Abstract
Background
The benefits of novel androgen receptor axis-targeted agents (ARATs) on oncological outcomes in patients with non-metastatic castration-resistant prostate cancer (nmCRPC) in real-world settings are unclear.
Methods
This multi-institutional retrospective study included 178 patients with nmCRPC treated between September 2003 and August 2022. Patients were divided into two groups: those who were treated with any novel ARATs, including apalutamide, enzalutamide, darolutamide, and abiraterone acetate, during any line of nmCRPC treatment (novel ARATs group) and those who were not (control group). Multivariable Cox proportional hazards regression analyses were performed to evaluate the effects of novel ARATs on metastasis-free survival (MFS) and overall survival (OS).
Results
The median age and follow-up period after nmCRPC diagnosis were 76 years and 37 months, respectively. Of the 178 patients, 122 (69%) were treated with novel ARATs after nmCRPC diagnosis. The MFS and OS in the novel ARATs group were significantly longer than those in the control group (P < 0.001 and P = 0.020, respectively). In multivariable analyses, a prostate-specific antigen doubling time (PSADT) of <3 months and novel ARATs were independently and significantly associated with MFS and OS. The effects of novel ARATs on MFS were consistently observed across subgroups stratified by age (<75 years or ≥75 years), history of radical treatment (no or yes), biopsy Gleason score (<9 or ≥9), clinical stage (≤cT3 and cN0, or cT4 or cN1), and PSADT (≥3 months or <3 months).
Conclusion
Novel ARATs were significantly associated with improved oncological outcomes in patients with nmCRPC in a real-world setting, regardless of tumor aggressiveness.
Keywords: nmCRPC, Novel ARATs, Oncological outcomes, PSA doubling time, Real-world
1. Introduction
Prostate cancer (PC) remains the second most common malignancy among men worldwide.1 Although most men are diagnosed with localized PC and undergo either prostatectomy or radiotherapy, approximately 15–30% experience recurrence.2,3 Androgen deprivation therapy (ADT) is the standard primary treatment for these patients, as well as for men unsuitable for radical treatment.4 However, the disease eventually progresses to castration-resistant PC (CRPC).
Non-metastatic CRPC (nmCRPC), defined as prostate-specific antigen (PSA) progression despite castrate levels of serum testosterone and no evidence of distant metastases on conventional imaging,5 frequently advances to metastatic CRPC (mCRPC) with poor clinical outcomes.6 Recently, three phase III trials have demonstrated that novel androgen receptor axis-targeted agents (ARATs), such as apalutamide, enzalutamide, and darolutamide, significantly improved metastasis-free survival (MFS) and overall survival (OS) compared to placebo.7, 8, 9 However, the effects of these novel ARATs on oncological outcomes in real-world settings remain unclear. Moreover, it is also unknown who can obtain benefits by novel ARATs treatments.
Thus, the present study aimed to investigate the real-world effects of novel ARATs on oncological outcomes and identify optimal candidates for novel ARATs treatment in patients with nmCRPC.
2. Materials and methods
2.1. Ethics statement
The current study followed the principles of the Declaration of Helsinki and was approved by the ethics committees of the Hirosaki University Graduate School of Medicine (authorization number: 2019-099-1) and all hospitals included in this study. Written informed consent was not obtained because of the public disclosure of study information (opt-out approach).
2.2. Patient selection
This multi-institutional retrospective study evaluated 829 patients with CRPC treated between September 2003 and August 2022 at one academic center and 10 general hospitals: Aomori Prefectural Central Hospital, Mutsu General Hospital, Hakodate Municipal Hospital, Towada City Central Hospital, Aomori Rosai Hospital, Aomori City Hospital, Tsugaru General Hospital, Odate Municipal General Hospital, Ageo Central General Hospital, and Oyokyo Kidney Research Institute Hirosaki Hospital. CRPC was defined as castrate serum testosterone <50 ng/dL and one of the following types of progression: (1) biochemical progression: three consecutive rises in PSA at least one week apart resulting in two 50% increases over the nadir, and a PSA level >2 ng/mL or (2) radiological progression: the appearance of new lesions, either two or more new bone lesions on bone scan or a soft tissue lesion using the Response Evaluation Criteria in Solid Tumors version 1.1.10 nmCRPC was defined as PSA >2 ng/mL, castrate testosterone levels <50 ng/dL, and the absence of metastatic lesions on conventional imaging (computed tomography [CT] or bone scintigraphy).11 Of the 829 patients with CRPC, we excluded 648 patients with non-metastatic or metastatic castration-sensitive PC who directly progressed to mCRPC and three patients with nmCRPC who had insufficient information for analysis. Finally, 178 patients with nmCRPC were included in this study (Fig. 1). Patients were divided into two groups: those who were treated with any novel ARATs, including apalutamide, enzalutamide, darolutamide, and abiraterone acetate, during any line of nmCRPC treatment (novel ARATs group) and those who were not (control group) (Fig. 1).
Fig. 1.
Patient selection. Numbers of patients included and excluded. CRPC, castration-resistant prostate cancer; nmCRPC, non-metastatic castration-resistant prostate cancer; mCSPC, metastatic castration-sensitive prostate cancer; nmCSPC, non-metastatic castration-sensitive prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; ARATs, androgen receptor axis-targeted agents.
2.3. Evaluation of variables
The following variables were analyzed: age at nmCRPC diagnosis, initial PSA level, clinical tumor (T) and node (N) stages at initial diagnosis, biopsy Gleason score (GS), history of radical treatment, PSA doubling time (PSADT), and time of nmCRPC diagnosis. The PSADT was calculated from the nadir time after the first hormone therapy until the diagnosis of nmCRPC.12
2.4. Treatment
All patients underwent ADT, including bilateral orchiectomy and luteinizing hormone-releasing hormone agonists or antagonists. In Japan, the clinical use of enzalutamide and abiraterone acetate has been available in patients with nmCRPC from when these agents were first introduced (May 2014 and September 2014, respectively). The agents used for nmCRPC treatment were administered at the discretion of the clinician.
2.5. Follow-up schedule
After nmCRPC progression, patients were followed up with complete blood counts, serum biochemistry tests, and serum PSA and testosterone once every one to three months, and chest, abdominal, and pelvic CT scans and bone scintigraphy once every three to six months.
2.6. Statistical analysis
SPSS version 29.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 9 (GraphPad Software, San Diego, CA, USA) were used for the statistical analyses. Quantitative variables were expressed as medians with interquartile ranges. Differences in quantitative variables between the two groups were analyzed using the Mann–Whitney U test. Categorical variables were compared using Fisher's exact test or the chi-squared test. The correlation between MFS and OS was analyzed using Spearman's rank correlation coefficients. The optimal cutoff point of PSADT for OS was calculated using the receiver operating characteristic (ROC) curve. MFS and OS were evaluated using the Kaplan–Meier method and compared using the log-rank test. Univariable and multivariable Cox proportional hazards regression analyses were performed to evaluate the effects of novel ARATs on MFS and OS. These outcomes were calculated from the date of nmCRPC diagnosis to the date of the first event or last follow-up. Statistical significance was set at P < 0.05.
3. Results
3.1. Patients’ background
The median age and follow-up period after nmCRPC diagnosis were 76 years and 37 months, respectively. Of the 178 patients, 122 (69%) were treated with novel ARATs after nmCRPC diagnosis (Fig. 1). No significant differences were found in the background characteristics of the patients between the two groups (Table 1).
Table 1.
Background of patients
| All n = 178 | Control group n = 56 | Novel ARATs group n = 122 | P value | |
|---|---|---|---|---|
| Age, years | 76 (72–81) | 76 (73–79) | 77 (72–82) | 0.807 |
| iPSA, ng/mL | 26 (11–100) | 29 (12–97) | 24 (10–100) | 0.819 |
| Biopsy Gleason score | 0.580 | |||
| ≤7 | 40 (22%) | 10 (18%) | 30 (25%) | |
| 8 | 29 (16%) | 9 (16%) | 20 (16%) | |
| ≥9 | 109 (61%) | 37 (66%) | 72 (59%) | |
| Clinical stage | ||||
| cT4 or cN1 | 68 (38%) | 22 (39%) | 46 (38%) | 0.840 |
| History of radical treatment | 0.378 | |||
| None | 79 (44%) | 29 (52%) | 50 (41%) | |
| Prostatectomy | 58 (33%) | 15 (27%) | 43 (35%) | |
| Radiation therapy | 41 (23%) | 12 (21%) | 29 (24%) | |
| PSADT, months | 3.5 (2.0–6.1) | 4.2 (2.2–6.4) | 3.2 (1.8–5.9) | 0.274 |
| Follow-up period, months | 37 (20–58 | 47 (29–74) | 35 (18–54) |
All data are presented as n (%) or medians (interquartile ranges). ARATs, androgen receptor axis-targeted agents; iPSA, initial prostate-specific antigen; PSADT, prostate-specific antigen doubling time.
Of the 56 patients in the control group, 7 (13%) were treated with docetaxel for the first-line treatment (Fig. S1). Of the 122 patients in the novel ARATs group, 7 (5.7%) and 77 (63%) were treated with docetaxel and novel ARATs for the first-line treatment (Fig. S2).
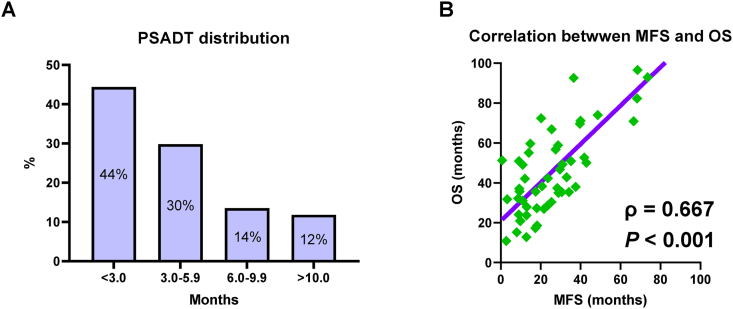
Almost all patients had a PSADT < 10 months. The optimal cutoff point of PSADT for OS was three months (Fig. S3), and 44% of the patients had a rapid PSADT (<3 months) (Fig. 2A).
Fig. 2.
Prostate-specific antigen doubling time (PSADT) distribution and correlation between metastasis-free survival (MFS) and overall survival (OS). The PSADT distribution in all patients (A). The correlation between MFS and OS was analyzed using Spearman's rank correlation coefficient (B).
3.2. Oncological outcomes
At the end of the follow-up period, 38 (68%) and 37 (30%) patients in the control and novel ARATs groups, respectively, had mCRPC progression. Median MFS was 35 and 74 months in the control and novel ARATs groups, respectively. Similarly, 35 (63%) and 30 (25%) patients died from any causes in the control and novel ARATs groups, respectively. The median OS was 55 and 93 months in the control and novel ARATs groups, respectively. MFS was significantly correlated with OS in patients who experienced mCRPC progression and died from any causes (Fig. 2B; ρ = 0.667 and P < 0.001).
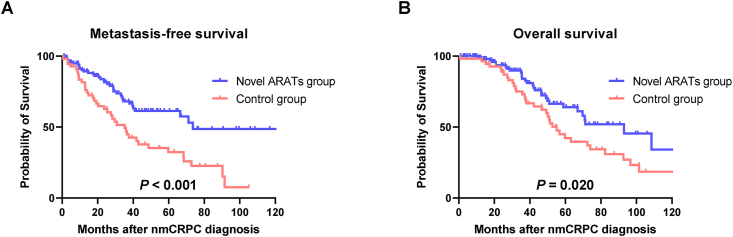
The MFS and OS in the novel ARATs group were significantly longer than those in the control group (Fig. 3A and B; P < 0.001 and P = 0.020, respectively). In univariable analyses, age, PSADT, and novel ARATs were significantly associated with MFS (Table S1). Similarly, in univariable analyses, PSADT and novel ARATs were significantly associated with OS (Table S2). After adjusting for age, GS, clinical T and N stages, and time of nmCRPC diagnosis, the PSADT and novel ARATs were independently and significantly associated with MFS (Table 2; P < 0.001, hazard ratio [HR] 3.039; P < 0.001, HR 0.324, respectively) and OS (Table 2; P < 0.001, HR 2.704; P < 0.001, HR 0.397, respectively).
Fig. 3.
Metastasis-free survival and overall survival. Metastasis-free survival (A) and overall survival (B) after non-metastatic castration-resistant prostate cancer (nmCRPC) diagnosis were evaluated using the Kaplan–Meier method and compared using the log-rank test. ARATs, androgen receptor axis-targeted agents.
Table 2.
Multivariable analyses for metastasis-free survival and overall survival
| Metastasis-free survival | Factor | P value | Hazard ratio | 95% CI |
|---|---|---|---|---|
| Age | Continuous | 0.214 | 0.979 | 0.946–1.012 |
| Time of nmCRPC diagnosis | Before 2014 | 0.768 | 1.077 | 0.657–1.767 |
| Gleason score | ≥9 | 0.297 | 1.296 | 0.796–2.109 |
| Clinical T and N stages | T4 or N1 | 0.512 | 0.841 | 0.502–1.409 |
| PSADT | <3 months | <0.001 | 3.039 | 1.780–5.188 |
| Novel ARATs |
Positive |
<0.001 |
0.324 |
0.196–0.538 |
| Overall survival |
Factor |
P value |
Hazard ratio |
95% CI |
| Age | Continuous | 0.049 | 1.045 | 1.000–1.092 |
| Time of nmCRPC diagnosis | Before 2014 | 0.710 | 0.899 | 0.511–1.580 |
| Gleason score | ≥9 | 0.665 | 1.121 | 0.668–1.882 |
| Clinical T and N stages | T4 or N1 | 0.548 | 1.182 | 0.685–2.039 |
| PSADT | <3 months | <0.001 | 2.704 | 1.536–4.758 |
| Novel ARATs | Positive | <0.001 | 0.397 | 0.231–0.682 |
CI, confidence interval; nmCRPC, non-metastatic castration-resistant prostate cancer; PSADT, prostate-specific antigen doubling time; ARATs, androgen receptor axis-targeted agents.
3.3. Subgroup analyses for MFS
When patients in each group were subdivided according to age, history of radical treatment, biopsy GS, clinical stage at initial diagnosis, and PSADT, MFS in the novel ARATs group were significantly longer than those in the control group across subgroups (Fig. 4).
Fig. 4.
Metastasis-free survival (MFS) in subgroup analyses. Subgroup analyses on MFS using Cox-proportional hazards regression analyses were performed. ARATs, androgen receptor axis-targeted agents; PSADT, prostate-specific antigen doubling time; NR, not reached.
4. Discussion
To the best of our knowledge, the present study is the first one to investigate the real-world effects of novel ARATs on oncological outcomes in patients with nmCRPC. The results of the present study showed that novel ARATs were significantly associated with improved MFS and OS. Moreover, the effects of novel ARATs were consistently observed across subgroups (Fig. 4). Although further prospective studies in a real-world setting are needed, the present study demonstrated the real-world effects of novel ARATs in patients with nmCRPC.
Although three recent phase III trials, SPARTAN, PROSPER, and ARAMIS, have demonstrated the effects of novel ARATs on MFS and OS in patients with nmCRPC,7, 8, 9 it remains unclear whether these novel ARATs contribute to improved oncological outcomes in real-world clinical practice because of a lack of evidence. In mCRPC and metastatic hormone-sensitive PC (mHSPC) settings, several studies have reported the real-world effects of novel ARATs on the prognosis. Narita et al. reported that upfront abiraterone promoted better OS compared with ADT alone or combined androgen blockade in patients with high-volume mHSPC after propensity score matching (P = 0.010, HR 0.49).13 Similarly, Lowentritt et al. evaluated approximately 1,500 patients with mHSPC treated with apalutamide, enzalutamide, or abiraterone acetate and demonstrated lower mCRPC progression rates (34%, 39%, and 45% by 24 months, respectively).14 Payne et al. conducted a real-world prospective observational study in patients with mCRPC prescribed enzalutamide (PREMISE study) and revealed that chemotherapy- and abiraterone-naïve patients in the study had longer time to PSA progression compared to the equivalent population in PREVAIL study (17.7 vs. 11.2 months, respectively).15,16 As described above, novel ARATs might be beneficial in patients with mCRPC or mHSPC in real-world clinical practice. However, no evidence exists regarding this association in patients with nmCRPC. The present real-world study showed that novel ARATs were significantly associated with prolonged MFS and OS, regardless of tumor aggressiveness, as well as the aforementioned phase III trials.7, 8, 9 Table S3 showed a side-by-side comparison between three phase III trials (SPARTAN, PROSPER, and ARAMIS) and the present real-world study. Because the application of clinical trial results to real-world clinical practice is not straightforward due to issues such as restrictive enrollment criteria, biological variability, experimental design limitations, and publication bias,17 our results might be helpful for clinicians in making treatment decisions for patients with nmCRPC.
Although shorter PSADT is a well-known strong predictor of metastasis progression and all-cause mortality in nmCRPC,6,18, 19, 20 its optimal cutoff point for predicting oncological outcomes and risk stratification remains unknown. Various cutoff points have been proposed to stratify patients into high- and low-risk metastasis progression.21, 22, 23 A PSADT of six months is often used as a cutoff point to determine the necessity of more aggressive treatment.23 Three recent phase III trials, SPARTAN, PROSPER, and ARAMIS, also used six months as a cutoff point of PSADT for subgroup analyses.7, 8, 9 However, our ROC analysis showed three months as an optimal cutoff point of PSADT for OS. These results are consistent with those of a previous study. Howard et al. categorized PSADT into groups every three months and then combined groups with similar hazard ratios in patients with nmCRPC. The study showed that the optimal cutoff point of PSADT to distinguish the highest-risk group was less than three months.24 However, their study included patients with relatively slower PSADT (median 13.3 months) compared to the present study (median 3.5 months) and aforementioned three phase III trials (median 3.7–4.7 months).7, 8, 9 Moreover, although our additional analyses showed that the predictive abilities of PSADT < 3 months for MFS and OS were superior to those of PSADT < 6 months, the differences were not statistically significant (Fig. S4). Therefore, further studies are needed to identify the optimal cutoff point of PSADT for predicting oncological outcomes and risk stratification in patients with nmCRPC.
The present study had several limitations. First, the retrospective study design prevented us from drawing definitive conclusions. We were unable to control for selection bias and other immeasurable confounders. Second, a relatively small number of patients were enrolled in this study. Third, we could not evaluate novel ARATs-related adverse events due to a lack of data. Finally, the time from nmCRPC diagnosis to the novel ARATs treatment varied for each patient.
5. Conclusions
Novel ARATs were significantly associated with improved oncological outcomes regardless of tumor aggressiveness in patients with nmCRPC in a real-world setting.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The authors thank Editage (www.editage.com) for the English language editing. This study was supported by a Grant-in-Aid for Scientific Research (No. 19K18603) from the Japan Society for the Promotion of Science.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2023.12.002.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Fig. S1 Treatment sequence in the control group. The treatment sequence in the control group was shown using a Sankey diagram. nmCRPC, non-metastatic castration-resistant prostate cancer; CA, chlormadinone acetate; ADT, androgen deprivation therapy; Bic, bicalutamide; EMP, estramustine phosphate; DTX, docetaxel; Flu, flutamide; mCRPC, metastatic castration-resistant prostate cancer; EE, ethinylestradiol; Pre, prednisolone; Fig. S2 Treatment sequence in the novel androgen receptor axis-targeted agents (ARATs) group. The treatment sequence in the novel ARATs group was shown using a Sankey diagram. nmCRPC, Vintage hormone therapy (Vin) included bicalutamide, flutamide, estramustine phosphate, ethinylestradiol, and chlormadinone acetate. non-metastatic castration-resistant prostate cancer; DTX, docetaxel; Apa, apalutamide; Abi, abiraterone acetate; Dar, darolutamide; Enz, enzalutamide; CBZ, cabazitaxel; mCRPC, metastatic castration-resistant prostate cancer; Fig. S3 Optimal cutoff point of prostate-specific antigen doubling time (PSADT) for overall survival (OS). The optimal cutoff point of PSADT for OS was calculated using the receiver operating characteristic curve; Fig. S4 Predictive abilities of prostate-specific antigen doubling time (PSADT) for metastasis-free survival (MFS) and overall survival (OS). The predictive abilities were evaluated using the area under the receiver operating characteristic curve (ROC) and then compared using the DeLong test.
References
- 1.Bergengren O., Pekala K.R., Matsoukas K., Fainberg J., Mungovan S.F., Bratt O., et al. 2022 Update on prostate cancer epidemiology and risk factors – a systematic review. Eur Urol. 2023;84:191–206. doi: 10.1016/j.eururo.2023.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zumsteg Z.S., Spratt D.E., Romesser P.B., Pei X., Zhang Z., Polkinghorn W., et al. The natural history and predictors of outcome following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67:1009–1016. doi: 10.1016/j.eururo.2014.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boorjian S.A., Thompson R.H., Tollefson M.K., Rangel L.J., Bergstralh E.J., Blute M.L., et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59:893–899. doi: 10.1016/j.eururo.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Kluth L.A., Shariat S.F., Kratzik C., Tagawa S., Sonpavde G., Rieken M., et al. The hypothalamic-pituitary-gonadal axis and prostate cancer: implications for androgen deprivation therapy. World J Urol. 2014;32:669–676. doi: 10.1007/s00345-013-1157-5. [DOI] [PubMed] [Google Scholar]
- 5.Mateo J., Fizazi K., Gillessen S., Heidenreich A., Perez-Lopez R., Oyen W.J.G., et al. Managing nonmetastatic castration-resistant prostate cancer. Eur Urol. 2019;75:285–293. doi: 10.1016/j.eururo.2018.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Smith M.R., Kabbinavar F., Saad F., Hussain A., Gittelman M.C., Bilhartz D.L., et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 7.Smith M.R., Saad F., Chowdhury S., Oudard S., Hadaschik B.A., Graff J.N., et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79:150–158. doi: 10.1016/j.eururo.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Sternberg C.N., Fizazi K., Saad F., Shore N.D., De Giorgi U., Penson D.F., et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020;382:2197–2206. doi: 10.1056/NEJMoa2003892. [DOI] [PubMed] [Google Scholar]
- 9.Fizazi K., Shore N., Tammela T.L., Ulys A., Vjaters E., Polyakov S., et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N Engl J Med. 2020;383:1040–1049. doi: 10.1056/NEJMoa2001342. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Scher H.I., Morris M.J., Stadler W.M., Higano C., Basch E., Fizazi K., et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arlen P.M., Bianco F., Dahut W.L., D'Amico A., Figg W.D., Freedland S.J., et al. Prostate Specific Antigen Working Group guidelines on prostate specific antigen doubling time. J Urol. 2008;179:2181–2185. doi: 10.1016/j.juro.2008.01.099. discussion 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narita S., Kimura T., Hatakeyama S., Hata K., Yanagisawa T., Maita S., et al. Real-world survival outcomes of adding docetaxel or abiraterone in patients with high-volume metastatic castration-sensitive prostate cancer: historically controlled, propensity score matched comparison with androgen deprivation therapy. World J Urol. 2022;40:1135–1141. doi: 10.1007/s00345-022-03963-y. [DOI] [PubMed] [Google Scholar]
- 14.Lowentritt B., Du S., Rossi C., Kinkead F., Waters D., Moore B., et al. Prostate-specific antigen response and time-to-castration resistance among patients with metastatic castration sensitive prostate cancer initiated on apalutamide, enzalutamide, or abiraterone acetate. J Urol. 2023;209:e387. [Google Scholar]
- 15.Payne H., Robinson A., Rappe B., Hilman S., De Giorgi U., Joniau S., et al. A European, prospective, observational study of enzalutamide in patients with metastatic castration-resistant prostate cancer: PREMISE. Int J Cancer. 2022;150:837–846. doi: 10.1002/ijc.33845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer T.M., Armstrong A.J., Rathkopf D.E., Loriot Y., Sternberg C.N., Higano C.S., et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarbin M. Real life outcomes vs. clinical trial results. J Ophthal Vis Res. 2019;14:88–92. doi: 10.4103/jovr.jovr_279_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitney C.A., Howard L.E., Freedland S.J., DeHoedt A.M., Amling C.L., Aronson W.J., et al. Impact of age, comorbidity, and PSA doubling time on long-term competing risks for mortality among men with non-metastatic castration-resistant prostate cancer. Prostate Canc Prostat Dis. 2019;22:252–260. doi: 10.1038/s41391-018-0095-0. [DOI] [PubMed] [Google Scholar]
- 19.Smith M.R., Cook R., Lee K.A., Nelson J.B. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer. 2011;117:2077–2085. doi: 10.1002/cncr.25762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith M.R., Saad F., Oudard S., Shore N., Fizazi K., Sieber P., et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J Clin Oncol. 2013;31:3800–3806. doi: 10.1200/JCO.2012.44.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonarakis E.S., Chen Y., Elsamanoudi S.I., Brassell S.A., Da Rocha M.V., Eisenberger M.A., et al. Long-term overall survival and metastasis-free survival for men with prostate-specific antigen-recurrent prostate cancer after prostatectomy: analysis of the Center for Prostate Disease Research National Database. BJU Int. 2011;108:378–385. doi: 10.1111/j.1464-410X.2010.09878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowski M.C., Chen Y., Feng Z., Cullen J., Trock B.J., Suzman D., et al. PSA doubling time and absolute PSA predict metastasis-free survival in men with biochemically recurrent prostate cancer after radical prostatectomy. Clin Genitourin Cancer. 2019;17:470–475.e1. doi: 10.1016/j.clgc.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caffo O., Maines F., Kinspergher S., Veccia A., Messina C. To treat or not to treat: is it acceptable to avoid active therapies in advanced prostate cancer today? Expert Rev Anticancer Ther. 2021;21:389–400. doi: 10.1080/14737140.2021.1856661. [DOI] [PubMed] [Google Scholar]
- 24.Howard L.E., Moreira D.M., De Hoedt A., Aronson W.J., Kane C.J., Amling C.L., et al. Thresholds for PSA doubling time in men with non-metastatic castration-resistant prostate cancer. BJU Int. 2017;120 doi: 10.1111/bju.13856. E80-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 Treatment sequence in the control group. The treatment sequence in the control group was shown using a Sankey diagram. nmCRPC, non-metastatic castration-resistant prostate cancer; CA, chlormadinone acetate; ADT, androgen deprivation therapy; Bic, bicalutamide; EMP, estramustine phosphate; DTX, docetaxel; Flu, flutamide; mCRPC, metastatic castration-resistant prostate cancer; EE, ethinylestradiol; Pre, prednisolone; Fig. S2 Treatment sequence in the novel androgen receptor axis-targeted agents (ARATs) group. The treatment sequence in the novel ARATs group was shown using a Sankey diagram. nmCRPC, Vintage hormone therapy (Vin) included bicalutamide, flutamide, estramustine phosphate, ethinylestradiol, and chlormadinone acetate. non-metastatic castration-resistant prostate cancer; DTX, docetaxel; Apa, apalutamide; Abi, abiraterone acetate; Dar, darolutamide; Enz, enzalutamide; CBZ, cabazitaxel; mCRPC, metastatic castration-resistant prostate cancer; Fig. S3 Optimal cutoff point of prostate-specific antigen doubling time (PSADT) for overall survival (OS). The optimal cutoff point of PSADT for OS was calculated using the receiver operating characteristic curve; Fig. S4 Predictive abilities of prostate-specific antigen doubling time (PSADT) for metastasis-free survival (MFS) and overall survival (OS). The predictive abilities were evaluated using the area under the receiver operating characteristic curve (ROC) and then compared using the DeLong test.