4, 15.6) | 0.30 |
| Current alcohol use (n = 40) | 4.8 (2.4, 10.8) | 27.4 (4.6, 57.7) | 0.18 |
Bold entries indicate statistical significance
Table presents the median (interquartile range) of PLMI for those with versus without a given demographic or clinical characteristic. Diabetes, included in Table 1, is not included in this table because only a single participant had diabetes, precluding statistical comparison
ESS Epworth Sleepiness Scale, PLMI periodic limb movement index, PSQI Pittsburgh Sleep Quality Index, RLS restless legs syndrome
Table 4.
Association between PLMI and demographic and clinical characteristics (continuous characteristics)
| F value for relationship with PLMI | p value | |
|---|---|---|
| Age (n = 40) | 0.87 | 0.36 |
| BMI (n = 34) | 2.06 | 0.16 |
| Log serum ferritin (n = 39) | 0.26 | 0.61 |
| Hemoglobin (n = 38) | 0.84 | 0.37 |
| Hematocrit (n = 38) | 1.34 | 0.25 |
| Creatinine (n = 39) | 0.0 | 0.97 |
| LDL, mg/dL (n = 38) | 0.78 | 0.38 |
| HDL, mg/dL (n = 38) | 3.20 | 0.08 |
| Log pAHI (n = 36) | 7.37 | 0.01 |
Bold entries indicate statistical significance
Table presents the univariate linear regression of PLMI with each continuous demographic and clinical variable
BMI body mass index, HDL high density lipoprotein, LDL low density lipoprotein, pAHI apnea–hypopnea index measured by peripheral arterial tonometry, PLMS periodic limb movements of sleep
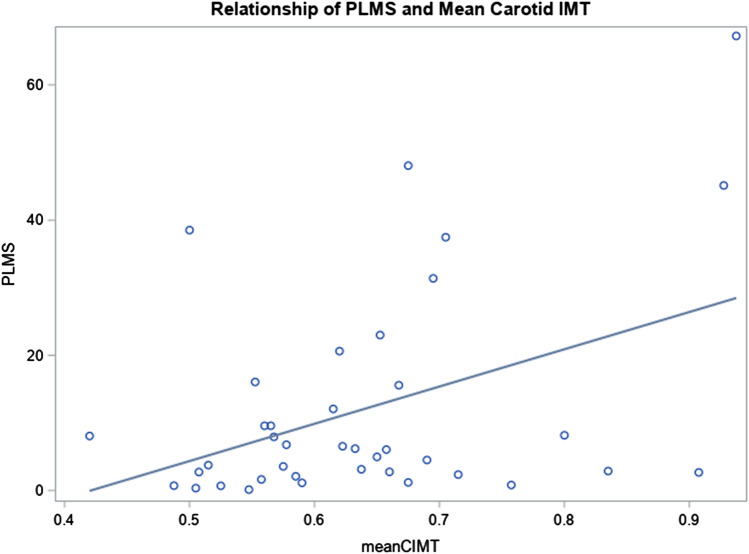
There were several significant associations between subclinical CVD measurements and PLMI in univariate analyses (Table 5), including reactive hyperemia index (F = 5.24, p = 0.03, i.e., increasing PLMS was associated with a lower hyperemic response), augmentation index (F = 4.20, p = 0.049, i.e., increasing PLMS associated with higher augmentation index), and carotid IMT (F = 8.0, p = 0.008, i.e., increasing PLMS were associated with greater IMT), all indicating increasing subclinical CVD with increasing number of PLMS. However, in the models adjusted for RLS status, age, hypertension, hyperlipidemia, and log pAHI, only the relationship between PLMI and carotid IMT remained significant (p value for model < 0.0006, β for PLMS = 0.003, p value for PLMS = 0.04; see Supplemental Table 1 for full model). Figure 1 presents the scatterplot and linear best fit line between carotid cIMT and PLMI.
Table 5.
Subclinical cardiovascular disease measures and association with PLMS
| F value for relationship with PLMI | Univariate p value* | Covariate adjusted p value** | |
|---|---|---|---|
| Reactive hyperemia index (n = 39) | 5.24 | 0.03 | 0.70 |
| Flow mediated dilation %, controlling for baseline diameter (n = 39) | 1.08 | 0.30 | |
| Augmentation index, controlling for height and heart rate (n = 39) | 4.20 | 0.049 | 0.89 |
| Pulse wave velocity, m/s (n = 38) | 1.23 | 0.27 | – |
| Mean carotid IMT, mm (n = 39) | 8.0 | 0.008 | 0.04 |
Bold entries indicate statistical significance
Table represents linear regression of PLMI predicting each subclinical cardiovascular disease measure
IMT intima-media thickness, PLMI periodic limb movement index
*Univariate p value reflects the relationship between PLMI and the variable of interest, in univariate linear regression for reactive hyperemia index, pulse wave velocity, and mean carotid IMT and multiple linear regression for augmentation index (including height, also controlled for heart rate) and flow mediated dilation (including baseline diameter)
**Covariate adjusted analyses were performed in the case of significant univariate associations, and were additionally controlled for age, hypertension, hyperlipidemia, log pAHI, and RLS status; p values reflect the p value for the relationship between PLMI and the variable of interest in the multivariate model
Fig. 1.
Carotid intima-media thickness versus periodic limb movements per hour. Scatter plot of the 39 participants with measured periodic limb movements index and carotid intima-media thickness (mm) and the line of best fit in the unadjusted model showing a positive correlation (r = 0.42, p = 0.008)
Discussion
In this work, we demonstrated a significant relationship between PLMI and increasing carotid IMT, controlling for relevant confounders. Epidemiological studies have previously implicated PLMS in the development of CVD [8, 24]. Observational studies have demonstrated a connection between PLMS and increased large artery stiffness as well as transient increases in blood pressure and heart rate (sympathetic microarousals) [25–27]. To our knowledge, this is the first study demonstrating a relationship between PLMS and cIMT. This relationship was independent of RLS status and remained significant after controlling for age, race, OSA severity measured simultaneously via pAHI, smoking, and hypertension. Increased cIMT has been implicated as strong predictor of future cardiovascular events[28] and there is biological plausibility for an association between PLMS and cIMT thickness, given the sympathetic activation that occurs with PLMS, not unlike the mechanism implicated in the association between OSA and cIMT thickness [29, 30].
We did not observe a significant association between cIMT and RLS status, although those participants with RLS had numerically higher average cIMT (0.66 ± 0.13 for those with RLS vs 0.63 ± 0.12 for those without, p = 0.07). Prior studies assessing a relationship between RLS and cIMT report mixed results. Park et al. found a negative association between RLS and cIMT when comparing 38 RLS patients and 64 controls [31]. In contrast, Zolfaghari et al. found an association between affirmative answers to the four RLS criteria (without specific exclusion of mimics) and cIMT in a study of 2,047 people meeting the four RLS criteria and 24,257 other participants [32]. In part, these findings may reflect different sample sizes, with smaller studies seeing a null association due to lesser power. They may also reflect differences in RLS symptom severity, which in our sample was relatively low compared to clinical populations. However, if PLMS are associated with cIMT independent of RLS, as observed in this study, differences in the prevalence and number of PLMS in RLS patients and controls across the respective RLS cohorts may also impact the ability to detect associations, especially in studies with smaller sample sizes. The different ages of the cohorts, approximately 10 years older in the Zolfaghari study than in our population, might also impact rate and severity of PLMS.
RLS was not significantly associated with any subclinical CVD in our multivariate analyses. It is possible that some previously demonstrated associations between RLS and CVD may occur via traditional CVD risk factors, such as age and sleep quality. The composition of our cohort, in which the CHDWB intentionally selected for healthy participants, might have contributed to this negative result. That being said, several previously shown RLS associations were observed in our cohort, including those with older age and worse sleep quality [33, 34].
Some inconsistencies with prior studies did appear. Notably, the relationship between RLS and iron deficiency is well-known. Our data showed no association between RLS and serum ferritin. This could be due, in part, to there being no “very severe” cases of RLS in the group and only two “severe” cases. Further, the strongest association with RLS and iron deficiency is with brain iron deficiency, which is not well-reflected in peripheral blood iron indices. Within the PLMS dataset, the relationship between PLMI and RLS had a marginal association, however, the sample size in the PLMS cohort may have reduced the power to detect a statistically significant association.
There were several limitations to this study. The sample size for the PLMS subgroup was 40 participants, which is small relative to the number of covariates in our multivariate model. Some clinical features were collected via questionnaires filled out by participants, including RLS status, albeit with a questionnaire validated for this purpose and designed to exclude common RLS mimics [12]. A lack of neck circumference measurement meant we were unable to utilize the full STOP-BANG criteria for OSA in the RLS group, which has been shown to have an increased sensitivity and specificity for the diagnosis of suspected OSA compared to the STOP score alone [35]. Most importantly, PLMS were measured via accelerometry, rather than polysomnography. Because accelerometry does not measure sleep time, PLMS are determined per hour of recording, rather than per hour of sleep, as in polysomnography. Although the PAM-RL cannot fully distinguish between PLMS and respiratory-related limb movements, we controlled for simultaneously-measured pAHI in our analyses, such that respiratory-related limb movements are unlikely to explain our observed association between PLMS and cIMT. Further, PLMS are known to have substantial night-to-night variability [36], such that our use of monitoring over multiple nights may have more accurately determined PLMS status than would a single night in-laboratory sleep study.
Despite these limitations, this study has clear strengths, including a large, diverse, randomly sampled population, objective measurement of PLMS and OSA in a subgroup, and use of validated questionnaires and CVD measures. We present a novel association between PLMS and cIMT. Importantly, no correlation was found in this group between RLS and cIMT, potentially implicating PLMS as a primary contributor to increased subclinical CVD, rather than sensory symptoms of RLS. Taken together, these results add to the growing literature implicating PLMS as a potentially modifiable risk factor for CVD [8]. Further, some work suggests improvement in surrogate outcomes, such as overnight blood pressure, with treatment of PLMS [37]. Future work is needed to determine the relationship between PLMS and CVD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work is based on information from the Emory Predictive Health Institute and Center for Health Discovery and Well Being (CHDWB) supported by Emory University and the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002378. CHDWB leadership approved the addition of RLS questions to the database and the collection of PLMS and OSA data in a subset of CHDWB participants, provided us access to RLS and CVD measures for the cohort, and approved the submission of this article for publication. Additional support for this work was provided by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number KL2 RR025009 (LMT) and the National Institute for Neurologic Disorders and Stroke under Award Number R01 NS111280 (LMT). We are grateful to Jane Clark for arranging access to the CHDWB database.
Declarations
Conflict of interest
The authors declare they have no financial conflicts of interest. Dr. Trotti is a member of the Board of Directors of the American Academy of Sleep Medicine (AASM); views expressed are those of the authors and do not necessarily reflect those of the AASM or the funding source (National Institutes of Health).
Ethical Committee Permission
Approval for this research was obtained from the Emory University Institutional Review Board. This work was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Human participants/consent to participate
All participants provided written, informed consent for use of information and samples.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Collaborators, G.B.D.C.o.D. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 3.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trotti LM. Restless legs syndrome and sleep-related movement disorders. CONTINUUM Lifelong Learn Neurol. 2017;23:1005–1016. doi: 10.1212/CON.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 5.Haba-Rubio J, Marti-Soler H, Marques-Vidal P, Tobback N, Andries D, Preisig M, Waeber G, Vollenweider P, Kutalik Z, Tafti M, Heinzer R. Prevalence and determinants of periodic limb movements in the general population. Ann Neurol. 2016;79:464–474. doi: 10.1002/ana.24593. [DOI] [PubMed] [Google Scholar]
- 6.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–65. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 7.Walters AS, Rye DB. Review of the relationship of restless legs syndrome and periodic limb movements in sleep to hypertension, heart disease, and stroke. Sleep. 2009;32:589–597. doi: 10.1093/sleep/32.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendzerska T, Kamra M, Murray BJ, Boulos MI. Incident cardiovascular events and death in individuals with restless legs syndrome or periodic limb movements in sleep: a systematic review. Sleep. 2017 doi: 10.1093/sleep/zsx013. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Somers VK, Punjabi NM, Winkelman JW. Restless legs syndrome and cardiovascular disease: a research roadmap. Sleep Med. 2017;31:10–17. doi: 10.1016/j.sleep.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brigham KL. Predictive health: the imminent revolution in health care. J Am Geriatr Soc. 2010;58(Suppl 2):S298–302. doi: 10.1111/j.1532-5415.2010.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johns MM, Brigham KL. Transforming health care through prospective medicine: the first step. Acad Med. 2008;83:706. doi: 10.1097/ACM.0b013e31817eb579. [DOI] [PubMed] [Google Scholar]
- 12.Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10:1097–1100. doi: 10.1016/j.sleep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, Trenkwalder C. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–132. doi: 10.1016/S1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 14.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 15.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie JD, Miller CK, Smiciklas-Wright H. Tanita foot-to-foot bioelectrical impedance analysis system validated in older adults. J Am Diet Assoc. 2005;105:1617–1619. doi: 10.1016/j.jada.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Sforza E, Johannes M, Claudio B. The PAM-RL ambulatory device for detection of periodic leg movements: a validation study [see comment] Sleep Med. 2005;6:407–413. doi: 10.1016/j.sleep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Tuisku K, Holi M, Wahlbeck K, Ahlgren A, Lauerma H. Quantitative rest activity in ambulatory monitoring as a physiological marker of restless legs syndrome: a controlled study. Mov Disord. 2003;18:442–448. doi: 10.1002/mds.10381. [DOI] [PubMed] [Google Scholar]
- 19.Chung F, Yegneswaran B, Liao P, Chung SA, Vairavanathan S, Islam S, Khajehdehi A, Shapiro CM. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–821. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 20.Choi JH, Kim EJ, Kim YS, Choi J, Kim TH, Kwon SY, Lee HM, Lee SH, Shin C, Lee SH. Validation study of portable device for the diagnosis of obstructive sleep apnea according to the new AASM scoring criteria: Watch-PAT 100. Acta Otolaryngol. 2010;130:838–843. doi: 10.3109/00016480903431139. [DOI] [PubMed] [Google Scholar]
- 21.Pang KP, Gourin CG, Terris DJ. A comparison of polysomnography and the WatchPAT in the diagnosis of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137:665–668. doi: 10.1016/j.otohns.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Gujral UP, Mehta A, Sher S, Uphoff I, Kumar S, Hayek SS, Ko YA, Martin GS, Gibbons GH, Quyyumi AA. Ethnic differences in subclinical vascular function in South Asians, Whites, and African Americans in the United States. Int J Cardiol Heart Vasc. 2020;30:100598. doi: 10.1016/j.ijcha.2020.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corrigan FE, 3rd, Kelli HM, Dhindsa DS, Heinl RE, Al Mheid I, Hammadah M, Hayek SS, Sher S, Eapen DJ, Martin GS, Quyyumi AA. Changes in truncal obesity and fat distribution predict arterial health. J Clin Lipidol. 2017;11(1354–1360):e3. doi: 10.1016/j.jacl.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuellar NG. The effects of periodic limb movements in sleep (PLMS) on cardiovascular disease. Heart Lung. 2013;42:353–360. doi: 10.1016/j.hrtlng.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Drakatos P, Higgins S, Pengo MF, Kent BD, Muza R, Karkoulias K, Leschziner G, Williams A. Derived arterial stiffness is increased in patients with obstructive sleep apnea and periodic limb movements during sleep. J Clin Sleep Med. 2016;12:195–202. doi: 10.5664/jcsm.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pennestri MH, Montplaisir J, Fradette L, Lavigne G, Colombo R, Lanfranchi PA. Blood pressure changes associated with periodic leg movements during sleep in healthy subjects. Sleep Med. 2013;14:555–561. doi: 10.1016/j.sleep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui F, Strus J, Ming X, Lee IA, Chokroverty S, Walters AS. Rise of blood pressure with periodic limb movements in sleep and wakefulness. Clin Neurophysiol. 2007;118:1923–1930. doi: 10.1016/j.clinph.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 29.Somuncu MU, Karakurt ST, Karakurt H, Serbest NG, Cetin MS, Bulut U. The additive effects of OSA and nondipping status on early markers of subclinical atherosclerosis in normotensive patients: a cross-sectional study. Hypertens Res. 2019;42:195–203. doi: 10.1038/s41440-018-0143-0. [DOI] [PubMed] [Google Scholar]
- 30.Guggisberg AG, Hess CW, Mathis J. The significance of the sympathetic nervous system in the pathophysiology of periodic leg movements in sleep. Sleep. 2007;30:755–766. doi: 10.1093/sleep/30.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, Han SW, Baik JS. Carotid intima-media thickness in patients with idiopathic restless legs syndrome. Eur Neurol. 2012;67:321–325. doi: 10.1159/000334869. [DOI] [PubMed] [Google Scholar]
- 32.Zolfaghari S, Dasgupta K, Postuma RB. Restless leg syndrome and objectively-measured atherosclerosis in the Canadian longitudinal study on aging. Mov Disord. 2020;35:2314–2318. doi: 10.1002/mds.28326. [DOI] [PubMed] [Google Scholar]
- 33.Phillips B, Young T, Finn L, Asher K, Hening W, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Int Med. 2000;160:2137–2141. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Malo C, Peralta SR, Garcia-Borreguero D. Restless legs syndrome and other common sleep-related movement disorders. Continuum (Minneap Minn) 2020;26:963–987. doi: 10.1212/CON.0000000000000886. [DOI] [PubMed] [Google Scholar]
- 35.Chung F, Abdullah HR, Liao P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. 2016;149:631–638. doi: 10.1378/chest.15-0903. [DOI] [PubMed] [Google Scholar]
- 36.Trotti LM, Bliwise DL, Greer SA, Sigurdsson AP, Gudmundsdottir GB, Wessel T, Organisak LM, Sigthorsson T, Kristjansson K, Sigmundsson T, Rye DB. Correlates of PLMs variability over multiple nights and impact upon RLS diagnosis. Sleep Med. 2009;10:668–671. doi: 10.1016/j.sleep.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Bauer A, Cassel W, Benes H, Kesper K, Rye D, Sica D, Winkelman JW, Bauer L, Grieger F, Joeres L, Moran K, Schollmayer E, Whitesides J, Carney HC, Walters AS, Oertel W, Trenkwalder C, S.P.s. Investigators Rotigotine's effect on PLM-associated blood pressure elevations in restless legs syndrome: an RCT. Neurology. 2016;86:1785–1793. doi: 10.1212/WNL.0000000000002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.