Abstract
Leontodon hispidulus Boiss is a wild annual plant growing in Egypt. The present study aims for the first time, to evaluate the phytochemical profile of the main secondary metabolites of the optimized ethanolic extract of the plant using Quadrupole Time-of-Flight Liquid chromatography-mass spectrometry and Gas chromatography-mass spectrometry. It also aims to assess the anticancer activity of its different fractions against the prostate carcinoma cell line. Moreover, an in-silico docking study was performed using the Hexokinase-two enzyme. LC-qToF-MS analysis revealed the tentative identification of 36 phenolic compounds including the glycosides of (luteolin, quercetin, kaempferol, apigenin, isorhamnetin, and daidzein), coumarines (esculin, esculetin, and daphnetin), and phenolic acids (chlorogenic, caffeic, quinic, P-coumaric, and rosmarinic). GC–MS/MS analysis revealed the presence of 18 compounds where palmitic acid, myristic acid, alpha-amyrin, and beta-amyrin were the major ones. The cytotoxic activity results revealed that methylene chloride and ethyl acetate fractions showed the highest cytotoxic activity against the PC3 cell line, with IC50 values of 19, and 19.6 μg/ml, respectively. Interestingly, the docking study demonstrated that apigenin-7-O-glucoside, luteolin-7-O-glucoside, kaempferol-3-O-glucuronide, quercetin-4′-O-glucoside, esculin, rosmarinic acid, chlorogenic acid, and α-amyrin exhibited high affinity to the selected target, HEK-2 enzyme.
Keywords: Leontodon hispidulus Boiss., Prostate carcinoma, LC-qToF-MS, GC–MS/MS, Hexokinase-2 enzyme
Subject terms: Pharmacology, Screening, Cancer therapy
Introduction
Leontodon is a rare wild plant of the family Asteraceae. It includes about 50 species that are geographically distributed through the Mediterranean, European, and Asian countries1. Leontodon hispidulus Boiss. is a member of the genus Leontodon that grows as a wild plant in Egypt. It is an annual plant, stemless with a rigid taproot, simple glandular hairs, rosette, oblong-oblanceolate leaves, and yellow–orange flowers1. Family Asteraceae is well-known for its flowering plants with a wide range of traditional uses. For example, Carduus species have often been used as anti-hemorrhoidal and cardiotonic remedies in traditional medicine, and Onopordum tauricum as a remedy for liver disease. The flowers and roots from Onopordum acanthium were used as antipyretic and diuretic agents, and Centaurea solstitalis is used in folk medicine to treat stomach problems, abdominal pain, herpes infections, and the common cold. Tanacetum parthenium, also known as feverfew in folk medicine and medieval aspirin, has been used as a remedy for headaches, migraine, nausea, vomiting, stomachaches, rheumatism, and other inflammations. Another plant with practical uses is Bidens pilosa, also known as Spanish needles, which grows mostly in subtropical and tropical regions. It has been used as a remedy for liver problems and to lower blood pressure. In addition, Carthamus tinctorius (safflower) is a treatment for rheumatism and osteoporosis in Korean herbal medicine. Cichorium intybus (chicory) is used in traditional medicine as a remedy for inflammation and liver disorders. Tonics from C. intybus have also been used to treat enlarged spleen and fever in Indian Ayurveda medicine, and a decoction from the leaves was used as a cure for rheumatism and gout. Tea prepared from Achillea aleppica and Achillea biebersteinii was recommended for abdominal pain. The aerial parts from Chrysophthalmum montanum were boiled and applied to wounds and other injuries. Matricaria aurea was recommended in the diet twice a day for bronchitis, sore throat, and cough2. Sesquiterpenoids especially guaiane-type compounds are the unique chemotaxonomic markers of Leontodon in the flower heads of L. autumnalis and the roots of L. hispidus3,4. Hypocretenolide glycoside was first isolated from L. hispidus, which had a potent cytotoxic activity against CD34( +) bone-marrow stem cell malignancy5 and anti-inflammatory activity6. In addition, other secondary metabolites had been reported in Leontodon taxa like phenolic acids (chicoric, chlorogenic, caffeoyl tartaric, and 3,5-dicaffeoyl quinic acids) as well as luteolin and its glycosides7,8. Moreover, a novel oleanene triterpene was isolated from the chloroform extract of the aerial parts of L. filii which showed a fungicidal activity9. In our previous work10 we reported that this plant species contains certain classes of secondary metabolites like sterols, flavonoids, coumarins, tannins, and terpenes through phytochemical screening. The High-performance liquid chromatography analysis showed the presence of chlorogenic acid, p-coumaric acid, rutin, quercetin, and kaempferol in L. hispidulus aerial parts 95% ethanolic extract. We also managed to optimize the plant extraction process of both the yield and total phenolic content by applying the Box-Behnken Design. The optimized extract produced the maximized concentration of phenolics (104.18 ± 0.37 μg/mg) with a minimum percentage of errors (− 0.27%) and flavonoid content (29.73 ± 0.0 μg/mg) using 201 ml of 74.5% ethanol/water at 72 h of extraction. The optimized extract showed good biological activities. The antioxidant activity using 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) was found to be 41.89 mg Trolox-equivalent/gm, with 80% free radicals inhibition. Moreover, 100 mg/kg of the extract inhibited the edema in rats by 83.5% after 4 h of carrageenan injection as compared to 81.7% inhibition by indomethacin. The extract also showed good anticancer activity on both prostate carcinoma (PC3) and cervical carcinoma (HELA) cell lines (IC50 = 16.5 and 23 µg/ml, respectively)10. More studies were required to unveil the types of secondary metabolites of L. hispidulus. Prostate carcinoma is the majorly recorded malignancy in men, leading to many complications and finally mortality. Its early detection could be achieved by screening for serum prostate-specific antigen. Despite the advanced research to allow early diagnosis, the onset of the disease is often asymptomatic, it develops slowly and spreads from the prostate to other parts of the body. The standard primary treatment includes radical prostatectomy, radiation, and androgen deprivation therapy11. Unfortunately, patients with an initial response to treatment will relapse within three years with androgen-independent prostate cancer (PC3) which is rapidly fatal12. Generally, cancer treatment includes chemotherapy, radiotherapy, surgery, the transformation of stem cells, photodynamics, and immunotherapy. Severe side effects result from such treatments as limited bioavailability, toxicity, nonspecificity, fast clearance, and metastasis13. There are also many undesirable side effects that cancer patients find difficult to tolerate such as nausea, vomiting, anemia, fatigue, hair loss, appetite changes, constipation, bleeding, and infection. Considering this, there is a global need to look for more selective active compounds of natural origin. Hexokinase-2 enzyme (HEK-2) is an important target that is over-expressed in most human cancers and has been proven to be a promising target for cancer therapy. Although HEK-2 has been a potential target for cancer treatment for many years, several challenges remain in the discovery and design of efficient and selective HEK-2 inhibitors. These challenges arise out of the structural properties of HEK-2, including the high polarity of the active site of the enzyme and the difficulty in specifically inhibiting the different isoenzymes14. Computational or in-silico docking studies are important to assess the drug-like properties of lead compounds to predict their biological activity in-vivo. These studies help in the fast prediction of relevant properties and important parameters that should be met by a compound for it to be considered as a potential drug candidate. This objective is achieved by the application of automated software which can be used for making various predictions. Drug uptake, its absorption, evacuation, and associated hazardous effects are important factors for consideration in drug design and should be known in the early stages of drug development. Several important physicochemical properties like molecular weight, polar surface area, molecular flexibility, etc. have to be taken into consideration in drug designing. Toxicological assessment is another important aspect of drug discovery which predicts the safety and adverse effects of a drug. Therefore, bioactivity scores of probable drug leads against various human receptors can be used to predict and evaluate the probability of them acting as potential drug candidates in-vivo15.
The study aims to identify the major active compounds in L. hispidulus optimized extract, including phenolic compounds and terpenes, evaluate the cytotoxic activity of its successive fractions against prostate carcinoma cell lines (PC3), and test the inhibitory effect of the main compounds against HEK-2 enzyme in-silico.
Materials
Plant material collection and identification
The aerial parts of Leontodon hispidulis Boiss. were collected in March 2022 at the flowering stage from the north coast of Egypt, it was kindly identified by Dr. Soad Abdallah Hassan, Professor of flowering plants, Botany Department, Faculty of Science, Ain Shams University, Cairo, Egypt. Voucher specimens of the plant was deposited in the herbarium of “Pharmacognosy department, Faculty of Pharmacy, Cairo University, Cairo, Egypt” with the code number (21-3-17). Plant collection and experimental protocol were achieved after permission from the “Desert Research Center, Cairo, Egypt”, and the “Ethical Committee of the Faculty of Pharmacy, Cairo University, Cairo, Egypt” (serial number of the protocol: MP 1841). Experimental research and field studies on plants (either cultivated or wild), including the collection of plant material, must comply with relevant institutional, national, and international guidelines and legislation.
Chemicals
Solvents for extraction and fractionation
Ethanol, petroleum ether, methylene chloride, ethyl acetate, butanol (all HPLC grade) were purchased from Sigma-Aldrich, Germany.
LC-qTOF-MS of the total extract
Mobile phase (negative mode): 5 mM ammonium formate buffer (pH = 8) containing 1% methanol. All solvents were HPLC grade and were kindly provided and prepared by Proteomics Laboratory of Children Cancer Hospital 57357, Cairo, Egypt.
GC–MS/MS of the petroleum ether fraction
Solvent: petroleum ether (HPLC grade) was purchased from Sigma Aldrich Chemicals, Germany.
Mobile phase: Helium gas at a flow rate of 1.0 ml/min at a splitless mode was provided by Central Laboratories Network, National Research Center, Giza, Egypt.
Silylation: 1% TMCS (trimethylchlorosilane) was provided by the National Research Center, Giza, Egypt.
Cytotoxic activity
Cell lines: Human prostate carcinoma (PC3) cell lines were purchased from the National Cancer Institute, Cairo, Egypt.
Doxorubicin: as a standard anticancer drug, it was purchased from Sigma Aldrich Chemicals, Germany.
Sulfo-Rhodamine B stain (SRB): for staining of the surviving cells, it was purchased from Sigma Aldrich Chemicals, Germany.
Acetic acid and Tris–EDTA buffer: were purchased from Sigma Aldrich Chemicals, Germany.
Methods
Preparation of the plant material
The powder of the aerial parts was extracted by maceration at room temperature using 201 ml of 74.5% ethanol/water at 72 h for 20 g powder to obtain the optimized extract with the maximum phenolic content10. The solvent was evaporated using a Rotatory Evaporator "BuchiRR-300, USA" at 45°. The resulting residue was subjected to fractionation with solvents of increasing polarity (petroleum ether, methylene chloride, ethyl acetate, butanol, and water).
LC-qTOF-MS of the total extract
The separation process was performed using a Shimadzu UPLC system (Kyoto, Japan) equipped with a binary solvent delivery system and an autosampler, coupled with ACQUITY UPLC BEH-C18 (150 × 2.1 mm, 1.7 um: Waters, USA). The column temperature was maintained at 25 °C and mass spectrometry was performed on a Triple TOF™ 5600 + system with a Duo-Spray™ source operating in the Negative Electron Spray Ionization mode (ESI) (AB SCIEX, CA, USA). The flow rate was kept at 0.3 ml/min. The mobile phase A (5 mM ammonium formate buffer (pH = 8) containing 1% methanol) and mobile phase B (100% acetonitrile) were applied according to the gradient elution program demonstrated in Table 1.
Table 1.
Gradient elution program for LC-qTOF-MS analysis.
| Time (min) | Flow (ml/min) | Mobile phase A | Mobile phase B |
|---|---|---|---|
| 0 | 0.3 | 90 | 10 |
| 1 | 0.3 | 90 | 10 |
| 21 | 0.3 | 10 | 90 |
| 25 | 0.3 | 10 | 90 |
| 25.01 | 0.3 | 90 | 10 |
| 28 | 0.3 | 90 | 10 |
Sample preparation for LC–MS/MS analysis
The optimized 74.5% ethanol/water extract of L. hispidulus was used in the LC–MS/MS analysis10. One ml mobile phase working solution (MP-WS) [DI-Water: Methanol: Acetonitrile—50: 25: 25] was added to 50 mg sample, followed by vortex for 2 min, then ultra-sonication for 10 min and centrifuge for 10 min at 10,000 rpm. 20 µl stock (50/1000 µl) was diluted with 1000 µl reconstitution solvent. Finally, the injected concentration was 1 µg/µl. The injection volume was 10 µl and 10 µl MP-WS as a blank.
Data processing
Respect Negative (1573 records) was the database used16. MasterView Program was used for feature (peaks) extraction from the Total ion chromatogram (TIC) based on the following criteria; features should have Signal-to-Noise > 5 (Non-targeted analysis), features intensities of the sample-to-blank > 5.
The following optimized parameters were applied; ion spray voltage (5.5 kV), ion source heater (550 °C), curtain gas [35 psi—gas 1 (nebulizer gas, 55 psi), gas 2 (heater gas, 55 psi)], declustering potential (60 eV), collision energy (30 eV), collision energy spread (15 eV). The scan range was from 50 to 800 m/z with a 250 ms accumulation time. An automated calibration delivery system (CDS) was applied for the regulation of the MS and the MS/MS automatically.
GC–MS/MS of the petroleum ether fraction
The GC–MS/MS system (Agilent Technologies) was equipped with a gas chromatograph (7890B) and mass spectrometer detector (5977A) at Central Laboratories Network, National Research Centre, Giza, Egypt. The GC was equipped with HP-5MS column (30 m × 0.25 mm internal diameter and 0.25 μm film thickness). The petroleum ether fraction was subjected to a silylation process using 1% trimethylchlorosilane (TMCS). Helium was the carrier gas (1.0 ml/min flow rate) in a splitless mode and the injection volume was (0.1 µl). The following temperature program was applied [80 °C for 2 min; rising at 5 °C/min to 300 °C and held for 10 min]. The injector and detector were held at 280 °C and 300 °C, respectively. Mass spectra were obtained by electron ionization (EI) at 70 eV; using a spectral range of m/z 25–550 and solvent delay of 3.7 min. Identification of different constituents was determined by comparing the spectrum fragmentation pattern with those stored in Wiley and NIST Mass Spectral Library data.
In-vitro cytotoxic activity
Measurement of the potential cytotoxic activity was performed on prostate carcinoma cell lines (PC3), using the successive fractions of the optimized ethanolic extract of L. hispidulus [petroleum ether, methylene chloride, ethyl acetate, butanol, and aqueous fractions], while doxorubicin was used as a positive control. The procedures were according to the Sulforhodamine-B assay (SRB)10. Each value is the mean of three replicates. Obtained values were presented as mean ± SD. Significant differences between the values were calculated using SPSS software (V.22) using One-way ANOVA and Tukey’s test. The difference is considered significant at (P ˂ 0.05).
In-silico docking study using hexokinase-2 enzyme
The molecular modeling studies were carried out using Molecular Operating Environment (MOE, 2019.0102) software. The X-ray crystallographic structure of Hexokinase-2 complexed with the reference compound [2-amido-6-benzenesulfonamide glucosamine derivative (PDB ID: 5HFU)] was downloaded from the protein data bank (https://www.rcsb.org/structure/5HFU). All the tested compounds were compared to the reference compound.
Results and discussion
LC-qTOF-MS of L. hispidulus total extract
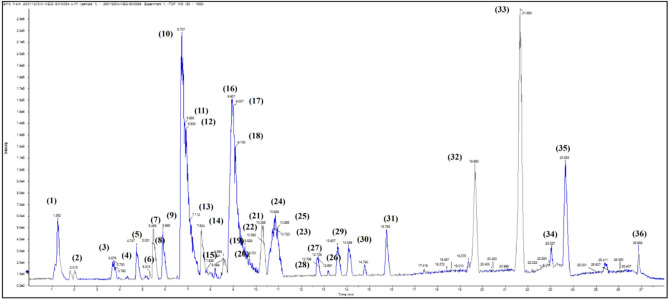
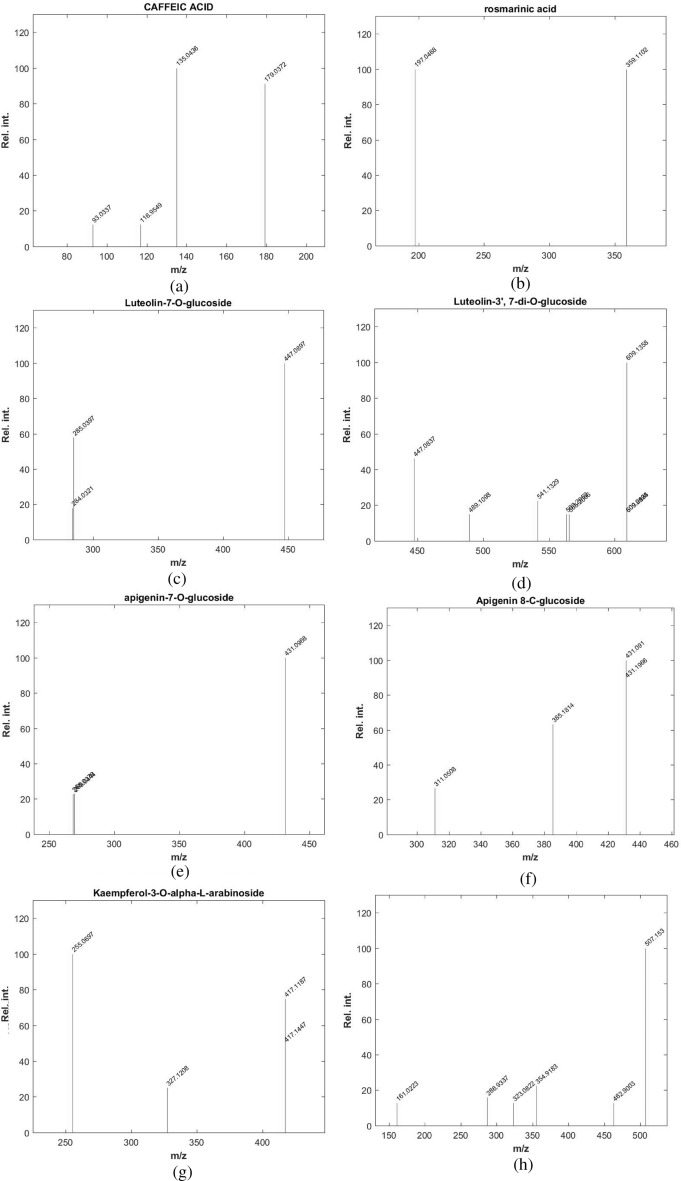
Table 2 shows in detail the different identified phenolic compounds in L. hispidulus optimized ethanolic extract in the negative mode, while the base peak chromatogram is demonstrated in Fig. 1. Figure 2a–n demonstrates MS/MS spectrum figures of the major identified phenolic compounds.
Table 2.
Tentative identification of the major secondary metabolites of Leontodon hispidulus Boiss. optimized extract in the negative mode.
| Peak no. | Rt (min) | M-H m/z | Molecular formula | Error of m/z | Ms/Ms | Compound name |
|---|---|---|---|---|---|---|
| 1 | 1.262 | 353 | C16H18O9 | 0 | 191, 179, 173, 93, 85 | Chlorogenic acid |
| 2 | 2.013 | 191 | C7H12O6 | 1 | 173, 135 | Quinic acid |
| 3 | 3.676 | 153 | C7H6O4 | 0.5 | 109, 94 | 3,4-dihydroxybenzoic acid |
| 4 | 3.730 | 163 | C9H8O3 | 1.2 | 119, 91 | P-coumaric acid |
| 5 | 4.747 | 339 | C15H16O9 | -0.5 | 337, 293, 271, 194, 178, 177, 173, 149, 131 | Esculin |
| 6 | 5.313 | 137 | C7H6O3 | 0.1 | 93 | P-hydroxybenzoic acid |
| 7 | 5.469 | 177 | C9H6O4 | -0.6 | 162, 133, 129, 105 | 6,7-dihydroxy coumarin (Esculetin) |
| 8 | 5.531 | 179 | C9H8O4 | -0.8 | 135, 79 | Caffeic acid |
| 9 | 5.885 | 153 | C7H6O4 | 0.5 | 136, 109, 67 | 2,5-dihydroxybenzoic acid |
| 10 | 6.737 | 609 | C27H30O16 | -3 | 607, 565, 563, 541, 489, 447, 285 | Luteolin-3ˋ,7-di-O-glucoside |
| 11 | 6.850 | 177 | C9H6O4 | 1.7 | 149, 133, 121, 105, 95, 89, 81 | 7,8-dihydroxy coumarin (Daphnetin) |
| 12 | 6.930 | 359 | C18H16O8 | 0.6 | 197 | Rosmarinic acid |
| 13 | 7.112 | 461 | C21H18O12 | -1 | 315, 285, 161, 132, 85 | Kaempferol-3-Glucuronide |
| 14 | 7.604 | 431 | C21H20O10 | -1.5 | 429, 363, 354, 295, 293, 285, 274, 179, 162, 158, 112 | Kaempferol-3-O-L-rhamnoside |
| 15 | 7.835 | 609 | C28H34O15 | 1.4 | 565, 563, 541, 448, 355, 301, 285 | Hesperetin-7-O-neohesperidoside |
| 16 | 8.931 | 463 | C21H20O12 | -1.1 | 458, 301, 300, 274, 256 | Quercetin-4ˋ-O-glucoside |
| 17 | 9.007 | 431 | C21H20O10 | -1 | 341, 311, 269 | Apigenin 8-C-glucoside (Vitexin) |
| 18 | 9.100 | 167 | C8H8O4 | 2 | 162, 152, 130, 124, 123, 108 | 5-Methoxysalicylic acid |
| 19 | 9.529 | 623 | C28H32O16 | 1.2 | 612, 577, 510, 477, 461, 357, 315, 314 | Isorhamnetin-3-O- rutinoside |
| 20 | 9.701 | 477 | C22H22O12 | -0.4 | 315, 300, 270, 180 | Isorhamnetin-3-O- glucoside |
| 21 | 10.265 | 577 | C27H30O14 | -1.2 | 576, 515, 269, 236 | Rhoifolin |
| 22 | 10.362 | 431 | C21H20O10 | 1.1 | 269 | Apigenin-7-O-glucoside |
| 23 | 10.723 | 433 | C20H18O11 | -0.3 | 301, 300, 271, 255 | Quercetin-3-D-xyloside |
| 24 | 10.839 | 507 | C23H24O13 | 0.6 | 463, 394, 345, 323, 286, 258, 179, 161, 153 | Syringetin-3-O-glucoside |
| 25 | 10.955 | 415 | C21H20O9 | -2.1 | 369, 304, 295, 253, 238, 192, 135 | Daidzein-8-C-glucoside |
| 26 | 12.691 | 417 | C20H18O10 | -1.6 | 327, 255 | Kaempferol-3-O-alpha-L-arabinoside |
| 27 | 12.738 | 593 | C30H26O13 | 2.1 | 548, 528, 188 | Kaempferol-3-O-(p-coumaroyl)-glucoside |
| 28 | 12.798 | 445 | C21H18O11 | -0.9 | 377, 308, 269, 193, 161, 133 | Baicalein-7-O-glucuronide |
| 29 | 13.607 | 299 | C16H12O6 | 0.1 | 284, 256, 227, 211, 135, 134, 107 | 3ˋ,5,7-trihydroxy-4ˋ-methoxyflavone (Diosmetin) |
| 30 | 14.098 | 447 | C21H20O11 | 0.6 | 285 | Luteolin-7-O-glucoside |
| 31 | 15.756 | 269 | C15H10O5 | 1 | 225, 201, 181, 151, 117, 107 | Apigenin |
| 32 | 19.660 | 267 | C16H12O4 | 0.5 | 266, 252, 239, 221, 183,131 | Formononetin |
| 33 | 21.656 | 301 | C15H10O7 | -1.3 | 301, 151, 107 | Quercetin |
| 34 | 23.027 | 285 | C15H10O6 | 0.8 | 241, 201, 175, 151, 133, 121 | Luteolin |
| 35 | 23.653 | 283 | C16H12O5 | 1 | 268, 240, 239, 212, 211, 151, 117, 107 | Acacetin |
| 36 | 26.869 | 271 | C15H12O5 | -0.8 | 151, 119 | Naringenin |
Peak no. Peak number, Rt (min) Retention time in minutes, M–H (m/z) Mass divided by Charge number in the Negative mode, MS/MS Mass fragmentation.
Figure 1.
Base Peak Chromatogram of Leontodon hispidulus Boiss. Optimized extract in the Negative mode.
Figure 2.
Fragmentation figures of the main identified compounds in Leontodon hispidulus Boiss. ethanolic extract (a) Ms/MS spectrum of caffeic acid, (b) Ms/Ms spectrum of rosmarinic acid, (c) Ms/Ms spectrum of luteolin-7-O-glucoside, (d) Ms/Ms spectrum of luteolin-3′,7-di-O-glucoside, (e) Ms/Ms spectrum of apigenin-7-O-glucoside, (f) MS/MS spectrum of apigenin 8-C-glucoside, (g) MS/MS spectrum of kaempferol-3-O-alpha-L-arabinoside, (h) MS/MS spectrum of syringetin-3-O-glucoside, (i) MS/MS spectrum of acacetin, (j) MS/MS spectrum of rhoifolin, (k) MS/MS spectrum of hesperetin-7-O-neohesperidoside, (l) MS/MS spectrum of quercetin-4′-glucoside, (m) MS/MS spectrum of 6,7-dihydroxy coumarin, (n) MS/MS spectrum of daphnetin.
Identification of phenolic acids
Many phenolic acids have been identified in the negative mode of LC-qTOF-MS of L. hispidulus ethanolic extract. Chlorogenic acid was identified by the deprotonated molecule [M-H]− at m/z = 353 and the fragment ion at m/z = 191 corresponding to the deprotonated quinic acid17. Quinic acid molecular ion (m/z = 191) was also detected17. The caffeic acid molecular ion was identified at m/z = 179 and its fragment ion m/z = 135 corresponding to (Caffeic acid—CO2)18. P-coumaric acid molecular ion appeared at m/z = 163 and its fragment ion (m/z = 119) corresponds to (quinic acid—CO2)19. Rosmarinic acid was identified by the molecular ion (m/z = 359), with its fragment ion (m/z = 197) which represents the loss of caffeic acid20. In the previous studies, few phenolic acids had been reported in Leontodon taxa (chicoric, chlorogenic, caffeoyl tartaric, and 3,5- dicaffeoyl quinic acids)7.
Identification of flavonoids and their glycosides
Different classes of flavonoids and their glycosides were identified in the LC-qTOF-MS spectrum of L. hispidulus optimized ethanolic extract including flavones, flavonols, isoflavones, and flavanones.
Flavones and their glycosides
Two flavones were detected, luteolin was tentatively assigned based on its parent ion at (m/z = 285) and by fragment ions (m/z = 151) for ring A and (m/z = 133) for ring B after ring C cleavage21. Similarly, apigenin at (m/z = 269) with the characteristic fragment ion (m/z = 117) for ring B as well as (m/z = 151) for ring A21. Two luteolin glycosides were identified. Luteolin-7-O-glycoside with its characteristic quasimolecular ion (m/z = 447) and fragment ion of the flavone aglycone (m/z = 285) with a diagnostic loss of hexose moiety (162 amu)17,22. The second one was luteolin -3ˋ,7- di-O-glycoside which was tentatively identified by its molecular ion (m/z = 609) as well as two characteristic fragment ions [(m/z = 447) after the loss of one hexose molecule and the distinct peak of the aglycone at (m/z = 285)]23. Two apigenin glycosides were tentatively identified. Apigenin-7-O-glycoside with molecular ion (m/z = 431) and fragment ion (m/z = 269) after loss of hexose molecule22. The other one was apigenin-8-C-glycoside (vitexin) with the same molecular ion and fragment ion of the aglycone, but with two characteristic fragment ions of the C-glycoside, (m/z = 341) indicating [M-H-90]− and (m/z = 311) indicating [M-H-120]−17. Rhoifolin was tentatively identified with its molecular ion (m/z = 577) and the fragment ion (m/z = 269) corresponding to [M-H-Rham-Glc]−24. A methoxyflavone compound, 3′,5,7-trihydroxy-4′-methoxyflavone (diosmetin) was identified by the molecular ion (m/z = 299) and the characteristic fragments [M-H-CH3]− with (m/z = 284), [M-H-CH3-CO]− with (m/z = 256) and A-ring system with (m/z = 135)25. Acacetin (4′-O-methylated flavone) was identified at (m/z = 283) and the fragment ions (107, 117, and 151) resulted from Retro-Diels–Alder cleavage. Also, the fragment ions (239 and 211) were detected after the loss of CO2 and CO, respectively. Moreover, the fragment ions (268, 240, and 212) were detected due to the loss of CH3, CO, and CO groups, respectively26. Baicalein-7-O-glucuronide (trihydroxyflavone) was tentatively identified with the molecular ion (m/z = 445) and baicalein fragment ion (m/z = 269) corresponding to [M-H-176]− due to loss of glucuronide moiety27. In the previous studies, luteolin and its glycosides were identified in Leontodon taxa7,8.
Flavonols and their glycosides
Quercetin (flavon-3-ol) was tentatively identified by its molecular ion (m/z = 301)28. Two quercetin glycosides were tentatively identified. The first one was quercetin-4ˋ-O-glycoside which was identified by its molecular ion (m/z = 463) and the fragment ion of the aglycone (m/z = 301) after loss of hexose molecule29. The other one was quercetin-3-D-xyloside and it was characterized by its molecular ion (m/z = 433) and the same peak of the aglycone (m/z = 301)30. Four kaempferol glycosides were tentatively identified. Kaempferol-3-Glucuronide was identified by its molecular ion (m/z = 461) and the characteristic loss of glucuronic acid moiety (176) leaving the aglycone peak at (m/z = 285)28. Similarly, kaempferol-3-O-alpha-L-arabinoside, kaempferol-3-O-(p-coumaroyl)-glucoside, and kaempferol-3-O-L-rhamnoside were tentatively identified using the online databases (RESPECT and MONA)16.
Two isorhamnetin (O-methoxy-flavonol) glycosides were also detected. Isorhamnetin-3-O-glucoside was identified by its molecular ion (m/z = 477), as well as the fragment ions of the aglycone (m/z = 315), [M-H-(Glc-H2O)-CH3]− with (m/z = 300) and [M-H-(Glc-H2O)-CH3-Co]− with (m/z = 270)31. The second one, isorhamnetin-3-O-rutinoside, was identified by its molecular ion (m/z = 623), the fragment ions [M-H-146]− with (m/z = 477) and [M-H-162]− with (m/z = 461) characteristic for the disaccharide moiety (rutinose: m/z = 308) and by the aglycone part (m/z = 315)32,33. Syringetin-3-O-glucoside (O-methylated-flavonol) was tentatively detected with the molecular ion (m/z = 507) and the aglycone (m/z = 345)34.
Isoflavones
Daidzein-8-C-glucoside was found with the molecular ion (m/z = 415), the fragment ion (m/z = 295) characteristic of C-glycoside [M-H-120]− and the aglycone (m/z = 253) corresponding to [M-H-160]−35. Formononetin (4′-O-methyl isoflavone) was detected based on (m/z = 267) and the fragment ion (m/z = 252) due to the loss of the CH3 group36.
Flavanones
Naringenin molecular ion (m/z = 271) appeared with the fragment ions (m/z = 151) for ring A and (m/z = 119) for ring B37. Hesperetin-7-O-neohesperidoside was detected with the molecular ion (m/z = 609) and the fragment ion of the aglycone hesperetin (m/z = 301) indicating the loss of neohesperidose [M-308]−38.
Identification of Coumarins and their glycosides
For the first time, three coumarins have been tentatively identified in L. hispidulus ethanolic extract. Daphnetin (7,8-dihydroxy coumarin) was identified with its molecular ion (m/z = 177) and characteristic fragment ions (m/z = 149) corresponding to [M-H–CO]−, (m/z = 121) corresponding to [M-H-CO-CO]− and (m/z = 133) corresponding to [M-H-CO2]− which were formed due to the lactone ring structure39. Esculetin (6,7-dihydroxy coumarin) was found with its molecular ion (m/z = 177) and fragment ion (m/z = 133)40. Esculin was identified with the molecular ion (m/z = 339) and the fragment ion (m/z = 177) due to loss of sugar40.
GC–MS/MS of the petroleum ether fraction of L. hispidulus extract
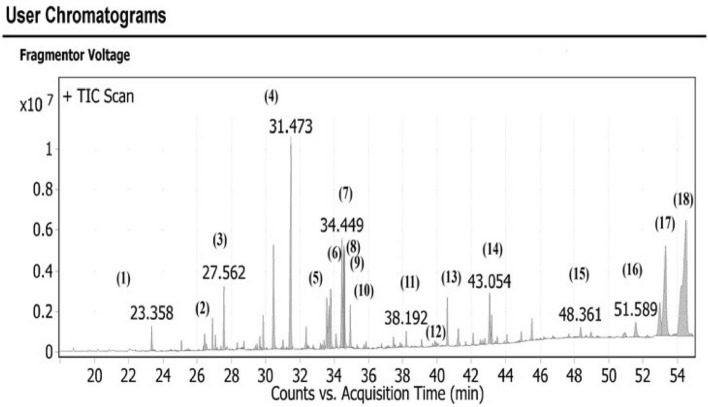
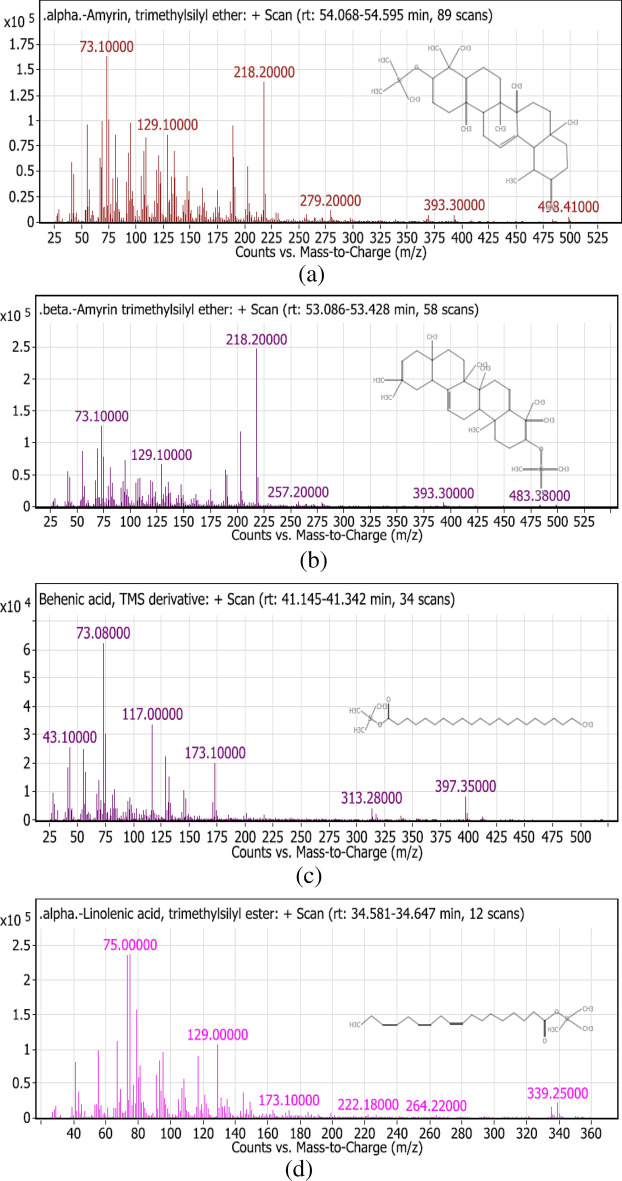
Table 3 summarizes the identified compounds in L. hispidulus petroleum ether fraction by GC–MS/MS analysis. The total ion chromatogram is demonstrated in Fig. 3. Figure 4a–g demonstrates the MS/MS spectrum figures of the major identified compounds.
Table 3.
The identified compounds in Leontodon hispidulus Boiss. petroleum ether fraction by GC–MS/MS analysis.
| Peak no. | Rt (min) | Molecular weight | Molecular formula | Error | Ms/Ms | Compound name | Peak area % |
|---|---|---|---|---|---|---|---|
| 1 | 23.316 | 272.49 | C15H32O2Si | 1.33 | 257, 201, 117, 73, 43 | Dodecanoic acid, TMS derivative | 1.10 |
| 2 | 26.436 | 256.42 | C16H32O2 | 1 | 326, 211, 157, 88, 43 | Tetradecanoic acid ethyl ester | 1.82 |
| 3 | 27.562 | 300.55 | C17H36O2Si | 0.67 | 285, 201, 145, 132, 117, 73, 43 | Myrestic acid, TMS derivative | 7.25 |
| 4 | 31.473 | 328.6 | C19H40O2Si | 0.97 | 313, 269, 201, 145, 132, 117, 73, 43 | Hexadecanoic acid TMS derivative | 8.49 |
| 5 | 33.569 | 308.5 | C20H36O2 | 1.03 | 339, 263, 67 | Ethyl linoleate | 1.72 |
| 6 | 33.791 | 368.7 | C23H48OSi | 1 | 213, 143, 73, 43 | Silane,[(3,7,11,15- tetramethyl-2-hexadecenyl)oxy]trimethyl | 0.77 |
| 7 | 34.449 | 352.6 | C21H40O2Si | 0.88 | 337, 262, 178, 145, 132, 129, 117,75,73 | 9,12-Octadecadienoic acid TMS derivative | 7.08 |
| 8 | 34.563 | 354.64 | C21H42O2Si | 0.34 | 339, 264, 185, 117, 73 | 9-Octadecenoic acid TMS derivative | 7.93 |
| 9 | 34.593 | 350.61 | C21H38O2Si | 1.42 | 335, 264, 222, 173, 145, 132, 129, 117, 75,73 | alpha-linolenic acid trimethylsilyl derivative | 9.02 |
| 10 | 34.94 | 356.65 | C21H44O2Si | 0.65 | 341, 297, 201, 73, 43 | Octadecanoic acid trimethylsilyl ester | 1.88 |
| 11 | 38.192 | 384.7 | C23H48O2Si | 0.15 | 369, 325, 201, 117, 73, 43 | Eicosanoic acid trimethylsilyl ester | 1.04 |
| 12 | 40.588 | 474.86 | C25H54O4Si2 | 0.89 | 401, 371, 313, 205, 147, 73, 43 | 1-Monopalmitin 2TMS derivative | 2.14 |
| 13 | 41.241 | 412.76 | C25H52O2Si | 1.61 | 397, 313, 173, 117, 73, 43 | Behenic acid TMS derivative | 1.82 |
| 14 | 43.054 | 500.9 | C27H56O4Si2 | 1.21 | 483, 395, 263, 203, 129, 73 | 2-Oleoylglycerol 2TMS derivative | 1.37 |
| 15 | 48.361 | 282.47 | C18H34O2 | 0.45 | 484, 394, 255, 129, 83, 55 | Oleic acid | 2.26 |
| 16 | 51.589 | 486.88 | C32H58OSi | 1.03 | 486, 357, 203, 129, 55 | Stigmast-5-ene, 3-beta (trimethylsiloxy) (24S) | 2.12 |
| 17 | 53.314 | 498.9 | C33H58OSi | 0.35 | 483, 393, 257, 218, 216, 129, 73 | beta-Amyrin trimethylsilyl derivative | 20.13 |
| 18 | 54.512 | 498.9 | C33H58OSi | 0.37 | 498, 483, 393, 279, 218, 203, 189, 129, 73 | alpha- Amyrin trimethylsilyl derivative | 20.38 |
Peak no. Peak number, Rt (min) Retention time in minutes, MS/MS Mass fragmentation.
Figure 3.
Chromatogram of the GC/MS of the petroleum ether fraction of Leontodon hispidulus Boiss.
Figure 4.
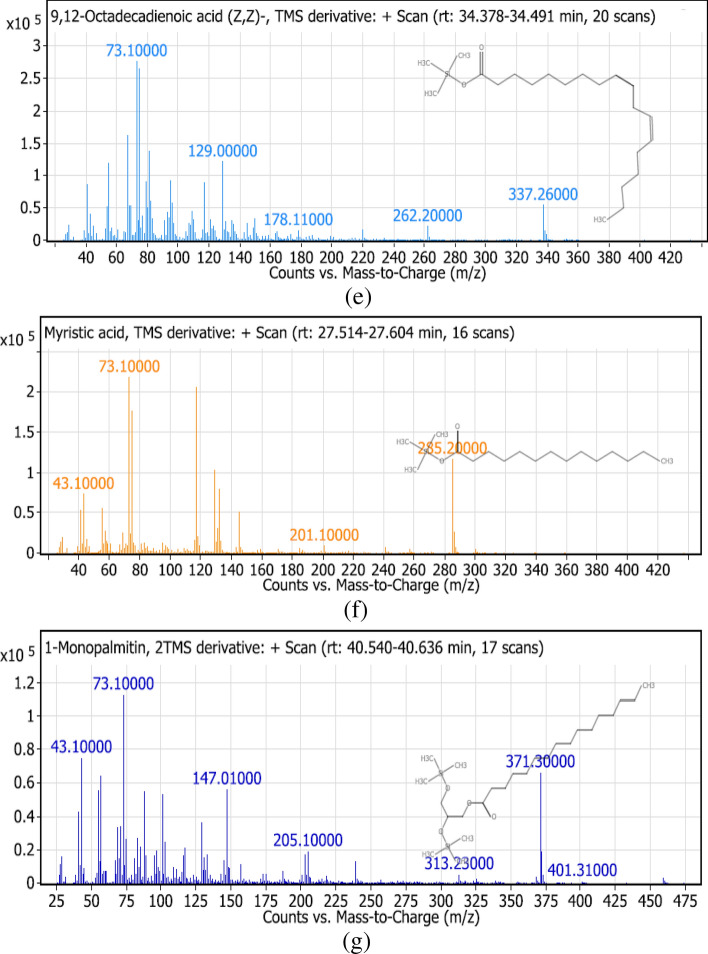
Fragmentation figures of the main identified compounds in Leontodon hispidulus Boiss. petroleum ether fraction, (a) Trimethylsilyl ether of Alpha-Amyrin, (b) Trimethylsilyl ether of Beta-Amyrin, (c) Trimethylsilyl derivative of Behenic acid, (d) Trimethylsilyl ester of Alpha-Linolenic acid, (e) Trimethylsilyl derivative of 9,12-Octadecadienoic acid, (f) Trimethylsilyl derivative of Myrestic acid, and (g) 2- trimethylsilyl derivative of 1-Monopalmitin.
Identification of the major compounds in L. hispidulus petroleum ether fraction.
Trimethylsilyl derivatives of the fatty acids show a characteristic fragmentation pattern. Hexadecanoic acid TMS derivative (palmitic acid) is the major saturated fatty acid that exists. A characteristic peak appears at m/z = 313 [M-15] due to the loss of methyl group from the silyl part which makes the cation more stable. A characteristic peak appears at m/z = 145 [(CH3)3SiCO2C2H4]+ due to the cleavage of the β, γ bond (relative to the carbonyl group). Another characteristic peak appears at m/z = 132 [(CH3)3SiCO2CH3]+ due to Mclafferty rearrangement. Generally, in TMS derivatives, the peak m/z = 73 [CH3)3Si]+ is the base peak. Another important peak appears at m/z = 117 [(CH3)3SiCO2]+. A similar fragmentation pattern applies to other saturated TMS derivatives of fatty acids as myristic acid41. 9,12-Octadecadienoic acid TMS derivative is the main unsaturated fatty acid detected. The peak m/z = 337 [M-15] appears due to the loss of methyl group from the silyl part. The peak m/z = 73 [CH3)3Si]+ is the base peak. The compound follows a similar fragmentation pattern to the saturated fatty acids except that the peaks at m/z = 132 [(CH3)3SiCO2CH3]+ and at m/z = 145 [(CH3)3SiCO2C2H4]+ are less prominent41. Two triterpenes were detected for the first time in the genus Leontodon, α-amyrin (ursane-type) and β-amyrin (oleanane-type). Both follow the Retro Diels–Alder fragmentation pattern. Similar to the fatty acids, the base peak is also at m/z = 73 [CH3)3Si]+ for both α- and β-amyrin. Also, the peak at m/z = 483 [M-15] due to the loss of methyl group from the silyl part appears in both compounds. The peak at m/z = 218 is prominent in both which is characteristic of Retro Diels–Alder fragmentation. The peak at m/z = 189 is characteristic of α-amyrin, while the peak at m/z = 216 is indicative of β-amyrin42. Guaiane-type sesquiterpenoid compounds were discovered previously in the flower heads of L. autumnalis3 and the roots of L. hispidus4. Moreover, a hypocretenolide glycoside was isolated from L. hispidus, which had a potent cytotoxic activity5 and anti-inflammatory activity6.
Cytotoxic activity of L. hispidulus successive fractions against Prostate carcinoma (PC3)
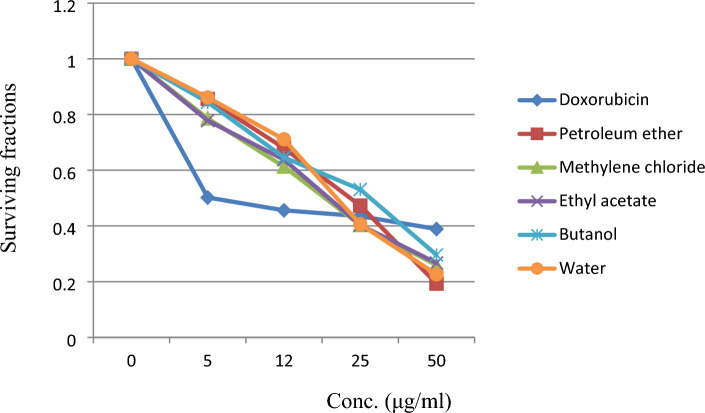
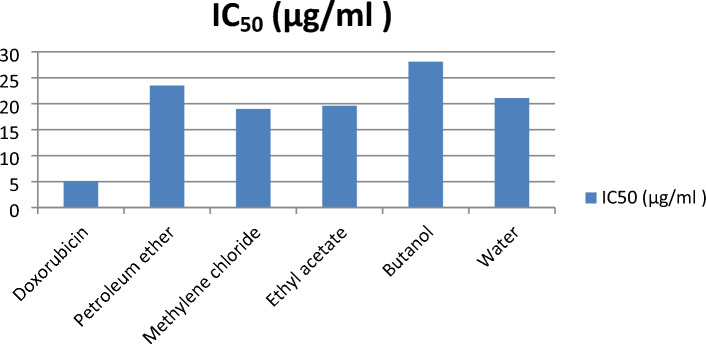
Results of the cytotoxic study are demonstrated in Figs. 5 and 6, where IC50 values of the different fractions (petroleum ether, methylene chloride, ethyl acetate, butanol, and aqueous) against prostate carcinoma were (23.5, 19, 19.6, 28.1, and 21.1 μg/ml, respectively). The most active fractions were methylene chloride and ethyl acetate with very close IC50 values (19 and 19.6 μg/ml, respectively), while the standard doxorubicin IC50 was 5.1 μg/ml. In our previous work10, the IC50 of L. hispidulus total optimized ethanolic extract against the PC3 cell line was 16.5 μg/ml which was more potent anticancer activity than each fraction alone. This result indicates a synergistic activity between the five fractions. Statistical analysis of the data of the surviving fractions was performed by SPSS software (V.22) using One-way ANOVA and Tukey’s test. Table 4 represents The One-way ANOVA results for the surviving fractions of Leontodon hispidulus Boiss. comparing the successive fractions to doxorubicin. Table 5 demonstrates The Multiple Comparison: Post Hoc Test—Tukey’s HSD results. Interestingly, both methylene chloride and ethyl acetate fractions showed a non-significant difference from the standard doxorubicin through the lowest concentrations (05.00, 12.50, and 25.00 µg/ml), while the difference was significant (higher in activity than doxorubicin) at the highest concentration (50.00 µg/ml). As demonstrated in Table 5, group (2) [methylene chloride fraction] showed a non-significant difference from group (6) [doxorubicin] through the first three concentrations [05.00 µg/ml, P-value = 0.077, 12.50 µg/ml, P-value = 0.319] and [25.00 µg/ml, P-value = 0.976]. While the difference was significant (higher in activity than doxorubicin) at the highest concentration [50.00 µg/ml, P-value = 0.007*]. Group (3) [ethyl acetate fraction] showed a similar pattern, the difference from group (6) [doxorubicin] was non-significant through the first three concentrations [05.00 µg/ml, P-value = 0.088, 12.50 µg/ml, P-value = 0.193] and [25.00 µg/ml, P-value = 0.975]. While the difference was significant (higher in activity than doxorubicin) at the highest concentration [50.00 µg/ml, P-value = 0.011*]. This result came in agreement with the close values of the IC50 of methylene chloride fraction, ethyl acetate fraction, and doxorubicin (19, 19.6, and 5.1, respectively), hence, the high anticancer activity of both fractions against the PC3 cell line. Group (5) [aqueous] came in the third position in activity after groups (2 and 3). It showed a significant difference (higher activity) from group (6) [doxorubicin] at the first two lowest concentrations [05.00 µg/ml, P-value = 0.019*, 12.50 µg/ml, P-value = 0.038*] and at the highest concentration [50.00 µg/ml, P-value = 0.001*], hence, the IC50 = 21.1 μg/ml. Group (1) [petroleum ether] showed a significant difference from group (6) [doxorubicin] at both the lowest concentration [05.00 µg/ml, P-value = 0.021*] and the highest concentration [50.00 µg/ml, P-value = 0.000*]. Finally, group (4) [butanol] showed a significant difference from group (6) [doxorubicin] at the lowest concentration [05.00 µg/ml, P-value = 0.026*]. It is worthy to mention, that all the successive fractions (except for the butanol fraction) showed a significantly higher anticancer activity against the PC3 cell line than doxorubicin at the highest concentration (50.00 µg/ml). In agreement with a previous study, the anticancer activity of L. hispidus was mainly linked to the isolated compound (hypocretenolide glycoside)5. Similarly, the anticancer potential of L. saxatilis43 was attributed to the presence of cichoric acid and three flavone glycosides [apigenin 4′-O-β-D-glucoside, luteolin 7-O-β-D-glucoside, and luteolin 4′-O-β-D-glucoside] against plasma cell myeloma (OPM2) cell line. Ultimately, the results suggest that, the promising anticancer activity of L. hispidulus Boiss. is due to the synergistic action of compounds of different chemical groups (flavonoid glycosides, phenolic acids, coumarins, and terpenes).
Figure 5.
Cytotoxic activity of Leontodon hispidulus Boiss. successive fractions and doxorubicin against prostate carcinoma cell line.
Figure 6.
IC50 of Leontodon hispidulus Boiss. successive fractions and Doxorubicin against prostate carcinoma cell line.
Table 4.
The One-way ANOVA results for the surviving fractions of Leontodon hispidulus Boiss. comparing the successive fractions to doxorubicin.
| Conc. (µg/ml) | Surviving fractions | Sum of squares | Degree of freedom (df) | Mean square | F-value | Significance |
|---|---|---|---|---|---|---|
| 05.00 | Between groups | 0.280 | 5 | 0.056 | 4.464 | 0.016* |
| Within groups | 0.151 | 12 | 0.013 | |||
| Total | 0.430 | 17 | ||||
| 12.50 | Between groups | 0.119 | 5 | 0.024 | 3.037 | 0.053 |
| Within groups | 0.094 | 12 | 0.008 | |||
| Total | 0.212 | 17 | ||||
| 25.00 | Between groups | 0.040 | 5 | 0.008 | 2.746 | 0.070 |
| Within groups | 0.035 | 12 | 0.003 | |||
| Total | 0.075 | 17 | ||||
| 50.00 | Between groups | 0.068 | 5 | 0.014 | 11.161 | 0.000* |
| Within groups | 0.015 | 12 | 0.001 | |||
| Total | 0.083 | 17 |
*The mean difference is significant at (P < 0.05).
Groups: petroleum ether, methylene chloride, ethyl acetate, butanol, aqueous and doxorubicin.
Table 5.
The Multiple Comparison: Post Hoc Test – Tukey’s HSD results for the surviving fractions of Leontodon hispidulus Boiss. comparing the successive fractions to doxorubicin.
| Conc. (µg/ml) | Significance (P-value) | ||||
|---|---|---|---|---|---|
| 05.00 | 12.50 | 25.00 | 50.00 | ||
| Group (I) versuss Group (J) | |||||
| 1 | 2 | 0.967 | 0.934 | 0.619 | 0.261 |
| 3 | 0.950 | 0.991 | 0.615 | 0.172 | |
| 4 | 1.000 | 0.997 | 0.771 | 0.033* | |
| 5 | 1.000 | 0.997 | 0.658 | 0.849 | |
| 6 | 0.021* | 0.079 | 0.947 | 0.000* | |
| 2 | 1 | 0.967 | 0.934 | 0.619 | 0.261 |
| 3 | 1.000 | 0.999 | 1.000 | 1.000 | |
| 4 | 0.985 | 0.997 | 0.105 | 0.788 | |
| 5 | 0.956 | 0.747 | 1.000 | 0.849 | |
| 6 | 0.077 | 0.319 | 0.976 | 0.007* | |
| 3 | 1 | 0.950 | 0.991 | 0.615 | 0.172 |
| 2 | 1.000 | 0.999 | 1.000 | 1.000 | |
| 4 | 0.974 | 1.000 | 0.104 | 0.908 | |
| 5 | 0.936 | 0.905 | 1.000 | 0.706 | |
| 6 | 0.088 | 0.193 | 0.975 | 0.011* | |
| 4 | 1 | 1.000 | 0.997 | 0.771 | 0.033* |
| 2 | 0.985 | 0.997 | 0.105 | 0.788 | |
| 3 | 0.974 | 1.000 | 0.104 | 0.908 | |
| 5 | 1.000 | 0.939 | 0.117 | 0.216 | |
| 6 | 0.026* | 0.163 | 0.310 | 0.059 | |
| 5 | 1 | 1.000 | 0.997 | 0.658 | 0.849 |
| 2 | 0.956 | 0.747 | 1.000 | 0.849 | |
| 3 | 0.936 | 0.905 | 1.000 | 0.706 | |
| 4 | 1.000 | 0.939 | 0.117 | 0.216 | |
| 6 | 0.019* | 0.038* | 0.984 | 0.001* | |
| 6 | 1 | 0.021* | 0.079 | 0.947 | 0.000* |
| 2 | 0.077 | 0.319 | 0.976 | 0.007* | |
| 3 | 0.088 | 0.193 | 0.975 | 0.011* | |
| 4 | 0.026* | 0.163 | 0.310 | 0.059 | |
| 5 | 0.019* | 0.038* | 0.984 | 0.001* | |
*The mean difference is significant at (P < 0.05).
Standard Error for conc. 05.00 µg/ml = 0.09144.
Standard Error for conc. 12.50 µg/ml = 0.07214.
Standard Error for conc. 25.00 µg/ml = 0.04401.
Standard Error for conc. 50.00 µg/ml = 0.02854.
Group 1: Petroleum ether fraction, Group 2: Methylene chloride fraction, Group 3: Ethyl acetate fraction, Group 4: butanol fraction, Group 5: Aqueous fraction, Group 6: Standard doxorubicin.
In-silico docking study using hexokinase-2 enzyme
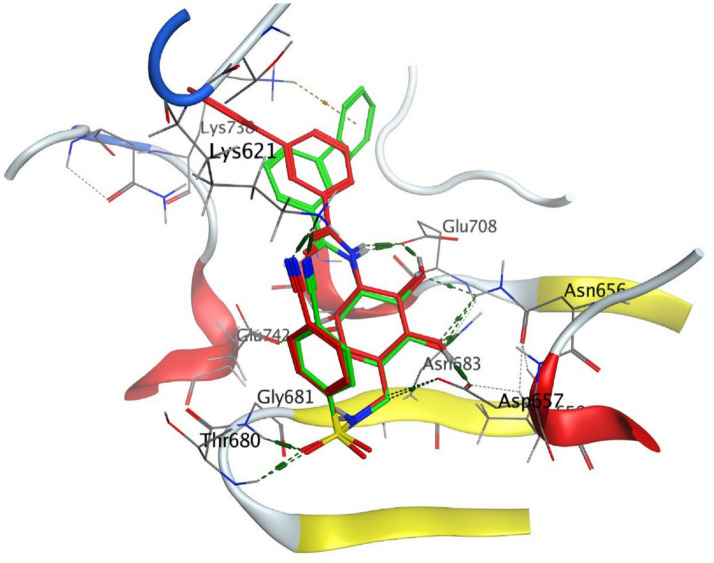
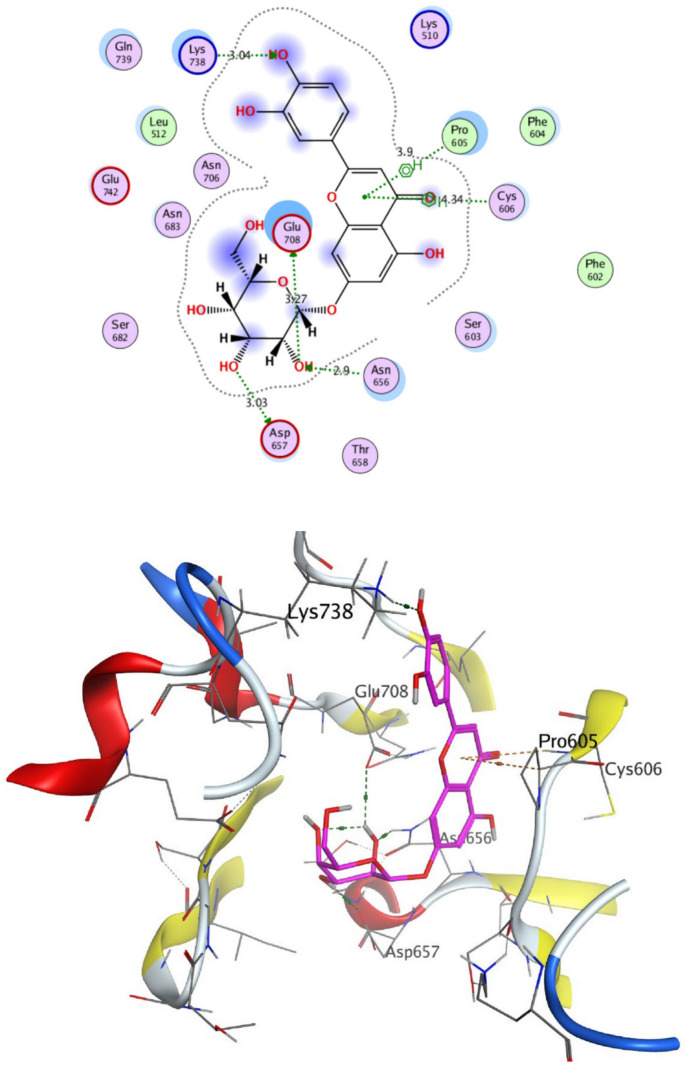
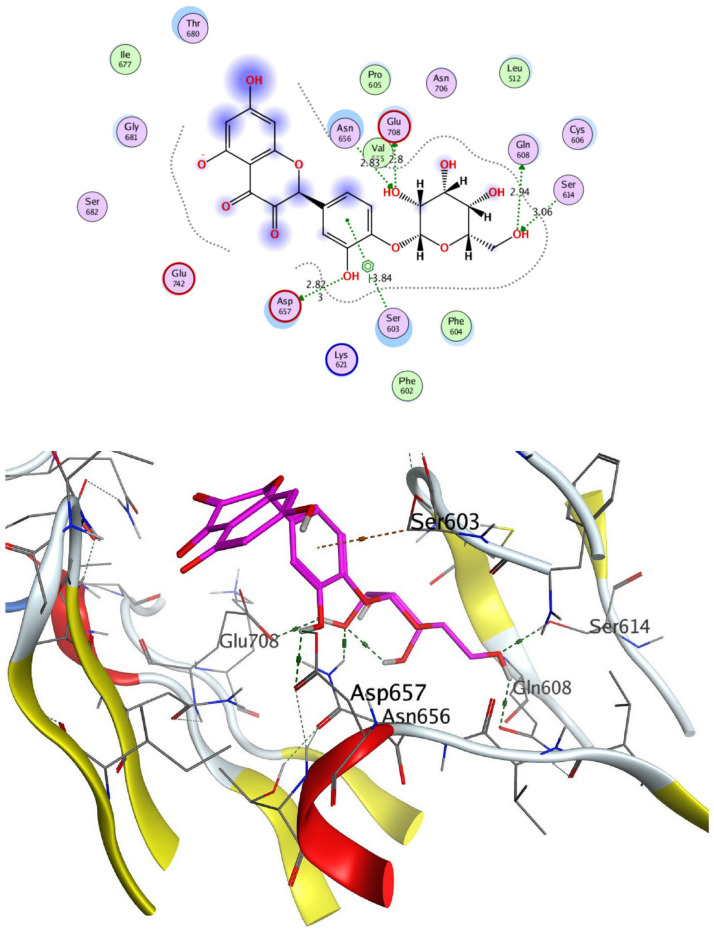
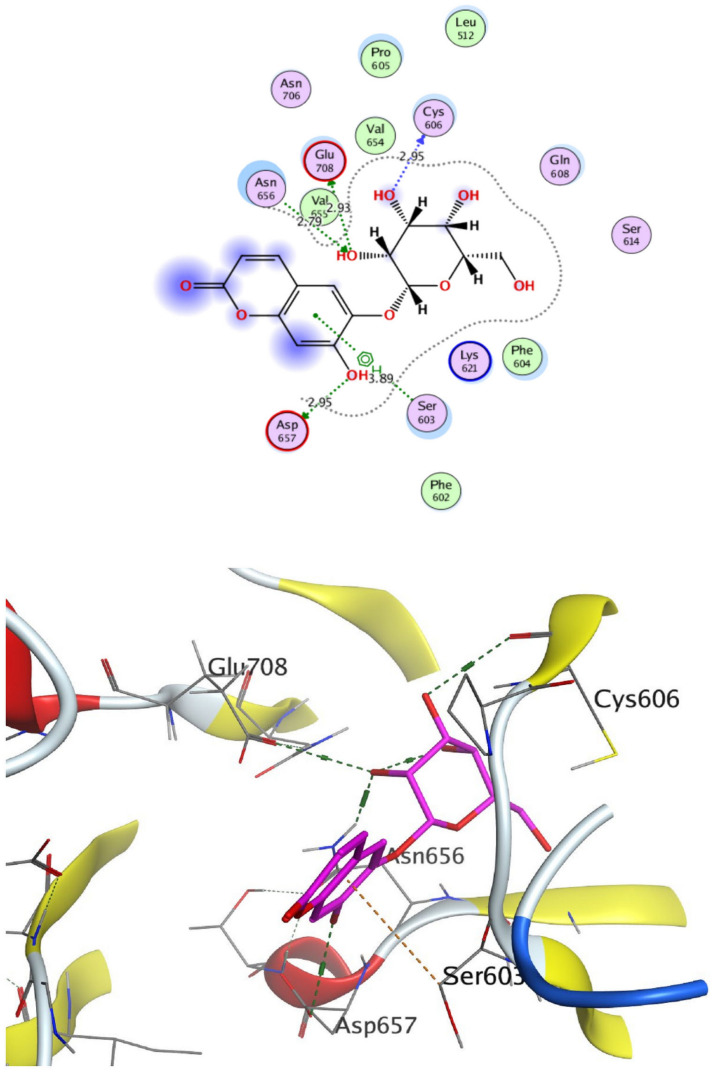
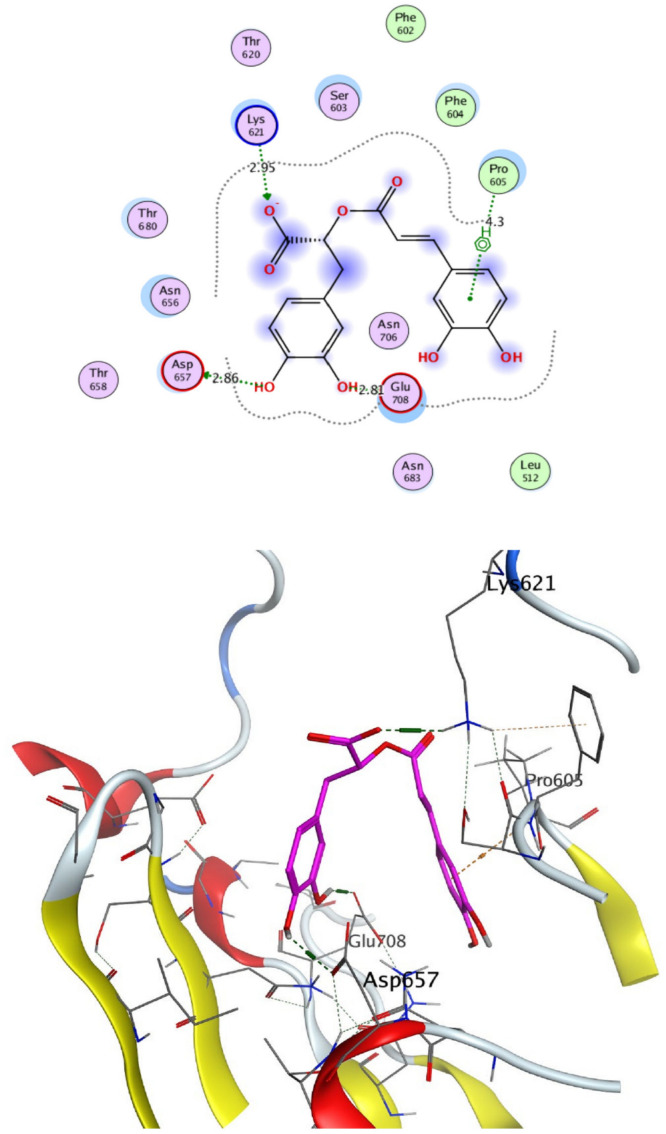
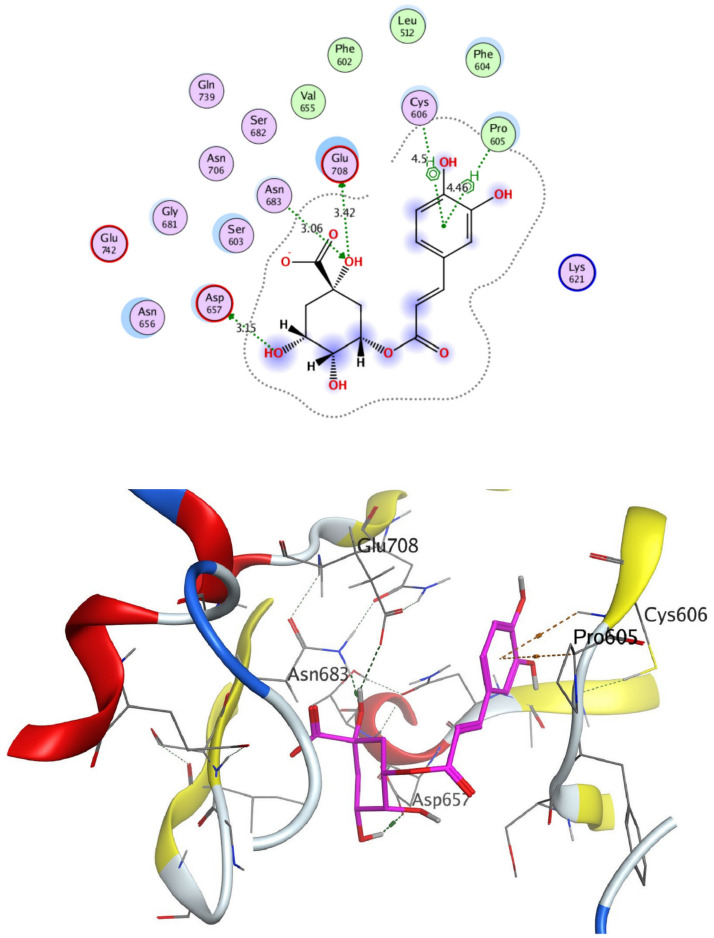
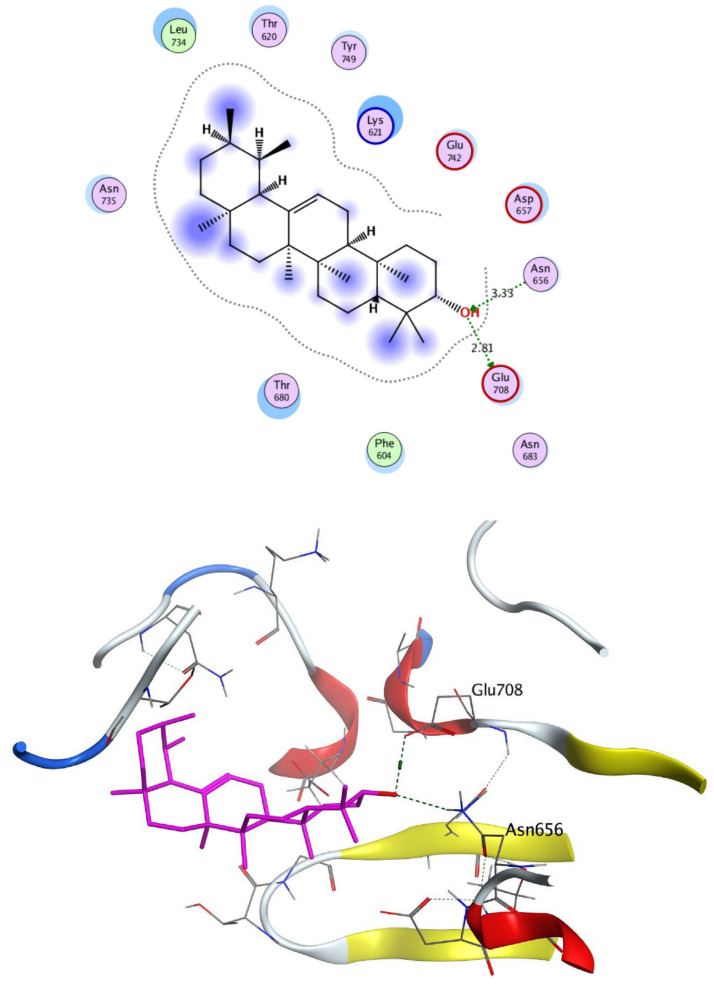
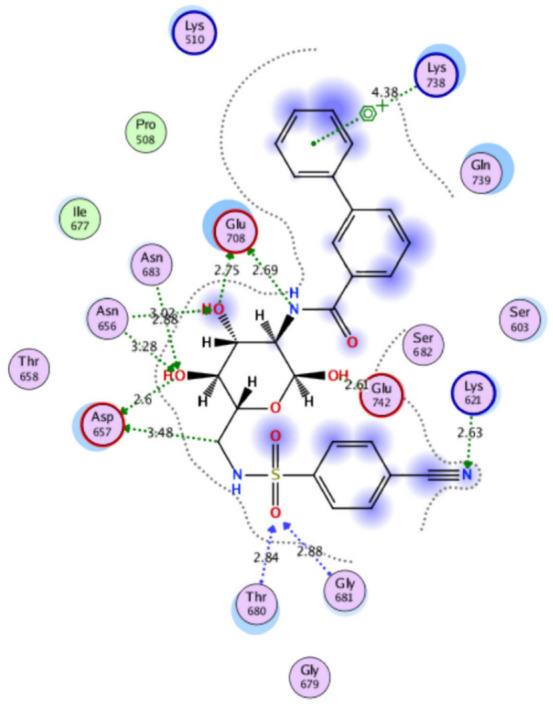
The structural properties of HEK-2 represent a challenge due to the high polarity of the active sites of the enzyme and the difficulty in specifically inhibiting the different isoenzymes14. Therefore, eight identified compounds of varying polarity were selected for the in-silico docking study. The molecular modeling study was carried out using Molecular Operating Environment (MOE, 2019.0102) software. The docking setup was first validated by self-docking of the co-crystallized ligand in the vicinity of the binding site of the enzyme, the docking score (S) was − 8.018 kcal/mol. and root mean square deviation (RMSD) was 1.931 Å Figs. 7 and 8. As demonstrated in (Table 6), all the tested eight compounds showed promising inhibitory effects on the HEK-2 enzyme. Apigenin-7-O-glucoside (Fig. 9) was the most active compound with a binding score of -7.412 through three hydrogen bonds with HEK-2. Two bonds as a H-bond donor with Asp657 and Glu742 amino acid residues and one bond as a H-bond acceptor with Lys621. luteolin-7-O-glucoside (Fig. 10) was found to be the second most active compound with a very close score of − 7.342. IT also formed three H-bonds with HEK-2, two as a H-bond donor with the amino acid residues Asp657 and Glu708, and one as a H-bond acceptor with Asn656. There were also two additional H–π interactions with Cys606 and Pro605 amino acid residues. Both compounds proved to possess an anticancer activity44. This result was in good agreement with the previous study of Palombo45 and a previous study on L. saxatilis43 that reported the anticancer activity of both apigenin 4′-O-β-D-glucoside, luteolin 7-O-β-D-glucoside. Kaempferol-3-O-glucuronide (Fig. 11) and quercetin-4′-O-glucoside (Fig. 12) were next in activity due to the similar structure to the first two compounds. Kaempferol-3-O-glucuronide showed anticancer activity using the Ehrlich ascites assay46, while quercetin-4′-O-glucoside proved its anticancer potential against hepatoblastoma cell line (HepG-2), PC3 and colorectal adenocarcinoma (HT-29) cell lines47. Importantly, esculin (Fig. 13) showed a high binding affinity with a score of -6.210 despite being smaller in size than the previous flavonoid glycosides. It formed three H-bonds as a H-bond donor, one H-bond as a H-bond acceptor, and one H-π interaction. Esculin was reported to have good anticancer activity via induction of apoptosis and autophagy in human glioblastoma multiforme cells (T98G) and human anaplastic astrocytoma cells48. Rosmarinic acid (Fig. 14) came very close to esculin with a score of − 6.144 through the formation of two H-bonds as a H-bond donor, one as a H-bond acceptor, and one as H-π interaction. Rosmarinic acid showed anticancer activity against many types of cancer as colon, skin, lung, and ovarian cancers49. Finally, came chlorogenic acid (Fig. 15) and the triterpene alpha-amyrin (Fig. 16) with scores of − 5.616 and − 5.272, respectively. Chlorogenic acid proved its activity against breast carcinoma50, while alpha-amyrin was reported to have anticancer activity against hepatocellular carcinoma via an in-silico docking study51. This result may explain the promising synergistic anticancer activity of the different fractions of L. hispidulus optimized extract against prostate carcinoma cell lines.
Figure 7.
2D interactions of the native ligand within the hexokinase-2 active site.
Figure 8.
3D representation of the superimposition of the co-crystallized (green) and the docking pose (red) of the native ligand in the active site of hexokinase-2.
Table 6.
Docking results with Hexokinase-2 enzyme.
| S (kcal/mol) | Amino acids | Interacting groups | Type of interaction | Length | |
|---|---|---|---|---|---|
| Native ligand | − 8.018 | Asp657 | OH | H-bond donor | 2.60 |
| Asp657 | CH2 | Non-classical H-bond | 3.48 | ||
| Asn656 | OH | H-bond acceptor | 3.02 | ||
| Asn656 | OH | H-bond acceptor | 3.28 | ||
| Glu708 | NH | H-bond donor | 2.69 | ||
| Glu708 | OH | H-bond donor | 2.75 | ||
| Asn683 | OH | H-bond acceptor | 2.88 | ||
| Gly681 | SO2 | H-bond acceptor | 2.88 | ||
| Thr680 | SO2 | H-bond acceptor | 2.84 | ||
| Lys621 | CN | H-bond acceptor | 2.63 | ||
| Lys738 | Phenyl | cation-Pi interaction | 4.38 | ||
| Alpha-amyrin | − 5.272 | Asn656 | OH | H-bond acceptor | 3.33 |
| Glu708 | OH | H-bond donor | 2.81 | ||
| Apigenin-7-O-glucoside | − 7.412 | Asp657 | OH | H-bond donor | 2.98 |
| Glu742 | OH | H-bond donor | 3.44 | ||
| Lys621 | O | H-bond acceptor | 3.89 | ||
| Chlorogenic acid | − 5.616 | Asp657 | OH | H-bond donor | 3.15 |
| Asn683 | OH | H-bond acceptor | 3.06 | ||
| Glu708 | OH | H-bond donor | 3.42 | ||
| Cys606 | Phenyl | H-Pi interaction | 4.50 | ||
| Pro605 | Phenyl | H-Pi interaction | 4.46 | ||
| Esculin | − 6.210 | Asp657 | OH | H-bond donor | 2.95 |
| Asn656 | OH | H-bond acceptor | 2.79 | ||
| Glu708 | OH | H-bond donor | 2.93 | ||
| Cys606 | OH | H-bond donor | 2.95 | ||
| Ser603 | Phenyl | H-Pi interaction | 3.89 | ||
| Kaempferol-3-O-glucuronide | − 6.858 | Asp657 | OH | H-bond donor | 3.10 |
| Glu708 | OH | H-bond donor | 2.89 | ||
| Pro605 | Pyran | H-Pi interaction | 4.31 | ||
| Pro605 | Phenyl | H-Pi interaction | 4.63 | ||
| Luteolin-7-O-glucoside | − 7.342 | Asp657 | OH | H-bond donor | 3.03 |
| Asn656 | OH | H-bond acceptor | 2.90 | ||
| Glu708 | OH | H-bond donor | 3.27 | ||
| Cys606 | Pyran | H-Pi interaction | 4.34 | ||
| Pro605 | Pyran | H-Pi interaction | 3.90 | ||
| Quercetin-4′-O-glucoside | − 6.289 | Asp657 | OH | H-bond donor | 2.82 |
| Asn656 | OH | H-bond acceptor | 2.83 | ||
| Glu708 | OH | H-bond donor | 2.80 | ||
| Ser603 | Phenyl | H-Pi interaction | 3.84 | ||
| Gln608 | OH | H-bond donor | 2.94 | ||
| Ser614 | OH | H-bond acceptor | 3.06 | ||
| Rosmarinic acid | − 6.144 | Asp657 | OH | H-bond donor | 2.86 |
| Glu708 | OH | H-bond donor | 2.81 | ||
| Lys621 | O | H-bond acceptor | 2.95 | ||
| Pro605 | Phenyl | H-Pi interaction | 4.30 |
S (kcal/mol), docking score in kilocalorie per mole; Asn, Asparagine; Asp, Aspartic acid; Cys, Cysteine; Glc, Glucose; Gln, Glutamine; Glu, Glutamic acid; Gly, Glycine; Lys, Lysine; Pro, Proline; Ser, Serine; Thr, Threonine.
Figure 9.
2D and 3D interactions of Apigenin-7-O-glucoside within hexokinase-2 active site.
Figure 10.
2D and 3D interactions of Luteolin-7-O-glucoside within hexokinase-2 active site.
Figure 11.

2D and 3D interactions of Kaempferol-3-O-glucuronide within hexokinase-2 active site.
Figure 12.
2D and 3D interactions of Quercetin-4ˋ-O-glucoside within hexokinase-2 active site.
Figure 13.
2D and 3D interactions of Esculin within hexokinase-2 active site.
Figure 14.
2D and 3D interactions of Rosmarinic acid within hexokinase-2 active site.
Figure 15.
2D and 3D interactions of Chlorogenic acid within hexokinase-2 active site.
Figure 16.
2D and 3D interactions of Alpha-Amyrin.
Conclusion
The comprehensive profiling of L. hispidulus optimized ethanolic extract revealed the presence of thirty-six phenolic compounds which were identified for the first time in this plant species using LC-qTOF-MS. These compounds include the glycosides of (luteolin, quercetin, kaempferol, apigenin, isorhamnetin, and daidzein), coumarines (esculin, esculetin and daphnetin), phenolic acids (chlorogenic, caffeic, quinic, P-coumaric and rosmarinic). GC–MS/MS also was performed for the first time revealing the presence of eighteen fatty acids and hydrocarbons as palmitic acid, myristic acid, α-linolenic acid, hexadecanoic acid, oleic acid, behenic acid, and triterpenes (α- and β-amyrin). Methylene chloride and ethyl acetate were the most active fractions against the PC3 cell line. They showed very close IC50 values (19 and 19.6), respectively. It is worthy to mention, that all the successive fractions (except for the butanol fraction) showed a significantly higher anticancer activity against the PC3 cell line than doxorubicin at the highest concentration (50.00 µg/ml). Apigenin-7-O-glucoside showed the highest inhibitory effect of HEK-2 enzyme with a binding score of − 7.412, followed by luteolin-7-O-glucoside, kaempferol-3-O-glucuronide, quercetin-4ˋ-O-glucoside, esculin, rosmarinic acid, chlorogenic acid and finally alpha-amyrin. The results of the docking study showed consistency with the results of the anticancer activity study on the successive fractions, as the synergistic activity could be due to the flavonoid glycosides, coumarins, phenolic acids, and triterpenes combined. This study could be a starting point for many future studies on L. hispidulus to further investigate its biological potential.
Abbreviations
- ABTS
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- Asn
Asparagine
- Asp
Aspartic acid
- CDS
Calibration delivery system
- Cys
Cysteine
- DI-water
Deionized water
- EDTA
Ethylenediaminetetraacetic acid
- EI
Electron ionization
- ESI
Electron spray ionization
- EV
Electronvolt
- GC/MS
Gas chromatography-mass spectrometry
- Glc
Glucose
- Gln
Glutamine
- Glu
Glutamic acid
- Gly
Glycine
- HEK-2
Hexokinase-2 enzyme
- 5HFU
Crystal structure of Human Hexokinase-2
- HELA
Human Cervical carcinoma cellline
- HepG-2
Hepatoblastoma cell line
- HPLC
High-performance liquid chromatography
- HT-29
Colorectal adenocarcinoma cell line
- IC50
Half-maximal inhibitory concentration
- Kcal/mol
Kilocalorie per mole
- KV
Kilovolt
- LC-qTOF-MS
Quadrupole Time-of-Flight Liquid chromatography-mass spectrometry
- Lautumnalis
Leontodon autumnalis
- L. filli
Leontodon filli
- L. hispidus
Leontodon hispidus
- L. hispidulus
Leontodon hispidulus
- L.saxatilis
Leontodon saxatilis
- Lys
Lysine
- MOE
Molecular operating environment
- MONA
Mass Bank of North America
- MP-WS
Mobile phase working solution
- NIST
National Institute of Standards and Technology
- OPM2
Plasma cell myeloma cell lines
- PC3
Human Prostate carcinoma cell line
- PDB
Protein data bank
- Pro
Proline
- Rham
Rhamnose
- RESPECT
Database for phytochemicals
- RMSD
Root mean square deviation
- Ser
Serine
- SRB
Sulforhodamine-B assay
- T98G
Human glioblastoma multiforme cells
- Thr
Threonine
- TIC
Total Ion Chromatogram
- TMCS
Trimethylchlorosilane
- TROLOX
6-Hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid
- UPLC
Ultra-performance liquid chromatography
Author contributions
N.M.A., M.S.H., R.A.L., and I.Y.Y. contributed to Conceptualization, Methodology, Investigation, analysis—discussing the data and Writing—reviewing the original article.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boulos FI. Flora of Egypt. Vol. 3. Flora of Egypt (Verbenaceae—Compositae) Nord. J. Bot. 2002;22(4):390. [Google Scholar]
- 2.Rolnik A, Olas B. The plants of the Asteraceae family as agents in the protection of human health. Int. J. Mol. Sci. 2021;22(6):3009. doi: 10.3390/ijms22063009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zidorn C, et al. New taxonomically significant sesquiterpenoids from Leontodon autumnalis. J. Natl. Prod. 2000;63(6):812–816. doi: 10.1021/np990554j. [DOI] [PubMed] [Google Scholar]
- 4.Michalska K, Pieron K, Stojakowska A. Sesquiterpene lactones and phenolics from roots of Leontodon hispidus subsp. hispidus. Natl. Prod. Commun. 2018;13(4):19345781801300403. [Google Scholar]
- 5.Zidorn C, et al. Cytotoxic activities of Hypocretenolides from Leontodon h ispidus. J. Natl. Prod. 1999;62(7):984–987. doi: 10.1021/np990058v. [DOI] [PubMed] [Google Scholar]
- 6.Zidorn C, et al. Anti-inflammatory activities of hypocretenolides from Leontodon hispidus. Planta medica. 1999;65(08):704–708. doi: 10.1055/s-1999-14046. [DOI] [PubMed] [Google Scholar]
- 7.Zidorn C, Stuppner H. Evaluation of chemosystematic characters in the genus Leontodon (Asteraceae) Taxon. 2001;50(1):115–133. doi: 10.2307/1224515. [DOI] [Google Scholar]
- 8.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Mini Rev. Med. Chem. 2009;9(1):31–59. doi: 10.2174/138955709787001712. [DOI] [PubMed] [Google Scholar]
- 9.Tostao Z, et al. A novel pentacyclic triterpene from Leontodon filii. Fitoterapia. 2005;76(2):173–180. doi: 10.1016/j.fitote.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Abd-El-Aziz NM, et al. Application of Box-Behnken design for optimization of phenolics extraction from Leontodon hispidulus in relation to its antioxidant, anti-inflammatory and cytotoxic activities. Sci. Rep. 2022;12(1):8829. doi: 10.1038/s41598-022-12642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanbrough M, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res.arch. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 12.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, Tu SM, Campbell ML. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 13.Iqbal J, et al. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017;7(12):1129–1150. doi: 10.1016/j.apjtb.2017.10.016. [DOI] [Google Scholar]
- 14.Liu Y, et al. Structure based discovery of novel hexokinase 2 inhibitors. Bioorganic Chem. 2020;96:103609. doi: 10.1016/j.bioorg.2020.103609. [DOI] [PubMed] [Google Scholar]
- 15.Khan T, et al. Computational drug designing and prediction of important parameters using in silico methods-A review. Curr. Comput. Aided Drug Des. 2019;15(5):384–397. doi: 10.2174/1573399815666190326120006. [DOI] [PubMed] [Google Scholar]
- 16.Sawada Y, et al. RIKEN tandem mass spectral database (ReSpect) for phytochemicals: A plant-specific MS/MS-based data resource and database. Phytochemistry. 2012;82:38–45. doi: 10.1016/j.phytochem.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Rabaneda F, et al. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao) J. Mass Spectrom. 2003;38(1):35–42. doi: 10.1002/jms.395. [DOI] [PubMed] [Google Scholar]
- 18.Lin L-Z, Harnly JM. Identification of hydroxycinnamoylquinic acids of arnica flowers and burdock roots using a standardized LC-DAD-ESI/MS profiling method. J. Agric. Food Chem. 2008;56(21):10105–10114. doi: 10.1021/jf802412m. [DOI] [PubMed] [Google Scholar]
- 19.Alberti, Á., LC-ESI-MS/MS methods in profiling of flavonoid glycosides and phenolic acids in traditional medicinal plants: Sempervivum tectorum L. and Corylus avellana L. (2014).
- 20.Benedec D, et al. Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pak. J. Pharm. Sci. 2015;28(6):2297–2303. [PubMed] [Google Scholar]
- 21.Végh K, et al. Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J. Pharm. Biomed. Anal. 2018;149:488–493. doi: 10.1016/j.jpba.2017.11.029. [DOI] [PubMed] [Google Scholar]
- 22.Ben Salah H, et al. LC-ESI-MS/MS Phenolic Profile of Volutaria lippii (L.) Cass. Extracts and evaluation of their in vitro antioxidant, antiacetylcholinesterase, antidiabetic, and antibacterial activities. Evid. Based Complement. Altern. Med. 2019;2019:1–13. doi: 10.1155/2019/9814537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques R, et al. Characterization of weld (Reseda luteola L.) and spurge flax (Daphne gnidium L.) by high-performance liquid chromatography–diode array detection–mass spectrometry in Arraiolos historical textiles. J. Chromatogr. A. 2009;1216(9):1395–1402. doi: 10.1016/j.chroma.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, et al. Quantitative and qualitative analysis of flavonoids in leaves of Adinandra nitida by high performance liquid chromatography with UV and electrospray ionization tandem mass spectrometry detection. Anal. Chim. Acta. 2005;532(1):97–104. doi: 10.1016/j.aca.2004.10.042. [DOI] [Google Scholar]
- 25.Justesen U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001;36(2):169–178. doi: 10.1002/jms.118. [DOI] [PubMed] [Google Scholar]
- 26.Yin J, et al. A systematic study of the metabolites of dietary acacetin in vivo and in vitro based on UHPLC-Q-TOF-MS/MS analysis. J. Agric. food Chem. 2019;67(19):5530–5543. doi: 10.1021/acs.jafc.9b00330. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Lin G, Zuo Z. Involvement of UDP-glucuronosyltransferases in the extensive liver and intestinal first-pass metabolism of flavonoid baicalein. Pharm. Res. 2007;24(1):81–89. doi: 10.1007/s11095-006-9126-y. [DOI] [PubMed] [Google Scholar]
- 28.Younis IY, et al. Untargeted metabolites profiling of volatile and non-volatile components of Egyptian lotus (Nelumbo nucifera Gaertn.) using UHPLC/PDA/ESI-MS and solid-phase microextraction (SPME) GC/MS in relation to its antiaging and anti-inflammatory effects. Ind. Crops Prod. 2023;197:116561. doi: 10.1016/j.indcrop.2023.116561. [DOI] [Google Scholar]
- 29.Lee J, Mitchell AE. Quercetin and isorhamnetin glycosides in onion (Allium cepa L.): varietal comparison, physical distribution, coproduct evaluation, and long-term storage stability. J. Agric. Food Chem. 2011;59(3):857–863. doi: 10.1021/jf1033587. [DOI] [PubMed] [Google Scholar]
- 30.Venkatalakshmi P, Vadivel V, Brindha P. Identification of flavonoids in different parts of Terminalia catappa L. Using LC-ESI-MS/MS and investigation of their anticancer effect in EAC Cell line model. J. Pharm. Sci. Res. 2016;8(4):176. [Google Scholar]
- 31.Chen F, et al. Analysis of phenolic acids of Jerusalem artichoke (Helianthus tuberosus L.) responding to salt-stress by liquid chromatography/tandem mass spectrometry. Sci. World J. 2014;2014:1–8. doi: 10.1155/2014/568043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abad-García B, et al. On line characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta. 2012;99:213–224. doi: 10.1016/j.talanta.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 33.Cuyckens F, et al. Tandem mass spectral strategies for the structural characterization of flavonoid glycosides. Analusis. 2000;28(10):888–895. doi: 10.1051/analusis:2000280888. [DOI] [Google Scholar]
- 34.Mattivi F, et al. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 2006;54(20):7692–7702. doi: 10.1021/jf061538c. [DOI] [PubMed] [Google Scholar]
- 35.Prasain JK, et al. Identification of isoflavone glycosides in Pueraria lobata cultures by tandem mass spectrometry. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2007;18(1):50–59. doi: 10.1002/pca.951. [DOI] [PubMed] [Google Scholar]
- 36.Guo P, et al. Simultaneous determination of linarin, naringenin and formononetin in rat plasma by LC-MS/MS and its application to a pharmacokinetic study after oral administration of Bushen Guchi Pill. Biomed. Chromatogr. 2015;29(2):246–253. doi: 10.1002/bmc.3267. [DOI] [PubMed] [Google Scholar]
- 37.Zeng X, et al. Simultaneous determination of rosuvastatin, naringin and naringenin in rat plasma by RRLC–MS/MS and its application to a pharmacokinetic drug interaction study. J. Chromatogr. Sci. 2018;56(7):611–618. doi: 10.1093/chromsci/bmy034. [DOI] [PubMed] [Google Scholar]
- 38.Roowi S, Crozier A. Flavonoids in tropical citrus species. J. Agric. Food Chem. 2011;59(22):12217–12225. doi: 10.1021/jf203022f. [DOI] [PubMed] [Google Scholar]
- 39.Su XL, et al. Identification and characterisation of the Chinese herb Langdu by LC-MS/MS analysis. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2003;14(1):40–47. doi: 10.1002/pca.685. [DOI] [PubMed] [Google Scholar]
- 40.El-Fadaly AA, et al. protective action mechanisms of Launaea mucronata extract and its nano-formulation against nephrotoxicity in rats as revealed via biochemical, histopathological, and UPLC-QTOF–MS/MS Analyses. Metabolites. 2023;13(7):786. doi: 10.3390/metabo13070786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.da Silva RAC, et al. Ximenia Americana: Chemical and spectral studies of extracts of seeds. Analysis of trimethylsilyl derivatives by gas chromatography and mass spectrometry. Am. J. Anal. Chem. 2016;7(2):192–202. doi: 10.4236/ajac.2016.72016. [DOI] [Google Scholar]
- 42.Yam-Puc A, et al. Pentacyclic triterpenes and other constituents in propolis extract from Melipona beecheii collected in Yucatan México. Revista Brasileira de Farmacognosia. 2019;29:358–363. doi: 10.1016/j.bjp.2019.01.006. [DOI] [Google Scholar]
- 43.Ҫiҫek SS, et al. Cytotoxic constituents and a new hydroxycinnamic acid derivative from Leontodon saxatilis (Asteraceae, Cichorieae) RSC Adv. 2021;11(18):10489–10496. doi: 10.1039/D0RA10973H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodarzi S, et al. Cuminum cyminum fruits as source of luteolin-7-O-glucoside, potent cytotoxic flavonoid against breast cancer cell lines. Nat. Prod. Res. 2020;34(11):1602–1606. doi: 10.1080/14786419.2018.1519824. [DOI] [PubMed] [Google Scholar]
- 45.Palombo R, et al. Luteolin-7-O-β-D-glucoside inhibits cellular energy production interacting with HEK2 in keratinocytes. Int. J. Mol. Sci. 2019;20(11):2689. doi: 10.3390/ijms20112689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Badria FA, Ameen M, Akl MR. Evaluation of cytotoxic compounds from Calligonum comosum L. growing in Egypt. Zeitschrift für Naturforschung C. 2007;62(9–10):656–660. doi: 10.1515/znc-2007-9-1005. [DOI] [PubMed] [Google Scholar]
- 47.Pan Y, Zheng YM, Ho WS. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018;15(4):4657–4661. doi: 10.3892/ol.2018.7893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li CX, et al. The pharmacological and pharmacokinetic properties of esculin: A comprehensive review. Phytother. Res. 2022;36(6):2434–2448. doi: 10.1002/ptr.7470. [DOI] [PubMed] [Google Scholar]
- 49.Hossan MS, et al. Rosmarinic acid: A review of its anticancer action. World J. Pharm. Pharm. Sci. 2014;3(9):57–70. [Google Scholar]
- 50.Zeng A, et al. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021;45(2):717–727. doi: 10.3892/or.2020.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kamaraj M, et al. In silico docking and anti-cancer activity of the isolated compounds (Alpha and Beta Amyrin) from methanolic bark extract of Shorea robusta. Int. J. Pure Med. Res. 2019;4(12):11–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.