Abstract
Importance
Sarcopenia, the age-related loss of muscle mass and strength/function, is an important clinical condition. However, no international consensus on the definition exists.
Objective
The Global Leadership Initiative in Sarcopenia (GLIS) aimed to address this by establishing the global conceptual definition of sarcopenia.
Design
The GLIS steering committee was formed in 2019–21 with representatives from all relevant scientific societies worldwide. During this time, the steering committee developed a set of statements on the topic and invited members from these societies to participate in a two-phase International Delphi Study. Between 2022 and 2023, participants ranked their agreement with a set of statements using an online survey tool (SurveyMonkey). Statements were categorised based on predefined thresholds: strong agreement (>80%), moderate agreement (70–80%) and low agreement (<70%). Statements with strong agreement were accepted, statements with low agreement were rejected and those with moderate agreement were reintroduced until consensus was reached.
Results
107 participants (mean age: 54 ± 12 years [1 missing age], 64% men) from 29 countries across 7 continents/regions completed the Delphi survey. Twenty statements were found to have a strong agreement. These included; 6 statements on ‘general aspects of sarcopenia’ (strongest agreement: the prevalence of sarcopenia increases with age (98.3%)), 3 statements on ‘components of sarcopenia’ (muscle mass (89.4%), muscle strength (93.1%) and muscle-specific strength (80.8%) should all be a part of the conceptual definition of sarcopenia)) and 11 statements on ‘outcomes of sarcopenia’ (strongest agreement: sarcopenia increases the risk of impaired physical performance (97.9%)). A key finding of the Delphi survey was that muscle mass, muscle strength and muscle-specific strength were all accepted as ‘components of sarcopenia’, whereas impaired physical performance was accepted as an ‘outcome’ rather than a ‘component’ of sarcopenia.
Conclusion and relevance
The GLIS has created the first global conceptual definition of sarcopenia, which will now serve to develop an operational definition for clinical and research settings.
Keywords: older people, conceptual, definitions, sarcopenia, GLIS
Key Points
Question: several societies and organisations have developed definitions of sarcopenia, which are region specific. At present, there is no international consensus on how to define sarcopenia.
Findings: the Global Leadership Initiative in Sarcopenia (GLIS), an international collaboration of experts from all major sarcopenia societies/organisations worldwide has developed the first global conceptual definition of sarcopenia. This conceptual definition of sarcopenia will now be used to develop an operational definition of sarcopenia for both clinical and research settings.
Meaning: this collaborative effort by the GLIS signifies a critical step towards advancing our understanding and management of sarcopenia on a global scale.
Introduction
Sarcopenia, the age-related loss of muscle mass and decline in strength and function, increases the risk of distinct clinical outcomes including disability, falls and mortality [1, 2]. Despite this advancement in knowledge, sarcopenia has received limited clinical recognition and currently no pharmacological treatment exists for this condition [3, 4]. Sarcopenia also lacks a global and widely accepted definition that can be routinely used in clinical settings, even though an ICD10-CM code for this condition exists [5].
The lack of a single, standard definition of sarcopenia has led to several issues. First, research into the prevalence, incidence, and causes and consequences of sarcopenia is often difficult to harmonise, as disparate definitions can lead to widely different estimates of prevalence [6] or can identify different important consequences of sarcopenia. Second, the lack of a single definition has clinical implications because those seeing patients may be uncertain as to which measures or cut-off points to use when evaluating patients. Third, the lack of a unified definition has impeded the development of clinical care pathways for sarcopenia. Indeed, while there is evidence that, even in the oldest old, non-pharmacological interventions such as resistance-based exercise can increase muscle strength [7–9] and multicomponent exercise interventions (aerobic, strength and balance/flexibility) can reduce the risk of mobility disability [10, 11], there are few specific exercise or dietary prescriptions in regular clinical use to treat sarcopenia. There is also a stymied development of pharmacological treatments for sarcopenia. A common definition may facilitate development of a well-defined path towards regulatory approval of novel therapeutics (e.g. help identify specific exercise and/or dietary protocols for sarcopenia or drug agents with a clear mechanism of action for sarcopenia). Although the ICD10-CM code is available in some countries such as Australia and the United States, the World Health Organization (WHO) has yet to include an ICD-10 code for sarcopenia in its version. A global definition of sarcopenia would ease the adoption of an ICD-10 code for sarcopenia by WHO, which, in turn, would increase clinical recognition of this condition worldwide.
Several groups have proposed definitions of sarcopenia, including the Asian Working Group for Sarcopenia (AWGS) [12, 13], the Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) [14, 15], the European Working Group for Sarcopenia in Older Persons (EWGSOP) that issued guidelines in 2010 [16] that were revised in 2019 [17]; the Foundation for the National Institutes of Health (FNIH) Sarcopenia Project [18], the International Working Group on Sarcopenia (IWGS) [19] and the Sarcopenia Definitions and Outcomes Consortium (SDOC) that issued operational cut-off points in several measures that characterise sarcopenia in 2020 [20, 21]. The Global Leadership Initiative in Sarcopenia (GLIS) was formed in an attempt to harmonise these competing definitions into one unifying common classification that would be used as the gold standard in sarcopenia assessment.
The aim of this study is to describe the formation of GLIS, and report on the findings of the Delphi process used to create a globally accepted conceptual definition of sarcopenia. The next step in the coming years of GLIS is to develop an operational definition for clinical and research settings from the agreed-upon conceptual definition formed by the Delphi process.
Methods
Development of the GLIS initiative
In 2019, when the last updates of sarcopenia definitions were being produced, leading members of the AWGS, EWGSOP and SDOC resolved to launch a collaborative initiative to produce a global definition of sarcopenia. A first meeting with members of the three working groups was planned in March 2020, but the initiative had to be postponed due to the COVID-19 pandemic. In the second half of 2021 a new meeting was convened, and a representative of the Australia and New Zealand consensus was added. A steering committee that represented the four groups was created and started meeting regularly, mostly virtually, and decided that an inclusive procedure was required. Accordingly, a large number of international societies related to the field of sarcopenia were contacted, and many agreed to support the initiative by sending a representative who was included in the steering committee (the list of societies and representatives in the steering group is listed in Supplementary File 1). A representative of the group that recently published a definition of sarcopenic obesity [22] and of the organisations that defined osteoporosis some decades ago were also invited to join the group. This global initiative was named GLIS, in alignment with a similar consensus process that had been used by international nutrition societies to produce the Global Leadership Initiative in Malnutrition (GLIM) global definition of malnutrition [23, 24]. The final steering committee, together with their Declaration of Interest (DOI) forms (declaring any potential conflict of interest) is available here: https://www.eugms.org/fileadmin/images/news/2022/GLIS_Steering_Committee_Rev3.pdf.
The WHO was also contacted, and we were informed that a global definition would facilitate inclusion of sarcopenia in the International Classification of Diseases (at present it is only included in the Clinical Modification version of ICD) [25].
To create the full GLIS group, all members of current working groups (ANZSSFR, AWGS, EWGSOP, SDOC) were invited to join. Members of these groups were also invited to name experts in sarcopenia (the only requirement was to have at least two or three articles published on sarcopenia or muscle), and all participating societies were invited to send additional names. In an effort to include under-represented groups, all were encouraged to increase the number of female members, and invite experts from Africa, Middle East, and Central/Latin-America. Some additional people with expertise in sarcopenia who learned about the initiative and wanted to join were also invited. The experts who sent their DOI forms, described later (Characteristics of the GLIS committee) were included as part of the GLIS initiative. Note, experts from industry were not required to complete the DOI form, as they were known employees of companies. This research follows the World Medical Association’s Declaration of Helsinki.
Initiating with a glossary and conceptual paper: a strategic approach for subsequent operationalization
In the first meetings, the steering committee explored the issues that had to be addressed in the search for a global consensus. It became evident that some of the differences in previous definitions were related to disagreements on whether muscle mass should be included in the definition partly due to measurement issues of available techniques and their association with outcomes, whether physical function should be considered as part of the definition or as an outcome, and around the concept of muscle quality [26, 27]. In addition, some terms were not well defined in the literature. As an example, many different definitions of muscle quality were used in previous studies, and the term ‘sarcopenia’ had been used to refer to low muscle mass alone in some studies and to the loss of muscle mass with ageing without consideration of function [28]. For this reason, the first product of the GLIS initiative was a glossary of terms used in sarcopenia research and practice [29].
The steering committee recognised that before any usable universal definition was derived, agreement on the concept of sarcopenia, including the elements that are key to defining the condition, was needed. Once these elements were agreed on, including these key framing concepts into a universal and usable definition would be facilitated [ 3, 26, 30].
It was agreed that this conceptual definition would use a modified Delphi process involving the GLIS group including several rounds until consensus was reached. A draft with statements related to the conceptual definition of sarcopenia was developed with several iterations and corrections until a final list of statements was agreed upon in the steering committee. To avoid the issue that answers might be skewed by the accuracy and lack of currently available measurement methods, experts were asked to assume that perfect, feasible assessments of each listed measure existed.
Statistical analysis
Experts were initially invited to join the GLIS initiative by email. Those who did not respond to the first email were subsequently emailed for two more attempts to join the initiative (spam and junk folders were manually checked to ensure emails were not lost to these folders). An 11-point Likert scale ranging from 0 (strongly disagree) to 10 (strongly agree) was used to rank respondents’ agreements with a list of statements on an online software program (SurveyMonkey Australia Pty limited). Each statement contained a comment box where respondents could suggest the rewording of statements, propose new statements or leave general comments regarding their motivation for choices. Respondents were reminded to refer to the glossary of terms on sarcopenia for clarifying statements [29]. All text responses were inspected offline by steering committee members and discussed at meetings. Thresholds for agreement with statements were pre-specified by the steering committee as follows: low agreement (<70% respondents scoring ≥7), moderate agreement (70 to 80% respondents scoring ≥7) or strong agreement (>80% respondents scoring ≥7). Statements with strong agreement were accepted, and statements with low agreement were rejected. Statements with moderate agreement were reintroduced in the subsequent round until consensus was reached. This modified Delphi approach has been previously utilised in the literature [14].
Results
Inclusion/exclusion
Two hundred and six participants were invited to take part in the Delphi Consensus; 5 were industry experts and 201 were non-industry experts. Of those industry experts, four (80%) accepted the invitation and one did not respond to emails. Of the non-industry experts, 104 (52%) accepted the invitation and completed the DOI form and 97 either did not respond to emails, declined the invitation, or did not complete the DOI form. A further 1 (0.5%) non-industry expert was lost to follow-up; that is, did not respond to email requests after completing the DOI form. This left 107 respondents (4 industry experts, 103 non-industry experts) with a total response rate of 52%.
Characteristics of the GLIS committee
A total of 107 participants (mean age: 54 ± 12 years [n = 1 missing age], 64% men) from 29 countries and crossing 7 continents/regions completed rounds 1 and 2 of the Delphi process. Forty percent of participants were from Europe, 22% from Asia, 19% from North America, 12% from Australia, 4% from South America, 3% from Africa and 1% from Antarctica/New Zealand. Of those 107 participants, 76 (71%) were academic professionals (e.g. University research and education), 23 (22%) were health professionals (e.g. hospital, rehabilitation or clinical setting) and 5 (5%) reported as either mixed academic and industry professional (n = 4) or Emeritus (n = 1). Regarding academic professionals, nearly two-thirds (71%) were professors and over two-thirds (78%) of health professionals were geriatricians. All industry professionals occupied senior roles (100% reported as either scientific director/executive director or programme leader). Table 1 shows the full characteristics of the GLIS committee.
Table 1.
Characteristics of the GLIS committee (n = 107 respondents)
| Variable | Sub-category | Overall (n = 107) |
|---|---|---|
| Age, years, mean (±SD)a | 54 ± 12 | |
| Sex, men, n (%) | 68 (64%) | |
| Race/Ethnicity, n (%) | ||
| White/Caucasian | 73 (68%) | |
| Asian/Pacific Islander | 26 (24%) | |
| Hispanic | 3 (3%) | |
| Black or African American | 1 (1%) | |
| Preferred not to say | 4 (4%) | |
| Continent/region, currently residing, n (%) | ||
| Europe | 43 (40%) | |
| Asia | 23 (22%) | |
| North America | 20 (19%) | |
| Australia | 13 (12%) | |
| South America | 4 (4%) | |
| Africa | 3 (3%) | |
| Antarctica/New Zealand | 1 (1%) | |
| Country, currently residing, n (%) | ||
| Australia | 13 (12%) | |
| Belgium | 5 (5%) | |
| Brazil | 3 (3%) | |
| Cameroon | 1 (1%) | |
| Canada | 4 (4%) | |
| Chile | 1 (1%) | |
| China | 3 (3%) | |
| Czech Republic | 1 (1%) | |
| Denmark | 1 (1%) | |
| Finland | 1 (1%) | |
| France | 2 (2%) | |
| Germany | 4 (4%) | |
| Italy | 5 (5%) | |
| Japan | 5 (5%) | |
| Mexico | 1 (1%) | |
| Netherlands | 6 (6%) | |
| New Zealand | 1 (1%) | |
| Poland | 2 (2%) | |
| Republic of Korea | 4 (4%) | |
| Saudi Arabia | 3 (3%) | |
| Singapore | 3 (3%) | |
| South Africa | 2 (2%) | |
| Spain | 2 (2%) | |
| Sweden | 1 (1%) | |
| Switzerland | 3 (3%) | |
| Taiwan | 5 (5%) | |
| Turkey | 1 (1%) | |
| United Kingdom of Great Britain and Northern Ireland | 9 (8%) | |
| United States of America | 15 (14%) | |
| Primary role, n (%) | ||
| Academic professional | 76 (71%) | |
| Professor | 54 (71%) | |
| Associate Professor | 11 (14%) | |
| Research Fellow (e.g. postdoctoral, senior, assistant professor) | 6 (8%) | |
| Lecturer | 2 (3%) | |
| Not specified | 3 (4%) | |
| Health professional | 23 (22%) | |
| Geriatrician | 18 (78%) | |
| Physician | 3 (13%) | |
| Rehabilitation | 1 (4%) | |
| Other (please specify)b | 1 (4%) | |
| Industry professional | 3 (3%) | |
| Scientific Director | 1 (33%) | |
| Other (please specify)c | 2 (67%) | |
| Other (please specify)d | 5 (5%) |
a n = 1 did not report age.
bHealth professional—Other (please specify): n = 1 Cardiologist.
cIndustry professional—Other (please specify): n = 1 Executive Director; n = 1 Programme leader.
dPrimary role—Other (please specify): n = 4 Academic and Health professional (Mixed); n = 1 Emeritus.
Round 1 of the Delphi process
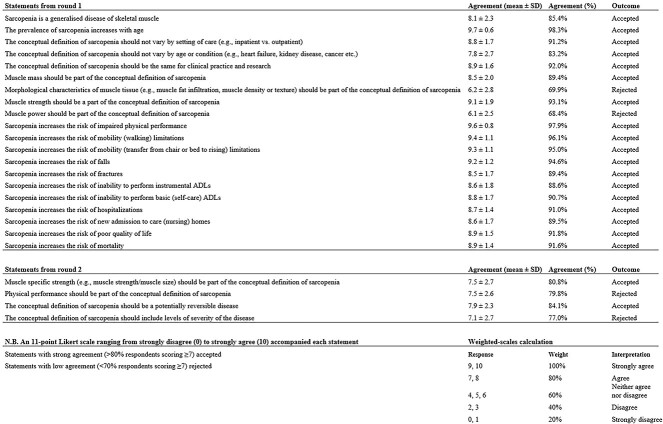
A total of 107 respondents completed round 1 of the Delphi Process—Supplementary File 2 shows the GLIS survey used in round 1. Of the 25 statements introduced in round 1, 18 (72%) were accepted with strong agreement and 2 (8%) were rejected with low agreement (Figure 1; Tables 2 and 3). The remaining five statements, four (16%) with moderate agreement and one (4%) with strong agreement that was just above the cut-off point for strong agreement, were reintroduced in round 2 and are listed below.
Figure 1.
List of accepted and rejected statements from round 1 and 2 of the Delphi Process (n = 107 respondents).
Table 2.
List of accepted statements from the GLIS Delphi (n = 107 respondents)
| Number | Statement | Agreement |
|---|---|---|
| General aspects of sarcopenia | ||
| 1 | Sarcopenia is a generalised disease of skeletal muscle | 85.4% |
| 2 | The prevalence of sarcopenia increases with age | 98.3% |
| 3 | The conceptual definition of sarcopenia should not vary by setting of care (e.g. inpatient vs. outpatient) | 91.2% |
| 4 | The conceptual definition of sarcopenia should not vary by age or condition (e.g. heart failure, kidney disease, cancer etc.) | 83.2% |
| 5 | The conceptual definition of sarcopenia should be the same for clinical practice and research | 92.0% |
| 6 | The conceptual definition of sarcopenia should be a potentially reversible disease | 84.1% |
| Components of sarcopenia | ||
| 7 | Muscle mass should be part of the conceptual definition of sarcopenia | 89.4% |
| 8 | Muscle strength should be a part of the conceptual definition of sarcopenia | 93.1% |
| 9 | Muscle-specific strength (e.g. muscle strength/muscle size) should be part of the conceptual definition of sarcopenia | 80.8% |
| Outcomes of sarcopenia | ||
| 10 | Sarcopenia increases the risk of impaired physical performance | 97.9% |
| 11 | Sarcopenia increases the risk of mobility (walking) limitations | 96.1% |
| 12 | Sarcopenia increases the risk of mobility (transfer from chair or bed to rising) limitations | 95.0% |
| 13 | Sarcopenia increases the risk of falls | 94.6% |
| 14 | Sarcopenia increases the risk of fractures | 89.4% |
| 15 | Sarcopenia increases the risk of inability to perform instrumental ADLs | 88.6% |
| 16 | Sarcopenia increases the risk of inability to perform basic (self-care) ADLs | 90.7% |
| 17 | Sarcopenia increases the risk of hospitalizations | 91.0% |
| 18 | Sarcopenia increases the risk of new admission to care (nursing) homes | 89.5% |
| 19 | Sarcopenia increases the risk of poor quality of life | 91.8% |
| 20 | Sarcopenia increases the risk of mortality | 91.6% |
Table 3.
List of rejected statements from the GLIS Delphi (n = 107 respondents)
| Number | Statement | Agreement |
|---|---|---|
| General aspects of sarcopenia | ||
| 1 | Morphological characteristics of muscle tissue (e.g. muscle fat infiltration, muscle density or muscle texture) should be part of the conceptual definition of sarcopenia | 69.9% |
| 2 | The conceptual definition of sarcopenia should include levels of severity of the disease | 77.0% |
| Components of sarcopenia | ||
| 3 | Muscle power should be part of the conceptual definition of sarcopenia | 68.4% |
| 4 | Physical performance should be part of the conceptual definition of sarcopenia | 79.8% |
1. ‘The conceptual definition of sarcopenia should include levels of severity of the disease’. Agreement: 77.9% (mean score: 7.3 ± 3.0).
2. ‘Muscle strength should be a marker of severity for the conceptual definition of sarcopenia’. Agreement: 79.1% (mean score: 7.3 ± 2.9).
3. ‘Muscle-specific strength (e.g., muscle strength/muscle size) should be part of the conceptual definition of sarcopenia’, Agreement: 72.3% (mean score: 6.5 ± 2.8).
4. ‘Physical performance should be part of the conceptual definition of sarcopenia’. Agreement: 79.4% (mean score: 7.3 ± 2.8).
5. Physical performance should be a marker of severity for the conceptual definition of sarcopenia: Agreement: 82.2% (mean score: 7.7 ± 2.5).
There was a discrepancy between statements 4 (moderate agreement) and 5 (strong agreement) above, both of which are related to ‘physical performance’. As such, both were reintroduced in round 2 for clarity.
Lastly, a new statement relating to ‘general aspects of sarcopenia’ was introduced in round 2 and is listed below.
1. ‘The conceptual definition of sarcopenia should be a potentially reversible disease’.
Round 2 of the Delphi process
All 107 (100%) respondents who completed round 1 of the Delphi process also completed round 2—Supplementary File 3 shows the GLIS survey used in round 2. Of the eight statements introduced in round 2, two (25%) were accepted with strong agreement, and two (25%) were rejected with low agreement (Figure 1; Tables 2 and 3). Statement 5 ‘The conceptual definition of sarcopenia should include levels of severity of the disease’ was rejected with an agreement of 77.0% (mean score: 7.1 ± 2.7, n = 107 respondents). As statement 5 did not meet the acceptance threshold (>80%), the remaining four (50%) statements relating to ‘markers of the severity of sarcopenia’ were rejected. Note that these four statements were optional as specified in the survey guidelines. This accounts for the smaller sample size with some respondents (n = 13–14) skipping these statements as highlighted below.
1. ‘Muscle mass should be a marker of severity for the conceptual definition of sarcopenia’. Agreement: 66.0% (mean score: 5.8 ± 3.1; answered = 93, skipped = 14).
2. ‘Muscle strength should be a marker of severity for the conceptual definition of sarcopenia’. Agreement: 79.8% (mean score: 7.5 ± 2.7; answered = 94, skipped = 13).
3. ‘Muscle-specific strength (e.g., muscle strength/muscle size) should be a marker of severity for the conceptual definition of sarcopenia’. Agreement: 68.6.% (mean score: 6.1 ± 2.9; answered = 93, skipped = 14).
4. ‘Physical performance should be a marker of severity for the conceptual definition of sarcopenia’. Agreement: 81.9% (mean score: 7.6 ± 2.4; answered = 93, skipped = 14) (Figure 2).
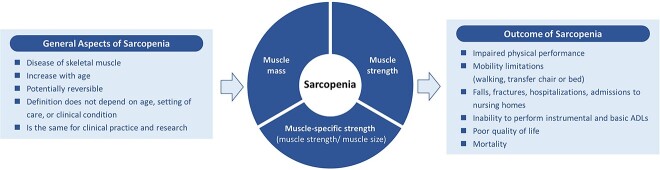
Figure 2.
A graphical representation of the conceptual definition of sarcopenia.
Discussion
We agreed that the conceptual definition of sarcopenia comprises the concurrent combination of reduced muscle mass and muscle strength. We also found broad agreement that sarcopenia is a generalised disease of skeletal muscle; that prevalence of sarcopenia increases with age; that the definition of sarcopenia should not vary by setting of care, age or condition, or use (clinic vs. research); and that sarcopenia is a potentially reversible disease. Moreover, muscle-specific strength should be considered part of the conceptual definition of sarcopenia (note: muscle-specific strength is defined as ‘strength standardised to muscle size’ e.g. leg extension maximal strength standardised to quadriceps muscle volume [29]). Respondents also agreed that there are many adverse health consequences of sarcopenia including impaired physical performance, mobility limitations (walking and transferring), inability to complete activities of daily living (ADLs) and instrumental activities of living (IADLs), and poor quality of life, and increased risk of falls, fractures, hospitalisation, nursing home admission and mortality.
Several statements were also rejected including whether a conceptual definition of sarcopenia should include levels of severity of disease (which were included in some of the former definitions) and specific statements relating to severity. Therefore, there was no support for a conceptual definition to include severity. Furthermore, impaired physical performance was accepted as an ‘outcome’ (Sarcopenia increases the risk of impaired physical performance (97.9%)) rather than a ‘component’ of sarcopenia (Physical performance should be part of the conceptual definition of sarcopenia (79.4%)).
Altogether, this suggests that a conceptual definition of sarcopenia would include muscle mass, strength and muscle-specific strength as components. However, inclusion of three overlapping components presents challenges for the next steps for GLIS, which are to translate this conceptual definition into an operational definition. These efforts will focus on which measure or measures of muscle mass, strength and specific strength to consider for inclusion and how to develop cut-points for these measures to differentiate older adults with sarcopenia from those without sarcopenia. A challenge that was not previously considered when developing the Delphi statements is how to consider these potentially overlapping components, where muscle mass and strength may be the same components included in the specific strength measure. The overall support for inclusion of specific strength in the conceptual definition for sarcopenia was only slightly higher than our threshold for agreement—80.8% supported its inclusion when the threshold is 80%, and this statement did not meet the threshold in the first round. Therefore, in the next steps of GLIS, we will carefully consider whether an operational definition includes all three measures (muscle mass, strength and specific strength) or just two measures (muscle mass and strength) or just one (muscle-specific strength). The concept of specific strength is interesting as an attempt to blend measurements of muscle mass and strength in a single component [ 29].
The next phase of GLIS will include the operationalization of the newly developed conceptual definition of sarcopenia. This work will be performed by a working group on muscle mass, one on muscle strength and one on clinical outcomes, and will include experts not only in the field of sarcopenia but also in the specific working group topic. As muscle-specific strength is defined by both muscle mass and muscle strength, the working groups on mass and strength will work in harmony to develop the muscle-specific strength component. The working groups will collaborate to agree on the operationalisation, and will closely balance the different factors that need to be considered such as measurement validity, measurement error, clinical accessibility, feasibility and availability of normative data for specific ethnicities and populations. Potentially competing factors may need to be considered. For example, in previous work, the SDOC found that there is substantial disagreement regarding the use of lean mass measured by DXA as a proxy of muscle mass: on one hand, lean mass is widely measured and clinically available; while on the other hand, it does not directly measure muscle mass, and its associations with sarcopenia-related outcomes (mortality, fractures, falls, disability) are weak or inconsistent [1, 20, 21].
A global operationalization of sarcopenia would galvanise clinical and observational research by allowing scientists worldwide to use common terminology, measures and cut-off points to describe the determinants and sequelae of an important condition of ageing and disease, similar to the development of ‘osteopenia’ and ‘osteoporosis’. A global operationalization could be used clinically to identify those who have the condition and would support the development and testing of exercise, nutritional and pharmacological interventions to effectively treat sarcopenia.
Our study has several strengths. The GLIS included many different groups that have previously published guidelines to define sarcopenia and used a transparent, rigorous Delphi process. The GLIS included individuals from all continents with diverse backgrounds including those from academia, clinical practice and pharmaceutical industry. The GLIS included reasonable representation of men and women, and geography, although most of the respondents were from Europe. The total response rate of GLIS was 52% (107/206 participants originally invited agreed to participate) which could have been higher. Nevertheless, we retained 100% (107/107) of participants through both rounds of the Delphi process which is another strength of our study.
In summary, the GLIS has created the first global conceptual definition of sarcopenia, through a rigorous and transparent International Delphi Consensus process, comprising experts from all major sarcopenia societies worldwide. This conceptual definition of sarcopenia will now be used to develop an operational definition of sarcopenia for use in both clinical and research settings. This collaborative effort signifies a critical step towards advancing our understanding and management of sarcopenia on a global scale.
Supplementary Material
Contributor Information
Ben Kirk, Department of Medicine, Western Health, Melbourne Medical School, University of Melbourne, Melbourne, VIC, Australia; Australian Institute for Musculoskeletal Science (AIMSS), University of Melbourne and Western Health, Melbourne, VIC, Australia.
Peggy M Cawthon, California Pacific Medical Center, Research Institute, 550 16th Street, Second Floor, San Francisco, CA 94143 USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, CA USA.
Hidenori Arai, National Center for Geriatrics and Gerontology, Obu, Aichi Japan.
José A Ávila-Funes, Department of Geriatrics, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, Mexico City, Mexico; Bordeaux Population Health Research Center, UMR 1219, University of Bordeaux, Inserm, Bordeaux F-33000, France.
Rocco Barazzoni, Department of Medical, Surgical and Health Sciences, University of Trieste, Trieste, Italy.
Shalender Bhasin, Boston Claude D. Pepper Older Americans Independence Center, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA USA.
Ellen F Binder, Division of General Medicine and Geriatrics, School of Medicine, Washington University in St. Louis, St. Louis MO, USA.
Olivier Bruyere, WHO Collaborating Center for Public Health Aspects of Musculo-Skeletal Health and Ageing, Division of Public Health, Epidemiology and Health Economics, University of Liège, Liège, Belgium; Department of Sport and Rehabilitation Sciences, University of Liège, Liège, Belgium.
Tommy Cederholm, Department of Public Health and Caring Sciences, Clinical Nutrition and Metabolism, Uppsala University, Uppsala, Sweden; Theme Inflammation and Ageing, Karolinska University Hospital, Stockholm, Sweden.
Liang-Kung Chen, Center for Geriatrics and Gerontology, Taipei Veterans General Hospital, Taipei, Taiwan; Center for Healthy Longevity and Aging Sciences, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Cyrus Cooper, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK; Department of Epidemiology, University of Oxford, Oxford, OX UK.
Gustavo Duque, Bone, Muscle & Geroscience Group, Research Institute of the McGill University Health Centre, Montreal, QC, Canada; Dr Joseph Kaufmann Chair in Geriatric Medicine, Department of Medicine, McGill University, Montreal, QC, Canada.
Roger A Fielding, Nutrition Exercise, Physiology, and Sarcopenia Laboratory, Jean Mayer U.S. Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, Boston, MA USA.
Jack Guralnik, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD USA.
Douglas P Kiel, Department of Medicine Beth Israel Deaconess Medical Center and Harvard Medical School, Hinda and Arthur Marcus Institute for Aging Research, Hebrew SeniorLife, Boston, MA USA.
Francesco Landi, Fondazione Policlinico Universitario “Agostino Gemelli” IRCCS, Rome 00168, Italy.
Jean-Yves Reginster, WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging, Liège, Belgium; Chair for Biomarkers of Chronic Diseases, College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia.
Avan A Sayer, AGE Research Group, NIHR Newcastle Biomedical Research Centre, Newcastle Hospitals and Faculty of Medical Sciences Newcastle University, Newcastle, UK.
Marjolein Visser, Department of Health Sciences, Faculty of Science, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands; The Amsterdam Public Health Research Institute, Amsterdam, The Netherlands.
Stephan von Haehling, Department of Cardiology and Pneumology, University Medicine Göttingen (UMG), Göttingen, Germany; German Centre for Cardiovascular Research (DZHK), Partner Site Göttingen, Göttingen, Germany.
Jean Woo, Department of Medicine & Therapeutics, The Chinese University of Hong Kong, Hong Kong, China.
Alfonso J Cruz-Jentoft, Servicio de Geriatría, Hospital Universitario Ramón y Cajal (IRYCIS), Madrid, Spain.
The Global Leadership Initiative in Sarcopenia (GLIS) group:
Alberto Frisoli Júnior, Andrea Britta Maier, Anne B Newman, Anton De Spiegeleer, Antoneta Granic, Antonio Cherubini, Assim AlAbdulKader, Charlotte Beaudart, Brian Clark, Todd Brown, Carla Prado, Carolyn Greig, Chang Won Won, Charlotte Suetta, Chih-Kuang Liang, Christopher Hurst, Daniel Rooks, David Le Couteur, David Scott, Debra Waters, Dolores Sanchez-Rodriguez, Esmee Reijnierse, Eva Topinková, Fanny Petermann, Finbarr Callaghan Martin, Gülistan Bahat, Haya Alhmly, Ivan Aprahamian, Jae-Young Lim, Jean-Pierre Michel, Jesse Zanker, John Batsis, John Kanis, Joshua Lewis, Juergen Bauer, Julie Pasco, Justin Keogh, Kaisu Pitkala, Ken Madden, Kenji Toba, Kristina Norman, Laura Schaap, Lin Kang, Li-Ning Peng, Lisa Micklesfield, Lisette CPGM de Groot, Lorenzo M Donini, Marc Sim, Maria Cristina Gonzalez, Marie-Josiane Ntsama Essomba, Masafumi Kuzuya, Mathis Grossmann, Matteo Cesari, Michael Tieland, Miles Witham, Ming-Yueh Chou, Minoru Yamada, Miranda Grounds, Pedro Abizanda Soler, Qianli Xue, Rachel Cooper, Rainer Wirth, Renuka Visvanathan, Reshma Aziz Merchant, Rene Rizzoli, Robin Daly, Sebastiana Kalula, Sian Robinson, Stany perkisas, Stéphane Schneider, Steven B Heymsfield, Steven Phu, Stuart Phillips, Sunyoung Kim, Suzette Pereira, Thomas Gill, Tomasz Grodzicki, Tomasz Kostka, Tungwai Auyeung, Wee-Shiong Lim, Wei-Ju Lee, Yasmin Algindan, Yosuke Yamada, Yunhwan Lee, Yves Boirie, and Yvette Luiking
Acknowledgements:
The list of societies who sent representatives for the GLIS steering committee:
Aging in Motion (AIM) coalition/Alliance for Aging Research (AAR)—Jack Guralnik
American Geriatrics Society (AGS)—Ellen F. Binder
American Society for Bone and Mineral Research (ASBMR)—Douglas P. Kiel
Asian Association for Frailty and Sarcopenia (AAFS) —Liang-Kung Chen
Australian and New Zealand Society for Sarcopenia and Frailty Research (ANZSSFR) —Gustavo Duque
European Association for the Study of Obesity (EASO)—Rocco Barazzoni
European Geriatric Medicine Society (EuGMS)—Alfonso J. Cruz-Jentoft
European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO)—Olivier Bruyere
European Society for Clinical Nutrition and Metabolism (ESPEN)—Tommy Cederholm
Gerontological Society of America (GSA)—Peggy M. Cawthon & Roger A. Fielding
International Association of Gerontology and Geriatrics (IAGG)—José A. Ávila-Funes
International Conference on Frailty and Sarcopenia Research (ICFSR)—Roger A. Fielding
International Osteoporosis Foundation (IOF)—Cyrus Cooper & Jean-Yves Reginster
Society on Sarcopenia, Cachexia and Wasting Disorders (SCWD)—Stephan von Haehling
The authors would like to extend their gratitude to Katrin Werner-Perez and Lindsay Clarke, both from the Alliance for Aging Research, for their administrative support which greatly assisted this Delphi study.
Declaration of Conflicts of Interest:
Before starting the GLIS initiative, all authors submitted a Declaration of Interest form. This declaration, which is more detailed than the standard COI form, was open for public scrutiny and can be viewed here: https://www.eugms.org/fileadmin/images/news/2022/GLIS_Steering_Committee_Rev3.pdf.
Declaration of Sources of Funding:
R.A.F. was supported by the U.S. Department of Agriculture, under agreement No. 58-1950-4-003. Any opinions or recommendations expressed in this publication are those of the author (s) and do not necessarily reflect the views of the U.S. Department of Agriculture. All authors declarations of sources of funding can be read here: https://www.eugms.org/fileadmin/images/news/2022/GLIS_Steering_Committee_Rev3.pdf.
References
- 1. Cawthon PM, Manini T, Patel SM et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc 2020; 68: 1429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cawthon PM, Blackwell T, Cummings SR et al. Muscle mass assessed by the D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community-dwelling older men. J Gerontol A Biol Sci Med Sci 2021; 76: 123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Guralnik JM, Cawthon PM, Bhasin S et al. Limited physician knowledge of sarcopenia: a survey. J Am Geriatr Soc 2023; 71: 1595–602. [DOI] [PubMed] [Google Scholar]
- 4. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet 2019; 393: 2636–46. [DOI] [PubMed] [Google Scholar]
- 5. Anker SD, Morley JE, von HaehlingS. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle 2016; 7: 512–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayhew AJ, Amog K, Phillips S et al. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing 2019; 48: 48–56. [DOI] [PubMed] [Google Scholar]
- 7. Kirk B, Mooney K, Amirabdollahian F, Khaiyat O. Exercise and dietary-protein as a countermeasure to skeletal muscle weakness: Liverpool Hope University – Sarcopenia Aging Trial (LHU-SAT). Front Physiol 2019; 10: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirk B, Mooney K, Cousins R et al. Effects of exercise and whey protein on muscle mass, fat mass, myoelectrical muscle fatigue and health-related quality of life in older adults: a secondary analysis of the Liverpool Hope University—Sarcopenia Ageing Trial (LHU-SAT). Eur J Appl Physiol 2020; 120: 493–503. [DOI] [PubMed] [Google Scholar]
- 9. Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians: effects on skeletal muscle. JAMA 1990; 263: 3029–34. [PubMed] [Google Scholar]
- 10. Pahor M, Guralnik JM, Ambrosius WT et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 2014; 311: 2387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernabei R, Landi F, Calvani R et al. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ 2022; 377: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen LK, Liu LK, Woo J et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. [DOI] [PubMed] [Google Scholar]
- 13. Chen L-K, Woo J, Assantachai P et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc 2020; 21: 300–307.e2. [DOI] [PubMed] [Google Scholar]
- 14. Zanker J, Sim M, Anderson K et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J Cachexia Sarcopenia Muscle 2023; 14: 142–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zanker J, Scott D, Reijnierse EM et al. Establishing an operational definition of sarcopenia in Australia and New Zealand: Delphi method based consensus statement. J Nutr Health Aging 2019; 23: 105–10. [DOI] [PubMed] [Google Scholar]
- 16. Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing 2010; 39: 412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cruz-Jentoft AJ, Bahat G, Bauer J et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Studenski SA, Peters KW, Alley DE et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014; 69: 547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fielding RA, Vellas B, Evans WJ et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhasin S, Travison TG, Manini TM et al. Sarcopenia definition: the position statements of the sarcopenia definition and outcomes consortium. J Am Geriatr Soc 2020; 68: 1410–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cawthon PM, Travison TG, Manini TM et al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: proceedings of the sarcopenia definition and outcomes consortium conference. J Gerontol A Biol Sci Med Sci 2020; 75: 1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donini LM, Busetto L, Bischoff SC et al. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO consensus statement. Clin Nutr 2022; 41: 990–1000. [DOI] [PubMed] [Google Scholar]
- 23. Jensen GL, Cederholm T, Correia MITD et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J Parenter Enteral Nutr 2019; 43: 32–40. [DOI] [PubMed] [Google Scholar]
- 24. Cederholm T, Jensen GL. To create a consensus on malnutrition diagnostic criteria: a report from the Global Leadership Initiative on Malnutrition (GLIM) meeting at the ESPEN Congress 2016. Clin Nutr 2017; 36: 7–10. [DOI] [PubMed] [Google Scholar]
- 25. Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. J Am Med Dir Assoc 2016; 17: 675–7. [DOI] [PubMed] [Google Scholar]
- 26. Rolland Y, Cruz-Jentoft AJ. Editorial: sarcopenia: keeping on search for the best operational definition. J Nutr Health Aging 2023; 27: 202–4. [DOI] [PubMed] [Google Scholar]
- 27. Sanchez-Rodriguez D, Marco E, Cruz-Jentoft AJ. Defining sarcopenia: some caveats and challenges. Curr Opin Clin Nutr Metab Care 2020; 23: 127–32. [DOI] [PubMed] [Google Scholar]
- 28. Cruz-Jentoft AJ, Gonzalez MC, Prado CM. Sarcopenia ≠ low muscle mass. Eur Geriatr Med 2023; 14: 225–8. [DOI] [PubMed] [Google Scholar]
- 29. Cawthon PM, Visser M, Arai H et al. Defining terms commonly used in sarcopenia research: a glossary proposed by the Global Leadership in Sarcopenia (GLIS) steering committee. Eur Geriatr Med 2022; 13: 1239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cruz-Jentoft AJ. Diagnosing sarcopenia: turn your eyes back on patients. Age Ageing 2021; 50: 1904–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.