Abstract
Background
It is unclear whether there are differences in benefits and harms between mobile and fixed prostheses for total knee arthroplasty (TKA). The previous Cochrane review published in 2004 included two articles. Many more trials have been performed since then; therefore an update is needed.
Objectives
To assess the benefits and harms of mobile bearing compared with fixed bearing cruciate retaining total knee arthroplasty for functional and clinical outcomes in patients with osteoarthritis (OA) or rheumatoid arthritis (RA).
Search methods
We searched The Cochrane Library, PubMed, EMBASE, CINAHL and Web of Science up to 27 February 2014, and the trial registers ClinicalTrials.gov, Multiregister, Current Controlled Trials and the World Health Organization (WHO) International Clinical Trials Registry Platform for data from unpublished trials, up to 11 February 2014. We also screened the reference lists of selected articles.
Selection criteria
We selected randomised controlled trials comparing mobile bearing with fixed bearing prostheses in cruciate retaining TKA among patients with osteoarthritis or rheumatoid arthritis, using functional or clinical outcome measures and follow‐up of at least six months.
Data collection and analysis
We used standard methodological procedures as expected by The Cochrane Collaboration.
Main results
We found 19 studies with 1641 participants (1616 with OA (98.5%) and 25 with RA (1.5%)) and 2247 knees. Seventeen new studies were included in this update.
Quality of the evidence ranged from moderate (knee pain) to low (other outcomes). Most studies had unclear risk of bias for allocation concealment, blinding of participants and personnel, blinding of outcome assessment and selective reporting, and high risk of bias for incomplete outcome data and other bias.
Knee pain
We calculated the standardised mean difference (SMD) for pain, using the Knee Society Score (KSS) and visual analogue scale (VAS) in 11 studies (58%) and 1531 knees (68%). No statistically significant differences between groups were reported (SMD 0.09, 95% confidence interval (CI) ‐0.03 to 0.22, P value 0.15). This represents an absolute risk difference of 2.4% points higher (95% CI 0.8% lower to 5.9% higher) on the KSS pain scale and a relative percent change of 0.22% (95% CI 0.07% lower to 0.53% higher). The results were homogeneous.
Clinical and functional scores
The KSS clinical score did not differ statistically significantly between groups (14 studies (74%) and 1845 knees (82%)) with a mean difference (MD) of ‐1.06 points (95% CI ‐2.87 to 0.74, P value 0.25) and heterogeneous results. KSS function was reported in 14 studies (74%) with 1845 knees (82%) as an MD of ‐0.10 point (95% CI ‐1.93 to 1.73, P value 0.91) and homogeneous results. In two studies (11%), the KSS total score was favourable for mobile bearing (159 vs 132 for fixed bearing), with MD of ‐26.52 points (95% CI ‐45.03 to ‐8.01, P value 0.005), but with a wide 95% confidence interval indicating uncertainty about the estimate.
Other reported scoring systems did not show statistically significant differences: Hospital for Special Surgery (HSS) score (seven studies (37%) in 1021 knees (45%)) with an MD of ‐1.36 (95% CI ‐4.18 to 1.46, P value 0.35); Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) total score (two studies (11%), 167 knees (7%)) with an MD of ‐4.46 (95% CI ‐16.26 to 7.34, P value 0.46); and Oxford total (five studies (26%), 647 knees (29%) with an MD of ‐0.25 (95% CI ‐1.41 to 0.91, P value 0.67).
Health‐related quality of life
Three studies (16%) with 498 knees (22%) reported on health‐related quality of life, and no statistically significant differences were noted between the mobile bearing and fixed bearing groups. The Short Form (SF)‐12 Physical Component Summary had an MD of ‐1.96 (95% CI ‐4.55 to 0.63, P value 0.14) and heterogeneous results.
Revision surgery
Twenty seven revisions (1.3%) were performed in 17 studies (89%) with 2065 knees (92%). In all, 13 knees were revised in the fixed bearing group and 14 knees in the mobile bearing group. No statistically significant differences were found (risk difference 0.00, 95% CI ‐0.01 to 0.01, P value 0.58), and homogeneous results were reported.
Mortality
In seven out of 19 studies, 13 participants (37%) died. Two of these participants had undergone bilateral surgery, and for seven participants, it was unclear which prosthesis they had received; therefore they were excluded from the analyses. Thus our analysis included four out of 191 participants (2.1%) who had died: one in the fixed bearing group and three in the mobile bearing group. No statistically significant differences were found. The risk difference was ‐0.02 (95% CI ‐0.06 to 0.03, P value 0.49) and results were homogeneous.
Reoperation rates
Thirty reoperations were performed in 17 studies (89%) with 2065 knees (92%): 18 knees in the fixed bearing group (of the 1031 knees) and 12 knees in the mobile group (of the 1034 knees). No statistically significant differences were found. The risk difference was ‐0.01 (95% CI ‐0.01 to 0.01, P value 0.99) with homogeneous results.
Other serious adverse events
Sixteen studies (84%) reported nine other serious adverse events in 1735 knees (77%): four in the fixed bearing group (of the 862 knees) and five in the mobile bearing group (of the 873 knees). No statistically significant differences were found (risk difference 0.00, 95% CI ‐0.01 to 0.01, P value 0.88), and results were homogeneous.
Authors' conclusions
Moderate‐ to low‐quality evidence suggests that mobile bearing prostheses may have similar effects on knee pain, clinical and functional scores, health‐related quality of life, revision surgery, mortality, reoperation rate and other serious adverse events compared with fixed bearing prostheses in posterior cruciate retaining TKA. Therefore we cannot draw firm conclusions. Most (98.5%) participants had OA, so the findings primarily reflect results reported in participants with OA. Future studies should report in greater detail outcomes such as those presented in this systematic review, with sufficient follow‐up time to allow gathering of high‐quality evidence and to inform clinical practice. Large registry‐based studies may have added value, but they are subject to treatment‐by‐indication bias. Therefore, this systematic review of RCTs can be viewed as the best available evidence.
Keywords: Humans; Knee Prosthesis; Arthritis, Rheumatoid; Arthritis, Rheumatoid/surgery; Arthroplasty, Replacement, Knee; Arthroplasty, Replacement, Knee/adverse effects; Arthroplasty, Replacement, Knee/instrumentation; Arthroplasty, Replacement, Knee/mortality; Bias; Health Status; Knee Joint; Osteoarthritis, Knee; Osteoarthritis, Knee/surgery; Prosthesis Design; Prosthesis Design/methods; Quality of Life; Randomized Controlled Trials as Topic; Range of Motion, Articular; Reoperation; Reoperation/statistics & numerical data
Plain language summary
Implants in knee replacement surgery for patients with osteoarthritis and rheumatoid arthritis
Review question
We reviewed the evidence on benefits and harms of mobile bearing compared with fixed bearing implants for cruciate retaining total knee replacement in patients with osteoarthritis (OA) or rheumatoid arthritis (RA). We found 19 relevant studies.
Background
In some people, damage and pain in the knee from arthritis are so severe that surgery is required. In these people, the joint can be replaced by a knee implant. In total knee replacement surgery, the ends of the long bones of the leg are usually replaced with metal ends, and a plastic insert is placed between them. Like fixed‐bearing implants, mobile‐bearing implants use three components to provide a relatively natural joint. In a mobile‐bearing knee, however, the polyethylene insert can rotate short distances inside the metal tibial tray. This design allows patients a few degrees of greater rotation to the medial and lateral sides of their knee. Compared with fixed bearing designs, mobile bearing knee implants require greater support from soft tissues, such as the ligaments surrounding the knee. If the soft tissues are not strong enough, mobile bearing knees are more likely to dislocate. They also may cost more than fixed bearing implants.
Study characteristics
On 27 February 2014, we found 19 studies that tested 1641 people with OA or RA. After surgery, these people were followed for at least six months. Most (98.5%) of the patients had OA. Seven out of 19 studies were funded by the prosthesis manufacturer; eight studies did not report their sources of funding.
Key results: at least six months after surgery
Knee pain (higher score means less pain)
• People in the fixed bearing group rated their pain as 0.09 points higher on the KSS scale of 0 to 50 than people in the mobile bearing group (absolute difference 2.4%).
• People in the mobile bearing group rated their pain at 41.4 points compared with 41.49 points in the fixed bearing group.
Clinical and functional scores (higher score means better function)
• People in the fixed bearing group rated their function as 0.10 points lower on the KSS scale of 0 to 100 than people in the mobile bearing group (absolute difference 0.1%).
• People in the mobile bearing group rated their function at 84.5 points compared with 84.4 points in the fixed bearing group.
Health‐related quality of life (higher score means better quality of life)
• People in the fixed bearing group rated their physical quality of life as 1.96 points lower on the Short Form (SF)‐12 scale of 0 to 100 than people in the mobile bearing group (absolute difference 1.96%).
• People in the mobile bearing group rated their physical quality of life to be 42.3 points compared with 40.34 points in the fixed bearing group.
Revision surgery
• 3 more knees per 1000 in the mobile bearing group needed further surgery than in the fixed bearing group. This may have happened by chance.
• 11 per 1000 knees in the fixed bearing group and 14 per 1000 knees in the mobile group needed further surgery to the knee (a revision).
Mortality
• 11 more people per 1000 in the mobile bearing group died than in the fixed bearing group. This may have happened by chance.
• 22 per 1000 people in the fixed bearing group and 33 per 1000 people in the mobile bearing group died.
Reoperation rate
• 12 per 1000 knees in both fixed bearing and mobile bearing groups needed a reoperation.
Other serious adverse events
• 1 more knee per 1000 in the fixed bearing group had another serious adverse event than in the mobile bearing group. This may have happened by chance.
• 7 per 1000 knees in the fixed bearing group and 6 per 1000 knees in the mobile bearing group had another serious adverse event.
Quality of the evidence
Mobile bearing implants probably cause little or no difference in pain compared with fixed bearing implants (moderate quality).
Mobile bearing implants may cause little or no difference in function, health‐related quality of life, revision surgery, mortality, reoperation rates and serious adverse events compared with fixed bearing implants (low quality).
Summary of findings
Summary of findings for the main comparison. Mobile bearing vs fixed bearing prosthesis for posterior cruciate retaining total knee arthroplasty for postoperative functional status in patients with osteoarthritis and rheumatoid arthritis.
| Mobile bearing vs fixed bearing prosthesis for posterior cruciate retaining total knee arthroplasty for postoperative functional status in patients with osteoarthritis and rheumatoid arthritis | ||||||
| Patient or population: patients with posterior cruciate retaining total knee arthroplasty for postoperative functional status in patients with osteoarthritis and rheumatoid arthritis Settings: hospital Intervention: fixed bearing Comparison: mobile bearing | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mobile bearing | Fixed bearing | |||||
| Pain ‐ measured as KSS pain Knee Society Score, subscore pain. Scale from 0 (severe pain) to 50 (no pain) Follow‐up: 1‐10.8 years | Mean SMD in the mobile bearing groups was 41.4 points | Standardised mean pain score in the fixed bearing groups was 0.09 higher (‐0.03 lower to 0.22 higher) | 1531 knees (68%) (11 studies, 58%) | ⊕⊕⊕⊝ Moderatea | Transformed into Knee Society score, subscore pain (range 0 to 50) Absolute difference: 2.4% higher (‐0.08% to 5.9%) Relative percent change: 0.22% (‐0.07% to 0.53% higher) Not statistically significant |
|
| Function ‐ measured as KSS function Knee Society Score, function. Scale from 0 to 100 (higher scores indicates better function) Follow‐up: 0.5‐10.8 years | Mean KSS function in the mobile bearing groups was 84.5 points | Mean KSS function in the fixed bearing groups was 0.1 lower (1.93 lower to 1.73 higher) | 1865 knees (83%) (14 studies, 74%) | ⊕⊕⊝⊝ Lowa,b | Absolute difference: 0.1% higher (‐1.93% to 1.73%) Relative percent change: 0.1% (‐2.28% to 2.05% higher) Not statistically significant |
|
| Health‐related quality of life ‐ measured as SF‐12 PCS SF‐12 PCS. Scale from 0 to 100 (higher scores indicate better health‐related quality of life) Follow‐up: 2‐2.5 years | Mean SF‐12 PCS in the mobile bearing groups was 42.3 points | Mean SF‐12 PCS in the fixed bearing groups was 1.96 lower (4.55 lower to 0.63 higher) | 498 knees (22%) (3 studies, 16%) | ⊕⊕⊝⊝ Lowa,b | Absolute difference: 1.96% lower (‐4.55% to 0.63%) Relative percent change: 4.63% (‐10.75% to 1.49% higher) Not statistically significant |
|
| Revision surgery Follow‐up: 1‐9.8 years | 14 per 1000 | 11 per 1000 (4 to 24) | See comment RR 0.80 (0.26‐1.74) |
2065 knees (92%) (17 studies) | ⊕⊕⊝⊝ Lowa,c | Risks were calculated from pooled risk differences Absolute risk difference: 0.00 (‐0.01 to 0.01) Relative percent change: 20% (I) (74% (W) to 74% (I) Not statistically significant |
| Mortality Follow‐up: 1‐2 years | 33 per 1000 | 22 per 1000 (‐18 to 58) | See comment RR 0.69 (‐0.55‐1.78) |
188 persons (12%) (4 studies) | ⊕⊕⊝⊝ Lowa,c | Risks were calculated from pooled risk differences Absolute risk difference: 0.02 lower (‐0.06 to 0.03) Relative percent change: 31% (I) (211% (W) to 78% (I) Not statistically significant |
| Reoperation rate Follow‐up: 1‐9.8 years | 12 per 1000 | 12 per 1000 (2 to 22) | See comment RR 1.01 (0.14‐1.86) |
2065 (92%) (17 studies) | ⊕⊕⊝⊝ Lowa,c | Risks were calculated from pooled risk differences Absolute risk difference 0.01 lower (‐0.01 to 0.01) Relative percent change: 1% (h) (86% (W) to 86% (I) Not statistically significant |
| Other serious adverse events Follow‐up: 1‐9.8 years | 6 per 1000 | 7 per 1000 (3 to 11) | See comment RR 1.16 (0.44‐1.84) |
1732 knees (77%) (17 studies) | ⊕⊕⊝⊝ Lowa,c | Risks were calculated from pooled risk differences Absolute risk difference: 0.00 (‐0.01 to 0.01) Relative percent change: 16% (h) (56% (W) to 84% (I) Not statistically significant |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aRisk of bias in individual studies, see 'Risk of bias' tables. bHeterogeneity is present. cTotal number of events is less than 300.
Background
Description of the condition
Osteoarthritis (OA) and rheumatoid arthritis (RA) are conditions that can affect the knee joints. OA and RA lead to pain, loss of function and a lower quality of life. In some people, damage and pain in the knee from arthritis are so severe that joint replacement is required. Approximately 10% of men and 18% of women older than 60 years have OA (World Health Organization 2013). Because of the ageing society as well as increasing obesity, the prevalence of knee OA continues to increase (Pereira 2011). The prevalence of RA varies between 0.3% and 1% (World Health Organization 2013).
Description of the intervention
Total knee arthroplasty (TKA) is a very common and reliable orthopaedic procedure for end‐stage arthritis of the knee. TKA has proved to be a successful surgical intervention that reduces pain and enhances physical function. It is a frequently performed procedure, and the number of TKAs is expected to increase exponentially in future years (Kurtz 2007).
Recent decennia have seen an expansion of technological developments in TKA, usually introduced into clinical practice without appropriate assessment (Gioe 2011). The mobile (meniscal or rotating) bearing TKA with a polyethylene insert has some freedom of movement and is an example of such a new development. The main goal of the mobile bearing insert is to decrease contact stresses at the implant interface (Matsuda 1998; Szivek 1996). Contradictory views exist as to whether the mobile bearing prosthesis will improve functionality as compared with the fixed bearing prosthesis for cruciate retaining TKA.
Why it is important to do this review
Previously, we performed a systematic review of the literature to assess whether mobile bearing total knee prostheses provide better functional outcomes in patients with OA and RA (Jacobs 2004). This previous review included two randomised controlled trials. Performing a meta‐analysis therefore was not possible. Since the time of that review, many trials have been performed to study the clinical and functional outcomes of mobile bearing TKA in comparison with fixed bearing TKA. Thus, an update of the previous review is warranted.
Objectives
To assess the benefits and harms of mobile bearing compared with fixed bearing cruciate retaining total knee arthroplasty for functional and clinical outcomes in patients with osteoarthritis or rheumatoid arthritis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing mobile and fixed bearing cruciate retaining TKA published as full text in a peer‐reviewed journal.
Types of participants
People who have had TKA for OA or RA.
Types of interventions
We included studies of primary, unconstrained, cruciate retaining, total (bi‐ or tricompartmental) knee arthroplasty with a mobile bearing (meniscal or rotational) or a fixed bearing polyethylene insert. We excluded studies with TKA after prior patellectomy and osteotomy.
Types of outcome measures
The outcome measurement in the studies had to be a functional or a clinical measure with a minimal follow‐up of six months.
Major outcomes
Knee pain (e.g. visual analogue score (VAS), Knee Society Score (pain), Western Ontario and McMaster Universities Arthritis Index (WOMAC) score (pain), Hospital for Special Surgery Score (HSS) (pain), Oxford Knee Score (OKS) (pain)).
Clinical and functional questionnaire scores (e.g. WOMAC, Knee Injury and Osteoarthritis Outcome Score (KOOS), OKS, HSS, Bristol Knee Score, International Knee Documentation Committee Subjective Knee Form (IKDC) or Performance Outcome (Knee Society (functional) Score, Knee Society (clinical) Score), Knee Society (total) Score)).
Health‐related quality of life (e.g. Short Form (SF)‐36, SF‐12).
Revision surgery.
Mortality.
Reoperation rate.
Serious adverse events (excluding revision surgery, mortality and reoperation rate).
Minor outcomes
Radiolucent lines.
Femorotibial alignment.
Performance outcome (flexion, extension, range of motion (ROM)).
Search methods for identification of studies
Electronic searches
In co‐operation with a trained medical librarian, we composed a new search strategy. We searched the following databases on 27 February 2014: The Cochrane Library (2014, Issue 1), PubMed (1944 to 27 February 2014), EMBASE (Ovid version) (1980 to 27 February 2014), Web of Science (1945 to 27 February 2014) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EbscoHost‐version) (1981 to 27 February 2014). In addition, we searched the following trial registries on 11 February 2014: ClinicalTrials.gov, Multi‐register, Current Controlled Trials, the World Health Organization (WHO) International Clinical Trials Registry Platform and the Dutch trial registry.
The search strategy consisted of the AND combination of two main concepts: rheumatoid arthritis or osteoarthritis, and knee arthroplasty. For the different concepts, we used all relevant keyword variations, not only keyword variations in the controlled vocabularies of the various databases, but free‐text word variations of these concepts as well. We optimised the search strategies for all consulted databases, taking into account differences in the various controlled vocabularies, as well as differences in database‐specific technical variations (e.g. use of quotation marks). We composed three different versions of the search strategy.
The intervention concept used as a major subject, the disease concept used both a major or minor subject.
The intervention concept and the disease concept used as both major and minor subjects, combined with the combination "mobile/fixed" as an additional concept.
A limited intervention concept combined with an extended "mobile/fixed" concept.
Finally, the results were limited to RCTs including human participants. All details of the queries are listed in Appendix 1.
Searching other resources
We screened the reference lists of included studies to look for additional studies with the same selection criteria and processed them as the primary search results.
Data collection and analysis
We managed publications with the aid of Reference Manager. In addition, we recorded relevant information pertaining to database source, reason for exclusion and consensus of review authors. We conducted statistical analyses using Review Manager (RevMan) software 5.
Selection of studies
Four review authors (KN, BP, SH, PM) conducted the literature search in co‐operation with a trained medical librarian and retrieved the references to be evaluated. Two review authors (KN, BP or SH, PM) independently selected trials for inclusion in the review. We resolved disagreements by consensus. When we could not reach consensus, we consulted a third review author (WJ) for the decisive vote.
We selected articles in two steps. In the first step, we excluded articles when it was apparent from either the title or the abstract that the study did not meet the criteria as mentioned in the Criteria for considering studies for this review. In the second step, we excluded articles when it was apparent from inspection of the printed article:
that it did not meet the inclusion criteria for the review; and
that the population had already been reported in another included study (most informative publication was included as primary reference, and additional publications as secondary reference).
We documented the reason for exclusion for each reference.
Data extraction and management
We closely examined articles that met all selection criteria with the aid of a checklist and a data extraction form (Appendix 2). One review author (SH or KN) entered data into RevMan 5, and another review author (PJ or WJ) checked the data.
Assessment of risk of bias in included studies
Two out of five possible review authors (KN, BP, WJ, SH, PM) assessed the risk of bias in duplicate independently. We assessed risk of bias using the tool of The Cochrane Collaboration (Higgins 2011), including the domains random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. In the domain 'other bias,' we checked for homogeneity of data and co‐interventions. We scored each domain as low, high or unclear. Under 'other bias,' we assessed co‐interventions and baseline imbalance such as group homogeneity and subgroup homogeneity, because heterogeneity is often encountered and accounts for lack of power in many orthopaedic surgery trials.
When two review authors could not reach consensus, we consulted a third review author until consensus was reached.
Measures of treatment effect
Studies eligible for the review were RCTs comparing a cruciate retaining mobile (rotating or meniscal) TKA against a fixed TKA.
Dichotomous data
For dichotomous outcomes, we calculated Mantel Haenszel random‐effects risk ratios (RRs). This RR refers to the risk of an event in the experimental group relative to the risk of an event in the control group. Therefore the RR can be calculated only when events are reported in the study groups. If the events were rare and empty cells were found in one of the groups in many studies, we calculated Mantel Haenszel random‐effects risk differences (RDs). Risk difference is the difference between observed risk in the two groups. The RD can be calculated even when no events are reported in one of the study groups.
Continuous data
For continuous outcomes, we calculated a random‐effects mean difference (MD) weighted by the inverse variance. The mean difference is a standard statistic that measures the absolute difference between mean values in two groups in a clinical trial while taking into account the precision by which this is estimated. It estimates the amount by which the experimental intervention on average changes the outcome compared with the control group. In addition, when the same outcome was reported on different scales, using differing units and methods of assessment (e.g. pain scales), we pooled the results by calculating a standardised mean difference (SMD). We corrected differences in the direction of the scale by subtracting mean values from the maximum value of the scale. To facilitate interpretation of the SMD, we transformed it back into a common scale, using data from the most representative study, with the largest weighting as mobile bearing group baseline and standard deviation.
Unit of analysis issues
An issue for studies on TKA is the possibility to perform bilateral surgery in which one knee is randomly assigned to receive mobile bearing and the other knee to fixed bearing prostheses. As not all studies have this design, we will analyse knee pain, clinical and functional scores and health‐related quality of life with and without including these studies performing bilateral knee surgery to assess whether this affects our results. For mortality, we excluded from the analysis participants who underwent bilateral surgery.
Dealing with missing data
Standard deviation (SD) was used when available, or we imputed it from ranges if available. If only the average was reported and no other information was available to calculate the SD, we imputed the average SD from other studies in the same meta‐analysis.
Assessment of heterogeneity
We tested heterogeneity by using the I2 statistic. The I2 statistic can be interpreted as the percentage of total variability in a set of effect sizes due to between‐studies variability.
Thresholds for interpretation of I2 of:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: show considerable heterogeneity.
Throughout this review, we considered results as heterogenous when I2 was 50% or greater.
Assessment of reporting biases
To determine publication bias, we searched the following trial registries: ClinicalTrials.gov, Multiregister, Current Controlled Trials, the WHO International Clinical Trials Registry Platform and the Dutch trial registry.
Data synthesis
We used a random‐effects model to pool data from each trial.
We conducted statistical analyses by using Review Manager 5.
Subgroup analysis and investigation of heterogeneity
We used the cutoff point of I2 ≥ 50% to indicate heterogeneity. If heterogeneity was present, we conducted subgroup analyses if possible. We intended to conduct subgroup analyses to investigate the effects of different follow‐ups (one year, two years and more than two years of follow‐up) on the observed effect.
Sensitivity analysis
We conducted sensitivity analyses to assess the effect on our results of including studies performing bilateral knee surgery. Therefore, we analysed knee pain, clinical and functional scores and health‐related quality of life with and without including these studies to assess whether this would affect our results. Furthermore, if possible, we planned to assess the effect of including only high quality studies.
'Summary of findings' table
We reported all major outcomes in the 'Summary of findings' table generated using GRADEpro version 3.2.2.
Grading strength of the evidence
We assessed the strength of the evidence by using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach, and added this information to the 'Summary of findings' table.
GRADE Working Group grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Downgrading strength of the evidence
We downgraded the quality of the evidence if any of these factors were present.
Limitations in the design and implementation of available studies suggesting high likelihood of bias.
Indirectness of evidence (indirect population, intervention, control, outcomes).
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses).
Imprecision of results (wide confidence intervals).
High probability of publication bias.
Results
Description of studies
We found 19 studies (with 1641 participants and 2247 knees ‐ 1616 participants with OA (98.5%) and 25 with RA (1.5%)), which were described in 22 articles. Seventeen of these studies were new since the time of the previous Cochrane review.
Results of the search
We searched the databases and identified 5660 references, of which 3290 were unique (Figure 1, PRISMA flowchart). Reference lists of studies selected for evaluation provided three additional titles, and citation tracking added two new references to the search. We screened 73 articles after removal of duplicates on the basis of title and abstract. We assessed the full text of 53 articles for eligibility. We excluded 34 articles, mostly because a posterior stabilised design was used for one or both types of prostheses in the study. This left 19 studies for inclusion in the review and three additional articles, of which one described follow‐up of an included study and two formed a subgroup of an included study.
1.
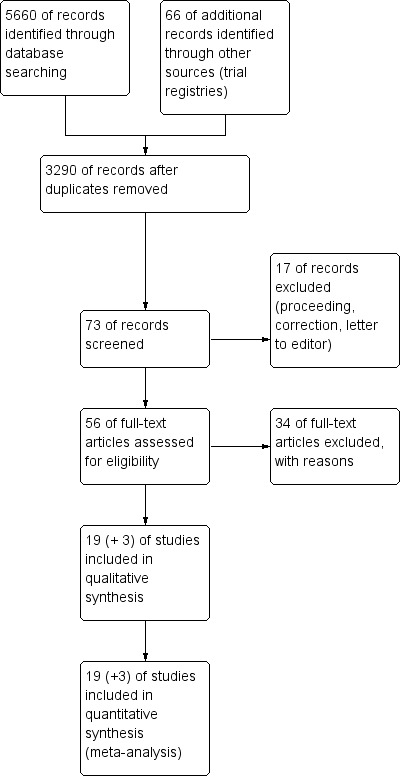
Study flow diagram (PRISMA).
Grey literature
We found nine proceedings that fulfilled the inclusion criteria. Five of these studies were later published as full text (Hanush 2008; Kim 2001a; Nilsson 2005; Tibesku 2006; Wylde 2008a). For one proceeding, no abstract was traceable (Suarez 2004). The study of Chatterji 2005 found higher levels of dissatisfaction and patellar‐femoral problems in the mobile bearing group. Jolles 2006 found better relative differences between preoperative and postoperative ROM and KSS scores at three months and six months for the fixed bearing TKA in comparison with the mobile bearing TKA. However, they did not describe postoperative comparisons of both prostheses. Tibesku 2009a found no functional advantage of mobile bearing TKA over fixed bearing TKA in a fluoroscopic study. Furthermore, we found two studies (NCT00208286; NCT01150929) in trial registries that may fulfil our inclusion criteria. However, no results were posted, and it was unclear whether these studies were cruciate retaining. In addition, we found one ongoing study (Characteristics of ongoing studies) without (complete) results.
Included studies
We included 22 reports of 19 studies in this review. See the Characteristics of included studies table for details. All studies were stated by their authors to be RCTs comparing mobile (rotating or meniscal) bearing versus fixed bearing, cruciate retaining, primary TKA.
Intervention
Nineteen studies compared mobile bearing versus fixed bearing prostheses. Of the mobile bearing group, 10 studies used a rotating design. Most prostheses were PFC Sigma systems (Bailey 2014; Hanush 2010; Higuchi 2009; Kim 2007; Kim 2009a; Möckel 2004; Munro 2010). Other prostheses were balanSys (Jacobs 2011), Columbus (Lampe 2011) and Trekking MB (Lizaur‐Utrilla 2012). Nine studies used a meniscal design, and three of these used the LCS (Grodzki 2001; Kim 2001; Kim 2009b). Other prostheses were Rotaglide (Hansson 2005; Watanabe 2005), MBK (Henricson 2006), e.motion‐FP (Kim 2010), TMK (Price 2003) and Genesis II (Tibesku 2011).
In the fixed bearing group, most prostheses were PFC Sigma (Bailey 2014; Grodzki 2001; Hanush 2010; Higuchi 2009; Kim 2007; Munro 2010). Other prostheses were Nuffield (Hansson 2005), NexGen (Henricson 2006; Watanabe 2005), balanSys (Jacobs 2011), AMK (Kim 2001; Kim 2009b), Medial Pivot (Kim 2009a), Genesis II (Kim 2010; Tibesku 2011), Columbus (Lampe 2011), Multigen Plus FB (Lizaur‐Utrilla 2012), Natural Knee (Möckel 2004) and AGC (Price 2003).
Six studies performed only bilateral knee surgeries (Kim 2001; Kim 2007; Kim 2009a; Kim 2010; Price 2003; Watanabe 2005). Five studies included some bilateral surgeries (Hansson 2005: 52 knees in 42 patients; Henricson 2006: 52 knees in 47 patients; Higuchi 2009: 76 knees in 68 patients; Lampe 2011: 100 knees in 96 patients; Munro 2010: 54 knees in 46 patients).
Participant characteristics
We have reported age and gender of study groups in Characteristics of included studies. Most studies included participants with osteoarthritis. Three studies included both participants with RA and those with OA (Kim 2001: six RA, 110 OA; Kim 2007: one RA, 173 OA; Watanabe 2005: 18 RA, four OA). In total, 98.5% of participants had OA.
In general we found participant populations from different studies to be comparable, especially in studies with bilateral TKA (Kim 2001; Kim 2007; Kim 2009a; Kim 2009b; Kim 2010; Price 2003; Watanabe 2005). Moreover, the groups are fairly homogeneous regarding etiology, with more than 90% of participants having OA. As we included only cruciate retaining TKA, the groups were homogeneous in this aspect.
However, selection criteria of included studies are sometimes absent, or they differ between studies, which might produce heterogeneous groups with regard to underlying disease (Hansson 2005; Kim 2001; Kim 2007; Munro 2010; Möckel 2004; Watanabe 2005).
Excluded studies
We excluded Aglietti 2005, Ball 2011, Bhan 2005, Breeman 2013, Breugem 2008, Chen 2013, Chiu 2001, Gioe 2009, Harrington 2009, Jawed 2012, Jolles 2012, KAT trial group 2009, Kim 2007b, Kim 2012, Kim 2012b, Läderman 2008, Li 2008, Matsuda 2010, Munoz 2008, Pagnano 2004, Pijls 2012, Rahman 2010, Saari 2003, Shemshaki 2012, Tienboon 2012, Uvehammer 2007, Vasdev 2009, Wohlrab 2009, Woolson 2004, Wylde 2008 and Zeng 2011 because one, both or some of the implants used in these studies were posterior stabilised and thus were not posterior cruciate ligament retaining. In the trial NCT00289094, other inflammatory arthritis and avascular necrosis of bone were included. See also Characteristics of excluded studies.
Risk of bias in included studies
The methodological quality scores of the individual studies are given in the 'Risk of bias' tables in the Characteristics of included studies section. Figure 2 and Figure 3 show the risk of bias graph and the methodological quality summary, respectively, of all included studies. The studies Bailey 2014,Kim 2009b,Lizaur‐Utrilla 2012 and Price 2003 did not have high risk of bias in any of the domain assessed.
2.
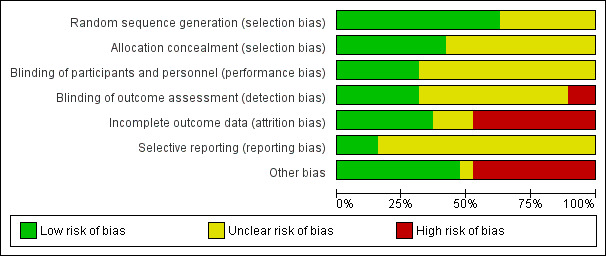
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
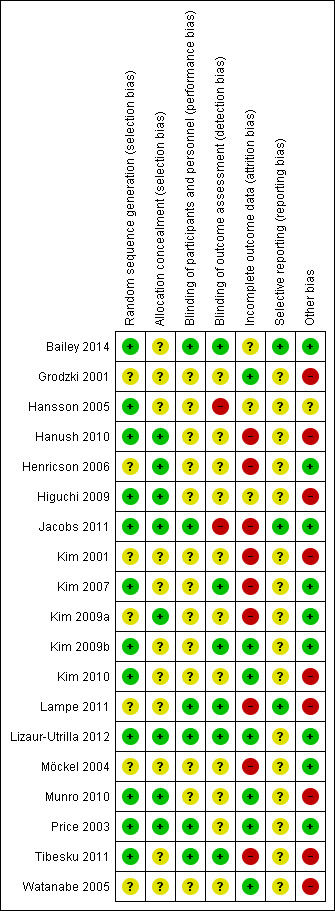
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
The randomisation technique is described in most studies but is unclear in the following studies: Grodzki 2001, Henricson 2006, Kim 2001, Kim 2009a, Lampe 2011, Möckel 2004 and Watanabe 2005. Methods of allocation sequences described include minimisation technique, computer‐generated random numbers and sequential pool of random numbers. Study authors describe concealment of allocation in Hanush 2010, Henricson 2006, Jacobs 2011, Kim 2009a, Lizaur‐Utrilla 2012, Munro 2010 and Price 2003. Methods described include sealed envelopes and telephone calls.
Blinding
Study authors describe use of patient blinding only in Bailey 2014, Jacobs 2011, Lampe 2011, Lizaur‐Utrilla 2012, Price 2003 and Tibesku 2011. They explain use of assessor blinding in Bailey 2014, Kim 2007, Kim 2009a, Lampe 2011, Lizaur‐Utrilla 2012 and Tibesku 2011.
Incomplete outcome data
Most studies reported the drop‐outs and had an acceptable drop‐out rate. One study (Möckel 2004) had too many (> 20%) participants lost to follow‐up, and another study (Jacobs 2011) excluded 30 participants (28% of the fixed bearing group) as the result of randomisation error. Higuchi 2009 and Tibesku 2011 did not describe the drop‐outs. The following studies used an intention‐to‐treat analysis: Grodzki 2001,Kim 2009b, Kim 2010, Lizaur‐Utrilla 2012, Möckel 2004, Munro 2010, Price 2003 and Watanabe 2005.
Selective reporting
We could find only online protocols for three included studies (Bailey 2014; Jacobs 2011; Lampe 2011), and this limited our assessment of reporting bias. Data are selectively available for time points in these studies. Fourteen studies report short‐term (up to one year) results (Bailey 2014; Grodzki 2001; Hansson 2005; Hanush 2010; Henricson 2006; Higuchi 2009; Jacobs 2011; Kim 2009a; Kim 2010; Lampe 2011; Lizaur‐Utrilla 2012; Möckel 2004; Munro 2010; Price 2003). Six studies report midterm (longer than one year to two years) results (Bailey 2014; Hansson 2005; Henricson 2006; Kim 2007; Lizaur‐Utrilla 2012; Tibesku 2011), and eight studies report long‐term (longer than two years) results (Kim 2001; Kim 2007; Kim 2009a; Kim 2009b; Kim 2010; Lizaur‐Utrilla 2012; Price 2003; Watanabe 2005). However, the outcomes that studies reported varied, as did follow‐up results. For example, Hansson 2005 reported HSS total only at two follow‐up points.
Other potential sources of bias
Other co‐interventions used during the procedure of the arthroplasty were frequently not reported. Hansson 2005 and Higuchi 2009 did not describe treatment of the patella. Cementing is unclear in Hansson 2005,Higuchi 2009 and Price 2003.
Effects of interventions
See: Table 1
See the 'Summary of findings' table for major outcome measures in the comparison of mobile versus fixed bearing prostheses (Table 1).
Major outcomes
Knee pain
We calculated the standardised mean difference (SMD) for pain, using the KSS pain and VAS scores for 11 studies (58%) and 1531 knees (68%). For studies that reported Oxford pain, HSS pain or WOMAC pain and also reported KSS pain, the KSS pain was used. The SMD was 0.09 (95% CI ‐0.03 to 0.22, P value 0.15). This represents an absolute risk difference of 2.4% points higher (95% CI 0.8% lower to 5.9% higher) on the KSS pain scale and a relative percent change of 0.22% (95% CI 0.07% lower to 0.53% higher) on the KSS pain scale, but these are not significant clinical or statistical differences.
All outcome measures for knee pain showed no statistically significant differences and wide confidence intervals, indicating considerable uncertainty in the estimates. Nine studies (47%) reported Knee Society pain score in 1392 (62%) knees. No significant differences were found; the mean difference was 0.41 (95% CI ‐0.06 to 0.88, P value 0.08) in favour of fixed bearing. The results are homogeneous (I2 = 0%, P value 0.57). Three studies (16%) reported VAS pain in 300 knees (13%) with a mean difference of ‐0.13 points (95% CI ‐0.96 to 0.69, P value 0.75). The results are heterogeneous (I2 = 77%, P = 0.01). Furthermore, Oxford pain was reported in two studies (11%) with 184 knees (8%) with a mean difference of ‐0.42 (95% ‐0.89 to 0.05, P value 0.08). Other pain outcomes are WOMAC pain and HSS pain, but these were not available for pooling. WOMAC pain was reported in only one study. HSS pain was reported in three studies, but two of these studies did not report ranges or SDs.
Clinical and functional scores
Given the differences in outcomes measured in different studies, calculating a single standardised mean difference was not appropriate.
The Knee Society score was reported in 14 studies (74%) (1845 knees (82%)). No significant differences between groups were found, and the mean difference in KSS clinical was ‐1.06 point (95% CI ‐2.87 to 0.74, P value 0.25). The mean difference in KSS function, as reported in 14 studies (1865 knees), was ‐0.10 points (95% CI ‐1.93 to 1.73, P value 0.91). KSS clinical showed heterogeneity (I2 = 77%, P value < 0.01) and, for KSS function, homogeneous results (I2 = 45%, P value 0.04). Furthermore, we found uncertainty in the estimate of the KSS total score based on two studies (Grodzki 2001; Tibesku 2011) with 71 knees. The mean difference between groups is ‐26.52 points (95% CI ‐45.03 to ‐8.01, P value 0.005). These results are homogeneous (I2 = 0%, P = 0.80).
Other reported scoring systems also showed uncertainty in their estimates, including HSS (seven studies (37%) in 1021 knees (45%)) with a mean difference of ‐1.36 (95% CI ‐4.18 to 1.46, P value 0.35) (I2 = 86%, P value < 0.01), WOMAC total score (two studies (11%) in 167 knees (7%)) with a mean difference of ‐4.46 (95% CI ‐16.26 to 7.34, P value 0.46) (I2 = 87%, P value < 0.01) and Oxford total (five studies (26%) in 647 knees (29%) with a mean difference of ‐0.25 (95% CI ‐1.41 to 0.91, P value 0.67) (I2 = 0%, P = 0.79). No other validated scoring systems (KOOS, WOMAC function, WOMAC stiffness, Oxford function) were available for pooling because no studies or just one study reported these outcomes.
Health‐related quality of life
Only the SF‐12 (PCS and MCS) was reported in three studies (16%) (Bailey 2014; Lizaur‐Utrilla 2012; Munro 2010) with 498 knees (22%). The mean difference in PCS was ‐1.96 (95% CI ‐4.55 to 0.63, P value 0.14). The mean difference in MCS was ‐1.26 points (95% CI ‐4.75 to 2.22, P = 0.48). Both results were heterogeneous (I2 = 61%, P value 0.09; I2 = 80%, P value 0.007), respectively).
Revision surgery
Orthopaedic surgeons performed a total of 27 revisions in 17 studies (89%) with 2065 knees (92%) ‐ 13 knees in the fixed bearing group (of the 1031 knees) and 14 knees in the mobile bearing group (of the 1034 knees). No significant differences between groups were found (RD 0.00, 95% CI ‐0.01 to 0.01, P value 0.58). Follow‐up time of the studies ranged from 0.5 year to 10 to 12 years, and 13 studies reported a follow‐up time less than three years. The groups were homogeneous (I2 = 0%, P value 1.00). Higuchi 2009 and Tibesku 2011 did not report the number of revisions. Reasons for revision surgery were polyethylene bearing dislocation (mobile bearing), ligamentous instability between the femur and the tibia (fixed bearing), complete wear of the tibial bearing polyethylene (mobile bearing and fixed bearing), infection (mobile bearing and fixed bearing), severe osteolysis (fixed bearing), patella component added (fixed bearing), tibial aseptic loosening (fixed bearing) and dislocation of the meniscal component (mobile bearing).
Mortality
Seven studies (37%) reported mortality. A total of 13 participants died. However, two of those who died (in two studies ‐ Price 2003; Watanabe 2005) had undergone bilateral surgery, so death could not be attributed to one particular group; they were thus excluded from the analysis. Hanush 2010 reported four deaths and Munro 2010 reported three deaths, but it was unclear whether these participants received a fixed bearing or a mobile bearing prosthesis. Therefore, in our analyses we included one participant who died (of the 96 participants) in the fixed bearing group and three who died (of the 95 participants) in the mobile bearing group. No significant difference was found between groups in terms of RD (‐0.02, 95% CI ‐0.06 to 0.03, P value 0.49). The groups were homogeneous (I² = 0%, P value 0.79). Kim 2010 stated that no deaths were related to surgery but did not report the number of persons who died. These studies thus were not included for this outcome.
Reoperation rate
A total of 30 reoperations were performed in 17 studies (89%) with 2065 knees (92%) ‐ 18 knees in the fixed bearing group (of the 1031 knees) and 12 knees in the mobile bearing group (of the 1034 knees). No significant difference was found between groups in terms of RD (‐0.01, 95% CI ‐0.01 to 0.01, P value 0.99). The groups were homogeneous (I2 = 0%, P = 0.81). Higuchi 2009 and Tibesku 2011 did not report the number of reoperations. Reasons for reoperation were patella resurfacing (mobile bearing and fixed bearing), femoral fracture (fixed bearing), infection (fixed bearing and mobile bearing), skin‐edge necrosis (mobile bearing and fixed bearing) and soft tissue revision for hematoma (mobile bearing).
Other serious adverse events
In all, 16 studies (84%) reported nine other serious adverse events in 1735 knees (77%) ‐ four in the fixed bearing group (of the 862 knees) and five in the mobile bearing group (of the 873 knees). No significant difference was found (mean RD 0.00, 95% CI ‐0.01 to 0.01, P value 0.88). The groups were homogeneous (I² = 0%, P = 1.00). Serious adverse events included deep vein thrombosis or pulmonary embolism (three mobile bearing and two fixed bearing), deep peroneal nerve palsy (two mobile bearing and one fixed bearing) and periprosthetic infection (not described whether a revision or a reoperation was needed) (one fixed bearing). Revision surgeries, reoperations and mortality were excluded from this rate of other serious adverse events because they are reported individually.
Minor outcomes
Five studies (26%) reported overall (not stratified by tibial or femoral) radiolucent lines in 978 knees (44%). A total of 90 events occurred in the fixed bearing group (of the 489 knees) and 75 events in the mobile bearing group (of the 489 knees). No significant difference was found between groups (RR 1.20, 95% CI 0.93 to 1.55, P value 0.16). The results were homogeneous (I2 = 0%, P value 0.84). Six studies (32%) reported tibial radiolucent lines in 1258 knees (56%). No significant difference was found between groups (RR 0.92, 95% CI 0.49 to 1.72, P value 0.79). The results were heterogeneous (I2 = 68%, P value 0.008). Four studies (21%) reported femoral radiolucent lines in 1095 knees (49%). No significant difference was found between groups (RR 0.92, 95% CI 0.46 to 1.85, P value 0.82). The results were homogeneous (I2 = 0%, P value 0.49).
Furthermore, six studies (32%) reported femorotibial alignment in 1047 knees (47%). No difference was found between groups; the mean difference was ‐0.40 (95% CI ‐0.86 to 0.06, P value 0.08). The results were heterogeneous (I2 = 60%, P value 0.03).
Nine studies (47%) in 838 knees (37%) reported flexion. A significant difference in flexion was found in favour of mobile bearing, but with uncertainty in the estimate. The mean difference was ‐1.84 ° (95% CI ‐3.48 to ‐0.20, P value 0.03). The results are homogeneous (I2 = 0%, P value 0.75). No significant difference was found regarding extension (four studies (21%), 291 knees (13%), 0.07 ° (95% CI ‐0.54 to 0.68, P value 0.82)). No heterogeneity was observed (I2 = 0%, P value 0.43). Range of motion was reported in 10 studies (53%) in 1361 knees (61%). No significant difference between groups was found; the mean difference was ‐0.67 ° (95% CI ‐3.26 to 1.90, P value 0.61). However, the results were heterogeneous (I2 = 77%, P value < 0.001) and the estimate is uncertain.
Subgroup analysis
We did not perform any subgroup analysis because the number of studies per subgroup would be too small.
Sensitivity analysis
Six studies performed only bilateral surgeries (Kim 2001; Kim 2007; Kim 2009b; Kim 2010; Price 2003; Watanabe 2005). We found similar results in outcomes if we excluded these studies from the analyses. The only exception was HSS, which became significant in favour of mobile bearing when these studies were excluded, with a mean difference of ‐3.68 (95% CI ‐7.18 to ‐0.17, P value 0.04) based on four studies. However, the results were heterogeneous (I2 = 72%, P value 0.01), with uncertainty in the estimate, and the difference is not clinically relevant. As very few studies had a low or unclear risk of bias, sensitivity analyses by quality of evidence were not possible.
Publication bias
We found two unpublished terminated trials (NCT00208286; NCT01150929) that may fulfil our inclusion criteria. However, no results were posted, and it was unclear whether these studies were cruciate retaining. It is thus possible that some selection bias could have occurred. In addition, we found one ongoing study (NCT00740376) without (complete) results.
Discussion
Summary of main results
In our search, we found 19 randomised trials and three additional articles about already included studies. Seventeen of these studies were new compared with studies included in the previous review (Jacobs 2004). In short, both types of prostheses do not show clinically important differences in benefits and harms. Although some studies found results in favour of the mobile bearing total knee arthroplasty (TKA), no clinically relevant differences were found between mobile bearing and fixed bearing posterior cruciate retaining total knee arthroplasty regarding knee pain, clinical and functional questionnaire scores and health‐related quality of life.
Knee pain was measured in 11 studies, but no clinically relevant differences were found. For clinical and functional scores, meta‐analyses showed statistically significant differences only for the Knee Society Score (KSS) total score. However, this finding was based on two studies (Grodzki 2001; Tibesku 2011) and includes a very large 95% confidence interval, indicating uncertainty in the estimate. Health‐related quality of life was measured in only three studies (Bailey 2014; Lizaur‐Utrilla 2012; Munro 2010), and no clinically relevant differences were found.
Furthermore, no significant differences between groups were seen in revision surgery, mortality, reoperation rates and other serious adverse event rates. Especially the numbers of serious adverse events and revision surgery procedures hardly differed. We could include only four of the 13 reported deaths in our analysis because of bilateral surgeries, and because some studies did not report which prosthesis participants received. Reoperations were reported in 18 of the 1031 knees in the fixed bearing group and in 12 of the 1034 knees in the mobile bearing group. The difference in number of reoperations was caused mainly by findings from the study of Kim 2009a. These investigators had a high incidence of infection in the fixed bearing group, and the study was temporarily stopped by the Infection Control Committee at their hospital, but no specific factors leading to the high incidence of infection were found. Furthermore, most studies reported follow‐up less than three years, so it is possible that there are differences in outcomes with longer follow‐up, especially for these outcomes. Large registry‐based studies with long‐term follow‐up may be of added value for further study potential differences in these outcomes. However, as these studies are subject to treatment‐by‐indication bias, findings must be interpreted cautiously.
The quality of the evidence, as assessed by the GRADE approach, ranged from moderate (knee pain) to low (other major outcomes) (Table 1).
Overall completeness and applicability of evidence
Results are frequently not split for different treatment modalities nor different patient categories. Although we can understand that the prime interest of some articles differs, we believe that reporting more detailed preoperative and postoperative data in orthopaedic surgery could greatly benefit interpretation of outcome results. Functional performance could be affected by patellar resurfacing. Resurfacing of the patella could increase the work line of the quadriceps tendon, thereby increasing muscle efficiency and thus walk ability capacity (e.g. staircase) of patients. Until the influence of such factors is known, it is paramount to have insight into the results per factor in each study, and thus to report data specifically for all subgroups. Otherwise it is impossible to draw conclusions about treatment efficacy within a particular study or to pool results from different studies.
Most of the included studies describe different types of prostheses for the comparison of mobile bearing versus fixed bearing TKA. It is therefore impossible to know whether observed results are due to use of a mobile bearing or fixed bearing TKA, or to differences in other design features or even preoperative patient characteristics. Accordingly, when such studies find a significant difference in outcomes between prostheses, this could be the result of these design differences rather than to use of a mobile bearing or fixed bearing TKA. Furthermore, it is currently unknown whether differences in outcome may change over time if either implant behaves differently with reference to survivorship. Applicability of the results of cohort‐based clinical studies to the general population has long been a topic of controversy. Such data are available in national arthroplasty registers and can thereby contribute substantial added value to an informed discussion of arthroplasty outcomes (Labek 2011), especially for outcomes that appear at long‐term follow‐up such as mortality and revision.
The KSS total was 26.52 points higher in favour of mobile bearing, but as mentioned before, but this finding was based on only two studies with a wide 95% CI (‐45.03 to ‐8.01), indicating uncertainty in the estimate. The probability of publication bias was high, as only two studies reported this outcome instead of the more commonly reported KSS functional and KSS clinical separately. It is possible that although the KSS functional and the KSS clinical separately showed no significant differences, a significant difference would have been shown if both scores were summed up.
Furthermore, most (98.5%) of the participants had osteoarthritis (OA), so the results primarily reflect results in individuals with OA.
Quality of the evidence
The quality of the evidence as graded by the GRADE approach ranged from moderate to low. This assessment was based on risk of bias of individual studies, indirectness, inconsistency of results, imprecision of results and high probability of publication bias, and provides the rationale or justification for downgrading the quality of the evidence.
The quality of knee pain, measured by KSS pain as moderate, and thus further research are likely to have an important impact on our confidence in the estimate of effect and may change the estimate. The quality of evidence of this outcome measure was downgraded because of the risk of bias of individual studies (see Figure 3). This risk of bias was also responsible for downgrading of the quality of evidence in all other major outcome parameters (Table 1).
We did not downgrade any of the outcomes because of indirectness of the evidence. Only randomised controlled trials (RCTs) comparing fixed versus mobile bearings were included in different settings. Clinical and functional scores (range of motion (ROM)) and health‐related quality of life measures (measured as Short Form (SF)‐12 Physical Components Summary (PCS)) were downgraded because of unexplained heterogeneity. This heterogeneity may affect interpretation of results.
The number of serious adverse events (SAEs) resulting in revision and mortality was less than 300; this was also downgraded because of imprecision of results. These outcomes are graded as low quality, which means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Potential biases in the review process
This review has several strengths and limitations. We composed a new search strategy in cooperation with a trained medical librarian, and, besides the search in databases, we also searched trial registries. We found two unpublished terminated trials (NCT00208286; NCT01150929) that may fulfil our inclusion criteria. However, no results were posted, and it was unclear whether these studies were cruciate retaining. It is thus possible that some selection bias could have occurred. In addition, we found one ongoing study (NCT00740376) without (complete) results. Two review authors independently selected trials for inclusion in the review and resolved disagreements by consensus. When no consensus could be found, a third review author was consulted for the decisive vote. Two review authors independently assessed in duplicate risk of bias. This reduces the possibility of observer bias. A limitation of this meta‐analysis is that many studies report outcomes of only one postoperative follow‐up moment, which limits the possibility of pooling intermediate results and may cause heterogeneity between studies. This also limits the possibility of analysing differences in follow‐up moments. Furthermore, in our selection, we found rotating bearing and meniscal bearing types of implants. Differences could be present because of the anterior movement possibility of the meniscal bearing type. In the Characteristics of included studies table, we have described each implant, so care providers can judge whether the results are applicable to their practice.
Agreements and disagreements with other studies or reviews
We found nine other systematic reviews on mobile bearing versus fixed bearing total knee arthroplasty.
Apostolopoulos 2011 reviewed clinical and basic scientific studies that compared clinical results, biomechanical features and kinematic patterns of fixed bearing versus mobile bearing knee designs. They concluded that clinical studies have not proved the superiority of mobile bearing.
Bo 2013 included 12 studies in a meta‐analysis. They included RCTs with bilateral mobile bearing and fixed bearing total knee replacements. We included six of these studies. The study did not include retainment of the posterior cruciate as one of the inclusion criteria. Investigators found no differences in clinical, functional, satisfaction, complication and radiological results.
Cheng 2013 included nine articles in a meta‐analysis; only two of these articles are included in our selection. Study authors selected RCTs comparing mobile bearing and fixed bearing, including posterior stabilised/PCL resection with a mean follow‐up > 5 years. Researchers reported no differences in radiological outcomes or general health results between groups.
van der Voort 2013 selected 41 studies; we included 14 of these articles. They included RCTs comparing mobile bearing and fixed bearing, regardless of whether or not they were cruciate retaining. Meta‐analyses showed no clinically relevant differences in terms of revision rates, clinical outcome scores or patient‐reported outcome measures between mobile bearing and fixed bearing total knee replacements (TKRs).
Smith 2011 identified 13 articles, but only four of these are included in our selection. This study did not have retainment of the posterior cruciate as one of the inclusion criteria. This could explain the difference in included studies in comparison with our review. Study authors used a limited search strategy, which might explain the additional trials in our review. Regardless, this study could find no significant differences in clinical outcome scores.
Wen 2011 identified 15 articles, of which five are included in our selection. This can be explained by the inclusion of posterior stabilised/posterior cruciate ligament (PCL) resection designs in this review. This review could not find differences between the two designs in terms of clinical and radiological outcomes.
Post 2010 identified seven non‐comparative long‐term follow‐up studies. They analysed not only functional outcomes, but also long‐term survivorship with both designs. This review found no differences in clinical outcome scores.
Bracht van der 2010 identified six articles, of which three are included in our selection. This can be explained by the inclusion of posterior stabilised/PCL resection designs and non‐randomised studies in this review. Moreover, study authors searched in six major journals on orthopaedic surgery instead of searching medical databases. They found no superiority in the clinical outcome of mobile‐bearing over fixed‐bearing TKA.
Oh 2009 identified 10 articles, but only four of these are included in our selection. This can be explained by the inclusion of posterior stabilised/PCL resection designs and non‐randomised studies in this review. Study authors used a limited search strategy, which might explain the additional trials in our review. Regardless, this review could find no differences in clinical outcome scores.
Although all of these reviews used different selection criteria to compare mobile bearing versus fixed bearing (e.g. uni/bilateral, posterior stabilised/cruciate retaining) and differed in outcome measures, their results are congruent with our findings. No clinically important differences were found regarding clinical, functional, complication and radiological outcomes.
Authors' conclusions
Implications for practice.
Current evidence suggests similar patient outcomes for mobile bearing total knee arthroplasty and fixed bearing total knee arthroplasty, regarding knee pain, clinical and functional questionnaire scores, health‐related quality of life, revision surgery, mortality, reoperation and other serious adverse events among patients. No statistically and clinically relevant differences were found for any of these outcomes. Also, given the moderate to low quality of the studies, we cannot draw firm conclusions for clinical practice.
Implications for research.
Since the time of preparation of the previous version of this review, many new publications have reported randomised trials on this subject. To be able to compare and pool outcomes from different studies, the outcomes must be presented at comparable assessment moments. The present review clearly identifies the need for trials to present data at final follow‐up, but also for intermediate follow‐ups. In the included studies, we could find no evidence of significant or clinically relevant differences in favour of mobile bearing total knee arthroplasty in comparison with fixed bearing total knee arthroplasty. However, specific patient groups may benefit from a certain prosthesis, such as athletes. This is a potential area for further research. In addition, future studies should report in greater detail on the outcomes presented in this systematic review, with sufficient follow‐up time to obtain high‐quality evidence and inform clinical practice. Large registry‐based studies may have added value, particularly for infrequent outcomes such as mortality, revision and serious adverse events. However, as these registry‐based studies are subject to treatment‐by‐indication bias (which is not the case in RCTs), the present systematic review of RCTs can be viewed as the best available evidence.
A specific problem related to comparing different types of prostheses is that the differences are small, and consequently the effect on patient performance for a given parameter is hard to detect and can be detected only with large sample sizes. Even more, clinical differences are strongly associated with preoperative functional capacity (Nelissen 1992). The effect of an outcome parameter is often important in itself but of limited influence on the rest of the patient's performance. For example, the extent of migration in a radiostereophotogrammetric analysis (RSA) study should always be accompanied by functional and clinical parameters. We welcome the development of guidelines, such as those published in the Journal of Bone and Joint Surgery (Poss 2002). Because of these methodological problems, more rigorous statistical methods must be performed so the coherence of several aspects of the outcome can be evaluated.
What's new
| Date | Event | Description |
|---|---|---|
| 27 February 2014 | New search has been performed | New search; a total of 19 studies included |
| 8 January 2014 | New citation required but conclusions have not changed | New authorship |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 2, 2004
| Date | Event | Description |
|---|---|---|
| 5 May 2008 | Amended | Converted to new review format. CMSG ID: C053‐R |
Acknowledgements
We would like to thank J. Schoones, MA, Librarian, for help provided in performing the search and in arranging reprints, as well as the editorial team of the Cochrane Musculoskeletal Group for providing valuable comments on early drafts and for assisting with the searches. We would like to thank Patricia Anderson and Jacques van Limbeek for help provided in earlier versions of this review.
Appendices
Appendix 1. Search strategy
| Source________________ | Search_strings______________________________________ | Number of references | Number of unique references |
| PubMed (1944 to 12‐11‐2013) | #1. "arthritis, rheumatoid"[mesh] OR (felty* AND syndrome) OR (caplan* AND syndrome) OR rheumatoid nodule OR (sjogren* AND syndrome) OR (sicca AND syndrome) OR still disease OR stills disease OR still's disease OR bechterew disease OR bechterews disease OR bechterew's disease OR (arthritis AND rheumat*) OR "osteoarthritis"[mesh] OR osteoarthr* OR (degenerative AND arthritis) OR gonarthrosis OR ("mobile‐bearing"[ti] AND "fixed‐bearing") #2. "Arthroplasty"[majr] OR "Joint Prosthesis"[majr] OR "Prostheses and Implants"[majr] OR arthroplast*[ti] OR joint prosthe*[ti] #3. "Knee"[majr] OR "Knee Joint"[majr] OR knee[ti] OR knees[ti] #4. "Arthroplasty, Replacement, Knee"[majr] OR "Knee Prosthesis"[ti] OR tka[ti] OR ((knee[ti] OR knees[ti] OR knee*[ti]) AND (replace*[ti] OR replacement[ti] OR replacing[ti] OR replaced[ti] OR arthroplast*[ti] OR arthroplasty[ti] OR arthroplastic[ti] OR prosthe*[ti] OR prosthesis[ti] OR prosthetic[ti] OR endoprosthe*[ti] OR implant*[ti] OR implant[ti] OR implants[ti] OR implanted[ti])) #5. (#2 AND #3) #6. (#5 OR #4) #7. (#1 AND #6) #8. randomized controlled trial[pt] OR randomized[ti] OR randomised[ti] OR RCT[ti] OR RCTs[ti] OR "random allocation"[mesh] OR "double‐blind method"[mesh] OR "single‐blind method"[mesh] OR randomised[ti] OR random*[ti] OR comparative study[pt] OR comparative[ti] OR "double‐blind"[ti] OR "single‐blind"[ti] OR "double‐blinded"[ti] OR "single‐blinded"[ti] OR "Comparative Study"[Publication Type] OR compar*[ti] OR "clinical outcomes" OR "clinical outcome" OR comparat* OR compare* OR compari* #9. (#7 AND #8) #10. "animals"[mesh] NOT "humans"[mesh] #11. (#9 NOT #10) #12. "arthritis, rheumatoid"[mesh] OR (felty* AND syndrome) OR (caplan* AND syndrome) OR rheumatoid nodule OR (sjogren* AND syndrome) OR (sicca AND syndrome) OR still disease OR stills disease OR still's disease OR bechterew disease OR bechterews disease OR bechterew's disease OR (arthritis AND rheumat*) OR "osteoarthritis"[mesh] OR osteoarthr* OR (degenerative AND arthritis) OR gonarthrosis OR ("mobile‐bearing"[ti] AND "fixed‐bearing") #13. "Arthroplasty"[mesh] OR "Joint Prosthesis"[mesh] OR "Prostheses and Implants"[mesh] OR arthroplast*[tw] OR joint prosthe*[tw] #14. "Knee"[mesh] OR "Knee Joint"[mesh] OR knee[tw] OR knees[tw] #15. (#13 AND #14). #16. "Arthroplasty, Replacement, Knee"[mesh] OR "Knee Prosthesis"[tw] OR tka[tw] OR ((knee[tw] OR knees[tw] OR knee*[tw]) AND (replace*[tw] OR replacement[tw] OR replacing[tw] OR replaced[tw] OR arthroplast*[tw] OR arthroplasty[tw] OR arthroplastic[tw] OR prosthe*[tw] OR prosthesis[tw] OR prosthetic[tw] OR endoprosthe*[tw] OR implant*[tw] OR implant[tw] OR implants[tw] OR implanted[tw])) #17. (#12 AND (#15 OR #16)) #18. "fixed‐bearing" OR "mobile‐bearing" OR "fixation" #19. (#17 AND #18) #20. randomized controlled trial[pt] OR randomized[ti] OR randomised[ti] OR RCT[ti] OR RCTs[ti] OR "random allocation"[mesh] OR "double‐blind method"[mesh] OR "single‐blind method"[mesh] OR randomised[ti] OR random*[ti] OR comparative study[pt] OR comparative[ti] OR "double‐blind"[ti] OR "single‐blind"[ti] OR "double‐blinded"[ti] OR "single‐blinded"[ti] OR "Comparative Study"[Publication Type] OR compar*[ti] #21. (#19 AND #20) #22. "animals"[mesh] NOT "humans"[mesh] #23. (#21 NOT #22) #24. total knee replacement OR total knee arthroplasty #25. mobile bearing OR mobile platform OR rotating platform OR meniscal bearing #26. fixed bearing OR fixed platform #27. randomized controlled trial[pt] OR randomized[ti] OR randomised[ti] OR RCT[ti] OR RCTs[ti] OR "random allocation"[mesh] OR "double‐blind method"[mesh] OR "single‐blind method"[mesh] OR randomised[ti] OR random*[ti] OR comparative study[pt] OR comparative[ti] OR "double‐blind"[ti] OR "single‐blind"[ti] OR "double‐blinded"[ti] OR "single‐blinded"[ti] OR "Comparative Study"[Publication Type] OR compar*[ti] #28. "animals"[mesh] NOT "humans"[mesh] #29. (#24 AND #25 AND #26 AND #27) #30. (#29 NOT #28) #31. (#11 OR #23 OR #30) |
2275 | |
| EMBASE (Ovid version) (1980 to 12‐11‐2013) | #1. exp rheumatoid arthritis/ OR ((felty* ADJ2 syndrome) OR (caplan* ADJ2 syndrome) OR rheumatoid nodule OR (sjogren* ADJ2 syndrome) OR (sicca ADJ2 syndrome) OR still disease OR stills disease OR still's disease OR bechterew disease OR bechterews disease OR bechterew's disease OR (arthritis ADJ2 rheumat*) OR exp osteoarthritis/ OR osteoarthr*.mp OR (degenerative ADJ2 arthritis) OR gonarthrosis).mp OR ("mobile‐bearing" AND "fixed‐bearing").mp #2. exp *Arthroplasty/ OR exp *Joint Prosthesis/ OR exp *"Prostheses and Orthoses"/ OR arthroplast*.ti OR joint prosthe*.ti #3 exp *Knee/ OR knee*.ti #4. (#2 AND #3) #5. exp *Knee Arthroplasty/ OR exp *Knee prosthesis/ OR tka.ti OR "Knee Prosthesis".ti OR (knee*.ti AND (replace* OR replacement OR replacing OR replaced OR arthroplast* OR arthroplasty OR arthroplastic OR prosthe* OR prosthesis OR prosthetic OR endoprosthe* OR implant* OR implant OR implants OR implanted).ti #6. (#4 OR #5) #7. (#1 AND #6) #8. randomized controlled trial/ OR randomization/ OR triple blind procedure/ OR double blind procedure/ OR single blind procedure/ OR placebo/ OR ("random allocation" OR "double‐blind*" OR "single‐blind*" OR placebo OR placebos OR random* OR ramdom* OR ramdon* OR randon* OR rct OR rct's OR rcts OR ((single OR double OR treble OR triple) AND (mask* OR blind*)) OR placebo* OR random*).mp OR compar*.ti OR versus.ti OR vs.ti OR exp comparative study/ #9. exp Human/ #10. (#7 AND #8 AND #9) #11. exp rheumatoid arthritis/ OR ((felty* ADJ2 syndrome) OR (caplan* ADJ2 syndrome) OR rheumatoid nodule OR (sjogren* ADJ2 syndrome) OR (sicca ADJ2 syndrome) OR still disease OR stills disease OR still's disease OR bechterew disease OR bechterews disease OR bechterew's disease OR (arthritis ADJ2 rheumat*) OR exp osteoarthritis/ OR osteoarthr*.mp OR (degenerative ADJ2 arthritis) OR gonarthrosis).mp OR ("mobile‐bearing".ti AND "fixed‐bearing".ti).mp #12. exp Arthroplasty/ OR exp Joint Prosthesis/ OR exp "Prostheses and Orthoses"/ OR arthroplast*.mp OR joint prosthe*.mp #13. exp Knee/ OR knee*.mp #14. (#12 AND #13) #15. exp Knee Arthroplasty/ OR exp Knee prosthesis/ OR tka.mp OR "Knee Prosthesis"mpi OR (knee*.mp AND (replace* OR replacement OR replacing OR replaced OR arthroplast* OR arthroplasty OR arthroplastic OR prosthe* OR prosthesis OR prosthetic OR endoprosthe* OR implant* OR implant OR implants OR implanted).mp) #16. (#14 OR #15) #17. (#11 AND #16) #18. ("fixed‐bearing" OR "mobile‐bearing" OR "fixation").mp #19. (#17 AND #18 ) #20. randomized controlled trial/ OR randomization/ OR triple blind procedure/ OR double blind procedure/ OR single blind procedure/ OR placebo/ OR ("random allocation" OR "double‐blind*" OR "single‐blind*" OR placebo OR placebos OR random* OR ramdom* OR ramdon* OR randon* OR rct OR rct's OR rcts OR ((single OR double OR treble OR triple) AND (mask* OR blind*)) OR placebo* OR random*).mp OR compar*.ti OR versus.ti OR vs.ti OR exp comparative study/ #21. exp Human/ #22. (#19 AND #20 AND #21) #23. (total knee replacement OR total knee arthroplasty).mp #24. (mobile bearing OR mobile platform OR rotating platform OR meniscal bearing).mp #25. (fixed bearing OR fixed platform).mp #26. (#23 AND #24 AND #25) #27. randomized controlled trial/ OR randomization/ OR triple blind procedure/ OR double blind procedure/ OR single blind procedure/ OR placebo/ OR ("random allocation" OR "double‐blind*" OR "single‐blind*" OR placebo OR placebos OR random* OR ramdom* OR ramdon* OR randon* OR rct OR rct's OR rcts OR ((single OR double OR treble OR triple) AND (mask* OR blind*)) OR placebo* OR random*).mp OR compar*.ti OR versus.ti OR vs.ti OR exp comparative study/ #28. exp Human/ #29. (#26 AND #27 AND #28) #30. (#10 OR #22 OR #29) |
1564 | 551 |
| Web of Science (1981 to 12‐11‐2013) | #1. TS=(rheumatoid arthritis OR felty syndrome OR sicca syndrome OR caplan syndrome OR still* disease OR sjogren* syndrome OR bechterew* disease OR rheumatoid nodule* OR osteoarthr* OR degenerative arthritis OR gonarthrosis OR "mobile‐bearing" AND "fixed‐bearing") #2. TI=(knee* and (replace* OR arthroplast* OR prosthe* OR endoprosthe* OR implant*) #3. TS=(randomized controlled trial OR randomization OR triple blind procedure OR double blind procedure OR single blind procedure OR placebo OR "random allocation" OR "double‐blind*" OR "single‐blind*" OR placebo OR placebos OR random* OR ramdom* OR ramdon* OR randon* OR rct OR rcts OR "comparative study" OR ((single OR double OR treble OR triple) AND (mask* OR blind*)) OR placebo* OR random*) #4. TI=(compar* OR versus OR vs) #5. (#1 AND #2 AND (#3 OR #4)) #6. TS=(rheumatoid arthritis OR felty syndrome OR sicca syndrome OR caplan syndrome OR still* disease OR sjogren* syndrome OR bechterew* disease OR rheumatoid nodule* OR osteoarthr* OR degenerative arthritis OR gonarthrosis OR "mobile‐bearing" AND "fixed‐bearing") #7. TS=(knee* and (replace* OR arthroplast* OR prosthe* OR endoprosthe* OR implant*) #8. TS=("fixed‐bearing" OR "mobile‐bearing" OR "fixation") #9. (#6 AND #7 AND #8 AND (#3 OR #4)) #10. TS=(total knee replacement OR total knee arthroplasty) #11. TS=(mobile bearing OR mobile platform OR rotating platform OR meniscal bearing) #12. TS=(fixed bearing OR fixed platform) #13. (#10 AND #11 AND #12 AND (#3 OR #4)) #14. (#5 OR #9 OR #13) |
709 | 186 |
| The Cochrane Library (1898 to 12‐11‐2013) | #1. ((felty* AND syndrome) OR (caplan* AND syndrome) OR rheumatoid nodule OR (sjogren* AND syndrome) OR (sicca AND syndrome) OR still disease OR stills disease OR still's disease OR bechterew disease OR bechterews disease OR bechterew's disease OR (arthritis AND rheumat*) OR "osteoarthritis"[mesh] OR osteoarthr* OR (degenerative AND arthritis) OR gonarthrosis OR ("mobile‐bearing"[ti] AND "fixed‐bearing")) #2. ((arthroplast* OR joint prosthe*) AND knee*):ti #3. ("Knee Prosthesis" OR tka OR ((knee OR knees OR knee*) AND (replace* OR replacement OR replacing OR replaced OR arthroplast* OR arthroplasty OR arthroplastic OR prosthe* OR prosthesis OR prosthetic OR endoprosthe* OR implant* OR implant OR implants OR implanted))):ti #4. (#2 OR #3) #5. (#1 AND #4) #6. ((arthroplast* OR joint prosthe*) AND knee*) #7. ("Knee Prosthesis" OR tka OR ((knee OR knees OR knee*) AND (replace* OR replacement OR replacing OR replaced OR arthroplast* OR arthroplasty OR arthroplastic OR prosthe* OR prosthesis OR prosthetic OR endoprosthe* OR implant* OR implant OR implants OR implanted))) #8. ("fixed‐bearing" OR "mobile‐bearing" OR "fixation") #9. (#1 AND ( #6 OR #7 ) AND #8) #10. (total knee replacement OR total knee arthroplasty) AND (mobile bearing OR mobile platform OR rotating platform OR meniscal bearing) AND (fixed bearing OR fixed platform) #11. (#5 OR #9 OR #10) |
718 | 160 |
| CINAHL (EbscoHost version) (1981 to 12‐11‐2014) | #1. rheumatoid arthritis OR felty syndrome OR sicca syndrome OR caplan syndrome OR still* disease OR sjogren* syndrome OR bechterew* disease OR rheumatoid nodule* OR osteoarthr* OR degenerative arthritis OR gonarthrosis OR ("mobile‐bearing" AND "fixed‐bearing") #2. knee* and (replace* OR arthroplast* OR prosthe* OR endoprosthe* OR implant*) #3. randomized controlled trial OR randomization OR triple blind procedure OR double blind procedure OR single blind procedure OR placebo OR "random allocation" OR "double‐blind*" OR "single‐blind*" OR placebo OR placebos OR random* OR ramdom* OR ramdon* OR randon* OR rct OR rcts OR "comparative study" OR ((single OR double OR treble OR triple) AND (mask* OR blind*)) OR placebo* OR random* #4. TI(compar* OR versus OR vs) #5. #1 AND #2 AND (#3 OR #4) |
313 | 118 |
| ClinicalTrials.gov (to 11‐2‐2014) | #1. (total knee replacement OR total knee arthroplasty) AND (mobile bearing OR mobile platform OR rotating platform OR meniscal bearing) AND (fixed bearing OR fixed platform) #2. (total knee OR knee replacement OR knee arthroplasty) AND (mobile bearing OR mobile platform OR rotating platform OR meniscal bearing) AND (fixed bearing OR fixed platform) #3.(knee OR tka OR tkr) AND (mobile OR rotating OR meniscal) AND fixed |
28 | |
| Multi‐register (to 11‐2‐2014) | #1. (total knee OR knee replacement OR knee arthroplasty) AND (mobile bearing OR mobile platform OR rotating platform OR meniscal bearing) AND (fixed bearing OR fixed platform) | 11 | |
| Current Controlled Trials (to 11‐2‐2014) | #1. (total knee OR knee replacement OR knee arthroplasty) AND (mobile bearing OR mobile platform OR rotating platform OR meniscal bearing) AND (fixed bearing OR fixed platform) #2. (knee OR tka OR tkr) AND (mobile OR rotating OR meniscal) AND fixed |
3 | 1 |
| WHO International Clinical Trials Registry Platform (to 11‐2‐2014) | #1. knee AND mobile AND fixed | 24 |
Appendix 2. Data extraction form
Article number:
Review author:
Date:
| General information | Instructions | Data extracted | ||||
| First author | ||||||
| Sponsorship trial | Copy any sponsorship mentioned in the article (usually bottom left, first page) | |||||
| Methods | ||||||
| Study design | RCT, quasi‐RCT, non‐randomised CCT | |||||
| Method of randomisation | Tables, coin, etc | |||||
| Allocation concealment | Closed envelopes, etc | |||||
| Blindedness | ||||||
| Population | ||||||
| Place | Hospital/City/Country | |||||
| Enrolment dates | Helps in finding out double‐reported populations | |||||
| Inclusion criteria | ||||||
| Exclusion criteria | ||||||
| Age | Describe the age of the included population | |||||
| Sex | Describe the sex distribution of the included population | |||||
| Ethnicity | ||||||
| Work status | ||||||
| Duration of symptoms | ||||||
| Previous treatments | ||||||
| Total number of patients recruited | ||||||
| Number of patients who met inclusion criteria | ||||||
| Total number of patients randomised | ||||||
| Total number of patients followed | ||||||
| Interventions: | ||||||
| Group 1 PCL (yes/no) Brand implant (manufacterer) Patella (yes/no) Bearing (mobile/fixed/rotating) Flexion space (how determined) |
You may copy the description of the intervention here, or simply indicate on which page and in which paragraph it can be found | PCL: Brand: Patella: Flexion space: |
||||
| Group 2 | Idem | PCL: CR Brand: Patella: Flexion space: |
||||
| Group 3 | Idem | PCL: Brand: Patella: Flexion space: |
||||
| Outcomes | ||||||
| Who carried out the measurement? | ||||||
| What were the follow‐up moments? | Preop | 6 Months | 12 Months | 24 Months | ___Months | |
| What was measured at each follow‐up and with which tool? ROM KSS functional KSS clinical VAS WOMAC KOOS OKS HSS IKDC Active flexion Passive flexion Revision Mortality SF‐36 Gait analysis Good Samaritan Knee Assessment Questionnaire |
||||||
| Analysis: | ||||||
| Statistical technique used: | Which tests? Alpha? Power? Sample size calculation? Software used? | Power: Sample size: Software: |
||||
| Does technique adjust for confounding? | ||||||
| Number (or %) followed‐up from each group: | ||||||
| Results: | ||||||
| Quantitative results | If no between‐group comparisons are given, then report here the general results | |||||
| Qualitative results | ||||||
| Adverse effects or complications | ||||||
| 1. Comparison: (A) .................. (N=) vs (B) .................... (N= ) Outcome: Follow‐up: preop (A): 0 (0) (B): 0 (0) 6 months (A): 0 (0) (B): 0 (0) 12 months (A): 0 (0) (B): 0 (0) 24 months (A): 0 (0) (B): 0 (0) X months (A): 0 (0) (B): 0 (0) 2. Comparison: (A) .................. (N=) vs (B) .................... (N= ) Outcome: Follow‐up: preop (A): 0 (0) (B): 0 (0) 6 months (A): 0 (0) (B): 0 (0) 12 months (A): 0 (0) (B): 0 (0) 24 months (A): 0 (0) (B): 0 (0) X months (A): 0 (0) (B): 0 (0) 3. Comparison: (A) .................. (N=) vs (B) .................... (N= ) Outcome: Follow‐up: preop (A): 0 (0) (B): 0 (0) 6 months (A): 0 (0) (B): 0 (0) 12 months (A): 0 (0) (B): 0 (0) 24 months (A): 0 (0) (B): 0 (0) X months (A): 0 (0) (B): 0 (0) 4. Comparison: (A) .................. (N=) vs (B) .................... (N= ) Outcome: Follow‐up: preop (A): 0 (0) (B): 0 (0) 6 months (A): 0 (0) (B): 0 (0) 12 months (A): 0 (0) (B): 0 (0) 24 months (A): 0 (0) (B): 0 (0) X months (A): 0 (0) (B): 0 (0) | ||||||
Data and analyses
Comparison 1. Mobile vs fixed bearing, major outcome: knee pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 KSS pain | 9 | 1392 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.06, 0.88] |
| 2 VAS pain | 3 | 200 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.96, 0.69] |
| 3 Oxford pain | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.89, 0.05] |
| 4 Knee pain (combined scores) | 12 | 1592 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.03, 0.22] |
1.1. Analysis.
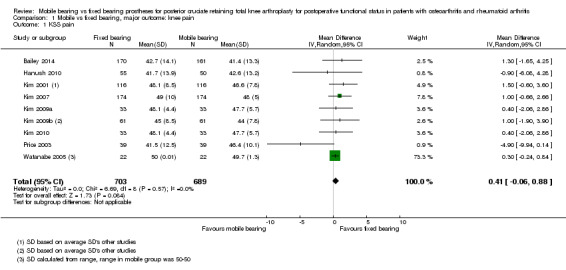
Comparison 1 Mobile vs fixed bearing, major outcome: knee pain, Outcome 1 KSS pain.
1.2. Analysis.
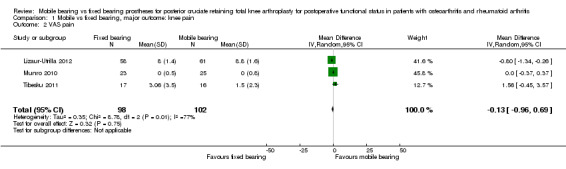
Comparison 1 Mobile vs fixed bearing, major outcome: knee pain, Outcome 2 VAS pain.
1.3. Analysis.
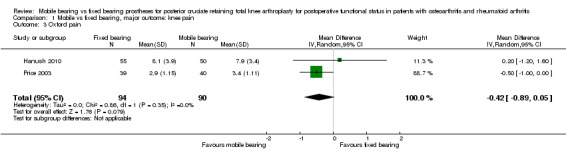
Comparison 1 Mobile vs fixed bearing, major outcome: knee pain, Outcome 3 Oxford pain.
1.4. Analysis.
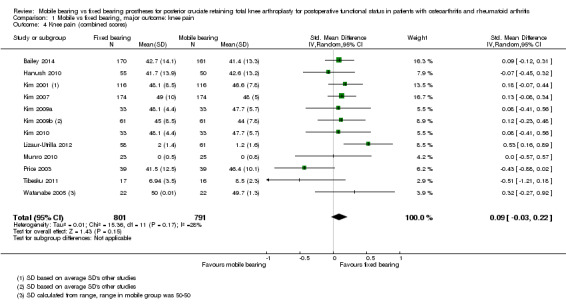
Comparison 1 Mobile vs fixed bearing, major outcome: knee pain, Outcome 4 Knee pain (combined scores).
Comparison 2. Mobile vs fixed bearing, major outcome: clinical and functional scores.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 KSS clinical | 14 | 1845 | Mean Difference (IV, Random, 95% CI) | ‐1.06 [‐2.87, 0.75] |
| 2 KSS function | 14 | 1865 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.93, 1.73] |
| 3 KSS total | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐26.52 [‐45.03, ‐8.01] |
| 4 HSS | 7 | 1021 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐4.18, 1.46] |
| 5 WOMAC total | 2 | 167 | Mean Difference (IV, Random, 95% CI) | ‐4.46 [‐16.26, 7.34] |
| 6 Oxford total | 5 | 647 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐1.41, 0.91] |
2.1. Analysis.
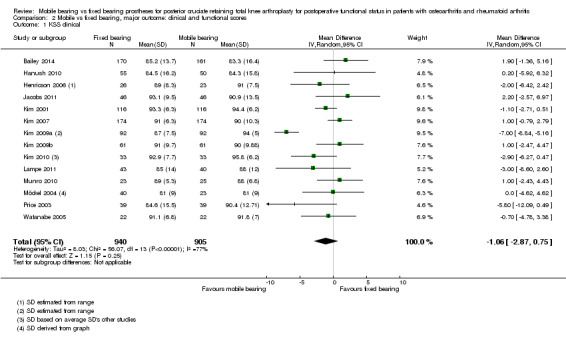
Comparison 2 Mobile vs fixed bearing, major outcome: clinical and functional scores, Outcome 1 KSS clinical.
2.2. Analysis.
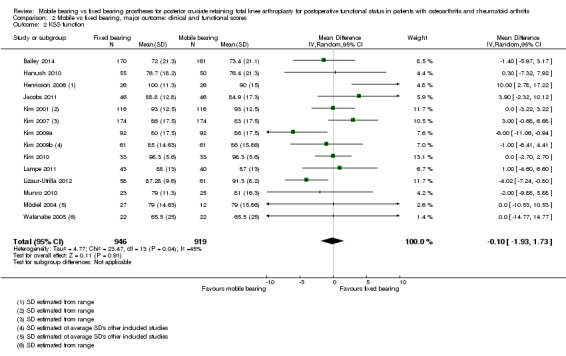
Comparison 2 Mobile vs fixed bearing, major outcome: clinical and functional scores, Outcome 2 KSS function.
2.3. Analysis.
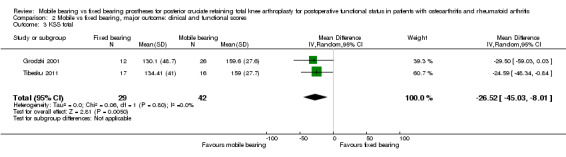
Comparison 2 Mobile vs fixed bearing, major outcome: clinical and functional scores, Outcome 3 KSS total.
2.4. Analysis.
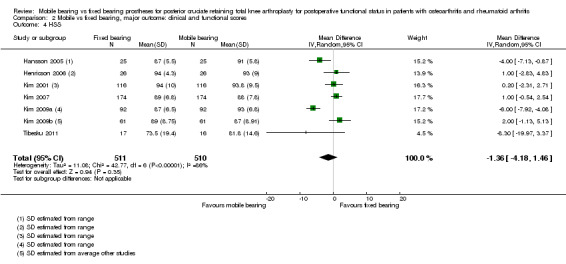
Comparison 2 Mobile vs fixed bearing, major outcome: clinical and functional scores, Outcome 4 HSS.
2.5. Analysis.
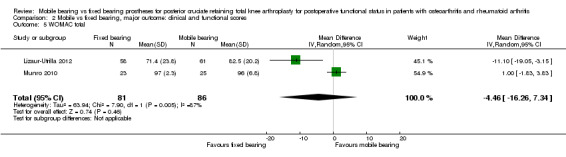
Comparison 2 Mobile vs fixed bearing, major outcome: clinical and functional scores, Outcome 5 WOMAC total.
2.6. Analysis.
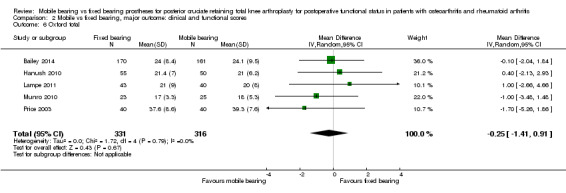
Comparison 2 Mobile vs fixed bearing, major outcome: clinical and functional scores, Outcome 6 Oxford total.
Comparison 3. Mobile vs fixed bearing, major outcome: health‐related quality of life.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SF‐12 PCS | 3 | 498 | Mean Difference (IV, Random, 95% CI) | ‐1.96 [‐4.55, 0.63] |
| 2 SF‐12 MCS | 3 | 498 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐4.75, 2.22] |
3.1. Analysis.
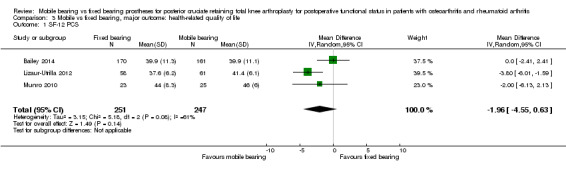
Comparison 3 Mobile vs fixed bearing, major outcome: health‐related quality of life, Outcome 1 SF‐12 PCS.
3.2. Analysis.
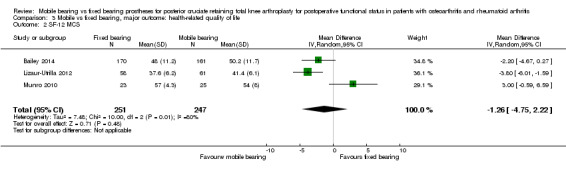
Comparison 3 Mobile vs fixed bearing, major outcome: health‐related quality of life, Outcome 2 SF‐12 MCS.
Comparison 4. Mobile vs fixed bearing, major outcome: revision surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Revision surgery | 17 | 2065 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
4.1. Analysis.
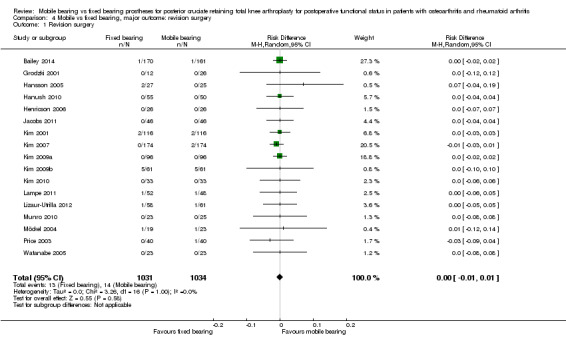
Comparison 4 Mobile vs fixed bearing, major outcome: revision surgery, Outcome 1 Revision surgery.
Comparison 5. Mobile vs fixed bearing, major outcome: mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 191 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.06, 0.03] |
5.1. Analysis.
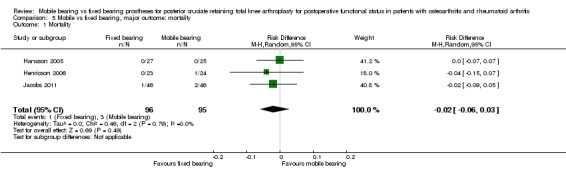
Comparison 5 Mobile vs fixed bearing, major outcome: mortality, Outcome 1 Mortality.
Comparison 6. Mobile vs fixed bearing, major outcome: reoperation rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reoperation rate | 17 | 2065 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.01, 0.01] |
6.1. Analysis.
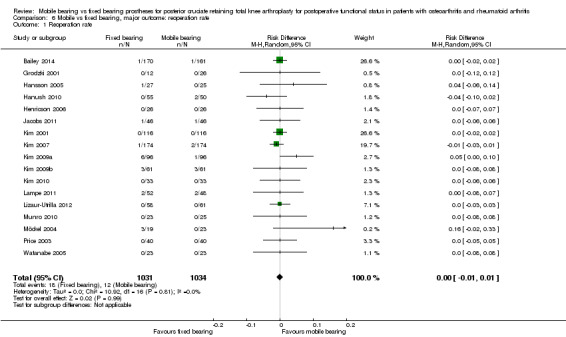
Comparison 6 Mobile vs fixed bearing, major outcome: reoperation rate, Outcome 1 Reoperation rate.
Comparison 7. Mobile vs fixed bearing, major outcome: other serious adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 16 | 1735 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
7.1. Analysis.
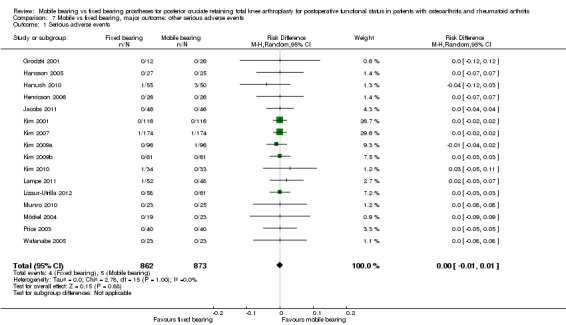
Comparison 7 Mobile vs fixed bearing, major outcome: other serious adverse events, Outcome 1 Serious adverse events.
Comparison 8. Mobile vs fixed bearing, minor outcomes: radiological outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Radiolucent lines (tibial) | 6 | 1258 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.49, 1.72] |
| 2 Radiolucent lines (femoral) | 4 | 1095 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.46, 1.85] |
| 3 Radiolucent lines (overall) | 5 | 978 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.93, 1.55] |
| 4 Femorotibial alignment | 6 | 1047 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.86, 0.06] |
8.1. Analysis.
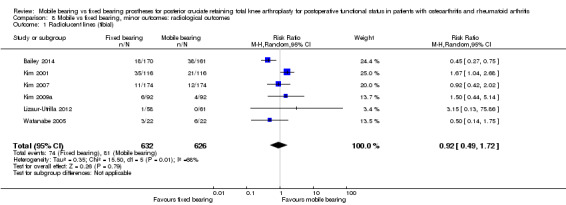
Comparison 8 Mobile vs fixed bearing, minor outcomes: radiological outcomes, Outcome 1 Radiolucent lines (tibial).
8.2. Analysis.
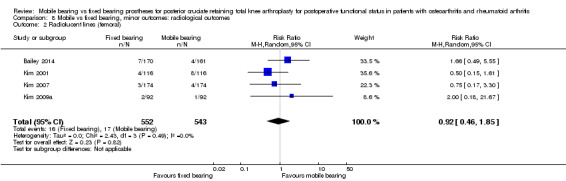
Comparison 8 Mobile vs fixed bearing, minor outcomes: radiological outcomes, Outcome 2 Radiolucent lines (femoral).
8.3. Analysis.
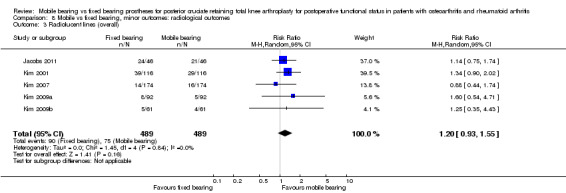
Comparison 8 Mobile vs fixed bearing, minor outcomes: radiological outcomes, Outcome 3 Radiolucent lines (overall).
8.4. Analysis.
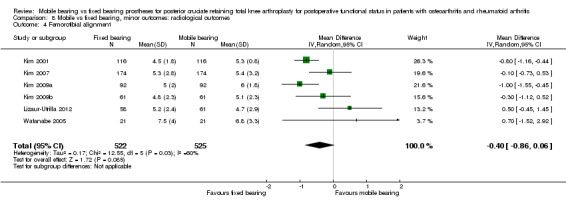
Comparison 8 Mobile vs fixed bearing, minor outcomes: radiological outcomes, Outcome 4 Femorotibial alignment.
Comparison 9. Mobile vs fixed bearing, minor outcomes: performance outcome.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Flexion | 9 | 838 | Mean Difference (IV, Random, 95% CI) | ‐1.84 [‐3.48, ‐0.20] |
| 2 Extension | 4 | 291 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.54, 0.68] |
| 3 Range of motion | 10 | 1456 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐3.21, 1.87] |
9.1. Analysis.
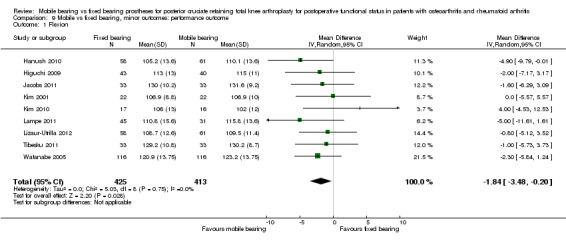
Comparison 9 Mobile vs fixed bearing, minor outcomes: performance outcome, Outcome 1 Flexion.
9.2. Analysis.
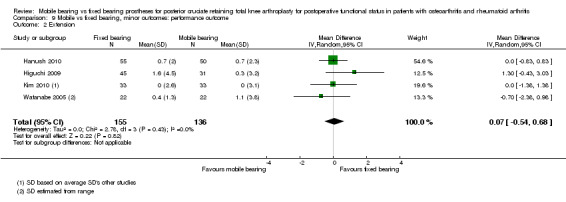
Comparison 9 Mobile vs fixed bearing, minor outcomes: performance outcome, Outcome 2 Extension.
9.3. Analysis.
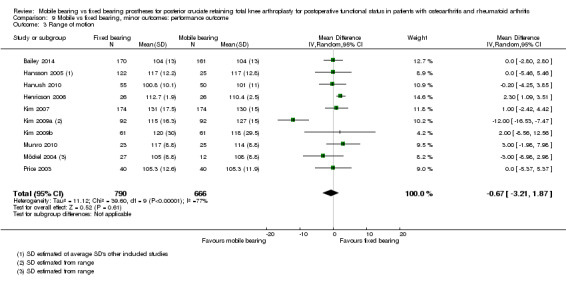
Comparison 9 Mobile vs fixed bearing, minor outcomes: performance outcome, Outcome 3 Range of motion.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bailey 2014.
| Methods | RCT stated Randomisation determined by a third party randomisation process to ensure similar demographics between the 2 groups Duration of the study: 2 years |
|
| Participants | Inclusion: primary knee OA requiring a primary TKA, age > 35 Exclusion: previous knee surgery, inflammatory arthroplasty, significant PMHx, complex surgery requiring bone grafting or revision prosthesis UK: 331 participants Fixed: n = 170, female 102, age 70.1 ± 7.9 years Mobile: n = 161, female 87, age 69.2 ± 8.6 years |
|
| Interventions | Fixed: PFC Sigma (Depuy) Mobile: PFC, rotating platform (Depuy) Decision to resurface the patella was made intraoperatively on the basis of intraoperative patellar tracking and clinical patellar wear Both the tibia and the femoral prosthesis were cemented |
|
| Outcomes | ROM, OKS, KSS, SF‐12 and radiolucency Assessments: preoperative and at 12 and 24 months Average and SD given |
|
| Notes | Study funded by DePuy International No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation determined by a third party randomisation process to ensure similar demographics between the 2 groups |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant was blinded; surgeon was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor was blinded; statistician who carried out the analysis was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rate was given and acceptable; not clear whether intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Low risk | Protocol available and prespecified outcomes reported |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Grodzki 2001.
| Methods | RCT stated Randomisation technique not stated (1:2 factor?) Duration of study: 1 year |
|
| Participants | Inclusion: primary gonarthrosis Exclusion: local infection near the knee joint, RA, insulin‐dependent diabetes mellitus, > 15 ° of varus/valgus, absolute medial or lateral collateral ligament instability Germany: 38 participants; sex ratio not stated Fixed: n = 12, age 73.9 (53‐89) years Mobile: n = 26, age 73.1 (55‐91) years |
|
| Interventions | Fixed: PFC Sigma (DePuy) Mobile: LCS, rotating platform (DePuy) Routine patellar resurfacing Tibial component cemented; femoral component cementless |
|
| Outcomes | KSS total, revision Assessments: preoperative and at 1 week, 2 weeks, 6 weeks, 3 months, 6 months and 1 year Average and standard deviation given |
|
| Notes | Funding not stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation. Probably with factor 1:2 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions described in sufficient detail |
Hansson 2005.
| Methods | RCT stated Randomisation technique not stated Duration of study: 2 years | |
| Participants | No selection criteria stated; selection resulted in knee arthrosis grade II to IV
Sweden: 42 participants (52 knees) Fixed: n = 27, 14 female, age 75 (64‐86) years Mobile: n = 25, 12 female, age 74 (60‐85) years |
|
| Interventions | Fixed: Niffield (Corin Medical) Mobile: Rotaglide, meniscal bearing (Corin Medical) Patellar resurfacing unclear Cementing unclear | |
| Outcomes | RSA, ROM, alignment, HSS RSA: postoperative at 6 weeks and at 3 months, 6 months, 1 year and 2 years. Clinical scores: preoperative and at 1 and 2 years Average and range scores given | |
| Notes | Study supported by Lund University and Corin Medical Ltd No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rate given and acceptable; not clear whether intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Unclear whether there was homogeneity in participant groups; co‐interventions described in sufficient detail |
Hanush 2010.
| Methods | RCT stated Randomisation based in part on minimisation technique, in part on schedule Duration of study: 13.4 months |
|
| Participants | Inclusion: patients with osteoarthritis, suitable for fixed bearing and mobile bearing Exclusion: patients with rheumatoid arthritis and those undergoing revision arthroplasty, requiring tibial component augmentation or a constrained prosthesis United Kingdom: 105 participants Fixed: n = 55, female 22, age 69.4 (± 7.9) years Mobile: n = 50, female 30, age 70 (± 8.4) years |
|
| Interventions | Fixed: PFC Sigma fixed bearing (DePuy) Mobile: PFC Sigma, rotating platform (DePuy) Routine patellar unresurfacing All components cemented |
|
| Outcomes | Flexion, extension, ROM, KSS pain (KSS), function (KSS); OKS pain (OKS), function (OKS); revision, osteolysis Assessments: preoperative and at 1‐year follow‐up Average and standard deviation given |
|
| Notes | Funded by DePuy International Study authors reported no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation: in part minimisation technique, in part schedule |
| Allocation concealment (selection bias) | Low risk | Closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given and acceptable; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions described in sufficient detail |
Henricson 2006.
| Methods | RCT stated Randomisation based on sealed envelopes opened during surgery Duration of study: 2 years | |
| Participants | Inclusion: primary gonarthrosis grade III‐IV; age between 60 and 85 years; body weight < 120 kg; no gonarthrosis secondary to arthritis or trauma; no previous knee surgery
Sweden: 47 participants (52 knees) Fixed: n = 26, 14 female, age 72 (62‐83) years Mobile: n = 26, 16 female, age 72 (62‐84) years |
|
| Interventions | Fixed: NexGen (Zimmer)
Mobile: MBK, meniscal bearing (Zimmer) Some participants with patellar component All components cemented |
|
| Outcomes | RSA, KSS, HSS Assessments: preoperative and at 3, 12 and 24 months Average, range or 95% CI given | |
| Notes | One of the study authors received funding from Zimmer Scandinavica | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes opened during operation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given and acceptable; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Higuchi 2009.
| Methods | RCT stated Randomisation based on computer‐generated random numbers Duration of study: 4 years |
|
| Participants | Inclusion: patients with osteoarthritis of the knee Exclusion: rheumatoid arthritis Japan: 68 participants (76 knees) 19 men and 49 women, age 68.4 (56‐81) years |
|
| Interventions | Fixed: PFC (DePuy) Mobile: PFC Sigma, rotating platform (DePuy) Treatment of patella unclear Cementing unclear Flexion space with knee balancer |
|
| Outcomes | Flexion, extension Assessments: preoperative and at 12 months and 48 months Average and standard deviation given |
|
| Notes | Funding not stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of withdrawals and dropouts; not clear whether intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; unsure whether co‐interventions are described in sufficient detail |
Jacobs 2011.
| Methods | RCT, multi‐centre Computer‐generated block‐stratified randomisation Duration of the study: 1 year |
|
| Participants | Inclusion: patient diagnosed with osteoarthritis; candidate for primary TKA; expected to undergo only 1 arthroplasty procedure within next 12 months; 60–75 years old; preoperative alignment (varus or valgus) < 10 °; BMI < 30; lives independently Exclusion: missing/insufficient PCL The Netherlands/Switzerland: 92 participants Fixed: n = 46, 32 female, age 67.6 (± 4.4) years Mobile: n = 46, 33 female, age 66.7 (± 4.6) years |
|
| Interventions | Fixed: balanSysTM type (Mathys Medical Ltd) Mobile: balanSysTM type (Mathys Medical Ltd) No patellar resurfacing Tibia and femur components cemented |
|
| Outcomes | Active flexion, KSS function, KSS clinical Assessments: preoperative and at 3 months, 6 months and 12 months Average and standard deviation given |
|
| Notes | Funded by Mathys Medical Ltd No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block‐stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding attempted at any of the assessments |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 centre with 30 participants was excluded from analysis because of randomisation error; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Protocol available and prespecified outcomes reported |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Kim 2001.
| Methods | RCT stated Randomisation technique not stated Duration of study: 7.4 years | |
| Participants | Inclusion: all patients with bilateral simultaneous TKA
No exclusion criteria; PCL status not considered, could be retained in all cases
Korea: 116 participants (232 knees) 80 female, 36 male, 110 OA, 6 RA, age 65 (33‐70) years |
|
| Interventions | Fixed: AMK (DePuy)
Mobile: LCS, meniscal bearing (DePuy) Routine patellar resurfacing All components cemented |
|
| Outcomes | KSS, HSS, VAS for severity, location and frequency of pain, functional benchmarks, overall well‐being and satisfaction, survival, radiolucency Short‐ (yearly) and long‐term (> 6 years) follow‐up stated, but only final follow‐up results given Only point estimates given; not specified for indication groups | |
| Notes | Funding not stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given and acceptable; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions described in sufficient detail |
Kim 2007.
| Methods | RCT stated Randomisation based on sequential pool derived from a table of randomised numbers Duration of study: 5.6 years | |
| Participants | Inclusion and exclusion criteria not described
Selection yielded bilateral procedures on 173 patients with osteoarthritis and on 1 patient with rheumatoid arthritis
Korea: 174 patients (348 knees) 112 female, 62 male, age 67 (45‐85) years |
|
| Interventions | Fixed: PFC Sigma (DePuy)
Mobile: PFC Sigma, rotating platform (DePuy)
Routine patellar resurfacing
All components cemented Flexion space with bone resection |
|
| Outcomes | KSS, HSS, alignment, component positions, radiolucent lines, lateral patellar tilt Only final, long‐term outcome (5.6 years) given Point estimates and ranges given | |
| Notes | No benefits received from any commercial party No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential pool based on a table of randomised numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given and acceptable; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Kim 2009a.
| Methods | RCT stated Randomisation technique not stated Duration of study: 2.6 years | |
| Participants | Inclusion: bilateral cases with degenerative osteoarthritis with prior non‐operative therapy
Exclusion: rheumatoid arthritis, septic arthritis history Korea: 92 participants (184 knees) 85 female, 7 male, age 69.5 (± 7.92) years |
|
| Interventions | Fixed: Medial Pivot (Wright Medical) Mobile: PFC Sigma, rotating platform (DePuy) Routine patellar resurfacing All components cemented Flexion space with various bone referenced techniques | |
| Outcomes | KSS, HSS, range of motion, satisfaction Only final follow‐up (2.6 years) given Point estimates and ranges given | |
| Notes | No commercial association of any of the study authors Study authors reported no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given and acceptable; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Kim 2009b.
| Methods | RCT stated Randomisation based on sequential pool derived from a table of randomised numbers Duration of study: 10‐12 years |
|
| Participants | Inclusion: patients younger than 55 requiring bilateral TKA Exclusion: criteria not mentioned Korea: 61 participants (122 knees) 45 female, 16 male, age 48.3 (34‐55) years |
|
| Interventions | Fixed: AMK (DePuy) Mobile: LCS, meniscal bearing (DePuy) Routine patellar resurfacing All components cemented |
|
| Outcomes | KSS total, KSS functional, KSS pain, ROM, HSS total, HSS pain, alignment, radiolucent lines Assessments preoperative and at final follow‐up 10 to 12 years postoperative Average given |
|
| Notes | No benefits or funds received in support of the study No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential pool based on a table of randomised numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observer blinded for radiographic findings |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Kim 2010.
| Methods | RCT stated Randomisation performed using a randomisation table Duration of study: 2 years |
|
| Participants | Inclusion: osteoarthritic patients scheduled for bilateral TKA with flexion contracture < 15 °; mechanical tibial femoral angle < 20 °; intraoperative intact PCL Korea: 66 participants (132 knees) Fixed: n = 33 CR, 33 PS Mobile: n = 66, 64 female, age 70 (55‐79) years |
|
| Interventions | Fixed: Genesis II (Smith and Nephew) Mobile: e.motion, meniscal bearing (BBraun‐Aesculap) All patellae resurfaced All components cemented |
|
| Outcomes | Flexion, extension, KKS pain, KKS knee, KKS function, WOMAC stiffness, WOMAC pain, WOMAC function, preferred knee Assessments preoperative and at 6 months, 12 months and 24 months Average and standard deviation given |
|
| Notes | No funding stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions described in sufficient detail |
Lampe 2011.
| Methods | RCT stated Randomisation technique not stated Duration of study: 1 year |
|
| Participants | Inclusion: osteoarthritic patients (40‐90) with failed non‐operative treatment, no previous ipsilateral bone or joint surgery, no deformity > 20° varus or 15° valgus, no option for osteotomy or unicompartmental implant Germany: 96 participants (100 knees) Fixed: n = 52, 39 female, age 69 (± 8) years Mobile: n = 48, 34 female, age 70 (± 7) years |
|
| Interventions | Fixed: Columbus (BBraun Aesculap) Mobile bearing: Rotating Platform (BBraun Aesculap) No patella resurfaced All components cemented |
|
| Outcomes | KSS knee, KSS function, KSS pain, flexion, Oxford, radiographic alignment Assessments preoperative and at 3 months, 6 months and 12 months Average, standard deviation and range given |
|
| Notes | Study was funded by BBraun Aesculap No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observer blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given and acceptable; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Protocol available and prespecified outcomes reported |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions described in sufficient detail |
Lizaur‐Utrilla 2012.
| Methods | RCT Randomisation based on computer‐generated random numbers table Duration of the study: 2.5 years |
|
| Participants | Inclusion: osteoarthritic patients with primary TKA, aged 70 years or older, without prior infection in the knee and with severe angular deformity or severe instability that required grafting, modular augmentation or a constrained design Spain: 119 participants Fixed: n = 58, 47 female, age 73.9 (± 3.2) years Mobile: n = 61, 47 female, age 74.6 (± 3.3) years |
|
| Interventions | Fixed: Trekking MB (Samo) Mobile: Multigen Plus FB (Lima) Patella resurfaced if there was degeneration Cementless femoral component design and a cemented tibial component |
|
| Outcomes | Maximum knee flexion assessments preoperative and at 3 months, 6 months, 12 months and 24 months KSS function, WOMAC, SF‐12, VAS, radiolucent lines assessments preoperative and at 3 months, 6 months and 12 months, and yearly thereafter, but only final follow‐up results given Average, standard deviation and range given |
|
| Notes | No funding stated Study authors reported no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables |
| Allocation concealment (selection bias) | Low risk | Office staff |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Munro 2010.
| Methods | RCT Randomisation based on computer‐generated sequence with sealed envelopes Duration of study: 2 years |
|
| Participants | Inclusion: patients with degenerative knee disease undergoing TKA Exclusion: severe deformity (requiring femoral or tibial augment), inflammatory arthritis, younger than 45 years or older than 85 years, refusal of consent, previous failed TKA or unicompartmental arthroplasty, previous high tibial osteotomy, TKA of the contralateral knee New Zealand: 41 participants (48 knees) Fixed: n = 23, 10 female, age 67.7 (50‐79) years Mobile: n = 25, 11 female, age 67.2 (47‐83) years |
|
| Interventions | Fixed: PFC Sigma fixed‐bearing (DePuy) Mobile: PFC Sigma, rotating‐platform (DePuy) Patella: resurfacing at indication Cement for femoral and tibial components Flexion space with ligament balancing tool |
|
| Outcomes | SF‐12 mental, SF‐12 physical, KSS clinical, KSS function, WOMAC total, ROM, OKS, VAS pain, revisions, cancellous bone mineral density change, cortical bone mineral density change Assessments: preoperative and at 6 weeks, 12 months and 24 months Average and range given |
|
| Notes | Study was partially funded by DePuy International No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor for clinical evaluations blinded to implant type |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions described in sufficient detail |
Möckel 2004.
| Methods | RCT stated Randomisation technique not stated Duration of study: 6 months | |
| Participants | Inclusion criteria: PCL sufficient
Exclusion criteria: other existing implants in lower extremities, factors influencing gait analysis, BMI > 35
Germany: 53 participants 45 female, 17 male, mean age 69 years |
|
| Interventions | Fixed: Natural Knee (Centerpulse) or Maxim (Biomet Merck) Mobile: PFC Sigma, rotating platform (DePuy) No patellar resurfacing All components cemented | |
| Outcomes | ROM, KSS, gait analysis, alignment
3 months and 6 months follow‐up given Average and some range given |
|
| Notes | No funding stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate given: > 20% lost at 6 months; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Price 2003.
| Methods | RCT, multi‐centre Randomisation based on computer‐generated randomisation to side of prosthesis Duration of study: 1 year | |
| Participants | Inclusion: osteoarthritis, bilateral procedures
Exclusion: no previous patellectomy or high tibial osteotomy, PCL status not clear as authors state AGC can be used in both sacrificing and retaining procedures; status of the PCL could not be identified. Study authors mention that the PCL is usually retained
United Kingdom and Australia: n = 40 (80 knees) 24 female, age 73.1 (54.8‐86.4) years |
|
| Interventions | Fixed: AGC (Biomet Merck)
Mobile: TMK, meniscal bearing (Biomet Merck)
No routine arthroplasty of patella Cementing unclear |
|
| Outcomes | KSS, KSS pain subscore, Oxford score, Oxford pain sub score, ROM
Only short‐term (1‐year) outcome Average and standard deviation given |
|
| Notes | 1 or more study authors have received benefits; benefits have been directed at affiliated non‐profit party | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation to side of prosthesis |
| Allocation concealment (selection bias) | Low risk | Telephone call |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded to implant type |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Some assessors potentially unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Homogeneity in participant groups on prognostic factors; co‐interventions described in sufficient detail |
Tibesku 2011.
| Methods | RCT stated Randomisation based on computer‐generated list Duration of study: 2 years |
|
| Participants | Inclusion: 50‐80 years, unilateral primary osteoarthritis, absence of mediolateral instability, deviation of the long leg axis of less than 10° Exclusion: any co‐morbidity that negatively influenced gait Germany: 33 participants Fixed: n = 17, 12 female, age 66 (± 10) years Mobile: n = 16, 9 female, age 65 (± 9) years |
|
| Interventions | Fixed: Genesis II (Smith and Nephew) Mobile: Genesis II, meniscal bearing (Smith and Nephew) No patellar resurfacing Cementing unclear |
|
| Outcomes | Flexion, KSS, HSS, SF‐36, Tegner, UCLA, VAS pain, gait analysis Assessments preoperative and at final follow‐up 24 months postoperative Average and standard deviation given |
|
| Notes | No funding stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participant blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observer blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No description of withdrawals and dropouts; no intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; co‐interventions not described in sufficient detail |
Watanabe 2005.
| Methods | RCT stated Randomisation technique not stated Duration of study: 98 months | |
| Participants | Selection criteria not described
Selection resulted in bilateral procedures in 18 patients with rheumatoid arthritis and 4 with osteoarthritis
Japan: 22 participants (44 knees) 21 female, age 59.6 (35‐78) years |
|
| Interventions | Fixed: NexGen CR (Zimmer) Mobile: Rotaglide, meniscal bearing (Corin) Patellar resurfacing in all knees 20 of 22 knees fully cemented, 2 hybrid | |
| Outcomes | KSS, flexion, extension, femorotibial angle, radiolucent lines
Only final follow‐up (98.6/96.2 months) results given Average and range given |
|
| Notes | No funding stated No declarations of interest reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised allocation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participant blinding not described; surgeon not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessor blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rate given and acceptable; intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | No homogeneity in participant groups on prognostic factors; no subgroups given that are homogeneous; unsure whether co‐interventions are described in sufficient detail |
AGC = Anatomic Graduated Components. BMI = Body mass index. CCT = Controlled clinical trial. CI = Confidence interval. CR = Cruciate retaining. HSS = Hospital Special Surgery score. KSS = Knee Society Score. OA = Osteoarthritis. OKS = Oxford Knee Score. PCL = Posterior cruciate ligament. preop = Preoperative. PS = Posterior stabilised. RA = Rheumatoid arthritis. RCT = Randomised clinical trial. ROM = Range of motion. RSA = Roentgen Stereophotogrammetric Analysis. SD = Standard deviation. SF‐12 = Short Form 12. TKA = Total knee arthroplasty. UCLA = University of California, Los Angeles scores WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aglietti 2005 | Fixed bearing type had a posterior stabilised design |
| Ball 2011 | Posterior stabilised implants |
| Bhan 2005 | Posterior stabilised implants |
| Breeman 2013 | Treatment of PCL dependents of individual surgeon’s preference (updated KAT trial) |
| Breugem 2008 | Posterior stabilised implants |
| Chen 2013 | Cruciate ligaments excised |
| Chiu 2001 | Fixed bearing type had a posterior stabilised design |
| Gioe 2009 | Posterior stabilised implants |
| Harrington 2009 | Posterior stabilised and cruciate retaining implants used |
| Jawed 2012 | Posterior cruciate ligament sacrificed in all cases |
| Jolles 2012 | Posterior stabilised implants |
| KAT trial group 2009 | Treatment of PCL dependents of individual surgeon’s preference |
| Kim 2007b | LCS stated as designed for implantation with resection of the PCL |
| Kim 2012 | Posterior stabilised implants |
| Kim 2012b | LCS stated as designed for implantation with resection of the PCL |
| Li 2008 | Posterior stabilised implants |
| Läderman 2008 | Posterior stabilised implants |
| Matsuda 2010 | Posterior stabilised implants |
| McGonagle 2012 | Treatment of PCL dependents of individual surgeon’s preference |
| Munoz 2008 | Posterior stabilised implants |
| NCT00289094 | Included also other inflammatory arthritis and avascular necrosis of bone |
| Pagnano 2004 | Posterior stabilised implants |
| Pijls 2012 | Posterior stabilised implants |
| Rahman 2010 | Posterior stabilised implants |
| Saari 2003 | Treatment of PCL dependents of individual surgeon’s preference |
| Shemshaki 2012 | Posterior stabilised implants |
| Tienboon 2012 | Posterior stabilised implants |
| Uvehammer 2007 | Treatment of PCL dependents of individual surgeon’s preference |
| Vasdev 2009 | Posterior stabilised implants |
| Wohlrab 2009 | Posterior stabilised implants |
| Woolson 2004 | Posterior stabilised implants |
| Woolson 2011 | Posterior stabilised implants |
| Wylde 2008 | Mixture of patients who had had the posterior cruciate sacrificed and retained |
| Zeng 2011 | Posterior stabilised implants |
Characteristics of ongoing studies [ordered by study ID]
NCT00740376.
| Trial name or title | Uniglide™ Mobile Bearing Unicondylar Knee System vs the Uniglide™ Fixed Bearing Unicondylar Knee System (MBK) |
| Methods | Randomised controlled trials |
| Participants | Patients with osteoarthritis, skeletally mature, need to obtain pain relief and improved function, moderate or severe pain with walking or at rest on the Hospital for Special Surgery score, preoperative medial tibiofemoral joint space narrowing on x‐rays (Kellgren Lawrence grade 3 or 4), preoperative Hospital for Special Surgery Knee Evaluation total score < 69, preoperative arc of motion > 90° in the affected knee |
| Interventions | Mobile: Uniglide Mobile Bearing Unicondylar Knee System (MBK) Fixed: Uniglide Fixed Bearing Unicondylar Knee System (FBK) |
| Outcomes | Primary outcome measures: composite clinical success (CCS), HSS success (month 24), HSS ≥ 70 (if preop HSS between 60 and 69), HSS > 70 plus a minimum 10‐point improvement No radiographic failure (month 24): no radiolucent lines > 1 mm in greater (time frame: month 24 postoperative) |
| Starting date | 2008 |
| Contact information | Study Director: K. Trier |
| Notes | January 2014 (final data collection date for primary outcome measure) Funded by Corin |
Differences between protocol and review
We changed the major outcome parameter from range of motion (ROM) to:
knee pain;
clinical and functional questionnaire scores;
health‐related quality of life;
revision surgery;
mortality;
reoperation rate; and
other serious adverse events
Furthermore, we have now used GRADE to assess the quality of the evidence, as well as the 'Risk of bias' tool.
Contributions of authors
W Jacobs, M van Hooff: writing the protocol. SN Hofstede, KA Nouta, W Jacobs, BG Pijls, RGHH Nelissen, P Marang‐van de Mheen: selecting studies, extracting data, writing the manuscript. SN Hofstede, BG Pijls, P Marang‐van de Mheen: statistics. AAB Wymenga, RGHH Nelissen: providing clinical interpretation of the results.
Sources of support
Internal sources
Sint Maartenskliniek, Netherlands.
Leiden University Medical Center, Netherlands.
External sources
Dutch Arthritis Association (project number: LLP13; 08‐1‐300), Netherlands.
Declarations of interest
The Department of Research, Development and Education, Sint Maartenskliniek, Nijmegen, Netherlands (affiliation of MH and AW, and former affiliation of WJ) received financial support from Mathys Medical Ltd, Bettlach, Switzerland, in advance for performing a literature review in the setup of a randomised controlled trial on the bearing aspect of two of the company's knee prosthesis types. Mathys did not take part in the design or conduct of the study; in collection, management, analysis or interpretation of data; nor in preparation, review or approval of the manuscript. Furthermore, WJ was the first author of one of the included studies, and AW was co‐author.
No funding was obtained to carry out the present review.
All review authors declare that they have no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bailey 2014 {published data only}
- Bailey O, Fergusson K, Crawfurd E, James P, May PA, Brown S, et al. No clinical difference between fixed‐ and mobile‐bearing cruciate retaining total knee arthroplasty: a prospective randomized study. Knee Surgery Sports Traumatology Arthroscopy 2014;Epub. [DOI] [PubMed] [Google Scholar]
Grodzki 2001 {published data only}
- Grodzki T, Haak H, Behrendt R, Merk H, Krauspe R. A prospective, randomized, comparative study on the early functional results of total knee arthroplasty systems: rotating tibia component versus fixed tibial PE‐inlay. [Prospektiv randomisierte vergleichsstudie frühfunktioneller ergebnisse zweier kniegelenksendoprothesensysteme rotationsplateau versu fixiertes polyethyleninlay]. Zeitschrift für Orthopädie 2001;139:393‐6. [DOI] [PubMed] [Google Scholar]
Hansson 2005 {published data only}
- Hansson U, Toksvig‐Larsen S, Jorn LP, Ryd L. Mobile vs. fixed meniscal bearing in total knee replacement: a randomised radiostereometric study. The Knee 2005;12(6):414‐8. [DOI] [PubMed] [Google Scholar]
Hanush 2010 {published data only}
- Hanush B, Lou TN, Warriner G, Hui A, Gregg P. Functional outcome of PFC Sigma fixed and rotating‐platform total knee arthroplasty. A prospective randomised controlled trial. International Orthopaedics (SICOT) 2010;34:349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henricson 2006 {published data only}
- Henricson A, Dalen T, Nilsson KG. Mobile bearings do not improve fixation in cemented total knee arthroplasty. Clinical Orthopaedics and Related Research 2006;448:114‐21. [DOI] [PubMed] [Google Scholar]
Higuchi 2009 {published data only}
- Higuchi H, Hatayama K, Shimizu M, Kobayashi A, Kobayashi T, Takagishi K. Relationship between joint gap difference and range of motion in total knee arthroplasty: a prospective randomised study between different platforms. International Orthopaedics (SICOT) 2009;33:997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 2011 {published data only}
- Jacobs WCH, Christen B, Wymenga AB, Schuster A, Schaaf DB, Ham A, et al. Functional performance of mobile versus fixed bearing total knee prostheses: a randomised controlled trial. Knee Surgery, Sports Traumatology, Arthroscopy 2012;20:1450‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2001 {published data only}
- Kim YH, Kook HK, Kim JS. Comparison of fixed‐bearing and mobile‐bearing total knee arthroplasties. Clinical Orthopaedics and Related Research 2001;392:101‐15. [DOI] [PubMed] [Google Scholar]
Kim 2007 {published data only}
- Kim YH, Kim DY, Kim JS. Simultaneous mobile‐ and fixed‐bearing total knee replacement in the same patients. A prospective comparison of mid‐term outcomes using a similar design of prosthesis. Journal of Bone and Joint Surgery (Brittish Volume) 2007;89(7):904‐10. [DOI] [PubMed] [Google Scholar]
Kim 2009a {published data only}
- Kim YH, Yoon SH, Kim JS. Early outcome of TKA with a medial pivot fixed‐bearing prosthesis is worse than with a PFC mobile‐bearing prosthesis. Clinical Orthopaedics and Related Research 2009;467:493‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2009b {published data only}
- Kim YH, Kim JS. Prevalence of osteolysis after simultaneous bilateral fixed‐ and mobile‐bearing total knee arthroplasties in young patients. The Journal of Arthroplasty 2009;24:932‐40. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Early clinical outcomes of floating platform mobile‐bearing TKA: longitudinal comparison with fixed‐bearing TKA. Knee Surgery, Sports Traumatology, Arthroscopy 2010;18:879‐88. [DOI] [PubMed] [Google Scholar]
Lampe 2011 {published data only}
- Lampe F, Sufi‐Siavach A, Bohlen KE, Hille E, Dries SPM. One year after navigated total knee replacement, no clinically relevant difference found between fixed bearing and mobile bearing knee replacement in a double‐blind randomized controlled trial. The Open Orthopaedics Journal 2011;5:201‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lizaur‐Utrilla 2012 {published data only}
- Lizaur‐Utrilla A, Sanz‐Reig J, Trigueros‐Rentero MA. Greater satisfaction in older patients with a mobile‐bearing compared with fixed‐bearing total knee arthroplasty. The Journal of Arthroplasty 2012;27(2):207‐11. [DOI] [PubMed] [Google Scholar]
Möckel 2004 {published data only}
- Möckel G, Perka C, Gabler J, Zippel H. Early postoperative functional differences between total knee arthroplasties supplied with mobile‐bearing platform or fixed‐bearing system ‐ an analysis of gait pattern [Fruhfunktionelle postoperative Unterschiede zwischen Knieendoprothesen mit rotierendem und festem Gleitlager ‐ eine ganganalytische Studie]. Zeitschrift fur Orthopaedie und Ihre Grenzgebiete 2004;142(1):40‐5. [DOI] [PubMed] [Google Scholar]
Munro 2010 {published data only}
- Munro JT, Pandit S, Walker CG, Clatworthy M, Pitto RP. Loss of tibial bone density in patients with rotating‐ or fixed‐platform TKA. Clinical Orthopaedics and Related Research 2010;468:775‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Price 2003 {published data only}
- Beard DJ, Pandit H, Price AJ, Butler‐Manuel PA, Dodd CA, Murray DW, et al. Introduction of a new mobile‐bearing total knee prosthesis: minimum three year follow‐up of an RCT comparing it with a fixed‐bearing device. The Knee 2007;14(6):448‐51. [DOI] [PubMed] [Google Scholar]
- Price AJ, Rees JL, Beard D, Juszczak E, Carter S, White S, et al. A mobile‐bearing total knee prosthesis compared with a fixed‐bearing prosthesis; a multicentre single blind randomised controlled trial. Journal of Bone and Joint Surgery (British Volume) 2003;85‐B(1):62‐7. [DOI] [PubMed] [Google Scholar]
- Rees JL, Beard DJ, Price AJ, Gill HS, McLardy‐Smith, Dodd CAF, et al. Real in vivo kinematic differences between mobile‐bearing and fixed‐bearing total knee arthroplasties. Clinical Orthopaedics and Related Research 2005;432:204‐9. [DOI] [PubMed] [Google Scholar]
Tibesku 2011 {published data only}
- Tibesku CO, Daniilidis K, Skwara A, Dierkes T, Rosenbaum D, Fuchs‐Winkelmann S. Gait analysis and electromyography in fixed‐ and mobile‐bearing total knee replacement: a prospective, comparative study. Knee Surgery, Sports Traumatology, Arthroscopy 2011;5:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Tibesku CO, Daniilidis K, Vieth V, Skwara A, Heindel W, Fuchs‐Winkelmann S. Sagittal plane kinematics of fixed‐ and mobile‐bearing total knee replacements. Knee Surgery, Sports Traumatology, Arthroscopy 2011;19:1488‐95. [DOI] [PubMed] [Google Scholar]
Watanabe 2005 {published data only}
- Watanabe T, Tomita T, Fujii M, Hashimoto J, Sugamoto K, Yoshikawa H. Comparison between mobile‐bearing and fixed‐bearing knees in bilateral total knee replacements. International Orthopaedics (SICOT) 2005;29(3):179‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aglietti 2005 {published data only}
- Aglietti P, Baldini A, Buzzi R, Lup D, Luca L. Comparison of mobile‐bearing and fixed‐bearing total knee arthroplasty: a prospective randomized study. Journal of Arthroplasty 2005;20:145‐53. [DOI] [PubMed] [Google Scholar]
Ball 2011 {published data only}
- Ball ST, Sanchez HB, Mahoney OM, Schmalzried TP. Fixed versus rotating platform total knee arthroplasty: a prospective, randomized, single‐blind study. The Journal of Arthroplasty 2011;26(4):531‐6. [DOI] [PubMed] [Google Scholar]
Bhan 2005 {published data only}
- Bhan S, Malhotra R, Kiran EK, Shukla S, Bijjawara M. A comparison of fixed‐bearing and mobile‐bearing total knee arthroplasty at a minimum follow‐up of 4.5 years. Journal of Bone and Joint Surgery (American Volume) 2005;87(10):2290‐6. [DOI] [PubMed] [Google Scholar]
Breeman 2013 {published data only}
- Breeman S, Campbell MK, Dakin H, Fiddian N, Fitzpatrick R, Grant A, et al. KAT Trial Group. Five‐year results of a randomised controlled trial comparing mobile and fixed bearings in total knee replacement. The Bone and Joint Journal 2013;95(B(4)):486‐92. [DOI] [PubMed] [Google Scholar]
Breugem 2008 {published data only}
- Breugem SJM, Sierevelt IN, Schafroth MU, Blankevoort L, Schaap GR, Dijk van CN. Less anterior knee pain with a mobile‐bearing prosthesis compared with a fixed‐bearing prosthesis. Clinical Orthopaedics and Related Research 2008;466:1959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen LB, Tan Y, Al‐Aidaros M, Wang H, Wang X, Cai SH. Comparison of functional performance after total knee arthroplasty using rotating platform and fixed‐bearing prostheses with or without patellar resurfacing. Orthopaedic Surgery 2013;5(1):112‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiu 2001 {published data only}
- Chiu KY, Ng TP, Tang WM, Lam P. Bilateral total knee arthroplasty: one mobile‐bearing and one fixed‐bearing. Journal of Orthopaedic Surgery 2001;9(1):45‐50. [DOI] [PubMed] [Google Scholar]
Gioe 2009 {published data only}
- Gioe TJ, Glynn J, Sembrano J, Suthers K, Santos ERG, Singh J. Mobile and fixed‐bearing (all‐polyethylene tibial component) total knee arthroplasty designs. A prospective randomized trial. Journal of Bone and Joint Surgery (American Volume) 2009;91:2104‐12. [DOI] [PubMed] [Google Scholar]
Harrington 2009 {published data only}
- Harrington MA, Hopkinson WJ, Hsu P, Manion L. Fixed‐ vs mobile‐bearing total knee arthroplasty. Does it make a difference? A prospective randomized study. The Journal of Arthroplasty 2009;24(6 Suppl 1):24‐7. [DOI] [PubMed] [Google Scholar]
Jawed 2012 {published data only}
- Jawed A, Kumar V, Malhotra R, Yadav CS, Bhan S. A comparative analysis between fixed bearing total knee arthroplasty (PFC Sigma) and rotating platform total knee arthroplasty (PFC‐RP) with minimum 3‐year follow‐up. Archives of Orthopaedic and Trauma Surgery 2012;132(6):875‐81. [DOI] [PubMed] [Google Scholar]
Jolles 2012 {published data only}
- Jolles BM, Grzesiak A, Eudier A, Dejnabadi H, Voracek C, Pichonnaz C, et al. A randomised controlled clinical trial and gait analysis of fixed‐ and mobile‐bearing total knee replacements with a five‐year follow‐up. The Bone and Joint Journal 2012;94(5):648‐55. [DOI] [PubMed] [Google Scholar]
KAT trial group 2009 {published data only}
- KAT Trial Group: Johnston L, MacLennan G, McCormack K, Ramsay C, Walker A. The Knee Arthroplasty Trial (KAT) design features, baseline characteristics, and two‐year functional outcomes after alternative approaches to knee replacement. Journal of Bone and Joint Surgery (American Volume) 2009;91:134‐41. [DOI] [PubMed] [Google Scholar]
Kim 2007b {published data only}
- Kim YH, Yoon SH, Kim JS. The long‐term results of simultaneous fixed‐bearing and mobile‐bearing total knee replacements performed in the same patient. Journal of Bone and Joint Surgery (British Volume) 2007;89(10):1317‐23. [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim D, Seong SC, Lee MC, Lee S. Comparison of the tibiofemoral rotational alignment after mobile and fixed bearing total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy 2012;20(2):337‐45. [DOI] [PubMed] [Google Scholar]
Kim 2012b {published data only}
- Kim YH, Kim JS, Choe JW, Kim HJ. Long‐term comparison of fixed‐bearing and mobile‐bearing total knee replacements in patients younger than fifty‐one years of age with osteoarthritis. Journal of Bone and Joint Surgery 2012;94(10):866‐73. [DOI] [PubMed] [Google Scholar]
Läderman 2008 {published data only}
- Läderman A, Saudan M, Riand N, Fritschy D. Fixed‐bearing versus mobile‐bearing total knee arthroplasty: a prospective randomised clinical and radiological study [Prothèse totale du genou: étude prospective randomisée comparant les plateaux tibiaux fixes et mobiles]. Revue de Chirurgie Orthopédique et Réparatrice de l'appareil Moteur 2008;94:247‐51. [DOI] [PubMed] [Google Scholar]
Li 2008 {published data only}
- Li Z‐J, Zhang K, Kim TK. Mobile‐ and fixed‐bearing total knee arthroplasty for knee osteoarthritis: comparisons of early clinical outcomes. Journal of Clinical Rehabilitative Tissue Engineering Research 2008;12(48):9589‐93. [Google Scholar]
Matsuda 2010 {published data only}
- Matsuda S, Mizu‐uchi H, Fukagawa S, Miura H, Okazaki K, Matsuda H, et al. Mobile‐bearing prosthesis did not improve mid‐term clinical results of total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy 2010;18:1311–6. [DOI] [PubMed] [Google Scholar]
McGonagle 2012 {published data only}
- McGonagle L, Bethell L, Byrne N, Bolton‐Maggs BG. The Rotaglide+ total knee replacement: a comparison of mobile versus fixed bearings. Knee Surgery, Sports Traumatology, Arthroscopy 2012;22(7):1626‐31. [DOI] [PubMed] [Google Scholar]
Munoz 2008 {published data only}
- Munoz AS, Herrero FA, Lozano RL, Linares FA. Comparison of mobile‐ and fixed‐bearing cemented total knee arthroplasty. Acta Orthopaedica Belgica 2008;74:801‐8. [PubMed] [Google Scholar]
NCT00289094 {unpublished data only}
Pagnano 2004 {published data only}
- Pagnano MW, Trousdale RT, Stuart MJ, Hanssen AD, Jacofsky D. Rotating platform knees did not improve patellar tracking. A prospective, randomized study of 240 primary total knee arthroplasties. Clinical Orthopaedics and Related Research 2004;428:221–7. [PubMed] [Google Scholar]
Pijls 2012 {published data only}
- Pijls BG, Valstar ER, Kaptein BL, Nelissen RG. Differences in long‐term fixation between mobile‐bearing and fixed‐bearing knee prostheses at ten to 12 years' follow‐up: a single‐blinded randomised controlled radiostereometric trial. The Bone and Joint Journal 2012;94(10):1366‐71. [DOI] [PubMed] [Google Scholar]
Rahman 2010 {published data only}
- Rahman WA, Garbuz DS, Masri BA. Randomized controlled trial of radiographic and patient‐assessed outcomes following fixed versus rotating platform total knee arthroplasty. The Journal of Arthroplasty 2010;25(8):1201‐7. [DOI] [PubMed] [Google Scholar]
Saari 2003 {published data only}
- Saari T, Uvehammer J, Carlsson LV, Herberts P, Regnér L, Kärrholm J. Kinematics of three variations of the Freeman‐Samuelson total knee prosthesis. Clinical Orthopaedics and Related Research 2003;410:235–47. [DOI] [PubMed] [Google Scholar]
Shemshaki 2012 {published data only}
- Shemshaki H, Dehghani M, Eshaghi MA, Esfahani MF. Fixed versus mobile weight‐bearing prosthesis in total knee arthroplasty. Knee Surgery, Sports Traumatology, Arthroscopy 2012;20(12):2519‐27. [DOI] [PubMed] [Google Scholar]
Tienboon 2012 {published data only}
- Tienboon P, Jaruwangsanti N, Laohasinnurak P. A prospective study comparing mobile‐bearing versus fixed‐bearing type in total knee arthroplasty using the free‐hand‐cutting technique. Journal of the Medical Association of Thailand 2012;95(Suppl 10):77‐86. [PubMed] [Google Scholar]
Uvehammer 2007 {published data only}
- Uvehammer J, Kärrholm J, Carlsson L. Influence of joint area design on tibial component migration. Comparison between fixed symmetrical, asymmetrical, and a moveable bearing. The Journal of Knee Surgery 2007;20(1):20‐6. [DOI] [PubMed] [Google Scholar]
Vasdev 2009 {published data only}
- Vasdev A, Kumar S, Chadha G, Mandal SP. Fixed‐ versus mobile‐bearing total knee arthroplasty in Indian patients. Journal of Orthopaedic Surgery 2009;17(2):179‐82. [DOI] [PubMed] [Google Scholar]
Wohlrab 2009 {published data only}
- Wohlrab D, Ditl J, Herrschelmann R, Schietsch U, Hein W, Hube R. Does the NexGen LPS flex mobile knee prosthesis offer advantages compared to the NexGen LPS? A comparison of clinical and radiological results. [Bietet das NexGen LPS Flex mobile‐knieprothesensystem vorteile gegenüber dem NexGen LPS? Ein vergleich klinischer und radiologischer ergebnisse.]. Archives of Orthopaedic and Trauma Surgery 2005;143(5):567‐72. [DOI] [PubMed] [Google Scholar]
Woolson 2004 {published data only}
- Woolson ST, Northrop GD. Mobile‐ vs. fixed‐bearing total knee arthroplasty. A clinical and radiologic study. The Journal of Arthroplasty 2004;19(2):135‐40. [DOI] [PubMed] [Google Scholar]
Woolson 2011 {published data only}
- Woolson ST, Epstein NJ, Huddleston JI. Long‐term comparison of mobile‐bearing vs fixed‐bearing total knee arthroplasty. Journal of Arthroplasty 2011;26(8):1219‐23. [DOI] [PubMed] [Google Scholar]
Wylde 2008 {published data only}
- Wylde V, Learmonth I, Potter A, Bettinson K, Lingard E. Patient‐reported outcomes after fixed‐ versus mobile‐bearing total knee replacement. A multicentre randomised controlled trial using the kinemax total knee replacement. Journal of Bone and Joint Surgery (British Volume) 2008;90‐B:1172‐9. [DOI] [PubMed] [Google Scholar]
Zeng 2011 {published data only}
- Zeng Y, Cao L, Liu Y, Peng GF, Peng LB, Yang DS, et al. Early clinical outcomes of fixed‐bearing versus mobile‐bearing total knee arthroplasty. Zhonghua yi Xue Za Zhi 2011;91(11):752‐6. [PubMed] [Google Scholar]
References to ongoing studies
NCT00740376 {unpublished data only}
- Uniglide™ Mobile Bearing Unicondylar Knee System vs the Uniglide™ Fixed Bearing Unicondylar Knee System (MBK). Ongoing study 2008.
Additional references
Apostolopoulos 2011
- Apostolopoulos AP, Michos IV, Mavrogenis AF, Chronopoulos E, Papachristou G, Lallos SN, et al. Fixed versus mobile bearing knee arthroplasty: a review of kinematics and results. Journal of Long‐Term Effects of Medical Implants 2011;21(3):197‐203. [DOI] [PubMed] [Google Scholar]
Bo 2013
- Bo Z, Liao LL, Zhao J, Wei Q, Ding X, Yang B. Mobile bearing or fixed bearing? A meta‐analysis of outcomes comparing mobile bearing and fixed bearing bilateral total knee replacements. The Knee 2013;13:192‐0. [DOI] [PubMed] [Google Scholar]
Bracht van der 2010
- Bracht van der H, Maele van G, Verdonk P, Almqvist KF, Verdonk R, Freeman M. Is there any superiority in the clinical outcome of mobile‐bearing knee prosthesis designs compared to fixed‐bearing total knee prosthesis designs in the treatment of osteoarthritis of the knee joint? A review of the literature. Knee Surgery, Sports Traumatology, Arthroscopy 2010;18:367‐74. [DOI] [PubMed] [Google Scholar]
Chatterji 2005
- Chatterji U, Lewis PL, Butcher C, Lekkas P. Comparison of the early results of fixed bearing and mobile bearing knee arthroplasties. The Journal of Bone and Joint Surgery (British Volume) 2005;87B:337. [Google Scholar]
Cheng 2013
- Cheng M, Chen D, Guo Y, Zhu C, Zhang X. Comparison of fixed‐ and mobile‐bearing total knee arthroplasty with a mean five‐year follow‐up: a meta‐analysis. Experimental and Therapeutic Medicine 2013;6(1):45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gioe 2011
- Gioe TJ, Sharma A, Tatman P, Mehle S. Do "premium" joint implants add value? Analysis of high cost joint implants in a community registry. Clinical Orthopaedics and Related Research 2011;469(1):48‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanush 2008
- Hanush BC, Patil S, Hui A, Gregg P. Randomized controlled trial comparing functional outcome for fixed and mobile bearing in total knee arthroplasty. The Journal of Bone and Joint Surgery 2008;90B:327. [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. [Google Scholar]
Jolles 2006
- Jolles BM, Aminian K, Dejnabadi H, Voracek C, Leyvraz PF. Ambulatory gait analysis results after total knee arthroplasty: arguments for mobile or fixed bearing?. The Journal of Bone and Joint Surgery (British Volume) 2006;88B:108. [Google Scholar]
Kim 2001a
- Kim Y, Kook H, Kim J. Comparison of fixed‐bearing and mobile‐bearing total knee arthroplasties. Clinical Orthopaedics & Related Research 2001;392:2‐329. [DOI] [PubMed] [Google Scholar]
Kurtz 2007
- Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. Journal of Bone and Joint Surgery (American Volume) 2007;89(4):780‐5. [DOI] [PubMed] [Google Scholar]
Labek 2011
- Labek G, Neumann D, Agreiter M, Schuh R, Bohler N. Impact of implant developers on published outcome and reproducibility of cohort‐based clinical studies in arthroplasty. Journal of Bone and Joint Surgery (British Volume) 2011;93 suppl 3:55‐61. [DOI] [PubMed] [Google Scholar]
Matsuda 1998
- Matsuda S, White SE, Williams VG, McCarthy DS, Whiteside LA. Contact stress analysis in meniscal bearing total knee arthroplasty. Journal of Arthroplasty 1998;13(6):699‐706. [DOI] [PubMed] [Google Scholar]
NCT00208286
- NCT00208286. P.F.C. Sigma Fixed versus P.F.C. RP Mobile Bearing Total Knee Systems. clinicaltrials.gov/ct2/show/NCT00208286 (accessed 24 February 2014).
NCT01150929
- NCT01150929. Rehabilitation after total knee arthroplasty ‐ Rotating platform versus fixed bearing polyethylene. clinicaltrials.gov/ct2/show/NCT01150929 (accessed 24 February 2014).
Nelissen 1992
- Nelissen RG, Brand R, Rozing PM. Survivorship analysis in total condylar knee arthroplasty. A statistical review. Journal of Bone and Joint Surgery (American Volume) 1992;74(3):383‐9. [PubMed] [Google Scholar]
Nilsson 2005
- Nilsson K, Dalen T, Henricson A. Fixed or mobile bearing in cemented total knee arthroplasty? A prospective and randomised study using RSA. Journal of Bone and Joint Surgery (British Volume) 2005;87B:337. [Google Scholar]
Oh 2009
- Oh KJ, Pandher DS, Lee SH, Sung Joon SD Jr, Lee ST. Meta‐analysis comparing outcomes of fixed‐bearing and mobile‐bearing prostheses in total knee arthroplasty. Journal of Arthroplasty 2009;24(6):873‐84. [DOI] [PubMed] [Google Scholar]
Pereira 2011
- Pereira D, Peleteiro B, Araujo J, Branco J, Santos RA, Ramos E. The effect of osteoarthritis definition on prevalence and incidence estimates: a systematic review. Osteoarthritis Cartilage 2011;19:1270‐85. [DOI] [PubMed] [Google Scholar]
Poss 2002
- Poss R, Clark CR, Heckman JD. A concise format for reporting the longer‐term follow‐up status of patients managed with total knee arthroplasty. Journal of Bone and Joint Surgery (American Volume) 2002;83:1779‐80. [DOI] [PubMed] [Google Scholar]
Post 2010
- Post ZD, Matar WY, Leur van de T, Grossman EL, Austin MS. Mobile‐bearing total knee arthroplasty. Better than a fixed‐bearing?. The Journal of Arthroplasty 2010;25(6):998‐1003. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith H, Jan M, Mahomed NN, Davey JR, Gandhi R. Meta‐analysis and systematic review of clinical outcomes comparing mobile bearing and fixed bearing total knee arthroplasty. Journal of Arthroplasty 2011;2:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
Suarez 2004
- Suarez SM, Murcia MA, Rodriguez LL, Cebal CG, Nuno MJ. Fixed conventional versus mobile bearing polyethylene in total knee arthroplasty. Journal of Bone and Joint Surgery 2004;86B:257‐8. [Google Scholar]
Szivek 1996
- Szivek JA, Anderson PL, Benjamin JB. Average and peak contact stress distribution evaluation of total knee arthroplasties. Journal of Arthroplasty 1996;11(8):952‐63. [DOI] [PubMed] [Google Scholar]
Tibesku 2006
- Tibesku CO, Diekres T, Skwara A, Rosenbaum D, Fuchs S. Gait analysis and electromyography in fixed and mobile bearing total knee replacement. A prospective randomized patient‐ and observer‐blinded clinical study. Journal of Bone and Joint Surgery (British Volume) 2006;29:71. [Google Scholar]
Tibesku 2009a
- Tibesku CO, Vieth V, Skwara A, Stuckmann V, Heindl W, Winkelmann S. Knee joint kinematics after fixed‐ and mobile‐bearing total knee replacement ‐ a fluoroscopic study of prospectively randomized patients. The Journal of Bone and Joint Surgery (British Volume) 2009;91B:71‐2. [Google Scholar]
van der Voort 2013
- Voort P, Pijls BG, Nouta KA, Valstar ER, Jacobs WCH, Nelissen RGHH. A systematic review and meta‐regression of mobile‐bearing versus fixed‐bearing total knee replacement in 41 studies. The Bone & Joint Journal 2013;95‐B:1209‐16. [DOI] [PubMed] [Google Scholar]
Wen 2011
- Wen Y, Liu D, Huang Y, Li B. A meta‐analysis of the fixed‐bearing and mobile‐bearing prostheses in total knee arthroplasty. Archives of Orthopaedic and Trauma Surgery 2011;131(10):1341‐50. [DOI] [PubMed] [Google Scholar]
World Health Organization 2013
- World Health Organization. Chronic diseases and health promotion: chronic rheumatic conditions. http://www.who.int/chp/topics/rheumatic/en/ (accessed 24 January 2014).
References to other published versions of this review
Jacobs 2004
- Jacobs W, Anderson P, Limbeek J, Wymenga A. Mobile bearing vs fixed bearing prostheses for total knee arthroplasty for post‐operative functional status in patients with osteoarthritis and rheumatoid arthritis. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003130.pub2] [DOI] [PubMed] [Google Scholar]


