Abstract
Background
Metastatic hormone-sensitive prostate cancer (mHSPC) treatment has changed drastically during the last years with the emergence of androgen receptor–targeted agents (ARTAs). ARTA combined with androgen deprivation therapy has demonstrated better oncological and survival outcomes in these patients. However, the optimal choice among different ARTAs remains uncertain due to their analogous efficacy.
Objectives
The objective of this study was to describe prostate-specific antigen (PSA) response and oncological outcomes of patients with mHSPC treated with apalutamide.
Material and methods
Medical records from three different hospitals in Spain were used to conduct this study. Patients diagnosed with mHSPC and under apalutamide treatment were included between March 2021 and January 2023. Data regarding PSA response, overall survival (OS), and radiographic progression-free survival (rPFS) were collected and stratified by metastasis volume, timing, and stating.
Results
193 patients were included; 34.2% of patients were de novo mHSPC, and the majority was classified as m1b. The 18-month OS and rPFS were 92.5% and 88.9%, respectively. Patients with PSA levels ≤0.2 ng/ml showcased an 18-month OS rate of 98.7%, contrasting with 65.3% for those with PSA >0.2 ng/ml. Similar trends emerged for rPFS (97.4% and 53.7%, respectively). When differentiating between low-volume and high-volume metastasis, the OS rate stood at 98.4% and 80.7%, respectively, while the rPFS rates were 93% and 81.6%, respectively. No significant differences were found between groups stratified by metastasis timing.
Conclusion
This real-world study on patients with mHSPC treated with apalutamide plus androgen deprivation therapy revealed robust oncological outcomes, aligning with the emerging evidence. The study's hallmark finding highlights the significance of rapid and deep PSA response as a predictor of improved oncological and survival outcomes.
Keywords: De novo, Metastatic hormone-sensitive prostate cancer, Metastatic volume, Prostate cancer
1. Introduction
Prostate cancer (PC) ranks as the second most common cancer among men worldwide, with Europe reporting the highest incidence at 10.8%, corresponding to 473,344 new cases annually, and a mortality rate of 5.5%, accounting for 108,088 deaths in a given year [1]. Spain shares a similar burden, witnessing an incidence of 12.3% (34,613 new cases) and a mortality rate of 5.1% (5,798 deaths) [1,2]. Of these, 4% were diagnosed as metastatic [3], leading to a significantly poorer prognosis than progressive PC cases [4].
Survival duration varies significantly due to the heterogeneous nature of the M1 population [5,6]. Several subgroups can be distinguished within the metastatic hormone-sensitive prostate cancer (mHSPC) patients, depending on metastasis volume (high-volume [HV] and low-volume [LV]) and the time of presentation (de novo and metachronous). These subgroups carry substantial prognostic implications. Notably, patients classified as metachronous and LV PC seemed to benefit the most from androgen deprivation therapy (ADT) with a prolonged median of overall survival (OS) and time to castration-resistant prostate cancer (CRPC) [6, 7, 8].
Traditionally, ADT has represented the standard of care for mHSPC until 2015 [9,10], when new evidence revealed the variability of ADT's efficacy and recognized primary resistance to ADT [11]. Even more, the STAMPEDE trial highlighted patients exhibiting prolonged responses to ADT[5]. Yet, the identification of these long-response ADT patients remains an unanswered question.
The integration of abiraterone [12,13], apalutamide [14,15], enzalutamide [16,17], and more recently, darolutamide [18] in combination with ADT and even docetaxel [18,19], has consistently demonstrated significant survival benefits over ADT alone. While several meta-analyses suggest that combination therapies surpass ADT monotherapy [20], a clear superiority among the combination options remains to be definitively established. Amid this dynamic landscape, the question of selecting the most appropriate androgen receptor–targeted agent (ARTA) for each patient remains. Medical decision is still guided by several patient factors, including individual characteristics (comorbidities, basal situation, and preferences), tumour characteristics (volume, risk, and presentation) and drug attributes (adverse effects, interactions, and management)[6]. It is worth noting that despite these indications, ADT monotherapy is still the treatment followed by several urologist, accounting for up to 56% of cases [21]. Importantly, apalutamide in combination with ADT presents a viable option for mHSPC patients all patients, except for the ones with a very bad basal status [22].
The aim of this study was to retrieve data from medical files of all patients diagnosed with mHSPC and under apalutamide treatment to evaluate their survival outcomes, prostate-specific antigen (PSA) levels, and treatment adverse effects.
2. Materials and Methods
2.1. Study design and participants
Following the approval from the ethical committee, the study encompassed a retrospective consecutive cohort of patients diagnosed with mHSPC between March 2021 and January 2023. The patient pool was drawn from three prominent medical centers in Spain: Virgen de la Arrixaca Hospital, Instituto Valenciano de Oncología, and Santa Lucía Hospital. mHSPC was defined in accordance with the CHAARTED criteria [23], with further categorization based on metastasis volume and timing, as outlined by the Francini and Gravis classification [7,8].
2.2. Data collection
Data were extracted from medical records and were continued to be monitored until January 2023 or the time of death, whichever came first. The assessment covered three distinct categories of data: baseline characteristics, post-apalutamide data, and survival data.
-
•
For baseline patient characteristics, individual records encompassed information such as age, prior medical conditions (diabetes mellitus, severe hypertension, ischemic cardiopathy, cardiac insufficiency, anticoagulant treatment, hypothyroidism, cognitive impairment, and severe renal failure), Eastern Cooperative Oncology Group Performance Status Performance Status, PSA levels, International Society of Urologic Pathologists grade, history of prior PC local treatment, volume of metastases, and localization of metastases.
-
•
Following the initiation of apalutamide, an evaluation was conducted on patient-reported adverse effects, instances of dosage reduction due to adverse effects, and PSA reduction metrics at 1, 3, 6, and 12 months.
-
•
OS and radiographic progression-free survival (rPFS) were reviewed.
2.3. Statistical analysis
A descriptive analysis was performed on patients' baseline characteristics, adverse effects, and PSA response. Categorical variables were expressed as percentages, whereas continuous variables were summarized using medians and ranges. The association between PSA decline, metastasis volume, localization and timing, and survival outcomes was evaluated using the Kaplan–Meier method. PSA decline was assessed based on 50% reduction, 90% PSA reduction, or PSA ≤0.2 ng/ml achieved at landmark times of 1, 3, 6, and 12 months under apalutamide treatment.
All statistical tests were two-sided, and statistical significance was established at P < 0.05. The statistical analyses were carried out using SPSS for Windows version 25.0.
3. Results
3.1. Patients’ characteristics
Our retrospective analysis encompassed a cohort of 193 patients drawn from three medical centers in Spain. The median age of the patients was 72 years. The predominant pre-existing medical condition was diabetes mellitus (30.3%). The Eastern Cooperative Oncology Group Performance Status Performance Status score was 0 for the majority of the cohort (65.5%).
In terms of PC characteristics, the median PSA level at diagnosis was 16 ng/mL (2.5-1410). Among these patients, 52.8% exhibited a Gleason score of less than 8. Notably, 34.2% of patients received a diagnosis of de novo mHSPC. Concerning metastases, the majority was classified as m1b (59.1%). A comprehensive breakdown of patients' characteristics is provided in Table 1.
Table 1.
Overview of the main characteristics of the included patients
| Variable | ||
|---|---|---|
| Median of age | 72 years (48–89) | |
| Median of follow-up | 10 months (0–23) | |
| Previous pathologies | Diabetes mellitus | 30.3% |
| Hypertension | 27.3% | |
| Isquemic Cardiopaty | 6.1% | |
| Cardiac Insufiency | 4.5% | |
| Anticoagulant treatment | 7.6% | |
| Hypotiroidism | 4.5% | |
| Cognitive impairment | 0% | |
| Severe renal failure | 1.5% | |
| ECOG | 0 | 65.5% (116) |
| 1 | 32.2% (57) | |
| 2 | 2.3% (4) | |
| Department | Virgen de la Arrixaca Hospital | 34.1% (66) |
| Instituto Valenciano de Oncología | 47.2% (91) | |
| Santa Lucía Hospital | 18.7% (36) | |
| Median of PSA | 16 (2.5-1410) | |
| ISUP | 1-3 (Gleason <8) | 52.8% (102) |
| 4-5 (Gleason ≥8) | 47.2% (91) | |
| Previous local treatment | Synchronic | 34.2% (66) |
| Radical prostatectomy (RP) | 13% (25) | |
| Radiotherapy (Rt) | 21.2% (41) | |
| RP + Rt | 31.6% (61) | |
| Metastases | M1a | 36.3% (70) |
| M1b | 59.1% (114) | |
| M1c | 4.7% (9) | |
| Francini groups | DN/LV | 18.7% (36) |
| DN/HV | 15.5% (30) | |
| PD/LV | 47.7% (92) | |
| PD/HV | 18.1% (35) | |
| Bone metastases | Oligometastatic (≤3) | 58.5% |
DN, de novo or synchronous; ECOG, Eastern Cooperative Oncology Group; HV, high volume; ISUP, International Society of Urologic Pathologists; LV, low volume; PD, progressive disease or metachronous; PSA, prostrate-specific antigen.
3.2. Adverse events
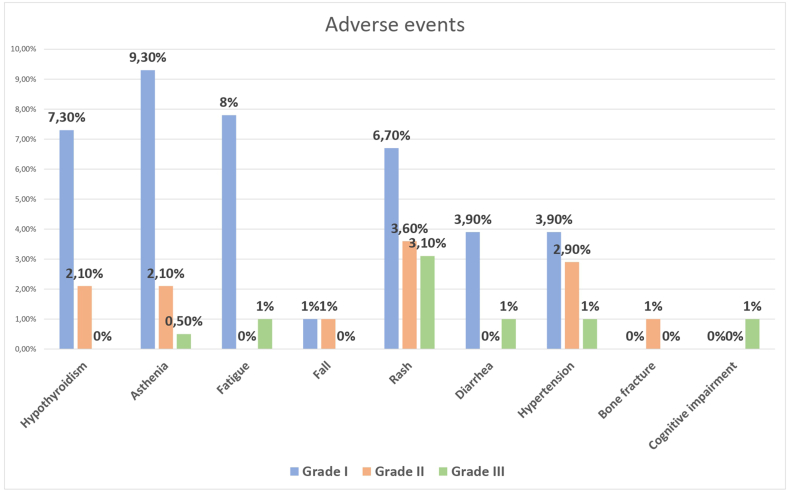
Our findings revealed an adverse events prevalence of 38.9%. Asthenia emerged as the principal adverse event at 9.3%. Within the realm of Grade II (23.2%) and III (19.60%) adverse events, rash took center stage, accounting for 3.6% and 3.1%, respectively (Fig. 1). Notably, 9.8% of patients needed a dosage reduction due to these adverse events.
Figure 1.
Overview of the main adverse events observed in the included patients.
3.3. PSA levels
Remarkably, a 50% reduction in PSA levels at 12 months was observed across the entirety of the patient cohort. Additionally, 90% reduction in PSA levels was evident in 88% of patients and a PSA decline to ≤0.2 ng/ml in 82.4%.
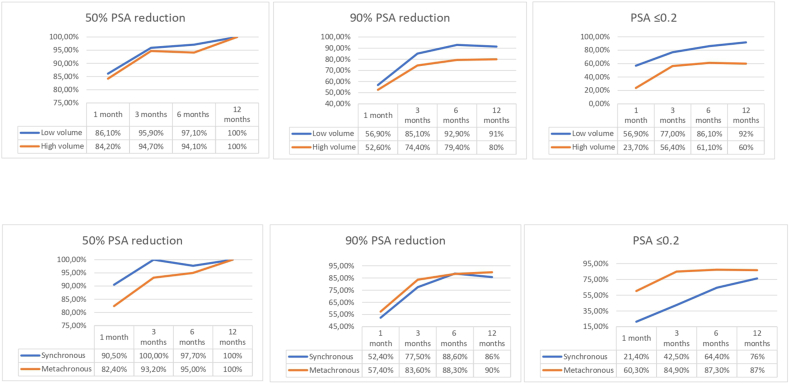
Comparisons of PSA levels were drawn between the groups categorized by metastasis volume, timing, and staging (“M” staging). As previously indicated, all patients exhibited a 50% reduction in PSA levels. Notably, the LV and metachronous groups displayed a higher prevalence of patients achieving a 90% PSA reduction (91% and 90%, respectively), in contrast to 80% in the HV and 86% in the synchronous groups. In parallel, the LV and metachronous groups demonstrated a greater percentage of patients achieving a PSA decline to ≤0.2 ng/ml at 12 months (Fig. 2).
Figure 2.
PSA levels graphics showing the 50% PSA reduction, 90% PSA reduction, and PSA ≤0.2 ng/ml achieved in our cohort, stratified by metastasis volume (high-volume and low-volume) and time of presentation (synchronous and metachronous). Abbreviation: PSA = prostrate-specific antigen.
Regarding “M” staging, all M1a patients exhibited a 90% PSA reduction, and up to 95.5% of them raised PSA levels ≤0.2 ng/ml at 12 months of follow-up. In contrast, only 72.4% of M1b patients reached PSA levels ≤0.2 ng/ml at 12 months.
3.4. Overall survival
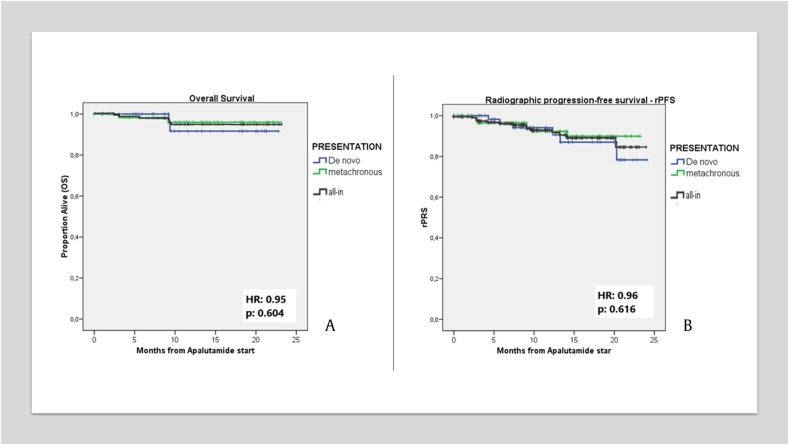
The 18-month OS was 92.5%. In addition, evaluating survival based on metastasis volume, we observed an OS rate of 98.4% at 18 months for the LV group, while the HV group exhibited an OS rate of 80.7% (hazard ratio [HR]: 0.82, P = 0.08) (Fig. 3A). Focusing on PSA levels, the OS rate at 18 months in PSA ≤0.2 ng/ml group was 98.7%, in comparison to 65.3% for those with PSA >0.2 ng/ml (HR: 0.66) (Fig. 3B). Differences were also noted in “M” staging: 97% in M1a, 92% in M1b, and 50% in M1c (p = 0.156). When assessing the OS stratified by the timing of metastasis, the “de novo” group showed an OS of 91.7%, whereas the metachronous group showed an OS of 96% (HR: 0.95). Importantly, these differences are not statistically significant (P = 0.604) (Fig. 4A).
Figure 3.
A. Kaplan–Meier Survival Curve for Overall Survival (OS) by Metastasis Volume. B. Kaplan–Meier Survival Curve for radiographic progression-free survival (rPFS) by Metastasis Volume. C. Kaplan–Meier Survival Curve for OS by PSA levels. D. Kaplan–Meier Survival Curve for rPFS by PSA levels.
Figure 4.
Kaplan Meir Survival Curve for OS (A) and rPFS (B) by time of metastasis. Abbreviations: OS = overall survival; PSA = prostrate-specific antigen.
3.5. Radiographic progression-free survival
The evaluation of rPFS at 18 months disclosed a rate of 88.9%. Diving into an analysis based on metastasis volume, the 18-month rPFS rates stood at 93% for the LV group and at 81.6% for the HV group (HR: 0.87) (Fig. 3C). Shifting our attention to the assessment based on PSA levels, the patients with PSA levels of ≤0.2 ng/ml presented an 18-month rPFS rate of 97.4%, and those with PSA levels >0.2 ng/ml, a rate of 53.7% (HR: 0.55 p = 0.000) (Fig. 3D). In terms of “M” staging, 18-month rPFS rates were 94.3% in M1a, 87.9% in M1b, and 43.8% in M1c (P = 0.061). Stratifying rPFS analysis by metastasis timing, we observed an rPFS 18-month rate of 87% in the “de novo” group, comparable to 89.9% of metachronous group (HR: 0.96). Consistent with the observations in the OS analysis, these differences were not statistically significant (P = 0.616) (Fig. 4B).
4. Discussion
From 2013, studies began to showcase improved OS in mHSPC patients by comparing ADT alone to ADT combined with other treatments. This journey started with docetaxel, where its addition to ADT was associated with clinical benefits in three trials—CHAARTED [23,24], STAMPEDE [25], and GETUG-AFU 15 [26]. The combined approach yielded a slight benefit in terms of OS compared to ADT alone treatment arm (median OS of 57.6, 81 and 58.9 months vs. 44, 71 and 54.2 months, respectively). In contrast, the introduction of ARTA such as abiraterone, apalutamide, enzalutamide and, recently darolutamide, demonstrated significant OS improvements in their respective randomized controlled trials.
Abiraterone acetate in combination with prednisone showed an OS HR of 0.66 (0.56–0.78) [12,13]. The TITAN trial demonstrated a benefit in apalutamide, with an HR of 0.65 (0.53–0.79) [14,15]. ARCHES [17] and ENZAMET [16] trials showed parallel outcomes in OS, with HRs of 0.66 (0.53–0.81) and 0.67 (0.52–0.86), respectively. The PEACE-1 trial published its results, comparing abiraterone plus ADT with or without docetaxel and with or without radiotherapy in the novo mHSPC patients, revealing improved OS (HR: 0.82) [19]. Although numerous meta-analyses indicate the superiority of combination therapies over ADT monotherapy, a conclusive determination regarding the most effective combination approach is still lacking.
The TITAN trial explored apalutamide versus placebo in addition to continuous ADT for patients with mHSPC. The study encompasses 81% of patients with de novo mHSPC and 63% with HV mHSPC [14]. In our cohort, our findings differed, revealing 34.2% of de novo mHSPC patients and 33.6% with HV mHSPC, as illustrated in Table 1. Unlike the TITAN trial, we included patients with lymph node metastasis (M1a).
Focusing on oncological outcomes, PSA levels have emerged as a key prognostic marker. The CHAARTED study identified PSA ≤0.2 ng/mL at 7 months as an independent prognostic marker in patients receiving ADT for metastatic disease, independent of the addition of docetaxel [27]. Additionally, the SWOG 9346 study stratified patients into three prognostic groups, based on PSA levels at 7 months post ADT initiation (ADT monotherapy). These groups displayed distinct median survival rates: 75 months for PSA <0.2 ng/mL, 44 months for PSA = 0.2–4 ng/mL, and 13 months for PSA >4 ng/mL [28]. These findings align with those observed in the SPARTAN trial, in nonmetastatic CRPC patients. In this trial, patients achieving deep PSA response at ≤0.2 ng/mL experienced prolonged times to metastasis, PSA progression, and death [29].
Recent observations highlight the association between a significant reduction in PSA levels and improved survival outcomes among mHSPC patients treated with apalutamide. Achieving a deep PSA decline was associated with improved OS, rPFS, time to PSA progression, and time to CRPC compared to those without deep PSA decline [30]. In our cohort, we identified a significant difference in 18-month OS and rPFS between patients with PSA levels ≤0.2 ng/mL and those with PSA levels >0.2 ng/mL (with corresponding HR values of 0.66 and 0.55 for OS and rPFS, respectively). Notably, the former exhibited an OS rate of 98.7% and an rPFS rate of 97.4%, while the latter displayed rates of 65.3% and 53.7%, respectively (Fig. 3B and D). Additionally, we explored the percentage of patients achieving PSA level reduction to ≤0.2 ng/mL based on volume and metastasis timing. At the 12-month mark, 92% of LV and 87% of metachronous mHSPC patients achieved PSA levels ≤0.2 ng/dL, in contrast to 60% of HV and 76% of de novo mHSPC patients (Fig. 2).
The TITAN trial's findings revealed a favorable impact of apalutamide combined with ADT on the OS. Over a follow-up of 44 months, the trial yielded an HR of 0.65 (0.53–0.79). This OS benefit extended across patients' subgroups, including metastases volume groups. Specifically, the HV group demonstrated an HR of 0.70 (0.56–0.88), while the LV group exhibited an even more pronounced HR of 0.52 (0.35–0.79) [15]. Within our own cohort, an 18-month OS rate of 92.5% was observed. Stratifying by volume, the LV group achieved an OS rate of 98.4% at 18 months, while the HV group displayed an OS rate of 80.7% (HR: of 0.82) (Fig. 3A). Similarly, this benefit extended to rPFS, as evidenced by an HR of 0.49 (0.40–0.61) in the TITAN trial. For HV and LV groups, the rPFS HR stood at 0.53 (0.41–0.67) and 0.36 (0.22–0.57), respectively [15]. Notably, our cohort disclosed a compelling 18-month rPFS rate of 88.9%, with specific 18-month rPFS rates of 93% for the LV and 81.6% for the HV groups (HR: 0.87) (Fig. 3C).
Several systematic reviews attempted to compare the oncological benefits of various ARTAs. Wenzel et al [31] observed that in the subset of HV mHSPC, relative to ADT alone, only abiraterone, apalutamide, and docetaxel were associated with extended OS. Utilizing a network meta-analysis approach to rank the likelihood of maximal OS benefit in HV mHSPC, abiraterone ranked first, followed by apalutamide and docetaxel. Furthermore, in a similar vein, Jian et al. [32] showed that ADT plus apalutamide was ranked first in OS among all therapies. Noteworthy was the observation that for LV disease, the combination of apalutamide plus ADT and enzalutamide plus ADT showed the best improvement in OS.
Turning our attention to patient subgroups categorized by the time of metastasis onset, the distinction between de novo and metachronous mHSPC groups yields varying oncological outcomes, favoring a more optimistic prognosis for the latter [6,33]. In their systematic review and meta-analysis, Menges et al [34], found no difference in survival benefit in patients with de novo mHSPC treated with different ARTA plus ADT combinations. By contrast, their findings only supported the efficacy of ADT plus apalutamide in metachronous mHSPC patients, compared to other ARTA options. However, our analysis did not yield statistically significant differences between the groups stratified by metastasis timing.
Assessing Treatment-Emergent Adverse Events (TEAEs) related to apalutamide represents a nuanced challenge. The existing literature primarily derives from the TITAN cohort, supplemented by meta-analyses comparing various TEAEs across different ARTA trials. It's essential to note that these assessments are potentially influenced by the association with ADT, complicating the distinction between apalutamide-specific effects and those potentiated by the combination. Additionally, the TITAN trial reported TEAE rates of 97.3% for the apalutamide combined with ADT arm and 96.8% in the ADT combined with placebo arm. Among these, TEAE of special interest reported were skin rash (29.2%), fracture (10.3%), fall (9.4%), ischemic heart disease (5.9%), ischemic cerebrovascular disorder (2.5%), and seizure (0.6%) [15]. Cutaneous adverse events, most notably skin rash, have emerged as the prevailing TEAE, reported as a key factor contributing to treatment discontinuation or dose reduction for up to 13% of patients in the apalutamide plus ADT arm [14,35]. However, the adverse event incidence rate ratio compared to ADT alone arm is similar to the other ARTA[34], and apalutamide is listed as the ARTA with the lowest ≥Grade III adverse events [32]. In our cohort, we observed a 38.9% incidence of adverse events, with 57.1% listed as Grade I. During our follow-up, no cardiovascular events were noted. The most prevalent adverse event in our cohort was asthenia (9.3%), followed by fatigue (8%), hypothyroidism (7.3%), and rash (6.7%) as depicted in Fig. 1. A total of 9.8% of our patients needed a dosage reduction due to Grade III adverse events.
While our study has yielded promising insights, it is essential to recognize and address the limitations inherent to retrospective observational research. The study design itself, derived from real-world clinical practice, carries inherent constraints. However, PSA levels, OS, and rPFS are factors that cannot have a different interpretation. Notably, our cohort featured a relatively smaller proportion of de novo and HV mHSPC patients than in the TITAN trial. Furthermore, unlike our cohort, apalutamide trial did not encompass mHSPC M1a patients. Prior to the introduction of apalutamide, the treatment path for these patients remained unclear. Fortunately, our institutions were able to provide this treatment to an overlooked population, and the outcomes have been encouraging. Nevertheless, this composition mirrors the intricate realities of actual clinical scenarios, where patients' characteristics vary considerably. It's worth highlighting that our cohort also included patients with M1a disease. Furthermore, our follow-up period is limited. Continued monitoring of these patients, coupled with the inclusion of broader and diverse patient groups, would provide a robust platform for corroborating trial outcomes within the contours of real-world clinical practice.
5. Conclusions
Our real-world study found that patients with mHSPC under apalutamide combined with ADT treatment had a PSA response, OS, and rPFS that was consistent with the TITAN trial and the different meta-analysis published. We should highlight the apalutamide obtains rapid and deep PSA response, which has been identified as an important prognostic factor. “The better the PSA response, the better the oncological and survival outcomes.”
Authors contribution
Conception and design: López González, Cao Avellaneda, Moreno Alarcón. Acquisition of data: Yago Giménez, Server Gómez, Ramírez Backhaus. Analysis and interpretation of data: López González, López-Abad. Drafting of the manuscript: López-Abad, López González. Critical revision of the manuscript for important intellectual content: López Cubillana, Cao Avellaneda, Moreno Alarcón, de Pablos Rodríguez, Juan Fita, Climent Durán, Guardiola Ruiz, Vidal Crespo, Artés Artés, Montoya Chinchilla, Moreno Avilés, Guzmán Martínez-Valls.
Ethical statement
This study adheres to the ethical standards and safeguards to ensure the confidentiality and anonymity of all patient data. All information collected and utilized in this research was handled in accordance with the established ethical guidelines and regulations. Patient data were de-identified prior to analysis to ensure the complete protection of their privacy. The study protocol was reviewed and approved by the ethical committee (Medical Research Ethics Committee Virgen de la Arrixaca Hospital), assuring that the research is conducted in a manner that respects the rights and well-being of the participants. The study was conducted in strict adherence to the principles outlined in the Declaration of Helsinki. The research team is committed to upholding the principles of integrity, transparency, and ethical responsibility throughout the entirety of this study.
Conflicts of interest
None.
Funding
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.prnil.2023.10.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.International Agency for Research on Cancer . Globocan; 2022. World Health Organization.https://gco.iarc.fr/ [Internet]. [cited 2023 May 21]. Available from: [Google Scholar]
- 2.Sociedad Española de Oncología Médica . 2021. Las cifras del cáncer en España.https://seom.org/images/Cifras_del_cancer_en_Espnaha_2021.pdf [Internet]. [cited 2023 May 21]. Available from: chrome-extension. [Google Scholar]
- 3.Cózar J.M., Miñana B., Gómez-Veiga F., Rodríguez-Antolín A., Villavicencio H., Cantalapiedra A., et al. Registro nacional de cáncer de próstata 2010 en España. Actas Urol Esp. 2013;37(1):12–19. doi: 10.1016/j.acuro.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Patrikidou A., Loriot Y., Eymard J.C., Albiges L., Massard C., Ileana E., et al. Who dies from prostate cancer? Prostate Cancer Prostatic Dis. 2014;17(4):348–352. doi: 10.1038/pcan.2014.35. [DOI] [PubMed] [Google Scholar]
- 5.James N.D., Spears M.R., Clarke N.W., Dearnaley D.P., De Bono J.S., Gale J., et al. Survival with Newly Diagnosed Metastatic Prostate Cancer in the “Docetaxel Era”: Data from 917 Patients in the Control Arm of the STAMPEDE Trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67(6):1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 6.Mottet N., Cornford P., van den Bergh R., Briers E. European Association of Urology; 2023. Expert Patient Advocate (European Prostate Cancer Coalition/Europa UOMO), Eberli D, et al. EAU - EANM - ESTRO ESUR - ISUP - SIOG Guidelines on Prostate Cancer 2023 [Internet]https://uroweb.org/guidelines/prostate-cancer [cited 2023 Jul 31]. Available from: [Google Scholar]
- 7.Francini E., Gray K.P., Xie W., Shaw G.K., Valença L., Bernard B., et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC) Prostate. 2018;78(12):889–895. doi: 10.1002/pros.23645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gravis G., Boher J.M., Chen Y.H., Liu G., Fizazi K., Carducci M.A., et al. Burden of Metastatic Castrate Naive Prostate Cancer Patients, to Identify Men More Likely to Benefit from Early Docetaxel: Further Analyses of CHAARTED and GETUG-AFU15 Studies. Eur Urol. 2018;73(6):847–855. doi: 10.1016/j.eururo.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mottet N., Bellmunt J., Briers E., van den Bergh R., Bolla M., van Casteren N.J., et al. 2015. EAU Guidelines on Prostate Cancer 2015 [Internet]https://d56bochluxqnz.cloudfront.net/documents/EAU-Guidelines-on-Prostate-Cancer-2015.pdf [cited 2023 Jul 31]. Available from: chrome-extension. [Google Scholar]
- 10.Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T., et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur Urol. 2017;71(4):630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Gravis G., Boher J.M., Joly F., Soulié M., Albiges L., Priou F., et al. Androgen Deprivation Therapy (ADT) Plus Docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 Trial. Eur Urol. 2016;70(2):256–262. doi: 10.1016/j.eururo.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Fizazi K., Tran N., Fein L., Matsubara N., Rodriguez-Antolin A., Alekseev B.Y., et al. Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2019;20(5):686–700. doi: 10.1016/S1470-2045(19)30082-8. [DOI] [PubMed] [Google Scholar]
- 13.James N.D., De Bono J.S., Spears M.R., Clarke N.W., Mason M.D., Dearnaley D.P., et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338–351. doi: 10.1056/NEJMoa1702900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chi K.N., Agarwal N., Bjartell A., Chung B.H., Pereira De Santana Gomes A.J., Given R., et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13–24. doi: 10.1056/NEJMoa1903307. [DOI] [PubMed] [Google Scholar]
- 15.Chi K.N., Chowdhury S., Bjartell A., Chung B.H., Pereira De Santana Gomes A.J., Given R., et al. Apalutamide in Patients With Metastatic Castration-Sensitive Prostate Cancer: Final Survival Analysis of the Randomized, Double-Blind, Phase III TITAN Study. J Clin Oncol. 2021;39(20):2294–2303. doi: 10.1200/JCO.20.03488. [DOI] [PubMed] [Google Scholar]
- 16.Davis I.D., Martin A.J., Stockler M.R., Begbie S., Chi K.N., Chowdhury S., et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019 Jul 11;381(2):121–131. doi: 10.1056/NEJMoa1903835. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong A.J., Szmulewitz R.Z., Petrylak D.P., Holzbeierlein J., Villers A., Azad A., et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974–2986. doi: 10.1200/JCO.19.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith M.R., Hussain M., Saad F., Fizazi K., Sternberg C.N., Crawford E.D., et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132–1142. doi: 10.1056/NEJMoa2119115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fizazi K., Foulon S., Carles J., Roubaud G., McDermott R., Fléchon A., et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695–1707. doi: 10.1016/S0140-6736(22)00367-1. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.S., Kim S.H., Tae J.H., Chang I.H., Kim T.H., Myung S.C., et al. Oral chemotherapeutic agents in metastatic hormone-sensitive prostate cancer: A network meta-analysis of randomized controlled trials. Prostate Int. 2023;11(3):159–166. doi: 10.1016/j.prnil.2023.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swami U., Hong A., El-Chaar N.N., Nimke D., Ramaswamy K., Bell E.J., et al. Real-world first-line (1L) treatment patterns in patients (pts) with metastatic castration-sensitive prostate cancer (mCSPC) in a U.S. health insurance database. J Clin Oncol. 2021;39(15_suppl) 5072–5072. [Google Scholar]
- 22.Borque-Fernando A., Calleja-Hernández M.A., Cózar-Olmo J.M., Gómez-Iturriaga A., Pérez-Fentes D.A., Puente-Vázquez J., et al. Consenso multidisciplinar sobre idoneidad farmacológica en cáncer de próstata hormono-sensible metastásico. Actas Urol Esp. 2023;47(2):111–126. doi: 10.1016/j.acuroe.2022.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Sweeney C.J., Chen Y.H., Carducci M., Liu G., Jarrard D.F., Eisenberger M., et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyriakopoulos C.E., Chen Y.H., Carducci M.A., Liu G., Jarrard D.F., Hahn N.M., et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080–1087. doi: 10.1200/JCO.2017.75.3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.James N.D., Sydes M.R., Clarke N.W., Mason M.D., Dearnaley D.P., Spears M.R., et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gravis G., Fizazi K., Joly F., Oudard S., Priou F., Esterni B., et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 27.Harshman L.C., Chen Y.H., Liu G., Carducci M.A., Jarrard D., Dreicer R., et al. Seven-Month Prostate-Specific Antigen Is Prognostic in Metastatic Hormone-Sensitive Prostate Cancer Treated With Androgen Deprivation With or Without Docetaxel. J Clin Oncol. 2018;36(4):376–382. doi: 10.1200/JCO.2017.75.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussain M., Tangen C.M., Higano C., Schelhammer P.F., Faulkner J., Crawford E.D., et al. Absolute Prostate-Specific Antigen Value After Androgen Deprivation Is a Strong Independent Predictor of Survival in New Metastatic Prostate Cancer: Data From Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24(24):3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 29.Saad F., Small E.J., Feng F.Y., Graff J.N., Olmos D., Hadaschik B.A., et al. Deep Prostate-specific Antigen Response following Addition of Apalutamide to Ongoing Androgen Deprivation Therapy and Long-term Clinical Benefit in SPARTAN. Eur Urol. 2022;81(2):184–192. doi: 10.1016/j.eururo.2021.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Chowdhury S., Bjartell A., Agarwal N., Chung B.H., Given R.W., Pereira De Santana Gomes A.J., et al. Deep, rapid, and durable prostate-specific antigen decline with apalutamide plus androgen deprivation therapy is associated with longer survival and improved clinical outcomes in TITAN patients with metastatic castration-sensitive prostate cancer. Ann Oncol. 2023 May;34(5):477–485. doi: 10.1016/j.annonc.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel M., Würnschimmel C., Nocera L., Collà Ruvolo C., Tian Z., Shariat S.F., et al. Overall Survival After Systemic Treatment in High-volume Versus Low-volume Metastatic Hormone-sensitive Prostate Cancer: Systematic Review and Network Meta-analysis. Eur Urol Focus. 2022;8(2):399–408. doi: 10.1016/j.euf.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Jian T., Zhan Y., Yu Y., Yu K., Hu R., Wang J., et al. Combination therapy for high-volume versus low-volume metastatic hormone-sensitive prostate cancer: A systematic review and network meta-analysis. Front Pharmacol. 2023;14 doi: 10.3389/fphar.2023.1148021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Preisser F., Chun F.K., Banek S., Wenzel M., Graefen M., Steuber T., et al. Management and treatment options for patients with de novo and recurrent hormone-sensitive oligometastatic prostate cancer. Prostate Int. 2021;9(3):113–118. doi: 10.1016/j.prnil.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menges D., Yebyo H.G., Sivec-Muniz S., Haile S.R., Barbier M.C., Tomonaga Y., et al. Treatments for Metastatic Hormone-sensitive Prostate Cancer: Systematic Review, Network Meta-analysis, and Benefit-harm assessment. Eur Urol Oncol. 2022;5(6):605–616. doi: 10.1016/j.euo.2022.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Tohi Y., Kato T., Fukuhara H., Kobayashi K., Ohira S., Ikeda K., et al. Real-world analysis of apalutamide-associated skin adverse events in Japanese patients with advanced prostate cancer:a multi-institutional study in the Chu-shikoku Japan Urological Consortium. Int J Clin Oncol. 2022;27(8):1348–1355. doi: 10.1007/s10147-022-02183-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.