Summary
Cellular energy metabolism analysis is complex, expensive, and indirect. We present a protocol to analyze relative contribution of metabolic pathways to ATP production by directly measuring ATP levels. We describe steps for cell counting and seeding in 96-well plate, treating with metformin, and systematic inhibition with metabolic inhibitors. We then detail procedures for a viability and ATP assay and calculating energy metabolism dependency. This high-throughput and accessible protocol works with any cell line and allows for flexible perturbation studies.
Subject areas: Cancer, Metabolism, Molecular Biology
Graphical abstract

Highlights
-
•
Protocol for measuring ATP levels in HepG2 cells
-
•
Steps for cell seeding, metabolic inhibitor treatment, and cell viability and ATP assays
-
•
Instructions for ATP level normalization and metabolic dependency calculation
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Cellular energy metabolism analysis is complex, expensive, and indirect. We present a protocol to analyze relative contribution of metabolic pathways to ATP production by directly measuring ATP levels. We describe steps for cell counting and seeding in 96-well plate, treating with metformin, and systematic inhibition with metabolic inhibitors. We then detail procedures for a viability and ATP assay and calculating energy metabolism dependency. This high-throughput and accessible protocol works with any cell line and allows for flexible perturbation studies.
Before you begin
Metabolic reprogramming is a hallmark of cancer cells.1 Eukaryotic cells produce adenosine triphosphate (ATP)-the energy currency of the cell primarily via glycolysis or oxidative phosphorylation, which may use reducing equivalents generated from glucose or amino acid metabolism or fatty acid oxidation (Figure 1A). Depending upon resources available as well as external and internal perturbations, cancer cells shift their dependency on these pathways. Understanding pathway-wise capacities and dependencies are essential to devise therapeutic strategies. Existing methods to deduce the differential reliance of cancer cells on these pathways include metabolic flux analysis by extracellular flux analyzer (Seahorse2), mass spectrometry-based metabolomics,3 and measuring metabolic enzyme activity.4 However, these methods are complex, expensive and require specialized technical proficiency as well. A comparison of the methods is enlisted in Table 1.
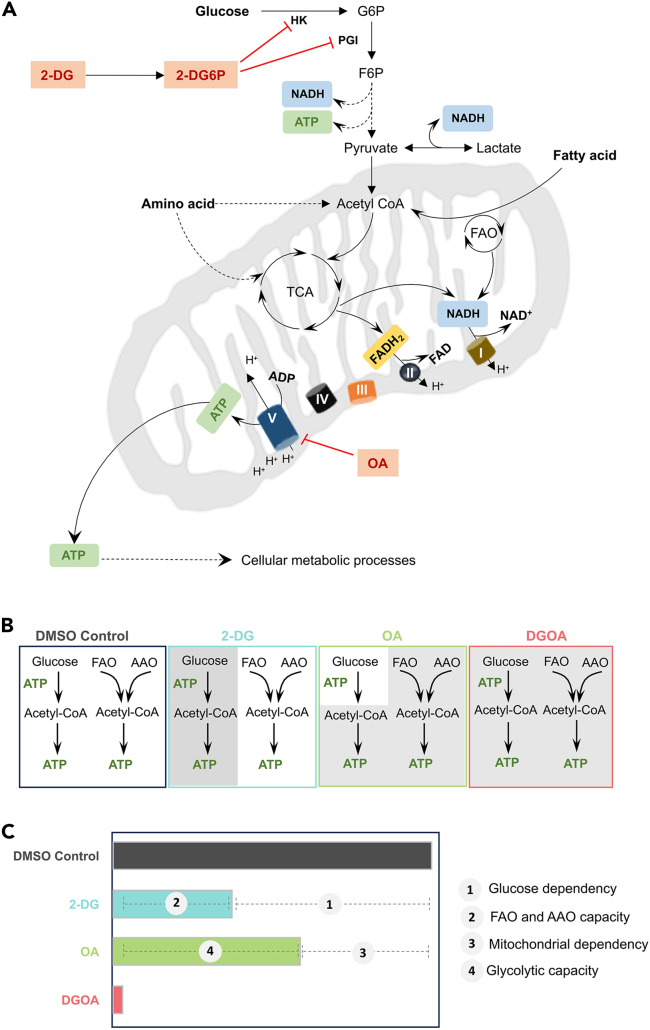
Figure 1.
Energy metabolic pathways in cancer cells and their systematic inhibition to delineate differential dependencies and capacities
(A) A schematic diagram of energy metabolic pathways in cancer cells that generate ATP. (Abbreviations: 2-DG- 2-deoxy-D-Glucose, 2-DG6P- 2-deoxy-D-Glucose-6-phosphate, HK- hexokinase, PGI- glucose-6-phosphate isomerase, G6P- glucose-6-phosphate, F6P- fructose-6-phosphate, TCA- tricarboxylic acid cycle, FAO- fatty acid oxidation, I-V- complex I to V of the electron transport chain, OA- oligomycin A, ADP- adenosine diphosphate, ATP- adenosine triphosphate).
(B) Systematic inhibition of energy metabolic pathways using either 2-deoxy-D-glucose (2-DG) or oligomycin A (OA) or both (DGOA) for estimating fractional contribution of each pathway towards ATP production. (Abbreviations: FAO - Fatty acid oxidation, AAO - amino acid oxidation, DGOA - simultaneous treatment with 2-DG and OA).
(C) A horizontal bar plot cartoon showing the calculations of differential dependencies and capacities from ATP assay after inhibition.
Table 1.
Comparative table of methods
| Current protocol | SCENITH | Seahorse | Mass spectrometry | |
|---|---|---|---|---|
| Consumable cost/samplea | Low (< 1 USD) | Medium (1–5 USD) | High (≈10 USD) | High (5–10 USD) |
| Instrumentation cost | Low | High | High | Very high |
| Technical expertise required | Less | High | High | Very high |
| High throughput amenability | Very high | Low | High | High |
| Read out for metabolic profiling | Luminescence-based ATP | Protein synthesis rate as a surrogate marker for ATP | Extracellular oxygen, and pH | Metabolites |
| Single cell resolution | No | Yes | No | Yesb |
| Time (Hrs.) for profiling a batch of 24 samples.c | 4 | 4–65 | 242 | 1–24d |
Estimated cost excluding cell culture. Costs are empirical, may differ according to the brands used.
Single-cell metabolomics platforms. e.g.- MALDI, SIMS, DESI etc.6
Time calculated from introduction of metabolic inhibitor to data acquisition.
Depending upon the platform (e.g., – MALDI, LC-MS, GC-MS etc.), run time of each sample is 5–60 min.
Notably, the flux analyzer estimates cellular energetics based on oxygen consumption rate (OCR) and extra cellular acidification rate (ECAR). However, neither all oxygen consumed by cell is used in electron transport chain (ETC) nor all oxygen consumed through electron transport chain ends up in ATP production.7 They may also result in reactive oxygen species,8 particularly, in mitochondria with leaky ETC machinery. Recently, Argüello et al. introduced a simple, flow cytometry-based method to profile energy metabolism at single cell resolution.5 While elegant, it used de novo protein synthesis rate as the surrogate for ATP production. However, de novo protein synthesis and proliferation may not always be a linear function of the ATP production of the cells. For example, targeting mammalian target of rapamycin (mTOR) signaling may alter protein synthesis irrespective of proportional change of ATP abundances.9 In fact, an earlier study showed that FCCP treatment, which leads to uncoupling, increased proliferations in cells with compromised NAD+/NADH ratio.10 Cyclin-dependent kinases (CDKs)-controlled quiescence and proliferative signals in cancer cells may also decouple ATP level and proliferation-associated protein synthesis.11 Additionally, discrepancy between de novo protein synthesis and ATP production rates may also arise due to utilization of ATP in several other biochemical pathways.12 A direct measurement of ATP production would not suffer from such limitations and give a more accurate assessment of the cellular energy metabolism dynamics. Hence, we employed a high-throughput luminescence-based ATP assay to enable measurement of average capacity and dependency of a cell population on different pathways involved in energy metabolism as depicted in Figures 1B and 1C.
Here, we describe analysis of the differential dependency of HepG2 cells on energy metabolic pathways upon metformin treatment (Figure 2). Cell viability-normalized ATP level was further used to calculate Glucose dependency, Fatty acid oxidation and Amino acid oxidation capacity, Mitochondrial dependency, and Glycolytic capacity. Thus, this protocol is able to provide a quick overview of the average bioenergetic status of these cells. In principle, this protocol is fit for application to any cancerous or non-cancerous cell line and drug of interest.
Figure 2.
Overview of the current protocol
Cell culture and maintenance
Timing: Approximately 7 days
-
1.Reviving the cells from stock.
-
a.Take out HepG2 cell stock from liquid nitrogen storage.
-
b.Thaw the stock in 37°C water bath 2–3 min or till the ice melts.Optional: Centrifuge the vial at 600X g for 5 min at 25°C–30°C to pellet down the cells to get rid of cytotoxic DMSO. Wipe the outer surface of the vial with 70% ethanol and transfer into the cell culture hood. Carefully discard the supernatant and resuspend the cells with pre-warmed DMEM media.
-
c.Wipe the outer surface of the vial with 70% ethanol and transfer into the mammalian cell culture hood.
-
d.Pour the contents in a 100 mm cell culture plate containing complete media (low glucose (1 g/L) DMEM medium supplemented with 10% FBS, 100 IU/mL penicillin, and 50 μg/mL Streptomycin antibiotics.).Note: Use appropriate cell culture plate for the cell type. Treated plates are for adherent cells and non-treated plates are for suspension culture. Ensure the use of appropriate culture medium and FBS concentration for the cell type of interest.
-
e.Incubate the plate in a humidified mammalian cell culture incubator at 37°C with 5% CO2 supply.
-
f.On the next morning, carefully wash the cell plates with 37°C warm Phosphate buffer saline (PBS) twice and add fresh complete media.
-
g.Change the media in every 48 h until the cell confluency reaches up to 70%–80%.
-
a.
-
2.Passaging and subculture.
-
a.Discard the media, wash twice with warm PBS.
-
b.Add 1 mL of 0.25% trypsin-EDTA solution and swirl the plate to make sure total surface is covered.
-
c.Keep the plate in the incubator for 5 min.Note: Incubation period may be different for different cell type. Incubate them accordingly.
-
d.Take out the plate and flush the cells with 3 mL fresh warm media.
-
e.Split the cells into 4 new plates containing 9 mL of complete media.
-
f.Subculture the cells likewise for another three passages before setting up the experiment.
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Dulbecco’s modified Eagle’s medium (DMEM) | HiMedia | AT006 |
| Penicillin G sodium salt | HiMedia | TC020 |
| Streptomycin sulphate | HiMedia | TC035 |
| Fetal bovine serum, certified, United States | Gibco | 16000044 |
| Trypsin-EDTA (0.25%), phenol red | Gibco | 25200072 |
| 2-deoxy-D-glucose | TCI | D0051 |
| DMSO | Sigma-Aldrich | D8418-100ML |
| Oligomycin A | Sigma-Aldrich | 75351 |
| 1,1-dimethylbiguanide hydrochloride (metformin) | Sigma-Aldrich | D150959 |
| Trypan blue solution, 0.4% | Gibco | 15250061 |
| Sodium chloride | Sigma-Aldrich | S9625 |
| Potassium chloride | Sigma-Aldrich | P9541 |
| Sodium phosphate dibasic | Sigma-Aldrich | S9763 |
| Potassium phosphate monobasic | Sigma-Aldrich | P0662 |
| Hydrochloric acid | Sigma-Aldrich | 258148 |
| Critical commercial assays | ||
| Cell proliferation kit II (XTT) | Sigma-Aldrich | 11465015001 |
| Luminescent ATP detection assay kit | Abcam | ab113849 |
| Experimental models: Cell lines | ||
| HepG2 cell line | Mukherjee et al.13 | NA |
| Software and algorithms | ||
| Microsoft Office | Microsoft | www.microsoft.com |
| GraphPad Prism 9.0 | Dotmatics | https://www.graphpad.com |
| BioRender | BioRender | https://www.biorender.com |
| Other | ||
| 100 mm cell culture dishes | Thermo Fisher Scientific | 150464 |
| Nunc serological pipettes | Thermo Fisher Scientific | 170356N |
| Microscopic cover glass | Blue Star | https://bluestarslides.com |
| Eppendorf conical tubes 15 mL | Eppendorf | 0030122151 |
| Counting chamber BLAUBRAND Neubauer improved, w/o clips double ruling | BRAND | 717805 |
| Nunc 96-well flat bottom, clear | Thermo Fisher Scientific | 161093 |
| Nunc 96-well flat bottom, white | Thermo Fisher Scientific | 136102 |
| BioTek Synergy HTX multimode reader | BioTek | 1623207 |
Materials and equipment
-
•
1X Phosphate Buffer Saline, pH- 7.4.
| Reagent | Final concentration | Amount |
|---|---|---|
| Sodium chloride | 0.137 M | 8 g |
| Potassium Chloride | 0.0027 M | 0.2 g |
| Sodium Phosphate Dibasic | 0.01 M | 1.44 g |
| Potassium Phosphate Monobasic | 0.0018 M | 0.245 g |
| Hydrochloric acid | N/A | Adjust pH to 7.4 |
| ddH2O | N/A | Up to 1 L |
| Total | N/A | 1 L |
Note on storage conditions: filter with 0.22 μ filter into a sterile bottle and store in 4°C, use within 3 months.
-
•
Complete DMEM media.
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM | 90% | 900 mL |
| Penicillin | 100 IU/mL | 0.06 g |
| Streptomycin | 50 μg/mL | 0.05 g |
| Fetal bovine serum | 10% | 100 mL |
| Total | N/A | 1 L |
Note on storage conditions: filter with 0.22 μ filter into a sterile bottle and store in 4°C, use within 1 month.
Step-by-step method details
Cell counting and seeding
Timing: 1 h
This step describes the counting of the cell and seeding 10000 cells in each well of the 96-well cell culture plate.
-
1.Harvesting of the cells.
-
a.Discard the media from the plate.
-
b.Wash the plate twice with warm PBS.
-
c.Add 1 mL Trypsin-EDTA solution to the plate and swirl to cover the whole plate.
-
d.Keep the plate in the incubator for approximately 5 min.
-
e.Flush the cells with 2 mL complete media.
-
f.Make a single cell suspension by gentle pipetting and transfer that to a 15 mL centrifuge tube.
-
a.
CRITICAL: Perform these steps as quickly as possible to avoid any unnecessary stress to the cells. Avoid over-pipetting to make cell suspension as they may rupture the cells.
Note: The method can also be adapted for a 384 well format. Number of seed cells, volume of media and subsequent buffers and reagents needs to be modified accordingly.
-
2.Cell counting using hemocytometer.Alternatives: Automated cell counter can be used for more accurate counting.
-
a.Take 100 μL of cell suspension to a fresh 1.5 mL microcentrifuge tube.
-
b.Add 100 μL trypan blue solution to the tube and incubate at 25°C–30°C for 3 min. Thus, making a 2-fold diluted cell suspension compared to original stock.Note: Dilute the cells higher than 2-fold if initial cell number is too high. Too much cells in the cell counting chamber may lead to error. Typically, it should be diluted to count 20–50 cells per square of the hemocytometer.
 CRITICAL: Incubation of cells with Trypan blue should not be more than 3–5 min.
CRITICAL: Incubation of cells with Trypan blue should not be more than 3–5 min. -
c.Now, clean the hemocytometer and 22 mm cover slip with lint free tissue paper.
-
d.Put the hemocytometer on a flat parallel surface and place the cover slip on the top of the counting area.
-
e.Take 10 μL of the diluted cell suspension and slowly expel to the counting surface underneath the cover slip.
-
f.Under the microscope, count the total cell present in the 4 large square at each corner containing 16 smaller squares.Note: Follow standard cell counting strategies for counting with hemocytometer detailed elsewhere.14 Counting cells in larger square by repeat sampling increase the accuracy of counted cell number.
-
g.Estimate the cell number per mL by the formula –
Total cells/mL = (Total cells counted / Number of squares counted) x Dilution factor x 10,000. Note: Dilution factor would be 2 as per step b above. Use appropriate dilution factor if the cell suspension is further diluted to adjust cell numbers per square. -
h.Use similar formula as above to count total live and dead cells separately. Only use the live cell number to adjust for seeding in 96-well plate.
-
a.
-
3.Seeding the cells in 96-well cell culture plate.
-
a.Adjust the cell number to 105 cells/ mL from the original cell stock of step 2a. with complete media.Note: Pre-determine the total number of wells required for the experiment and adjust the volume of diluted cell suspension.
-
b.Add 100 μL to each well of white and clear cell culture treated flat bottom 96-well plate so that each well contains 10000 cells.Note: White plate is for luminescence-based assay and clear plate is used for colorimetric assay.
-
c.Seed the cells to 12 wells in a row for each treatment condition. (Figure 3).Note: Seed more wells according to experimental conditions of interest.
-
d.Also add only media without any cell in 5 wells for use as blank.
-
e.Keep the plates 12–16 h in the incubator for attachment of cells to the plate surface. After successful attachment of the cells, proceed to the next step.Note: Different cell types have different attachment times. Proceed to the next step accordingly.
-
a.
Figure 3.
Experimental setup on a 96-well cell culture plate
Row A: control groups; Row B: metformin treatment groups treated with 2.5 mM metformin for 48 h. Afterward both were incubated with 100 mM 2-deoxy-D-glucose (2-DG) or 1 μM Oligomycin A (OA), or both, for 1 h. Dimethyl sulfoxide (DMSO) used as a vehicle control. Color codes for each treatment indicated in the figure.
Metformin treatments
Timing: 48 h
This step summarizes the intervention that we add to the cells and profile the metabolic dependency based on our research interest. Here we used metformin as our drug of interest.
-
4.Metformin treatment.
-
a.Prepare fresh media containing 2.5 mM metformin from the 1 M metformin stock enough for 24 wells. For preparation of control media add equal volume of sterile distilled water to the fresh media.Note: Use freshly prepared drug stocks and sterilize it by passing through 0.22 μ syringe filter.Use compatible filter membrane according to solvent used for preparing drug solution.
-
b.Discard the media of the plate carefully without disturbing the cells.
-
c.Wash the cells twice with warm PBS.Note: Use multichannel pipettes to discard media and washing. Take utmost care not to scratch the surface of the wells.
-
d.Add 100 μL metformin containing media to each well designated as ‘treatment’ of both the plates.
-
e.Add 100 μL control media to the ‘control’ wells in both plates.
-
f.Add 100 μL complete media to the ‘blank’ wells in both plates.
-
g.Keep the plate in the incubator for 48 h.Note: Preferably use multichannel pipettes to dispense media quickly. Incubation time would vary according to the experimental design.
-
a.
Specific pathway inhibition by inhibitors
Timing: 1.30 h
This step introduces the metabolic inhibitors to acutely block the specific energy metabolism pathway.
-
5.Incubation with specific metabolic inhibitor (problem 1).
-
a.Directly add 2DG or OA or both to the same media used in step 4 to achieve a final concentration of 100 mM 2-DG or 1 μM OA or both (2-DG+OA) in the dedicated wells for each condition in both plates.Note: Prepare drug stock concentration in such a way that the volume of stock (e.g.- 10 μL) do not exceed 10% of the culture media volume to achieve the working concentration.
-
b.Calculate the final volume of DMSO that was introduced with the OA and accordingly add the same volume of DMSO in the vehicle control wells in both the plate.
-
c.Keep the plates in the incubator for 1 h.
-
a.
Cell viability assay
Timing: 4.30 h
This step describes the estimation of the viability of the cells in each condition after treatment and pathway inhibition. The purpose of this step is to estimate the viability, which will be used to normalize the ATP assay data to estimate per cell ATP level.
-
6.XTT assay for estimation of viability (problem 3).
-
a.Take out the clear 96-well plate from incubator after 1 hr. incubation with pathway inhibitors in the previous step.
-
b.Perform the viability assay with Cell Proliferation Kit II (XTT) from Roche according to manufacturer’s protocol with slight modification.
-
c.Just before the assay thaw and mix sufficient amount of XTT labeling reagent and coupling reagent in 50:1 ratio.
-
d.Add 10 μL of the XTT mixture in each well including blank wells.Alternatives: MTT based assay can also be used instead of XTT assay. However, XTT-based assay has the advantage of producing soluble dye making the assay more convenient.Note: Optimize the volume according to cell type and cell number.
-
e.Incubate the plate in the incubator for 4 hr.Note: Visually inspect the color development. Incubate for longer duration if needed.
-
f.Take the absorbance in a multi-well spectrophotometer at 492 nm wavelength.
-
a.
ATP assay
Timing: 30 min
This step summarizes the in-situ cell lysis and release of ATP by mild detergent followed by incubation with substrate solution containing luciferin and luciferase. The luciferase in the presence of cellular ATP catalyzes conversion of luciferin to oxyluciferin that luminescent at 560 nm wavelength. In this step, Luminescent ATP Detection Assay Kit from Abcam was utilized following the manufacturer’s protocol.
Alternatives: Other colorimetric method can be applied. However, luminescence-based assay kit is recommended due to its superior sensitivity and cost effectiveness.
-
7.Cell lysis and stabilization of ATP.
-
a.Take out the white 96-well plate from incubator after 1 hr. incubation with EM pathway inhibitor in the step 5c.
-
b.Thaw and add 50 μL detergent solution to each well and shake in an orbital shaker for 20 min at 25°C–30°C.
-
a.
Note: Shaking duration should be optimized for different cell types.
Optional: Additionally at this step a series of standard ATP dilutions can be added to the unused wells as directed by the manufacturers instruction to make a calibration curve for quantitation of ATP.
-
8.ATP detection (problem 2, problem 3).
-
a.Add 50 μL substrate solution to each well and cover the plate in aluminum foil.Note: Reduce the substrate volume if luminescence is too high.
-
b.Shake the plate in an orbital shaker for 5 min at 25°C–30°C.
-
c.Measure the luminescence with a microplate reader with 560 nm filter set.
-
a.
Calculations
Timing: 1–2 h
Estimation of cell viability, ATP level, normalization of ATP level with viability, and metabolic dependency profile calculations are described.
-
9.Estimation of cell viability.
-
a.Export the raw absorbance values of XTT assay in an excel sheet. (Table 2).
-
b.Annotate the conditions (e.g.- Control, Metformin treated) and groups (DMSO control, 2-DG, OA, and DGOA) properly.
-
c.Take average of blank absorbance values.
-
d.Subtract the average blank value from each well of the samples. (Table 3).
-
e.Now, calculate average value of the control wells (M) (control, DMSO control).
-
f.Calculate the relative viability of each well respective to A by the formula –where V is relative viability, x is value of each well, and M is average of the control value. (Table 4).
-
g.Average the viability of each group. (Table 5).
-
a.
-
10.Estimation and normalization of ATP level.
-
a.Export the raw luminescence values of ATP assay in an excel sheet. (Table 6).
-
b.Annotate the conditions (e.g.- Control, Metformin treated) and groups (DMSO control, 2-DG, OA, and DGOA) properly.
-
c.Take average of the blank values.
-
d.Subtract the average blank value from each well. (Table 7).
-
e.Normalize the ATP level by dividing the each well of the group with the group specific viability. (Table 8).
-
f.Now you have cell viability-normalized ATP level in each group of respective conditions.
-
a.
-
11.
Metabolic capacity and dependency calculations.
Here, with the normalized ATP level, the degree of reliance of the cells on different metabolic pathway to produce energy currency (i.e.- ATP) was calculated.-
a.Use the following formulas to calculate the metabolic dependency and capacity in each condition (Table 9).
-
i.Glucose dependence (%) = [(DMSO control – 2-DG)/ (DMSO control – DGOA)] X 100.
-
ii.FAO & AAO capacity (%) = 100 – Glucose dependence.
-
iii.Mitochondrial dependence (%) = [(DMSO control – OA)/ (DMSO control – DGOA)] X 100.
-
iv.Glycolytic capacity (%) = 100 – Mitochondrial dependence.Note: Cellular ATP is produced via glycolytic and TCA cycle mediated glucose oxidation or fatty acid and amino acid oxidation followed by oxidative phosphorylation as depicted in Figure 1A. The glucose metabolism is inhibited by 2-deoxyglucose (2-DG), whereas the oxidative phosphorylation is inhibited by oligomycin A (OA). The decrease in ATP level upon simultaneous use of 2-DG and OA (DGOA) indicates total amount of ATP produced via these pathways. Stepwise inhibition of ATP production using 2-DG or OA can, thus, be used to delineate the fractional contribution of the individual modes of ATP production as schematically shown in Figures 1B and 1C. The fraction of ATP produced by utilizing glucose in aerobic glycolysis and TCA cycle is termed as glucose dependence, which can be obtained by 2-DG mediated inhibition of glucose metabolism. The rest is basically the fractional contribution of oxidation of fatty acids and amino acids to generate ATP via oxidative phosphorylation. This is called fatty acid oxidation and amino acid oxidation capacity (FAO & AAO capacity), which can be obtained by subtracting the fractional contribution from glucose metabolism (using 2-DG). Use of OA inhibits mitochondrial metabolism of glucose as well as fatty acids and amino acids towards ATP production. Thus, the fractional contribution of mitochondrial metabolism can be calculated from the relative decrease in ATP level upon OA treatment, which is defined as mitochondrial dependence. The remaining fraction is basically from glycolysis, which is defined as the glycolytic capacity.
-
i.
-
a.
Table 2.
Raw absorbance values of XTT assay measured on a plate reader
| DMSO control | 2-DG | OA | DGOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.484 | 0.48 | 0.545 | 0.378 | 0.437 | 0.435 | 0.508 | 0.524 | 0.588 | 0.577 | 0.537 | 0.557 |
| Metformin | 0.286 | 0.301 | 0.335 | 0.332 | 0.345 | 0.348 | 0.361 | 0.326 | 0.348 | 0.393 | 0.427 | 0.32 |
| Blank | 0.056 | 0.054 | 0.052 | 0.053 | 0.051 | 0.052 | ||||||
Table 3.
Blank subtracted XTT absorbance values
| DMSO control | 2-DG | OA | DGOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0.431 | 0.427 | 0.492 | 0.325 | 0.384 | 0.382 | 0.455 | 0.471 | 0.535 | 0.524 | 0.484 | 0.504 |
| Metformin | 0.233 | 0.248 | 0.282 | 0.279 | 0.292 | 0.295 | 0.308 | 0.273 | 0.295 | 0.34 | 0.374 | 0.267 |
Table 4.
XTT values normalized with respect to DMSO control group of Control arm
| DMSO control | 2-DG | OA | DGOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 95.78 | 94.89 | 109.33 | 72.22 | 85.33 | 84.89 | 101.11 | 104.67 | 118.89 | 116.44 | 107.56 | 112.00 |
| Metformin | 51.78 | 55.11 | 62.67 | 62.00 | 64.89 | 65.56 | 68.44 | 60.67 | 65.56 | 75.56 | 83.11 | 59.33 |
Table 5.
Mean relative viability derived from XTT assay
| DMSO control | 2-DG | OA | DGOA | |
|---|---|---|---|---|
| Control | 100 | 80.81481 | 108.2222 | 112 |
| Metformin | 56.5185185 | 64.14815 | 64.88889 | 72.66667 |
Table 6.
Raw luminescence values of ATP assay measured on a plate reader
| DMSO control | 2-DG | OA | DGOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 11724 | 17913 | 13637 | 3622 | 2856 | 2970 | 13936 | 12023 | 14025 | 265 | 485 | 400 |
| Metformin | 4789 | 4964 | 4274 | 512 | 491 | 613 | 2939 | 1087 | 1479 | 236 | 288 | 283 |
| Blank | 33 | 42 | 52 | 52 | 68 | |||||||
Table 7.
Blank subtracted ATP luminescent values
| DMSO control | 2-DG | OA | DGOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 11674.6 | 17863.6 | 13587.6 | 3572.6 | 2806.6 | 2920.6 | 13886.6 | 11973.6 | 13975.6 | 215.6 | 435.6 | 350.6 |
| Metformin | 4739.6 | 4914.6 | 4224.6 | 462.6 | 441.6 | 563.6 | 2889.6 | 1037.6 | 1429.6 | 186.6 | 238.6 | 233.6 |
Table 8.
ATP luminescent values after normalization with respective viability
| DMSO control | 2-DG | OA | DGOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 116.75 | 178.64 | 135.88 | 44.21 | 34.73 | 36.14 | 128.32 | 110.64 | 129.14 | 1.93 | 3.89 | 3.13 |
| Metformin | 83.86 | 86.95 | 74.75 | 7.98 | 7.65 | 9.56 | 44.53 | 15.99 | 22.03 | 2.58 | 3.30 | 3.23 |
Table 9.
Energy metabolism pathway dependency calculated from normalized ATP luminescent values
| Control | Metformin | |||||
|---|---|---|---|---|---|---|
| Glucose dependency | 70.18574 | 77.94879 | 76.52491 | 93.1908 | 94.46181 | 91.95957 |
| FAO & AAO capacity | 29.81426 | 22.05121 | 23.47509 | 6.8092 | 5.538192 | 8.040429 |
| Mitochondrial Dependency | 10.88252 | 23.67409 | 10.39097 | 47.08214 | 83.84894 | 76.09105 |
| Glycolytic Capacity | 89.11748 | 76.32591 | 89.60903 | 52.91786 | 16.15106 | 23.90895 |
Expected outcomes
This protocol describes a simple, cost-effective, high throughput technique to profile energy metabolism pathway dependency. Following this protocol, one is expected to obtain a per cell ATP abundance. From that ATP abundance, the relative contribution of energy metabolic pathways to produce ATP can be derived. Thus, a differential energy metabolic pathway activity profile upon perturbation can be analyzed.
We applied this protocol to analyze the change of energy pathway dependency due to metformin treatment in HepG2 cell line. We found that 48 h of 2.5 mM metformin exposure decreased the viability (Figure 4A) compared to respective controls. However, viability was not significantly affected in either control or metformin treated group after 1 h incubation with metabolic pathway inhibitors (Figure 4A). Parallelly, we found that the relative ATP level was reduced significantly upon metformin treatment (Figure 4B). Also, as expected the metabolic pathway inhibitors reduced ATP levels compared to the respective controls. In order to account for the contribution of changes in cell viability to the ATP production, the ATP levels were normalized with respect to cell viability of respective groups. This gives an estimate of the cellular ATP level per viable cell under different conditions. Thus, the changes of ATP level upon inhibition of different EM pathways in control and metformin-treated cells were calculated and plotted as shown in Figure 4C. These were used to calculate the Glucose dependency, Fatty acid oxidation & Amino acid oxidation capacity, Mitochondrial dependency, and Glycolytic capacity. Results (Figure 4D) showed that metformin, increased glucose dependency of HepG2 cells while reducing mitochondrial fatty acid and amino acid oxidation capacity. A heightened mitochondrial dependency and concomitant reduction of glycolytic capacity following metformin administration was also observed. An increased shuttling of cytosolic NADH via glycerol phosphate shuttling to deliver FADH2 in the mitochondrial matrix can increase ATP production using complex-II of electron transport chain supported with the fact that metformin inhibits complex-I15 but not mitochondrial glycerol phosphate dehydrogenase (mGPD).16 These results are in line with earlier observations that metformin increases glycolytic glucose metabolism17 while perturbing TCA cycle18 and reducing amino acid uptake and metabolism.19
Figure 4.
Energy metabolism pathway dependency profile of control and 2.5 mM metformin treated HepG2 cells
(A) Relative viability of the control (gray bars) or 48-h metformin-treated (orange bars) HepG2 cells treated with DMSO or 100 mM 2-deoxy-D-glucose (2-DG) or 1 μM Oligomycin A (OA) or both for 1 h estimated by XTT assay.
(B) Relative levels of ATP in respective groups.
(C) Viability-normalized relative cellular ATP levels in respective groups estimated by luminescent-based ATP assay.
(D) Energy metabolism pathway dependency profile calculated from normalized ATP levels in respective groups. Data shown as mean ± SD. n = 3 biological replicates. Two tailed Student’s t test was applied for significance testing, ∗ indicates p < 0.05.
Quantification and statistical analysis
As detailed in the previous steps, the absorbance and luminescence values were acquired and processed. Mean relative viability was calculated (Table 5) from raw absorbance values (Table 2). At first, mean ‘Blank’ value were subtracted from each data point (Table 3) followed by normalization with ‘DMSO control’ group of ‘Control’ arm (Table 4). Similarly, the raw luminescence values (Table 6) were subtracted with mean ‘Blank’ luminescence value (Table 7). The blank-subtracted values were then divided with the corresponding mean viability values to get viability-normalized ATP abundance (Table 8). Finally, the metabolic dependence and capacity were calculated from the normalized ATP values as described in the step 11. a. (Table 9). Mean and standard deviations were calculated for statistical analysis. Two tailed Student’s t test was applied for testing significant difference of metabolic dependencies and capacities between control and metformin treated groups.
Limitations
This article describes a protocol for profiling energy metabolism pathway dependency in cancer cell line, that is easy to perform, high-throughput compatible, and requires minimal technical expertise. However, this protocol doesn’t have the ability to obtain data at single cell resolution. Therefore, this protocol can’t be applied to profile co-culture or mixed cell culture or metabolic heterogeneity within the sample.
MTT and XTT-based viability assays reflect cellular NADPH oxidoreductase activity. Thus, normalization with respect to viability measured by MTT or XTT assay is not exactly same as normalization with respect to cell number. However, it is expected to give quite reasonable estimate of relative cellular ATP levels in most cases and amenable to be included in a high-throughput setup. Nonetheless, protein quantification, and viable cell counting is more accurate to perform normalization.
Another key limitation of this study is associated with the in vitro cell culture media. This study uses widely used DMEM media that contains nutrients at non-physiological concentrations. Use of more physiologically relevant media (e.g.- human plasma-like medium, HPLM20) would provide potentially more accurate assessment of dependency and capacity of the energy metabolic pathways.
Troubleshooting
Problem 1
Excessive cell death in control arm after acute exposure to metabolic inhibitors. (step 5).
Potential solution
-
•
Use healthy maintained cells.
-
•
Use lower concentration of inhibitors.
Problem 2
Very high variability of luminescence within the group. (step 8).
Potential solution
-
•
Use healthy maintained cells preferably within passage 3 to 10.
-
•
Cell lysis is incomplete. Shake longer in orbital shaker.
-
•
Handle pipette carefully to avoid erroneous volume dispersion. Use multichannel pipette.
-
•
Use the reagents after thawing in 25°C–30°C.
Problem 3
Blank wells have higher or similar absorbance or luminescence. (step 6 & 8).
Potential solution
-
•
Use higher number of cells per well.
-
•
Avoid cross contamination among the wells.
-
•
Use fresh and properly thawed reagents.
-
•
Incubate for longer duration.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Sk Ramiz Islam (ramizs82@gmail.com).
Technical contact
For further technical information associated with this method should be directed to and will be fulfilled by the technical contact, Sk Ramiz Islam (ramizs82@gmail.com), and Sebabrata Maity (seba.brata7070@gmail.com).
Materials availability
Not applicable as this protocol is not associated with newly generated materials.
Data and code availability
Not applicable.
Acknowledgments
We are thankful to Dr. Partha Chakrabarti, CSIR-Indian Institute of Chemical Biology, Kolkata, for providing HepG2 cell line. The authors acknowledge divisional members for their helpful co-operation for instrument and facility access. Graphical abstract and illustrations were created using Microsoft PowerPoint, Adobe Illustrator, and BioRender.com. Statistical analysis and data visualization were done using GraphPad Prism 9 and Microsoft Excel. This work was supported by intramural funding from the Department of Atomic Energy, Govt. of India. S.R.I. and S.M. are supported by UGC and CSIR junior research fellowship, respectively.
Author contributions
S.R.I., conceptualization, methodology, validation, formal analysis, investigation, data curation, writing – original draft, and visualization. S.M., methodology, validation, formal analysis, investigation, writing – original draft, and visualization. O.C., resources, writing – review and editing, supervision, project administration, and funding acquisition. S.K.M., resources, writing – review and editing, supervision, project administration, and funding acquisition.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Sk Ramiz Islam, Email: ramizs82@gmail.com.
Sebabrata Maity, Email: seba.brata7070@gmail.com.
References
- 1.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J., Nuebel E., Wisidagama D.R.R., Setoguchi K., Hong J.S., Van Horn C.M., Imam S.S., Vergnes L., Malone C.S., Koehler C.M., Teitell M.A. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nat. Protoc. 2012;7:1068–1085. doi: 10.1038/nprot.2012.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danzi F., Pacchiana R., Mafficini A., Scupoli M.T., Scarpa A., Donadelli M., Fiore A. To metabolomics and beyond: a technological portfolio to investigate cancer metabolism. Signal Transduct. Target. Ther. 2023;8:137. doi: 10.1038/s41392-023-01380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller A., Nagy C., Knapp B., Laengle J., Ponweiser E., Groeger M., Starkl P., Bergmann M., Wagner O., Haschemi A. Exploring Metabolic Configurations of Single Cells within Complex Tissue Microenvironments. Cell Metab. 2017;26:788–800.e6. doi: 10.1016/J.CMET.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 5.Argüello R.J., Combes A.J., Char R., Gigan J.P., Baaziz A.I., Bousiquot E., Camosseto V., Samad B., Tsui J., Yan P., et al. SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab. 2020;32:1063–1075.e7. doi: 10.1016/J.CMET.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders K.D.G., Lewis H.M., Beste D.J., Cexus O., Bailey M.J. Spatial single cell metabolomics: Current challenges and future developments. Curr. Opin. Chem. Biol. 2023;75 doi: 10.1016/J.CBPA.2023.102327. [DOI] [PubMed] [Google Scholar]
- 7.Wagner B.A., Venkataraman S., Buettner G.R. The Rate of Oxygen Utilization by Cells. Free Radic. Biol. Med. 2011;51:700–712. doi: 10.1016/J.FREERADBIOMED.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newsholme P., Cruzat V.F., Keane K.N., Carlessi R., De Bittencourt P.I.H. Molecular mechanisms of ROS production and oxidative stress in diabetes. Biochem. J. 2016;473:4527–4550. doi: 10.1042/BCJ20160503C. [DOI] [PubMed] [Google Scholar]
- 9.Zou Z., Tao T., Li H., Zhu X. MTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020;10:31. doi: 10.1186/S13578-020-00396-1/TABLES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luengo A., Li Z., Gui D.Y., Sullivan L.B., Zagorulya M., Do B.T., Ferreira R., Naamati A., Ali A., Lewis C.A., et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell. 2021;81:691–707.e6. doi: 10.1016/J.MOLCEL.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weston W.A., Barr A.R. A cell cycle centric view of tumour dormancy. Br. J. Cancer. 2023;129:1535–1545. doi: 10.1038/s41416-023-02401-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn J., Grider M.H. StatPearls; 2023. Physiology, Adenosine Triphosphate. [PubMed] [Google Scholar]
- 13.Mukherjee M., Das D., Sarkar J., Banerjee N., Jana J., Bhat J., Reddy G J., Bharatam J., Chattopadhyay S., Chatterjee S., Chakrabarti P. Prion-derived tetrapeptide stabilizes thermolabile insulin via conformational trapping. iScience. 2021;24 doi: 10.1016/J.ISCI.2021.102573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green M.R., Sambrook J. Estimation of Cell Number by Hemocytometry Counting. Cold Spring Harb. Protoc. 2019;2019:732–734. doi: 10.1101/PDB.PROT097980. [DOI] [PubMed] [Google Scholar]
- 15.Cameron A.R., Logie L., Patel K., Erhardt S., Bacon S., Middleton P., Harthill J., Forteath C., Coats J.T., Kerr C., et al. Metformin selectively targets redox control of complex I energy transduction. Redox Biol. 2018;14:187–197. doi: 10.1016/J.REDOX.2017.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald M.J., Ansari I.U.H., Longacre M.J., Stoker S.W. Metformin’s Therapeutic Efficacy in the Treatment of Diabetes Does Not Involve Inhibition of Mitochondrial Glycerol Phosphate Dehydrogenase. Diabetes. 2021;70:1575–1580. doi: 10.2337/DB20-1143. [DOI] [PubMed] [Google Scholar]
- 17.Cai L., Jin X., Zhang J., Li L., Zhao J. Metformin suppresses Nrf2-mediated chemoresistance in hepatocellular carcinoma cells by increasing glycolysis. Aging. 2020;12:17582–17600. doi: 10.18632/AGING.103777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrzejewski S., Gravel S.-P., Pollak M., St-Pierre J. Metformin directly acts on mitochondria to alter cellular bioenergetics. Cancer Metab. 2014;2:12. doi: 10.1186/2049-3002-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islam S.R., Manna S.K. Identification of glucose-independent and reversible metabolic pathways associated with anti-proliferative effect of metformin in liver cancer cells. Metabolomics. 2024;20 doi: 10.1007/s11306-024-02096-0. [DOI] [PubMed] [Google Scholar]
- 20.Cantor J.R., Abu-Remaileh M., Kanarek N., Freinkman E., Gao X., Louissaint A., Lewis C.A., Sabatini D.M. Physiologic Medium Rewires Cellular Metabolism and Reveals Uric Acid as an Endogenous Inhibitor of UMP Synthase. Cell. 2017;169:258–272.e17. doi: 10.1016/j.cell.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.