Summary
Lethal intergroup encounters occur in many species because of sexual selection. While documented in mountain gorillas, they are absent in western gorillas as, instead, it is predicted by their higher feeding (frugivory) and mate competition (single-vs. multi-male groups). We investigate whether the injuries on three dead silverbacks and one adult female from four groups of western gorillas in the Central African Republic, resulted from interactions with gorillas or leopards. We identified two distinct injury patterns caused by gorillas (isolated lacerations, round wounds) and leopards (punctures clustered on head/neck) by analyzing injuries caused by mountain gorillas and leopards to gorillas and non-gorilla species, respectively. The western gorilla injury pattern is similar to that of mountain gorillas suggesting that lethal encounters occur, albeit infrequently, as predicted by sexual selection in a one-male society. While sexual dimorphism and polygynous sociality favored the evolution of violent encounters, multiple males in groups may influence their frequency.
Subject areas: Wildlife behavior, Ecology, Zoology
Graphical abstract

Highlights
-
•
We show lethal aggressions in western gorillas as predicted by sexual selection
-
•
Injury pattern from gorilla and leopard attacks differ for wound types and location
-
•
Both one- and multi-male society favor the evolution of violent encounters
-
•
Male age and multiple males in groups instead influence their frequency
Wildlife behavior; Ecology; Zoology
Introduction
Intraspecific, intergroup aggressive interactions are frequent in many social animals (e.g., insects,1 birds,2 and mammals3,4,5,6). In extreme cases, they can lead to the death of one or more of the individuals involved (e.g., insects7 and mammals8). In general, such agonistic interactions serve to defend territories, food resources, or access to sexual partners (e.g., poisonous frogs,9 birds,10 and mammals11,12,13,14).
Males of polygynous species are particularly exposed to intergroup agonistic interactions to gain access to sexual partners: a group of males or solitary males will confront the dominant males of a group including coveted females, like in Przewalski’s horses and meerkats.15,16 In some species, such as lions, bachelor males can attempt to attract females of a pride and kill their offspring in order to accelerate female sexual receptivity, a social mechanism known as infanticide.17,18 Infanticides and aggression between adult males to gain access to females are frequently attributed to sexual selection pressures.19,20,21,22 Sexual selection involves two mechanisms: intersexual selectivity23 and intrasexual competition, or the competition between individuals of the same sex for reproduction with the other sex.22
Different primate species show aggressive behavior during intergroup encounters (e.g., vervet monkeys,24 common marmoset,25 chacma baboons,26 and blue monkeys27). Some species lead coalitionary attacks on conspecifics, often proving fatal for some individuals (white-faced capuchin,28 mandrills,29 crested macaques,30 and chimpanzees31) or their infants (blue monkeys,32 northern plains gray langur,33 and ursine colobus34).
Among the great apes, the level of aggression during intergroup interactions takes different forms, even among closely related species. In chimpanzees, there are numerous records of hostile inter-community interactions, most often led by and carried out by males35,36,37,38,39,40,41,42 (but see Samuni, L., Preis, A., Mundry, R., Deschner, T., Crockford, C., & Wittig, R.M.,43 for an exception to the male sex bias). Some attacks and kills are carried out by coalitions, including what researchers have defined as wars between communities.44,45,46 Rarely, these coalitions may also target other ape species, such as the case of chimpanzees observed attacking gorillas (and killing two infants) in Gabon.47 When compared to chimpanzees, bonobos generally display more peaceful inter-community encounters44,48,49,50,51 with no reported cases of lethal intergroup aggression.31
Among gorillas, aggressive intergroup encounters are common and are mainly conducted by silverback males, with the occasional involvement of other group members.52,53 While the majority of these intergroup encounters entail bluff displays and vocalizations,53,54,55 in mountain gorillas they can also involve physical aggression, resulting in infanticide and/or death of one of the adult males involved.55,56,57
Most of our knowledge of intergroup aggression encounters and infanticides in gorillas comes from the well-studied mountain gorillas (Gorilla b. beringei). This subspecies relies on food which is predominantly widely spread, available year-round and non-monopolizable (with some exceptions of populations experiencing some degree of seasonal frugivory).54,58 Therefore, since gorillas are also non-territorial,59 aggressive intergroup encounters among mountain gorillas have been explained by group and mate defense, as opposed to ecological factors.60,61,62 This is not surprising, considering that competition between males to access females is known to be particularly high in sexually dimorphic mammals such as gorillas.63,64,65
However, through time, the mountain gorilla population has experienced an increase in population density (with limited habitat available), which has been accredited to increasing the rate of aggressive encounters (leading to the death of seven adult males).55 This recent increase is rather explained as an ecological consequence (i.e., carrying capacity reached) rather than the result of reproductive competition.55
Despite the recent increase in observations of elusive western gorillas (Gorilla gorilla), violent and lethal intergroup encounters have not been observed in this gorilla species, as would be predicted by the potential higher intergroup competition for both food and mates in this species. Across their range, western gorillas greatly rely on seasonally available fruits,66,67,68,69 which can attract several gorilla groups being simultaneously present at a given feeding site.70,71 Therefore, western gorillas may experience greater encounter rates between groups because of intergroup feeding competition, when compared to mountain gorillas (based on baseline data before the recent population growth of mountain gorillas72 and more recent datas73,74,75). In addition, while mountain gorillas may have several adult males in a reproductive group, only one-male groups have been observed in western gorillas.63,76,77,78 The presence of more than one silverback male in a group should discourage more extra-group males from attacking reproductive groups, when compared to the less protective potential of one-male groups in western gorillas.
Due to difficulties in habituating western gorillas in the dense lowland forests,79 researchers have not been able to follow and observe them in a comparable way to the long term, close range studies of mountain gorillas. The possibility of intergroup lethal encounters in western gorillas was only briefly mentioned once in an unrelated study.80 Rather, predation by leopards has often been reported as a possible cause of wounds observed in western gorillas, or even death,81,82,83 mainly because of records of traces of gorilla’s body parts and DNA found in leopard feces.81,84,85
Here, we present records from the long-term data on four habituated groups of western gorillas, detailing the death of three silverbacks and the injury of a young immigrant adult female. Since there were no direct observations of the events leading to the wounds and deaths, we examined the wound patterns on the dead and injured individuals. We compare the observed wound patterns of two datasets: (1) injuries caused by mountain gorillas during intergroup conflicts (the only dataset available for gorillas) and (2) injuries caused by leopards, the largest carnivores present at the study site.81,86,87 Since no detailed information is available on wounds made by leopards on apes, we used wound information of leopard attacks on other animals (i.e., monkeys and duikers) or humans, as described in the literature and/or on specialized websites (see STAR methods and supplemental information for details).
Given their anatomical differences and their different aggressive strategies and purposes of attack (i.e., predation vs. competition), we expect some specificity between the features of injury patterns inflicted by leopards and by gorillas on their victims (i.e., size, shape, body localization, and single or numerous wounds in a given area). We also predict that, as expert predators attacking with the intention to kill and consume, leopards will inflict lethal wounds on vital parts of the victim’s body, such as the neck, as in typical effective felid hunting.88 On the contrary, we do not expect gorilla opponents to target vital body parts but rather secondary sexual traits (i.e., silverback’s crest or the silver back), clear visual signals of their reproductive potential and success,64,89,90 as gorilla fights are expected to be triggered by sexual selection.22 Identifying the injury patterns of both gorilla and leopard attacks will help us to shed light on the nature of aggression or deaths in our study as well as in future observations on apes or other wild animals.
Results
Since the beginning of the Gorilla Habituation Program in 1997 till 2022, during our daily monitoring of four western gorilla groups in the Dzanga-Sangha Protected Areas, Central African Republic, we recorded the death of three silverbacks and the injury of a young immigrant adult female (see Table S1 for the start of the habituation and group compositions of each group). Using field observations, pictures and videos of these individuals, we classified the number, the type of wounds, and their location on the victim bodies (see details in Table 1).
Table 1.
Wound categories and definitions for comparison
| Category | Modes | Definitions |
|---|---|---|
| Location | Body location: Forelimbs (shoulders, arms, forearms, hands) - Chest - Back - Hindlimbs (hips, pelvic area, thighs, legs, feet). Head location: Face - Top of the head – Neck (throat and nape) - Back of the head. | Where the wound is located on the body (based on a mammal body). |
| General shape | Elongated – Circular – Irregular - Unclear | Elongated = longer than it is wide; circular = rounded in shape; irregular = it is difficult to distinguish the edges of the wound, there may be bits of hanging skin; unclear = the description or photo does not allow the shape of the wound to be distinguished. |
| Length | Small - Medium - Large | Longer side of the wound (according to its location): small <1/8 of the location (according to the first sign) medium = between 1/8 and 1/6 of the location; large = more than 1/6 of the location. |
| Width | Small - Medium – Large or similar to length | Smaller side of the wound. Small = at least 2 times smaller than the length; medium = between 2 times smaller and size of the length; Large or similar to length (confused with length) = there is no visibly smaller width/side to the wound (square, circular, etc.). |
| Apparent depth | Superficial - Deep | Estimation of the depth of the wound: superficial = no flesh/bone seen; deep = dark inside or flesh/bone seen. |
| Category of wounds | Puncture wound | General shape = circular; length = small; width = same as length; apparent depth = deep (caused by penetration of fatty tissue; e.g.,: bite). |
| Laceration | General shape = elongated, such as a cut caused by a claw or tooth. Length = small to large; width = small; depth = superficial or deep. | |
| Large round wound | General shape = circular; length = large; width = same as length; apparent depth = deep (it can be a large tear in the skin, e.g.,: a deep bite). | |
| Tear | General shape = irregular, with skin hanging; length = small or medium; width = small, medium or same as length. | |
| Other wounds | Other kinds of wounds difficult to describe because of their irregular forms or because pictures were unclear. These wounds did not fit into any other class. | |
| Score of number of wounds (on a same location) | 1-4, 5–10, >10 | Number of same-category wounds in a given location. |
Cases of injury or death observed
Group 1, two attacks
In November 1999, Group 1, consisting of approximately eight individuals, split up due to an event occurring over the night and in which the silverback was injured. The field team found blood and trampled vegetation at the nest site, inferring that a fight had occurred. At least two adult females, one infant and one or two juveniles disappeared after this event, though the group composition was not yet clear at that time since the group was only partially habituated. After this event, the group was thus reduced from 7-8 individuals to 3: the injured silverback, one adult female, and their infant. The silverback was observed from a distance as he spent the day apparently recuperating and barely moving. We thus do not have detailed information about the wounds on the silverback, and this event contributes to provide information only on the frequency of such attacks in this species.
In July 2004, a new female joined Group 1; at that time, the group consisted only of the silverback and his juvenile male son. One month later, the silverback and the new female were observed copulating. During this period, the silverback vocalized more than before the arrival of the female, possibly attracting other gorillas.91
On August 3, 2004, we did not find Group 1 at its nest site, instead, we observed another silverback who ran away upon our arrival. Following from there the traces of Group 1, we found only the silverback of group 1 with a large round wound in the middle of his back (Figure 1A; see Table 1 for wound definition according to their general shape, length, width, and apparent depth). He was still alive but moved very slowly. The silverback rested on the ground until we returned to camp around 5:00 p.m. On the following morning, he was found dead. The female and the juvenile were not present. We assumed that he died due to the wounds from the fight with the male found at his nest site. The juvenile of group 1 was later observed (December 10, 2004) with wounds on his back, a broken left arm, and a swollen finger on his right hand. Since he was a male juvenile, approximately six years of age, it is likely that he helped the father in the fight with the other male.
Figure 1.
Different type of wounds found on the western gorillas in this study
Large round wounds on the back of the silverback of group 1 (© C. Cipolletta) (A) and on the side of the young adult female of group 2 (© G. Bardino) (B). Laceration on the jaw (C), tear on the lower lip (D) and examples of the class “other wounds” on the forearm, all found of the silverback of group 4 (© F.S. Niatou Singa).
Group 2, injured young adult female
On the morning of October 22, 2016, we found Group 2 only 200m from where we left them the previous day. The recently immigrated female had a deep large round wound bleeding on the right side of her hip close to the groin (Figure 1B). She could hardly walk because she was unable to properly use her right leg and left hand (she used rather her wrist to walk). Furthermore, another young juvenile female could not properly walk, being also injured (although less seriously) on the right side of her hip. Both of them spent most of their time resting, and they did not feed. They stayed close to the silverback and next to each other (<2m). The team found fresh leopard feces 2m from the gorillas’ fresh traces, leading to speculations about a possible leopard attack on the females.
Group 3, dead silverback with fractures but no wounds
Group 3 was lost on August 27, 2022. The Group 3 silverback was found dead four days later, on August 31. The other group members were seen nearby. According to the state of decomposition of his body and the presence of flies and ants, we estimated he died on August 28, 2022. There were no signs of hunting by humans or fighting with other animals (gorilla or other) and there were no observable wounds on his body. Numerous fractures to the thighs and arms were noted, with some bones protruding under the skin on the thighs (but no wounds; Figure S2). The field team hypothesized that he may have fallen from a tree.
Group 4, dead silverback with several wounds and signs of a violent attack
On September 20, 2022, the silverback of Group 4 was found seriously injured, but still alive. Signs of a fight with another animal were present, including blood spilled all around the site and trampled vegetation (similarly to Group 1). He had wounds on his whole body: one wound on his lower lip (ripped off lip found on the ground), one big laceration on his right cheek, one laceration on the right side of his chest, one wound on the inside of his left arm, one on a forearm and one laceration on a knee (Figure 1C; Table 2; Figure S2). The trackers suggested he may have been the victim of a leopard attack. That day, he moved only a couple of meters from the fight site and rested for the rest of the day. The next day he was found less than 50m from the fight site and did not move from this place. On September 22, he died on the same spot.
Table 2.
Wound classification for the three western gorilla cases
| Study Case |
Cause of injuries | Localisation | Length | Width | Apparent depth | Category | Nb of wounds |
|---|---|---|---|---|---|---|---|
| Group1 | Silverback | Back | Large | Same as length | Deep | Large round wound | 1 |
| Group2 | Unknown | Back (next to hip) | Large | Same as length | Deep | Large round wound | 1 |
| Group4 | Unknown | Face (right cheek) | Large | Medium | Deep | Laceration | 1 |
| Face (lower lips) | Small | Same as length | Deep | Tear | 1 | ||
| Chest (right) | Large | Medium | Deep | Laceration | 1 | ||
| Upper limbs (arm) | Small | Same as length | Superficial | Other wound | 1 | ||
| Upper limbs (arm) | Large | Same as length | Deep | Other wound | 2 | ||
| Lower limbs (knee) | Large | Medium | Deep | Laceration | 1 | ||
| Lower limbs (leg) | Small | Small | Deep | Other wound | 3 |
Injury patterns from mountain gorilla and leopard attacks
To assess and compare gorilla and leopard injury patterns, we used the definitions in Table 1 and classified the wounds found on published or available pictures of the injuries made (1) by mountain gorillas (Nvictims = 15) found on specialized websites (i.e., Gorilla Doctors, Weathers, Berg Gorilla, see supplemental information), because no scientific literature was available, and (2) by leopards on different animals and humans, found in the literature (Nvictims = 8; on one blue duiker92; one gelada93; six humans94,95,96,97,98) or on websites (Nvictims = 3; on three baboons, i.e., Altuna, Nature on PBS, TrTube). No detailed information on wound features from leopards were available in the literature on apes (only some qualitative descriptions of the wound location in chimpanzees, see Boesch99).
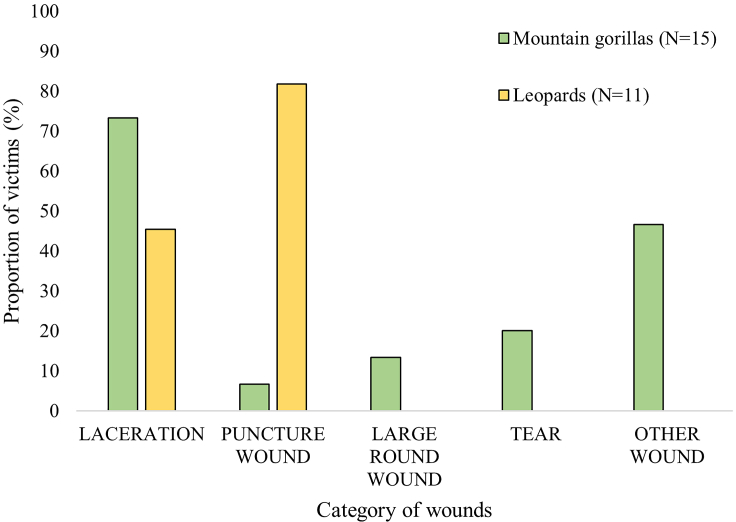
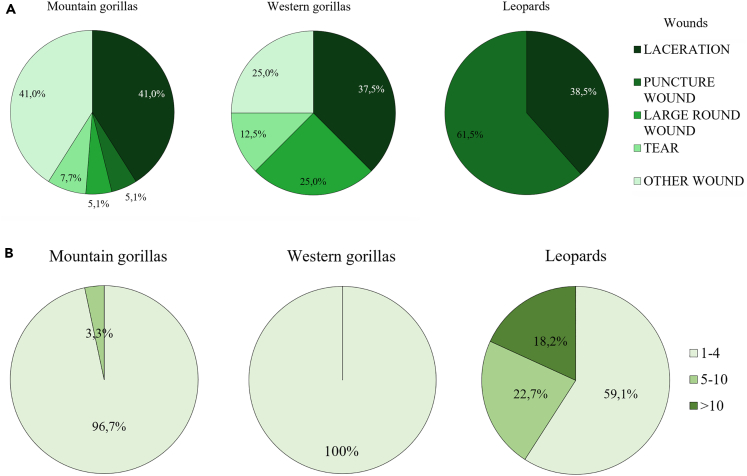
From this classification, wounds caused by mountain gorillas to mountain gorillas were found across the whole body, mostly on the forelimbs and head (both 65% of the victims; Nvictims = 15, Figure 2A). For the individuals having wounds on the head (Nvictims = 10), half of them had some on their face (Figure 2B). Mountain gorillas mainly caused lacerations (73.0% of the victims; Nvictims = 15; 40.0% of the scored wounds, Nwounds = 36; Figures 3 and 4A). In addition to lacerations, mountain gorilla victims also had tears and large round wounds, and only sometimes isolated punctures (Figures 3 and 4A). The majority of the victims had other types of wounds (Figure 1E) not found in the leopard victims (Figures 3 and 4A).
Figure 2.
Comparison of wound locations between mountain gorilla and leopard victims
Percentage of victims with wounds in a given body area (A) or part of the head (B).
Figure 3.
Comparison of wound category between mountain gorilla and leopard victims
Percentage of victims with wounds of a given category.
Figure 4.
Comparison of wounds recorded on victims of mountain gorillas, western gorillas and leopards
Percentage of wounds in a given category in each kind of attack (A) and number of similar wounds in a same location in each kind of attack (B).
All leopard victim (Nvictims = 11) had wounds on their head (Figure 2A), mainly on their neck (91.0% of the victims) and less on their face (36.0%; Figure 2B). A few victims had wounds on their back and chest (both 18.0% of victims; Figure 2A). The type of wounds found in leopard victims were mainly puncture wounds (82.0%, Nvictims = 11, 61.5%, Nwounds = 26) and lacerations (45% of the victims and 38.46% of scored wounds; Figures 3 and 4A). While injuries caused by mountain gorillas were rather isolated (rarely more than four in the same area), leopard victims had often multiple, similar wounds in a same targeted area, i.e., all classes of wound clusters of 0–4, 5–10 and >10 wounds (Figures 4B and S1). Specifically, human victims had several claw scratches on the back or head (Figure S1).
In the mountain gorilla victims, we found a strong positive correlation between the total number of wounds recorded on the entire body and the diversity of the wound types (i.e., different type of wounds such as laceration, tears, large round wounds etc.; Spearman correlation: rs = 0.971, N = 15, p < 0.001). This was not the case in the leopard victims (rs = 0.577, N = 11, p < 0.063). Among mountain gorilla victims with clusters of less than five wounds, there was a positive correlation between wound diversity and the number of clustered wounds (in other words these clusters showed diversity of wounds), which was not the case for leopard victims (gorilla: rs = 0.871, N = 15, P≪0.001; leopard: rs = 0.5847, N = 8, p = 0.128). For clusters containing >5 and >10 wounds, the correlation between wound diversity and the number of clustered wounds was not feasible in mountain gorillas, as victims had almost none of these two larger clusters of wounds. In leopard victims, the correlation between wound diversity and the number of these larger clusters of wounds was still not significant (class 5–10 wounds: rs = 0.370, p = 0.366; >10 wounds: rs = 0.221, N = 8, p = 0.598).
We thus could highlight two clearly distinct patterns of wound type and location between leopard and gorilla victims (Table 3). We also conducted a multiple correspondence analysis (MCA) on all cases that had clear wound images or descriptions (N = 71; this excludes two videos of the leopard attacks on baboons). The MCA shows clear clustering that helps to define the different wound patterns (Figures 5A and 5B; Table S1). The first two axes explain 15.54% of the total variation. The variables “puncture wound”, “laceration”, “tear”, “other wound” (for the category of wound), and “leopard” and “mountain gorilla” (for the attacking species) are most represented on the first axis (Dim.1, 8.55%; Figures 5A and 5B; Table S1). The variables “large round wound” and “western gorillas” (hypothetic attacker) are most represented on the second axis (Dim.2, 6.91%: Figures 5A and 5B; Table S1). The MCA confirmed distinct groups of attacking species, with specific wound categories for each: puncture wounds for leopard victims, and principally tears, lacerations and other wounds for gorilla victims, with large round wound for western gorillas.
Table 3.
Highlights of the gorilla and leopard injury comparison
| Gorilla’s attack | Leopard’s attack | |
|---|---|---|
| Location | Whole body | Forelimbs and head |
| Lacerations | Large and located on the whole body, mostly on the head (top of head, back of the head, jaw) | Small and large, especially in the neck area |
| Large round wounds | Circular shape, large size and located on the back | Not reported |
| Puncture wounds | Only one unclear wound recorded (but no photo was available) | The most frequent wounds. Circular shape but small size, located on the head, back and chest |
| Isolated wounds/clusters of wounds | Mostly isolated wounds | Majority of isolated wounds; clusters of wounds of the same type in a same location (e.g.,: scratches) |
This is a comparative summary of injury patterns from gorillas and leopards.
Figure 5.
Multiple Correspondence Analysis of the study cases
Multiple Correspondence Analysis showing plots (A) by attacking species (LEO = leopard, MGOR = mountain gorillas, and WGOR = western gorillas) and (B) by wound categories.
Furthermore, when comparing wound patterns, all cases of wounded western gorillas seemed more aligned with a gorilla attack, rather than a leopard attack (on non-gorilla victims; this excludes the case 3 in which no wounds were present; Figures 4A and 4B). From our observations, wounded western gorillas (1) presented always at least one large round wound, (2) did not have puncture wounds nor long slashes from claws, (2) their wounds were isolated and present on different body parts, and (3) lacerations on jaws or chest were very similar to those found in mountain gorilla victims, likely the result of tissue tearing damage (Figure S2). The lacerations on the silverback of Group 4 were on the side of the head, on the jaw, rather than the neck where leopards commonly focus their attack (Figures 1A and S1). Overall, the wounds were too large to be made from leopard canines, which cause small, round, deep wounds (Figure S1).
Discussion
While disease,100,101,102,103 human hunting,104 and predation by leopards81,83 have been suggested as reasons of death among adult gorillas, to date, intraspecies aggression among western gorillas has not been conclusively recorded. Here, we investigated the possible cause of death and injury of (four) habituated western gorillas based on their general injury patterns, and how these injuries compared with those from wounds suffered from mountain gorilla and leopard attacks. Compiling the wound information from the three type of victims (i.e., mountain gorillas, western gorillas, and leopard victims), we found wound categories and their locations to be rather species specific (confirmed also by our clustering analysis). Based on the wound type and their presence, isolated or clustered, in different body parts, the injury pattern of western gorillas clearly resembled that of mountain gorillas, when compared to that of leopards (on non-gorilla species). This information strongly implies the presence of deadly combat among western gorillas, which has previously only been observed in mountain gorillas.55 In the absence of direct observations, our suggestions are not irrefutable proof, but they do provide new evidence supporting the existence of lethal encounters among western gorillas.
Specifically, some wounds, such as the large round wounds, were typical of gorilla attacks and were absent in the leopard victims. Injuries caused by the gorillas were located over the entire body of the victim. On the contrary, all leopard victims primarily had wounds on their head, and most of them on their forelimbs (Table 3). While lacerations occurred both in gorilla and leopard attacks, those caused by leopards were rather localized around the neck (on the throat or on the base of the nape). This is for instance the case of the human victims attacked by leopards, who have the most similar body size and shape to gorillas, among the leopard victims analyzed here. Furthermore, wild chimpanzees attacked by leopards also suffered wounds on their throat.99 In mountain gorillas, lacerations on the head region were mainly found on top of the head, the back of the head or jaws. In both gorilla and leopard victims, lacerations may indicate that the victim resisted or fought back, likely occurring when the opponent is of similar strength and size (as in the gorilla fights) or when the victim is large and strong (as could be the case of the adult men killed by leopards).
While gorillas caused rather isolated wounds (Figure 4B), the typical wounds from leopards were small, deep, and with multiple punctures present in the same body location.96 Similarly, wild chimpanzees attacked by leopards also had several claw stripes covering their trunk, particularly on the belly and perforations into the lungs.99 This is likely because leopards immobilize victims by holding them with claws. While leopard canines typically cause small and deeply penetrating wounds, gorilla canines cause rather large tears. In addition, the strong positive correlation between wound diversity and both the number of wounds and of the clustered wounds (of <5 wounds) found in mountain gorillas but not in the leopard victims, corroborates such difference in the gorilla and leopard injury patterns. Being expert predators, this difference may highlight that leopards are more efficient in their attacking techniques (or at least in the successful cases analyzed here), when compared with gorillas. In the gorilla fights, the two male opponents had often likely similar techniques and body size, as opposed to the reported attacks from leopards in which victims were always a different species and often more vulnerable. Mountain gorillas likely had to try different ways to attack the opponent, resulting in a higher variety of wound type in relation to the higher number of reported wounds or cluster of wounds. Since wound features can also likely be influenced by the size, morphology and defensive fighting abilities of the victim, it would be crucial to have more reports on wound patterns caused by leopards to apes.
Our study thus suggests that the death of the silverbacks of Group 1 and 4 were likely due to an aggressive encounter with an extra-group silverback. In the case of the female of Group 2, it seems likely that the injuries were rather the result of within-group contests, given that the silverback was not injured (i.e., he would have rather intervened in the case of an external attack). Since the injured female was quite young and recently immigrated into the group, this may have been the result of a physical attack from the other resident female who often aggressed her (Masi, personal observation). This incident underscores the likelihood that severe physical fights can also occur among group members, as observed in mountain gorillas105,106,107 and other mammals (mongooses108; marmosets109; macaques110; spider monkeys111).
Silverbacks are more than twice the size of females.112 They will protect their females from other silverbacks’ efforts to acquire them.60,61,62,76,91,113 To attract females, secondary sexual traits are notable in gorillas, such as the role and size of their sagittal crests, which correlate with the number of adult females in the group.64,89,90,114 Probably for this reason, the silverback crest (located at the top of the head) often has wounds after physical or lethal encounters with an opponent silverback (this study). It is likely that targeting the opponent’s head would in itself be a successful tactic to reduce the opponent’s defensive capabilities. On the other hand, these secondary sexual traits can also contribute to avoiding physical encounters, as in the case of silverback chest-beating or emitting pungent odor, signaling to warn group members of a danger or to signal his presence to far away males.91,115
In one-male species, factors determining whether an aggressive intergroup encounter leads to death of the dominant male can be multiple (e.g., blue-banded goby116). For instance, our findings suggest the characteristics of the contenders (e.g., age and size) may also play an important role. Both deadly wounded silverbacks were estimated as ‘relatively old’ (both silverbacks of the Group 1 and 4 having established large groups when habituation started and having died 5 and 20 years after, respectively).
As in other species with a one-male social structure (e.g., horses117), other factors can contribute to reduce aggression during intergroup encounters, such as the familiarity with the other group53,91,118 or the group composition (e.g., presence of young silverbacks or blackbacks who can help in the fight62). Indeed, both Group 1 and 4 included young males in the group and this may have encouraged the silverback to engage in a deadly combat. Similarly, but in a larger perspective, the higher number of males, i.e., multi-male groups, may also help to explain the higher frequency of violent fights in mountain gorilla compared to western gorillas. Indeed, this study underlines that lethal and physical encounters among western gorillas are rare (two and three cases, respectively, from four habituated groups since 1997), despite the potential higher intergroup feeding competition (because of higher frugivory) and the lower defensive ability of one-male groups (as opposed to multi-male groups), when compared to mountain gorillas.63,66,67,68,69,76,77,78,119,120 In addition, it is conceivable that the low frequency of lethal and aggressive encounters in western gorillas when compared to mountain gorillas, is also due to the more densely distributed mountain species in comparison to the large area of continuous forest habitat of the less dense western gorillas across several Central African countries (beyond the recent increase in mountain gorilla population).
Strategies for avoiding highly aggressive and costly intergroup physical interactions are however particularly crucial in species having one-male polygynous societies. Indeed, deadly intergroup encounters for the only adult male can have dramatic consequences on the group. The most obvious is on their social structure; for example, group members and particularly female can disperse, increasing the risk of infanticide upon attempting to join another group (e.g., lions,121 hippos,122 and primates82; 123; 124). During such encounters, infanticide risk is already high, even when the male does not die. Another consequence is the impact on the activity budget of group members; e.g., spending more time traveling and less time resting during post-conflict periods.107
During our 26-year long-term monitoring of the study groups, we did not observe any leopard attacks on gorillas. However, the daily presence of human observers may also discourage leopards from approaching gorilla groups, though we acknowledge that leopards are mainly active at night. Leopards are known to prey on several species, including many primates and even chimpanzees.93,99,125,126,127,128 Chimpanzees can also attack leopards as an anti-predator reaction.99,129 However, the risk to attack a group defended by a silverback western gorilla, which can weigh up to 180kg112 may be too high for leopards. Gorillas thus may have evolved to benefit from a size advantage in order to decrease their predation risk.130 Even though some felid species are likely capable of killing large primates such as orangutans,131 gorillas are larger when compared to the size of preferred leopard prey species.85 Moreover, since gorillas mostly live in groups, this increases their capacity to defend themselves,132 particularly with the presence of immature males in the group. Furthermore, contrary to chimpanzees, gorillas typically make their night nests most often on the ground rather than in trees,133 indicating a potential reduced risk perception of leopard attacks. However, leopard predation on gorillas seems to exist as gorilla DNA was consistently found in leopard feces.81,84,85 Given the high frequency of these findings, scavenger behavior from leopard on already dead gorillas may be unlikely. Young and isolated individuals may be at higher risk for leopard attacks.
Two other types of lethal attacks on gorillas exist. Very recently, lethal coalitionary attacks by chimpanzees have been reported twice on western gorillas.47 The victims in these cases were infants, but their mothers have been also aggressed by a number of chimpanzees leaving the possibility of a lethal attack from chimpanzees still open. However, it seems unlikely that among all individuals of a cohesive gorilla group, the large silverback, who is double the size of a large male chimpanzee, could be severely injured during these incidents. Human predation is another persistent threat for gorillas, who can be snared or shot;134 however, snare-related injuries or those caused by weapons are easily recognizable.135
Overall, our findings contribute to reinforce the evidence of physical and deadly encounters between males, in terms of sexual selection in sexually dimorphic animal species with one-male social structures (Przewalski’s horses,15 lions,136 and meerkats16). However, rather than one- or multi-male sociality, polygynous sociality and large sexual dimorphism in body size seem to have favored the evolution of violent and lethal encounters in animal species. The presence of one or more males may be more likely to influence their frequency in the individual species.
Finally, ecological factors, such as population density increase or the reduction of resources, may also exacerbate the intensity and frequency of such aggressive encounters,55,137 all factors potentially exacerbated by the alarming global climate change trends and anthropogenic destruction of the forest and oceans.138,139
Limitations of the study
Our conclusion may be taken with caution because they are based on a small sample size for western gorillas (three wounded individuals) and on the inference of a similar injury pattern between western and mountain gorillas. Although we have observed numerous intergroup aggressive interactions between western gorilla silverbacks, our conclusions are based on indirect evidence. In addition, due to the lack of detailed injury records of leopard attacks on either great apes or gorillas, our comparison of injury patterns between gorillas and leopards may be biased by the smaller body size of the leopard victims for whom we have found photographs and descriptions of wounds.
Finally, in our multiple correspondence analysis the first two dimensions account only for 15.53% of the total inertia of the dataset. Therefore, the first level represents only a small portion of the variability in the data. Caution should be used in interpreting these data, although the different injury patterns between gorillas and leopards appear to be quite consistent across the different methods used.
STAR★Methods
Key resources table
This study did not use any reagents, materials or similar, therefore no KRT is applicable.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Shelly Masi (shelly.masi@mnhn.fr).
Materials availability
This study did not generate new unique material or reagents.
Data and code availability
-
•
The Leading contact is Shelly Masi, shelly.masi@mnhn.fr. Data reported in this manuscript will be shared by the lead contact upon request.
-
•
This manuscript does not report original code. All used codes are available in this paper’s supplemental information.
-
•
Any additional information required to reanalyze the data reported in this manuscript is available from the lead contact upon request.
Experimental model and study participant details
Study site and study animals
This study is based on long-term data from 1997 to 2022 (Table S1) collected at two field research stations within the Dzanga-Ndoki National Park, part of the Dzanga-Sangha Protected Area (DSPA) in the south-western part of the Central African Republic (CAR): 1) Bai Hokou (N 2° 51.574′, E 16° 28.045’; Datum: WGS84) and 2) Mongambe (N 2° 55.077’; E 16° 23.324’; Datum: WGS84). The DSPA covers 4579 km2 and it is characterized by a pristine tropical rainforest comprised primarily of Gilbertiodendron monodominant forest and mixed forest with scattered secondary forest patches. The DSPA has a seasonal rainy tropical climate with a dry season (<100 mm monthly rainfall) between December and February.
Ethical statement
Four groups of habituated western gorillas have been monitored daily for decades, since the beginning of their habituation (see Table S1 for start of the habituation and the group compositions of the study groups). When an adult gorilla was found severely injured or dead, we took videos and pictures of wounds and of the whole body (although, we acknowledge that some wounds may have been hidden due to the long and thick hair of the gorillas).
This research adhered to ethics and health protocols and legal requirements of the government of the Central African Republic. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Given the non-invasive observation on wild animals, ethic committee approval was not required.
Method details
Identification of wound patterns
Using field observations, pictures and videos of the western gorilla study groups, HP classified the location of wounds on their bodies as following: head (further subdivided into face, top of the head, neck (including throat and nape), back of the head), forelimbs (shoulders, arms, forearms, hands), chest, back, hindlimbs (hips, pelvic area, thighs, legs, feet). HP then identified the different wound features based on their general shape (elongated, circular, irregular, unclear), length (small, medium, large), width (small, medium, large, or similar to length), and apparent depth (superficial, deep; see Table 1 for specific definitions of these scores). Wounds were then independently assessed by SM for approval. If several similar injuries were found in the same area, we grouped them together and specified their number for each specific body area. When comparing wound patterns in victims, a group of injuries counted as single injury.
Gorilla and leopard injury patterns
To distinguish between the injury patterns made during gorilla-gorilla fights from those made during leopard attacks, we used the same classification used for the western gorilla wounds. We classified published or available pictures and descriptions of injuries made a) among mountain gorillas (Nvictims = 15) found on specialized websites (i.e., Gorilla Doctors, Weathers, Berg Gorilla, see supplemental information), as no scientific literature was available, and b) by leopards on different animals found either in the literature (Nvictims = 8; on one blue duiker92; one gelada93; six humans94,95,96,97,98) or on websites (Nvictims = 3; on three baboons, i.e., Altuna, Nature on PBS, TrTube). We selected only cases that were sufficiently described by reporting all wounds and providing clear pictures of the injuries made either by mountain gorillas or leopards. We thus discarded cases of injuries having too vague descriptions or pictures that did not allow us to identify the injuries.
Quantification and statistical analysis
We compared the injury patterns made by mountain gorillas and leopards using two-tailed Spearman correlations ran in R (R Development Core Team, version 1.4.1103 released on 2021-15-02) between the total number of wounds and the wound diversity (number of different type of wounds such as laceration, tears, large round wounds, etc.) found in each of the victims. We also tested the Spearman correlation between the wound diversity in a victim and the number of clustered wounds found on a victim. This last one was possible only for a) a subsample of leopard victims for which pictures allowed wound number counting (N = 8), and b) for mountain gorillas only when the clusters had less than five wounds, because the two other classes (5–6 wounds and >10) were almost zeros. In these analyses and in the graphical comparisons, we considered proportions of the victims having wounds at a given location in each of these two groups and those having wounds of each wound category (knowing that victims could have multiple different wounds on their body).
Finally, to discern whether the western gorillas in the study were likely to have been attacked by another gorilla or a leopard, we compared these injury patterns to the pattern of wounds observed in the western gorillas. Using the FactominR package in R software, we also conducted a Multiple Correspondence Analysis (MCA) to identify these patterns, based on two qualitative variables: the body location of the wounds and wound categories for each wound collected on the study cases (N = 71 wounds).
Acknowledgments
We are grateful to the Ministry of Higher Education and Scientific Research of Central African Republic (CAR) for permission to conduct this research, the Dzanga-Sangha Protected Areas and WWF CAR for allowing us to carry out fieldwork at their sites and the sharing of information included in this manuscript. Special thanks go to the Bai-Hokou and Mongambe staff for assistance in the field, especially the local Aka trackers, particularly Ngbanda Barthèlemy, Ngombo Diedonné, Nyele Didas, Ndiki Alphonse, Bwanga and Mbosa for their exceptional tracking skills and incredible forest knowledge. This study is part of the long-term Studies in Ecology and Evolution (SEE-Life) program of the CNRS. We thank Luigi Boitani for his support. To finish, we greatly thank the University of Rome “La Sapienza”, the long-term SEE-Life program of the CNRS, the WWF CAR, the UMR 7206, and the National Museum of Natural History in Paris, for institutional and financial support. We are very grateful to the anonymous reviewers who helped to greatly improve our manuscript and to David Greer for his invaluable English corrections and comments on the manuscript.
This study was financially supported by the Action Transversal, the Federation Project Programs of the Department of Humans and Environment, and the UMR 7206 of the National Museum of Natural History (MNHN), France, by the WWF CAR and by the long-term SEE-Life program of the CNRS.
Author contributions
S.M. led and designed the study; S.M., C.C., F.S.N.S., T.N.F., G.B., and E.K. collected the data; S.M. and H.P. wrote the manuscript; C.C., P.S., and P.H. contributed to the writing of the final versions of the manuscript; H.P. and S.M. coded the data; H.P. conducted the statistical analyses; All authors approved the manuscript.
Declaration of interests
The authors declare no competing interests and that they, or their immediate family members, have no financial interests to declare, no positions or related patents to declare, and are not members of the journal’s advisory board.
Published: March 6, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109437.
Supplemental information
References
- 1.Batchelor T.P., Briffa M. Fight tactics in wood ants: Individuals in smaller groups fight harder but die faster. Proc. Biol. Sci. 2011;278:3243–3250. doi: 10.1098/rspb.2011.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radford A.N., Fawcett T.W. Conflict between Groups Promotes Later Defense of a Critical Resource in a Cooperatively Breeding Bird. Curr. Biol. 2014;24:2935–2939. doi: 10.1016/j.cub.2014.10.036. [DOI] [PubMed] [Google Scholar]
- 3.White P.C., Harris S. Encounters between Red Foxes (Vulpes vulpes): Implications for Territory Maintenance, Social Cohesion and Dispersal. J. Anim. Ecol. 1994;63:315. doi: 10.2307/5550. [DOI] [Google Scholar]
- 4.Cassidy K.A., MacNulty D.R., Stahler D.R., Smith D.W., Mech L.D. Group composition effects on aggressive interpack interactions of gray wolves in Yellowstone National Park. Behav. Ecol. 2015;26:1352–1360. doi: 10.1093/beheco/arv081. [DOI] [Google Scholar]
- 5.Okamoto K., Matsumura S. Intergroup encounters in wild moor macaques (Macaca maurus) Primates. 2002;43:119–125. doi: 10.1007/BF02629671. [DOI] [PubMed] [Google Scholar]
- 6.Korstjens A.H., Nijssen E.C., Noë R. Intergroup Relationships in Western Black-and-White Colobus, Colobus polykomos polykomos. Int. J. Primatol. 2005;26:1267–1289. doi: 10.1007/s10764-005-8853-y. [DOI] [Google Scholar]
- 7.Plowes N.J.R., Adams E.S. An empirical test of Lanchester’s square law: Mortality during battles of the fire ant Solenopsis invicta. Proc. Biol. Sci. 2005;272:1809–1814. doi: 10.1098/rspb.2005.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson F.J., Marshall H.H., Vitikainen E.I., Cant M.A. Causes and consequences of intergroup conflict in cooperative banded mongooses. Anim. Behav. 2017;126:31–40. doi: 10.1016/j.anbehav.2017.01.017. [DOI] [Google Scholar]
- 9.Pröhl H. Territorial Behavior in Dendrobatid Frogs. J. Herpetol. 2005;39:354–365. doi: 10.1670/162-04A.1. [DOI] [Google Scholar]
- 10.Stiles F.G., Wolf L.L. Hummingbird Territoriality at a Tropical Flowering Tree. Auk. 1970;87:467–491. doi: 10.2307/4083791. [DOI] [Google Scholar]
- 11.Henschel J.R., Skinner J.D. Territorial Behaviour by a Clan of Spotted Hyaenas Crocuta crocuta. Ethology. 1991;88:223–235. doi: 10.1111/j.1439-0310.1991.tb00277.x. [DOI] [Google Scholar]
- 12.Sillero-Zubiri C., Macdonald D.W. Scent-marking and territorial behaviour of Ethiopian wolves Canis simensis. J. Zool. 1998;245:351–361. doi: 10.1111/j.1469-7998.1998.tb00110.x. [DOI] [Google Scholar]
- 13.Fashing P. Male and female strategies during intergroup encounters in guerezas (Colobus guereza): Evidence for resource defense mediated through males and a comparison with other primates. Behav. Ecol. Sociobiol. 2001;50:219–230. doi: 10.1007/s002650100358. [DOI] [Google Scholar]
- 14.Watts D., Mitani J. Boundary patrols and intergroup encounters in wild chimpanzees. Beyond Behav. 2001;138:299–327. doi: 10.1163/15685390152032488. [DOI] [Google Scholar]
- 15.Keiper R., Receveur H. Social interactions of free-ranging Przewalski horses in semi-reserves in the Netherlands. Appl. Anim. Behav. Sci. 1992;33:303–318. doi: 10.1016/S0168-1591(05)80068-1. [DOI] [Google Scholar]
- 16.Dyble M., Houslay T.M., Manser M.B., Clutton-Brock T. Intergroup aggression in meerkats. Proc. Biol. Sci. 2019;286 doi: 10.1098/rspb.2019.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertram B.C.R. Social factors influencing reproduction in wild lions. J. Zool. 1975;177:463–482. doi: 10.1111/j.1469-7998.1975.tb02246.x. [DOI] [Google Scholar]
- 18.Palombit R.A. Infanticide as Sexual Conflict: Coevolution of Male Strategies and Female Counterstrategies. Cold Spring Harbor Perspect. Biol. 2015;7 doi: 10.1101/cshperspect.a017640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartlett T.Q., Sussman R.W., Cheverud J.M. Infant Killing in Primates: A Review of Observed Cases with Specific Reference to the Sexual Selection Hypothesis. Am. Anthropol. 1993;95:958–990. doi: 10.1525/aa.1993.95.4.02a00090. [DOI] [Google Scholar]
- 20.Clutton-Brock T.H., Hodge S.J., Spong G., Russell A.F., Jordan N.R., Bennett N.C., Sharpe L.L., Manser M.B. Intrasexual competition and sexual selection in cooperative mammals. Nature. 2006;444:1065–1068. doi: 10.1038/nature05386. [DOI] [PubMed] [Google Scholar]
- 21.Lindenfors P., Tullberg S. Evolutionary Aspects of Aggression. Adv. Genet. 2011;75:7–22. doi: 10.1016/B978-0-12-380858-5.00009-5. [DOI] [PubMed] [Google Scholar]
- 22.Hosken D.J., House C.M. Sexual selection. Curr. Biol. 2011;21:R62–R65. doi: 10.1016/j.cub.2010.11.053. [DOI] [PubMed] [Google Scholar]
- 23.Campbell B.G. AldineTransaction; 1972. Sexual Selection and the Descent of Man: The Darwinian Pivot. [Google Scholar]
- 24.Cheney D.L. Intergroup Encounters among Free-Ranging Vervet Monkeys. Folia Primatol. 1981;35:124–146. doi: 10.1159/000155970. [DOI] [PubMed] [Google Scholar]
- 25.Lazaro-Perea C. Intergroup interactions in wild common marmosets, Callithrix jacchus: Territorial defence and assessment of neighbours. Anim. Behav. 2001;62:11–21. doi: 10.1006/anbe.2000.1726. [DOI] [Google Scholar]
- 26.Kitchen D., Seyfarth R., Cheney D. Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus) Beyond Behav. 2004;141:197–218. doi: 10.1163/156853904322890816. [DOI] [Google Scholar]
- 27.Roth A.M., Cords M. Effects of group size and contest location on the outcome and intensity of intergroup contests in wild blue monkeys. Anim. Behav. 2016;113:49–58. doi: 10.1016/j.anbehav.2015.11.011. [DOI] [Google Scholar]
- 28.Gros-Louis J., Perry S., Manson J.H. Violent coalitionary attacks and intraspecific killing in wild white-faced capuchin monkeys (Cebus capucinus) Primates. 2003;44:341–346. doi: 10.1007/s10329-003-0050-z. [DOI] [PubMed] [Google Scholar]
- 29.Setchell J.M., Knapp L.A., Wickings E.J. Violent coalitionary attack by female mandrills against an injured alpha male. Am. J. Primatol. 2006;68:411–418. doi: 10.1002/ajp.20234. [DOI] [PubMed] [Google Scholar]
- 30.Martínez-Íñigo L., Engelhardt A., Agil M., Pilot M., Majolo B. Intergroup lethal gang attacks in wild crested macaques, Macaca nigra. Anim. Behav. 2021;180:81–91. doi: 10.1016/j.anbehav.2021.08.002. [DOI] [Google Scholar]
- 31.Wilson M.L., Boesch C., Fruth B., Furuichi T., Gilby I.C., Hashimoto C., Hobaiter C.L., Hohmann G., Itoh N., Koops K., et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513:414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 32.Butynski T.M. Harem-male replacement and infanticide in the blue monkey (Cercopithecus mitus stuhlmanni) in the Kibale Forest, Uganda. Am. J. Primatol. 1982;3:1–22. doi: 10.1002/ajp.1350030102. [DOI] [PubMed] [Google Scholar]
- 33.Sommer V. Infanticide among the langurs of Jodhpur: testing the sexual selection hypothesis with a long-term record. Infanticide and parental care. 1994:155–198. [Google Scholar]
- 34.Sicotte P., Teichroeb J. Infanticide in ursine colobus monkeys (Colobus vellerosus) in Ghana: New cases and a test of the existing hypotheses. Beyond Behav. 2008;145:727–755. doi: 10.1163/156853908783929160. [DOI] [Google Scholar]
- 35.Goodall J. Belknap Press of Harvard Univ. Press; 1986. The Chimpanzees of Gombe: Patterns of Behavior. [Google Scholar]
- 36.Wilson M.L., Wrangham R.W. Intergroup Relations in Chimpanzees. Annu. Rev. Anthropol. 2003;32:363–392. doi: 10.1146/annurev.anthro.32.061002.120046. [DOI] [Google Scholar]
- 37.Wilson M.L., Wallauer W.R., Pusey A.E. New Cases of Intergroup Violence Among Chimpanzees in Gombe National Park, Tanzania. Int. J. Primatol. 2004;25:523–549. doi: 10.1023/B:IJOP.0000023574.38219.92. [DOI] [Google Scholar]
- 38.Watts D.P., Muller M., Amsler S.J., Mbabazi G., Mitani J.C. Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. Am. J. Primatol. 2006;68:161–180. doi: 10.1002/ajp.20214. [DOI] [PubMed] [Google Scholar]
- 39.Mitani J.C., Watts D.P., Amsler S.J. Lethal intergroup aggression leads to territorial expansion in wild chimpanzees. Curr. Biol. 2010;20:507–508. doi: 10.1016/j.cub.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Boesch C., Crockford C., Herbinger I., Wittig R., Moebius Y., Normand E. Intergroup conflicts among chimpanzees in Taï National Park: Lethal violence and the female perspective. Am. J. Primatol. 2008;70:519–532. doi: 10.1002/ajp.20524. [DOI] [PubMed] [Google Scholar]
- 41.Boesch C., Wittig R., Crockford C., Vigilant L., Deschner T., Leendertz F., editors. The Chimpanzees of the Taï Forest: 40 Years of Research. 1st ed. Cambridge University Press; 2019. [DOI] [Google Scholar]
- 42.Martínez-Íñigo L., Baas P., Klein H., Pika S., Deschner T. Intercommunity interactions and killings in central chimpanzees (Pan troglodytes troglodytes) from Loango National Park, Gabon. Primates. 2021;62:709–722. doi: 10.1007/s10329-021-00921-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samuni L., Preis A., Mundry R., Deschner T., Crockford C., Wittig R.M. Oxytocin reactivity during intergroup conflict in wild chimpanzees. Proc. Natl. Acad. Sci. USA. 2017;114:268–273. doi: 10.1073/pnas.1616812114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wrangham R.W. Evolution of coalitionary killing. Am. J. Phys. Anthropol. 1999;110:1–30. doi: 10.1002/(sici)1096-8644(1999)110:29+<1::aid-ajpa2>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 45.Watts D.P. Intracommunity Coalitionary Killing of an Adult Male Chimpanzee at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 2004;25:507–521. doi: 10.1023/B:IJOP.0000023573.56625.59. [DOI] [Google Scholar]
- 46.Sandel A.A., Watts D.P. Lethal Coalitionary Aggression Associated with a Community Fission in Chimpanzees (Pan troglodytes) at Ngogo, Kibale National Park, Uganda. Int. J. Primatol. 2021;42:26–48. doi: 10.1007/s10764-020-00185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Southern L.M., Deschner T., Pika S. Lethal coalitionary attacks of chimpanzees (Pan troglodytes troglodytes) on gorillas (Gorilla gorilla gorilla) in the wild. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-93829-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furuichi T. Female contributions to the peaceful nature of bonobo society. Evol. Anthropol. 2011;20:131–142. doi: 10.1002/evan.20308. [DOI] [PubMed] [Google Scholar]
- 49.Clay Z., Furuichi T., de Waal F.B. Obstacles and catalysts to peaceful coexistence in chimpanzees and bonobos. Beyond Behav. 2016;153:1293–1330. doi: 10.1163/1568539X-00003335. [DOI] [Google Scholar]
- 50.Sakamaki T., Ryu H., Toda K., Tokuyama N., Furuichi T. Increased Frequency of Intergroup Encounters in Wild Bonobos (Pan paniscus) Around the Yearly Peak in Fruit Abundance at Wamba. Int. J. Primatol. 2018;39:685–704. doi: 10.1007/s10764-018-0058-2. [DOI] [Google Scholar]
- 51.Tokuyama N., Sakamaki T., Furuichi T. Inter-group aggressive interaction patterns indicate male mate defense and female cooperation across bonobo groups at Wamba, Democratic Republic of the Congo. Am. J. Phys. Anthropol. 2019;170:535–550. doi: 10.1002/ajpa.23929. [DOI] [PubMed] [Google Scholar]
- 52.Watts D.P. Agonistic Interventions in Wild Mountain Gorilla Groups. Beyond Behav. 1997;134:23–57. doi: 10.1163/156853997X00269. [DOI] [Google Scholar]
- 53.Mirville M.O., Ridley A.R., Samedi J.P.M., Vecellio V., Ndagijimana F., Stoinski T.S., Grueter C.C. Factors influencing individual participation during intergroup interactions in mountain gorillas. Anim. Behav. 2018;144:75–86. doi: 10.1016/j.anbehav.2018.08.003. [DOI] [Google Scholar]
- 54.Robbins M., Sawyer S. Intergroup encounters in mountain gorillas of Bwindi Impenetrable National Park, Uganda. Beyond Behav. 2007;144:1497–1519. doi: 10.1163/156853907782512146. [DOI] [Google Scholar]
- 55.Caillaud D., Eckardt W., Vecellio V., Ndagijimana F., Mucyo J.-P., Hirwa J.-P., Stoinski T. Violent encounters between social units hinder the growth of a high-density mountain gorilla population. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aba0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watts D.P. Infanticide in Mountain Gorillas: New Cases and a Reconsideration of the Evidence. Ethology. 1989;81:1–18. doi: 10.1111/j.1439-0310.1989.tb00754.x. [DOI] [Google Scholar]
- 57.Robbins A.M., Gray M., Basabose A., Uwingeli P., Mburanumwe I., Kagoda E., Robbins M.M. Impact of Male Infanticide on the Social Structure of Mountain Gorillas. PLoS One. 2013;8 doi: 10.1371/journal.pone.0078256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watts D.P. Agonistic relationships between female mountain gorillas (Gorilla gorilla beringei) Behav. Ecol. Sociobiol. 1994;34:347–358. doi: 10.1007/BF00197005. [DOI] [Google Scholar]
- 59.Robira B., Benhamou S., Fuh T.N., Masi S. In: Movement Ecology of Afrotropical Forest Mammals, 151–170. Reyna-Hurtado R., Chapman C.A., Melletti M., editors. Springer International Publishing; 2023. Do Seasonal Frugivory and Cognition Shape Foraging Movements in Wild Western Gorillas? [DOI] [Google Scholar]
- 60.Sicotte P. Inter-group encounters and female transfer in mountain gorillas: Influence of group composition on male behavior. Am. J. Primatol. 1993;30:21–36. doi: 10.1002/ajp.1350300103. [DOI] [PubMed] [Google Scholar]
- 61.Rosenbaum S., Vecellio V., Stoinski T. Observations of severe and lethal coalitionary attacks in wild mountain gorillas. Sci. Rep. 2016;6 doi: 10.1038/srep37018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cooksey K., Sanz C., Ebombi T.F., Massamba J.M., Teberd P., Magema E., Abea G., Peralejo J.S.O., Kienast I., Stephens C., Morgan D. Socioecological Factors Influencing Intergroup Encounters in Western Lowland Gorillas (Gorilla gorilla gorilla) Int. J. Primatol. 2020;41:181–202. doi: 10.1007/s10764-020-00147-6. [DOI] [Google Scholar]
- 63.Gatti S., Levréro F., Ménard N., Gautier-Hion A. Population and group structure of western lowland gorillas (Gorilla gorilla gorilla) at Lokoué, Republic of Congo: Population Structure of Western Gorillas. Am. J. Primatol. 2004;63:111–123. doi: 10.1002/ajp.20045. [DOI] [PubMed] [Google Scholar]
- 64.Caillaud D., Levréro F., Gatti S., Ménard N., Raymond M. Influence of male morphology on male mating status and behavior during interunit encounters in western lowland gorillas. Am. J. Phys. Anthropol. 2008;135:379–388. doi: 10.1002/ajpa.20754. [DOI] [PubMed] [Google Scholar]
- 65.Breuer T., Robbins A.M., Olejniczak C., Parnell R.J., Stokes E.J., Robbins M.M. Variance in the male reproductive success of western gorillas: Acquiring females is just the beginning. Behav. Ecol. Sociobiol. 2010;64:515–528. doi: 10.1007/s00265-009-0867-6. [DOI] [Google Scholar]
- 66.Rogers M.E., Abernethy K., Bermejo M., Cipolletta C., Doran D., Mcfarland K., Nishihara T., Remis M., Tutin C.E.G. Western gorilla diet: A synthesis from six sites. Am. J. Primatol. 2004;64:173–192. doi: 10.1002/ajp.20071. [DOI] [PubMed] [Google Scholar]
- 67.Masi S., Cipolletta C., Robbins M.M. Western lowland gorillas (Gorilla gorilla gorilla) change their activity patterns in response to frugivory. Am. J. Primatol. 2009;71:91–100. doi: 10.1002/ajp.20629. [DOI] [PubMed] [Google Scholar]
- 68.Masi S., Mundry R., Ortmann S., Cipolletta C., Boitani L., Robbins M.M. The Influence of Seasonal Frugivory on Nutrient and Energy Intake in Wild Western Gorillas. PLoS One. 2015;10 doi: 10.1371/journal.pone.0129254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masi S., Breuer T. Dialium seed coprophagy in wild western gorillas: Multiple nutritional benefits and toxicity reduction hypotheses. Am. J. Primatol. 2018;80 doi: 10.1002/ajp.22752. [DOI] [PubMed] [Google Scholar]
- 70.Bermejo M. Home-range use and intergroup encounters in western gorillas (Gorilla g. gorilla) at Lossi forest, North Congo. Am. J. Primatol. 2004;64:223–232. doi: 10.1002/ajp.20073. [DOI] [PubMed] [Google Scholar]
- 71.Walsh P.D., Breuer T., Sanz C., Morgan D., Doran-Sheehy D. Potential for Ebola Transmission between Gorilla and Chimpanzee Social Groups. Am. Nat. 2007;169:684–689. doi: 10.1086/513494. [DOI] [PubMed] [Google Scholar]
- 72.Doran-Sheehy D.M., Greer D., Mongo P., Schwindt D. Impact of ecological and social factors on ranging in western gorillas. Am. J. Primatol. 2004;64:207–222. doi: 10.1002/ajp.20075. [DOI] [PubMed] [Google Scholar]
- 73.Robbins M.M., Gray M., Fawcett K.A., Nutter F.B., Uwingeli P., Mburanumwe I., Kagoda E., Basabose A., Stoinski T.S., Cranfield M.R., et al. Extreme Conservation Leads to Recovery of the Virunga Mountain Gorillas. PLoS One. 2011;6 doi: 10.1371/journal.pone.0019788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caillaud D., Ndagijimana F., Giarrusso A.J., Vecellio V., Stoinski T.S. Mountain gorilla ranging patterns: Influence of group size and group dynamics: Mountain Gorilla Ranging Patterns. Am. J. Primatol. 2014;76:730–746. doi: 10.1002/ajp.22265. [DOI] [PubMed] [Google Scholar]
- 75.Granjon A.-C., Robbins M.M., Arinaitwe J., Cranfield M.R., Eckardt W., Mburanumwe I., Musana A., Robbins A.M., Roy J., Sollmann R., et al. Estimating abundance and growth rates in a wild mountain gorilla population. Anim. Conserv. 2020;23:455–465. doi: 10.1111/acv.12559. [DOI] [Google Scholar]
- 76.Robbins M.M. A Demographic Analysis of Male Life History and Social Structure of Mountain Gorillas. Beyond Behav. 1995;132:21–47. doi: 10.1163/156853995X00261. [DOI] [Google Scholar]
- 77.Bradley B.J., Robbins M.M., Williamson E.A., Steklis H.D., Steklis N.G., Eckhardt N., Boesch C., Vigilant L. Mountain gorilla tug-of-war: Silverbacks have limited control over reproduction in multimale groups. Proc. Natl. Acad. Sci. USA. 2005;102:9418–9423. doi: 10.1073/pnas.0502019102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stoinski T.S., Rosenbaum S., Ngaboyamahina T., Vecellio V., Ndagijimana F., Fawcett K. Patterns of male reproductive behaviour in multi-male groups of mountain gorillas: examining theories of reproductive skew. Beyond Behav. 2009;146:1193–1215. doi: 10.1163/156853909X419992. [DOI] [Google Scholar]
- 79.Doran-Sheehy D.M., Derby A.M., Greer D., Mongo P. Habituation of western gorillas: The process and factors that influence it. Am. J. Primatol. 2007;69:1354–1369. doi: 10.1002/ajp.20442. [DOI] [PubMed] [Google Scholar]
- 80.Tutin C.E.G. In: Great Ape Societies. 1st ed. McGrew W.C., Marchant L.F., Nishida T., editors. Cambridge University Press; 1996. Ranging and social structure of lowland gorillas in the Lopé Reserve, Gabon; pp. 58–70. [DOI] [Google Scholar]
- 81.Fay J., Carroll R., KerbisPeterhans J.C., Harris D. Leopard attack on and consumption of gorillas in the Central African Republic. J. Hum. Evol. 1995;29:93–99. doi: 10.1006/jhev.1995.1048. [DOI] [Google Scholar]
- 82.Robbins M.M., Bermejo M., Cipolletta C., Magliocca F., Parnell R.J., Stokes E. Social structure and life-history patterns in western gorillas (Gorilla gorilla gorilla) Am. J. Primatol. 2004;64:145–159. doi: 10.1002/ajp.20069. [DOI] [PubMed] [Google Scholar]
- 83.Klailova M., Casanova C., Henschel P., Lee P., Rovero F., Todd A. Non-Human Predator Interactions with Wild Great Apes in Africa and the Use of Camera Traps to Study Their Dynamics. Folia Primatol. 2012;83:312–328. doi: 10.1159/000342143. [DOI] [PubMed] [Google Scholar]
- 84.Henschel P., Abernethy K.A., White L.J.T. Leopard food habits in the Lope National Park, Gabon, Central Africa. Afr. J. Ecol. 2005;43:21–28. doi: 10.1111/j.1365-2028.2004.00518.x. [DOI] [Google Scholar]
- 85.Hayward M.W., Henschel P., O’Brien J., Hofmeyr M., Balme G., Kerley G.I.H. Prey preferences of the leopard (Panthera pardus) J. Zool. 2006;270:298–313. doi: 10.1111/j.1469-7998.2006.00139.x. [DOI] [Google Scholar]
- 86.Ray J., Sunquist M. Trophic relations in a community of African rainforest carnivores. Oecologia. 2001;127:395–408. doi: 10.1007/s004420000604. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson A.P., Gerngross P., Lemeris J.R., Jr., Schoonover R.F., Anco C., Breitenmoser-Würsten C., Durant S.M., Farhadinia M.S., Henschel P., Kamler J.F., et al. Leopard (Panthera pardus) status, distribution, and the research efforts across its range. PeerJ. 2016;4 doi: 10.7717/peerj.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bailey T.N. Blackburn Press; 2005. The African Leopard: Ecology and Behavior of a Solitary Felid; p. 476p. [Google Scholar]
- 89.Breuer T., Robbins M.M., Boesch C. Using photogrammetry and color scoring to assess sexual dimorphism in wild western gorillas (Gorilla gorilla) Am. J. Phys. Anthropol. 2007;134:369–382. doi: 10.1002/ajpa.20678. [DOI] [PubMed] [Google Scholar]
- 90.Breuer T., Robbins A.M., Boesch C., Robbins M.M. Phenotypic correlates of male reproductive success in western gorillas. J. Hum. Evol. 2012;62:466–472. doi: 10.1016/j.jhevol.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Masi S., Bouret S. Odor signals in wild western lowland gorillas: An involuntary and extra-group communication hypothesis. Physiol. Behav. 2015;145:123–126. doi: 10.1016/j.physbeh.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura M., Hosaka K., Itoh N., Matsumoto T., Matsusaka T., Nakazawa N., Nishie H., Sakamaki T., Shimada M., Takahata Y., et al. Wild chimpanzees deprived a leopard of its kill: Implications for the origin of hominin confrontational scavenging. J. Hum. Evol. 2019;131:129–138. doi: 10.1016/j.jhevol.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 93.Lin B., Foxfoot I.R., Miller C.M., Venkatamaran V.V., Kerby J.T., Bechtold E.K., Kellogg B.S., Nguyen N., Fashing P.J. Leopard predation on gelada monkeys at Guassa, Ethiopia. Am. J. Primatol. 2020;82:e23098. doi: 10.1002/ajp.23098. [DOI] [PubMed] [Google Scholar]
- 94.Bahram R., Burke J.E., Lanzi G.L. Head and neck injury from a leopard attack: Case report and review of the literature. J. Oral Maxillofac. Surg. 2004;62:247–249. doi: 10.1016/j.joms.2003.04.015. [DOI] [PubMed] [Google Scholar]
- 95.Nabi D.G., Tak S.R., Kangoo K.A., Halwai M.A. Injuries from leopard attacks in Kashmir. Injury. 2009;40:90–92. doi: 10.1016/j.injury.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 96.Hejna P. A Fatal Leopard Attack. J. Forensic Sci. 2010;55:832–834. doi: 10.1111/j.1556-4029.2010.01329.x. [DOI] [PubMed] [Google Scholar]
- 97.Pawar S.R., Kshirsagar R.A., Raut P.H., Patankar A.P. Maxillofacial injury from a leopard attack. Natl. J. Maxillofac. Surg. 2018;9:96–99. doi: 10.4103/njms.NJMS_41_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Agarwal P., Dhiman A., Rashid N., Kataria R. Head and neck injuries after leopard attack: Presentation and management. Chin. J. Traumatol. 2021;24:389–393. doi: 10.1016/j.cjtee.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boesch C. The Effects of Leopard Predation On Grouping Patterns in Forest Chimpanzees. Beyond Behav. 1991;117:220–241. doi: 10.1163/156853991X00544. [DOI] [Google Scholar]
- 100.Blakeslee J.R., Mcclure H.M., Anderson D.C., Bauer R.M., Huff L.Y., Olsen R.G. Chronic fatal disease in gorillas seropositive for Simian T-lymphotropic virus I antibodies. Cancer Lett. 1987;37:1–6. doi: 10.1016/0304-3835(87)90139-X. [DOI] [PubMed] [Google Scholar]
- 101.Rouquet P., Froment J.-M., Bermejo M., Kilbourn A., Karesh W., Reed P., Kumulungui B., Yaba P., Délicat A., Rollin P.E., Leroy E.M. Wild Animal Mortality Monitoring and Human Ebola Outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg. Infect. Dis. 2005;11:283–290. doi: 10.3201/eid1102.040533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Spelman L.H., Gilardi K.V.K., Lukasik-Braum M., Kinani J.-F., Nyirakaragire E., Lowenstine L.J., Cranfield M.R. Respiratory disease in mountain gorillas (Gorilla beringei beringei) in Rwanda, 1990–2010: Outbreaks, clinical course, and medical management. J. Zoo Wildl. Med. 2013;44:1027–1035. doi: 10.1638/2013-0014R.1. [DOI] [PubMed] [Google Scholar]
- 103.Murray S., Kishbaugh J.C., Hayek L.-A.C., Kutinsky I., Dennis P.M., Devlin W., Hope K.L., Danforth M.D., Murphy H.W. Diagnosing cardiovascular disease in western lowland gorillas (Gorilla gorilla gorilla) with brain natriuretic peptide. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Harcourt A.H., Stewart K.J. Gorilla society: What we know and don’t know. Evol. Anthropol. 2007;16:147–158. doi: 10.1002/evan.20142. [DOI] [Google Scholar]
- 105.Yamagiwa J. Intra- and inter-group interactions of an all-male group of virunga mountain gorillas (Gorilla gorilla beringei) Primates. 1987;28:1–30. doi: 10.1007/BF02382180. [DOI] [Google Scholar]
- 106.Watts D.P. Harassment of Immigrant Female Mountain Gorillas by Resident Females. Ethology. 1991;89:135–153. doi: 10.1111/j.1439-0310.1991.tb00300.x. [DOI] [Google Scholar]
- 107.Mirville M.O., Ridley A.R., Samedi J.P.M., Vecellio V., Ndagijimana F., Stoinski T.S., Grueter C.C. Intragroup Behavioral Changes Following Intergroup Conflict in Mountain Gorillas. Int. J. Primatol. 2020;41:382–400. doi: 10.1007/s10764-020-00130-1. [DOI] [Google Scholar]
- 108.Rasa O.A.E. The Effects of Crowding on the Social Relationships and Behaviour of the Dwarf Mongoose (Helogale undulata rufula) Z. Tierpsychol. 2010;49:317–329. doi: 10.1111/j.1439-0310.1979.tb00295.x. [DOI] [Google Scholar]
- 109.Sutcliffe A.G., Poole T.B. Intragroup agonistic behavior in captive groups of the common marmoset Callithrix jacchus jacchus. Int. J. Primatol. 1984;5:473–489. doi: 10.1007/BF02692270. [DOI] [Google Scholar]
- 110.Boccia M.L., Laudenslager M., Reite M. Food distribution, dominance, and aggressive behaviors in bonnet macaques. Am. J. Primatol. 1988;16:123–130. doi: 10.1002/ajp.1350160203. [DOI] [PubMed] [Google Scholar]
- 111.Asensio N., Aureli F., Schaffner C., Korstjens A. Intragroup Aggression, Fission-Fusion Dynamics and Feeding Competition in Spider Monkeys. Beyond Behav. 2008;145:983–1001. JSTOR. 40295874. [Google Scholar]
- 112.Jungers W.L., Susman R.L. In: The Pygmy Chimpanzee. Susman R.L., editor. Springer US; 1984. Body Size and Skeletal Allometry in African Apes; pp. 131–177. [DOI] [Google Scholar]
- 113.Stokes E.J., Parnell R.J., Olejniczak C. Female dispersal and reproductive success in wild western lowland gorillas (Gorilla gorilla gorilla) Behav. Ecol. Sociobiol. 2003;54:329–339. doi: 10.1007/s00265-003-0630-3. [DOI] [Google Scholar]
- 114.Balolia K.L., Soligo C., Wood B. Sagittal crest formation in great apes and gibbons. J. Anat. 2017;230:820–832. doi: 10.1111/joa.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Klailova M., Lee P.C. Response to Masi and Bouret—Commentary on Klailova and Lee’s (2014) “Wild western lowland gorillas signal selectively using odor.”. Physiol. Behav. 2015;145:127–128. doi: 10.1016/j.physbeh.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 116.Solomon-Lane T.K., Willis M.C., Pradhan D.S., Grober M.S. Female, but not male, agonistic behaviour is associated with male reproductive success in stable bluebanded goby (Lythrypnus dalli) hierarchies. Beyond Behav. 2014;151:1367–1387. doi: 10.1163/1568539X-00003188. [DOI] [Google Scholar]
- 117.Rutberg A.T. Inter-group transfer in Assateague pony mares. Anim. Behav. 1990;40:945–952. [Google Scholar]
- 118.Mirville M.O., Ridley A.R., Samedi J.P.M., Vecellio V., Ndagijimana F., Stoinski T.S., Grueter C.C. Low familiarity and similar ‘group strength’ between opponents increase the intensity of intergroup interactions in mountain gorillas (Gorilla beringei beringei) Behav. Ecol. Sociobiol. 2018;72:178. doi: 10.1007/s00265-018-2592-5. [DOI] [Google Scholar]
- 119.Watts D.P. Composition and variability of mountain gorilla diets in the Central Virungas. Am. J. Primatol. 1984;7:323–356. doi: 10.1002/ajp.1350070403. [DOI] [PubMed] [Google Scholar]
- 120.Ganas J., Robbins M.M., Nkurunungi J.B., Kaplin B.A., McNeilage A. Dietary Variability of Mountain Gorillas in Bwindi Impenetrable National Park, Uganda. Int. J. Primatol. 2004;25:1043–1072. doi: 10.1023/B:IJOP.0000043351.20129.44. [DOI] [Google Scholar]
- 121.Pusey A.E., Packer C. In: Infanticide and parental care. Parmigiani S., vom Saal F., editors. Harwood, Chur; Switzerland: 1994. Infanticide in lions: Consequences and counterstrategies; pp. 277–299. [Google Scholar]
- 122.Lewison R. Infanticide in the hippopotamus: evidence for polygynous ungulates. Ethol. Ecol. Evol. 1998;10:277–286. [Google Scholar]
- 123.Beehner J.C., Bergman T.J. Infant mortality following male takeovers in wild geladas. Am. J. Primatol. 2008;70:1152–1159. doi: 10.1002/ajp.20614. [DOI] [PubMed] [Google Scholar]
- 124.Li W., Dong S., Niu F., Li N., Su Z., Wang C., Huang K., Zhao H., Pan R., Zhang P., et al. Infanticide in golden snub-nosed monkeys with multilevel society. Curr. Zool. 2023;1 doi: 10.1093/cz/zoad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matsuda I., Tuuga A., Higashi S. Clouded leopard (Neofelis diardi) predation on proboscis monkeys (Nasalis larvatus) in Sabah, Malaysia. Primates. 2008;49:227–231. doi: 10.1007/s10329-008-0085-2. [DOI] [PubMed] [Google Scholar]
- 126.Jooste E., Pitman R.T., Van Hoven W., Swanepoel L.H. Unusually High Predation on Chacma Baboons (Papio ursinus) by Female Leopards (Panthera pardus) in the Waterberg Mountains, South Africa. Folia Primatol. 2012;83:353–360. doi: 10.1159/000339644. [DOI] [PubMed] [Google Scholar]
- 127.McLester E., Sweeney K., Stewart F.A., Piel A.K. Leopard (Panthera pardus) predation on a red-tailed monkey (Cercopithecus ascanius) in the Issa Valley, western Tanzania. Primates. 2019;60:15–19. doi: 10.1007/s10329-018-0700-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nakazawa N., Hanamura S., Inoue E., Nakatsukasa M., Nakamura M. A leopard ate a chimpanzee: First evidence from East Africa. J. Hum. Evol. 2013;65:334–337. doi: 10.1016/j.jhevol.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 129.Hiraiwa-Hasegawa M., Byrne R.W., Takasaki H., Byrne J.M. Aggression toward Large Carnivores by Wild Chimpanzees of Mahale Mountains National Park, Tanzania. Folia Primatol. 1986;47:8–13. doi: 10.1159/000156259. [DOI] [PubMed] [Google Scholar]
- 130.Isbell L.A. Predation on primates: Ecological patterns and evolutionary consequences. Evol. Anthropol. 2005;3:61–71. doi: 10.1002/evan.1360030207. [DOI] [Google Scholar]
- 131.Sunderland-Groves J.L., Tandang M.V., Patispathika F.H., Marzec A., Knox A., Nurcahyo A., Husson S.J., Sihite J. Suspected Sunda clouded leopard (Neofelis diardi) predation attempts on two reintroduced Bornean orangutans (Pongo pygmaeus wurmbii) in Bukit Batikap Protection Forest, Central Kalimantan, Indonesia. Primates. 2021;62:41–49. doi: 10.1007/s10329-020-00842-1. [DOI] [PubMed] [Google Scholar]
- 132.Van Schaik C.P. Why Are Diurnal Primates Living in Groups? Beyond Behav. 1983;87:120–144. doi: 10.1163/156853983X00147. [DOI] [Google Scholar]
- 133.Tutin C.E.G., Williamson E.A., Rogers M.E., Fernandez M. A case study of a plant-animal relationship: Cola lizae and lowland gorillas in the Lopé Reserve, Gabon. J. Trop. Ecol. 1991;7:181–199. doi: 10.1017/S0266467400005320. Cambridge Core. [DOI] [Google Scholar]
- 134.Estrada A., Garber P.A., Rylands A.B., Roos C., Fernandez-Duque E., Di Fiore A., Nekaris K.A.-I., Nijman V., Heymann E.W., Lambert J.E., et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017;3 doi: 10.1126/sciadv.1600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Haggblade M.K., Smith W.A., Noheri J.B., Usanase C., Mudakikwa A., Cranfield M.R., Gilardi K.V.K. Outcomes of snare-related injuries to endangered mountain gorillas (Gorilla beringei beringei) in Rwanda. J. Wildl. Dis. 2019;55:298–303. doi: 10.7589/2018-01-008. [DOI] [PubMed] [Google Scholar]
- 136.West P.M., MacCormick H., Hopcraft G., Whitman K., Ericson M., Hordinsky M., Packer C. Wounding, mortality and mane morphology in African lions, Panthera leo. Anim. Behav. 2006;71:609–619. doi: 10.1016/j.anbehav.2005.06.009. [DOI] [Google Scholar]
- 137.Morrison R.E., Hirwa J.P., Ndagijimana F., Vecellio V., Eckardt W., Stoinski T.S. Cascading effects of social dynamics on the reproduction, survival, and population growth of mountain gorillas. Anim. Conserv. 2022;26:398–411. doi: 10.1111/acv.12830. acv.12830. [DOI] [Google Scholar]
- 138.Díaz S., Settele J., Brondízio E.S., Ngo H.T., Agard J., Arneth A., Balvanera P., Brauman K.A., Butchart S.H.M., Chan K.M.A., et al. Pervasive human-driven decline of life on Earth points to the need for transformative change. Science. 2019;366 doi: 10.1126/science.aax3100. [DOI] [PubMed] [Google Scholar]
- 139.Bush E.R., Whytock R.C., Bahaa-El-Din L., Bourgeois S., Bunnefeld N., Cardoso A.W., Dikangadissi J.T., Dimbonda P., Dimoto E., Edzang Ndong J., et al. Long-term collapse in fruit availability threatens Central African forest megafauna. Science. 2020;370:1219–1222. doi: 10.1126/science.abc7791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The Leading contact is Shelly Masi, shelly.masi@mnhn.fr. Data reported in this manuscript will be shared by the lead contact upon request.
-
•
This manuscript does not report original code. All used codes are available in this paper’s supplemental information.
-
•
Any additional information required to reanalyze the data reported in this manuscript is available from the lead contact upon request.