Abstract
Background and Purpose
The hypofluorescence of fundus lesions observed during the late phase of indocyanine green angiography (ICGA) in various diseases has often been overlooked or misinterpreted. This article explores the significance of fundus lesions that are initially isofluorescent during the early phase of ICGA but become hypofluorescent later in the examination.
Findings
Pathologies such as multiple evanescent white spot syndrome, acute posterior placoid syphilitic chorioretinitis, chronic central serous chorioretinopathy, choroidal hemangioma, and some fundus with drusen, present this phenomenon of late hypofluorescence.
Interpretation
The interpretation of ICGA images and the role of indocyanine green (ICG) uptake by the retinal pigment epithelium (RPE) in late fundus fluorescence is debated. Experimental evidence suggests that ICG accumulates progressively in the RPE after intravenous injection of the dye or after direct contact in vitro, making it a potential marker of RPE activity. Although the exact mechanisms of ICG diffusion through the choroid and its binding to the RPE require further investigation, the late hypofluorescence observed in certain ICGA diseases provides information on different modalities of RPE dysfunction.
Financial Disclosure(s)
The author has no proprietary or commercial interest in any materials discussed in this article.
Keywords: Hypofluorescence, Indocyanine green angiography, Retinal pigment epithelium
The significance of the hypofluorescence of fundus lesions appearing only during the very late phase of indocyanine green angiography (LP-ICGA) in various diseases has often been overlooked or misinterpreted.
Lesions that are hypofluorescent during the early phase of angiography and remain hypofluorescent throughout the sequence can be ascribed to a choroidal perfusion defect.
However, this interpretation does not apply to fundus lesions that are isofluorescent during the early phase of indocyanine green angiography (ICGA) and only become hypofluorescent later during the examination. This is the case in very different diseases such as multiple evanescent white dot syndrome (MEWDS), acute syphilitic posterior placoid chorioretinitis (ASPPC), chronic central serous chorioretinopathy (CSCR), and choroidal hemangiomas.
The use of ICGA was introduced in clinical practice in the 1970s to image the choroidal circulation because the near-infrared light used to stimulate its fluorescence penetrates, better than the blue light, through the retinal pigment epithelium (RPE) and choroidal pigment. The fact that the fluorophore did not freely leak out of the choriocapillaris allowed visualization of the main choroidal vessels, which was considered important; in contrast, fluorescein sodium leaks freely, masking the outer choroid (for a review of the basic properties of indocyanine green [ICG] and the historical contributions of ICGA, see Flower et al1).
Scanning laser ophthalmoscopy has improved the acquisition speed of fundus images and their resolution, making ICGA a routine examination. More recently, the use of scanning laser ophthalmoscopy with ultrawidefield ICGA has enhanced its value.
Indocyanine green angiography was initially used to improve the detection of choroidal new vessels (CNVs), in particular type 1 CNVs and polypoidal choroidal vasculopathy. In comparison, its use to diagnose choroidal hemangiomas, a choroidal inflammatory disease, or CSCR was marginal.2
As OCT has become the first-line method for the diagnosis of CNV and even polypoidal choroidal vasculopathy, the value of ICGA in age-related macular degeneration (AMD) has been reduced. However, it is regaining increasing interest in inflammatory and infectious fundus diseases, CSCR and pachychoroid, various rare diseases, and is still useful for diagnosing choroidal hemangioma.3
The interpretation of ICGA images is mainly focused on the presence of an early or late hyperfluorescence. The presence of an early hypofluorescence has been considered a good sign of choriocapillaris hypoperfusion. Here, we discussed the interpretation of fundus lesions that are specifically hypofluorescent only on LP-ICGA in various diseases.
For example, acute fundus diseases such as MEWDS and ASPPC are characterized by an ICGA sequence with unremarkable fundus fluorescence during the first few minutes after intravenous dye injection, but the fundus lesions become progressively hypofluorescent compared with the surrounding fundus, with contrast peaking after 30 minutes. In some chronic or long-standing diseases such as CSCR or choroidal hemangioma, the lesion is hyperfluorescent during the middle phase of ICGA but becomes progressively hypofluorescent. Finally, in some cases of intermediate AMD with drusen, the posterior pole becomes progressively hypofluorescent on LP-ICGA.
This occurrence of a late hypofluorescence on ICGA in such disparate diseases is intriguing, given that an early fluorescence is normal, and no consensus explanation has been proposed. The aim of this article was to try to elucidate the pathogenesis of this late hypofluorescence.
ICG Uptake by the RPE
Indocyanine green internalization by RPE cells has been investigated in vivo in human and monkey eyes by Chang et al in 1998.4 Frozen histological samples were examined under an x-ray fluorescence microscope with a barrier filter to record ICG fluorescence. A human eye enucleated for melanoma 40 minutes after intravenous injection of ICG showed a strongly fluorescent RPE–Bruch’s membrane complex. Three monkey eyes were also studied histologically 7, 15, and 50 minutes after ICG injection.
During the early phase, the lumen of the retinal and choroidal vessels was fluorescent, and the vascular endothelium was strongly fluorescent. Bruch’s membrane and the basal surface of the RPE were brightly fluorescent, whereas the fluorescence did not reach the apical pole of RPE cells. A faint fluorescence was also seen in the extravascular choroidal stroma. During the middle phase, the Bruch’s membrane–RPE complex was uniformly fluorescent. During the late phase, the Bruch’s membrane–RPE complex was brightly fluorescent, whereas no fluorescence was seen in the vascular lumen, and only a faint fluorescence was observed in the vascular endothelium or choroidal stroma.
The authors concluded for the first time that ICG accumulation in the RPE contributes to the late fluorescence of the fundus seen on ICGA.
In another publication, a CNV sample surgically excised from the subretinal space was studied after intravenous injection of ICG. Only the RPE was strongly fluorescent, whereas it was not the case for the new vessels.5
Pankova et al6 studied rat fundus fluorescence and the histological location of ICG between 2 and 28 days after its intravenous injection. They noted that the fundus remained fluorescent for up to 28 days, and that the higher the initial dose of ICG used for angiography was, the brighter the fluorescent signal was. On histology, the RPE layer was strongly fluorescent. Interestingly, when sodium iodate (NaIO3), known to be toxic for RPE cells, was injected before ICG injection, no fundus fluorescence appeared. The authors hypothesized that the RPE fluorescence seen on LP-ICGA could be due to the binding of the dye to lipoproteins and its uptake by lipoprotein receptors at the basolateral surface of the RPE.
Tam et al7 have used noninvasive adaptive optics enhanced ICG ophthalmoscopy to localize ICG fluorescence in human RPE cells in vivo. They observed fluorescent hexagonal structures 20 to 120 minutes after intravenous injection of ICG, consistent with the expected size of RPE cells. Adaptive optics dark-field imaging confirmed that these cells were indeed RPE cells (Fig 1). This imaging technique has also been used in mice, in which the histology confirmed that ICG accumulates in the RPE after systemic injection.
Figure 1.
In vivo imaging of the retinal pigment epithelium (RPE) using adaptive optics enhanced indocyanine green (ICG) ophthalmoscopy. The same region was imaged at 2 different times after intravenous injection of ICG dye (20 and 120 minutes, respectively), using different fields of view, showing the mosaic formed by the individual fluorescence of the RPE cells. Dye uptake was stable and lasted ≥ 120 minutes. Scale bar: 50 μm. Images courtesy of Tam et al.7
The affinity of ICG for RPE cells has also been confirmed ex vivo. Chang et al8 incubated cultured human RPE cells with ICG and showed that they were fluorescent by infrared fluorescence microscopy. Pankova et al6 incubated fresh RPE monolayers with ICG ex vivo and showed ICG internalization by RPE cells. Interestingly, after its permeabilization by sodium iodate, the RPE released ICG into solution and reverted to colorless.
Thus, a large body of experimental data obtained in humans and animals supports the fact that the RPE progressively internalizes ICG after intravenous injection. The RPE staining persists for several days to weeks. Histological findings confirmed a specific RPE staining, whereas ICG rapidly disappears from the choroidal circulation and stroma. Ex vivo, the RPE staining in culture identified some mechanisms underlying the entry of ICG into RPE cells.
Indocyanine green is a large molecule with a molecular weight of 775 daltons that emits fluorescence when excited at 790 to 805 nm. It is an amphiphilic molecule that binds strongly to high-density lipoproteins (HDLs) but moderately to low-density lipoproteins (LDLs). Indocyanine green does not bind to esterified cholesterol and unesterified cholesterol, or triacylglycerol.9 It was initially thought that ICG bound to serum albumin.10 Subsequently, Yoneya et al9 confirmed a previous work showing that 80% of ICG was bound to lipoproteins, and only 20% to albumin, in the blood;11 they also showed that ICG bound strongly to HDL and moderately to LDL, with phospholipids being the binding site, in the blood.
Circulating LDLs are internalized by the RPE. It has been shown that HDLs and LDLs enter the basolateral surface of the RPE via the class B scavenger receptors and low-density lipoprotein receptor at the RPE plasma membrane.12 When ICG is bound to lipoproteins, it is likely that it is internalized in the RPE together with these lipoproteins. It could be assumed that ICGA allows monitoring of the intracellular trafficking of albumin and HDL, which are the 2 main proteins bound to ICG. Retinal pigment epithelium cells could internalize HDL through vesicular transports after binding to class B scavenger receptors, which is a mechanism to deliver xanthophylls to the retina.13 Plasma lipoproteins that deliver lipophilic essentials, including vitamins E and A, lutein, and unesterified cholesterol, enter the basolateral RPE through caveolin-mediated transcytosis because caveolin-1 is highly expressed by RPE cells.14 Moreover, Tserentsoodol et al15 have observed fluorescently labeled LDL and HDL in the photoreceptor outer segments 4 hours after intravenous injection in rats. Thus, the failure of the RPE to uptake ICG in some pathological conditions could be a marker of RPE dysfunction.
This knowledge about the physical properties and diffusion of ICG through the choroid, and its binding to the RPE during angiography in normal eyes, could help to better understand the hypofluorescence occurring during the late phase of ICGA in various diseases. Thus, it is possible to revisit the significance of the late hypofluorescence seen on ICGA based on these experimental and biochemical findings.
MEWDS
Multiple evanescent white dot syndrome was first described by Lee Jampol et al16 in 1984 as the presence of multiple white dots at the RPE or the deep retina, with a markedly abnormal cone and rod function early in the course of the disease. Indocyanine green angiography findings were first reported by Ie et al17 who reported a hypofluorescence of the spots, which appeared about 10 minutes after ICG injection and persisted throughout the 40-minute phase. They noted the discrepancy between the absence of a hypofluorescence on fluorescein angiography (FA) and the presence of a hypofluorescence on ICGA, as well as the unexpected delay in the onset of this hypofluorescence. Nevertheless, they concluded that these hypofluorescent spots represented inflammatory lesions in the choroid. Since this initial observation, the interpretation of the hypofluorescence of MEWDS spots on LP-ICGA has alternated between a choriocapillaris nonperfusion and the primary involvement of the RPE and photoreceptors.
Until 2006, publications based on ICGA have supported the explanation that these hypofluorescent dots were due to a choroidal hypoperfusion,17, 18, 19, 20 or to an initial RPE impairment with secondary choriocapillaris hypoperfusion.21,22
In 2008, Sikorski et al23 were the first to suggest that MEWDS lesions did not represent inflammatory choroidal lesions, but instead an alteration of the RPE-photoreceptor complex, based on multimodal imaging, including FA, ICGA, and spectral-domain OCT. A well-argued study by Pichi et al24 in 2016, based on multimodal imaging, including OCT angiography, came to the same conclusion. The authors stressed that the hypofluorescence seen on LP-ICGA was difficult to explain given the normal appearance of the choriocapillaris on OCT angiography. They suggested that MEWDS could be a “photoreceptoritis,” with secondary involvement of the RPE, resulting in a deficit in ICG uptake by RPE cells that accounted for the hypofluorescent spots. The absence of choriocapillaris nonperfusion on OCT angiography was confirmed by others.25, 26, 27, 28 Gaudric and Mrejen29 compared fluorescein and ICG dynamics to OCT findings in acute posterior multifocal placoid epitheliopathy and MEWDS and came to the conclusion that MEWDS is a primary pigment epitheliopathy, which appears as a reversible, nondestructive dysfunction of the RPE. The dark spots seen on LP-ICGA could be due to the lack of internalization of ICG by the RPE from the choroid at the dots, this type of dysfunction being detrimental to the photoreceptors (Fig 2).
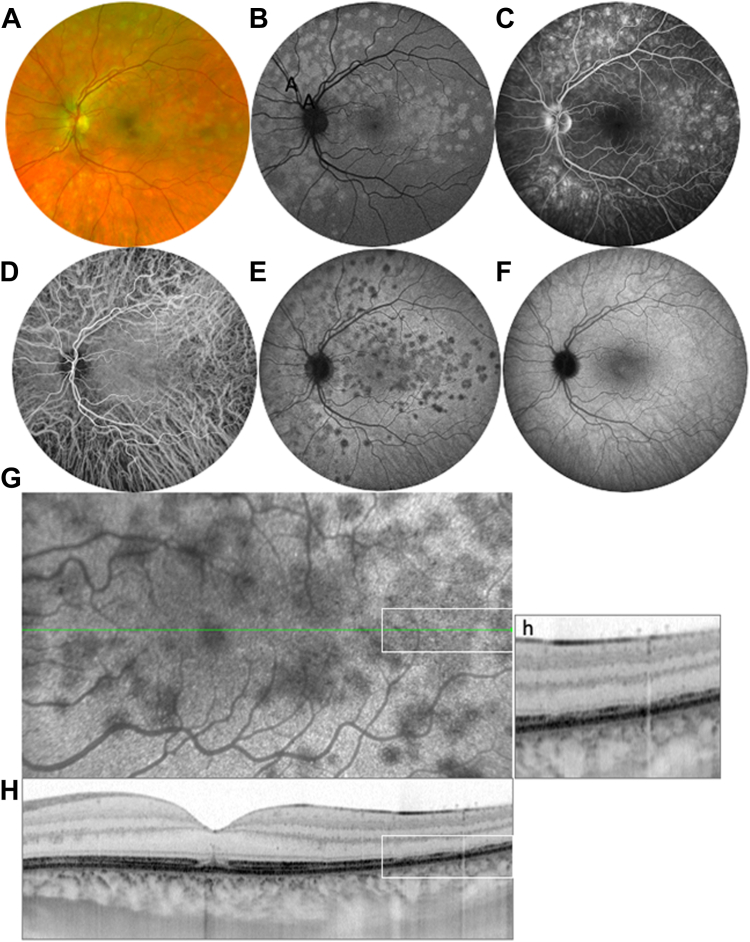
Figure 2.
Multiple evanescent white dot syndrome. A, Color photography showing the white dots. B, Blue fundus autofluorescence showing the autofluorescence of the dots, corresponding to the attenuation or loss of the photoreceptor (PR) outer segments shown in (H). C, Midphase fluorescein angiography by hypertransmission through altered outer segments. D, Normal early indocyanine green angiography (ICGA). E, Late-phase (LP-ICGA, 34 minutes) showing the hypofluorescence of the white dots. F, Three months later, normalization of the late phase of ICGA, without retinal pigment epithelium (RPE) scarring. G, H, Correlation between the dark dots seen on LP-ICGA (G) and on the OCT B-scan. In the foveal center, interruption of the ellipsoid zone (EZ) and interdigitation zone (IZ), and vertical linear hyperreflectivity in the outer nuclear layer (arrow), corresponding to a dark spot seen on LP-ICGA in G. h, Magnification of a detail of the OCT B-scan corresponding to the white inserts seen in G and H, showing the dislocation of the EZ and the loss of the IZ in the areas of dark dots seen on ICGA. Acute RPE dysfunction results in visible disturbances of the PR on OCT, while the RPE appears normal, although dysfunctional, on ICGA. It cannot be excluded that photoreceptors are primarily impaired, and they are indeed altered on OCT, but the typical hypofluorescence of the dots on LP-ICGA implies an RPE impairment.
Interestingly, Zicarelli et al28 found that the dark dots were still visible without dye reinjection 48 hours after the initial intravenous injection of ICG. They also detected hypofluorescent dots by near-infrared autofluorescence at the same location as the dark dots seen on LP-ICGA. They suggested that the photoreceptor alterations characterizing MEWDS could be secondary to RPE dysfunction involving melanosome rearrangements.
To date, there is no consensus on the initially damaged cell layer in MEWDS, nor an explanation for the unusual ICGA sequence with isofluorescent spots during the initial phase that become progressively hypofluorescent to reach a maximum on LP-ICGA. It cannot be excluded that photoreceptors are primarily impaired, and they are indeed altered on OCT, but the typical hypofluorescence of the dots on LP-ICGA implies an RPE impairment.
We therefore hypothesized that the RPE transiently loses its ability to internalize ICG, which is spontaneously restored after a few weeks, without scar formation in the RPE, indicating the absence of RPE cell death. The presence of a blue hyperautofluorescence could be explained by the loss of photoreceptor outer segments unmasking the normal fluorescence of the RPE that has not lost its autofluorescent bisretinoid load.
ASPPC
Acute syphilitic posterior placoid chorioretinitis is a rare manifestation of ocular syphilis. First described by De Souza et al30 in 1988, it has been characterized by Gass31 as “a large yellowish placoid lesion in the RPE in the macula and juxtapapillary zone.” Although ASPPC has been described in detail in recent years, its pathogenesis is not fully understood, and the question of whether the choriocapillaris, the RPE, or the photoreceptors are primarily involved remains unclear. It has been hypothesized that the presence of Treponema pallidum in the choriocapillaris could lead to an inflammatory reaction or antibody release and secondary focal ischemia in the choriocapillaris.32, 33, 34 However, although a choriocapillaris occlusion or hypoperfusion is often cited as a possible cause in the literature, this hypothesis is poorly supported. As suggested by Matsumoto and Spaide,35 the choriocapillaris perfusion is normal on FA, and the presence of some hypofluorescent foci could be due to a blocked fluorescence caused by clumps of yellowish material at the RPE rather than a choriocapillaris nonperfusion.
The use of ICGA has not contributed significantly to elucidating the pathogenesis of ASPPC until recently.36 After a first publication by Bellmann in 1999,37 a few cases have been reported. All reports have noted a hypofluorescence of the lesion, but often during both the early and late phases of ICGA.33,38, 39, 40, 41 In a large series of 53 eyes, Yang et al42 in 2015 distinguished cases with a hypofluorescence only seen on LP-ICGA from other cases. Hussnain et al43 in 2019 were the first to note that the placoid lesions were remarkable in terms of late hypofluorescence on ICGA, and a comparison has been made subsequently with the late hypofluorescence observed in MEWDS.27,44 In all these publications, the interpretation of ICGA suggested that a choroidal hypoperfusion was the cause of the RPE disturbances and photoreceptor impairment seen on OCT in ASPPC.
However, in a series of 15 eyes studied based on multimodal imaging, including systematically with LP-ICGA, Villaret et al36 showed that placoid lesions were all hyperautofluorescent, and that the ellipsoid zone as well as the interdigitation zone were disrupted in the placoid area. This suggests that the hyperautofluorescence of the placoid could be due to the unmasking of the RPE autofluorescence caused by the absence of photoreceptor outer segments.45 A normal choroidal filling was seen during the early phase of FA and ICGA. The dark spots observed in the placoid area were due to RPE granulations, observed on OCT, that were focally masking the choroidal fluorescence. A delayed choriocapillaris filling would have induced an early, patchy hypofluorescence on FA that would have also been visible on ICGA.29,46, 47, 48, 49
Although ASPPC and MEWDS are different in nature, they share similar characteristics on multimodal imaging: white/yellow patches on color fundus photography, hyperautofluorescence on blue-light fundus autofluorescence (BAF), normal choriocapillaris perfusion on FA and ICGA, ellipsoid zone disruption and outer retinal hyperreflectivity on spectral-domain OCT, and hypofluorescence on LP-ICGA. However, these diseases differ during the late stage of FA, with no leakage seen in MEWDS, whereas a leakage, or at least RPE staining, is seen in ASPPC, meaning that the RPE involvement in ASPPC is associated with some degree of outer blood-retinal barrier alteration. It is likely that, as in MEWDS, the RPE transiently loses its ability to internalize ICG (Fig 3), a function that is restored after antibiotic treatment. With prompt treatment, no or minimal scarring is observed in ASPPC.
Figure 3.
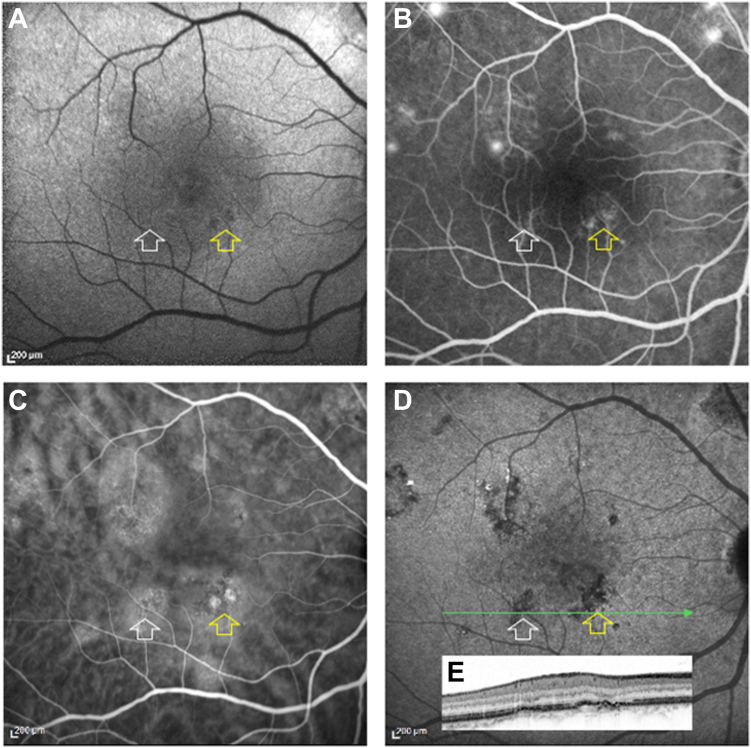
Syphilitic placoid. A, Blue fundus autofluorescence showing a hyperautofluorescent plaque occupying the entire posterior pole. B, Fluorescein angiography, venous phase, showing a homogeneous filling of the choriocapillaris. Fluorescein angiography, late phase, showing a staining of the plaque and a disc hyperfluorescence. D, E, Indocyanine green angiography (ICGA). The early phase in D, only shows a blockage of the fluorescence at the border of the plaque. During the late phase (30 minutes) in E, the plaque is mainly hypofluorescent. F, OCT B-scan passing through the macula corresponding to late-phase ICGA (green arrow) showing the loss of ellipsoid zone (arrowhead) and retinal pigment epithelium irregularities.
CSCR
Indocyanine green angiography was first used in CSCR by Hayashi et al50 in 1986. They showed what they assumed to be a hyperpermeability of the choroidal circulation at the RPE leaks, visible on FA or elsewhere, and focal areas of choroidal hypoperfusion. Similar observations have subsequently been reported.51, 52, 53 Of note, Guyer et al52 reported an additional interesting finding, namely, lesions showing early hyperfluorescence and late hypofluorescence on ICGA which could correspond to occult pigment epithelium detachments. Prünte et al54 first pointed out that CSCR could be caused by choroidal vein congestion, resulting in foci of choriocapillaris filling delay. They noted that early/midphase patches of choroidal hyperfluorescence disappeared during the ICGA sequence, but they did not discuss the foci of late hypofluorescence. Little attention was paid to these hypofluorescent foci or plaques seen on LP-ICGA until the publications by Shinojima et al,55,56 who noted that these foci corresponded to discrete irregularities or atrophic changes in the RPE. The absence of late ICG fluorescence of these plaques has been attributed to an irregular accumulation of lipids, such as neutral lipids and phospholipids in the Bruch’s membrane, preventing inward diffusion of ICG toward the RPE cells.55 Another explanation could involve a focal dysfunction of a cluster of RPE cells that were unable to internalize ICG, and thus remain hypofluorescent compared with the surrounding healthy RPE that becomes fluorescent on LP-ICGA.56 More recently, Bousquet et al57 studied the factors associated with midphase hyperfluorescent plaques (MPHPs). They found that areas with MPHPs were more frequently associated with abnormalities in the RPE layer on spectral-domain OCT than with the presence of dilated veins, and assumed that MPHPs could result from an excessive accumulation of ICG either within the abnormal RPE, or in sub-RPE deposits. Finding an explanation is even more difficult, as the aspect of these hypofluorescent plaques varies depending on the imaging technique used. On BAF, they may correspond to a hyper-, iso- or hypofluorescence, whereas on FA, they may or may not correspond to a leaking point. At this time, there is no consensus explanation for why the MPHPs become deeply hypofluorescent on LP-ICGA. However, the more likely explanation is that during the middle phase, the hyperfluorescence is due to a hyperpermeability or dilation of the choriocapillaris associated with choroidal vein dilation. Throughout the ICGA sequence, the dye disappears from the choroidal vascular lumen and the choroidal stroma. The nature of the progressive, late hypofluorescence is different. It could be due to a functional alteration of the RPE secondary to foci of choroidal hyperpermeability. Note that at this level, the RPE is often slightly detached from the Bruch’s membrane, a sign referred to as flat irregular pigment epithelium detachment58 (Fig 4). On BAF, they rarely correspond to a hypofluorescent area, which could suggest localized RPE atrophy. In fact, these hypofluorescent plaques seen on LP-ICGA more likely correspond to a localized RPE dysfunction.59
Figure 4.
Chronic central serous chorioretinopathy (CRSC). A, Blue fundus autofluorescence (BFAF): the yellow arrow shows 2 small foci of hypoautofluorescence; the white arrow shows no change in BFAF. B, Midphase fluorescein angiography where the yellow arrow shows faint hyperfluorescent spots, while the white arrow shows the absence of abnormal fluorescence. C, Midphase indocyanine green angiography (ICGA; 15 minutes) showing dilated choroidal veins, several hyperfluorescent choroidal plaques, 2 of them being marked by arrows. D, Late-phase ICGA (30 minutes) showing that the hyperfluorescent plaques seen in (C) became hypofluorescent (arrows) and correspond to shallow focal pigment epithelium detachments on the OCT B-scan shown in (E), corresponding to the green line in (D).
Choroidal Hemangioma
The ICGA pattern of choroidal hemangioma was first described by C. Shields et al.60 They reported that during LP-ICGA, the tumor that was initially hyperfluorescent became hypofluorescent, as if the dye had “washed out.” The term “washout” was used again by Arevalo et al61 and is now systematically used to describe the specific ICGA sequence characteristic of choroidal hemangiomas. However, this concept may be questioned. Indeed, there is no doubt that the vascular network of the choroidal hemangioma fills more slowly than the adjacent choriocapillaris, and that the dye persists for longer and is eliminated very progressively. Ultimately, the tumor should be isofluorescent with the rest of the choroid. There is no valid reason for it to be less fluorescent. Another explanation should therefore be proposed. When LP-ICGA findings are compared with the autofluorescence of these cases, it is striking to note that the “washout” image superimposes perfectly with the hyperautofluorescence of the tumor. It is likely that during the middle phase of ICGA, the hemangioma is brightly fluorescent, probably because the dye circulates slowly through the dense and thick volume of abnormal vessels forming the tumor and probably the staining of their wall. The washout is thus progressive, but this does not in itself explain the late hypofluorescence. Indeed, at best, the hemangioma should become isofluorescent with the rest of the fundus once the dye has been completely cleared. The most likely hypothesis is that the RPE overlying the tumor could be too damaged to be able to uptake ICG (Fig 5). The term “washout” is thus inadequate to describe this aspect on LP-ICGA.
Figure 5.
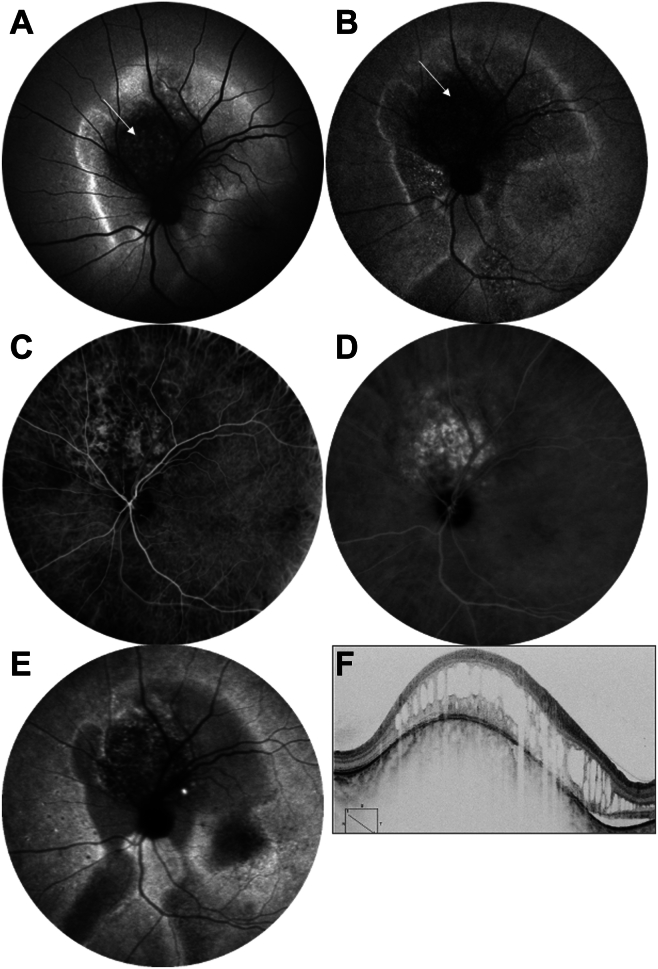
Choroidal hemangioma. A, Blue fundus autofluorescence showing the hyperautofluorescence of the hemangioma (arrow), surrounded by an area of hyperautofluorescence. B, Near-infrared autofluorescence showing a hypofluorescence at the hemangioma (arrow) and in the surrounding area, suggesting a loss of melanin in the retinal pigment epithelium (RPE). C, Indocyanine green angiography (ICGA), arterio-venous time, showing a delayed filling of the hemangioma vasculature. D, Midphase ICGA (10 minutes) showing an intense staining of the hemangioma while the surrounding choroid is normally fluorescent. E, Late-phase ICGA (45 minutes). The late hypofluorescence of the tumor could be due to the lack of ICG uptake by the RPE, as for the surrounding hypofluorescence of the serous retinal detachment and gravitational tracks. F, OCT B-scan showing the volume of the choroidal hemangioma overlaid by a cystoid retinal degeneration and surrounded by a shallow serous retinal detachment.
Drusen
In 1992, Scheider and Neuhauser62 were the first to show in elderly patients that some confluent drusen were hypofluorescent on LP-ICGA. Arnold et al63 subsequently showed that hard drusen were hyperfluorescent, whereas soft drusen were hypofluorescent, on LP-ICGA, and have hypothesized that this could be due to a masking of the underlying choroidal circulation. Shiraki et al64 examined 91 eyes of 85 patients aged ≥ 50 years without soft drusen and found hypofluorescent spots on LP-ICGA across the posterior pole in 20% of cases. In 2002, Mori et al65 used ultralate-phase ICGA (24 hours after dye injection) and observed hypofluorescent geographic lesions in the macula. They suggested for the first time that they could delineate the biodistribution of neutral lipids in the Bruch’s membrane. Thereafter, ICGA has been little used in drusen, until the hypofluorescence of certain drusen seen on ICGA drew attention again. Balaratnasingam et al66 noted that the center of large, cuticular drusen was hypofluorescent on LP-ICGA. Furthermore, Chen et al67,68 showed in a series of 70 fellow eyes of AMD patients with soft drusen that all eyes showed concomitant, age-related, scattered hypofluorescent spots on LP-ICGA. In another series of 87 patients with untreated polypoidal choroidopathy, 62% of them showed hypofluorescent spots which could be confluent on LP-ICGA, and which did not correspond to any other anomaly on multimodal imaging of the fundus.69 The authors suggested that this hypofluorescence could be due to the presence of basal linear deposits in the Bruch’s membrane preventing ICG to reach the RPE. A similar explanation has been proposed in a review by Chen et al,70 who suggested that the hypofluorescent spots seen on LP-ICGA in AMD could correspond to an exclusion of the dye that did not bind to the main hydrophobic lipids in the Bruch’s membrane, preventing access to the RPE. They hypothesized that the presence of basal linear deposits containing neutral lipids (esterified cholesterol, unesterified cholesterol, or triacylglycerol) in the Bruch’s membrane could form a barrier preventing ICG internalization by the RPE70 (Fig 6).
Figure 6.
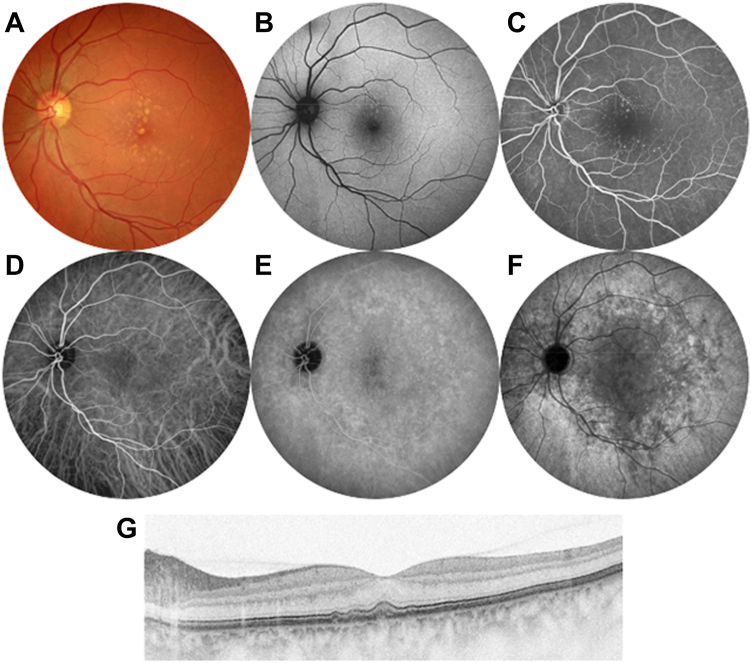
Drusen. A, Color photography showing medium size drusen without pigment nor atrophy. B, Blue fundus autofluorescence showing a faint hyperautofluorescence of some drusen. C, Fluorescein angiography showing a discrete hyperfluorescence in the drusen center. D–F, Indocyanine green angiography showing a normal early phase (A), and a discrete widespread hypofluorescence at the mid-phase (E), being more visible in the whole posterior pole at 30 mn. G, Drusen shown on OCT B-scan.
However, basal linear deposits and prebasal linear deposits contain lipoproteins to which ICG can bind.71,72 In any case, in these cases of drusen, either ICG cannot reach the RPE, or the RPE, the function of which is impaired, is unable to internalize ICG.
Thus, we found that very different diseases, whether acute or chronic, show a hypofluorescence on LP-ICGA, while the early phase is unremarkable. Other imaging modalities, including color fundus photography, BAF, and FA, may show different findings. We suggest not to interpret the hypofluorescence occurring only during LP-ICGA as a sign of choriocapillaris flow deficit, but rather as the inability of the RPE to internalize ICG bound to proteins, in particular lipoproteins. In several diseases, it could be considered a marker of RPE dysfunction. Further studies are needed to better assess its usefulness.
Acknowledgment
The author thanks Professor Alan Bird (London) for his careful reading and relevant suggestions.
Manuscript no. XOPS-D-23-00134R1.
Footnotes
Disclosure(s):
All authors have completed and submitted the ICMJE disclosures form.
The authors have no proprietary or commercial interest in any materials discussed in this article.
HUMAN SUBJECTS: No human subjects were included in this study.
No animal subjects were used in this study.
Author Contributions:
Conception and design: Gaudric
Data collection: Gaudric
Analysis and interpretation: Gaudric
Obtained funding: N/A
Overall responsibility: Gaudric
References
- 1.Flower R.W. Evolution of indocyanine green dye choroidal angiography. Opt Eng. 1995;34:727–736. [Google Scholar]
- 2.Yannuzzi L., Flower R., Slakter J. Mosby; 1997. Indocynanine Green Angiography; p. 359. [Google Scholar]
- 3.Cohen S.Y., Dubois L., Quentel G., Gaudric A. Is indocyanine green angiography still relevant? Retina. 2011;31:209–221. doi: 10.1097/IAE.0b013e31820a69db. [DOI] [PubMed] [Google Scholar]
- 4.Chang A.A., Morse L.S., Handa J.T., et al. Histologic localization of indocyanine green dye in aging primate and human ocular tissues with clinical angiographic correlation. Ophthalmology. 1998;105:1060–1068. doi: 10.1016/S0161-6420(98)96008-0. [DOI] [PubMed] [Google Scholar]
- 5.Chang A.A., Zhu M., Billson F.A., et al. Indocyanine green localisation in surgically excised choroidal neovascular membrane in age related macular degeneration. Br J Ophthalmol. 2004;88:307–309. doi: 10.1136/bjo.2003.024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pankova N., Zhao X., Liang H., et al. Delayed near-infrared analysis permits visualization of rodent retinal pigment epithelium layer in vivo. J Biomed Opt. 2014;19 doi: 10.1117/1.JBO.19.7.076007. [DOI] [PubMed] [Google Scholar]
- 7.Tam J., Liu J., Dubra A., Fariss R. In vivo imaging of the human retinal pigment epithelial mosaic using adaptive optics enhanced indocyanine green ophthalmoscopy. Invest Ophthalmol Vis Sci. 2016;57:4376–4384. doi: 10.1167/iovs.16-19503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang A.A., Zhu M., Billson F. The interaction of indocyanine green with human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2005;46:1463–1467. doi: 10.1167/iovs.04-0825. [DOI] [PubMed] [Google Scholar]
- 9.Yoneya S., Saito T., Komatsu Y., et al. Binding properties of indocyanine green in human blood. Invest Ophthalmol Vis Sci. 1998;39:1286–1290. [PubMed] [Google Scholar]
- 10.Cherrick G.R., Stein S.W., Leevy C.M., Davidson C.S. Indocyanine green: observations on its physical properties, plasma decay, and hepatic extraction. J Clin Invest. 1960;39:592–600. doi: 10.1172/JCI104072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker K.J. Binding of sulfobromophthalein (BSP) sodium and indocyanine green (ICG) by plasma alpha-1 lipoproteins. Proc Soc Exp Med. 1966;122:957–963. doi: 10.3181/00379727-122-31299. [DOI] [PubMed] [Google Scholar]
- 12.Tserentsoodol N., Gordiyenko N.V., Pascual I., et al. Intraretinal lipid transport is dependent on high density lipoprotein-like particles and class B scavenger receptors. Mol Vis. 2006;12:1319–1333. [PubMed] [Google Scholar]
- 13.Thomas S.E., Harrison E.H. Mechanisms of selective delivery of xanthophylls to retinal pigment epithelial cells by human lipoproteins. J Lipid Res. 2016;57:1865–1878. doi: 10.1194/jlr.M070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enyong E.N., Gurley J.M., De Ieso M.L., et al. Caveolar and non-Caveolar Caveolin-1 in ocular homeostasis and disease. Prog Retin Eye Res. 2022;91 doi: 10.1016/j.preteyeres.2022.101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tserentsoodol N., Sztein J., Campos M., et al. Uptake of cholesterol by the retina occurs primarily via a low density lipoprotein receptor-mediated process. Mol Vis. 2006;12:1306–1318. [PubMed] [Google Scholar]
- 16.Jampol L.M., Sieving P.A., Pugh D., et al. Multiple evanescent white dot syndrome. I. Clinical findings. Arch Ophthalmol. 1984;102:671–674. doi: 10.1001/archopht.1984.01040030527008. [DOI] [PubMed] [Google Scholar]
- 17.Ie D., Glaser B.M., Murphy R.P., et al. Indocyanine green angiography in multiple evanescent white-dot syndrome. Am J Ophthalmol. 1994;117:7–12. doi: 10.1016/s0002-9394(14)73008-9. [DOI] [PubMed] [Google Scholar]
- 18.Cimino L., Auer C., Herbort C.P. Sensitivity of indocyanine green angiography for the follow-up of active inflammatory choriocapillaropathies. Ocul Immunol Inflamm. 2000;8:275–283. doi: 10.1076/ocii.8.4.275.6462. [DOI] [PubMed] [Google Scholar]
- 19.Yen M.T., Rosenfeld P.J. Persistent indocyanine green angiographic findings in multiple evanescent white dot syndrome. Ophthalmic Surg Lasers. 2001;32:156–158. [PubMed] [Google Scholar]
- 20.Gross N.E., Yannuzzi L.A., Freund K.B., et al. Multiple evanescent white dot syndrome. Arch Ophthalmol. 2006;124:493–500. doi: 10.1001/archopht.124.4.493. [DOI] [PubMed] [Google Scholar]
- 21.Ikeda N., Ikeda T., Nagata M., et al. Location of lesions in multiple evanescent white dot syndrome and the cause of the hypofluorescent spots observed by indocyanine green angiography. Graefes Arch Clin Exp Ophthalmol. 2001;239:242–247. doi: 10.1007/s004170100276. [DOI] [PubMed] [Google Scholar]
- 22.Yi C., Zhao G., Ou J., Yan H. Changes of indocyanine green and fluorescein angiography in multiple evanescent White-dot Syndrome: a case report. Yan Ke Xue Bao. 2003;19:171–173. [PubMed] [Google Scholar]
- 23.Sikorski B.L., Wojtkowski M., Kaluzny J.J., et al. Correlation of spectral optical coherence tomography with fluorescein and indocyanine green angiography in multiple evanescent white dot syndrome. Br J Ophthalmol. 2008;92:1552–1557. doi: 10.1136/bjo.2007.135863. [DOI] [PubMed] [Google Scholar]
- 24.Pichi F., Srvivastava S.K., Chexal S., et al. En face optical coherence tomography and optical coherence tomography angiography of multiple evanescent white dot syndrome: new insights into pathogenesis. Retina. 2016;36:S178–S188. doi: 10.1097/IAE.0000000000001255. [DOI] [PubMed] [Google Scholar]
- 25.Gal-Or O., Priel E., Rosenblatt I., et al. Multimodal imaging in an unusual cluster of multiple evanescent white dot syndrome. J Ophthalmol. 2017;2017 doi: 10.1155/2017/7535320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Veronese C., Maiolo C., Morara M., et al. Bilateral multiple evanescent white dot syndrome. Int Ophthalmol. 2018;38:2153–2158. doi: 10.1007/s10792-017-0673-5. [DOI] [PubMed] [Google Scholar]
- 27.Russell J.F., Pichi F., Scott N.L., et al. Masqueraders of multiple evanescent white dot syndrome (MEWDS) Int Ophthalmol. 2020;40:627–638. doi: 10.1007/s10792-019-01223-4. [DOI] [PubMed] [Google Scholar]
- 28.Zicarelli F., Mantovani A., Preziosa C., Staurenghi G. Multimodal imaging of multiple evanescent white dot syndrome: a new interpretation. Ocul Immunol Inflamm. 2020;28:814–820. doi: 10.1080/09273948.2019.1635169. [DOI] [PubMed] [Google Scholar]
- 29.Gaudric A., Mrejen S. Why the dots Are black only in the late phase of the indocyanine green angiography in multiple evanescent white dot syndrome. Retin Cases Brief Rep. 2017;11:S81–S85. doi: 10.1097/ICB.0000000000000422. [DOI] [PubMed] [Google Scholar]
- 30.de Souza EC Jalkh A.E., Trempe C.L., et al. Unusual central chorioretinitis as the first manifestation of early secondary syphilis. Am J Ophthalmol. 1988;105:271–276. doi: 10.1016/0002-9394(88)90009-8. [DOI] [PubMed] [Google Scholar]
- 31.Gass J.D., Braunstein R.A., Chenoweth R.G. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990;97:1288–1297. doi: 10.1016/s0161-6420(90)32418-1. [DOI] [PubMed] [Google Scholar]
- 32.Lima L.H., de Andrade G.C., Vianello S., et al. Multimodal imaging analyses of hyperreflective dot-like lesions in acute syphilitic posterior placoid chorioretinopathy. J Ophthalmic Inflamm Infect. 2017;7:1. doi: 10.1186/s12348-016-0119-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pichi F., Ciardella A.P., Cunningham E.T., Jr., et al. Spectral domain optical coherence tomography findings in patients with acute syphilitic posterior placoid chorioretinopathy. Retina. 2014;34:373–384. doi: 10.1097/IAE.0b013e3182993f11. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama M., Cao K., Ogata S., Ohno-Matsui K. Detection of posterior vortex veins in eyes with pathologic myopia by ultra-widefield indocyanine green angiography. Br J Ophthalmol. 2017;101:1179–1184. doi: 10.1136/bjophthalmol-2016-309877. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto Y., Spaide R.F. Autofluorescence imaging of acute syphilitic posterior placoid chorioretinitis. Retin Cases Brief Rep. 2007;1:123–127. doi: 10.1097/01.iae.0000242759.80833.39. [DOI] [PubMed] [Google Scholar]
- 36.Villaret J., Errera M.H., Sahel J.A., et al. Indocyanine green angiography features in acute syphilitic posterior placoid chorioretinitis. Am J Ophthalmol. 2022;241:40–46. doi: 10.1016/j.ajo.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Bellmann C., Holz F.G., Breitbart A., Völcker H.E. Bilateral acute syphilitic posterior placoid chorioretinopathy – angiographic and autofluorescence characteristics. Der Ophthalmologe. 1999;96:522–528. doi: 10.1007/s003470050448. [DOI] [PubMed] [Google Scholar]
- 38.Joseph A., Rogers S., Browning A., et al. Syphilitic acute posterior placoid chorioretinitis in nonimmuno-compromised patients. Eye (Lond) 2007;21:1114–1119. doi: 10.1038/sj.eye.6702504. [DOI] [PubMed] [Google Scholar]
- 39.Meira-Freitas D., Farah M.E., Höfling-Lima A.L., Aggio F.B. Optical coherence tomography and indocyanine green angiography findings in acute syphilitic posterior placoid choroidopathy: case report. Arq Bras Oftalmol. 2009;72:832–835. doi: 10.1590/s0004-27492009000600019. [DOI] [PubMed] [Google Scholar]
- 40.Puech C., Gennai S., Pavese P., et al. Ocular manifestations of syphilis: recent cases over a 2.5-year period. Graefes Arch Clin Exp Ophthalmol. 2010;248:1623–1629. doi: 10.1007/s00417-010-1481-z. [DOI] [PubMed] [Google Scholar]
- 41.Eandi C.M., Neri P., Adelman R.A., et al. International Syphilis Study G. Acute syphilitic posterior placoid chorioretinitis: report of a case series and comprehensive review of the literature. Retina. 2012;32:1915–1941. doi: 10.1097/IAE.0b013e31825f3851. [DOI] [PubMed] [Google Scholar]
- 42.Yang B., Xiao J., Li X., et al. Clinical manifestations of syphilitic chorioretinitis: a retrospective study. Int J Clin Exp Med. 2015;8:4647–4655. [PMC free article] [PubMed] [Google Scholar]
- 43.Hussnain S.A., Gal-Or O., Daccache A., et al. Multimodal imaging of atypical acute syphilitic posterior placoid chorioretinitis mimicking a white dot syndrome. Ophthalmic Surg Lasers Imaging Retina. 2019;50:e52–e55. doi: 10.3928/23258160-20190129-20. [DOI] [PubMed] [Google Scholar]
- 44.Azar G., Wolff B., Azam S., Mauget-Faysse M. Acute syphilitic posterior placoid chorioretinopathy presenting as atypical multiple evanescent white dot syndrome. Eur J Ophthalmol. 2021;31:NP141–NP144. doi: 10.1177/1120672120957589. [DOI] [PubMed] [Google Scholar]
- 45.Freund K.B., Mrejen S., Jung J. Increased fundus autofluorescence related to outer retinal disruption. JAMA Ophthalmol. 2013;131:1645–1649. doi: 10.1001/jamaophthalmol.2013.5030. [DOI] [PubMed] [Google Scholar]
- 46.Young N., Bird A., Sehmi K. Pigment epithelial diseases with abnormal choroidal perfusion. Am J Ophthalmol. 1980;90:607–618. doi: 10.1016/s0002-9394(14)75127-x. [DOI] [PubMed] [Google Scholar]
- 47.Gaudric A., Coscas G., Bird A.C. Choroidal ischemia. Am J Ophthalmol. 1982;94:489–498. doi: 10.1016/0002-9394(82)90242-2. [DOI] [PubMed] [Google Scholar]
- 48.Gaudric A., Sterkers M., Coscas G. Retinal detachment after choroidal ischemia. Am J Ophthalmol. 1987;104:364–372. doi: 10.1016/0002-9394(87)90226-1. [DOI] [PubMed] [Google Scholar]
- 49.Mrejen S., Sarraf D., Chexal S., et al. Choroidal involvement in acute posterior multifocal placoid pigment epitheliopathy. Ophthalmic Surg Lasers Imaging Retina. 2016;47:20–26. doi: 10.3928/23258160-20151214-03. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi K., Hasegawa Y., Tokoro T. Indocyanine green angiography of central serous chorioretinopathy. Int Ophthalmol. 1986;9:37–41. doi: 10.1007/BF00225936. [DOI] [PubMed] [Google Scholar]
- 51.Scheider A., Nasemann J., Lund O.-E. Fluorescein and indocyanine green angiographies of central serous choroidopathy by scanning laser ophthalmoscopy. Am J Ophthalmol. 1993;115:50–56. doi: 10.1016/s0002-9394(14)73524-x. [DOI] [PubMed] [Google Scholar]
- 52.Guyer D.R., Yannuzzi L.A., Slakter J.S., et al. Digital indocyanine green videoangiography of central serous chorioretinopathy. Arch Ophthalmol. 1994;112:1057–1062. doi: 10.1001/archopht.1994.01090200063023. [DOI] [PubMed] [Google Scholar]
- 53.Piccolino F.C., Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina. 1994;14:231–242. doi: 10.1097/00006982-199414030-00008. [DOI] [PubMed] [Google Scholar]
- 54.Prunte C., Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol. 1996;121:26–34. doi: 10.1016/s0002-9394(14)70531-8. [DOI] [PubMed] [Google Scholar]
- 55.Shinojima A., Fujita K., Mori R., et al. Investigation of the etiology of central serous chorioretinopathy using en-face optical coherence tomography and indocyanine green angiography. Ophthalmologica. 2016;236 doi: 10.1159/000448342. [DOI] [PubMed] [Google Scholar]
- 56.Shinojima A., Mehanna C., Lavia C.A., et al. Central serous chorioretinopathy: risk factors for serous retinal detachment in fellow eyes. Br J Ophthalmol. 2020;104:852–856. doi: 10.1136/bjophthalmol-2019-314970. [DOI] [PubMed] [Google Scholar]
- 57.Bousquet E., Provost J., Zola M., et al. Mid-phase hyperfluorescent plaques seen on indocyanine green angiography in patients with central serous chorioretinopathy. J Clin Med. 2021;10:4525. doi: 10.3390/jcm10194525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hage R., Mrejen S., Krivosic V., et al. Flat irregular retinal pigment epithelium detachments in chronic central serous chorioretinopathy and choroidal neovascularization. Am J Ophthalmol. 2015;159:890–903.e3. doi: 10.1016/j.ajo.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 59.Zola M., Bousquet E., Favard C., et al. Indocyanine green angiography of type 1 macular neovascularization in age-related macular degeneration and central serous chorioretinopathy reveals different disease mechanisms. Retina. 2023;43:1255–1263. doi: 10.1097/IAE.0000000000003833. [DOI] [PubMed] [Google Scholar]
- 60.Shields C.L., Shields J.A., De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–245. doi: 10.1136/bjo.79.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arevalo J.F., Shields C.L., Shields J.A., et al. Circumscribed choroidal hemangioma: characteristic features with indocyanine green videoangiography. Ophthalmology. 2000;107 doi: 10.1016/s0161-6420(99)00051-2. [DOI] [PubMed] [Google Scholar]
- 62.Scheider A., Neuhauser L. Fluorescence characteristics of drusen during indocyanine-green angiography and their possible correlation with choroidal perfusion. Ger J Ophthalmol. 1992;1:328–334. [PubMed] [Google Scholar]
- 63.Arnold J.J., Quaranta M., Soubrane G., et al. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997;124:344–356. doi: 10.1016/s0002-9394(14)70826-8. [DOI] [PubMed] [Google Scholar]
- 64.Shiraki K., Moriwaki M., Kohno T., et al. Age-related scattered hypofluorescent spots on late-phase indocyanine green angiograms. Int Ophthalmol. 1999;23:105–109. doi: 10.1023/a:1026571327117. [DOI] [PubMed] [Google Scholar]
- 65.Mori K., Gehlbach P.L., Nishiyama Y., et al. The ultra-late phase of indocyanine green angiography for healthy subjects and patients with age-related macular degeneration. Retina. 2002;22:309–316. doi: 10.1097/00006982-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 66.Balaratnasingam C., Cherepanoff S., Dolz-Marco R., et al. Cuticular drusen: clinical phenotypes and natural history defined using multimodal imaging. Ophthalmology. 2018;125:100–118. doi: 10.1016/j.ophtha.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 67.Chen L., Zhang X., Li M., et al. Drusen and age-related scattered hypofluorescent spots on late-phase indocyanine green angiography, a candidate correlate of lipid accumulation. Invest Ophthalmol Vis Sci. 2018;59:5237–5245. doi: 10.1167/iovs.18-25124. [DOI] [PubMed] [Google Scholar]
- 68.Chen L., Zhang X., Liu B., et al. Age-related scattered hypofluorescent spots on late-phase indocyanine green angiography: the multimodal imaging and relevant factors. Clin Exp Ophthalmol. 2018;46:908–915. doi: 10.1111/ceo.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen L., Zhang X., Li M., et al. Age-related scattered hypofluorescent spots on late-phase indocyanine green angiography as precursor lesions of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2019;60:2102–2109. doi: 10.1167/iovs.19-26968. [DOI] [PubMed] [Google Scholar]
- 70.Chen L., Yang P., Curcio C.A. Visualizing lipid behind the retina in aging and age-related macular degeneration, via indocyanine green angiography (ASHS-LIA) Eye (Lond) 2022;36:1735–1746. doi: 10.1038/s41433-022-02016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curcio C.A. Soft drusen in age-related macular degeneration: biology and targeting via the oil spill strategies. Invest Ophthalmol Vis Sci. 2018;59:AMD160–AMD181. doi: 10.1167/iovs.18-24882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schultz R., Gamage K.C.L.K., Messinger J.D., et al. Fluorescence lifetimes and spectra of RPE and sub-RPE deposits in histology of control and AMD eyes. Invest OphthalmolVis Sci. 2020;61:9. doi: 10.1167/iovs.61.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]