Abstract
The risks of concomitant benzodiazepine (BZ) and opioid use are significant. Despite the urgent need to reduce BZ use among patients taking opioids, no treatment intervention research to our knowledge has addressed treatment for this concurrent, high-risk use. The current study will evaluate the efficacy of augmenting BZ taper procedures with CBT for anxiety disorders that has been adapted specifically for patients with concomitant BZ and opioid use (either use as prescribed or misuse), a high-risk patient population. Research combining rapidly scalable behavioral interventions ancillary to pharmacological approaches delivered via telehealth in primary care settings is innovative and important given concerning trends in rising prevalence of BZ/opioid co-prescription, BZ-associated overdose deaths, and known barriers to implementation of behavioral health interventions in primary care. CBT delivery using telehealth has the potential to aid adherence and promote access and dissemination of procedures in primary care. Lastly, the current study will utilize an experimental therapeutics approach to preliminarily explore the mechanism of action for the proposed interventions. The overall aim of the present pilot randomized controlled trial is to examine the feasibility and preliminary efficacy of a BZ taper with CBT for anxiety disorders adapted for patients with concomitant BZ (BZT+CBT) and opioid use to a BZ taper with a control health education program (BZT+HE) in a sample of individuals (N=54) who have been prescribed and are taking benzodiazepines and opioids for at least 3 months prior to baseline and experience anxious distress. Screening and outcome measures, methods, and implications are described.
Keywords: benzodiazepines, opioids, anxiety disorders, taper, cognitive behavioral therapy
Background
The opioid crisis has intensified concerns about benzodiazepine (BZ) use because: (1) anxiety disorders are prevalent among individuals with opioid use disorder (OUD) (Compton et al., 2007; Grant et al., 2004) and many patients with OUD receive concomitant BZ prescriptions (Hawkins et al., 2019; Mooney et al., 2022); (2) BZ use while taking opioids greatly increases risk of serious complications including overdose (Cho et al., 2020; Nielsen et al., 2007; Nielsen et al., 2015; McCane-Katz et al., 2010; Yeh et al., 2011) with a dose-response relationship between BZ daily dose and risk of death (Garg et al., 2017; Park et al., 2015); (3) there is a high level of co-prescribing BZs for anxiety and opioids for pain, and this co-use is associated with fatal overdose and all-cause mortality (Calcaterra et al., 2013; Hawkins et al., 2019; Mooney et al., 2022); and (4) overdose deaths involving BZs have been on the rise, with a 10-fold increase between 1999–2017 (Overdose Death Rates, 2020). Taken together, individuals who co-use BZs and opioids, either as prescribed or misused are a population at high risk of significant negative medical consequences and for the onset of OUD (Cragg et al., 2019), which brings with it additional negative consequences, including overdose risk (Taylor & Samet, 2022). Therefore, addressing this prevalent and alarming concomitant use can have a significant impact.
Although BZ-associated harms are amplified in combination with opioids and that BZ use is known to amplify opioid-related harms (Lembke et al., 2018; Limandri et al., 2018), clinical guidelines addressing treatment of problematic BZ use, particularly in relation to opioid use, are generally lacking. Clinical tools to provide guidance on BZ taper exist (e.g., The Royal Australian College of General Practitioners, 2023) but are not widely distributed and are primarily based on clinical input/consensus, rather than research. Further, there has been minimal research to inform clinical practice, and general healthcare settings may lack time and resources to address this need. This pilot randomized controlled trial (RCT) addresses this critical need by providing initial efficacy data on a treatment strategy for reducing BZ use in adults taking this medication in combination with opioids.
Prior Research on Strategies to Reduce Benzodiazepine Use
Cognitive behavioral therapy (CBT) that includes exposure to feared stimuli is a first-line treatment for anxiety disorders (Deacon & Abramowitz, 2004; Stewart & Chambless, 2009), and as supported by a series of studies, this treatment has been adapted to focus on the task of helping outpatients, particularly those with panic disorder, discontinue their BZs (Bruce et al., 1999; Otto et al., 1993; Otto et al., 2010). Through exposure-based CBT, patients can learn: (1) more adaptive ways of managing and responding to anxiety than using BZs (Otto et al., 2002); (2) to become less fearful of physiological sensations associated with BZ taper and withdrawal (Otto et al., 2002); and (3) to decrease catastrophic cognitions about physical sensations, including pain (Stewart & Asmundson, 2006). Exposure-based CBT has been shown to facilitate BZ tapering and enhance longer term outcomes in patients with panic disorder, where difficulties discontinuing BZs have been particularly challenging (Bruce et al., 1999; Otto et al., 1993; Otto et al., 2010).
Problems with Access to High Quality Interventions to Reduce Benzodiazepine Use
Interventions to reduce BZ use among patients with anxiety disorders who are prescribed opioids for pain (and thus are at risk of developing OUD or may have OUD) are urgently needed. Facilitating BZ taper with CBT for anxiety disorders may augment the efficacy of BZ tapering by helping patients learn strategies for responding adaptively to anxiety without the use of fast-acting medications to alleviate symptoms. Primary care represents a setting that treats a significant proportion of these at-risk patients. Notably, even patients without OUD prescribed both opioids and BZs are nearly four times as likely to die than those who receive only one of these prescriptions (Mooney et al., 2022).
Although efforts to embed mental health staff into primary care clinics have significantly increased over time (Roy-Byrne et al., 2010; Unützer et al., 2005), most primary care clinics are not equipped to provide high quality, evidence-based treatment for psychopathology, particularly behavioral interventions that typically include weekly sessions for 2–4 months (Dickinson, 2015; Kathol et al., 2010). Consequently, primary care physicians (PCPs) may struggle with following recommendations to taper BZs when they do not have the tools, appropriate staff, or scheduling flexibility to provide care for the underlying psychopathology that is being treated with BZs (Grazier et al., 2016). Telehealth offers a unique opportunity to utilize trained clinicians to deliver medication and behavioral health treatments, while partnering closely with PCPs to provide virtual “face-to-face” treatment to their patients, reducing the burden of travel and resource allocation in diverse clinical settings. Telehealth is an effective mode of treatment delivery for substance use and psychiatric disorders (Fortney et al., 2007; Frueh et al., 2007; Lin et al., 2019).
The Present Study
The current trial aims to shift clinical practice paradigms by establishing the feasibility, acceptability, and preliminary efficacy of an intervention that can be delivered by mental health professionals via telehealth, in collaboration with PCPs. This takes the burden of the treatment off the PCPs and creates a collaborative approach that reduces barriers to specialized mental health care for this understudied, undertreated, at-risk population. The intervention under investigation will include standardized BZ tapering facilitated by targeted CBT for anxiety and taper-related symptoms among patients with or at high risk of OUD who take BZs. The present study will utilize a two-arm RCT design examining the efficacy of a BZ taper + an 11-session CBT program (BZT+CBT) delivered via telehealth, compared to the BZ taper + a health education control (BZT+HE). This RCT is the first study to our knowledge to evaluate the efficacy of augmenting BZ taper procedures with CBT for anxiety disorders that has been adapted specifically for patients with concomitant BZ and opioid use.
Methods
Study Site
Participants will be recruited from large university-based primary care clinics. Clinical services will be provided remotely through telehealth visits by psychiatry and psychology study clinicians at the University of California, Los Angeles.
Study Design and Objectives
The overall aim of the pilot RCT is to examine the feasibility/acceptability and preliminary efficacy of a BZ taper with CBT for anxiety disorders adapted for patients with concomitant BZ (BZT+CBT) and opioid use to a BZ taper with a control health education program (BZT+HE). Although not powered to detect between-group differences on clinical outcomes, this study aims to evaluate a preliminary signal for an effect favoring BZT+CBT over BZT+ HE on our primary and secondary outcomes of interest (see below), and will lay the groundwork for a larger, more sufficiently powered efficacy trial. Figure 1 displays the RCT design. Study aims and hypotheses are:
Figure 1.
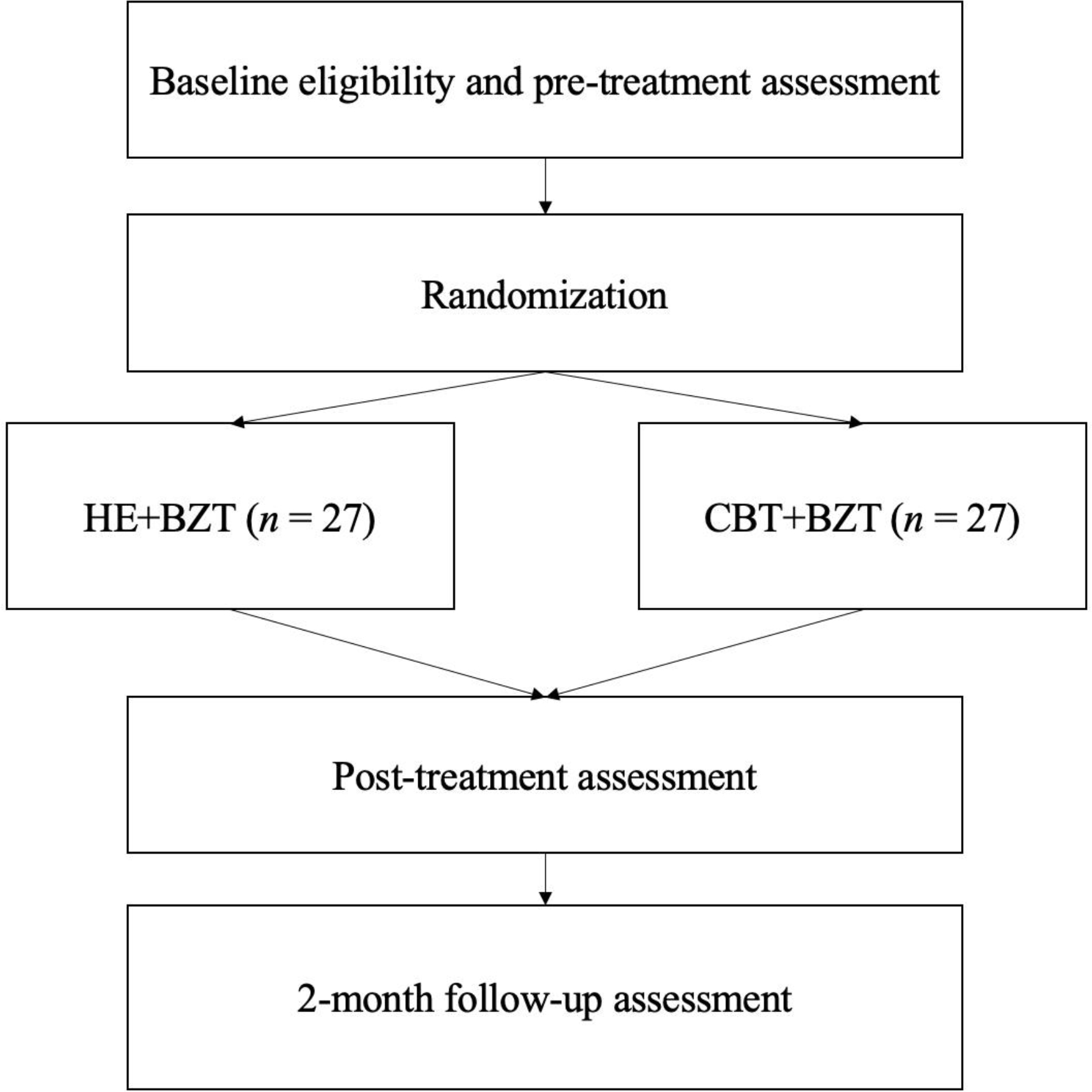
Pilot RCT Design
Evaluate patient and clinic provider acceptability of the interventions. We hypothesize that participants in BZT+CBT will show greater adherence (i.e., number of sessions attended, treatment completion) and satisfaction ratings than those in BZT+HE. We expect participants and prescribing physicians to report high satisfaction with telehealth delivery of the CBT+BZT protocol.
Determine whether participants assigned to BZT+CBT show greater reductions in BZ use compared to those in BZT+HE. We hypothesize that reductions in daily BZ dose will be greater in the BZT+CBT group compared to BZT+HE, as measured by self-report during and after the intervention and at follow-up assessment, and prescribed dose at 2-month follow-up. We hypothesize that a greater proportion of individuals in BZT+CBT will fully taper off BZs compared to BZT+HE, as measured by self-report and urine drug screen (UDS).
Examine whether participants in BZT+CBT show greater reductions in anxiety symptoms compared to BZT+HE. We hypothesize that anxiety symptom reduction will be greater in the BZT+CBT group versus the BZT+HE group, as measured by the Depression and Anxiety Severity Scale (DASS) at the 2-month follow-up.
Explore whether treatment effects extend to opioid use (prescription and non-prescription). Due to the focus on anxiety sensitivity in the CBT protocol, which is implicated in both anxiety disorders and pain conditions, we hypothesize that the BZT+CBT group versus the BZT+HE group may show less opioid use as measured by self-report and UDS during the intervention and at follow-up, and prescribed opioid dose by the final 2-month follow-up.
In line with an experimental medicine approach (Nielsen et al., 2018; Sumner et al., 2018), we seek to explore mechanisms of treatment effects, examining whether there is a signal indicating that anxiety sensitivity (Hearon et al., 2011; McHugh et al., 2017) and pain catastrophizing (Edwards et al., 2011; Morasco et al., 2013) mediate treatment outcomes. We hypothesize that anxiety sensitivity and pain catastrophizing (which we predict will decrease more in BZT+CBT compared to BZT+HE) will mediate treatment outcomes. The identification and validation of mechanistic targets can help hone treatments to more directly engage these mechanisms in order to improve efficacy.
Participants
The current study will recruit 54 patients (n=27 per condition) from university-based primary care clinics for an expected total of 40 completers. We anticipate that many patients will have pain-related distress and anxiety-related distress.
Participants are included who: (1) have been taking prescribed BZs for ≥3 months prior to baseline (as indicated in the medical record and corroborated by the patient) and have a positive UDS for BZs at baseline; (2) currently experience significant distress or impairment due to their anxiety symptoms (i.e., score ≥8 on the Overall Anxiety Severity and Impairment Scale (OASIS) during screening; Campbell-Sills et al., 2009); (3) have been prescribed opioids for ≥ 3 months for pain management (as indicated in the medical record and corroborated by the patient) and have a positive UDS for prescribed opioids at baseline; (4) are between 18–85 years old; (5) are fluent in English; (6) have access to a digital device with internet access for telehealth; and (7) are willing to reduce BZ use.
Exclusion criteria include: (1) pregnancy; (2) psychiatric symptoms requiring a higher level of care (i.e., severe suicidality, manic or psychotic symptoms not stabilized on medication); (3) presence of any substance use disorder other than OUD, sedative/hypnotic use disorder, or tobacco use disorder (evaluated by modules from a structured clinical diagnostic interview); (4) medical conditions that require ongoing treatment with BZs (e.g., certain seizure disorders); (5) use of drugs other than BZs and opioids in the past 30 days (as indicated by UDS and self-report via Timeline Followback), with the exception of intermittent cannabis use, i.e., more than 1x per week; (6) use of alcohol above at-risk drinking cutoffs per US Dietary Guidelines (Dietary Guidelines Advisory Committee, 2020); (7) marked cognitive impairment (i.e., MMSE <24; described below).
Study Assessments
See Table 1 for the assessment schedule. Clinician-administered assessments will be conducted via telehealth by assessors who are blind to condition through screening and baseline assessment. Assessors will be trained to 80% reliability in conducting the diagnostic interview (First et al., 2016) and will be supervised via audio recording by a psychologist. Reliability will be determined by having a fully trained assessor and the trainee assessor independently score three of the same diagnostic interviews. The trainee will need to match the diagnoses given by the fully trained assessor with ≥ 80% inter-rater reliability prior to conducting independent interviews. Random spot-checks throughout the study will be conducted to assess for drift and re-training will occur as needed. Self-report assessments will be administered online via HIPAA-compliant Qualtrics survey management.
Table 1.
Measure administration schedule
| Measure | Abbreviation | Assessment Points | Measure Type |
|---|---|---|---|
| Overall Anxiety Severity and Impairment Scale | OASIS | Screening | Assessor administered |
| Mini Mental State Examination | MMSE | Screening | Assessor administered |
| Structured Clinical Interview for DSM-5 | SCID-5 | Screening | Assessor administered |
| Demographics | N/A | Baseline | Self-report |
| Pain, Enjoyment, General Activity Scale | PEG | Baseline | Self-report |
| Timeline Followback | TLFB | Baseline (past 30 days), Weekly (past 7 days), Post-treatment (past 30 days), 2-mo follow-up (past 30 days) | Self-report |
| Urine Drug Screen | UDS | Baseline, Post-treatment, 2-mo follow-up | Biological sample, collected over video call |
| Credibility and Expectancy Questionnaire | CEQ | Week 2 | Self-report |
| Depression, Anxiety, and Stress Scale | DASS-21 | Weekly | Self-report |
| Adverse Events | N/A | Weekly | Self-report |
| Anxiety Sensitivity Index | ASI-3 | Biweekly | Self-report |
| Pain Catastrophizing Scale | PCS | Biweekly | Self-report |
| Treatment Satisfaction Questionnaire | TSQ | Post-treatment | Self-report |
| Yale Adherence and Competence Scale | YACS | Following treatment completion | Completed by rater |
Screening
Anxiety symptom severity will be assessed using the OASIS (Campbell-Sills et al., 2009). DSM-5 diagnoses will be assessed using select modules of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders (SCID), 5th ed. (First et al., 2016). Cognitive impairment will be assessed using the Mini Mental State Examination (MMSE; Folstein et al., 1975). Study staff will review the electronic medical record to identify prescription information relevant for inclusion/exclusion, and use of the prescription (or other sources of BZs or opioids) will be reported by the participant during the baseline assessment via the Timeline Followback (TLFB; Sobell & Sobell, 1995).
Demographics
Demographic information collected includes sex, gender, age, race, and ethnicity.
Measures of benzodiazepine use
BZ dose at each assessment period will be assessed via self-report using the TLFB (Sobell & Sobell, 1995), and facilitated by a dose diary card that participants will be given to track substance use (i.e., days of use of each drug including BZs) and dose of BZ on each day of BZ use. TLFB administration will also be enhanced by asking participants to show their BZ pill bottles and will be corroborated by UDS1 at baseline and 2-month follow-up. TLFB will be used to document self-reported use of substances for each day (including BZ dose, if any, taken each day) since the last TLFB assessment during the acute treatment phase (i.e., past 7 days during weekly study visits) and in the past 30 days for baseline, post-treatment, and 2-month follow-up visits.
BZ dose will be operationalized as the average daily dose taken over the past week. BZ use will be operationalized as the percentage of days of use over the assessment period (i.e., # days of use/# days in assessment period). Secondary measures of BZ use will include point of care UDS, and examination of EHR data to determine BZ prescriptions filled (and doses) since the last assessment, including at the 2-month follow-up. UDS will be used to corroborate reported abstinence at 2-month follow-up.
Measures of mental health
The anxiety subscale of the 21-item Depression and Anxiety Severity Scale (DASS-21; Lovibond & Lovibond, 1993) will be used as the primary anxiety disorder outcome measure. The depression subscale of the DASS will be used as a secondary measure to assess changes in depression symptoms. The Anxiety Sensitivity Index-3 (Taylor et al., 2007) will be used as another outcome measure.
Measures of opioid use
Like the BZ use assessment methods, opioid use will be assessed via TLFB, with abstinence corroborated by UDS at 2-month follow-up. A UDS panel that includes cannabis, cocaine, morphine, methamphetamine, amphetamines, benzodiazepines, barbiturates, methadone, buprenorphine, MDMA (ecstasy), oxycodone, phencyclidine, tramadol, opioids, fentanyl, and ETG (alcohol) will be administered as a secondary measure of substance use at baseline and follow-up. EHR data will be examined to determine opioid prescriptions and doses filled since the last assessment, with additional chart review if clinicians report concerns about opioid obtained from external providers during their routine prescription drug monitoring database. Participants will also be asked to bring opioid prescription bottles at weekly telemedicine visits with research staff.
Measures of putative mediators
The Anxiety Sensitivity Index-3 (ASI-3; Taylor et al., 2007) will be used in secondary/exploratory analyses to examine whether anxiety sensitivity is a mediator of treatment outcomes in the CBT+BZT condition. The Pain Catastrophizing Scale (Sullivan et al., 1995) will also be used to examine whether pain catastrophizing is a potential mediator of treatment outcomes.
Measures of medical history
We will assess chronicity of pain, medical comorbidities, and pain at baseline. Pain severity and interference will be measured at baseline and at each major assessment period using the Pain, Enjoyment, General Activity (PEG) Scale, which includes three items to assess pain and function by rating (0 to 10) of pain and interference in the past week (Krebs et al., 2009).
Measures related to treatment
Treatment Credibility.
The Treatment Expectancy and Credibility Questionnaire (Devilly & Borkovec, 2000) is administered after Session 1 in both conditions to assess whether participants in both conditions believe the intervention to be credible.
Acceptability and Feasibility.
Acceptability will be approximated by participant adherence variables. Participant adherence variables will include number of CBT sessions and BZ taper sessions attended and percentage of participants per condition who complete the course of intervention. Participant satisfaction will be measured using an abbreviated and adapted version of the Treatment Satisfaction Questionnaire (Cox et al., 1994). Participants will be asked separately on a 0 (not at all) to 7 (extremely) point scale how useful they found the intervention to be, how much it improved their anxiety, BZ use, opioid use, and quality of life, and how likely they would be to recommend the program to a friend. Average scores of 4 out of 7 will indicate at least moderate satisfaction with the intervention.
Satisfaction and feedback about the intervention will also be assessed via focus groups with participants (aiming for 1–2 groups of n=6–8 participants each) and PCPs (aiming for 1 group of n=6–8 PCPs in total) who had patients participate in each condition. Patients who complete the protocol will be offered the option to participate at the end of their follow-up assessment. We will also outreach to patients who dropped out prior to completing the protocol to offer them focus group participation. Physicians will be recruited via email announcements sent to the participating clinics. Physicians will be asked about their attitudes toward, and satisfaction with the protocol (including the coordinated care approach). Participants will be asked about attitudes, satisfaction, barriers, and facilitators to carrying out the taper, and telehealth platform usability. Focus group data will be coded and thematically summarized using qualitative data analytic approaches. Additional measures of feasibility include (1) meeting at least 80% of recruitment and enrollment target (including descriptive information about reasons for exclusion) and (2) number of technology-related issues interfering with treatment (with >20% of participants unable to participate in one or more study visits due to technological issues indicating the protocol may not be feasible for delivery via telehealth).
Treatment Fidelity and Therapist Competence.
Fidelity in CBT and HE conditions will be assessed by independent doctoral-level raters who will rate 20% of the recorded sessions on adherence (% of session components delivered) and competence (extent to which provider skillfully delivered each component). Competence ratings for each component of treatment that was delivered in a session being rated will be given using the Yale Adherence and Competence Scale (Corvino et al., 2000).
Treatment Conditions
Participants will be randomized to treatment conditions following screening and enrollment. The treatment conditions are (1) BZ taper + an 11-session CBT program (BZT+CBT) and (2) BZ taper + a health education control (BZT+HE). Randomization will employ a 1:1 with stratification by high/low BZ dose, operationalized as daily BZ equivalent doses greater than/less than or equivalent to 15mg diazepam. Participants in both conditions will receive care through the research clinic at the UCLA Department of Psychiatry from therapists, who are doctoral-level clinicians, and psychiatry residents. Therapists will receive standardized training on the CBT and HE protocols. Adherence to therapy techniques will be monitored using audio recordings and weekly supervision by a licensed psychologist. Psychiatry residents will receive training on the BZ taper protocol by the supervising psychiatrist and will receive weekly supervision. All appointments will take place via a secure video platform. Table 2 shows the weekly study visit schedule.
Table 2.
Weekly study visit schedule
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | CBT/HE | CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT+CBT/HE | BZT | BZT | BZT |
BZ Taper (BZT)
The BZT protocol will be identical in both conditions. Resident physicians will recommend a graduated BZT at approximately 10%−20% reduction per week for a total of 12 weeks, as supported in prior literature (Jones et al., 2012). Dose reduction will be recommended and will be adjusted based on participant response and tolerability. The psychiatry resident will communicate to the PCPs each week regarding recommendations and progress. Participants will receive their prescriptions from their PCPs, who will work in conjunction with the study team to report on progress and encourage that the recommendations are followed. The study team will not prescribe the BZs. Participants will have the option to cease study-related reduction, and the PCPs will have flexibility to adjust or decide against tapered doses at clinical visits. In line with standard coordination of care practices PCPs will provide clinical updates to the study physicians about their patients’ tolerability and symptom severity. The study team will join the existing monthly primary care provider meeting to discuss taper progress and clinical issues that arise for participants, including any concerns about diversion, non-prescribed use, or new onset of substance use.
Cognitive Behavioral Therapy (BZT+CBT)
A manualized and evidence-based, 11 session CBT protocol (8 weekly sessions and 3 booster sessions) to assist panic-disordered patients with BZ tapering (Otto & Pollack, 2009) was adapted to meet the needs of the current patient population. Specific modifications included (1) a focus on BZ discontinuation for individuals with any number of anxiety-related conditions rather than a targeted focus on panic disorder, (2) an similar focus on applying lessons learned from BZ discontinuation to the management of a broader set of anxiety difficulties, (3) application of skills to distress management around chronic pain, and (4) a principle-based rather than a protocol-based approach to manualized treatment. The protocol includes psychoeducation, interoceptive exposure, somatic skills, and cognitive restructuring (Table 3). CBT will be initiated for 2 weeks prior to the BZ taper, providing patients with skills for responding to anxiety.
Table 3.
CBT Content
| Session # | Psycho-Educational Content | Intervention Content |
|---|---|---|
| 1 | Benefits of and difficulties associated with BZ taper, including symptoms Anxiety, Fear, and Exposure |
Enhance motivation for treatment Assess interoceptive/symptom sensitivities Initial training in “relaxation non-response” to anxiety signals |
| 2 | Review of changing experience relative to anxiety Exposure therapy AND vs. OR perspective regarding comfort with anxiety Fear of Symptoms Cycle |
Rehearsal of anxiety non-response Interoceptive Exposure: Head-rolling Consolidate learning |
| 3 | AND vs. OR perspective regarding comfort with anxiety (review) Anxiety Cycle vs. Relative Comfort (review) Fear of Symptom Cycle (review) |
Interoceptive Exposure: Head-rolling Interoceptive Exposure: Hyperventilation Interoceptive Exposure: Jumping Jacks/Jogging in Place /Stair Climbing Consolidate learning |
| 4 | Anxiety non-response (review) Symptom maintenance and management (review) Benzodiazepine Flu Model |
Assess “What If” Thoughts associated with BZ Taper Interoceptive Exposure in relation to patient’s symptom sensitivities |
| 5 | Benzodiazepine Flu Model (review) Symptoms associated with taper (review) Nature of Anxiety-Related (What If) Thoughts (review) Cognitive Coaching |
Interoceptive Practice Review Interoceptive Exposure in relation to patient’s symptom sensitivities |
| 6 | Symptoms associated with taper (review) Cognitive Coaching (review) |
Interoceptive Practice Review Interoceptive Exposure in relation to patient’s symptom sensitivities Interventions for Sleeping Difficulties (optional) |
| 7 | Symptoms associated with taper (review) Cognitive Coaching (review) “Do Not Be Careful With Yourself Around Your Symptoms” discussion (i.e., relapse prevention start) |
Interventions for Sleeping Difficulties (optional) Interoceptive Practice Review Interoceptive Exposure in relation to patient’s symptom sensitivities Planning Pleasant Events |
| 8 | Symptoms associated with taper (review) Cognitive Coaching (review) Naturalistic Exposure Every Other Week Care Transition |
Review Pleasant Events Interoceptive Exposure in relation to patient’s symptom sensitivities |
| 9–10 | Nature of Anxiety-Related (What If) Thoughts (review, as needed) | Review Pleasant Events Rehearsal of anxiety non-response (as needed) Interoceptive exposure (as needed) Naturalistic Exposure Intervention for underlying anxiety disorder (as needed) |
| 11 | Nature of Anxiety-Related (What If) Thoughts (review, as needed) | Review Pleasant Events Rehearsal of anxiety non-response (as needed) Interoceptive exposure (as needed) Naturalistic Exposure Intervention for underlying anxiety disorder (as needed) Skill review and relapse prevention |
Health Education (BZT+HE)
Patients in the BZT Control condition will receive 11 weeks of an interactive health education (HE) program to match for therapy time. HE was originally developed for a group format but was adapted for 11 individual telehealth sessions. HE is a published multimedia program (e.g., psychoeducational videos, web page review) addressing various health, wellness, and lifestyle topics such as nutrition, dental care, and health screening, and has been used as a control condition in prior clinical trials (Kinnunen et al., 2008; Mooney et al., 2014) (full topic list available upon request). The clinician’s role in this condition is to lead participants through the educational video and web page components, field questions, and lead discussions related to content.
Data Analysis Plan
Acceptability and patient adherence analyses (Aim 1) will include descriptive statistics and between-groups analysis of variance (ANOVA) to examine differences between conditions on adherence indices in each intervention. Qualitative PCP and patient acceptability will be evaluated via analyses of the focus group using text management software (Fielding & Lee, 1991). After examining transcripts and extracting themes (Glaser & Strauss, 1967; Ryan, 1999; Strauss & Corbin, 1990), we will reach consensus about which themes should be examined in detail (MacQueen et al., 1988).
Clinical outcome analyses (Aims 2–4) are considered preliminary given the pilot nature of the study and will include examining between-condition effects over time on the dependent BZ reduction, anxiety reduction, and opioid use. Categorical variables will be examined with χ2 tests (i.e., +/− UDS for each BZ and opioid; and “success” of BZ taper, operationalized as at least 50% reduction in BZ use at end of intervention). Multiple imputation procedures using logistic regression will be utilized to handle missing dichotomous data. Continuous data will be analyzed using multilevel modeling (MLM), with each continuous dependent variable as the outcome variable in a separate analysis, time as the level 1 predictor, and condition as the level 2 predictor. MLM analyses will use hierarchical linear modeling software to examine change in outcome measures over time (pre-treatment, post-treatment, follow-up) within and between groups. Given that the study is likely to be underpowered to detect statistically significant differences between two active treatment conditions, we will also calculate between-group effect sizes (including 95% confidence intervals) to provide information about effect magnitudes.
The exploratory mediation aim will be addressed using a series of MLMs to examine within-group change slopes over time on each mechanism variable (i.e., anxiety sensitivity; pain catastrophizing) and between-group differences in slopes. Mediation will be tested using the MacArthur guidelines (Glaser & Strauss, 1967) and using time-lagged analyses to examine whether change in putative mediators during treatment (using the weekly data) predicts change in the anxiety, BZ use, and opioid use outcomes. Although possibly underpowered to detect mediation effects, we have observed significant mediation with similar sample sizes (e.g., Wolitzky-Taylor et al., 2018b; Wolitzky-Taylor et al., 2012).
Sample Size and Power Analysis
The present study is supported by a pilot funding mechanism. Thus, sample size (N=54 randomized; N=40 completers expected) was guided by appropriateness for pilot study objectives rather than by statistical power computations for formal hypothesis testing (Becker et al., 2008; Herzog, 2008; Lancaster et al., 2004). However, we expect a small-to-medium sized effect based on evidence from other trials. Specifically, effects from the RCT comparing CBT+BZT for panic disorder to relaxation control+BZT were moderate (d range: .38–.65) (Otto et al., 2010). Between-group effect sizes for an intervention targeting anxiety and substance use comorbidity were small-to-moderate (d range: .29–.56) (Wolitzky-Taylor et al., 2018). Effect sizes in a large trial of CBT for anxiety in primary care yielded small-to-medium effect sizes (Bruce et al., 1995).
Using the range of effect sizes in the prior CBT protocol for BZ tapering in patients with panic disorder (above), estimated power if N=54 is achieved (using G Power 3.1.9.7 for between-group effects using repeated measures ANOVA with 2 groups across three measurement points and a small correlation between repeated measurements = 0.2) ranges from .52 to .93. A medium effect size (Cohen’s d = .54; equating to f = .27) would result in sufficient power of .80. Therefore, despite the pilot trial de-emphasizing sufficient statistical power, a moderate effect size is likely to achieve sufficient power to detect differences between treatment conditions with the proposed sample size. Moreover, our MLM approach will allow us to include anyone who completed at least a baseline assessment in intent-to-treat analyses, thus retaining power.
Trial Status
The randomized trial of BZT+CBT vs. BZT+HE is underway as of March 2023. We anticipate recruitment will be completed by August 2024. Treatment will conclude by January 2025. The project was approved by the UCLA Institutional Review Board (IRB #22–001451) and registered with ClinicalTrials.gov (NCT05573906).
Conclusion
Substantial evidence supports the FDA recommendation for cautious use of BZs among those prescribed opioids (Jones et al., 2012), but safe and sustainable solutions to address this concurrent use, including treatment of underlying anxiety symptomatology in patients taking opioids, are still urgently needed. The current study will evaluate the efficacy of augmenting BZ tapering with CBT for anxiety disorders that has been adapted specifically for patients with concomitant BZ and opioid use. Research combining scalable behavioral interventions ancillary to pharmacological approaches delivered via telehealth in primary care settings is important given concerning trends in rising prevalence of BZ/opioid co-prescription (Mooney et al., 2022), BZ-associated overdose deaths (Overdose Death Rates, 2020), and known barriers to implementation of behavioral health interventions in primary care (Dickinson, 2015). Delivery of CBT using a telehealth platform has the potential to aid adherence and promote access and dissemination of procedures in primary care.
This innovative study lays the groundwork for a larger scale clinical trial to investigate the efficacy of a coordinated telehealth intervention to reduce BZ use in a population of individuals who use opioids in primary care who are at higher risk of morbidity and mortality, including fatal overdose (Mooney et al., 2022). Achievement of aims for the study will encourage large-scale study of the impact of reducing BZ use among patients taking opioids, thereby making a significant public health impact. Although there are no rigid go/no-go criteria milestones to move forward with a larger-scale trial, support for the public health impact of conducting a larger-scale trial of the protocol will include a demonstration in this project that delivery of the intervention is feasible, it is considered at least moderately acceptable by patients, and that there is some signal for greater efficacy in BZT+CBT compared to BZT+HE.
Acknowledgements:
We thank UCLA Toluca Lake Health Center and the UCLA Family Medicine Clinic for participating as recruitment sites.
Funding:
1 R21 DA053394-01A1
Footnotes
Trial Registration: ClinicalTrials.gov (NCT05573906)
UDS test kits will be mailed to participants. During the assessment, participants will (1) be given instructions for collecting the sample, (2) mute audio/turn off video and step away from the screen to use the UDS cup; (3) return with cup and turn on audio/video; (4) wait 5 min, after which the research assistant asks the participant to hold up the cup close to the camera in order to read the results. The research assistant (RA) checks for evidence of tampering by inspecting the adulteration section of the results. Once it is confirmed that there was no tampering or adulteration, the RA asks the participant to turn the cup to reveal the remaining results of the urine drug screen and RA records the results.
References
- Becker A, Leonhardt C, Kochen MM, Keller S, Wegscheider K, Baum E, ... & Chenot JF (2008). Effects of two guideline implementation strategies on patient outcomes in primary care: a cluster randomized controlled trial. Spine, 33(5), 473–480. 10.1097/BRS.0b013e3181657e0d [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Spiegel DA, Gregg SF, & Nuzzarello A (1995). Predictors of alprazolam discontinuation with and without cognitive behavior therapy in panic disorder. The American Journal of Psychiatry, 152(8): 1156–60. 10.1176/ajp.152.8.1156 [DOI] [PubMed] [Google Scholar]
- Bruce TJ, Spiegel DA, & Hegel MT (1999). Cognitive-behavioral therapy helps prevent relapse and recurrence of panic disorder following alprazolam discontinuation: a long-term follow-up of the Peoria and Dartmouth studies. Journal of Consulting and Clinical Psychology, 67(1):151–6. 10.1037//0022-006x.67.1.151 [DOI] [PubMed] [Google Scholar]
- Calcaterra S, Glanz J, & Binswanger IA (2013). National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug and Alcohol Dependence, 131(3), 263–270. 10.1016/j.drugalcdep.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Sills L, Norman SB, Craske MG, Sullivan G, Lang AJ, Chavira DA, ... & Stein MB (2009). Validation of a brief measure of anxiety-related severity and impairment: the Overall Anxiety Severity and Impairment Scale (OASIS). Journal of Affective Disorders, 112(1–3), 92–101. 10.1016/j.jad.2008.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Spence MM, Niu F, Hui RL, Gray P, & Steinberg S (2020). Risk of Overdose with Exposure to Prescription Opioids, Benzodiazepines, and Non-benzodiazepine Sedative-Hypnotics in Adults: A Retrospective Cohort Study. Journal of General Internal Medicine, 35(3) 696–703. 10.1007/s11606-019-05545-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, & Grant BF (2007). Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry, 64(5), 566–576. 10.1001/archpsyc.64.5.566 [DOI] [PubMed] [Google Scholar]
- Corvino J, Carroll K, Nuro K, Nich C, Sifry R, & Frankforter T (2000). Yale adherence and competence scale guidelines. West Haven, CT: Yale University Psychotherapy Development Center. [Google Scholar]
- Cox BJ, Fergus KD, & Swinson RP (1994). Patient satisfaction with behavioral treatments for panic disorder with agoraphobia. Journal of Anxiety Disorders, 8(3), 193–206. 10.1016/0887-6185(94)90001-9 [DOI] [Google Scholar]
- Cragg A, Hau JP, Woo SA, Kitchen SA, Liu C, Doyle-Waters MM, & Hohl CM (2019). Risk factors for misuse of prescribed opioids: a systematic review and meta-analysis. Annals of emergency medicine, 74(5), 634–646. 10.1016/j.annemergmed.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Deacon BJ, & Abramowitz JS (2004). Cognitive and behavioral treatments for anxiety disorders: A review of meta-analytic findings. Journal of Clinical Psychology, 60(4), 429–441. 10.1002/jclp.10255 [DOI] [PubMed] [Google Scholar]
- Devilly GJ & Borkovec TD (2000). Psychometric properties of the credibility/expectancy questionnaire. Journal of Behavior Therapy and Experimental Psychiatry, 31(2), 73–86. 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- Dickinson WP (2015). Strategies to support the integration of behavioral health and primary care: what have we learned thus far?. The Journal of the American Board of Family Medicine, 28(Supplement 1), S102–S106. 10.3122/jabfm.2015.S1.150112 [DOI] [PubMed] [Google Scholar]
- Dietary Guidelines Advisory Committee (2020). Part D. Chapter 11: Alcoholic Beverages. Retrieved from https://www.dietaryguidelines.gov/sites/default/files/2020-07/PartD_Ch11_AlcoholicBev_first-print.pdf
- Edwards RR, Wasan AD, Michna E, Greenbaum S, Ross E, & Jamison RN (2011). Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. The Journal of Pain, 12(9), 953–963. 10.1016/j.jpain.2011.02.357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding NG & Lee RM (1991). Using computers in qualitative research. Newbury Park, CA: Sage. [Google Scholar]
- First MB, Williams JB, Karg RS, & Spitzer RL (2016). SCID-5-CV: Structured clinical interview for DSM-5 disorders: Clinician version. American Psychiatric Association Publishing; Washington, DC. [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Fortney JC, Pyne JM, Edlund MJ, Williams DK, Robinson DE, Mittal D, & Henderson KL (2007). A randomized trial of telemedicine-based collaborative care for depression. Journal of General Internal Medicine, 22(8), 1086–1093. 10.1007/s11606-007-0201-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frueh BC, Monnier J, Yim E, Grubaugh AL, Hamner MB, & Knapp RG (2007). A randomized trial of telepsychiatry for post-traumatic stress disorder. Journal of Telemedicine and Telecare, 13(3), 142–147. 10.1258/135763307780677604 [DOI] [PubMed] [Google Scholar]
- Garg RK, Fulton-Kehoe D, & Franklin GM (2017). Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Medical care, 55(7), 661–668. 10.1097/MLR.0000000000000738 [DOI] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, … & Kaplan K (2004). Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry, 61(8), 807–816. 10.1001/archpsyc.61.8.807 [DOI] [PubMed] [Google Scholar]
- Glaser BG & Strauss AL (1967). The discovery of grounded theory: Strategies for qualitative research. Chicago, IL: Aldine. [Google Scholar]
- Grazier KL, Smiley ML, & Bondalapati KS (2016). Overcoming barriers to integrating behavioral health and primary care services. Journal of Primary Care & Community Health, 7(4), 242–248. 10.1177/2150131916656455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins EJ, Goldberg SB, Malte CA, & Saxon AJ (2019). New Coprescription of Opioids and Benzodiazepines and Mortality Among Veteran Affairs Patients with Posttraumatic Stress Disorder. The Journal of Clinical Psychiatry, 80(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon BA, Calkins AW, Halperin DM, Kathryn McHugh R, Murray HW, & Otto MW (2011). Anxiety sensitivity and illicit sedative use among opiate-dependent women and men. The American Journal of Drug and Alcohol Abuse, 37(1), 43–47. 10.4088/JCP.18m12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog M (2008). Considerations in Determining Sample Size for Pilot Studies. Research in Nursing & Health, 31, 180–191. 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- Jones JD, Mogali S, & Comer SD (2012). Polydrug abuse: A review of opioid and benzodiazepine combination use. Drug and Alcohol Dependence, 125(1), 8–18. 10.1016/j.drugalcdep.2012.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathol RG, Butler M, McAlpine DD, & Kane RL (2010). Barriers to physical and mental condition integrated service delivery. Psychosomatic Medicine, 72(6), 511–518. 10.1097/PSY.0b013e3181e2c4a0 [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Leeman RF, Korhonen T, Quiles ZN, Terwal DM, Garvey AJ, & Hartley LH (2008). Exercise as an adjunct to nicotine gum in treating tobacco dependence among women. Nicotine & Tobacco Research, 10(4), 689–703. 10.1080/14622200801979043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs EE, Lorenz KA, Bair MJ, Damush TM, Wu J, Sutherland JM, ... & Kroenke K (2009). Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. Journal of General Internal Medicine, 24(6), 733–738. 10.1007/s11606-009-0981-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster S, Dodd PR, & Williamson GA (2004). Design and analysis of pilot studies: recommendations for good practice. Journal of Evaluation in Clinical Practice, 10(2), 307–312. 10.1111/j..2002.384.doc.x [DOI] [PubMed] [Google Scholar]
- Limandri BJ (2018). Benzodiazepine use: the underbelly of the opioid epidemic. Journal of Psychosocial Nursing and Mental Health, 56(6): 11–15. 10.3928/02793695-20180521-03 [DOI] [PubMed] [Google Scholar]
- Lin LA, Casteel D, Shigekawa E, Weyrich MS, Roby DH, & McMenamin SB (2019). Telemedicine-delivered treatment interventions for substance use disorders: A systematic review. Journal of Substance Abuse Treatment, 101, 38–49. 10.1016/j.jsat.2019.03.007 [DOI] [PubMed] [Google Scholar]
- Lovibond SH & Lovibond PF (1993). Manual for the Depression Anxiety Stress Scales (DASS). Psychology Foundation Monograph. (Available from The Psychology Foundation, Room 1005 Mathews Building, University of New South Wales, NSW 2052, Australia). [Google Scholar]
- MacQueen K, McLellan E, Kay K, & Milstein B (1988). Code book development for team-based qualitative analysis. CAM Journal, 10, 31–36. 10.1177/1525822X980100020301 [DOI] [Google Scholar]
- McCance-Katz EF, Sullivan LE, & Nallani S (2010). Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. The American Journal on Addictions, 19(1), 4–16. 10.1111/j.1521-0391.2009.00005.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Votaw VR, Bogunovic O, Karakula SL, Griffin ML, & Weiss RD (2017). Anxiety sensitivity and nonmedical benzodiazepine use among adults with opioid use disorder. Addictive Behaviors, 65, 283–288. 10.1016/j.addbeh.2016.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney LJ, Cooper C, London ED, Chudzynski J, Dolezal B, Dickerson D, ... & Rawson, R. A. (2014). Exercise for methamphetamine dependence: Rationale, design, and methodology. Contemporary Clinical Trials, 37(1), 139–147. 10.1016/j.cct.2013.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney LJ, Yoo C, Zhu Y, Wolitzky-Taylor K, & Hser YH. (2022). Association between benzodiazepine prescription, opioid use disorder, and mortality among patients in a large healthcare system. Journal of Addiction Medicine. 16(1), 65–71. 10.1097/ADM.0000000000000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morasco BJ, Turk DC, Donovan DM, & Dobscha SK (2013). Risk for prescription opioid misuse among patients with a history of substance use disorder. Drug and Alcohol Dependence, 127(1–3), 193–199. 10.1016/j.drugalcdep.2012.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen S, Dietze P, Lee N, Dunlop A, & Taylor D (2007). Concurrent buprenorphine and benzodiazepines use and self-reported opioid toxicity in opioid substitution treatment. Addiction, 102(4), 616–622. 10.1111/j.1360-0443.2006.01731.x [DOI] [PubMed] [Google Scholar]
- Nielsen S, Lintzeris N, Bruno R, Campbell G, Larance B, Hall W, ... & Degenhardt L (2015). Benzodiazepine use among chronic pain patients prescribed opioids: Associations with pain, physical and mental health, and health service utilization. Pain Medicine, 16(2), 356–366. 10.1111/pme.12594 [DOI] [PubMed] [Google Scholar]
- Nielsen L, Riddle M, King JW, Atklin WM, Chen W, … Weber W (2018). The NIH Science of Behavior Change Program: Transforming the science through a focus on mechanisms of change. Behaviour Research and Therapy, 101, 3–11. 10.1016/j.brat.2017.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, Hong JJ, Safren SA (2002). Benzodiazepine discontinuation difficulties in panic disorder: conceptual model and outcome for cognitive-behavior therapy. Current Pharmaceutical Design, 8(1):75–80. 10.2174/1381612023396726 [DOI] [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Simon NM, Farach FJ, Worthington JJ, & Pollack MH (2010). Efficacy of CBT for benzodiazepine discontinuation in patients with panic disorder: Further evaluation. Behaviour Research and Therapy, 48(8), 720–727. 10.1016/j.brat.2010.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto MW, & Pollack MH (2009). Stopping anxiety medication (Therapist guide, 2nd ed.). New York: Oxford University Press. [Google Scholar]
- Otto MW, Pollack MH, Sachs GS, Reiter SR, Meltzer-Brody S, & Rosenbaum JF (1993). Discontinuation of benzodiazepine treatment: Efficacy of cognitive-behavioral therapy for patients with panic disorder. American Journal of Psychiatry, 150(10), 1485–1490. 10.1176/ajp.150.10.1485 [DOI] [PubMed] [Google Scholar]
- Overdose Death Rates (Revised March 2020). Retrieved from https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates.
- Lembke A, Papac J, & Humphreys K (2018). Our other prescription drug problem. The New England Journal of Medicine, 378, 693–695. 10.1056/NEJMp1715050 [DOI] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, & Bohnert AS (2015). Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. British Medical Journal, 350, h2698. 10.1136/bmj.h2698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy-Byrne P, Craske MG, Sullivan G, Rose RD, Edlund MJ, Lang AJ, ... & Campbell-Sills L (2010). Delivery of evidence-based treatment for multiple anxiety disorders in primary care: a randomized controlled trial. JAMA, 303(19), 1921–1928. 10.1001/jama.2010.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Royal Australian College of General Practitioners (2023). Discontinuing benzodiazepines. Retrieved from https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/drugs-of-dependence/part-b/discontinuing-benzodiazepines
- Ryan G (1999). Measuring the typicality of text: using multiple coders for more than just reliability and validity checks. Human Organization, 58, 313–322. 10.17730/humo.58.3.g224147522545rln [DOI] [Google Scholar]
- Sobell LC, & Sobell MB (1995). Alcohol timeline followback users’ manual. Toronto, Canada: Addiction Research Foundation. [Google Scholar]
- Stewart SH, Asmundson GJG (2006). Anxiety sensitivity and its impact on pain experiences and conditions: a state of the art. Cognitive Behavioral Therapy, 35(4), 185–188. 10.1080/16506070601090457 [DOI] [PubMed] [Google Scholar]
- Stewart RE, & Chambless DL (2009). Cognitive–behavioral therapy for adult anxiety disorders in clinical practice: A meta-analysis of effectiveness studies. Journal of Consulting and Clinical Psychology, 77(4), 595–606. 10.1037/a0016032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss AL, & Corbin J (1990). Basics of qualitative research: Grounded theory procedures and techniques. Newbury Park, CA: Sage. [Google Scholar]
- Sullivan MJ, Bishop SR, & Pivik J (1995). The pain catastrophizing scale: development and validation. Psychological Assessment, 7(4), 524–532. 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- Sumner JA, Carey RN, Michie S, Johnston M, Edmondson D, & Davidson KW (2018). Using rigorous methods to advance behaviour change science. Nature Human Behaviour, 2, 797–799. 10.1038/s41562-018-0471-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, & Samet JH (2022). Opioid use disorder. Annals of Internal Medicine, 175(1), ITC1–ITC16. 10.7326/AITC202201180 [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, ... & Coles M (2007). Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment, 19(2), 176–188. 10.1037/1040-3590.19.2.176 [DOI] [PubMed] [Google Scholar]
- Unützer J, Powers D, Katon W, & Langston C (2005). From establishing an evidence-based practice to implementation in real-world settings: IMPACT as a case study. Psychiatric Clinics, 28(4), 1079–1092. 10.1016/j.psc.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Craske MG, Labus J, Mayer E, & Naliboff B (2012). Visceral sensitivity as a mediator of treatment outcome for irritable bowel syndrome. Behaviour Research and Therapy, 50, 647–650. 10.1016/j.brat.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Krull JL, Rawson R, Roy-Byrne P, Ries R & Craske MG (2018). Randomized clinical trial evaluating the preliminary effectiveness of an integrated anxiety disorder treatment in substance use disorder specialty clinics. Journal of Consulting and Clinical Psychology, 86 (1), 81–88. 10.1037/ccp0000276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitzky-Taylor K, Drazdowski T, Rawson R & Craske MG (2018). Anxiety sensitivity and coping motives as putative mediators of outcome in an integrated treatment for comorbid anxiety and substance use disorders. Behaviour Research and Therapy, 107, 34–41. 10.1016/j.brat.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HH, Chen CY, Fang SY, Chang IS, Wu ECH, & Lin KM (2011). Five-year trajectories of long-term benzodiazepine use by adolescents: Patient, provider, and medication factors. Psychiatric Services, 62(8), 900–907. 10.1176/ps.62.8.pss6208_0900 [DOI] [PubMed] [Google Scholar]


