Summary
Studies on sensory information processing typically focus on whisker-related tactile information, overlooking the question of how sensory inputs from other body areas are processed at cortical levels. Here, we present a protocol for stimulating specific rodent limb receptive fields while recording in vivo somatosensory-evoked activity. We describe steps for localizing cortical-hindlimb coordinates using acute peripheral stimulation, electrode placement, and the application of electrical stimulation. This protocol overcomes the challenge of inducing a reproducible and consistent stimulation of specific limbs.
For complete details on the use and execution of this protocol, please refer to Miguel-Quesada et al.1
Subject areas: Health Sciences, Neuroscience, Behavior
Graphical abstract

Highlights
-
•
Steps for preparation of electrodes for peripheral limb stimulation
-
•
Instructions for craniotomy and placement of peripheral stimulation electrodes
-
•
Steps for locating somatotopic cortical regions in response to peripheral stimulation
-
•
Quantitative analysis of layering sensory-evoked cortical responses
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Studies on sensory information processing typically focus on whisker-related tactile information, overlooking the question of how sensory inputs from other body areas are processed at cortical levels. Here, we present a protocol for stimulating specific rodent limb receptive fields while recording in vivo somatosensory-evoked activity. We describe steps for localizing cortical-hindlimb coordinates using acute peripheral stimulation, electrode placement, and the application of electrical stimulation. This protocol overcomes the challenge of inducing a reproducible and consistent stimulation of specific limbs.
Before you begin
The primary somatosensory cortex (S1) receives information from four different sensory modalities (i.e., proprioception, temperature, touch and nociception). Prior in vivo electrophysiology studies have provided insight into some neural mechanisms involved in information processing. However, most of these studies have focused on structures like the barrel cortex due to the feasibility of stimulating specific whiskers in a rapid and reliable manner. Nonetheless, the study of information processing from the somatosensory representation of other body parts (e.g. peripheral extremities, trunk) represents a scientific interest due to several sensory pathologies associated with Central Nervous System (CNS) injuries (e.g., neuropathic pain, spasticity, phantom limb sensation),2,3 neurodegenerative disorders as well as development.4,5 In pursuit of this, different methods of peripheral stimulation have been used, including brush, pinch, prick, and electrical stimulation using fixed parameters. However, due to the difficulty of selection and consistent stimulation of specific receptive fields in the limbs, reproducible results are difficult to obtain due to inconclusive data among different experimental works. Here, we describe technical steps employed to set up a peripheral stimulation of the limbs of rodents to activate ascending sensory pathways related to tactile/proprioception and temperature/nociception. In addition, we describe how to identify and record evoked-sensory potentials from the S1 hindlimb representation. These steps include the assembly of stimulation electrodes and the configuration of signal acquisition for obtaining simultaneous cortical evoked potentials from S1 layers 2/3 to 6 (L2/3–6) in anesthetized mice.
Institutional permissions
Experiments were approved by the Ethical Committee for Animal Research at the Hospital Nacional de Parapléjicos (Toledo, Spain, 25-OH/2018) and carried out in accordance with the ARRIVE guidelines, the International Council for Laboratory Animal Science and the European Union 2010/63/EU Guidelines.
Assembly of peripheral bipolar stimulation electrodes
Timing: 1 h
Peripheral stimulation of the hindlimb is applied by two electrodes placed in the animal's ankle. This section describes the steps to fabricate the electrode required for the stimulation.
-
1.Prepare the bipolar electrodes for hindlimbs electrical stimulation:
-
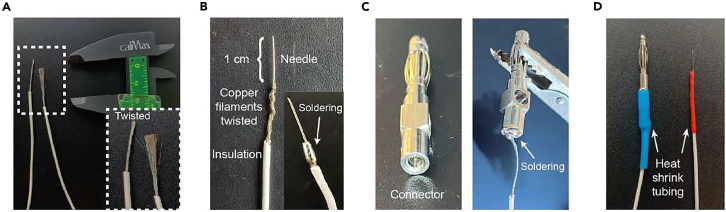
a.Separate the needle adapter and the 30G needle (0.3 × 8 mm) from the barrel of a three-piece insulin syringe (Omnican 20 0.5 mL/20 I.U., B. Braun, Germany) (Figures 1A and 1B).
-
b.Extract the needle from the plastic adapter by using the toothed part of a universal plier (RS Amidata S.A.U., Spain) to press the sides of the adapter and with another flat plier, pull out the needle (Figure 1C).
-
c.Use a wire stripper to carefully remove the silicone cover of the needle towards the blunt ending (Figure 1D).
-
d.Under a surgical microscope (M300, Leica, Germany), check that the beveled tip of the needle is undamaged (Figure 1E).
-
a.
CRITICAL: Handle the needle carefully to prevent any damage to the beveled tip that will be inserted subcutaneously in the animal's hindlimb (Figure 1E, undamaged needle). Additionally, ensure complete removal of the silicone cover, which acts as an electrical insulator, under the microscope (Figure 1F).
-
2.Electrode-connector assembly:
-
a.Peel the endings of a flexible multicore copper cable (1.5 mm outer diameter, RS Amidata S.A.U., Spain) by removing, with a wire stripper, 4–8 cm of the insulation cover on each side (Figure 2A).Note: Usually a 70 cm cable length is used, but it will depend on the distance between the stereotaxic apparatus where the animal is placed, and the stimulation set up connected to the recording system. Use short wires for the stimulus output to minimize stimulus artifact and other noise, therefore prepare cables for the shortest distance allowed in the setup.
-
b.Twist the multicore filaments from one end of the cable to the electrode needle, leaving 1–1.5 cm of the needle tip free (Figure 2B).
-
i.Begin by intertwining the filaments around each other.
-
ii.Position the blunt end of the needle against the multicore filament and proceed to twist them around the needle as shown in Figure 2B.
-
i.
-
c.Use a universal soldering tool at ∼400°C (ERSA, Germany) to melt an alloy thread of tin (60%) and lead (40%, thread: 250G weight, 1.2 mm diameter, MultiCore, Malaysia). This process involves coating the multicore strands with the tin-lead alloy and soldering them to the needle (Figure 2B).
-
d.Solder the other cable ending to a banana connector (diameter 5 mm, length 15 mm, RS Amidata S.A.U., Spain) (Figure 2C).Note: Connector type will depend on stimulus isolator output.
-
e.Use heat shrink tubing (2 mm diameter, RS Amidata S.A.U., Spain) to cover and protect both welds (Figure 2D).Note: We recommend performing this step in a fume hood due to potential lead exposure. For added protection against hand burns, wear leather safety gloves (Figures 2B–2D).Note: As bipolar electrodes are inserted subcutaneously on each side of the animal's ankle, two electrodes are required. Hence, repeat steps 1–2 to fabricate an additional stimulation electrode. It is advisable to have a spare set of electrodes ready for use. Additionally, we recommend cleaning the needle surface with soapy water to remove skin particles, followed by a wipe with 70% ethanol (Panreac Química S.L.U., Spain) after each experiment.
 CRITICAL: Verify the resistance and conductivity of the fabricated electrode using a multimeter (Amprobe 5XP-A, USA) after assembling the stimulation electrode and before the experiments. Resistance should be ≤ 0.5 MΩ to ensure proper conductivity. Troubleshooting 1.
CRITICAL: Verify the resistance and conductivity of the fabricated electrode using a multimeter (Amprobe 5XP-A, USA) after assembling the stimulation electrode and before the experiments. Resistance should be ≤ 0.5 MΩ to ensure proper conductivity. Troubleshooting 1.
-
a.
Figure 1.
Electrode needle extraction from the syringe
(A) Three-piece insulin syringe used for obtaining the electrical stimulation needle.
(B) Parts of the insulin syringe (cap, barrel and needle).
(C) Picture of the needle after removing it from the plastic adapter.
(D) Electrode needle completely extracted, and silicone cover removed. White square showing the needle tip.
(E) Picture of a damaged beveled ending (top) that does not work for stimulation and an undamaged/functional needle with an intact beveled ending (bottom).
(F) Picture showing a partial (top) and complete (bottom) removal of the silicone covering the needle.
Figure 2.
Steps for the electrode-connector assembly
(A) Left: Multicore copper cable used for assembling the stimulation needle. Right: zoom image of the white square on the left showing the multicore cable (the left one shows the twisting result).
(B) Twisted multicore filaments around the electrode needle. Inset image shows the multicore filament soldered with the needle.
(C) Left, banana connector showing where the cable ending will be soldered. Right, the cable ending soldered on a banana connector.
(D) Both cable endings soldered and isolated with heat shrink tubing.
Set up: Stimulation and recording system
Timing: 1 h
This section describes how to connect the stimulation electrodes to the recording system and how to set the stimulation parameters (Figure 3).
-
3.Connect stimulation electrodes.
-
a.Connect each banana plug from the two stimulation electrodes to the output of a stimulus isolator unit (Figure 3A1-2).Note: The isolation unit will be used to control the stimulus intensity (in voltage or current mode).
-
i.Use a BNC cable (50 Ω, RS Amidata S.A.U., Spain) to connect the output from the stimulus isolator to a digital stimulator (Figure 3A-3) to control frequency and pulse duration.
-
ii.Connect the digital stimulator to a recording system (Figure 3A-4).Note: In our case, we use an ISO-Flex (A.M.P.I., Israel) as an isolator unit and a DS8000 (WPI, USA) as a digital stimulator. Nonetheless, other commercial brands can be used to control the parameters of the electrical stimulation. If this is the case, we advise checking the specifications of each apparatus for a proper configuration of the stimulus parameters (ISO-Flex and DS8000).Note: Before connecting stimulation electrodes, it is advisable to verify that the output current from the stimulation unit corresponds to the desired one. For that, set the stimulus isolator to a series of random currents or voltages and use a multimeter to check the mA or V values, respectively. If reading values do not match the ones set in the stimulation unit, try fabricating other electrodes, checking the battery (see CRITICAL below) or calibrating the output with the corrected value. Note that for activation of ascending sensory pathways in mice, stimulation values from 0.2 mA to 5 mA are used.1
 CRITICAL: Before initiating a new set of experiments, verify the voltage of the isolator unit's supply battery with a multimeter. To ensure proper functioning of the ISO-flex isolator unit, the 9 V inner battery (used for the operation of the isolator) should remain above 8.5 V, and the 90 V high-voltage supply battery (used to drive the output pulses) should be maintained above 85 V. It is advisable to routinely verify that both batteries are within the optimal range to ensure a consistent stimulus amplitude. This checking process is usually performed at the initiation of a "set of experiments", which guarantee a perfect function for next 6 months. If reading limits are lower than necessary, the 90 V battery can be replaced with any kind of battery in the range of 9–100 V.
CRITICAL: Before initiating a new set of experiments, verify the voltage of the isolator unit's supply battery with a multimeter. To ensure proper functioning of the ISO-flex isolator unit, the 9 V inner battery (used for the operation of the isolator) should remain above 8.5 V, and the 90 V high-voltage supply battery (used to drive the output pulses) should be maintained above 85 V. It is advisable to routinely verify that both batteries are within the optimal range to ensure a consistent stimulus amplitude. This checking process is usually performed at the initiation of a "set of experiments", which guarantee a perfect function for next 6 months. If reading limits are lower than necessary, the 90 V battery can be replaced with any kind of battery in the range of 9–100 V.
-
i.
-
a.
-
4.Setting up the recording system.Note: Different recording systems can be used to acquire in vivo simultaneous extracellular recordings from mice somatosensory cortex. In our case, we use a multichannel neural data acquisition system (Omniplex D System, Plexon Inc, USA) (Figure 3A-4) equipped with a digital input, an auxiliary analog input (up to 512 channels), a digital headstage processor (DHP) subsystem (Figure 3A-5) connected to the Omniplex D System chassis and auxiliary analog input ribbons that connect DHP to headstages (Figure 3A-6).Note: Use the fabricant manual to configure the OmniPlex System hardware and software. Different configurations will depend on the recording probe and other acquisition properties such as sampling rate of acquisition, filtering and other characteristics determined by the experimental design.
-
a.Mount the SM-15 stereotaxic micromanipulator controlled by a MO-10 oil hydraulic micromanipulator (Narishige, Japan) on the stereotaxic frame (SR-6M-HT Narishige Scientific Instruments, Japan, Figures 3A-7).
-
b.Fix the adapter (MOLC-110-01-S-Q, SAMTEC, USA) for the recording probe on the micromanipulator as shown in Figures 3A-6.
-
c.Connect the four 8-channel digital headstages (HST/8o25-9P-GR, Omnetics Connector Corporation, USA) to the adapter.Note: Different adapters might be required depending on the probe and headstage design/alignment (e.g. adapter for 32 to 16ch (Adpt-A16-A32, NeuroNexus Technologies Inc., USA)). In our case, we use a 32 iridium contacts with a site area of 177 μm2 and spaced at 50 μm, impedance 1–4 MΩ at 1 kHz (A1x32-6mm-50-177-A32, NeuroNexus Technologies Inc., USA). The reference electrode was built in the probe, 0.5 mm above the most superficial recording site and outside the cortex (diameter 4200 μm2). For more information about the acquisition system and neural probes used in this Star Protocol, please visit https://plexon.com/plexon-systems/omniplex-neural-recording-system/ and https://www.neuronexus.com/products/electrode-arrays/.
-
a.
Figure 3.
Schematic of the stimulation and recording set up
(A) Different parts of the recording setup showing the acquisition system and the experimental table. Numbers represent: 1. stimulation unit; 2. stimulation unit battery; 3. digital stimulator; 4. Omniplex system; 5. DHP Amplifier; 6. Micromanipulator with electrode-headstage-adapter assemble; 7. stereotaxic frame; 8. ventilation mask for anesthetic influx; 9. lamp and surgical microscope; 10. homeothermic blanket.
(B) Illustrative representation of the stimulation and recording setup. Numbers represent the same from panel A.
Prepare the experimental table and surgical tools
Timing: 20 min
This step describes surgical supplies and materials required for the craniotomy surgery. It is recommended to prepare the experimental table 30 min before the beginning of the experiment.
-
5.
Sterilize surgical tools in 70% ethanol: fine scissors-sharp (F.S.T., Germany), dumont #7 forceps (F.S.T., Germany), delicate suture tying forceps (F.S.T., Germany), scalpel (Swann-Morton, UK), surgical caliper (F.S.T., Germany), bulldog clamp (F.S.T., Germany), drill and drill bit (Figure 4, F.S.T., Germany).
-
6.
Prepare the surgery setup consisting of a stereotaxic frame with a mouse nose mask connected to an anesthesia system (Figures 3A-8 and 5A-1, Medical Supplies & Services, Int. Ltd, UK), a light source coupled to a surgical microscope (CLS 150 MR, Leica, Germany; Figure 3A-9) and an automatic homoeothermic blanket (Figures 3A-10 and 5A-2, RTC-1, Cibertec SL, Spain) placed on top of the stereotaxic base.
-
7.
Cover the homoeothermic blanket with aluminum foil to maintain the temperature, and use a paper towel on top to place the animal (Figures 3A-10 and 5A-2).
-
8.
Check that the anesthetic vaporizer contains the appropriate amount of isoflurane (Aerrane, Baxter, USA) allowed by the system.
Figure 4.
Surgical material for stereotaxic surgery
(A) Fine scissors sharp.
(B) Dumont #7 forceps.
(C) Delicate suture tying forceps.
(D) Scalpel.
(E) Surgical caliper.
(F) Bulldog clamp.
(G) Drill bit.
(H) Drill.
Figure 5.
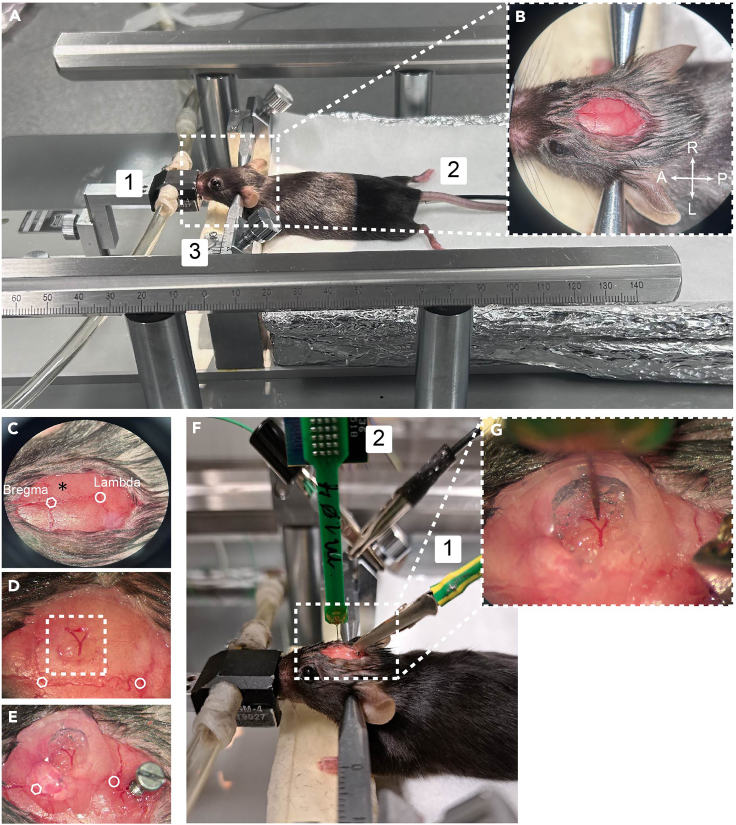
Cranial window and ground electrode placement
(A) Stereotaxic frame showing the animal on a homeothermic blanket held with ear bars on a ventilation mask. Note that aluminum foil is used to wrap the blanket to maintain animal temperature and decrease electrical noise. Numbers represent: 1. ventilation mask; 2. temperature sensor; 3. ear bars.
(B) Expanded image of the animal head showing the ear bars and cranial sutures. Note that both lambda and bregma are easily observed after removing cranial skin.
(C) Representation of bregma, lambda and the anatomical coordinate of the hindlimb somatosensory cortex on the right hemisphere (asterisk).
(D) Visible cranial window (3 × 3 mm) within the white square.
(E) View of the ground electrode (1 × 4 mm screw) placed posterior to lambda and the cranial window with the agar barrier.
(F) General view of the electrode array and the ground cable. Numbers represent: 1. ground cable; 2. recording electrode assembled to the headstage.
(G) Electrode array over the cortical surface of the right hindlimb cortex.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Acetic acid, 99.7%, ACS reagent | Sigma-Aldrich | Cat# 00761AJ |
| Agar | Sigma-Aldrich | Cat# A7002-250G |
| Chicago Sky Blue 6B | Sigma-Aldrich | Cat# 8679 |
| DAPI Fluoromount-G | Southern Biotech | Cat# 0100-20 |
| DPX mountant for histology | Sigma-Aldrich | Cat#06522-100ML |
| Ethanol | Panreac Química S.L.U. | Cat# 131086.1214 |
| Fluoromount-G | Southern Biotech | Cat# 0100-01 |
| Formalin (4% formaldehyde) | Sigma-Aldrich | Cat# 1.00496 |
| Gelatin powder | Merck | Cat# 1.04078.1000 |
| Heparin | Laboratorio Reig Jofré, S.A. | Cat# 608737.4 |
| Hoechst 33342 dye | Sigma-Aldrich | Cat# B2261 |
| Isoflurane (AErrane) | Baxter | Cat# FDG9623 |
| Lidocaine 2% | Normon | Cat# P06B1 |
| Methanol | Sigma-Aldrich | Cat# 34860-1L-R |
| Pentobarbital sodium (Dolethal) | Vetoquinol Group | N/A |
| Phosphate buffer solution 0.1 M (PB) | Sigma-Aldrich | Cat# P5244 |
| Phosphate-buffered saline (PBS) | Sigma-Aldrich | Cat# P7059 |
| Povidone iodine 10% | Laboratorio Reig Jofré, S.A. | Cat# 838151.7 |
| Saline solution 0.9% | B. Braun Medical, S.A. | Cat# 607189.2 |
| Sucrose | Sigma-Aldrich | Cat# S9378 |
| Thionine acetate salt | Sigma-Aldrich | Cat# T7029-5G |
| Tissue freezing medium | Leica Biosystems | Cat#14020108926 |
| Xylene | Sigma-Aldrich | Cat# 534056 |
| Experimental models: Organisms/strains | ||
| C57BL6/J male or female mice (8–16 weeks) | Jackson Laboratory | JAX:000664, RRID:IMSR_JAX:000664 |
| Software and algorithms | ||
| Igor Pro, Wavemetrics | WaveMetrics | RRID:SCR_000325 https://www.wavemetrics.com |
| ImageJ | Schneider et al.6 | RRID:SCR_003070 https://imagej.nih.gov/ij/ |
| MATLAB | The MathWorks, Inc. | RRID:SCR_001622 https://jp.mathworks.com/ |
| OmniPlex Server and PlexControl software | Plexon Inc | RRID:SCR_014803 |
| SARFIA (Igor Pro, Wavemetrics) | Dorostkar et al.7 | https://www.wavemetrics.com/project/SARFIA |
| Spike2 v7 | Cambridge Electronic Design (CED) | RRID:SCR_000903 |
| Deposited data | ||
| Custom-written scripts for processing data in Spike2 | This study | https://doi.org/10.5281/zenodo.10365406 |
| Other | ||
| 8-channel digital headstages | Omnetics Connector Corporation | HST/8o25-9P-GR |
| Adapter for recording probe (32–16 channels) | NeuroNexus Technologies Inc. | Adpt-A16-A32 |
| Alloy thread of tin (60%) and lead (40%) | Multicore | Cat# 295-4836 |
| Anesthesia system | Medical Supplies & Services, Int. Ltd | N/A |
| Banana connector | RS Amidata S.A.U. | N/A |
| BNC cable | RS Amidata S.A.U. | Cat#426-2038 |
| Bulldog clamp | F.S.T | Cat# 18039-45 |
| Cranial screws M1 x 4 mm | F.S.T. | Cat# 39886 |
| Cryostat CM1520 | Leica Biosystems | N/A |
| Delicate suture tying forceps | F.S.T | Cat# 11063-07 |
| Digital stimulator DS8000 | World Precision Instruments | RRID:SCR_008593 |
| Drill | F.S.T | Cat# 097034 |
| Drill bit | F.S.T | Cat# 19007-05 |
| Dumont #7 forceps | F.S.T | Cat# 91197-00 |
| Gelfoam sponge | Ferrosan Medical Device | Cat# 4547333 |
| Fine scissors-sharp | F.S.T | Cat# 14060-10 |
| Hair clipper | Aesculap Inc. | Cat# GT606 |
| Heat shrink tubing | RS Amidata S.A.U. | Cat# 481-1775 |
| Homoeothermic blanket | Cibertec SL | Cat# RTC-1 |
| Hypoallergenic non-wave tape CPK TAPE | Farmaban | Cat# 1436 |
| Leukosan adhesive | Leukoplast | Cat# 730-585 |
| Linear vertical array of 32 iridium contact | NeuroNexus Technologies Inc. | A1X32-6mm-50-177-A32 |
| Linear vertical arrays (A1x32) adaptor | Samtec | MOLC-110-01-S-Q |
| Lubrithal eye gel | Dechra | N/A |
| Microscope TCS SP5 | Leica Microsystems | N/A |
| Microtome Microm HM450 | Thermo Fisher Scientific | N/A |
| Multicore copper cable | RS Amidata S.A.U. | Cat# 285-7713 |
| Multimeter | Amprobe | Cat# 5XP/A |
| OmniPlex System | Plexon | RRID:SCR_014803 |
| One-axis oil hydraulic micromanipulator | Narishige International Limited | Cat# MO-10 |
| Plastic pipette | VWR | Cat# 612-2846P |
| Scalpel | Swann-Morton | Cat# 0305 |
| Soldering station (24 V, SMT unit 60 A) | ERSA | N/A |
| Stimulus isolator unit | ISO-Flex, A.M.P.I. | RRID:SCR_018945 |
| Stereotaxic frame and ear bar for mice | Narishige International Limited | Cat# SR-6M-HT and Cat# EB-5N |
| Stereotaxic micromanipulator | Narishige International Limited | Cat# SM-15R/L |
| Surgical microscope M300 and light source CLS 150 MR | Leica Microsystems | N/A |
| Surgical caliper | F.S.T | Cat# 18000-35 |
| Syringe 0.5 mL | B. Braun Medical, S.A. | Cat# 9161619S |
| Tissue multiwell plate | VWR | Cat# 734-2779 |
| Tweezers | F.S.T | Cat# 11008-13 |
| Universal pliers | RS Amidata S.A.U. | Cat# 161-164 and Cat# 221-4946 |
| Vaseline | Laboratorio Reig Jofré, S.A. | Cat# 845008-EFP |
| Vibratome VT1000S | Leica Biosystems | RRID:SCR_016495 |
Materials and equipment
All materials and equipment referenced in this protocol are listed in the key resources table. Unless otherwise noted, all materials and equipment can be substituted for equivalent products from different vendors.
Agar solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Agar | 3% | 3 g |
| Saline | 0.9% | 10 mL |
| Total | NA | 10 mL |
Note: Dilute 3 g of agar (Sigma-Aldrich, USA) in 10 mL of 0.9% saline solution (B. Braun Medical, S.A., Germany) within a glass beaker. Use a microwave to heat the solution for brief periods of time (10–20 sec) until it is totally dissolved. As agar will be used for making a barrier around the craniotomy, leave the solution at 22°C–24°C until it is warm (around 36°C). Keep in mind that the agar solution solidifies with a decrease in temperature. Therefore, it is advisable to prepare the solution just before use (Step 10).
Step-by-step method details
Electrical hindlimb stimulation will activate S1 neuronal circuits in a somatotopic manner. This section describes the steps to perform an acute cranial window over the hindlimb cortex followed by a precise verification of the cortical location through electrophysiological recordings.
Note: This experiment is designed for using 2–3 months old mice (both genders, weight > 20 g). If experiments will be performed in younger or smaller mice, anesthesia and electrode placement might be adjusted.
Note: No habituation to the setup is required as experiments will be performed acutely and under anesthesia.
Stereotaxic surgery for cranial window and ground electrode placement
Timing: 30–40 min
-
1.Anesthesia:
-
a.Deeply anesthetize the animal by first placing it in an induction chamber with a 3% isoflurane in 0.8–1 L O2/min for about 2–3 min until breathing rate is about 50–60 breaths/min.
-
b.After complete loss of movement, gently transfer the animal from the induction chamber to the ventilator mask (Figure 5A-1) coupled to the stereotaxic frame.
-
c.Check again that the breathing rate is about 50–60 breaths/min and that the animal does not exhibit response to tail or extremities (fore- or hindlimb) pinch.
 CRITICAL: If the animals start to gasp, it indicates that the anesthesia level is too high and should be decreased. For more information about monitoring vital signs in mice under anesthesia without electroencephalogram recording, see.8
CRITICAL: If the animals start to gasp, it indicates that the anesthesia level is too high and should be decreased. For more information about monitoring vital signs in mice under anesthesia without electroencephalogram recording, see.8
-
d.Insert the homoeothermic blanket's rectal thermal probe (Figure 5A-2, 2 mm) to maintain the animal's core temperature at 36.5°C.Note: To avoid harming the animal, first use a hyssop to apply vaseline (Laboratorio Reig Jofré, S.A., Spain) to the 2 cm tip of the rectal thermal. This helps ensure a smooth and safe insertion of the probe while minimizing any potential discomfort for the animal. Next, gently lift the mouse's tail and slowly insert ⅓ of the probe into the rectum. Finally, secure the flexible cable of the probe on the blanket using a piece of hypoallergenic tape (non-woven CPK TAPE, Farmaban, Spain).
 CRITICAL: During the experiment, regularly ensure that the probe is correctly inserted to prevent skin burns caused by inaccurate temperature readings.
CRITICAL: During the experiment, regularly ensure that the probe is correctly inserted to prevent skin burns caused by inaccurate temperature readings. -
e.Keep isoflurane to 1.5–2% with 0.8–1 L O2/min to maintain anesthesia.
-
a.
-
2.Head-fix the mouse using two ear-bars (EB-5N, Narishige, Japan) (Figure 5A-3).
-
a.Gently insert the ear bars into the ear canals, taking care not to damage the ears or ear canals. For that, insert one ear bar into the ear canal while holding the mouse's head and then do the same with the other ear bar.
-
b.Ensure that the ear bars are positioned symmetrically and securely.
-
c.Use the stereotaxic instrument’s adjustments to align the head properly. The bregma and lambda points should be level and centered as well as left and right hemispheres.
-
d.Secure ear bars by tightening the screws or knobs on the ear bars gradually and evenly to secure them in place. Be cautious not to overtighten, as it may cause injury to the mouse.
-
a.
Note: Choose ear bars that are appropriate for the size of the mouse and compatible with the stereotaxic instrument.
CRITICAL: Make sure that the head is securely fixed and does not move with breathing. This is critical as the quality of neuronal recordings and the presence of spike activity highly depend on tissue movement.
-
3.
Cover eyes with a drop of ophthalmic eye gel (Lubrithal, Dechra, UK) to retain moisture and avoid cornea dryness.
-
4.Prepare the mouse head for the craniotomy (Figure 5B):
-
a.Shave the mouse’s head with a hair clipper (Akkurata, Aesculap Inc., USA) specifically for small animals and remove any remaining hair residues using cotton-swabs or soft paper.
-
b.Clean and disinfect the area with a 70% ethanol-soaked cotton swab and then 10% povidone iodine (Laboratorio Reig Jofré, S.A., Spain).
-
c.Apply 2% lidocaine solution (Normon, Spain) subcutaneously into the scalp and wait about 5–10 min to induce local anesthesia.
-
d.Use a scalpel to make a straight incision of approximately 12 mm and to expose the skull.
-
e.Remove any periosteum/connective tissue on the dorsal surface of the skull with a saline-soaked cotton swab or tweezers.
-
f.Verify that the alignment of the mouse’s head is correct (Step 2c).
-
a.
-
5.Perform the craniotomy:
-
a.Use a surgical caliper to identify the anatomical coordinate of the somatosensory hindlimb cortex (for adult mice: AP -1; ML 1.5) and mark the position with a pen or using the same surgical tool (Figure 5C).
-
b.Use a drill with a drill bit of 0.5 mm to circularly thin the bone around the marked coordinate making a 3 × 3 mm cranial window (stereotaxic coordinates in anteroposterior and mediolateral axis: AP 0 to -3 mm; ML 1–4 mm, respectively, Figure 5D).
 CRITICAL: Drill cranium carefully. Any tissue damage to the cortical surface will directly affect the quality of the in vivo electrophysiological data. Troubleshooting 2.
CRITICAL: Drill cranium carefully. Any tissue damage to the cortical surface will directly affect the quality of the in vivo electrophysiological data. Troubleshooting 2. -
c.Keep the cortex wet with 0.9% saline solution. In case of bleeding, stop the hemorrhage with small pieces of espongostan film (Ferrosan Medical Devices, Denmark).
 CRITICAL: Avoiding dryness of cortical surface is crucial for the tissue integrity and quality of recordings.
CRITICAL: Avoiding dryness of cortical surface is crucial for the tissue integrity and quality of recordings.
-
a.
-
6.Ground electrode placement. (Figure 5E).
-
a.Use a drill with a 0.5 mm drill bit to create a small hole with a diameter of 0.8 mm and a depth of 0.4 mm, ideally along the midline just posterior to lambda. However, this location is not crucial, and it can vary to any place posterior to the lambda line.
-
b.Use a drill to tighten a 1 × 4 mm screw (F.S.T., Germany), leaving about 0.6 mm outside.Note: To ensure the fixation of the screw, add two drops of Leukosan Adhesive (Leukoplast, Spain) between the screw base and the skull.
 CRITICAL: Be careful not to insert the cranial screw too deeply, as it may damage the surface of the cerebellum.
CRITICAL: Be careful not to insert the cranial screw too deeply, as it may damage the surface of the cerebellum. -
c.Connect the ground cable of the acquisition system to the ground electrode (Figure 5F-1).
 CRITICAL: The cranial screw used for grounding must be well fixed to the bone and avoid touching any musculature or skin, otherwise, it will add physiological noise to the recording.
CRITICAL: The cranial screw used for grounding must be well fixed to the bone and avoid touching any musculature or skin, otherwise, it will add physiological noise to the recording.
-
a.
Insertion of the vertical probe and somatotopic verification of cortical limb region
Timing: 2 h
This section describes how to proceed with the cortical insertion of the recording electrode and to verify - by means of evoked neuronal activity - the correct somatotopic representation of the hindlimb cortex.
-
7.
Switch on the acquisition recording system (amplifier, preamplifier, computer) and stimulation devices (isolator unit, digital stimulator).
Note: Follow manufacturer's guidelines for the proper sequence (if any) to switch on all devices.
-
8.
Connect the recording probe to the headstage adapter mounted on the hydraulic micromanipulator and make sure that it is set to 0 mm (Figure 5F-2).
-
9.
Use the surgical caliper to visually localize the S1 hindlimb coordinate (AP -1; ML 1.5).
Note: An alternative is to calculate anatomical coordinates once the probe is held on the micromanipulator. Without touching the cranium, locate the probe over bregma and use this reference as zero point. Then, use the micromanipulator X-Z axis coarse control to reach the desired stereotaxic coordinates.
-
10.Make a 3% agar barrier around the cortical area where the electrode array will be inserted (Figures 5E and 5G).
-
a.Prepare agar solution (see materials and equipment section).
-
b.Use a plastic pipette (1 mL, VWR, USA) to place the agar solution around the borders of the craniotomy.
-
a.
Note: If the agar solution spills over the cortical surface, wait for a complete solidification of the agar and shape it carefully with the help of a needle under a surgical microscope.
-
11.Insert the recording electrode under visual guidance of the surgical microscope. Figure 5G.
-
a.Remove the dura mater to facilitate electrode insertion.Note: Dura mater is usually removed during the craniotomy when working with mice. Nonetheless, use the surgical microscope to verify the presence of the dura mater. If it is still attached to the cortical surface, elevate the dura with a surgical tweezer (F.S.T., Germany) and create an incision, thereby separating the dura mater toward the periphery of the craniotomy.
-
b.Position the recording electrode array over the cortical surface of the hindlimb cortex.
-
c.Use the Y coarse control of the micromanipulator to slowly insert the array a few micrometers into the cortical tissue until observing with the surgical microscope the penetration of the electrode tip or a slight release of pressure due to the rupture of meninges such as pia and arachnoid.
-
d.Slowly withdraw the probe to the cortical surface.Note: Use the surgical microscope to observe the cortical surface until it returns to a flat position and no pressure and/or tissue deformity is visible.
-
e.Add a few drops of saline solution inside the agar barrier to keep the cortical surface in optimal conditions and the probe moist to reduce electrical noise.Note: Background noise in the recording is increased when the reference electrode within the linear probe is dry. Therefore, continuously check that the electrode is moist and add saline solution when needed.
-
f.Continue lowering the electrode slowly while observing the patterns of neuronal activity on the acquisition system software (Figures 6A and 6B).Note: Verify that background noise is at minimum. Troubleshooting 3.
 CRITICAL: Maintenance of cortical tissue integrity is key for the quality of the recordings. Therefore, we recommend keeping the velocity of electrode insertion to 0.001 mm/s as reported in9 and the cortical surface always moist with saline solution.
CRITICAL: Maintenance of cortical tissue integrity is key for the quality of the recordings. Therefore, we recommend keeping the velocity of electrode insertion to 0.001 mm/s as reported in9 and the cortical surface always moist with saline solution. -
g.When reaching 200–300 μm depth, check that the pattern of neuronal activity is in slow wave oscillation (< 1 Hz, Figure 6A) and some spontaneous action potentials can be observed on top of the up-states (Figure 6B). Troubleshooting 4.Note: Sometimes, a reversal in the polarity of the signal is found when entering S1 (100 μm depth).
-
h.Introduce the recording probe to about 300–500 μm depth to reach pyramidal neurons in layer 2/3 and 4 and wait for about 10 min to stabilize the neuronal recording.
-
a.
-
12.
Once cortical activity is stable and spontaneous action potentials are observed during up-states, use the tip of a cotton swab or a small brush to gently apply repeated tactile stimulation on the plantar surface of the hindlimb (Figure 6C).
Note: If the electrode is placed in the somatotopic representation of the hindlimb, tactile stimulation of the receptive field will evoke a response in the local potential (LFP) containing action potentials (multiunit activity, MUA) during stimulation (Figures 6C and 6D).
Note: The evoked neuronal activity in S1 layer 4 neurons is due to the direct sensory inputs from the ventral posterolateral nucleus of the somatosensory thalamus carrying mainly tactile and proprioceptive information.10
Note: Changes in the voltage induced by action potentials produce an audible noise that can be heard by connecting the output of the amplifier to a loudspeaker. This audible noise can be used to facilitate the somatotopic verification of the electrode at the hindlimb representation.
CRITICAL: Make sure that tactile stimulation evokes action potentials on L2/3-L4. This is the confirmation that electrode recording is in the correct somatotopic place. Troubleshooting 5.
CRITICAL: Double-check that the animal's body temperature is between 36°C–37°C. Cortical activity is strongly affected by body temperatures lower than 36°C or higher than 37°C.
-
13.
After finding the correct somatotopic region, continue lowering the electrode as described in Step 11-d until 1.1–1.2 mm and then retrieve it 100–150 μm to release cortical pressure.
Note: The total depth of the electrode placement is 1.1 mm, which is usually the cortical thickness of the somatosensory hindlimb cortex in adult mice. Nonetheless, this measure may differ depending on the age, weight or strain of mice strain. We recommend performing a post-hoc checking of the electrode track to verify the electrode is covering the entire cortical depth (see Step 28).
Figure 6.
Spontaneous neuronal activity during slow wave activity and evoked tactile response
(A) Spontaneous local field potentials (LFP) from electrodes placed up to L4 showing neuronal activity during up-states (shaded squares) and down-states.
(B) Multi-unit activity (MUA) from the respective LFP traces shown in panel A. Note the presence of action potentials during up-states.
(C) Top: Schematic of the tactile stimulation performed with a cotton swab on the plantar surface of a mouse hindlimb. Bottom: Tactile-evoked response from electrodes placed on the somatosensory hindlimb cortex. Shaded squares are the onset of the evoked response.
(D) Top: Magnification of a sensory-evoked response showing the voltage deflection in response to tactile stimulation. Bottom: Filtered local field potentials showing multi-unit activity (MUA) from different electrodes in response to tactile stimulation. Note the onset response of action potentials starting in L4 showing the correct location of the recording electrode. Shaded squares are the onset of the evoked response.
Placement of stimulation electrodes and in vivo acquisition of cortical evoked potentials
Timing: 3–4 h
Steps describing how to place the peripheral stimulation electrodes on animal's ankles and further acquisition of evoked cortical potential in response to electrical stimulation.
-
14.
After total insertion of the recording probe, allow recovery of the cortical activity for about 30–60 min before starting the electrical stimulation.
Note: Maintain online visualization of the neuronal activity during the recovery time to continuously check the cortical state (Figures 6A and 6B). Also, continuously check the animal's temperature.
-
15.
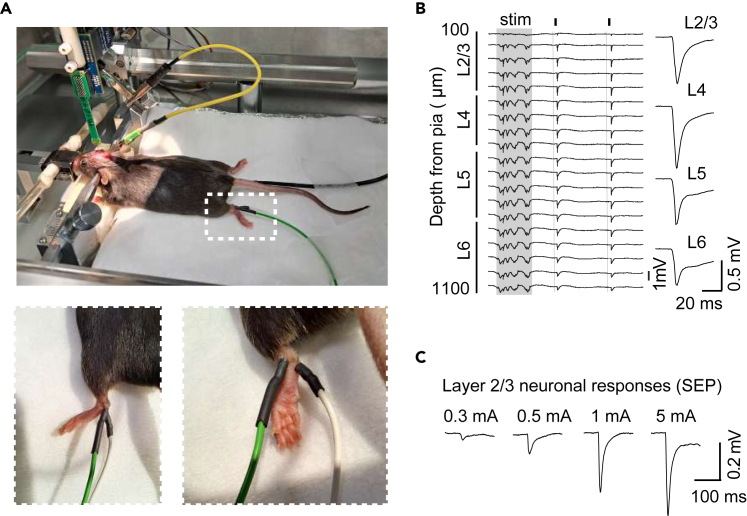
Meanwhile, place each stimulation electrode subcutaneously on each side of the contralateral ankle (Figure 7A) to generate an electric current from one pole to another to activate the ascending sensory fibers.
Note: Check the electrical stimulation by setting the stimulus unit to 5 mA and verifying that the hindlimb elicits a slight movement generated by the muscle contraction to the high stimulus intensity.
Note: Also optimize the electrical stimulation by changing the polarity of the stimulus unit and verifying the magnitude of the sensory-evoked potential (SEP).
-
16.
Set the intensity of the isolation unit to 0.2 mA (current mode, range 0.001–10 mA) and the frequency on the digital stimulator to 0.5 Hz, 0.2 ms.
-
17.
Begin the continuous recording and after 1 min of baseline start the stimulation protocol on the digital stimulator.
-
18.
Record continuous cortical activity for about 2 min (that is, 50–60 stimuli).
-
19.
Once finishing the first stimulus intensity, sequentially increase the amplitude of the stimulation in the isolation unit to the next intensity and repeat 17 and 18.
Note: To obtain an I:O curve with enough data points, use the following intensities: 0.2, 0.3, 0.4, 0.5, 0.75, 1, 1.5, 2, 3 and 5 mA. Figures 7B and 7C. Note that lower intensity (less than 1 mA) activates low threshold fibers and higher intensities stimulate all ascending sensory pathways.11
Note: For intensities lower than 1 mA, we recommend setting the multiplier knob to x0.1 and the amplitude knob to one digital unit for the right conversion (e.g. 0.2 mA: multiplier ×0.1 and amplitude 2).
Note: Wait for about 2–3 min between each stimulus intensity to allow cortical neurons to recover from the previous stimulation and thus avoid synaptic adaptation, especially when high intensity is applied (≥ 1 mA). Troubleshooting 6.
Note: The neuronal activation due to stimulation can induce a shift in cortical state from slow wave activity (0.5–1 Hz) to delta (1–4 Hz), so it is important to check the cortical state before each stimulation protocol. Troubleshooting 4.
Note: We recommend using - eventually - a random stimulation protocol (that is, scrambled order of stimulus intensities) to verify that cortical responses as a function of stimulus is not due to stimulus order.
-
20.Proceed to clean the recording electrode.
-
a.At the end of the in vivo electrophysiological experiment, completely remove the electrode array.
-
b.Rinse the electrode with distilled water and then soak the probe in a protein-dissolving detergent such as contact lens solution for 6–10 h.
-
c.Use isopropyl alcohol (e.g., 70% IPA) to rinse the electrode and store the probe until next use.
-
a.
Note: Follow the cleaning instructions from each electrode producer. In our case, all information about cleaning can be found at Neuronexus website.
Note: The usual lifetime of an electrode is about 10 uses, depending on the care taken while inserting the probe at the tissue and cleaning conditions. After that, recording signals from some channels (usually the ones at the tip of the array) will be noisy and inconsistent, indicating that the probe should be replaced.
-
21.Staining of the electrode track. Figure 8A.
-
a.After finishing the experiment and removing the electrode, dip a spare non-functional electrode in a Chicago Sky Blue immunofluorescent solution (2% in methanol from a 4% in saline stock solution, Sigma-Aldrich, USA).
-
b.Let it dry at 22°C–24°C for 30–40 min.
-
c.Mount the electrode back at the hydraulic manipulator.
-
d.Lower the electrode again into the same cortical location and leave it for about 10 min.Note: Since the lowering of the soaked electrode is used only for anatomical purposes, the velocity of the electrode insertion can be increased to 1 mm/s.9
-
e.Remove electrode and proceed with the post-hoc verification of electrode track described in Step 28.Note: For reusing the non-functional electrode for anatomical purposes, we recommend following Step 20.
-
a.
Figure 7.
Neuronal activity evoked by electrical stimulation of hindlimbs
(A) Top: Schematic of the experimental conditions showing the placement of the stimulation electrode on the contralateral hindlimb. Bottom: magnification of the hindlimb image (white square) showing in two views the placement of the electrodes.
(B) LFP traces showing sensory-evoked potentials in response to 2 mA stimulation (small black squares and vertical lines) with the averaged response across layers on the right. Shaded area indicates an up-state. Adapted from ref.1
(C) Original traces showing L2/3 sensory-evoked responses in response to different intensities. Adapted from ref.1
Figure 8.
Expected outcomes
Input-output curves of sensory-evoked responses.
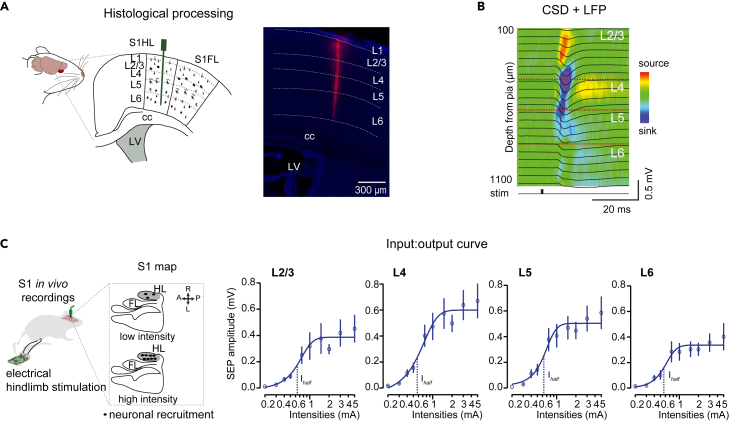
(A) Left: Schematic showing the recording electrode covering all layers of the hindlimb cortex. Right: Chicago Sky Blue staining showing the electrode track (magenta) and the layering delimitations (dotted lines). DAPI staining (blue) was used to visualize the cortical layering. Scale: 300 μm. Adapted from ref.1
(B) In vivo electrophysiological recordings displaying sensory-evoked responses overlapped on a CSD heatmap in response to 2 mA electrical stimulation of the contralateral hindlimb. Adapted from ref.1
(C) Left: Schematic of the in vivo electrophysiological recordings. Right: Input-output curves showing the averaged sensory-evoked potentials in response to different intensities across layers. (n = 7). Dots are mean ± SEM. Solid line is the Sigmoid Fitting of the averaged values. Dashed line indicates Ihalf (mA). Adapted from Miguel-Quesada et al.1
Analysis of sensory evoked potentials
Timing: 2–3 days
This section summarizes the steps for the offline analysis of the continuous wide-band waveform signals using Spike2 software (Spike2 v7, Cambridge Electronics, UK), although other analysis software as Matlab (The MathWorks Inc., USA) can be used. Since sensory responses in vivo are strongly dependent on cortical states, the analysis of the sensory-evoked potentials is performed only from stimuli occurring during down-states.12
-
22.
Import recording data from plexon (.plx file) to spike format file (.smr) using the default script from Spike2 named “Batch import plexon”.
Alternatives: In case of importing one single file, use the import option (file menu).
-
23.Signal processing of the in vivo recordings: use the built-in “1_Process” script from Spike2 to perform a process of multiple data files in a folder.
-
a.Apply a down-sampling from 40 kHz to 20 kHz to all channels.
-
b.Generate multi-unity activity (MUA) channels from the local field potentials (LFP) recordings by applying a Finite Impulse Response (FIR) band pass filter of 0.35–3 kHz with a gap = 0.12 kHz.
-
a.
Note: Depending on the system acquisition and the electrode mapping, recording channels will need to be ordered. Follow manufacturer guidelines.
Note: If the ground electrode is not well fixed to the cranium, a noticeable stimulus artifact can be recorded during the experiment. In this case, and for visualization purposes, use the Optional_NoArtf script to remove the artifact removal.
-
24.Manually select sensory-evoked potentials (SEP) during down-states periods of the slow wave activity (SWA). These periods correspond to a hyperpolarized cortical state during SWA and they are selected in order to decrease the response variability occurring during up states. Figure 7B.
-
a.Use vertical cursors on Spike2 software to go over the event channel while displaying a few electrode signals and manually select those sensory stimuli occurring during down-states.
-
b.Generate a new event channel containing only the stimuli occurring during down-states.
 CRITICAL: The minimum number of sensory responses during down-states to obtain an optimal quantitative analysis is about 15–20 stimuli. Two minutes of recording with a 0.5 Hz stimulation (60 stimuli) should be enough to generate this number of sensory responses. If not, double-check the recording to determine the frequency of the cortical state during recording (i.e. delta 1–4 Hz instead of slow-wave activity < 1 Hz).
CRITICAL: The minimum number of sensory responses during down-states to obtain an optimal quantitative analysis is about 15–20 stimuli. Two minutes of recording with a 0.5 Hz stimulation (60 stimuli) should be enough to generate this number of sensory responses. If not, double-check the recording to determine the frequency of the cortical state during recording (i.e. delta 1–4 Hz instead of slow-wave activity < 1 Hz).
-
a.
-
25.Determine the electrodes corresponding to each cortical layer. We recommend using both following methods to accurately define the cortical depth.
-
a.Use the multi-unit activity channels to define the electrodes showing the shortest response latency following sensory stimulation.Note: Since L4 is the thalamic-recipient layer, it must show the shortest response latency when compared to the other cortical layers.
- b.
-
a.
Note: Several methods can be used for the CSD analysis. In this protocol, we used the second spatial derivative of the local field potentials to quantify the differences between sinks and sources from the averaged sensory responses for each layer.
Note: We use Matlab Software to run CSD analysis. However, CSD is easily applied using the following mathematical computation:
For each temporal point, this formula is computed for LFP channel i.
Note: For visualization purposes, CSD can be smoothed using the function “spline” from Matlab.
-
26.Response data generation.
-
a.Use the built-in “2_Average” script from Spike2 to generate and save a new result view file (.srf) and a text file (.txt) containing the averaged LFP responses and SEP amplitude magnitude (mV), respectively, for cortical layers 2/3 to 6. The built-in script performs the following:
-
i.Creates a new virtual channel with the averaged LFP signal of electrodes within the same layer (according to the depth indicated in the file name).
-
ii.Generates an average per layer by using the previously created event channel (stimulus in down-state) as a trigger.
-
iii.Generates a new file (result view file, .srf type) with the averaged SEPs (± SD).
-
iv.Generates a text file (.txt) with the amplitude values of SEP from 5 to 25 ms after the stimulation for each layer.
-
i.
-
a.
Note: The usual cortical depth for each layer of of S1HL in mice is the following: L2/3: 100–350 μm, L4: 350–500 μm, L5: 500–850 μm, L6: 850–1100 μm.15,16 Cortical layers width may vary depending on the cortical column, age and weight of the animal.
CRITICAL: For the script to work, depth of the electrode should be indicated in the file title in cm and using “’” as separator (example: “1′1” mm).
-
27.Data visualization of sensory-evoked potentials. Figure 8C.
-
a.Once all the data are obtained, generate a data pool with the response to each stimulation intensity (response curve) for each animal.
-
b.Normalize each data to the maximum response in the corresponding layer of the same animal to obtain data about the recruitment and to avoid the inter subject variability.Note: As ensured with the CSD, the maximum raw response should be in layer 4 if the localization of the electrode is correct.Note: The normalization of the dataset can only be used when comparing differences in the neuronal recruitment (i.e. input gain).
-
c.Plot the magnitude of the SEPs as a function of stimulus intensity to generate an input:output (I:O) curve for each animal.Note: For graphical representation, use a logarithmic representation in the “X” axis (stimulus intensities, Figure 8C).
-
d.Use the Sigmoid function to fit the I:O curve into a “S”-shaped curve.Note: We use SARFIA23 package within Igor Pro Wavemetrics Software (Wavemetrics, USA) to generate the I:O curve with the Sigmoid fitting. Nonetheless, the data fitting functions are easily found in other Graphical or Mathematical Softwares.Note: I:O curve can also be adjusted with a Hill-equation fitting. We recommend trying both fittings to determine the one that better adjusts to the population data.
-
e.Obtain the Ihalf value from the Curve fitting for each animal to generate a bar plot. The Ihalf value indicates the intensity by which half of the cortical neuronal population is recruited following a sensory stimulation and it is used as a measure of input gain17 (Figure 8C).Optional: Matlab version 2017b or higher, or other software used for visualization and processing signals is optional for data analysis.
-
a.
Post-hoc verification of electrode implantation
Timing: 3 days
This section describes the steps to perform a post-hoc verification of the electrode track at the cortical column using Chicago Sky Blue (CSB) staining (Figure 8A).
-
28.Tissue preparation.
-
a.While maintaining the animal under inhalatory anesthesia, inject pentobarbital sodium (i.p. 100 mg/kg, Dolethal, Vetoquinol Group, Spain).
-
b.Move the animal to a fume hood.
-
c.After complete loss of reflex, transcardially perfuse the animal with 10 mL of phosphate-buffered saline (PBS, Sigma-Aldrich, USA) with heparin solution at 0.1% (Laboratorio Reig Jofré S.A., Spain) followed by 10 mL of formalin (4% formaldehyde, Sigma-Aldrich, USA) using one syringe for each solution.
-
d.Extract the brain and keep it in formalin for 6–12 h.Note: If immediate processing and analysis of the tissue is not possible, immerse the brain in sucrose solution (30% in PB 0.1 M, Sigma-Aldrich, USA) for 24 h and store it in −20°C in tissue freezing medium (Leica, Germany) until the tissue is ready to be processed.
 CRITICAL: Be careful when decapitating and excising the brain to avoid tissue damage.
CRITICAL: Be careful when decapitating and excising the brain to avoid tissue damage. -
e.Obtain coronal slices of 30–50 μm thickness and collect them in PBS in a multiwell plate (VWR, USA).Note: Different techniques including frozen sectioning using a microtome (e.g. Microm HM450, Thermo Fisher Scientific, Germany) or cryostat (e.g. Leica Biosystems CM1520, Germany), and sectioning without freezing by using a vibratome (Leica Biosystems, VT1000S, Germany) can be used to cut the brain. Follow the appropriate methodology for each cutting technique.Note: Brain slices can be stored in a cryoprotective solution (e.g. glycerol 3% and ethylene glycol 3% in PBS) at −20°C until use.
-
f.Select brain slices of the somatotopic hindlimb representation and recording site and wash them 3 times (20 min each) with PBS.
-
g.Mount brain slices on gelatinized (4% Gelatin powder, MERCK, Germany) slides using DAPI Fluoromount-G (Southern Biotech, USA) and let them dry in the dark at 22°C–24°C for 24 h.Alternatives: Hoechst (1:1000, Sigma-Aldrich, USA) in PBS (10 min) can also be used for identifying cell somas. In this case apply only Fluoromount-G (Southern Biotech, USA) for mounting brain slices.
-
h.Visualize brain sections containing CSB staining corresponding to the probe track:
-
i.Use a light microscope (i.e. TCS SP5, Leica, Germany) equipped with a resonant scanner and 10×/0.4 NA objective.
-
ii.Take mosaic images from the entire cortical region from all brain slices containing CSB fluorescence signals.
- iii.
-
i.
-
i.Perform Nissl staining:
-
i.Hydrate brain slices in subsequent immersion (1 min each) of decreasing graded alcohols (100%, 95%, 70% and 50%).Note: Distilled water for 2 min can also be used for the hydration process.
-
ii.Stain brain slices in 0.1% thionine solution (thionine acetate salt, Sigma-Aldrich, USA) for 2 min.
-
iii.Rinse 2× with distilled water for 1 min.
-
iv.Dehydrate in increasing alcohols gradients (50%, 70%, 95%, 3 min each) followed by 95% alcohol with acetic acid (1%, Sigma-Aldrich, USA; 3 min) and then 100% alcohol (2 × 3 min).
-
v.Clear in xylene (2 × 5 min, Sigma-Aldrich, USA).
-
vi.Mount the slices using DPX mountant (Sigma-Aldrich, USA) and let them dry inside the fume hood.
 CRITICAL: Nissl staining must be done in a fume hood due to the toxicity of some of the products (e.g. xylene and DPX).
CRITICAL: Nissl staining must be done in a fume hood due to the toxicity of some of the products (e.g. xylene and DPX).
-
i.
-
j.Use a magnifying microscope (4×) to check marks of the electrode track in the Nissl-stained slices. The electrode track can be visualized either as a brown stain (i.e., related to blood marks) or as a white mark (i.e., related to the loss of neuronal cells and therefore Nissl bodies induced by the electrode).
-
a.
Expected outcomes
The successful completion of this protocol enables the recording and analysis of cortical network activity in the somatosensory cortex, induced by precise stimulation of ascending sensory pathways (Figure 8). Due to the possibility to stimulate at different intensities the same spatial location, this protocol allows a standardization of stimulus response from limbs to the somatosensory cortex, which can be used to make comparisons between individuals in different physiological conditions and pathologies. Through an in-depth analysis of sensory-evoked potentials and its input:ouput curve, valuable information about network sensitivity and gain is generated, which can be used to determine the dynamic range of activation of cortical sensory neurons. Moreover, the protocol facilitates simultaneous information gathered from all cortical layers, enabling a detailed study of neuronal activity within specific local circuits (i.e., intra-layer) of the hindlimb cortex as well as investigation of their connectivity and information flow (i.e., inter-layer, inter-column).
Additionally, the protocol can be utilized to evaluate the latency of cortical responses induced by peripheral stimulation, particularly in conditions where sensory transmission is affected, such as sensory pathologies resulting from trauma, infections, neurodegenerative conditions, and metabolic diseases. In such cases, this procedure proves especially useful when comparing circuit-level data with subsequent behavioral assessments of sensory responses, as demonstrated in Miguel-Quesada, Zaforas et al., 2023.
Limitations
The use of anesthesia has been described to affect the strength of evoked neuronal responses by changing the extracellular ions including reduction of [K+]o.18 as well as by decreasing the level of neuromodulatory transmission in vivo.12 Both effects of anesthesia may affect neuronal membrane properties, spiking activity, and neuronal/glial intracellular pathways, which could potentially interfere in the neuronal processing of sensory information.19,20,21,22 However, the limitation of using anesthesia is considerably decreased when using experimental designs in which different conditions are compared in the same animal (i.e., before/after a drug application; before and immediately after a peripheral or central nervous system damage). Importantly, the use of anesthesia also allows the maintenance of the cortical activity in a stable state of cortical slow-wave oscillation (or other state such as delta), which permits the comparison of how modifications of local circuits (by means of pharmacology or cell-specific modulation) impact the cortical network properties.11,23
The electrical stimulation is designed to activate all ascending pathways in a graded manner, from lower threshold fibers of proprioception and tactile to higher threshold fibers of thermal, and nociception. Therefore, care should be taken in the interpretation of the results when considering the effect specificity [of a condition] on the sensory ascending signals. Nonetheless, by using the described protocol of electrical stimulation of increasing intensities, the researcher can record the specific cortical activity of tactile information in response to light mechanical stimuli when using low-intensity stimulation.6 On the other hand, high-intensity stimulation will activate simultaneously all ascending pathways involved in the somatosensory processing. Therefore, the graded activation of different amounts of peripheral fibers and all different modalities provides a structured protocol to study cortical dynamics, which cannot be implemented by other stimulation protocols in which only one or two modalities can be activated from a given receptive field.
Troubleshooting
Problem 1
Stimulation electrode resistance higher than expected. Related to Step 2 - before you begin.
Potential solution
Disassemble the electrode by first removing the heat shrink tubing with a scalpel and then melting the alloy thread with the chip welding to remove the copper cable. Cut the ending of the copper cable and repeat step 2.
Problem 2
Avoid tissue damage during craniotomy. Related to Step 5.
Potential solution
One possibility is to avoid touching the cortical surface either with the drill or surgical tools. For that, once skull bone is thin enough, use one side of a curved surgical forceps to stick the bone into it and then lift the skull within the cranial window without touching the dura surface. This will maintain the cortical surface tissue integrity. If a visible damage was encountered during the procedure, it is better to perform another craniotomy in the other hemisphere to assure an optimal recording.
Problem 3
Background noise is too high. Related to Step 11.
Potential solution
Electrical noise is the most prominent source of background noise during electrophysiological recordings. Therefore, first identify the source of noise by switching off electrical devices such as homeothermic blanket, stimulus isolator, digital stimulator and light source. Then, start switching on one-by-one while observing the electrophysiological recording on the acquisition system. Use a cable with both endings with a crocodile connector to ground the electrical device to reduce the noise. After that, verify with the help of another crocodile-connected cable other possible noise sources like grounding electrode, stereotaxic frame, stimulus electrode and ground as much as possible to improve at maximum the signal-to-noise ratio. We also recommend having a faraday cage of about 60 × 40 cm with a lift window at the front to cover the stereotaxic frame and recording system.
Problem 4
Neuronal activity is not in slow wave state. Related to Step 11 and Step 19.
Potential solution
Adjust the level of anesthesia until reaching a state of slow wave activity of the neuronal population.
Problem 5
No neuronal action potential is evoked following manual tactile stimulation. Related to Step 12.
Potential solution
First, double-check temperature and anesthesia level. Temperature should be around 36–37oC and isoflurane should be minimum to maintain a slow-wave activity of cortical networks (around 1 Hz). If temperature and anesthesia is correct, the problem might be that the electrode was not lowered in the correct representation of the hindlimbs. To verify that, use the cotton swab to gently apply tactile stimulation on different parts of the body (i.e., trunk, legs, tail, forelimbs). After identifying the body representation in which the electrode was placed – the one exhibiting evoked response after tactile stimulation, use a mouse Brain Atlas15,16 to determine where the electrode should be moved. For example, if cortical activity is evoked following tactile stimulation of the trunk, slowly retrieve the electrode and move it to a more anterior and medial position. Once in the new coordinate, repeat Steps 11 and 12.
Problem 6
Decrease of the sensory evoked amplitudes over time (i.e., synaptic adaptation). Related to Step 19.
Potential solution
Repeated stimulation can induce synaptic adaptation of cortical networks. If reduction in the sensory-evoked potential amplitude is observed during the time course of the stimulation protocol, decrease the frequency of the stimulus application.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Juliana M. Rosa (jmartinsd@sescam.jccm.es).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Marta Zaforas mzaforas@externas.sescam.jccm.es.
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Codes for data analysis in Spike2 have been deposited at Zenodo and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Acknowledgments
This work was funded by the Spanish Ministry of Science and Innovation MCIN/AEI/10.13039/501100011033 to J.M.R. (grant PID2021-126609NA-I00, co-funded by ‘‘ERDF A way of making Europe,’’ and grant RYC2019-026870-I, co-funded by ‘‘ESF Investing in your future’’), to J.A. (grant PID2019-105020GB-100), and to M.Z. (grant BES2017-082029, co-funded by ‘‘ESF Investing in your future’’). J.M.R. was also funded by the European Union’s Horizon 2020 research and innovation programme under Marie Sklodowska-Curie grant agreement 794926. The authors thank the Microscopy and Image Analysis Services of Hospital Nacional de Parapléjicos (Toledo, Spain) for technical support with acquisition and processing of immunofluorescence images.
Author contributions
Conceptualization, J.A. and J.M.R.; methodology, M.Z., C.M.-Q., E.F.-L., E.A.-C., A.M.-O., V.B.-M., J.A., and J.M.R.; investigation, M.Z., C.M.-Q., E.F.-L., E.A.-C., A.M.-O., V.B.-M., J.A., and J.M.R.; visualization, M.Z., E.F.-L., E.A.-C., A.M.-O., V.B.-M., and J.M.R.; funding acquisition, J.A. and J.M.R.; writing – original draft, M.Z. and J.M.R.; writing – review and editing, all authors; supervision, J.M.R.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Marta Zaforas, Email: mzaforas@externas.sescam.jccm.es.
Juliana M. Rosa, Email: jmartinsd@sescam.jccm.es.
References
- 1.Miguel-Quesada C., Zaforas M., Herrera-Pérez S., Lines J., Fernández-López E., Alonso-Calviño E., Ardaya M., Soria F.N., Araque A., Aguilar J., Rosa J.M. Astrocytes adjust the dynamic range of cortical network activity to control modality-specific sensory information processing. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112950. [DOI] [PubMed] [Google Scholar]
- 2.Jain N., Florence S.L., Kaas J.H. Reorganization of somatosensory cortex after nerve and spinal cord injury. News Physiol. Sci. 1998;13:143–149. doi: 10.1152/physiologyonline.1998.13.3.143. [DOI] [PubMed] [Google Scholar]
- 3.Sanganahalli B.G., Chitturi J., Herman P., Elkabes S., Heary R., Hyder F., Kannurpatti S.S. Supraspinal Sensorimotor and Pain-Related Reorganization after a Hemicontusion Rat Cervical Spinal Cord Injury. J. Neurotrauma. 2021;38:3393–3405. doi: 10.1089/neu.2021.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seelke A.M.H., Dooley J.C., Krubitzer L.A. The Emergence of Somatotopic Maps of the Body in S1 in Rats: The Correspondence Between Functional and Anatomical Organization. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giannì C., Belvisi D., Conte A., Tommasin S., Cortese A., Petsas N., Baione V., Tartaglia M., Millefiorini E., Berardelli A., Pantano P. Altered sensorimotor integration in multiple sclerosis: A combined neurophysiological and functional MRI study. Clin. Neurophysiol. 2021;132:2191–2198. doi: 10.1016/j.clinph.2021.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorostkar M.M., Dreosti E., Odermatt B., Lagnado L. Computational processing of optical measurements of neuronal and synaptic activity in networks. J. Neurosci. Methods. 2010;188:141–150. doi: 10.1016/j.jneumeth.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ewald A.J., Werb Z., Egeblad M. Monitoring of vital signs for long-term survival of mice under anesthesia. Cold Spring Harb. Protoc. 2011;2011 doi: 10.1101/pdb.prot5563. pdb.prot5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiáth R., Márton A.L., Mátyás F., Pinke D., Márton G., Tóth K., Ulbert I. Slow insertion of silicon probes improves the quality of acute neuronal recordings. Sci. Rep. 2019;9:111. doi: 10.1038/s41598-018-36816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamawaki N., Raineri Tapies M.G., Stults A., Smith G.A., Shepherd G.M. Circuit organization of the excitatory sensorimotor loop through hand/forelimb S1 and M1. Elife. 2021;10 doi: 10.7554/eLife.66836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilja J., Endo T., Hofstetter C., Westman E., Young J., Olson L., Spenger C. Blood oxygenation level-dependent visualization of synaptic relay stations of sensory pathways along the neuroaxis in response to graded sensory stimulation of a limb. J. Neurosci. 2006;26:6330–6336. doi: 10.1523/JNEUROSCI.0626-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Civillico E.F., Contreras D. Spatiotemporal properties of sensory responses in vivo are strongly dependent on network context. Front. Syst. Neurosci. 2012;6:25. doi: 10.3389/fnsys.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez-Zabalza M., Reig R., Manrique J., Jercog D., Winograd M., Parga N., Sanchez-Vives M.V. Modulation of Cortical Slow Oscillatory Rhythm by GABA B Receptors: An in Vitro Experimental and Computational Study. J. Physiol. 2020;598:3439–3457. doi: 10.1113/JP279476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wójcik D.K. Encyclopedia of Computational Neuroscience. Springer; 2014. Current Source Density (CSD) Analysis. [Google Scholar]
- 15.Paxinos G., Franklin K.B. Academic press; 2019. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- 16.Allen Institute for Brain Science. Allen Brain Atlas: Mouse Brain. 2011. https://mouse.brain-map.org/
- 17.Ferguson K.A., Cardin J.A. Mechanisms underlying gain modulation in the cortex. Nat. Rev. Neurosci. 2020;21:80–92. doi: 10.1038/s41583-019-0253-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding F., O'Donnell J., Xu Q., Kang N., O’Donnell J., Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016;352:550–555. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellot-Saez A., Cohen G., van Schaik A., Ooi L., Buskila Y., Buskila Y., Buskila Y. Astrocytic modulation of cortical oscillations. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-30003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slezak M., Kandler S., Van Veldhoven P.P., Van den Haute C., Bonin V., Holt M.G. Distinct Mechanisms for Visual and Motor-Related Astrocyte Responses in Mouse Visual Cortex. Curr. Biol. 2019;29:3120–3127.e5. doi: 10.1016/j.cub.2019.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takata N., Mishima T., Hisatsune C., Nagai T., Ebisui E., Mikoshiba K., Hirase H. Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 2011;31:18155–18165. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarrete M., Perea G., Fernandez de Sevilla D., Gómez-Gonzalo M., Núñez A., Martín E.D., Perea G., Fernández de Sevilla D., Gómez-Gonzalo M., Araque A., et al. Astrocytes mediate in vivo cholinergic induced synaptic plasticity. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Humanes-Valera D., Aguilar J., Foffani G. Reorganization of the Intact Somatosensory Cortex Immediately after Spinal Cord Injury. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
-
•
Codes for data analysis in Spike2 have been deposited at Zenodo and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.