Abstract
Hemoglobin variants are often discovered when hemoglobin A1c (HbA1c) levels measured with a high-performance liquid chromatography (HPLC) system in fast mode are found to be low. The HA-8180V HPLC analyzer by Arkray offers two measurement modes: fast mode (FM) and variant mode (VM). Two Japanese patients with α-chain variant Hb Q-Iran detected incidentally after analyses with the HA-8180V in VM showed an abnormal peak, are presented. The first patient was a man in his 70 s, and the second patient was a man in his 50 s. Both were non-diabetic, but their results from HbA1c measurement in VM showed an abnormal peak. The VM–HbA1c, FM–HbA1c, and HbA1c measured by enzymatic assay and glycated albumin levels of the two patients were all within the reference ranges. They were diagnosed as having Hb Q-Iran (α2-75Asp → His) by globin gene analysis. It is difficult to detect α-chain hemoglobin variants based on abnormal FM–HbA1c levels, but measuring HbA1c in VM is useful for efficiently detecting hemoglobin variants.
Keywords: Hemoglobin variant, Hemoglobin A1c, Glycated albumin, Hb Q-Iran
Introduction
Hemoglobin A1c (HbA1c) reflects the mean plasma glucose level over the previous 2 months and has been widely used as an index of glycemic control in patients with diabetes mellitus and as an indicator for diagnosing diabetes mellitus [1]. However, HbA1c does not accurately reflect glycemic control in patients with hemoglobin variants [2]. Most patients with hemoglobin variants showing abnormal HbA1c levels due to various mechanisms may have falsely low levels because of differences in HbA1c mobility on high-performance liquid chromatography (HPLC) in fast mode (FM) for glycated variant hemoglobin (HbX1c) [3]. However, α-chain hemoglobin variants are easier to miss than β-chain variants, because the HbA1c level decreases are milder [4].
The HbA1c analyzer HA-8180V (Arkray, Kyoto, Japan) operates on the HPLC assay principle and provides two measurement modes, the regular fast mode (FM) and the variant mode (VM), allowing the detection of hemoglobin variants such as HbE, HbD, HbC, and HbS as abnormal peaks. Our hospital uses VM routinely to measure HbA1c to avoid missing hemoglobin variants.
The cases of two patients with α-chain variant Hb Q-Iran that were detected incidentally after chromatographic results of routine HbA1c tests with HA-8180V in VM showed an abnormal peak are reported. In both patients, the FM–HbA1c levels were within the reference range, and chromatographic abnormalities were absent, making it difficult to detect Hb Q-Iran in the measurement of FM–HbA1c.
Results
Patient 1
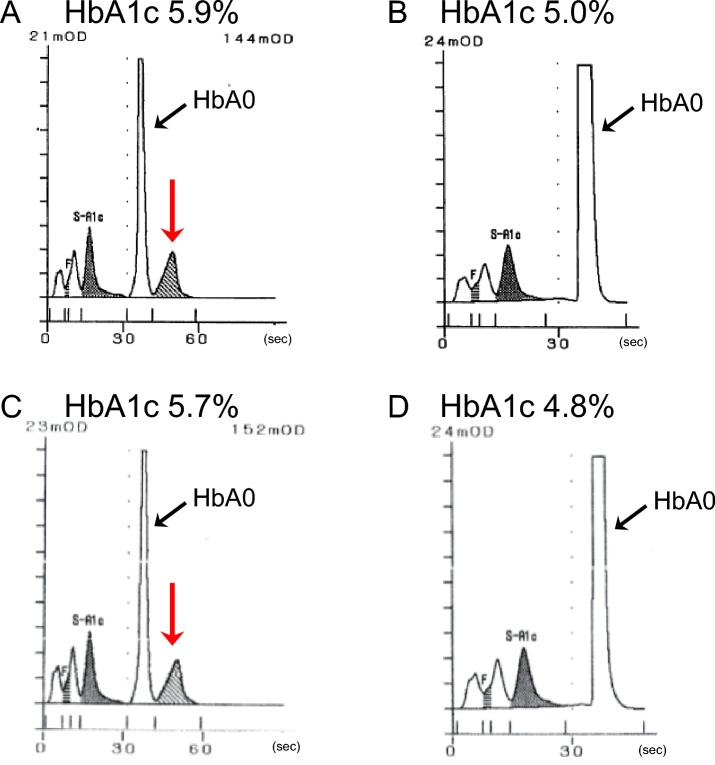
The patient was a man in his 70 s with a body mass index (BMI) of 20.9 kg/m2. The patient was undergoing follow-up after percutaneous angioplasty for arteriosclerosis obliterans in the cardiology department of our hospital. Serum biochemical tests showed no abnormalities, and randomly measured plasma glucose was 113 mg/dL (Table 1). The measurement of VM–HbA1c with the HA-8180V showed an abnormal peak that eluted later than that of HbA0 (Fig. 1A). The VM–HbA1c level was 5.9%. The presence of a hemoglobin variant was suspected, and additional blood tests were performed. The measurement of FM–HbA1c with HA-8180V gave a level of 5.0%, with no chromatographic abnormalities (Fig. 1B). The HbA1c level measured by enzymatic assay (CinQ HbA1c, Arkray) was 5.9%, and the glycated albumin (GA) level was 14.2%, both within the reference ranges. The ratios of GA/VM–HbA1c, GA/FAM–HbA1c, and GA/EA–HbA1c in this patient were 2.4, 2.8, and 2.4, respectively, each within the reference range (2.1–3.1).
Table 1.
Results of hematological and biochemical examinations in the present two patients with Hb Q-Iran
| Parameter | Patient 1 | Patient 2 | Reference range |
|---|---|---|---|
| WBC (× 104/μL) | 5.28 | 4.83 | 3.3–8.6 |
| RBC (× 106/μL) | 4.51 | 5.17 | 4.35–5.55 |
| Hb (g/dL) | 13.9 | 15.8 | 13.7–16.8 |
| Ht (%) | 41.1 | 46.3 | 40.7–50.1 |
| PLT (× 104/μL) | 26.6 | 20.7 | 15.8–34.8 |
| UN (mg/dL) | 22.4 | 14.9 | 8–20 |
| CRE (mg/dL) | 1.1 | 1.0 | 0.7–1.1 |
| eGFR (mL/min/1.73 m2) | 50.8 | 60.8 | ≥ 60 |
| T-Bil (mg/dL) | 0.7 | 1.0 | 0.4–1.5 |
| AST (U/L) | 25 | 22 | 13–30 |
| ALT (U/L) | 20 | 29 | 10–42 |
| γ-GTP (U/L) | 17 | 102 | 13–64 |
| LDH (U/L) | 231 | 164 | 124–222 |
| TP (g/dL) | 7.4 | 6.4 | 6.6–8.1 |
| ALB (g/dL) | 4.1 | 4.1 | 4.1–5.1 |
| LDL-C (mg/dL) | 92 | 123 | 65–163 |
| HDL-C (mg/dL) | 55.1 | 50.3 | 38–90 |
| TG (mg/dL) | 90 | 263 | 40–234 |
| RMPG (mg/dL) | 113 | 121 | – |
WBC white blood cell, RBC red blood cell, Hb hemoglobin, Ht hematocrit, PLT platelet, UN urea nitrogen, CRE creatinine, eGFR estimated glomerular filtration rate, T-Bil total bilirubin, AST aspartate aminotransferase, ALT alanine aminotransferase, γ-GTP γ-glutamyl transpeptidase, LDH lactate dehydrogenase, TP total protein, Alb albumin, LDL-C low-density lipoprotein cholesterol, HDL-C high-density lipoprotein cholesterol, TG triglyceride, RMPG randomly measured plasma glucose
Fig. 1.
HPLC chromatograms of two patients with Hb Q-Iran. HPLC chromatograms of patient 1 (A, B) and patient 2 (C, D) are shown. Chromatograms measured by VM (A, C) and FM (B, D) of HA-8180V are shown. In A and C, abnormal peaks indicated by red arrows, which eluted later than HbA0, are observed. VM variant mode, FM fast mode
Isoelectric focusing (IEF) showed abnormal bands (Fig. 2, lane B): a dark band indicated by a red arrow between HbA0 and HbA2, and a faint band indicated by a green arrow on the cathode side of HbA2. A globin gene analysis showed a GAC (aspartic acid) to CAC (histidine) substitution at position 75 of the α2-globin gene, leading to the diagnosis of Hb Q-Iran. No abnormalities were observed in the α1- and β-globin genes.
Fig. 2.
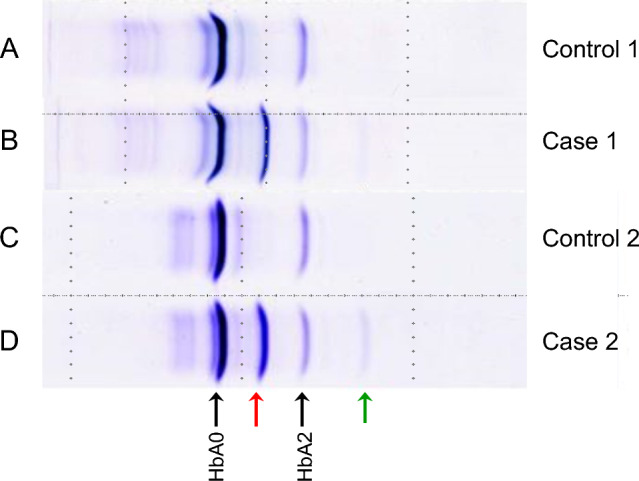
Results of isoelectric focusing (IEF) analysis. On IEF analysis, in patient 1 (lane B) and patient 2 (lane D), a dark band indicated by a red arrow between HbA0 and HbA2 and a faint band indicated by a green arrow on the cathode side of HbA2 are observed, which are not observed in the controls (lanes A and C)
Patient 2
The patient was a man in his 50 s with a BMI of 27.0 kg/m2. After an electrocardiogram in a physical examination showed an abnormality (negative T-wave), the patient presented to the cardiology department of our hospital for a work-up, but coronary computed tomography showed no significant coronary stenosis, resulting in the patient being placed on follow-up. Serum biochemical tests showed no abnormalities, and randomly measured plasma glucose was 121 mg/dL (Table 1). As with Patient 1, the chromatogram from a measurement of VM–HbA1c with HA-8180V showed an abnormal peak that eluted later than that of HbA0 (Fig. 1C). The HbA1c level was 5.7%. The measurement of FM–HbA1c with the HA-8180V gave a level of 4.8%, with no chromatographic abnormalities (Fig. 1D). The EA–HbA1c level was 5.7%, and the GA level was 13.9%, both within the reference ranges. The ratios of GA/VM–HbA1c, GA/FAM–HbA1c, and GA/EA–HbA1c in this patient were 2.4, 3.0, and 2.4, respectively, each within the reference range.
As with Patient 1, IEF showed a similar pattern of abnormal bands (Fig. 2, lane D). A gene analysis showed a GAC (aspartic acid) to CAC (histidine) substitution at position 75 of the α2-globin gene, leading to the diagnosis of Hb Q-Iran. No abnormalities were observed in the α1- and β-globin genes. Patient 1 and Patient 2 did not have a consanguineous relationship.
The above globin gene analyses of the two patients were performed after the patients were fully informed of the significance and method of the analyses and gave their written, informed consent and after the analyses were reviewed and approved by our institutional ethical committee (institutional ethical committee approval No. 937-2).
Relationship between VM–HbA1c and FM–HbA1c in the two patients with Hb Q-Iran
The relationship between VM–HbA1c and FM–HbA1c levels was examined in the two patients with Hb Q-Iran (Fig. 3). The VM–HbA1c and FM–HbA1c levels in the two patients were plotted on the x-axis and y-axis, respectively. Since the FM–HbA1c levels were lower than the VM–HbA1c levels, the HbA1c plots as measured by the two assay methods were located below the line represented by y = x. Moreover, given the mild severity of decreases in the FM–HbA1c level compared with the VM–HbA1c level, the FM–HbA1c level remained within the reference range (4.6–6.2%) in both patients.
Fig. 3.
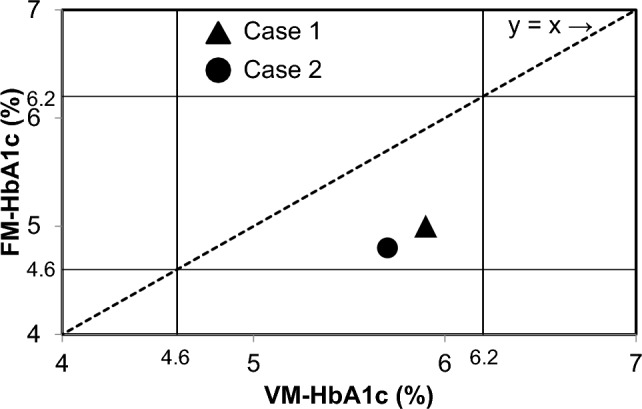
Relationship between VM–HbA1c and FM–HbA1c in two patients with Hb Q-Iran. VM–HbA1c (x-axis) and FM–HbA1c (y-axis) of two patients with Hb Q-Iran are plotted. The reference ranges of HbA1c (4.6–6.2%) are indicated by the solid line, and the straight line of y = x is indicated by the dotted line. VM variant mode, FM fast mode
Discussion
The α-chain variant Hb Q-Iran was incidentally detected in two patients after chromatographic results of routine HbA1c tests with the HPLC analyzer HA-8180V in VM showed an abnormal peak. These patients with Hb Q-Iran would not have been identified had the measurement of HbA1c been performed in FM, which would have shown no chromatographic abnormalities and HbA1c levels within the reference range. Although patients with Hb Q-Iran have been reported in countries such as Iran, these are the first reported patients in Japan, as far as we were able to ascertain. Moreover, they are the first patients with Hb Q-Iran whose HbA1c levels were measured.
Hb Q-Iran is a hemoglobin variant first reported in Iranians by Lorkin et al. [5] in 1970. Subsequently, patients have been reported primarily in the region from south Asia to west Asia [6]. The reported patients were asymptomatic, without hematological abnormalities such as anemia. Hb Q variants exhibit a molecular characteristic in which an aspartic acid to histidine substitution occurs at different positions in the amino acid sequence. A substitution at position 75 of the α-chain is seen in the patients with Hb Q-Iran reported here, whereas a substitution at position 74 of the α-chain has been identified as Hb Q-Thailand, and a substitution at position 64 of the α-chain, Hb Q-India [6, 7]. Hb Q-Thailand has been reported to be associated with α-thalassemia [8], but there are no reports of Hb Q-Iran or Hb Q-India being associated with hemoglobin depletion or impaired hemoglobin synthesis [9]. The present two patients with Hb Q-Iran also had no clinical symptoms or hematological abnormalities. Furthermore, the two present patients have no non-Japanese blood relationships and no consanguineous relationship with each other.
HbA1c may be measured by HPLC assay, immunoassay, enzymatic assay, and affinity chromatography. HPLC is the most frequently used assay at present due to its superiority over the other assays in terms of accuracy and reliability. With HPLC, separation is achieved by taking advantage of the charge differences in the amino acids that make up hemoglobin, and each component is measured with an absorbance detector. The HPLC system offers two modes of measurement: fast mode (FM), which is used commonly in Japan, and variant mode (VM), which allows the accurate measurement of HbA1c by compensating for the effect of hemoglobin variants. Hemoglobin variant (HbX0) elutes at a different time than normal hemoglobin (HbA0) due to a charge difference between them. HbX0, which carries a higher negative charge, elutes later than HbA0; however, in FM, HbX0 and HbA0 elute together. Due to the difference in elution times between glycosylated hemoglobin variant (HbX1c) and HbA1c, the area under the peak of HbA1c becomes smaller, giving an apparently low level. Meanwhile, in VM, in patients with a hemoglobin variant involving HbS, HbC, or HbD, which elutes later than HbA0, HbA1c is measured with the area under the HbX0 peak excluded, thereby allowing the accurate measurement of HbA1c. Moreover, HbX0 would appear as an abnormal peak on the chromatogram, which suggests a strong suspicion of a hemoglobin variant. However, when a hemoglobin variant that elutes earlier than HbA0 is involved, HbA1c cannot be measured accurately. In Europe and the United States, HPLC systems have been operated in VM due to a high prevalence of hemoglobin variants that elute later than HbA0. In Japan, however, HPLC systems have been operated exclusively in FM because of a low prevalence of hemoglobin variants. In recent years, the chance of encountering a patient with a hemoglobin variant in Japan has increased along with the growing population of foreign residents. Thus, even in Japan, HPLC systems with the VM function have become available for measurements.
There is one pair of globin genes encoding the β-chain compared with two pairs of genes encoding the α-chain. Thus, compared with β-chain hemoglobin variants, α-chain hemoglobin variants have been known to be associated with a milder degree of low HbA1c levels [4]. In the present two patients as well, the difference between FM–HbA1c and VM–HbA1c was as small as about 1.0%, and the HbA1c levels measured by both modes were within the reference range, making it difficult to detect the presence of a suspected α-chain hemoglobin variant based on the HbA1c level. We believe that this is the reason that Hb Q-Iran, which involves an α-chain hemoglobin variant, has not been detected in Japan previously. Since FM–HbA1c in non-diabetic patients with α-chain variant hemoglobin showed a decrease of about 1%, it is estimated that FM–HbA1c in diabetic patients with α-chain variant hemoglobin would show a decrease of about 1–2%. This discrepancy in HbA1c may cause patients with poor glycemic control to be mistakenly judged as good glycemic control, and the poor glycemic state may be left unaddressed. Therefore, it is important to measure HbA1c in VM in order not to overlook α-chain variant hemoglobin. Since the HPLC HA-8180V that our hospital uses does not have an automatic switching function between FM and VM, we measure all samples using VM. Therefore, the cost is relatively high and it takes about twice as much time. However, Arkray's new HPLC HA-8190V allows automatic switching between FM and VM when combined with a system. As a result, it is now possible to measure in VM to measure only patients measuring HbA1c for the first time and patients in which abnormal peaks were detected last time, and measure the rest in FM, saving costs and measurement time.
Given that GA is not subject to the effect of various factors that affect the measurement of HbA1c in those with a hemoglobin variant [2], we have established a reference range of GA/HPLC–HbA1c ratios and prepared a diagnostic flowchart [10] for individuals who show levels of FM–HbA1c that appear abnormally low compared with plasma glucose levels. The reference range of GA/HPLC–HbA1c ratios that we established is 2.1–3.3, but both the GA/VM–HbA1c and GA/FM–HbA1c ratios in these patients were within the reference range. For this reason, comparing FM–HbA1c to GA still does not facilitate the detection of hemoglobin variants.
The present two patients with Hb Q-Iran reported were detected incidentally after HPLC in VM showed an abnormal peak, which would have been difficult to detect based on abnormal levels of FM–HbA1c. Measuring HbA1c routinely with HPLC in VM can facilitate the efficient detection of hemoglobin variants including α-chain hemoglobin variants, thereby preventing erroneous reports of HbA1c levels. We expect that measurements of VM–HbA1c will lead to an increasing number of α-chain hemoglobin variants being reported.
Funding
This research received no specific grant.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Human rights and informed consent
All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (the ethics committee of Tenri Hospital [approval number of Ethics Committee: 937-2, date of approval: October 20, 2021]) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients prior to their participation in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Diabetes Association Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S83–S96. doi: 10.2337/dc22-S006. [DOI] [PubMed] [Google Scholar]
- 2.Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2021;433:96–104. doi: 10.1016/j.cca.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;47:153–163. doi: 10.1093/clinchem/47.2.153. [DOI] [PubMed] [Google Scholar]
- 4.Miyazaki A, Kohzuma T, Kasayama S, et al. Classification of variant forms of haemoglobin according to the ratio of glycated haemoglobin to glycated albumin. Ann Clin Biochem. 2012;49:441–444. doi: 10.1258/acb.2012.011192. [DOI] [PubMed] [Google Scholar]
- 5.Lorkin PA, Charlesworth D, Lehmann H, et al. Two haemoglobins Q, alpha-74 (EF3) and alpha-75 (EF4) aspartic acid to histidine. Br J Haematol. 1970;19:117–125. doi: 10.1111/j.1365-2141.1970.tb01607.x. [DOI] [PubMed] [Google Scholar]
- 6.Yadav AK. Comparative analysis of protein structure of common Hb Q variants. Indian J Pathol Microbiol. 2010;53:696–698. doi: 10.4103/0377-4929.72039. [DOI] [PubMed] [Google Scholar]
- 7.Wajcman H. Human hemoglobin variants, the electric versions. http://globin.cse.psu.edu. Accessed 1 July 2023.
- 8.Panyasai S, Pornprasert S. Hemoglobin Q-Thailand and its combinations with other forms of thalassemia or hemoglobinopathies in northern Thailand. Clin Lab. 2014;60:1099–1103. doi: 10.7754/Clin.Lab.2013.130513. [DOI] [PubMed] [Google Scholar]
- 9.Rahimi Z, Muniz A, Mozafari H. Abnormal hemoglobins among Kurdish population of Western Iran: hematological and molecular features. Mol Biol Rep. 2010;37:51–57. doi: 10.1007/s11033-009-9516-4. [DOI] [PubMed] [Google Scholar]
- 10.Koga M, Murai J, Soga S, et al. Study on five patients with variant hemoglobin discovered based on abnormally low HbA1c values during routine medical examinations. J Japan Diab Soc. 2013;56:841–848. [Google Scholar]