Abstract
Previous studies have suggested that the UL17 gene of herpes simplex virus type 1 (HSV-1) is essential for virus replication. In this study, viral mutants incorporating either a lacZ expression cassette in place of 1,490 bp of the 2,109-bp UL17 open reading frame [HSV-1(ΔUL17)] or a DNA oligomer containing an in-frame stop codon inserted 778 bp from the 5′ end of the UL17 open reading frame [HSV-1(UL17-stop)] were plaque purified on engineered cell lines containing the UL17 gene. A virus derived from HSV-1(UL17-stop) but containing a restored UL17 gene was also constructed and was designated HSV-1(UL17-restored). The latter virus formed plaques and cleaved genomic viral DNA in a manner indistinguishable from wild-type virus. Neither HSV-1(ΔUL17) nor HSV-1(UL17-stop) formed plaques or produced infectious progeny when propagated on noncomplementing Vero cells. Furthermore, genomic end-specific restriction fragments were not detected in DNA purified from noncomplementing cells infected with HSV-1(ΔUL17) or HSV-1(UL17-stop), whereas end-specific fragments were readily detected when the viruses were propagated on complementing cells. Electron micrographs of thin sections of cells infected with HSV-1(ΔUL17) or HSV-1(UL17-stop) illustrated that empty capsids accumulated in the nuclei of Vero cells, whereas DNA-containing capsids accumulated in the nuclei of complementing cells and enveloped virions were found in the cytoplasm and extracellular space. Additionally, protein profiles of capsids purified from cells infected with HSV-1(ΔUL17) compared to wild-type virus show no detectable differences. These data indicate that the UL17 gene is essential for virus replication and is required for cleavage and packaging of viral DNA. To characterize the UL17 gene product, an anti-UL17 rabbit polyclonal antiserum was produced. The antiserum reacted strongly with a major protein of apparent Mr 77,000 and weakly with a protein of apparent Mr 72,000 in wild-type infected cell lysates and in virions. Bands of similar sizes were also detected in electrophoretically separated tegument fractions of virions and light particles and yielded tryptic peptides of masses characteristic of the predicted UL17 protein. We therefore conclude that the UL17 gene products are associated with the virion tegument and note that they are the first tegument-associated proteins shown to be required for cleavage and packaging of viral DNA.
During herpes simplex virus type 1 (HSV-1) infection, individual viral genomes are cleaved from intranuclear concatameric viral DNA and packaged into preassembled capsids. Three types of capsids, which differ in density and electron microscopic appearance, accumulate in the nuclei of infected cells. Type A capsids consist of an approximately 120-nm-diameter proteinaceous shell, type B capsids contain the shell surrounding an internal core or scaffold, and type C capsids lack the scaffold but contain genomic viral DNA (18). Cleavage of the scaffold of large-cored B capsids (also called procapsids) by a packaged protease likely initiates conversion to small-cored B capsids (22, 43). It is believed that either large-cored or small-cored B capsids receive viral DNA, whereas type A capsids are believed to arise from an unsuccessful DNA packaging reaction in which the scaffold is expelled but DNA is not inserted (19, 27, 39). Coexpression of the UL18, UL19, UL26.5, UL26, and UL38 genes is necessary and sufficient for production of B capsids (36, 38). However, at least the UL6, UL15, UL25, UL28, UL32, and UL33 genes are necessary for production of type C capsids, and cells infected with viruses bearing mutations in these genes contain intranuclear capsids that appear morphologically indistinguishable from B capsids by electron microscopy (1–4, 26, 37, 42, 44). In cells infected with viral mutants lacking functional UL6, UL15, UL28, UL32, or UL33 gene products, unit-length genomes are not cleaved from concatameric viral DNA (2–4, 26, 33, 37, 44).
Also pertinent to this study is the observation that at least two different types of enveloped particles are detectable in the extracellular spaces of cells infected with wild-type herpesviruses (35). Virions contain type C-like capsids wrapped within an envelope containing a number of virus-encoded integral membrane proteins. Additional virion proteins are located between the capsid surface and the inner side of the virion envelope in a region termed the tegument (29). In contrast, light particles lack capsids but contain most of the proteins associated with the envelope and tegument (35).
The UL16 and UL17 genes are unique among known HSV genes because they lie within the intron of another gene, UL15 (20). Previous studies have indicated that the UL16 gene encodes a virion-associated protein that is dispensable for replication in cultured cells (6, 21). In contrast, attempts to purify viral mutants lacking a functional UL17 gene on cells not expressing UL17 were unsuccessful, suggesting that the UL17 gene is essential for virus replication (6). The current studies were undertaken to characterize the function and product of the UL17 gene.
MATERIALS AND METHODS
Viruses and cells.
G5 transformed cells were derived from Vero cells and contain HSV-1 DNA from genes UL16 to UL21 (16). Vero, rabbit skin, G5, and HEp-2 cells were maintained in Dulbecco’s medium supplemented with 10% newborn calf serum, penicillin, and streptomycin, as previously described (5, 9, 16). The human melanoma cell line (MeWo) (10) was grown in Dulbecco’s medium supplemented with 8% (vol/vol) fetal calf serum, 2 mM glutamine, nonessential amino acids, 100 U of penicillin per ml, and 100 μg of streptomycin per ml.
The wild-type herpes simplex viruses HSV-1(F) and HSV-1(17) have been described previously (11, 17). An HSV-1(17) mutant with a lesion in UL47 was constructed by cosmid recombination as described previously (14). The mutation consists of a duplication of 4 bp within a KpnI site at bp 105 to 108 in the 2,079-bp UL47 coding region and results in a frameshift near the 5′ end of the gene. HSV-1(R7224), which contains a cDNA copy of UL15 in the native position of exon I of UL15, and K23Z, which contains a lacZ cassette inserted in UL18, have been described previously (7, 16). Stocks of HSV-1(F) and HSV-1(R7224) were grown and titered on Vero cell monolayers. The viral mutant lacking UL47 protein expression was grown and titered on MeWo cells. Stocks of K23Z were propagated on G5 cells.
Plasmids and cosmids.
pRB208 has been described previously and contains the HSV-1 HindIII J fragment (genes UL14 to UL18) (6). SV2Neo contains the neomycin resistance gene under the control of the simian virus 40 (SV40) early promoter. To create a recombinant virus with a lesion in UL17, the lacZ gene driven by the SV40 promoter and terminated by the SV40 polyadenylation signal (kindly provided by Donald Holschu, Ohio University) was inserted into pRB457, a plasmid containing the UL17 gene and other DNA sequences between a BglII site near the 5′ end of the UL16 gene and a BamHI site in the second exon of UL15. The resulting plasmid, pJB67, contains a lacZ expression cassette in place of 1,490 bp of the 2,109-bp UL17 open reading frame. The lacZ cassette extends from a NotI site 105 bp from the 5′ end of UL17 to an XhoI site 516 bp from the 3′ end of UL17 and is transcribed in the opposite direction from UL17 transcription.
A bacterial cosmid (S.675) was constructed such that a DNA oligomer encoding termination codons in all three potential open reading frames and containing an XbaI site was inserted into the Sau3AI site at position 32718 in the HSV-1 genome (20), thus truncating the UL17 open reading frame from 703 to 261 codons. Insertion of the 16-bp DNA oligomer TAA TC TAG AT TAG ATC (stop codons underlined) and its complement was confirmed by sequencing. Other cosmids used for reconstitution of the HSV-1(17) viral genome have been described previously (14). The plasmid used to repair the lesion in HSV-1(UL17-stop) was designated pJB114 and contains 1,828 bp of HSV-1 DNA from a SacI site near the 5′ end of UL16 to a SacI site within the UL17 gene.
Electron microscopy.
Vero and clone G5 cells were infected with HSV-1(ΔUL17) and fixed in 2.5% glutaraldehyde in 0.07 M sodium cacodylate buffer (pH 7.4). The cells were embedded in Epon and prepared for electron microscopy essentially as described previously (12). Thin sections were viewed with a Phillips EM 201 electron microscope with an accelerated voltage of 80 kV and a 20-μm objective aperture. Vero and clone G5 cells were infected with HSV-1(UL17-stop) for 14 hours, washed twice in phosphate-buffered saline (PBS), and fixed in 3% gluteraldehyde in 100 mM sodium phosphate buffer. After 1 h, the fixative was removed and replaced with buffer containing 1% bovine serum albumin, and cells were embedded, sectioned, and stained for electron microscopic examination.
Capsid purification.
Vero cells in roller bottles were infected at a multiplicity of infection of 5.0 PFU per cell and incubated at 34°C for 20 h. Virus capsids were isolated and purified from nuclear lysates of infected cells on a 20 to 50% sucrose gradient, essentially as described previously (28). Following centrifugation, the capsid bands were pelleted and loaded onto a second gradient, collected, repelleted, and resuspended in TNE (0.5 M NaCl, 20 mM Tris-HCl [pH 7.4], 1 mM EDTA). Protein profiles were analyzed by separation on a denaturing polyacrylamide gel and viewed by silver stain (Bio-Rad).
Mass spectrometry.
Virions and light particles were purified from MeWo cells infected with HSV-1(17) or UL47− virus by centrifugation on 5 to 15% (wt/vol) Ficoll gradients as described previously (35). Virions or light particles were incubated on ice in 1% (vol/vol) Nonidet P-40 (NP-40) in PBS (140 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.4 mM KH2PO4 [pH 7.2]) for 15 min and subjected to centrifugation at 11,000 × g for 5 min. The supernatant (envelope fraction) was clarified, and the pellets (capsid-tegument or tegument fractions from NP-40-treated virions or light particles, respectively) were resuspended in 1% NP-40 in PBS by sonication, pelleted in a microcentrifuge for 5 min, and resuspended in PBS by sonication in a volume equal to that of the envelope fraction. Proteins were separated on denaturing polyacrylamide gels and were either stained with Coomassie blue or electrically transferred to a polyvinylidene difluoride membrane, stained with sulforhodamine, and digested with trypsin, as described previously (13, 24). The masses of resulting tryptic peptides were determined by a Finnigan Lasermat laser desorption mass spectrometer (15, 23). Peptide masses were compared by using the Massmap program (Thermo Bioanalysis Ltd.) with tryptic peptides predicted from version 14 of the National Center for Biotechnology Information Entrez database, which contains several large protein databases.
Production of UL17-specific polyclonal antiserum and immunoblotting.
The UL17 gene was inserted into pCDNA3 (Invitrogen) under transcriptional control of the human cytomegalovirus promoter. One hundred micrograms of the resulting plasmid, pJB66, was injected intramuscularly into a New Zealand White rabbit. The rabbit was boosted three subsequent times with 100 μg of plasmid each time. Antiserum was diluted 1:1,000 in PBS supplemented with 1% bovine serum albumin, and electrophoretically separated proteins transferred to nitrocellulose were probed as described previously (8). Bound antibody was localized by reaction with donkey alkaline phosphatase conjugate (obtained from Jackson Immunoresearch) followed by fixation of colored substrate as described by the manufacturer (Bio-Rad).
RESULTS
Construction of a cell line capable of rescuing UL17 mutants.
Because no virus bearing a mutation in the UL17 gene was available, we were unable to directly assess the ability of engineered cell lines to support replication of viral mutants bearing lethal mutations in UL17. We reasoned that cells capable of supporting growth of UL18 viral mutants and containing both the UL17 and UL18 genes might also express UL17 and thus support the replication of UL17 viral mutants. Therefore, plasmid DNAs from pRB208, which contains HSV-1 DNA sequences from UL14 to UL19, and pSV2Neo, which contains a gene encoding neomycin resistance, were cotransfected into rabbit skin cells. Cells expressing neomycin resistance were selected by growth in medium containing Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum and 50 μg of G418 per ml. Cells that were able to support the replication of K23Z, which has a lacZ cassette inserted into a truncated UL18 gene (16), were selected for further study and designated 171 cells.
Construction of a virus bearing a deletion in UL17.
The studies described herein were complicated by the fact that the UL17 gene normally lies within the UL15 intron. To ensure that introduced mutations affected UL17 solely and not UL15 RNA splicing, clone 171 cells were cotransfected with HSV-1(R7224) viral DNA and pJB67, which contains a lacZ expression cassette in the opposite orientation from UL17. The HSV-1(R7224) virus contains a cDNA copy of the complete UL15 gene in the native position of UL15 exon I, as well as a second copy of exon II in its native position; thus, UL17 mutations introduced into viral genomes by recombination with cotransfected pJB67 plasmid DNA should not interfere with expression of UL15 because they should lie outside the UL15 gene expressed as a complete cDNA. UL15.5, a gene contained within UL15 exon II and transcribed in the same direction as UL15, should also be expressed in such recombinant viruses because the cDNA of UL15 is sufficient for production of the UL15.5-encoded protein (4, 5) (Fig. 1).
FIG. 1.
Schematic representation of collinear HSV sequences relevant to the production and documentation of UL17 insertion and deletion viruses. (A) Line 1, representation of the HSV-1 genome (open rectangles represent inverted repeat regions flanking the UL and US components); line 2, schematic representation of sequences within the genome of recombinant virus R7224 relevant to these studies; lines 3 and 4, schematic representation of the construction of a plasmid containing the lacZ gene driven by an SV40 promoter in the reverse orientation from UL17 and the relevant sequences in the resulting recombinant HSV genome (filled rectangles, SV40 promoter; open rectangles, lacZ sequences); line 5, schematic representation of the BglII O and P fragments in HSV-1(R7224) and HSV-1(ΔUL17) DNAs, as shown in Fig. 2; line 6, schematic collinear diagram of the UL17 probe used in the experiment in Fig. 2. The probe contains both UL17 and UL15 exon II-specific sequences and hybridizes to both the BglII O fragment, which contains UL15 exon II-specific sequences, and the BglII P fragment, which contains both UL17-specific sequences and UL15 exon II-specific sequences. (B) Line 1, representation of the HSV-1(17) genome (open rectangles represent inverted repeat regions flanking the UL and US components); line 2, collinear representation of a set of cosmid DNAs cotransfected into cells for production of the HSV-1(UL17-stop) recombinant virus (the position of the oligomer inserted into UL17 encoding stop codons in all three potential open reading frames is indicated); line 3, schematic representation of sequences in the BglII P fragment of HSV-1 DNA (the arrows represent the direction and approximate lengths of the indicated open reading frames, and the position of the XbaI site within the DNA oligomer inserted within UL17 is indicated); line 4, collinear representation of relevant DNA sequences within the probe used in the experiment illustrated in Fig. 2; line 5, collinear representation of the BglII P fragment with the XbaI and BglII restriction enzyme sites which were used to generate the hybridizing band shown in Fig. 2; line 6, collinear representation of the HSV-1 DNA sequences used to restore the oligomer insertion to wild-type sequences.
Plaques induced by viral progeny of the cotransfection were overlayed with Dulbecco modified Eagle medium supplemented with 1.0% agarose, 1.0% newborn calf serum and 100 mg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) per ml. Plaques staining blue under the X-Gal overlay were plaque purified on 171 cells five times and then four subsequent times on G5 cells, which contain UL16 to UL21 sequences (16). This yielded a recombinant virus which grew to titers of 5.0 × 109 PFU/ml on complementing cells and less than 1.0 × 104 PFU/ml on noncomplementing cells. This virus, designated HSV-1(ΔUL17), was selected for growth of stock virus and was used for further studies.
Viral DNAs of HSV-1(F), HSV-1(R7224), and HSV-1(ΔUL17) were purified from infected cells and digested with BglII. The DNA fragments were electrophoretically separated on an agarose gel and transferred to nitrocellulose. The nitrocellulose was probed with radiolabeled HSV-1(F) DNA delimited by a BglII site near the 5′ end of UL16 and a BamHI site within UL15 exon II (pRB457). A schematic representation of the HSV DNA sequences contained in the probe is shown in Fig. 1A, line 6. The results, shown in Fig. 2, were as follows.
FIG. 2.
Scanned digital images of the autoradiographs of the electrophoretically separated viral DNAs. Lanes contain viral DNA purified from cells infected with the indicated viruses digested with BglII (lanes 1 to 3) and with BglII and XbaI (lanes 4 to 6) and probed with radiolabeled UL17 sequences (shown schematically in Fig. 1A, line 6, and Fig. 1B, line 4, respectively). The sizes (in kilobase pairs) of the DNA fragments are indicated to the right of each panel.
The pRB457 probe hybridized with the BglII P fragment of approximately 4.6 kbp in HSV-1(F) DNA (Fig. 2, lane 1) and in HSV-1(R7224) DNA (lane 2). The BglII P fragment contains the UL17 gene and should therefore hybridize with the pRB457 probe. The probe also recognized a band of approximately 5.2 kbp in HSV-1(R7224) DNA. A fragment of this size was expected to hybridize with UL15 exon II sequences because HSV-1(R7224) DNA contains such sequences within a UL15 cDNA inserted into the 4.2-kbp BglII O fragment (7).
In HSV-1(ΔUL17) DNA, the pRB457 probe recognized the 5.2-kbp DNA band also present in R7224 DNA but not the 4.6-kbp BglII P fragment. Instead, the probe hybridized to a novel band of approximately 7.5 kbp (Fig. 2, lane 3). A band electrophoretically indistinguishable from the 7.5-kbp band hybridized with the lacZ probe (data not shown).
We conclude that HSV-1(ΔUL17) DNA, like the HSV-1(R7224) virus from which it was derived, contains a cDNA copy of UL15 inserted into the position occupied by UL15 exon I in native viruses. We also conclude that this virus contains a lacZ expression cassette inserted into a truncated UL17 gene as designed in plasmid pJB67.
Construction of a virus with stop codons within the UL17 gene and a virus bearing a restored UL17 gene.
To ensure that any phenotype attributed to HSV-1(ΔUL17) was not a consequence of the presence of the cDNA copy of UL15 within the HSV-1(ΔUL17) genome, a second mutant was constructed with a lesion in UL17. To this end, a DNA oligomer containing stop codons in all three potential reading frames was inserted into the UL17 gene within a cosmid (cos 28) (see Materials and Methods), truncating the UL17-coding region from 703 to 260 codons. The cosmid was designated S.675. HSV-1 DNA inserts within this cosmid and four other cosmids [schematically represented in Fig. 1B and comprising the entire HSV-1(17) genome] were released by digestion with PacI and cotransfected into 171 cells. Viral progeny that arose by recombination between the cotransfected cosmid DNAs were plaque purified three times on clone 171 cells and twice on G5 cells. One virus, designated HSV-1(UL17-stop), was selected for further study.
To ensure that the phenotype attributed to HSV-1(UL17-stop) was due to the mutation in the UL17 gene, cells were (i) transfected with plasmid pJB114, delimited by a SacI site near the 5′ end of UL16 and a SacI site within UL17 (a schematic diagram of this fragment is shown in Fig. 1B, line 6), and (ii) infected with HSV-1(UL17-stop). Viral progeny were plaque purified five times on noncomplementing cells. One virus, expected to contain a UL17 gene restored by recombination with pJB114 plasmid DNA, was designated HSV-1(UL17-restored). Untransfected cells infected with HSV-1(UL17-stop) failed to produce viral progeny capable of plaque formation on noncomplementing cells. Thus, HSV-1(UL17-restored) arose from recombination between viral and plasmid DNA rather than reversion of the mutation in the UL17 gene of HSV-1(UL17-stop).
Viral DNAs were purified from HSV-1(F)-, HSV-1(UL17-stop)-, and HSV-1(UL17-restored)-infected cells, digested with BglII and XbaI, transferred to nitrocellulose, and probed with radiolabeled plasmid pRB457 DNA. A schematic diagram of DNA sequences within the probe is illustrated in Fig. 1B, line 4. As shown in Fig. 2, the introduction of the XbaI site into the UL17 gene of HSV-1(UL17-stop) genomic DNA caused a division of the 4.6-kbp BglII P fragment of HSV-1(F) DNA to 3.0 and 1.6 kbp upon digestion with BglII and XbaI (Fig. 2, lane 6). As expected, elimination of the XbaI site in HSV-1(UL17-restored) DNA caused the BglII P fragment to migrate at a position electrophoretically indistinguishable from that of the wild-type BglII P fragment (Fig. 2; compare lanes 4 and 5). These data indicate that HSV-1(UL17-stop) DNA contains a novel XbaI site within UL17; we therefore deduce that stop codons were inserted into UL17 as designed. Inasmuch as the HSV-1(UL17-restored) BglII P fragment was not cleaved upon digestion of HSV-1(UL17-restored) DNA with XbaI, we conclude that HSV-1(UL17-restored) bears a restored UL17 gene lacking the inserted oligomer.
UL17 mutants synthesize but do not cleave or package viral DNA.
To test the possibility that a lesion in UL17 would prevent cleavage and packaging of viral DNA, Vero cells and G5 cells were infected with either HSV-1(F), HSV-1(R7224), HSV-1(ΔUL17), HSV-1(UL17-stop), or HSV-1(UL17-restored). Viral DNAs were purified and were digested with BamHI. DNA fragments were then electrophoretically separated on an agarose gel, transferred to nitrocellulose, and probed with a radiolabeled HSV DNA containing the long terminal BamHI fragment of the genome (BamHI S). The results were as follows.
(i) As expected, the BamHI S probe recognized the BamHI S-P fragments of approximately 6.0 kbp in all of the tested viral DNAs. These fragments are present at the junctions of the long and short components within cleaved genomes and concatemeric viral DNA (32, 40) (Fig. 3, lanes 1 to 8).
FIG. 3.
Digitally scanned images of autoradiographs of electrophoretically separated viral DNA probed with end-specific sequences. Vero cells (or G5 cells where specified) were infected with the indicated viruses. Viral DNAs were purified, digested with BamHI, transferred to nitrocellulose, and hybridized with radiolabeled BamHI S DNA. The positions of the BamHI S fragments representing the termini of the long components in linear viral genomes and the S-P fragment derived from the junction of the long and short components in linear and concatemeric viral genomes are indicated.
(ii) BamHI digestion of viral DNAs purified from Vero cells infected with HSV-1(F), HSV-1(R7224), or HSV-1(UL17-restored) and viral DNAs purified from G5 cells infected with HSV-1(ΔUL17) or HSV-1(UL17-stop) produced several BamHI S fragments of around 3 to 4 kbp that hybridized with the end-specific probe. Multiple bands of approximately this size were expected to hybridize with the probe because the BamHI S fragment at the terminus of the long component of the HSV genome contains one or more copies of the approximately 400-bp a sequence (41). The presence of these end-specific DNA fragments indicated that all of the tested viruses cleaved concatameric DNA when propagated on Vero cells or G5 cells, respectively (Fig. 3, lanes 1 to 3 and 5 to 7).
(iii) BamHI S fragments were not detected in DNAs purified from Vero cells infected with HSV-1(ΔUL17) or HSV-1(UL17-stop) (Fig. 3, lanes 4 and 8). Thus, both viruses synthesized but did not cleave genomic viral DNA into unit-length molecules upon infection of noncomplementing Vero cells. In Vero cells infected with the HSV-1(UL17-restored) virus, however (Fig. 3, lane 7), end-specific BamHI S fragments were readily detected. Results similar to those shown were obtained upon analysis of cells infected with less than 1 PFU per cell with the various viruses; thus, varying the amount of input viral DNA did not alter the results (data not shown).
Since replicated DNA was readily detected in all of the above experiments but neither UL17 mutant produced cleaved genomic ends upon infection of noncomplementing Vero cells, we conclude that the UL17 gene is dispensable for DNA replication but is required for cleavage of unit-length genomes from concatameric viral DNA. Parenthetically, the G5 cell line does not contain an intact UL15 gene and does not support the replication of UL15 mutants (16). Thus, the inability of HSV-1(ΔUL17) and HSV-1(UL17-stop) mutants to produce unit-length genomes in G5 cells is not a consequence of an inadvertent mutation preventing UL15 gene expression or function. The observation that noncomplementing Vero cells infected with HSV-1(UL17-restored) derived from HSV-1(UL17-stop) but containing a restored UL17 gene contained readily detectable unit-length genomes further indicates that the mutation within UL17 is solely responsible for the restricted phenotype.
Capsids lacking DNA remain in the nuclei in cells infected with UL17 mutants.
Given the absence of detectable unit-length genomes in noncomplementing cells infected with UL17 mutants, it was of interest to determine the fate of capsids in cells infected with viruses lacking UL17. Vero cells or clone G5 cells were infected with HSV-1(ΔUL17) and HSV-1(UL17-stop) at 5.0 PFU per cell. At 14 h after infection, the cells were fixed and thin sections were prepared for electron microscopy. The results were as follows.
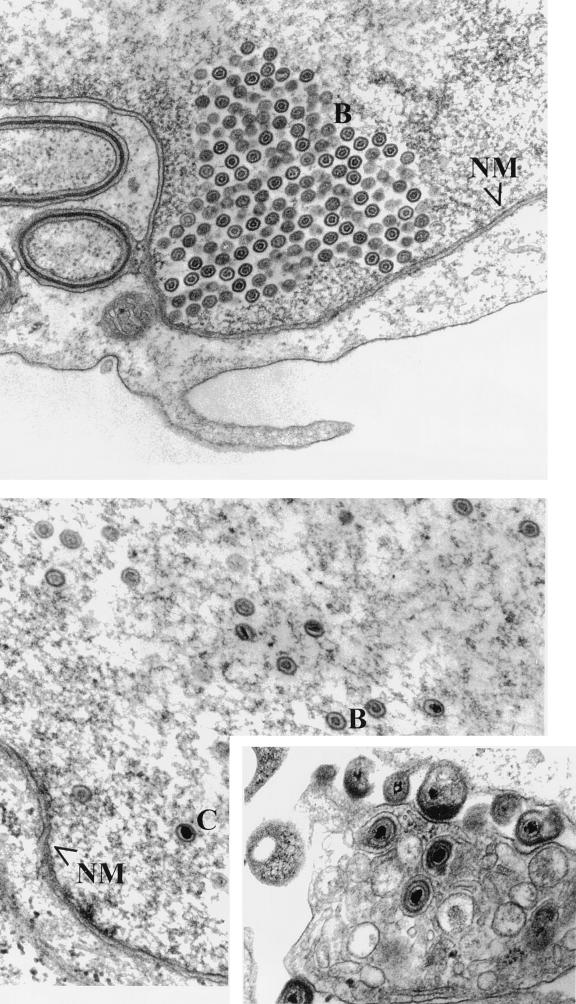
(i) In Vero cells infected with HSV-1(ΔUL17) and HSV-1(UL17-stop), enveloped particles were not detected, whereas capsids morphologically resembling type B capsids were present within infected cell nuclei (Fig. 4 and 5). The capsids contained an outer electron-dense shell surrounding an inner circular core that varied in diameter among different capsids. This observation suggested that the UL17 gene is dispensable for maturation of large-cored B capsids to small-cored capsids. Capsids were often observed in paracrystalline arrays near, but not abutting, the nuclear envelope. Such accumulations of capsids are sometimes detected late in infection in cells infected with wild-type virus (30). As might be expected under conditions in which genomic DNA is not cleaved from concatameric viral DNA, the absence of electron-dense cores in capsids within the nuclei of Vero cells infected with HSV-1(ΔUL17) or HSV-1(UL17-stop) is consistent with the conclusion that these capsids lacked packaged DNA.
FIG. 4.
Scanned digital images of electron micrographs. Vero (top) and G5 (bottom) cells were fixed 14 h after infection with HSV-1(ΔUL17). Thin sections were prepared and viewed with a Phillips EM 201 electron microscope. For size comparisons, HSV capsids are 120 nm in diameter. The left inset contains an image of the extracellular space of G5 cells infected with HSV-1(ΔUL17). RM delineates the reticular meshwork referred to in the text; NM delineates the nuclear membrane; A, B, and C indicate A, B, and C capsids, respectively.
FIG. 5.
Scanned digital images of electron micrographs. Vero (top) and G5 (bottom) cells were fixed 14 h after infection with HSV-1(UL17-stop). The inset shows an image of the cytoplasm and extracellular space of G5 cells infected with HSV-1(UL17-stop). NM delineates the nuclear membrane; B and C indicate B and C capsids, respectively.
One noteworthy feature of the appearance of Vero cells infected with the UL17 mutants was the lack of a reticular meshlike network of filaments near capsid aggregates. This network was readily detected in nuclear regions that lacked capsids and resembled intranuclear filaments that comprise the nuclear matrix (Fig. 4, top panel). It is not known whether the reticular meshwork was depolymerized in regions associated with capsids or was physically displaced from the plane of the section in these regions.
(ii) Type A, B, and C capsids were observed within the nuclei of G5 cells infected with HSV-1(ΔUL17) and HSV-1(UL17-stop). Type C capsids were also seen in the cytoplasm as enveloped particles. The appearance of these particles within G5-infected cells resembled that of cells infected with wild-type viruses and indicates that the inability of UL17 mutants to produce C capsids and virions was restored upon propagation in G5 cells containing the UL17 gene.
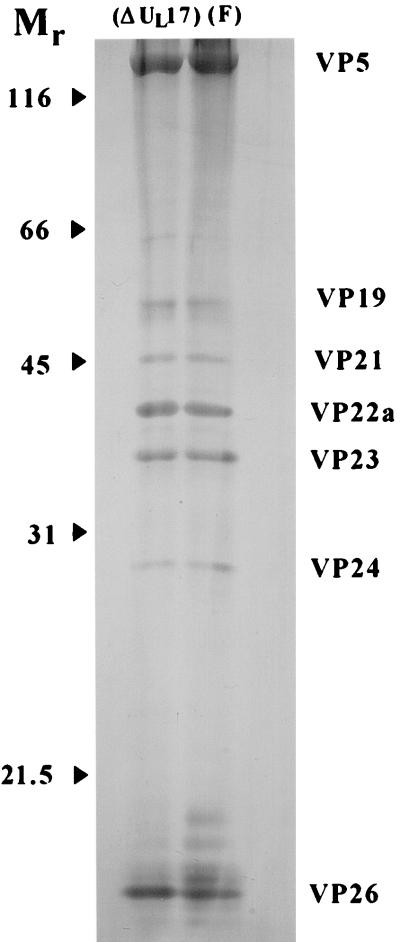
Electrophoretic profiles of capsids purified from HSV-1(ΔUL17)-infected cells are indistinguishable from those of wild-type capsids.
Vero cells were infected with HSV-1(F) or HSV-1(ΔUL17) at a multiplicity of infection of 5.0 PFU/cell. Twenty hours after infection, capsids were banded on two consecutive 20 to 50% sucrose gradients, pelleted, and lysed in sodium dodecyl sulfate-containing buffer (28). The denatured proteins were separated on a denaturing polyacrylamide gel and visualized by silver staining. The results, shown in Fig. 6, demonstrate that the electrophoretic profiles of proteins associated with capsids purified from HSV-1(ΔUL17)-infected cells appear virtually identical to those of HSV-1(F). These data, therefore, further indicate that the UL17 gene is dispensable for assembly of B-type capsids.
FIG. 6.
Scanned digital image of a denaturing polyacrylamide gel containing silver-stained capsid-associated proteins. Capsids were purified from cells infected with HSV-1(ΔUL17) or HSV-1(F). Positions of the major capsid proteins are indicated. Relative molecular weights are indicated in thousands.
Identification of the UL17 gene product in HSV-1-infected cells.
The UL17 gene was cloned into pCDNA3 (Invitrogen) under the control of the strong human cytomegalovirus promoter-enhancer, and a rabbit was immunized intramuscularly with purified plasmid DNA. To characterize the specificity of the putative anti-UL17 antisera, HEp-2 cells were mock infected or infected with 5.0 PFU of HSV-1(F) or HSV-1(ΔUL17) per cell. Twenty hours after infection, cells were lysed and proteins were electrophoretically separated on a denaturing polyacrylamide gel and transferred to nitrocellulose. The nitrocellulose was then reacted with the rabbit antiserum taken from the immunized rabbit, and bound antibody was visualized by the addition of alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin followed by the addition of chromogenic substrate. The results are shown in Fig. 7.
FIG. 7.
Scanned digital image of an immunoblot probed with UL17-specific antibody. Lanes 1 to 3, HEp-2 cells mock infected or infected with the indicated virus; lane 4, virions purified from cells infected with HSV-1(F). The proteins in lysates were electrophoretically separated, transferred to nitrocellulose, and probed with the antibody directed against UL17. The apparent Mr of the proteins reacting with the antibody are indicated.
Proteins of approximate Mr 85,000 and 82,000 were recognized by the polyclonal anti-UL17 antiserum in lanes containing lysates of mock-infected and HSV-1(F)- and HSV-1(ΔUL17)-infected HEp-2 cells. The presence of these bands in all tested cell lysates indicates that a host cell protein, upon denaturation in sodium dodecyl sulfate contains epitopes that are recognized by the rabbit antiserum. The antiserum also reacted strongly with a protein of apparent Mr 77,000 (Fig. 7, lane 2) and weakly with a protein of apparent Mr 72,000 in lanes containing HSV-1(F)-infected cell lysates (data not shown). Bands corresponding to these proteins were not apparent in lanes containing lysates of mock-infected or HSV-1(ΔUL17)-infected cells (Fig. 7, lanes 1 and 3). Because these proteins were detectable only in lysates of cells infected with wild-type virus containing an intact UL17 gene, we conclude that the antiserum recognizes two products of the UL17 open reading frame of apparent Mr 77,000 and 72,000.
The UL17 gene products are virion components.
To determine whether the UL17-encoded proteins were components of virions, two separate approaches were taken. In the first approach, Vero cells were infected with 3.0 PFU of HSV-1(F) and virions were purified as described previously (8, 34). Virion-associated polypeptides were separated on a denaturing polyacrylamide gel, transferred to nitrocellulose, and reacted with the UL17-specific antiserum. The results (Fig. 7, lane 4) indicated that the host protein of apparent Mr 85,000 recognized by the rabbit polyclonal antibody was not detectable in preparations of purified virions, whereas the UL17-specific protein band of apparent Mr 77,000 was readily detected.
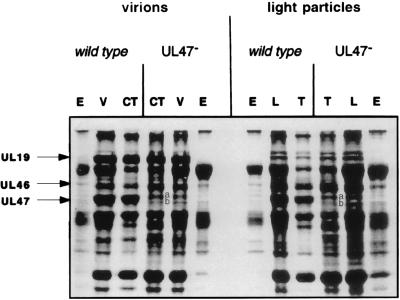
In the second approach, initial attempts to visualize a UL17-specific protein band in stained, electrophoretically separated virion polypeptides were unsuccessful due to the presence of the abundant, tegument-associated UL47 gene product that migrated in denaturing gels at a position expected to contain UL17-encoded proteins (Fig. 8). To overcome this difficulty, virions were purified from cells infected with a virus containing a mutation within the UL47 gene (UL47−) and the associated polypeptides were electrophoretically separated and stained with Coomassie blue. It was apparent that a major band corresponding to the UL47 gene product, present in wild-type virus-infected cells, was absent from protein profiles of virions purified from cells infected with the UL47− virus (compare lanes entitled V and L in wild-type and UL47− lanes [Fig. 8]). The absence of this band enabled the detection of two minor bands, designated a and b (Fig. 8), of apparent Mr 77,000 and 72,000, respectively. Of the two bands, the band of apparent Mr 77,000 was more readily detected.
FIG. 8.
Digitally scanned image of a denaturing polyacrylamide gel containing Coomassie blue-stained virion- and light particle-associated proteins. Virions (V) and light particles (L) were purified from cells infected with HSV-1(17) (wild type) or a virus lacking the UL47 gene (UL47−). Electrophoretic profiles of proteins associated with envelope (E), capsid-tegument (CT), or tegument (T) fractions are also shown. The position of the UL19 major capsid protein is indicated to show its presence in greatly reduced amounts in light particles which lack capsids; the small amount present likely originated from contaminating virions. Positions of the UL46 and UL47 proteins and bands a and b (containing products of the UL17 gene, as discussed in the text) are also indicated.
To localize the apparent Mr 77,000 and 72,000 proteins within virions, wild-type and UL47− virions were purified and treated with NP-40 to solubilize the virion envelope, associated integral membrane proteins, and some tegument proteins. Capsids with some associated tegument proteins were then pelleted by centrifugation. The soluble fraction was designated envelope (lanes E in Fig. 8), and pelleted fractions were designated capsid-tegument (lanes CT in Fig. 8). Light particles, containing tegument proteins but lacking capsid proteins, were also purified from cells infected with wild-type and UL47− viruses and treated with NP-40, and the tegument fractions were pelleted by centrifugation. Proteins associated with the various fractions of virions and light particles were then electrophoretically separated and stained with Coomassie blue.
The results indicated that proteins of apparent Mr 77,000 and 72,000 were visible in electrophoretic profiles of light particles and the capsid-tegument fractions of virions purified from UL47− virus-infected cells. These are designated bands a and b, respectively (Fig. 8). To confirm that band a contained UL17 protein, mass spectrometric analysis of 25 peptides produced by tryptic digestion of band a from the capsid-tegument fraction of UL47− virions was performed. Eleven peptides were characteristic of the UL46 protein; the major forms of this protein migrated more slowly than band a (as shown in Fig. 8). When the masses of the remaining 14 peptides (786.71, 1,046.2, 1,209.8, 1,255.0, 1,332.8, 1,400.0, 1,429.1, 1,490.1, 1,671.4, 1,705.6, 1,987.1, 2,420.7, 2,937.5, and 3,011.3 Da) were compared with those of tryptic peptides predicted from all proteins in the Entrez database with masses of 50,000 to 100,000 Da, the UL17 protein was the best match. Whether a peptide mass error of 3, 4, or 5 Da was allowed, the UL17 protein scored highest, with 10, 12, and 14 matches, respectively (some measured masses matched more than one predicted peptide). Band a, from the tegument fraction of light particles, yielded the same mass spectrum. Although the total number of detected peptide masses was less because of the smaller amount of material in band b, peptides from this band in the capsid-tegument fraction of virions and the tegument fraction of light particles exhibited similar spectra, characteristic of the presence of the UL17 and UL46 proteins.
Taken together, these data indicate that bands a and b consist of a mixture of the UL17 protein and minor forms of the UL46 protein and that the UL17 proteins are minor components of the virion tegument.
DISCUSSION
These studies indicate that truncation of the HSV-1 UL17 gene, whether by insertion of a lacZ expression cassette or insertion of a stop codon, eliminates viral DNA cleavage and packaging. Restoration of the wild-type phenotype upon recombination of the mutant UL17 gene in HSV-1(UL17-stop) DNA with wild-type UL17 sequences confirmed that the mutation in the UL17 gene of HSV-1(UL17-stop) was responsible for the null phenotype. Although DNA used for this marker rescue experiment also contained 198 bp of the UL16 open reading frame, recombination with UL16 sequences is unlikely to restore mutations that prevent DNA cleavage and packaging because the UL16 gene is dispensable for replication of HSV-1 (and hence, cleavage and packaging of viral DNA) in Vero cells (6). We therefore deduce that UL17 joins a group of genes including UL6, UL15, UL28, UL32, and UL33 that are dispensable for assembly of type B-like capsids but are required for cleavage of concatameric viral DNA (2–4, 14, 26, 33, 37, 42, 44).
A rabbit antiserum directed against a plasmid expression vector containing the UL17 gene recognized proteins of approximate Mr 77,000 and 72,000, which are close to the molecular weight (74,577) predicted from the amino acid sequence of the UL17 open reading frame (20). These proteins were not detected in lysates of mock-infected cells or cells infected with the UL17 deletion virus, indicating that they are products of the UL17 gene. Furthermore, bands of similar electrophoretic mobility were detectable in electrophoretically separated proteins from virions and light particles and yielded tryptic peptides predicted of the UL17 protein. Because light particles contain a variety of membrane- and tegument-associated proteins but lack capsid-associated proteins (35), these data imply that UL17 proteins are not integral components of capsids. In support of this conclusion, attempts to detect the UL17 gene products in immunoblots of polypeptides associated with highly purified B capsid have not been successful (data not shown). In conjunction with the detection of UL17 proteins in fractions of virions and light particles devoid of envelopes, these observations indicate that UL17-encoded proteins reside within the of virion tegument.
The UL17 proteins are the first herpesvirus tegument-associated proteins shown to be required for cleavage and packaging of viral DNA. The observations that at least UL6 and UL15 encode minor capsid components required for DNA cleavage and packaging suggest that these proteins and UL17 play distinct roles in the cleavage-packaging reaction (25, 31).
ACKNOWLEDGMENTS
We thank Stan Person for the UL18 deletion virus and the G5 transformed cells necessary for its propagation and Jarek Okulicz-Kozaryn for excellent technical assistance. We also thank Paul Webster, of the Center for Cell Imaging at Yale University, and the Center for Integrated Microscopy at Cornell University for production of the HSV-1(UL17-stop) and HSV-1(ΔUL17) electron micrographs, respectively.
The studies at Cornell University were supported by grant R01 GM-50740 from the National Institutes of Health.
REFERENCES
- 1.Addison C, Rixon F J, Palfreyman J W, O’Hara M, Preston V G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984;138:246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- 2.Addison C, Rixon F J, Preston V G. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J Gen Virol. 1990;71:2377–2384. doi: 10.1099/0022-1317-71-10-2377. [DOI] [PubMed] [Google Scholar]
- 3.Al-Kobashi M F, Rixon F J, McDougall I, Preston V G. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology. 1991;180:380–388. doi: 10.1016/0042-6822(91)90043-b. [DOI] [PubMed] [Google Scholar]
- 4.Baines J D, Cunningham C, Nalwanga D, Davison A J. The UL15 gene of herpes simplex virus type 1 contains within its second exon a novel open reading frame that is translated in frame with the UL15 gene product. J Virol. 1997;71:2666–2673. doi: 10.1128/jvi.71.4.2666-2673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines J D, Poon A P W, Rovnak J, Roizman B. The UL15 gene of herpes simplex virus encodes two proteins and is required for cleavage of viral DNA. J Virol. 1994;68:8118–8124. doi: 10.1128/jvi.68.12.8118-8124.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines J D, Roizman B. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J Virol. 1991;65:938–944. doi: 10.1128/jvi.65.2.938-944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baines J D, Roizman B. The cDNA of UL15, a highly conserved herpes simplex virus 1 gene, effectively replaces the two exons of the wild-type virus. J Virol. 1992;66:5621–5626. doi: 10.1128/jvi.66.9.5621-5626.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baines J D, Roizman B. The UL10 gene of herpes simplex virus 1 encodes a novel glycoprotein, gM, which is present in the virion and in the plasma membrane of infected cells. J Virol. 1993;67:1441–1452. doi: 10.1128/jvi.67.3.1441-1452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baines J D, Koyama A H, Huang T, Roizman B. The UL21 gene products of herpes simplex virus 1 are dispensable for growth in cultured cells. J Virol. 1994;68:2929–2936. doi: 10.1128/jvi.68.5.2929-2936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bean M A, Bloom B R, Heberman R B, Old L J, Oettgen H F, Klein G, Terry W D. Cell-mediated cytotoxicity for bladder carcinoma: evaluation of workshop. Cancer Res. 1975;35:2902–2913. [PubMed] [Google Scholar]
- 11.Brown S M, Ritchie D A, Subak-Sharpe J H. Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J Gen Virol. 1973;18:329–346. doi: 10.1099/0022-1317-18-3-329. [DOI] [PubMed] [Google Scholar]
- 12.Campadelli-Fiume G, Farabegoli F, Gaeta S D, Roizman B. Origin of unenveloped capsids in the cytoplasm of cells infected with herpes simplex virus 1. J Virol. 1991;65:1589–1595. doi: 10.1128/jvi.65.3.1589-1595.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coull J M, Pappin D J C. A rapid fluorescent staining procedure for proteins electroblotted on PVDF membranes. J Protein Chem. 1990;9:259–260. [Google Scholar]
- 14.Cunningham C, Davison A J. A cosmid-based system for constructing mutants of herpes simplex virus type 1. Virology. 1993;197:116–124. doi: 10.1006/viro.1993.1572. [DOI] [PubMed] [Google Scholar]
- 15.Davison A J, Davison M D. Identification of structural proteins of channel catfish virus by mass spectrometry. Virology. 1995;206:1035–1043. doi: 10.1006/viro.1995.1026. [DOI] [PubMed] [Google Scholar]
- 16.Desai P, DeLuca N A, Glorioso J C, Person S. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J Virol. 1993;67:1357–1364. doi: 10.1128/jvi.67.3.1357-1364.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ejercito P M, Kieff E D, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behavior of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 18.Gibson W, Roizman B. Proteins specified by herpes simplex virus. VIII. Characterization and composition of multiple capsid forms of subtypes 1 and 2. J Virol. 1972;10:1044–1052. doi: 10.1128/jvi.10.5.1044-1052.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J Y, Irmiere A, Gibson W. Primate cytomegalovirus assembly: evidence that DNA packaging occurs subsequently to B capsid assembly. Virology. 1988;167:87–96. doi: 10.1016/0042-6822(88)90057-8. [DOI] [PubMed] [Google Scholar]
- 20.McGeoch D J, Dalrymple M A, Davison A J, Dolan A, Frame M C, McNab D, Perry L J, Scott J E, Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988;69:1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- 21.Nalwanga D, Rempel S, Roizman B, Baines J D. The UL16 gene product of herpes simplex virus is a virion protein that colocalizes with intranuclear capsid proteins. Virology. 1996;226:236–242. doi: 10.1006/viro.1996.0651. [DOI] [PubMed] [Google Scholar]
- 22.Newcomb W W, Homa F L, Thomsen D R, Booy F P, Trus B L, Steven A C, Spencer J V, Brown J C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid formation. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 23.Pappin D J C, Hojrup P, Bleasby A J. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- 24.Pappin D J C, Rahman D, Hansen H F, Jeffrey W, Sutton C W. Peptide-mass fingerprinting as a tool for the rapid identification and mapping of cellular proteins. In: Atassi M, Apella E, editors. Methods in protein structural analysis. New York, N.Y: Plenum Press; 1995. pp. 161–173. [Google Scholar]
- 25.Patel A H, Maclean J B. The product of the UL6 gene of herpes simplex virus type 1 is associated with virus capsids. Virology. 1995;206:465–478. doi: 10.1016/s0042-6822(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 26.Patel A H, Rixon F J, Cunningham C, Davison A J. Isolation and characterization of herpes simplex virus type 1 mutants defective in the UL6 gene. Virology. 1996;217:111–123. doi: 10.1006/viro.1996.0098. [DOI] [PubMed] [Google Scholar]
- 27.Perdue M L, Cohen J C, Kemp M C, Randall C C, O’Callaghan D J. Characterization of three species of nucleocapsids of equine herpesvirus type 1 (EHV-1) Virology. 1975;64:187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- 28.Perdue M L, Kemp M C, Randall C C, O’Callaghan D J. Studies of the molecular anatomy of the L-M cell strain of equine herpes virus type 1: proteins of the nucleocapsid and intact virion. Virology. 1974;59:201–216. doi: 10.1016/0042-6822(74)90216-5. [DOI] [PubMed] [Google Scholar]
- 29.Roizman B, Furlong D. The replication of herpesviruses. In: Fraenkel-Conrat H, Wagner R R, editors. Comprehensive virology. New York, N.Y: Plenum Press; 1974. pp. 229–403. [Google Scholar]
- 30.Roizman B, Sears A. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 31.Salmon B, Baines J D. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of UL15-encoded proteins with B capsids requires at least the UL6, UL17, and UL28 genes. J Virol. 1998;72:3045–3050. doi: 10.1128/jvi.72.4.3045-3050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheldrick P, Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harbor Symp Quant Biol. 1975;39:667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- 33.Sherman G, Bachenheimer S L. DNA processing in temperature-sensitive morphogenetic mutants of HSV-1. Virology. 1987;158:427–430. doi: 10.1016/0042-6822(87)90214-5. [DOI] [PubMed] [Google Scholar]
- 34.Spear P G, Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972;9:143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szilagyi J F, Cunningham C. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J Gen Virol. 1991;72:661–668. doi: 10.1099/0022-1317-72-3-661. [DOI] [PubMed] [Google Scholar]
- 36.Tatman J D, Preston V G, Nicholson P, Elliott R M, Rixon F J. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J Gen Virol. 1994;75:1101–1113. doi: 10.1099/0022-1317-75-5-1101. [DOI] [PubMed] [Google Scholar]
- 37.Tengelsen L A, Pedersen N E, Shaver P R, Wathen M W, Homa F L. Herpes simplex virus type 1 DNA cleavage and capsidation require the product of the UL28 gene: isolation and characterization of two UL28 deletion mutants. J Virol. 1993;67:3470–3480. doi: 10.1128/jvi.67.6.3470-3480.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomsen D R, Roof L L, Homa F L. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994;68:2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19C and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 40.Wadsworth S, Jacob R J, Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975;15:1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner M J, Summers W C. Structures of the joint region and the termini of the DNA of herpes simplex virus type 1. J Virol. 1978;27:374–387. doi: 10.1128/jvi.27.2.374-387.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weller S K, Carmichael E P, Aschman D P, Goldstein D J, Schaffer P A. Genetic and phenotypic characterization of mutants in four essential genes that map to the left half of HSV-1 UL DNA. Virology. 1987;161:198–210. doi: 10.1016/0042-6822(87)90186-3. [DOI] [PubMed] [Google Scholar]
- 43.Wilson D W, Church G A. Study of herpes simplex virus maturation during a synchronous wave of assembly. J Virol. 1997;71:3603–3612. doi: 10.1128/jvi.71.5.3603-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu D, Shaeffer A K, Tenney D J, Weller S K. Characterization of ICP6::lacZ insertion mutants of the UL15 gene of herpes simplex virus type 1 reveals the translation of two proteins. J Virol. 1997;71:2656–2665. doi: 10.1128/jvi.71.4.2656-2665.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]