Abstract
Background:
Fluoroquinolones, one of the most commonly prescribed antibiotic classes, have been implicated in cases of central nervous system (CNS) and peripheral nervous system (PNS) adverse events, which highlights the need for epidemiologic studies of the neurological safety of fluoroquinolones.
Purpose:
To evaluate the safety of fluoroquinolones with regard to risk of diagnosed neurological dysfunction.
Methods:
We conducted a propensity score-matched inception cohort study using claims data from a commercially insured population. Our study included adults prescribed an oral fluoroquinolone or comparator antibiotic between January 2000 and September 2015 for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, uncomplicated urinary tract infection, or acute bronchitis. Our outcomes were CNS dysfunction, and four separate but complementary PNS dysfunction outcomes. Cox proportional hazards models were estimated after matching on propensity scores fitted using the variables age, sex, epilepsy, hereditary peripheral neuropathy, renal dysfunction, diabetes, gabapentinoid use, statin use, isoniazid use, and chemotherapy use.
Results:
Our cohort contained 976 568 individuals exposed to a fluoroquinolone antibiotic matched 1:1 with a comparator. Matching produced balance (standardized mean difference <0.1) on all variables included in the propensity score. The hazard ratio associated with fluoroquinolone exposure was 1.08 (95% confidence interval 1.05–1.11) for CNS dysfunction, and 1.09 (95% CI 1.07–1.11) for the most commonly occurring PNS dysfunction outcome.
Conclusions:
Fluoroquinolone antibiotic use was associated with the development of neurological dysfunction versus comparator antibiotic use in the adult population.
Keywords: comparative study, fluoroquinolones, neurologic manifestations, pharmacoepidemiology, propensity score, survival analysis
1 |. BACKGROUND
Fluoroquinolones are one of the most highly prescribed class of antibiotics, with around 30 million outpatient prescriptions dispensed each year in the United States.1–3 In 2004, the US Food and Drug Administration (FDA) required that peripheral neuropathy (PN) be added to fluoroquinolone warning labeling4 because of an increase in reported cases of this severe peripheral nervous system (PNS) disorder characterized by burning, numbness, pain, and muscle weakness.5–7 Since then, FDA has extended its drug safety communications relating to fluoroquinolones8,9 to include symptoms and disorders of the central nervous system (CNS). Few epidemiologic studies have examined the association between fluoroquinolones and nervous system dysfunction. One meta-analysis of randomized controlled trials (RCTs) found that fluoroquinolones were associated with CNS-related adverse events, with an odds ratio of 1.40 (95% confidence interval, 1.12–1.75). However, the trials captured only 198 CNS events in 4511 fluoroquinolone users,10 and some important CNS dysfunction symptoms such as seizure, intracranial hypertension, and altered mental status were not included in the meta-analysis. No large epidemiologic studies have examined the association between fluoroquinolone use and a wide breadth of CNS symptoms, and only two studies have evaluated the association with PNS dysfunction. The first, a US-based case–control study conducted in men, found an odds ratio of 2.07 (95% confidence interval, 1.56–2.74).11 The second, a UK-based nested case–control study comparing fluoroquinolones to comparator antibiotics, found an odds ratio of 1.47 (95% confidence interval, 1.13–1.92).12 Unfortunately the first study did not include women, yet the majority of fluoroquinolone prescriptions are for genitourinary infections that predominantly affect women.1,13 Additionally, both studies relied on identification codes that might not be sufficiently broad or may be inappropriate for identifying PN. Therefore, we sought to evaluate the neurological safety of fluoroquinolones in men and women by comparing occurrences of CNS and PNS dysfunction in users of fluoroquinolones versus comparator antibiotics, using propensity score matching and time-to-event analyses.
2 |. METHODS
The Institutional Review Board (IRB) at the University of Pennsylvania determined that this research met eligibility criteria for IRB review exemption.
2.1 |. Overview and study population
We conducted a propensity-score matched cohort study of adult new users of fluoroquinolones vs. comparator antibiotics. Data came from 2000 to 2015 Optum’s de-identified Clinformatics® Data Mart Database, which is comprised of billing claims from a US commercially insured population. Clinformatics includes de-identified data on more than 55 million unique members, including both medical and pharmacy benefits, encompassing 12–13 million annual covered lives.14
2.2 |. Study cohort
The cohort consisted of adults age 18 years and older dispensed an oral fluoroquinolone (ciprofloxacin, levofloxacin, ofloxacin, moxifloxacin, or gemifloxacin) or a comparator antibiotic (azithromycin, amoxicillin, amoxicillin plus clavulanic acid, or cefixime; Tables S1 and S2) used in temporal proximity to a diagnosis of one of four infectious indications for which fluoroquinolones are highly prescribed: acute bronchitis, acute bacterial sinusitis, uncomplicated urinary tract infection (UTI), and acute bacterial exacerbation of chronic bronchitis. This inclusion criterion was operationalized by requiring individuals to have had an International Classification of Diseases Ninth Revision Clinical Modification (ICD-9-CM) diagnosis code for any of the four infections recorded within 14 days before or after the dispensing date of the first prescription for an antibiotic of interest (referred to as the index prescription). These ICD-9-CM code sets, which have been used in prior studies,13,15–18 are listed in Table S3.
These comparator antibiotics were chosen because they are appropriate therapeutic alternatives for fluoroquinolones, are commonly used to treat the four study indications, and are not known to be associated with the neurological outcomes of interest (see Box S1 for further information). We defined new users as having no prescription dispensed for a fluoroquinolone or a comparator antibiotic for at least 180 days prior to entering the cohort. If an individual was eligible for inclusion multiple times, we included only the first instance. We conducted our analysis in two separate eras: pre- and post-September 15, 2004 (era 1: January 1, 2000 to September 14, 2004; era 2: September 15, 2004 to September 30, 2015) to examine the possibility of diagnostic suspicion bias, since in the fall of 2004 the FDA required that PN be added to all fluoroquinolone labels.
Patients were excluded if the days’ supply of the index prescription exceeded 30 days to preclude prescriptions for antibiotic prophylaxis, or if the subject had a recorded diagnosis of any of the outcomes of interest within the 180 days prior to their index prescription date. Individuals were also excluded if they were dispensed a fluoroquinolone and a comparator antibiotic on the same date. Patients had to have at least 180 days of uninterrupted claims data available before their index prescription, and were excluded if their enrollment terminated on the same date as their index prescription. Individuals were also excluded if their index prescription occurred on the last date of data availability (September 30, 2015).
Follow-up began on the day after the index prescription date, since events occurring on the index prescription date may have preceded dispensing of the antibiotic prescription, and continued until the first occurrence of the following: (a) outcome of interest (described below); (b) the last day of follow up (120 days after the date of the index prescription); (c) the last day of enrollment if this day occurred before 120 days; and (d) the last day of data availability (September 30, 2015) if this day occurred before 120 days. Fluoroquinolone associated symptoms have been reported to arise for up to 3 months after initiation of exposure.19,20 We used a follow-up period of 120 days (4 months) in order to capture any outcomes occurring just after 3 months and allow for any delays in diagnosis.
2.3 |. Exposure and covariate ascertainment
Exposure was defined as a dispensed prescription for an antibiotic of interest (fluoroquinolone vs. comparator antibiotic) in oral form and with a 30 day supply or less.
We measured potential confounders in the 180 days prior to and including the index prescription date. Demographic variables included age and sex. Drugs considered as potential confounders included select chemotherapy agents,21,22 statins, gabapentinoids, and isoniazid. Potentially confounding diseases included diabetes,23–25 hereditary PN, renal dysfunction,25 and epilepsy. All covariates were used to estimate a propensity score.
To identify the antibiotics of interest and confounding drugs of interest, we used drug-specific national drug codes (NDC) compiled from the Cerner Multum database. For identifying confounding diseases of interest, we used ICD-9-CM discharge diagnosis codes in any position of an inpatient claim or outpatient medical claim. A complete listing of covariate ICD-9-CM codes are available in Tables S3 and S4.
2.4 |. Outcome ascertainment
CNS dysfunction was defined by the occurrence of any one of the following CNS diagnoses during follow-up: seizures/convulsions, intracranial hypertension, psychosis/delirium, or altered mental status/encephalopathy. We used ICD-9-CM discharge diagnosis codes in any position of an outpatient medical claim or inpatient claim to identify these CNS symptoms (complete list of ICD-9-CM codes available in Table S6). These CNS diagnoses are listed as potential adverse reactions in the FDA prescribing information for fluoroquinolones, and have ICD-9 codes with good face validity.
Four separate but complementary PNS dysfunction outcomes were defined: (a) Symptoms (occurrence of one or more diagnoses consistent with PNS symptoms, including muscle weakness, sensory disturbance, gait dysfunction, or PN); (b) symptoms + PN diagnosis (symptoms as described in definition 1 plus a diagnosis of PN); (c) symptoms + EPT (symptoms as described in definition 1 plus the performance of electrophysiological testing of the PNS); and (4) symptoms + PN diagnosis + EPT (symptoms and PN diagnosis as described in definition 2 plus performance of electrophysiological testing). Electrophysiological testing, typically electromyography and nerve conduction studies, is a useful and definitive way of identifying PNs,26–28 and is often the gold standard for evaluating nerve function.27,28 We used ICD-9-CM discharge diagnosis codes in any position of an outpatient medical claim or inpatient claim to identify these PNS symptoms as well as any PN diagnoses, and we used Current Procedural Terminology (CPT) codes to identify electrophysiological testing of peripheral nerves (complete list of ICD-9-CM and CPT codes listed in Table S6). The algorithms used to define these outcomes were guided by a neurologist-epidemiologist, but have not been validated.
2.5 |. Statistical analysis
In each era, we stratified the cohort by our four infectious indications to yield four separate cohorts per era. For those individuals who experienced more than one indication of interest in the 14 days prior to or after the index prescription, we assigned them to the indication cohort with the smallest sample size. We then tabulated frequencies of baseline covariates by exposure group. We examined balance in potential confounding variables before matching by calculating standardized differences in proportions29 for each covariate (Table S7), and considered covariate distributions to be balanced if standardized differences were <0.1.29,30
We next used logistic regression to estimate a separate propensity score, representing the probability of receiving either a fluoroquinolone or comparator antibiotic, in each of the eight cohorts (four indications × two eras) using all baseline covariates. We then examined overlap in propensity scores between our exposure groups graphically using kernel density plots (Figure S1). Next, we ordered individuals randomly within each cohort and performed 1:1 nearest neighbor matching on the propensity score, without replacement. For five of the eight cohorts, we used a caliper size of 0.2 times the standard deviation of the logit of the propensity score. For acute bacterial exacerbation of chronic bronchitis in era 1, we used a caliper size of 0.01 times the standard deviation of the logit of the propensity score, and for both uncomplicated UTI cohorts, we used a caliper size of 0.001 times the standard deviation of the logit of the propensity score. We used tighter calipers for these three cohorts to achieve closer matches, because there were fewer comparator antibiotic users available to match to fluoroquinolone users. Additionally, for the uncomplicated UTI cohorts in era 1 and era 2, the fluoroquinolone group was much larger than the comparator antibiotic group, precluding the possibility of 1:1 matching for all individuals in the fluoroquinolone group. We therefore took a random sample of fluoroquinolone users equal in size to the comparator antibiotic group and used this subsample in matched analyses.
After matching, antibiotic indication cohorts were combined within each era. The cumulative incidence of each outcome and in each era was estimated at 120 days using the Kaplan–Meier estimator. We used Cox proportional-hazards regression models including only the exposure variable and a variance estimator used to more accurately estimate standard error by accounting for intragroup correlation between matched pairs in each era and for each of our CNS and PNS outcomes. Propensity-score matched hazard ratios (HRs) and 95% CIs were calculated for all outcomes in both eras, as well as in the combined cohort if there was no more than a 5% difference between HRs in era 1 and era 2. The proportional hazards assumption was assessed via graphical inspection of the correlation between Schoenfeld residuals and time (Figures S2 and S3). For those outcomes where the proportional hazards assumption did not hold, we incorporated an interaction term between a categorical time variable and exposure, to allow for time-varying HRs.
Statistical analyses were conducted using Stata MP v16.0 (StataCorp LP, College Station, TX).
3 |. RESULTS
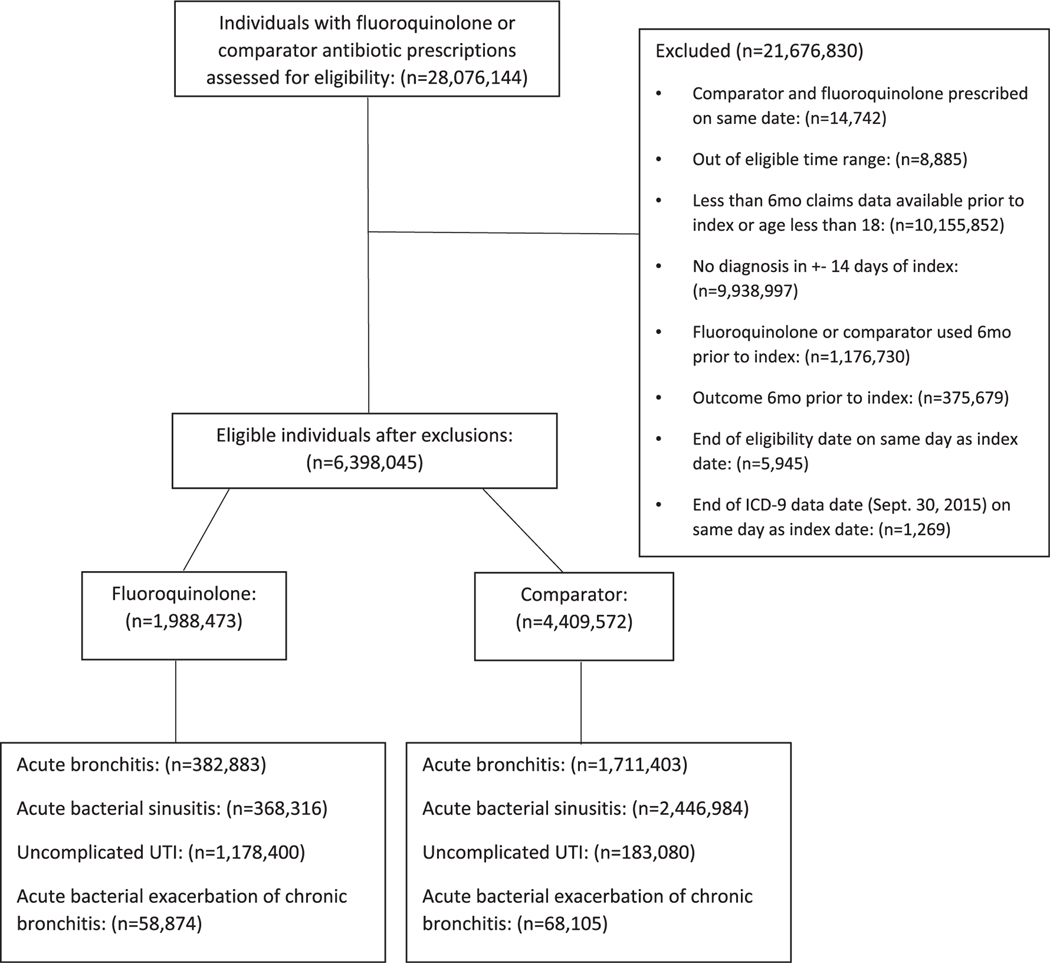
We identified 6 398 045 fluoroquinolone and comparator antibiotic users who met our inclusion and exclusion criteria (see Figure 1), of whom 1 988 473 were prescribed a fluoroquinolone and 4 409 572 were prescribed a comparator antibiotic index prescription. Prior to matching, 19, 19, 59, and 3% of fluoroquinolone users, and 39, 56, 4, and 2% of comparator antibiotic users had a diagnosis of acute bronchitis, acute bacterial sinusitis, uncomplicated UTI, and acute bacterial exacerbation of chronic bronchitis, respectively (Figure 1).
FIGURE 1.
Study cohort and exclusions
Standardized differences for covariates before matching, stratified by treatment and era, are shown in Table S7. Prior to propensity score matching, the majority of covariates were balanced (standardized difference <0.1); pre-matching standardized differences larger than 0.1 were found for age, sex, and diabetes in era 1 and age, sex, renal dysfunction, diabetes, and statin exposure in era 2. The fluoroquinolone group had a higher percentage of female subjects, and diabetes, renal dysfunction, hereditary PN, gabapentinoid exposure, and statin exposure were more prevalent. Using Kernel density plots (Figure S1), we observed substantial overlap in propensity scores between the fluoroquinolone and comparator groups before matching, indicating that the propensity score positivity assumption was met. After matching, around 60% of the era 1 cohort and era 2 cohort were female, and around 50–60% of individuals in the combined cohort were between the ages of 35 and 59. After matching, all measured covariates were balanced across the two treatment groups (all standardized differences <0.1; Table 1).
TABLE 1.
Comparison of baseline characteristics between fluoroquinolone and comparator antibiotic users after propensity score matching
| Combined cohort | Era 1 (January 1, 2000-September 14, 2004) | Era 2 (September 15, 2004-September 30, 2015) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| FQ N = 976 568 | Comparator N = 976 568 | SD | FQ N = 195 207 | Comparator N = 195 207 | SD | FQ N = 781 361 | Comparator N = 781 361 | SD | |
| Indication | |||||||||
| Acute bronchitis | 382 829 (39.2%) | 382 829 (39.2%) | <0.01 | 73 878 (37.8%) | 73 878 (37.8%) | <0.01 | 308 951 (39.5%) | 308 951 (39.5%) | <0.01 |
| Acute bacterial sinusitis | 368 274 (37.7%) | 368 274 (37.7%) | <0.01 | 81 234 (41.6%) | 81 234 (41.6%) | <0.01 | 287 040 (36.7%) | 287 040 (36.7%) | <0.01 |
| Uncomplicated UTI | 167 352 (17.1%) | 167 352 (17.1%) | <0.01 | 33 738 (17.3%) | 33 738 (17.3%) | <0.01 | 133 614 (17.1%) | 133 614 (17.1%) | <0.01 |
| Acute bacterial exacerbation of chronic bronchitis | 58 113 (6.0%) | 58 113 (6.0%) | <0.01 | 6357 (3.3%) | 6357 (3.3%) | <0.01 | 51 756 (6.6%) | 51 756 (6.6%) | <0.01 |
| Variable | |||||||||
| Age | |||||||||
| 18–24 | 55 019 (5.6%) | 55 008 (5.6%) | <0.01 | 12 097 (6.2%) | 12 092 (6.2%) | <0.01 | 42 922 (5.5%) | 42 916 (5.5%) | <0.01 |
| 25–29 | 59 660 (6.1%) | 59 659 (6.1%) | <0.01 | 14 400 (7.4%) | 14 403 (7.4%) | <0.01 | 45 260 (5.8%) | 45 256 (5.8%) | <0.01 |
| 30–34 | 80 325 (8.2%) | 80 328 (8.2%) | <0.01 | 20 998 (10.8%) | 20 997 (10.8%) | <0.01 | 59 327 (7.6%) | 59 331 (7.6%) | <0.01 |
| 35–39 | 99 386 (10.2%) | 99 387 (10.2%) | <0.01 | 25 669 (13.1%) | 25 665 (13.1%) | <0.01 | 73 717 (9.4%) | 73 722 (9.4%) | <0.01 |
| 40–44 | 109 935 (11.3%) | 109 929 (11.3%) | <0.01 | 28 000 (14.3%) | 28 006 (14.3%) | <0.01 | 81 935 (10.5%) | 81 923 (10.5%) | <0.01 |
| 45–49 | 114 122 (11.7%) | 114 090 (11.7%) | <0.01 | 26 457 (13.6%) | 26 455 (13.6%) | <0.01 | 87 665 (11.2%) | 87 635 (11.2%) | <0.01 |
| 50–54 | 110 173 (11.3%) | 110 262 (11.3%) | <0.01 | 23 278 (11.9%) | 23 315 (11.9%) | <0.01 | 86 895 (11.1%) | 86 947 (11.1%) | <0.01 |
| 55–59 | 99 546 (10.2%) | 99 644 (10.2%) | <0.01 | 19 155 (9.8%) | 19 155 (9.8%) | <0.01 | 80 391 (10.3%) | 80 489 (10.3%) | <0.01 |
| 60–64 | 75 882 (7.8%) | 76 096 (7.8%) | <0.01 | 12 124 (6.2%) | 12 156 (6.2%) | <0.01 | 63 758 (8.2%) | 63 940 (8.2%) | <0.01 |
| 65–69 | 58 288 (6.0%) | 58 541 (6.0%) | <0.01 | 4898 (2.5%) | 4892 (2.5%) | <0.01 | 53 390 (6.8%) | 53 649 (6.9%) | <0.01 |
| 70–74 | 43 793 (4.5%) | 43 861 (4.5%) | <0.01 | 5353 (2.7%) | 5281 (2.7%) | <0.01 | 38 440 (4.9%) | 38 580 (4.9%) | <0.01 |
| 75–79 | 38 075 (3.9%) | 37 688 (3.9%) | <0.01 | 2778 (1.4%) | 2790 (1.4%) | <0.01 | 35 297 (4.5%) | 34 898 (4.5%) | <0.01 |
| 80–84 | 25 614 (2.6%) | 25 310 (2.6%) | <0.01 | - | - | - | 25 614 (3.3%) | 25 310 (3.2%) | <0.01 |
| 85+ | 6750 (0.7%) | 6765 (0.7%) | <0.01 | - | - | - | 6750 (0.9%) | 6765 (0.9%) | <0.01 |
| Female Sex | 595 883 (61.0%) | 595 839 (61.0%) | <0.01 | 123 570 (63.3%) | 123 402 (63.2%) | <0.01 | 472 313 (60.4%) | 472 437 (60.5%) | <0.01 |
| Renal Dysfunction | 21 781 (2.2%) | 21 056 (2.2%) | <0.01 | 925 (0.5%) | 914 (0.5%) | <0.01 | 20 856 (2.7%) | 20 142 (2.6%) | <0.01 |
| Diabetes | 120 639 (12.4%) | 120 179 (12.3%) | <0.01 | 14 655 (7.5%) | 14 570 (7.5%) | <0.01 | 105 984 (13.6%) | 105 609 (13.5%) | <0.01 |
| Hereditary Peripheral Neuropathy | 4703 (0.5%) | 4629 (0.5%) | <0.01 | 403 (0.2%) | 373 (0.2%) | <0.01 | 4300 (0.6%) | 4256 (0.5%) | <0.01 |
| Epilepsy | 1937 (0.2%) | 1858 (0.2%) | <0.02 | 206 (0.1%) | 198 (0.1%) | <0.01 | 1731 (0.2%) | 1660 (0.2%) | <0.01 |
| Chemotherapy Medication Exposure | 205 (<1%) | 192 (<1%) | <0.01 | 11(<1%) | 8 (<1%) | <0.01 | 194 (<1%) | 184 (<1%) | <0.01 |
| Statin Exposure | 178 484 (18.3%) | 178 838 (18.3%) | <0.01 | 20 553 (10.5%) | 20 593 (10.5%) | <0.01 | 157 931 (20.2%) | 158 245 (20.3%) | <0.01 |
| Gabapentinoid Exposure | 27 587 (2.8%) | 27 479 (2.8%) | <0.01 | 2515 (1.3%) | 2489 (1.3%) | <0.01 | 25 072 (3.2%) | 24 990 (3.2%) | <0.01 |
| Isoniazid Exposure | 201 (<1%) | 173 (<1%) | <0.01 | 44 (<1%) | 29 (<1%) | <0.01 | 157 (<1%) | 144 (<1%) | <0.01 |
Note: Exposure to all medications assessed during the 6 months prior to the index prescription date.
Abbreviations: SD, standardized difference (vs. comparator).
The frequency of CNS and PNS dysfunction outcomes in our propensity score matched cohorts, stratified by treatment and era, are presented in Table 2. Among fluoroquinolone users, the 120-day cumulative incidence of CNS dysfunction was 0.41% in era 1 (January 1, 2000 to September 14, 2004), 1.09% in era 2 (September 15, 2004 to September 30, 2015), and 0.95% in the combined cohort. Of the four PNS dysfunction outcomes, the highest cumulative incidences among fluoroquinolone users were for the symptoms outcome (1.42% in era1 and 2.99% in era 2) and symptoms + EPT outcome (0.26% in era 1 and 0.31% in era 2). For all CNS and PNS dysfunction outcomes across both eras, cumulative incidence was similar but numerically greater in the fluoroquinolone group vs. the comparator antibiotic group (Table 2).
TABLE 2.
Frequency and incidence rates for outcomes by exposure group
| Combined cohort | Era 1 (January 1, 2000–September 14, 2004) | Era 2 (September 15, 2004–September 30, 2015) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| FQ N = 976 568 | Comparator N = 976 568 | FQ N = 195 207 | Comparator N = 195 207 | FQ N = 781 361 | Comparator N = 781 361 | |
| CNS dysfunction | ||||||
| Number of events (cumulative incidence) | 8814 (0.95%) | 8181 (0.88%) | 755 (0.41%) | 689 (0.37%) | 8059 (1.09%) | 7492 (1.01%) |
| Incidence rate per 100 000 person-days | 8.08 | 7.50 | 3.45 | 3.14 | 9.24 | 8.59 |
| PNS 1: Symptoms | ||||||
| Number of events (cumulative incidence) | 24 660 (2.68%) | 22 728 (2.47%) | 2604 (1.42%) | 2365 (1.29%) | 22 056 (2.99%) | 20 363 (2.77%) |
| Incidence rate per 100 000 person-days | 22.81 | 20.73 | 11.94 | 10.83 | 25.55 | 23.57 |
| PNS 2: Symptoms + peripheral neuropathy diagnosis | ||||||
| Number of events (cumulative incidence) | 736 (0.08%) | 638 (0.07%) | 54 (0.03%) | 44 (0.02%) | 682 (0.09%) | 594 (0.08%) |
| Incidence rate per 100 000 person-days | 0.67 | 0.58 | 0.25 | 0.20 | 0.78 | 0.68 |
| PNS 3: Symptoms + EPT | ||||||
| Number of events (cumulative incidence) | 2756 (0.30%) | 2504 (0.27%) | 474 (0.26%) | 473 (0.26%) | 2282 (0.31%) | 2031 (0.28%) |
| Incidence rate per 100 000 person-days | 2.52 | 2.29 | 2.16 | 2.16 | 2.61 | 2.32 |
| PNS 4: Symptoms + peripheral neuropathy diagnosis + EPT | ||||||
| Number of events (cumulative incidence) | 252 (0.03%) | 206 (0.02%) | 28 (0.02%) | 27 (0.01%) | 224 (0.03) | 179 (0.02%) |
| Incidence rate per 100 000 person-days | 0.23 | 0.19 | 0.13 | 0.12 | 0.26 | 0.20 |
Note: Cumulative incidences were calculated at 120 days.
Abbreviations: CNS, central nervous system; EPT, electrophysiological testing; FQ, fluoroquinolone; PN, peripheral neuropathy; PNS, peripheral nervous system.
Propensity score-matched HRs for CNS and PNS dysfunction are presented in Table 3. Analyses were conducted in the combined cohorts for the CNS dysfunction and PNS symptoms outcomes because the difference in era 1 and era 2 HRs for these two outcomes, but not the other outcomes (PNS symptoms + PN diagnosis, symptoms + EPT, and symptoms + PN diagnosis + EPT), differed by less than 5%. The HR for CNS dysfunction in the combined cohort was 1.08 (95% CI: 1.05, 1.11) and that for PNS symptoms in the combined cohort was 1.09 (95% CI: 1.07, 1.11). HRs for the PNS symptoms + PN diagnosis outcome were 1.23 (95% CI: 0.83, 1.83) in era 1 and 1.15 (95% CI: 1.03, 1.28) in era 2. For the symptoms + EPT outcome, HRs were 1.00 (95% CI: 0.88, 1.14) in era 1 and 1.12 (95% CI: 1.06, 1.19) in era 2. And for the symptoms + PN diagnosis + EPT outcome, HRs were 1.04 (95% CI: 0.61, 1.76) in era 1 and 1.25 (95% CI: 1.03, 1.52) in era 2.
TABLE 3.
Cox proportional-hazards regression results for central and peripheral nervous system dysfunction
| Combined cohort | Era 1 (January 1, 2000-September 14, 2004) | Era 2 (September 15, 2004-September 30, 2015) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Outcome | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value |
| CNS Dysfunction | 1.08a | 1.05, 1.11 | <0.001 | 1.10 | 0.99, 1.22 | 0.08 | 1.08a | 1.04, 1.11 | <0.001 |
| PNS 1: Symptoms | 1.09 | 1.07, 1.11 | <0.001 | 1.10 | 1.04, 1.17 | <0.001 | 1.08 | 1.06, 1.10 | <0.001 |
| PNS 2: Symptoms + PN | - | - | - | 1.23a | 0.83, 1.83 | 0.31 | 1.15a | 1.03, 1.28 | 0.01 |
| PNS 3: Symptoms + EPT | - | - | - | 1.00a | 0.88, 1.14 | 0.97 | 1.12 | 1.06, 1.19 | <0.001 |
| PNS 4: Symptoms + PN + EPT | - | - | - | 1.04a | 0.61, 1.76 | 0.89 | 1.25a | 1.03, 1.52 | 0.03 |
Note: In combined cohort, Cox proportional-hazards regression analysis was only conducted for CNS dysfunction outcome and PNS symptoms outcome.
Abbreviations: CNS, central nervous system; EPT, electrophysiological testing; PN, peripheral neuropathy; PNS, peripheral nervous system.
Proportional hazards assumption failed and categorical time-varying analysis was performed (Table S8).
The Cox proportional-hazards assumption was satisfied in both eras and in the combined cohort for the PNS symptoms outcome, but the assumption failed in one or both eras for all other outcomes (Figures S2 and S3). Time-varying HRs are presented in Table S8.
4 |. DISCUSSION
This study found that fluoroquinolones were associated with elevated hazards of both CNS and PNS dysfunction. Specifically, we found an association between fluoroquinolones and CNS dysfunction across both eras and in the combined cohort. Fluoroquinolones were also associated with the PNS symptoms outcome across both eras and in the combined cohort, and the PNS symptoms + PN diagnosis, PNS symptoms + EPT, and PNS symptoms + PN diagnosis + EPT outcomes in era 2 only (combined cohort analyses were not performed).
Consistent with prior studies, we found a modest elevated risk (HR: 1.08 and 95% CI: 1.05–1.11 in combined cohort) of CNS dysfunction with fluoroquinolone exposure. A meta-analysis of RCTs found that fluoroquinolones were associated with CNS adverse outcomes with an odds ratio of 1.49 versus macrolides and 1.90 versus amoxicillin plus clavulanic acid.10 Also consistent with prior studies, mentioned in the background section, we found an elevated risk of PNS dysfunction among fluoroquinolone users (ex. HR of 1.09 for symptoms in the combined cohort).
The magnitude of the absolute risk difference associated with fluoroquinolone exposure may be informative for clinical decision making. We found that fluoroquinolones were associated with an increased hazard of CNS dysfunction of 8% in the combined cohort and an increased hazard of 9% for the PNS symptoms outcome in the combined cohort. Given a 120-day cumulative incidence of CNS dysfunction in the combined cohort of 8.8 per thousand individuals (0.88%) in users of comparator antibiotics and 9.5 per thousand individuals (0.95%) in users of fluoroquinolones (Table 2), an 8% relative increase in the incidence rate results in an absolute risk difference of 0.68 per thousand, and thus a number needed to harm (NNH) of 1471. This NNH measure indicates that if the observed association is causal, for every 1471 people receiving a fluoroquinolone instead of a comparator antibiotic, one additional individual will experience CNS dysfunction. Similarly, given the 120-day incidence of PNS symptoms of 24.7 per thousand (2.47%) in users of comparator antibiotics and 26.8 per thousand (2.68%) in users of fluoroquinolones (Table 2) and a relative increase of 9% gives a risk difference of 2.1 per thousand and a NNH of 476.
We performed analyses allowing for time-varying HRs for all outcomes in one or both eras except for our PNS symptoms outcome. For CNS dysfunction, the time-varying analysis revealed a null association in the earliest time window (0–30 days) in era 2 and the combined cohort. This might suggest either a delay in the onset of CNS symptoms after fluoroquinolone exposure, or a delayed diagnosis of CNS symptoms. We also found a positive association in era 2 with the PNS symptoms + PN diagnosis outcome, and the PNS symptoms + PN diagnosis + EPT outcome. HRs for these outcomes varied widely and inconsistently across time-windows in era 1, although there was suggestion of a possible association in era 1 of our original analysis with all point estimates being one or greater (Table 3). These inconsistent results might be explained by the very low number of events captured for PNS outcomes. That is, there may be too few events to permit detection of an association between fluoroquinolone exposure and study outcomes in this smaller era. The differences seen in relative associations by era might also point to the possibility of surveillance bias. For example, fluoroquinolones were associated with the PNS symptoms + EPT outcome in era 2, but not in era 1. Perhaps individuals in era 2, post-FDA safety communication, were screened more for PNS symptoms using EPT or diagnosed with symptoms more than individuals in era 1, prior to the FDA safety communication.
Our study has limitations. We used claims data, so some outcomes may not have been captured due to errors in coding. However, we do not have reason to believe that coding errors would differ between fluoroquinolone and the comparator groups. Diagnostic suspicion may have led to differential outcome ascertainment between the fluoroquinolone and comparator groups; therefore, we stratified our analyses into two different eras in order to evaluate the possibility of this bias. After stratification, we found potential evidence of this bias and observed differences in association in PNS but not the CNS outcomes between the two eras. Additionally, we did not have data on antibiotic adherence so we cannot be sure that individuals adhered to their prescribed medications. Our CNS and PNS outcomes codes have also not been validated. However, our outcome definitions were determined with guidance from a neurologist-epidemiologist, and our PNS dysfunction outcomes were designed to complement each other with varying sensitivity and specificity. We also note that individuals with an index prescription for one of our comparator antibiotics could have been prescribed a fluoroquinolone antibiotic during follow-up, which was not reflected in our analysis. However, in order to minimize bias due to post-treatment characteristics, we have attempted to emulate an intention-to-treat analysis, with individuals analyzed according to the exposure group that they were originally part of. Finally, while we were only able to capture variables available from insurance billing information, we were able to note important confounders of interest using our data source.
5 |. CONCLUSION
This study provides evidence that fluoroquinolone users are at an increased risk of being diagnosed with acute CNS and PNS disorders relative to comparator antibiotic users prescribed antibiotics for the same clinical indication. However, the absolute risk is modest, and the absolute cumulative incidence of these disorders are generally low. Use of fluoroquinolone antibiotics should be guided by both clinical data on microbial susceptibility and the goal of avoiding adverse neurological and other outcomes, although further research is required in order to understand those fluoroquinolone-users who are at highest risk.
Supplementary Material
Key Points.
We evaluated the association between fluoroquinolone antibiotics and central (CNS) and peripheral nervous system (PNS) dysfunction in a commercially insured U.S. adult population.
We compared fluoroquinolone users to comparator antibiotic users prescribed antibiotics for the same clinical indications using propensity score matching.
Fluoroquinolone users were at an increased risk of being diagnosed with acute CNS and PNS disorders relative to comparator antibiotic users.
ACKNOWLEDGMENTS
The authors thank Q. Liu from the University of Pennsylvania for her help with preparation of the analytic dataset. This project was supported by NIH F31 grant 1F31 NS103445 from the National Institute of Neurological Disorders and Stroke. This study was approved by the Institutional Review Board (IRB) at the University of Pennsylvania. No prior submissions or presentations have been done on this study.
Funding information
National Institute of Neurological Disorders and Stroke, Grant/Award Number: NIH F31 grant 1 F31 NS103445
Footnotes
CONFLICT OF INTEREST
Dr. Hennessy has received grant support from Pfizer, Sanofi, and Johnson and Johnson, and has consulted for Mallinckrodt, Sage, Medullary Thyroid Cancer Consortium (Novo Nordisk, AstraZeneca, GlaxoSmithKline, Eli Lilly), Merck, Nektar Therapeutics, Esteve Pharmaceuticals, Novo Nordisk, Arbor Pharmaceuticals, Biogen and Intercept Pharmaceuticals, all unrelated to this submitted work. Dr. Hubbard has received grant support from Pfizer, Merck, and Johnson and Johnson unrelated to this work. All other authors listed have no disclosures or conflicts of interest to declare.
ETHICS STATEMENT
The Institutional Review Board (IRB) at the University of Pennsylvania determined that this research met eligibility criteria for IRB review exemption.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Kabbani S, Hersh AL, Shapiro DJ, Fleming-Dutra KE, Pavia AT, Hicks LA. Opportunities to improve Fluoroquinolone prescribing in the United States for adult ambulatory care visits. Clin Infect Dis.2018;67(1):134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hicks LA, Taylor TH Jr, Hunkler RJ. US outpatient antibiotic prescribing, 2010. N Engl J Med. 2013;368(15):1461–1462. [DOI] [PubMed] [Google Scholar]
- 3.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316. [DOI] [PubMed] [Google Scholar]
- 4.FDA requires label changes to warn of risk for possibly permanent nerve damage from antibacterial fluoroquinolone drugs taken by mouth or by injection. Silver Spring, MD: U.S. Food and Drug Administration; 2013. https://www.fda.gov/media/86575/download. [Google Scholar]
- 5.Wang SH, Xie YC, Jiang B, et al. Fluoroquinolone associated myasthenia gravis exacerbation: clinical analysis of 9 cases. Zhonghua Yi Xue Za Zhi. 2013;93(17):1283–1286. [PubMed] [Google Scholar]
- 6.Francis JK, Higgins E. Permanent peripheral neuropathy: a case report on a rare but serious debilitating side-effect of Fluoroquinolone administration. J Investig Med High Impact Case Rep. 2014;2(3):2324709614545225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peripheral Neuropathy Information Page. 2019; https://www.ninds.nih.gov/disorders/all-disorders/peripheral-neuropathyinformation-page.
- 8.Joint Meeting of the Antimicrobial Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. Silver Spring, MD: U.S. Food and Drug Administration; 2015. http://wayback.archive-it.org/7993/20170113234749/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Anti-InfectiveDrugsAdvisoryCommittee/UCM472655.pdf. [Google Scholar]
- 9.FDA Updates Warnings for Fluoroquinolone Antibiotics. Silver Spring, MD: U.S. Food and Drug Administration; 2016. https://www.fda.gov/news-events/press-announcements/fda-updates-warningsfluoroquinolone-antibiotics. [Google Scholar]
- 10.Tandan M, Cormican M, Vellinga A. Adverse events of fluoroquinolones vs. other antimicrobials prescribed in primary care: a systematic review and meta-analysis of randomized controlled trials. Int J Antimicrob Agents. 2018;52(5):529–540. [DOI] [PubMed] [Google Scholar]
- 11.Etminan M, Brophy JM, Samii A. Oral fluoroquinolone use and risk of peripheral neuropathy: a pharmacoepidemiologic study. Neurology. 2014;83(14):1261–1263. [DOI] [PubMed] [Google Scholar]
- 12.Morales D, Pacurariu A, Slattery J, Pinheiro L, McGettigan P, Kurz X. Association between peripheral neuropathy and exposure to oral Fluoroquinolone or Amoxicillin-Clavulanate therapy. JAMA Neurol. 2019; 76(7):827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee MT et al. Comparative effectiveness of different oral antibiotics regimens for treatment of urinary tract infection in outpatients: an analysis of national representative claims database. Medicine (Baltimore). 2014;93(28):e304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Retrospective Database Analysis. Eden Prairie, MN: Optum; 2013. https://www.optum.com/content/dam/optum/resources/productSheets/Retrospective-Database-Analysis.pdf. [Google Scholar]
- 15.Copp HL, Yiee JH, Smith A, Hanley J, Saigal CS, Urologic Diseases in America Project. Use of urine testing in outpatients treated for urinary tract infection. Pediatrics. 2013;132(3):437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suskind AM, Saigal CS, Hanley JM, et al. Incidence and management of uncomplicated recurrent urinary tract infections in a national sample of women in the United States. Urology. 2016;90:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piccirillo JF, Mager DE, Frisse ME, Brophy RH, Goggin A. Impact of first-line vs second-line antibiotics for the treatment of acute uncomplicated sinusitis. JAMA. 2001;286(15):1849–1856. [DOI] [PubMed] [Google Scholar]
- 18.Rothberg MB, Pekow PS, Lahti M, Brody O, Skiest DJ, Lindenauer PK. Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303(20):2035–2042. [DOI] [PubMed] [Google Scholar]
- 19.Cohen JS. Peripheral neuropathy associated with fluoroquinolones. Ann Pharmacother. 2001;35(12):1540–1547. [DOI] [PubMed] [Google Scholar]
- 20.Ali AK. Peripheral neuropathy and Guillain-Barre syndrome risks associated with exposure to systemic fluoroquinolones: a pharmacovigilance analysis. Ann Epidemiol. 2014;24(4):279–285. [DOI] [PubMed] [Google Scholar]
- 21.Staff NP et al. Chemotherapy-induced peripheral neuropathy: a current review. Ann Neurol. 2017;81(6):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brewer JR, Morrison G, Dolan ME, Fleming GF. Chemotherapy-induced peripheral neuropathy: current status and progress. Gynecol Oncol. 2016;140(1):176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khokhar B, Jette N, Metcalfe A, et al. Systematic review of validated case definitions for diabetes in ICD-9-coded and ICD-10-coded data in adult populations. BMJ Open. 2016;6(8):e009952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amaize AEA. Emergency Department Visits for Children and Young Adults With Diabetes, 2012. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs 2016. [Google Scholar]
- 25.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 26.Chung T, Prasad K, Lloyd TE. Peripheral neuropathy: clinical and electrophysiological considerations. Neuroimaging Clin N Am. 2014;24(1):49–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghtaderi A, Bakhshipour A, Rashidi H. Validation of Michigan neuropathy screening instrument for diabetic peripheral neuropathy. Clin Neurol Neurosurg. 2006;108(5):477–481. [DOI] [PubMed] [Google Scholar]
- 28.Bril V, Ellison R, Ngo M, et al. Electrophysiological monitoring in clinical trials. Roche Neuropathy Study Group. Muscle Nerve. 1998;21(11): 1368–1373. [DOI] [PubMed] [Google Scholar]
- 29.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.